Abstract
Wild rocket (Diplotaxis tenuifolia) has become a very popular salad leaf due to its peppery taste. It is part of the Brassicaceae family and thus has a high level of homology at the DNA level to other Brassica species including Arabidopsis thaliana. The vernalization and photoperiodic requirements of wild rocket have not been reported to date. Photoperiodic experiments described here demonstrate that rocket is a facultative long day plant. To investigate the vernalization requirement, both seed and young plants were given vernalization treatments at 4 °C for different lengths of time. A rocket homologue of FLOWERING LOCUS C (DtFLC) was isolated and shown to functionally complement the Arabidopsis FRI+flc3 null mutant. Whilst the expression of DtFLC was significantly reduced after just one week of cold treatment, cold treatments of two to eight weeks had no significant effect on bolting time of wild rocket indicating that rocket does not have a vernalization requirement. These findings illustrate that important fundamental differences can exist between model and crop plant species, such as in this case where down-regulation of DtFLC expression does not enable earlier flowering in wild rocket as it does in Arabidopsis and many other Brassica species.
Keywords: Bolting time, Diplotaxis tenuifolia, Photoperiod, Vernalization, Wild rocket
1. Introduction
Wild rocket (Diplotaxis tenuifolia) has increased in popularity over the last 20 years in the leafy salads market (Chun et al., 2013), in the UK alone over 80 tons of rocket is consumed per week (Gill, 2008). The genus Diplotaxis is found in the Brassicaceae family in the Oleracea clade (Arias and Pires, 2012) and is therefore closely related to species Brassica rapa, Brassica juncea, Brassica napus and Brassica oleracea, as well as Arabidopsis thaliana (Arabidopsis). Flowering is undesirable in commercial rocket production as pre-harvest flowering can lead to the crop being unsaleable (Fig. 1a), despite this very little work has been reported on the control of flowering in wild rocket. Here we investigate the vernalization and photoperiodic requirements of this relatively new salad crop species.
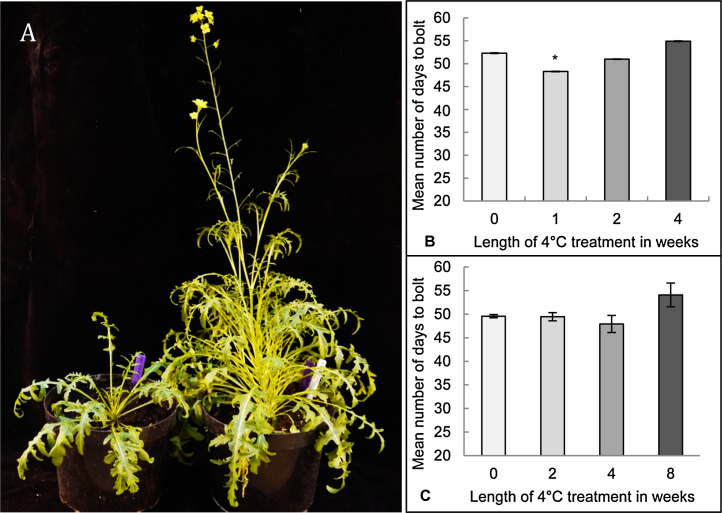
Fig. 1.
Effect of vernalization at 4 °C on D.tenuifolia.
A) Photo of a non-bolting rocket plant (left) and one that has bolted and flowered (right).
B) The mean number of days to bolt of plants where the seed was subjected to cold treatment at 4 °C for one, two or four weeks. Bars show mean ± SE (n = 14 (0 weeks), n = 15 (1 week and 2 weeks), n = 10 (4 weeks)). Student’s t-test was used to compare the number of days to bolt of each vernalization treatment (one, two and four weeks) against the ambient conditions (0 weeks). *Statistical significance of p < 0.05
C) Mean number of days to bolt for 4 week old plants subjected to cold treatment at 4 °C for two, four and eight weeks. The length of the vernalization treatment was subtracted from the number of days to bolt. Bars show mean ± SE (n = 14 (0 weeks), n = 15 (2 weeks and 4 weeks), n = 13 (8 weeks)). Student’s t-test was used to compare the number of days to bolt of each vernalization treatment (2, 4 and 8 weeks) against the ambient conditions (0 weeks). There was no significant difference in number of days to bolt between vernalization treatments and ambient conditions (p < 0.05)
Regulation of flowering time involves a complex gene network which allows a plant to respond to both internal and external signals in order to control the timing of the transition from the vegetative phase to the reproductive phase (Srikanth and Schmid, 2011). The timing of this transition is crucial to enable reproduction to occur when the plant is at its fittest and the environmental conditions are most favorable (Thomas et al., 2006). Most research into flowering time has been conducted in the model plant Arabidopsis through the generation and testing of many mutant and transgenic overexpression lines to elucidate gene function (Corbesier and Coupland, 2005). Using the knowledge gained from Arabidopsis and applying it to crop species is helping our understanding of the control of flowering in crop plants. Currently there are six key pathways that regulate flowering time: the photoperiodic, autonomous, vernalization, gibberellic acid, age dependent and ambient temperature pathways (Brambilla and Fornara, 2013, Fornara et al., 2010, Jarillo and Pineiro, 2011, Srikanth and Schmid, 2011). All of these pathways converge on the floral pathway integrator genes such as FLOWERING LOCUS T and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 which activate the downstream floral meristem identity genes. When flowering is induced the shoot apical meristem changes from forming vegetative tissues such as leaves to form flowers.
Vernalization is the attainment or acceleration of floral competence as a consequence of prolonged exposure to cold temperature (Chouard, 1960). In vernalization-responsive plant species, vernalization conveys the ability of the plant to flower and can greatly reduce the time it takes to flower (Guo et al., 2004, Hackett and Hartmann, 1967, Jung and Mueller, 2009, Strange et al., 2011). The cold treatment causes epigenetic changes that reduce levels of a floral repressor gene FLOWERING LOCUS C (FLC). When the plant is returned to a higher temperature these epigenetic changes are stable enough to maintain the repression of FLC expression and the reduced levels of this repressor thus enables flowering to be induced (Boss et al., 2004). Studies of Arabidopsis ecotypes show that there are differences in the requirement for vernalization. Rapid cycling ecotypes will flower without the need for a vernalization treatment, whereas winter annual ecotypes will flower very late unless exposed to a period of vernalization (Nordborg and Bergelson, 1999, Wang, 2014). It was found that these vernalization response phenotypes all relate to the levels of expression of FLC (Song et al., 2012). Plants which have a requirement for vernalization tend to be those from seasonal climates where adaptation to extended periods of cold can be advantageous. The stage in the plant’s lifecycle which is responsive to vernalization varies between species as does the length of treatment needed in order to fully satisfy the vernalization requirement. Arabidopsis is able to respond to vernalization as both seed and young plants (Nordborg and Bergelson, 1999, Strange et al., 2011), whereas many Brassica species only respond to a vernalization treatment as young plants (Jung and Mueller, 2009). Rocket is closely related to both Arabidopsis and Brassica and it is not currently known at which stage of its lifecycle it is able to respond, what the optimum vernalization period might be, or even if it has a vernalization response at all.
The ability of a plant to measure and respond to the length of day, or photoperiod, is often critical to its growth and development, particularly in the timing of the floral transition. Most plants fall into one of three main photoperiodic categories; long day (LD) plants which flower when the photoperiod is longer than a critical length, short day (SD) plants which flower when the photoperiod is shorter than a critical length, and day neutral plants which flower regardless of the photoperiod (Matsoukas et al., 2012). LD plants flower quite quickly in LD, but if subjected to SD conditions they will flower much more slowly (facultative response) or not at all (obligate response), the opposite being true for SD plants.
Understanding the vernalization and photoperiod requirements of commercial crops, and the underlying genes that control these responses in these crops, is of huge benefit to crop breeding programs aiming to create varieties that can be grown successfully in a range of latitudes and climatic conditions. In this paper, we investigate the photoperiodic and vernalization responses of D.tenuifolia. We show that wild rocket is a facultative long day plant and that it does not respond to vernalization treatments of several weeks duration even though the expression of the rocket FLC orthologue is strongly reduced by these treatments.
2. Materials and methods
2.1. Plant material
Elsoms Seeds Ltd provided seed of Diplotaxis tenuifolia bred through four generations of single seed descent for uniformity of leaf shape, color, and bolting time. Arabidopsis thaliana mutant FRI+flc3 null (Col0 background) seed was originally sourced from NASC (http://arabidopsis.info/) and used in the complementation experiments.
2.2. DNA and RNA extraction
Rocket genomic DNA was extracted using CTAB extraction method adapted from Stewart and Via (1993) on frozen leaf material, which was ground to a powder using a Dremel Drill with a 1.5 ml Eppendorf tube drill bit. 300 μl CTAB B buffer (100 mM Tris/Cl pH 8.0, 1.4 M NaCl, 20 mM EDTA, 2% hexadecyltrimethyl ammoniumbromide) was added and homogenized. Samples were incubated at 65 °C for 30 min and centrifuged. The supernatant was removed into a new tube and twice extracted using 300 μl Chloroform:Isoamyl alcohol (24:1) and centrifugation. The aqueous top phase was transferred each time. 300 μl CTAB C buffer (1% hexadecyltrimethyl ammoniumbromide, 10 mM EDTA, 50 mM Tris/Cl pH 8.0) was added and left overnight at room temperature. On day two, the tubes were centrifuged and the pellet dissolved in 400 μl 1 M CsCl. DNA is then precipitated using 100% Ethanol and centrifuged. The pellet was then washed twice using 70% Ethanol before drying and resuspending in 25 μl TE buffer (pH 8) with RNase A (Invitrogen) (20 μg/ml).
RNA extraction was done using the Z6 extraction buffer (containing 8 M guanidine hydrochloride) method (Logemann et al., 1987). Frozen leaf, or imbibed seed, samples were ground to a powder using liquid N2 and a pestle and mortar. The powder was transferred to a 1.5 ml Eppendorf tube and Z6 buffer plus 2-mecaptoethanol (50 mM final concentration) added. This was homogenized using a Dremel Drill with a 1.5 ml Eppendorf tube drill bit before the rest of the published method was followed. 5 μg RNA was DNase treated using Ambion® Turbo DNA-free™ DNase and then resuspended in 12 μl DEPC treated H2O. 1 μg of DNase treated RNA was synthesized into cDNA using an Invitrogen Thermoscript cDNA synthesis kit or BioRad iScript cDNA synthesis kit. Arabidopsis RNA extraction was performed using the same method, but with a reduction in the volume of reagents used due to the smaller amount of starting leaf material.
2.3. Vernalization experiments
D. tenuifolia seed was sown onto damp paper towels and covered with aluminum foil and placed at 4 °C for one, two or four weeks. At the end of the vernalization period, seeds were removed from the paper and put onto F2S soil (Levington) in P40 trays at one seed per cell and placed in the glasshouse at 20 °C with a 16 h photoperiod. The germination date was recorded. Control seed to stay at ambient temperature was sown in the same way but was placed into the glasshouse at 20 °C for one week by which time the seeds had germinated. These were then transferred to F2S soil in P40 trays at one seed per cell. At four weeks post germination, plants were transplanted into 5 inch pots of M2 soil (Levington) to continue growing. In the plant experiment, seed was sown directly to F2S soil (Levington) in P40 cells and put in a controlled environment cabinet (MLR-352, Panasonic Co. Ltd) at 20 °C to aid germination with 16 h photoperiod for five days (light level 104 μmol m−2 s−1). The germination date was recorded and plants grown until three weeks old in a glasshouse at 20 °C with 16 h photoperiod. These were then transplanted to 5 inch pots of M2 soil (Levington). After one week (four weeks after germination), the plants were moved to 4 °C with 16 h photoperiod to vernalize for two, four or eight weeks. The plants were then returned to glasshouse conditions. Control plants were kept at 20 °C with 16 h photoperiod (ambient conditions) for the entirety of the experiment. Plant material was harvested at the end of each week at 9 h after lights on. The number of days from germination to bolting was recorded, bolting was taken as the point when the height of the bolt was about 10 cm and is the measurement normally used by growers and industry to tell whether a plant has bolted. As the plants were grown in controlled environment most plants in a given treatment bolted around the same time. It is known that in Arabidopsis FLC is expressed in seed, shoot and root tissue (Chiang et al., 2009, Michaels and Amasino, 1999) so for analysis of FLC expression all material above soil level was sampled from up to six plants for the seed vernaliszation experiment and for weeks 1–3 of the plant experiment,. From week 4 onwards of the plant vernalization experiment when the plants were larger newly expanded leaves were collected from five plants and pooled together.
2.4. Photoperiod experiments
Rocket seeds were sown onto F2S soil (Levington) in p24 trays and topped with vermiculite. These were placed into a controlled environment cabinet (MLR-352, Panasonic Co. Ltd) at 22 °C with 16 h light, 8 h dark. The germination date was recorded and at 10 days after sowing, plants were moved into the different photoperiods. These were short day conditions with 8 h light and 16 h dark, intermediate day conditions with 12 h light and 12 h dark, and long day conditions with 16 h light and 8 h dark. Plants were grown in the controlled environment cabinets under fluorescent light (light levels 124 μmol m−2 s−1). Plants were transplanted at three weeks from sowing into 5 inch pots of M2 soil (Levington) and returned to the correct photoperiod and time to bolting was recorded for each plant. A second experiment was carried out using incandescent bulbs (Bell Striplite, 30 W, light levels 33 μmol m−2 s−1) to provide the day extension lighting. The experimental design was as described above but with short day (8:16) conditions with 8 h fluorescent bulb light and 16 h dark, intermediate day conditions (12:12) with 8 h fluorescent bulb light + 4 h incandescent bulb day extension and 12 h dark, and long day conditions (16:8) with 8 h fluorescent bulb light +8 h incandescent bulb day extension and 8 h dark.
2.5. Isolation of the DtFLC gene from D. tenuifolia
Degenerate primers for FLC were designed using aligned sequences for Arabidopsis and Brassica FLC genes and used to PCR amplify DtFLC using D. tenuifolia cDNA as the template. Specific DtFLC primers (Supplementary data Table 1A) were then designed to isolate the remaining sequence using RLM RACE (Invitrogen). 5′ and 3′ UTR primers specific to DtFLC (see supplementary data for sequences) were used to amplify the gene from rocket genomic DNA and cDNA. The gene sequence data has been submitted to the GenBank database under accession number KX148480.
2.6. Functional complementation in Arabidopsis
The DtFLC coding sequence was isolated from cDNA using primers which incorporated the start and stop codons. This was cloned into pGEM-T easy and amplified out using GATEWAY™ (Invitrogen) adapter primers specific to DtFLC (see supplementary data for sequences). Using BP clonase (Invitrogen), this was cloned into pDONR 207 and then pB2GW7 using LR clonase (Invitrogen). The sequence was verified before DtFLC containing pB2GW7 plasmid DNA was transformed into Agrobacterium tumefaciens strain c58pGV3101. 500 ml LB (10:5:5) plus 25 μg/ml Gentamycin, 100 μg/ml Spectinomycin and 12.5 μg/ml Rifampicin was inoculated using 5 ml cell culture of A. tumefaciens strain c58pGV3101 containing the DtFLC-pB2GW7 plasmid. This was incubated at 28 °C for 16 h. The culture was centrifuged and the supernatant removed. 500 ml 5% (w/v) sucrose solution was used to resuspend the cells and 100 μl silwet L-77 added before dipping the inflorescences of the Arabidopsis FRI+flc3 null plants (Clough and Bent, 1998). The plants were sealed in a bag for 24 h before putting at 22 °C with 16 h light, 8 h dark in the glasshouse facility. T1 seed was harvested and sown onto soil (Levington F2S:sand:vermiculite fine grade 6:1:1). BASTA (Ammonium glyfosinate (150 g/L)) soil soaking was used at 1:1000 as the selection method. The first treatment was given and the trays were covered and placed at 4 °C for three days. These were removed and put under a propagator lid in a 16 h photoperiod at 22 °C. Four further treatments were done before transplanting. Transformed plants were transplanted into individual pots and leaf number and number of days to bolt were recorded when the primary bolt was 1 cm. Seed was collected from plants showing the expected complementation phenotype and sown onto soil. The pots were stratified at 4 °C for 3 days before being placed at 22 °C with 16 h photoperiod in controlled environment cabinets (MLR-352, Panasonic Co. Ltd). The number of days to flower and rosette leaf number at 1 cm bolt were recorded. Leaf material was collected and RNA extracted as outlined above.
2.7. Real time RT-PCR
Real time RT-PCR was performed on samples from plants during the rocket vernalization experiments and also on the Arabidopsis DtFLC complementation T2 plants. To measure the expression of DtFLC in rocket from the vernalization experiments, a mixture of RNA from samples where DtFLC expression was likely to be highest, were combined to synthesize cDNA to make the standard for the real time reactions. The standard was diluted 10-fold from 100–10−4 to make the standard curve. All real time RT-PCR carried out for the vernalization samples used DtTIP41, DtCACS and Dtα-tubulin as housekeeping genes for normalization (Supplementary data Table 2A). To measure the expression of the DtFLC gene in Arabidopsis FRI + flc3 null plants, AtActin, AtTIP41 and Atβ-tubulin were used as housekeeping genes for normalization. The standard was made from PCR products amplified from cDNA using real time primer pairs for the housekeeping genes, and DtFLC (see supplementary data for sequences). These were combined and purified using a PCR purification kit (Qiagen) before diluting from 100 to 10−10 for the standard curve. cDNA synthesized for each sample was used with the reaction mix iTaq™Universal SYBR® Green Supermix (Bio-Rad Laboratories Ltd., UK). All real time PCR experiments were run on a CFX384 TouchTM Real-time PCR machine (Bio-Rad Laboratories Ltd., UK) using Bio-Rad CFX manager 3.0 software. Results were analyzed using Biogazelle qBase Plus software version 2.5 (http://www.biogazelle.com/qbaseplus).
3. Results
3.1. The effect of vernalization on flowering of D.tenuifolia
To investigate the vernalization response of wild rocket, both seed and young plants were tested for their response to different lengths of vernalization treatment at 4 °C. D.tenuifolia seed were subjected to a vernalization treatment of one, two or four weeks at 4 °C in the dark before being grown under ambient conditions (20 °C, 16 h photoperiod) in the glasshouse until they initiated bolting. The number of days to bolting was recorded starting from when the seed were taken out of the vernalization treatment (i.e. the time the seed spent in the vernalization treatment was not included). Control seed were not vernalized and were grown in ambient conditions for the duration of the experiment. There was no great effect on bolting time of the different lengths of vernalization treatment at 4 °C, except for a slight reduction of 4 days after one week of treatment which was not observed after two or four weeks of treatment (Fig. 1b). The effect of a 4 °C vernalization treatment on young four week old plants was also tested. The plants were subjected to two, four or eight weeks of cold treatment whilst a subset of control plants remained at constant ambient temperature throughout the experiment. There was no significant reduction in bolting time between the non-vernalized plants kept at ambient temperatures to those subjected to the different vernalization treatments (Fig. 1c). The plants which had been subjected to eight weeks of vernalization in fact bolted slightly later (approximately five days) than the control plants and the other vernalization treatments.
As wild rocket is a Mediterranean plant it may have a higher optimum vernalization temperature. To test this, a higher vernalization temperature of 10 °C was used for the vernalization treatment of both seed and young four week old plants. Overall the results mirror those subjected to a 4 °C vernalization as there was no great difference in bolting time between those kept under ambient conditions and the different vernalization treatments, although a small reduction in bolting time was observed following the six week treatment of seed (Supplementary data Fig. S1a). Vernalization of young plants at 10 °C did not reduce bolting time compared to non-vernalized plants kept at ambient temperature; indeed this treatment seemed to cause a delay in bolting (Supplementary data Fig. S1b).
3.2. Isolation of a rocket FLC gene
We isolated an FLC homologue from wild rocket to investigate whether expression of this gene changed in response to the vernalization treatment. PCR using primers designed to Arabidopsis and Brassica FLC genes gave fragments that had a sequence of an FLC-like gene, so specific RLM RACE (Invitrogen) primers were designed. This allowed the isolation of the full genomic sequence of the DtFLC gene revealing that it contained 7 exons and 6 introns (Fig. 2a). The intron sizes differ to that of Arabidopsis FLC but the exon sizes and placement are very similar (Michaels and Amasino, 1999).
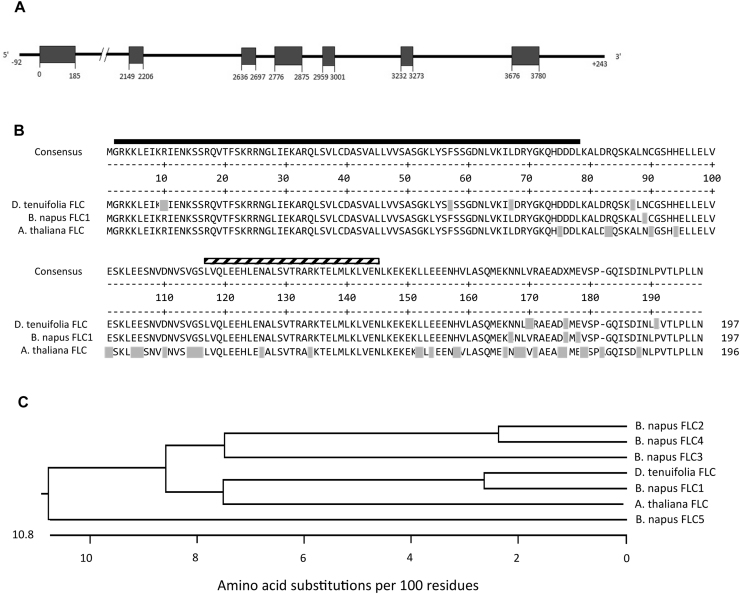
Fig. 2.
DtFLC gene and protein.
A) Structure of DtFLC gene from D.tenuifolia. Boxes represent exons and lines represent introns and UTR.
B) DtFLC protein alignment with B.napus FLC1 and A.thaliana FLC proteins. Grey shading identifies the residues that differ to the consensus sequence shown above. The black line denotes the MADS box domain and the hatched bar denotes the K-box motif.
C) Phylogenetic tree showing the relationship between DtFLC, AtFLC and the five B.napus FLC proteins (1–5). DtFLC is most closely related to B.napus FLC1.
The full length coding sequence for DtFLC has 94% identity to the B.napus FLC1 and Sinapis alba FLC genes and 86% identity to Arabidopsis thaliana FLC. The translated protein sequence has 95% identity to B.napus FLC1 and S.alba FLC, and 84% identity to A.thaliana FLC, and contains a MADS box at the N-terminus and a K-box domain in the middle of the sequence (Fig. 2b). Phylogenetic comparison of DtFLC protein sequence with that of AtFLC and the five known FLC-like proteins of B.napus (FLC1-5) shows that DtFLC is most closely related to B.napus FLC1 (Fig. 2c).
3.3. Functional complementation of the Arabidopsis FRIGIDA (FRI)+flc3 null mutant with DtFLC
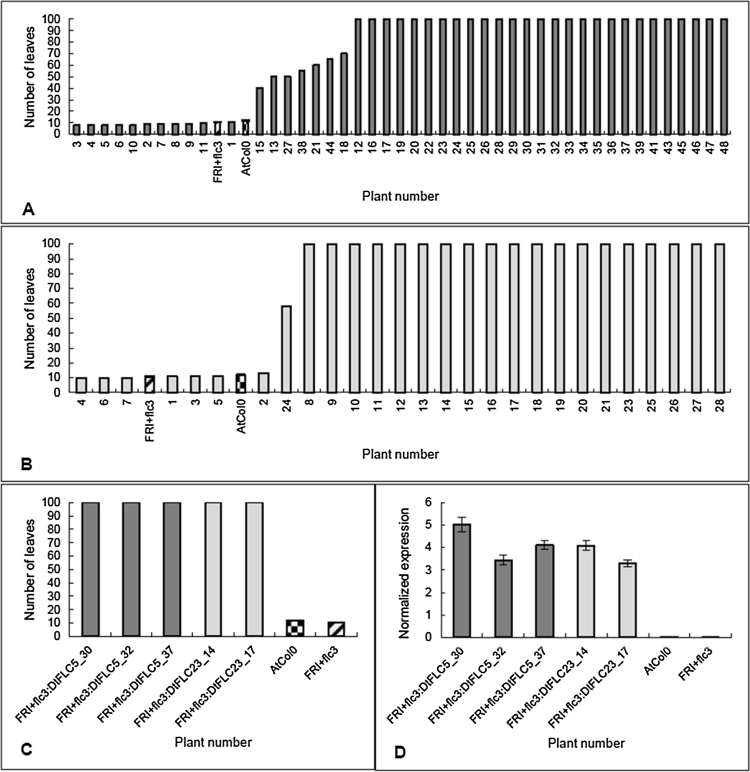
The coding sequence for DtFLC was cloned into the vector pB2GW7 containing a 35S promoter and Bar gene which was transformed into Arabidopsis FRI+flc3 null mutant plants by the floral dip method. The transformed seed were selected using BASTA and the T1 seed were grown and scored for bolting. The expression of the FLC floral repressor should result in delayed flowering/bolting and so T1 plants which were late bolting compared to the untransformed Arabidopsis Col0 and FRI+flc3 null mutant plants were harvested for seed. The T2 seed were sown out for the FRI+flc3:DtFLC 5 and FRI+flc3:DtFLC 23 lines and grown in a 16 h photoperiod to screen for the flowering phenotype. The T2 population for each line showed the expected segregation of 3:1 late:early flowering phenotype associated with the insertion of one copy of the transgene (Fig. 3a & b). A Chi-squared test was performed on the data and shows no significant difference to the 3:1 ratio. Overall the data suggest that DtFLC is able to complement the lack of function of AtFLC in FRI+flc3 null mutant plants. RNA was extracted from a few T2 plants of the FRI+flc3:DtFLC 5 and FRI+flc3:DtFLC 23 lines to investigate the levels of expression of the transgene present in the transformed lines. Fig. 3c shows the number of leaves at flowering for the plants tested from both the FRI+flc3:DtFLC 5 and FRI+flc3:DtFLC 23 lines compared to the non-transformed AtCol0 wild type and FRI+flc3 mutant plants, and Fig. 3d shows the expression level of the DtFLC transgene in each of these plants. As expected there is no transgene expression detectable in the non-transformed AtCol0 wild type and FRI+flc3 mutant plants.
Fig. 3.
Flowering time and DtFLC expression data of FRI+flc3:DtFLC T2 lines.
A) Flowering time data for line FRI+flc3:DtFLC 5. Rosette leaf number was recorded when bolt reached 1 cm.
B) Flowering time data for line FRI+flc3:DtFLC 23. Checked bar shows mean number of rosette leaves at flowering (1 cm bolt) for AtCol0 wild type (mean 11.8 ± 1.5 n = 24). Hatched bar shows mean number of leaves at flowering for FRI+flc3 mutant (mean 10.7 ± 1.2 n = 21). Black bars at 100 leaves show plants that did not bolt before the end of the experiment. Error bars denote the standard error of the mean. Chi-squared tests assessing goodness of fit for data to an expected 3:1 late:early bolting phenotype show that lines FRI+flc3:DtFLC 5 and FRI+flc3:DtFLC 23 were not significantly different to the 3:1 ratio (p < 0.05). C) Number of rosette leaves at 1 cm bolt of T2 plants from lines FRI + flc3:DtFLC 5 (dark grey bars) and FRI + flc3:DtFLC 23 (light grey bars) alongside WT AtCol0 (checked bar) and FRI + flc3 null mutant (hatched bar). Bars with 100 leaves did not bolt before the end of the experiment. Error bars denote standard error of the mean. D) Transgene expression analysis using real time PCR for T2 plants of lines FRI + flc3:DtFLC 5 (dark grey bars) and FRI + flc3:DtFLC 23 (light grey bars). Expression was normalized to the combined average expression of Atβ-tubulin, AtActin2 and AtTIP41. Error bars denote standard error of three technical replicates.
3.4. Expression of DtFLC during cold treatment of D.tenuifolia
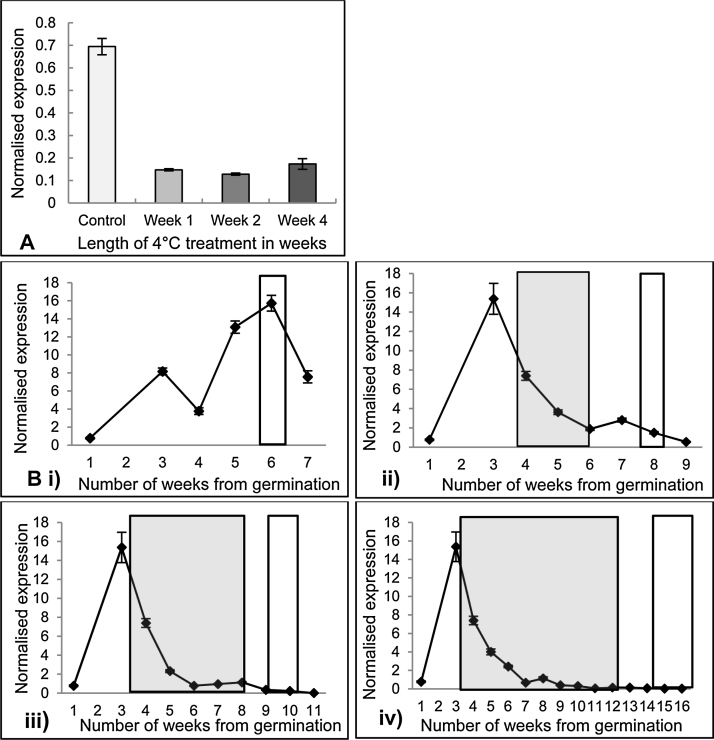
The isolation of DtFLC allowed the expression of the gene to be investigated in wild rocket following vernalization. In the 4 °C seed vernalization experiments, DtFLC expression was analyzed in the seed or seedlings collected at the end of each vernalization treatment (Fig. 4a) and the results revealed that those subjected to one week of ambient temperature conditions (control) had a much higher level of expression than in seed subjected to the vernalization treatments. This demonstrates that one week of cold treatment is sufficient to dramatically reduce the levels DtFLC expression. There is little change in the level of DtFLC expression during subsequent vernalization treatments indicating that once the expression level has been reduced in the first week the length of the vernalization treatment thereafter has no further effect on DtFLC expression.
Fig. 4.
Effect of vernalization at 4 °C on DtFLC expression in D.tenuifolia.
A) Expression of DtFLC where seed was subjected to vernalization at 4 °C for one, two or four weeks. Error bars denote the standard error of three biological replicates. B) Expression of DtFLC over development where 4 week old plants were subjected to vernalization at 4 °C for two, four or eight weeks. Change in levels of DtFLC expression over the course of the experiment under i) ambient conditions, ii) two weeks vernalization, iii) four weeks vernalization, iv) eight weeks vernalization. Error bars denote the standard error of three biological replicates. Range of bolting time of plants shown by white boxes and the respective 4 °C vernalization treatments shown by the grey boxes.
The expression of DtFLC throughout plant development was investigated for each of the 4 °C vernalization treatments of four week old plants, and the non-vernalized control. In non-vernalized plants maintained in ambient conditions DtFLC expression increased up until three weeks after germination, fell slightly at four weeks, then increased again up until bolting and fell after the transition to flowering had occurred (Fig. 4bi). In plants that had been given the vernalization treatment, the pattern of DtFLC expression was the same as under the ambient conditions up to the point the vernalization treatment started (at four weeks after germination). When the vernalization treatment began, the levels of DtFLC fell to a low level and the level remained low, even when plants were returned to ambient conditions (Fig. 4bii, iii, iv). This shows that all of the cold treatments tested were effective in reducing the expression of DtFLC and maintaining it at a low level after vernalization.
3.5. Photoperiodic requirements of D. tenuifolia
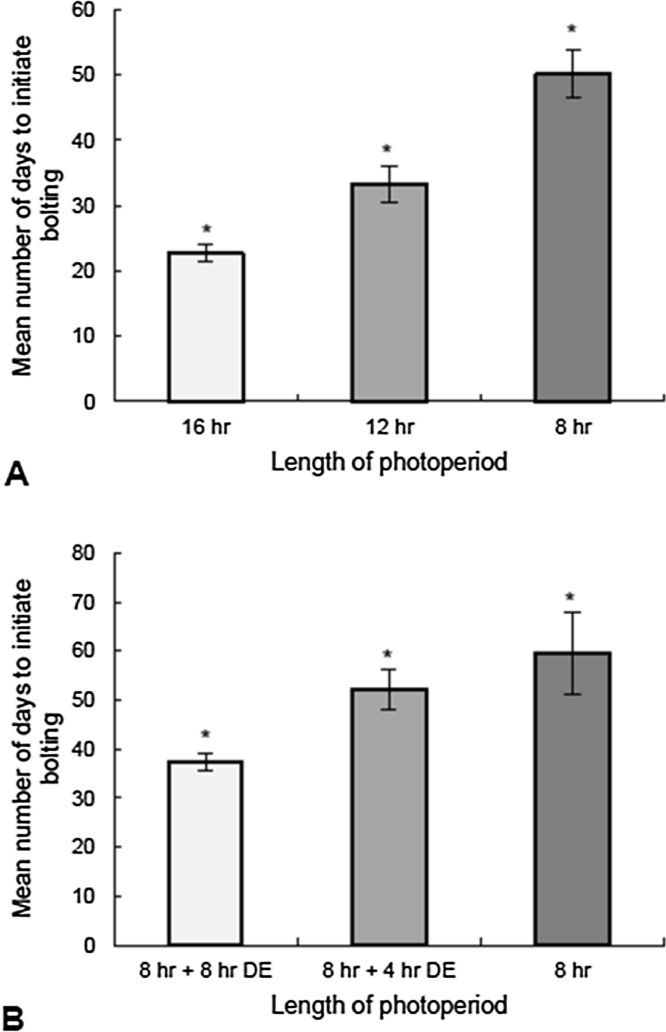
Three different photoperiods were used to investigate the effect of photoperiod on the number of days to initiate bolting in wild rocket. The experiment was carried out using fluorescent bulbs to provide photoperiods of 8 h, 12 h or 16 h (light levels 124 μmol.m−2.s−1). The data show that the plants respond differently to each photoperiod, with shorter photoperiods delaying bolting (Fig. 5a). There was a significant difference in the number of days to bolting between plants in each photoperiod (p < 0.05) (Fig. 5a). The experiment was repeated using fluorescent bulbs to provide a standard SD photoperiod of 8 h, with low levels of incandescent light (33 μmol m−2 s−1) used to provide the additional 4 and 8 h day extensions for the 12 h and 16 h photoperiods respectively. Low light levels were used for the day extension treatments to reduce photosynthesis levels therefore reducing any effects on flowering time that may be caused by photosynthesis/growth due to the additional 4 or 8 h light in the 12 h and 16 h photoperiodic treatments respectively. Incandescent bulbs rather than the fluorescent bulbs were used for the day extension treatments as these contain higher amounts of far-red light and are much more effective than fluorescent light for photoperiodic responses (Johnson et al., 1994). This experiment gave the same result as the first experiment in that shorter photoperiods caused a delay in bolting with there being a significant difference in the number of days to bolt between each photoperiod length (p < 0.05) (Fig. 5b).
Fig. 5.
Effect on bolting time of varying photoperiod lengths in Diplotaxis tenuifolia plants.
Mean number of days to initiate bolting for plants grown in 8 h, 12 h and 16 h photoperiods. A) Photoperiod experiment with light provided by fluorescent bulbs. Bars show mean ± SE (n = 9 (16 h + 12 h), n = 6 (8 h)). B) Photoperiod experiment with 8 h light provided by fluorescent bulbs and the 4 h or 8 h day extensions by incandescent bulbs. Bars show mean ± SE (n = 5 (8 h + 8 h DE, 8 h + 4 h DE), n = 6 (8 h)). Error bars denote the standard error of the mean. Ordinary one-way ANOVA showed a significant difference (*) between all photoperiod lengths on the initiation of bolting time (p < 0.05).
4. Discussion
The vernalization and photoperiodic pathways are two key pathways involved in the perception of, and response to, environmental conditions. Different responses to vernalization and photoperiod are observed in different ecotypes of Arabidopsis (Strange et al., 2011) and in different varieties of crop plants such as wheat and oilseed rape (Bluemel et al., 2015, Srikanth and Schmid, 2011). Understanding the vernalization and photoperiodic requirements of wild rocket is important for commercial production in different parts of the world, and for breeding of new varieties with different flowering behaviours.
Prior to this set of experiments it was not known whether wild rocket has a vernalization requirement, and if so at which developmental stage and temperature the response is greatest. Our data shows that wild rocket does not respond to vernalization neither as seed nor as four week old plants, at either 4 °C or 10 °C. Vernalization of vernalization-responsive Arabidopsis accessions results in a large difference in flowering time between non-vernalized plants and vernalized plants, which flower much earlier (Sanchez-Bermejo et al., 2012), and if wild rocket has a vernalization requirement, we would expect to see similar large differences in bolting time. Although there is a small decrease in the mean number of days to bolt after vernalizing wild rocket seed for one week at 4 °C, it clearly doesn’t satisfy a putative vernalization requirement as longer periods of vernalization of two or four weeks at 4 °C resulted in a later bolting time (Fig. 1b). Similarly, whilst a small reduction in the number of days to bolt was observed following vernalization of seed at 10 °C for six weeks (Supplementary data Fig. S1a), a much greater reduction in bolting time would be expected if wild rocket possessed a vernalization response. Experiments with young four week old plants showed that there is no decrease in the mean number of days to bolt in response to vernalization treatments at either 4 °C or 10 °C (Figs. 1 c & S1b). In some cases, the vernalized plants take slightly longer to bolt than the non-vernalized controls grown under constant ambient conditions, which may reflect the need for a recovery period from the cold temperature which is likely to have slowed metabolic and physiological processes in the treated seed/young plants.
A homologue of the Arabidopsis FLC (AtFLC) gene was isolated from D.tenuifolia (DtFLC), the predicted protein sequence was found to have a higher identity to B.napus FLC1 than to AtFLC and other Brassica FLC proteins. AtFLC functions as a repressor of flowering and Kim et al. (2007) investigated how candidate FLC genes from Chinese cabbage (B.rapa L. ssp. Pekinensis) behaved when used to complement Arabidopsis flc mutants. They found that all BrFLC genes tested caused a late flowering phenotype showing that these genes were able to complement the flc mutant and were repressors of flowering. A similar approach was taken to investigate the function of DtFLC. The Arabidopsis FRI+flc3 null mutant was transformed with the DtFLC coding sequence and late flowering lines were obtained in the T1 and T2 generations showing that DtFLC was able to complement the lack of functional AtFLC in the mutant flc lines. Analysis of transgene expression levels confirmed that the complemented lines were expressing the transgene (Fig. 3d). These results showed that DtFLC is a functional orthologue of AtFLC and enabled us to investigate the pattern of DtFLC gene expression during the various vernalization treatments used in our experiments.
When expression levels of DtFLC were examined in seed vernalized at 4 °C the results showed a large drop in expression during the first week of cold treatment and that levels remained low during additional weeks of vernalization (Fig. 4a), this reflects results obtained in Arabidopsis (Sheldon et al., 2000). The expression of DtFLC observed in the four week old plants subjected to vernalization at 4 °C showed a similar pattern in that one week of cold treatment was sufficient to cause a large reduction in expression levels. The levels of DtFLC were shown to remain low during longer periods of 4 °C cold, and were still low after the plants were returned to ambient conditions for the remainder of the experiment (Fig. 4b). Together, these results show that just one week of 4 °C cold treatment is effective in reducing the levels of DtFLC expression but that this does not have an effect on reducing the time to bolting.
The DtFLC expression data showed that the vernalization-induced reduction in expression levels of DtFLC is maintained even when plants are returned to ambient conditions (Fig. 4b). This suggests that wild rocket may have genes homologous to the Arabidopsis VERNALIZATION INSENSITIVE 3, VERNALIZATION 1 and VERNALIZATION 2 genes which reduce the expression of FLC and maintain the low levels after vernalization (Gendall et al., 2001, Levy et al., 2002, Sung and Amasino, 2004). However, as the reduction of DtFLC expression during and post-vernalization treatment does not affect bolting time in rocket, this suggests that the relationship between FLC expression and flowering in wild rocket may not be the same as in Arabidopsis. This may be due to some disruption of the flowering time pathway between DtFLC and the floral integrator genes meaning that the latter are unaffected by changes in DtFLC expression. It could also possibly be that in non-vernalized wild rocket DtFLC is expressed at levels which are insufficient to actively repress flowering and so the observed reduction in expression of DtFLC during vernalization would not have any significant effect on flowering. This would be similar to Arabidopsis ecotypes Ler, Da and Shakhdara which have a low expression of AtFLC but flower rapidly and are classified as summer annuals. It was found that the AtFLC gene in Ler, Da and Shakhdara did not contain any mutations in the coding sequence compared to Col0, but the low expression was caused by mutations in intron 1 (Ler) or the 5′ UTR (Da and Shakhdara) sequences (Michaels et al., 2003). Overall the results demonstrate that rocket does not have a vernalization requirement as the cold treatments (either 4 °C or 10 °C) did not reduce the time the plants took to bolt compared to non-vernalized plants. This could be due to its origins in the Mediterranean where a vernalization response would be a disadvantage as has been found in B.napus varieties grown in Mediterranean climates where the lack of a vernalization requirement is important for its early flowering phenotype (Dahanayake and Galwey, 1999). Other studies in B.rapa have also shown that vernalization isn’t a requirement for plants bred in warmer climates (Franks et al., 2015).
Studies in Arabidopsis have explored the pattern of ecotype distribution and flowering time response particularly in relation to photoperiod and vernalization responses (Caicedo et al., 2004, Lempe et al., 2005, Stinchcombe et al., 2004). They linked the different degrees of response to differences in the activity of FRI (which promotes FLC expression) and FLC. Further investigation, for example the identification of a FRI homologue in rocket, may provide a greater understanding of vernalization responses in Mediterranean crops.
The photoperiodic requirement of wild rocket has not been properly defined and so experiments were set up to test the response of D.tenuifoila to different photoperiods. Plants were able to flower in LD (16 h), intermediate (12 h) and SD (8 h) photoperiods but flowered much more rapidly in longer photoperiods (Fig. 5), this shows that wild rocket is a facultative LD plant. Results of photoperiodic experiments using day extensions of low levels of incandescent light day to minimise the effects of additional photosynthesis on the control of bolting in the longer photoperiods were consistent with the first experiment, confirming the conclusion that rocket is a facultative LD plant.
5. Conclusions
A rocket homologue of FLOWERING LOCUS C (DtFLC) has been isolated and shown to functionally complement the Arabidopsis FRI+flc3 null mutant. The expression of DtFLC is significantly reduced by one week of cold, however vernalization treatments of up to eight weeks had no significant effect on bolting time of rocket demonstrating that rocket does not have a vernalization requirement. The flowering response of rocket to different photoperiods show that flowering is promoted by LD and that rocket is a facultative long day plant.
Author contributions
J.T., A.M. and S.J. designed the experiments, J.T. performed the experiments, J.T. and S.J wrote the manuscript, A.M., S.J., S.K. and Y.H. supervised the study and revised the manuscript.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
We acknowledge the technical assistance of Mrs. Anushree Choudhary on the 10 °C vernalization experiments and advice from Professor Brian Thomas for the photoperiod experiments. We thank Elsoms Seeds Ltd for the D.tenuifolia seed stocks and Nottingham Arabidopsis Stock Centre for the Arabidopsis Col0 and FRI+flc3 mutant seeds.
This work was supported by a BBSRC CASE award (BB/I016120/1) in partnership with Elsoms Seeds Ltd., and BBSRC grant no. BB/G007330/1.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jplph.2017.03.015.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Arias T., Pires J.C. A fully resolved chloroplast phylogeny of the brassica crops and wild relatives (Brassicaceae: Brassiceae): novel clades and potential taxonomic implications. Taxon. 2012;61:980–988. [Google Scholar]
- Bluemel M., Dally N., Jung C. Flowering time regulation in crops – what did we learn from Arabidopsis? Curr. Opin. Biotechnol. 2015;32:121–129. doi: 10.1016/j.copbio.2014.11.023. [DOI] [PubMed] [Google Scholar]
- Boss P.K., Bastow R.M., Mylne J.S., Dean C. Multiple pathways in the decision to flower: enabling, promoting, and resetting. Plant Cell. 2004;16:S18–S31. doi: 10.1105/tpc.015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brambilla V., Fornara F. Molecular control of flowering in response to day length in rice. J. Integr. Plant Biol. 2013;55:410–418. doi: 10.1111/jipb.12033. [DOI] [PubMed] [Google Scholar]
- Caicedo A.L., Stinchcombe J.R., Olsen K.M., Schmitt J., Purugganan M.D. Epistatic interaction between Arabidopsis FRI and FLC flowering time genes generates a latitudinal cline in a life history trait. Proc. Natl. Acad. Sci. U. S. A. 2004;101:15670–15675. doi: 10.1073/pnas.0406232101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang G.C., Barua D., Kramer E.M., Amasino R.M., Donohue K. Major flowering time gene, FLOWERING LOCUS C, regulates seed germination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 2009;106:11661–11666. doi: 10.1073/pnas.0901367106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouard P. Vernalization and its relation to dormancy. Annu. Rev. Plant Physiol. 1960;11:191–238. [Google Scholar]
- Chun J.-H., Arasu M.V., Lim Y.-P., Kim S.-J. Variation of major glucosinolates in different varieties and lines of rocket salad. Hortic. Environ. Biotechnol. 2013;54:206–213. [Google Scholar]
- Clough S.J., Bent A.F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- Corbesier L., Coupland G. Photoperiodic flowering of Arabidopsis: integrating genetic and physiological approaches to characterization of the floral stimulus. Plant Cell Environ. 2005;28:54–66. [Google Scholar]
- Dahanayake S.R., Galwey N.W. Diallel analysis of vernalisation responses in spring rape (Brassica napus L.): a basis for adaptation to a Mediterranean environment. Aust. J. Agr. Res. 1999;50:1417–1423. [Google Scholar]
- Fornara F., de Montaigu A., Coupland G. SnapShot: control of flowering in arabidopsis. Cell. 2010;141:50–550. doi: 10.1016/j.cell.2010.04.024. [DOI] [PubMed] [Google Scholar]
- Franks S.J., Perez-Sweeney B., Strahl M., Nowogrodzki A., Weber J.J., Lalchan R., Jordan K.P., Litt A. Variation in the flowering time orthologs BrFLC and BrSOC1 in a natural population of Brassica rapa. PEER J. 2015;3:e1339. doi: 10.7717/peerj.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendall A.R., Levy Y.Y., Wilson A., Dean C. The VERNALIZATION 2 gene mediates the epigenetic regulation of vernalization in Arabidopsis. Cell. 2001;107:525–535. doi: 10.1016/s0092-8674(01)00573-6. [DOI] [PubMed] [Google Scholar]
- Gill M. The Vegetable Farmer. ACT Publishing; 2008. Wild rocket sales on the up; pp. 27–28. [Google Scholar]
- Guo D.P., Shah G.A., Zeng G.W., Zheng S.J. The interaction of plant growth regulators and vernalization on the growth and flowering of cauliflower (Brassica oleracea var. botrytis) Plant Growth Reg. 2004;43:163–171. [Google Scholar]
- Hackett W.P., Hartmann H.T. The influence of temperature on floral initiation in the olive. Physiol. Plant. 1967;20:430–436. [Google Scholar]
- Jarillo J.A., Pineiro M. Timing is everything in plant development. The central role of floral repressors. Plant Sci. 2011;181:364–378. doi: 10.1016/j.plantsci.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Johnson E., Bradley M., Harberd N.P., Whitelam G.C. Photoresponses of light-grown phyA mutants of Arabidopsis (phytochrome A is required for the perception of daylength extensions) Plant Physiol. 1994;105:141–149. doi: 10.1104/pp.105.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C., Mueller A.E. Flowering time control and applications in plant breeding. Trends Plant Sci. 2009;14:563–573. doi: 10.1016/j.tplants.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Kim S.-Y., Park B.-S., Kwon S.-J., Kim J., Lim M.-H., Park Y.-D., Kim D.Y., Suh S.-C., Jin Y.-M., Ahn J.H., Lee Y.-H. Delayed flowering time in Arabidopsis and Brassica rapa by the overexpression of FLOWERING LOCUS C (FLC) homologs isolated from Chinese cabbage (Brassica rapa L. ssp pekinensis) Plant Cell Rep. 2007;26:327–336. doi: 10.1007/s00299-006-0243-1. [DOI] [PubMed] [Google Scholar]
- Lempe J., Balasubramanian S., Sureshkumar S., Singh A., Schmid M., Weigel D. Diversity of flowering responses in wild Arabidopsis thaliana strains. PLoS Genet. 2005;1:109–118. doi: 10.1371/journal.pgen.0010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy Y.Y., Mesnage S., Mylne J.S., Gendall A.R., Dean C. Multiple roles of Arabidopsis VRN1 in vernalization and flowering time control. Science. 2002;297:243–246. doi: 10.1126/science.1072147. [DOI] [PubMed] [Google Scholar]
- Logemann J., Schell J., Willmitzer L. Improved method for the isolation of RNA from plant-tissues. Anal. Biochem. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- Matsoukas I.G., Massiah A.J., Thomas B. Florigenic and antiflorigenic signaling in plants. Plant Cell Physiol. 2012;53:1827–1842. doi: 10.1093/pcp/pcs130. [DOI] [PubMed] [Google Scholar]
- Michaels S.D., Amasino R.M. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. The Plant Cell. 1999;11(5):949–956. doi: 10.1105/tpc.11.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels S.D., He Y.H., Scortecci K.C., Amasino R.M. Attenuation of FLOWERING LOCUS C activity as a mechanism for the evolution of summer-annual flowering behavior in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10102–10107. doi: 10.1073/pnas.1531467100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordborg M., Bergelson J. The effect of seed and rosette cold treatment on germination and flowering time in some Arabidopsis thaliana (Brassicaceae) ecotypes. Am. J. Bot. 1999;86:470–475. [PubMed] [Google Scholar]
- Sanchez-Bermejo E., Mendez-Vigo B., Xavier Pico F., Martinez-Zapater J.M., Alonso-Blanco C. Novel natural alleles at FLC and LVR loci account for enhanced vernalization responses in Arabidopsis thaliana. Plant Cell Environ. 2012;35:1672–1684. doi: 10.1111/j.1365-3040.2012.02518.x. [DOI] [PubMed] [Google Scholar]
- Sheldon C.C., Rouse D.T., Finnegan E.J., Peacock W.J., Dennis E.S. The molecular basis of vernalization: the central role of FLOWERING LOCUS C (FLC) Proc. Natl. Acad. Sci. U. S. A. 2000;97:3753–3758. doi: 10.1073/pnas.060023597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Angel A., Howard M., Dean C. Vernalization – a cold-induced epigenetic switch. J. Cell Sci. 2012;125:3723–3731. doi: 10.1242/jcs.084764. [DOI] [PubMed] [Google Scholar]
- Srikanth A., Schmid M. Regulation of flowering time: all roads lead to Rome. Cell Mol. Life Sci. 2011;68:2013–2037. doi: 10.1007/s00018-011-0673-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart C.N., Via L.E. A rapid CTAB DNA isolation technique useful for rapid fingerprinting and other PCR applications. Biotechniques. 1993;14:748–751. [PubMed] [Google Scholar]
- Stinchcombe J.R., Weinig C., Ungerer M., Olsen K.M., Mays C., Halldorsdottir S.S., Purugganan M.D., Schmitt J. A latitudinal cline in flowering time in Arabidopsis thaliana modulated by the flowering time gene FRIGIDA. Proc. Natl. Acad. Sci. U. S. A. 2004;101:4712–4717. doi: 10.1073/pnas.0306401101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange A., Li P., Lister C., Anderson J., Warthmann N., Shindo C., Irwin J., Nordborg M., Dean C. Major-effect alleles at relatively few loci underlie distinct vernalization and flowering variation in Arabidopsis accessions. PLoS One. 2011;6:1–11. doi: 10.1371/journal.pone.0019949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung S.B., Amasino R.M. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature. 2004;427:159–164. doi: 10.1038/nature02195. [DOI] [PubMed] [Google Scholar]
- Thomas B., Carre I., Jackson S. Molecular Biology and Biotechnology of Flowering. 2nd edition. 2006. Photoperiodism and flowering; pp. 3–25. [Google Scholar]
- Wang J.W. Regulation of flowering time by the miR156-mediated age pathway. J. Exp. Bot. 2014;65:4723–4730. doi: 10.1093/jxb/eru246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.