Abstract
A new proposal has been created for establishing medical criteria for organ allocation in recipients receiving simultaneous liver-kidney transplants. In this article, we describe the new policy, elaborate on the points of greatest controversy, and offer a perspective on the policy going forward. Although we applaud the fact that simultaneous liver-kidney transplant activity will now be monitored and appreciate the creation of medical criteria for allocation in simultaneous liver-kidney transplants, we argue that some of the criteria proposed, especially those for allocating a kidney to a liver recipient with AKI, are too liberal. We call on the nephrology community to follow the consequences of this new policy and push for a re-examination of the longstanding policy of allocating kidneys to multiorgan transplant recipients before all other candidates. The charge to protect our system of equitable organ allocation is very challenging, but it is a challenge that we must embrace.
Keywords: Simultaneous Liver Kidney, Organ allocation, UNOS guidelines, Acute Kidney Injury, Humans, kidney, kidney transplantation, Liver, Liver Transplantation, nephrology, Prospective Studies, Renal Insufficiency, Chronic, Thiadiazines, Transplant Recipients, buprofezin
Introduction
A new proposal has been created for establishing medical criteria for organ allocation in recipients receiving simultaneous liver-kidney transplants (SLKs) 1). Practicing nephrologists should be aware of this new policy, because SLK removes kidneys from the pool available to patients awaiting kidney transplant alone (KA) at a time when our waiting list for this precious resource continues to grow. Furthermore, the medical criteria for SLK allocation in liver patients with CKD and AKI will likely create controversy, because they are less stringent than many nephrologists would want. By way of full disclosure, it needs to be stated at the outset that, although the authors of this article had nothing to do with creation of this policy, the Chair of the SLK Committee of the United Network for Organ Sharing (UNOS) who did is the Medical Director of the Kidney Pancreas Program at the authors’ institution.
We describe the new policy, elaborate on the points of greatest controversy, and offer a perspective on the policy going forward.
Background
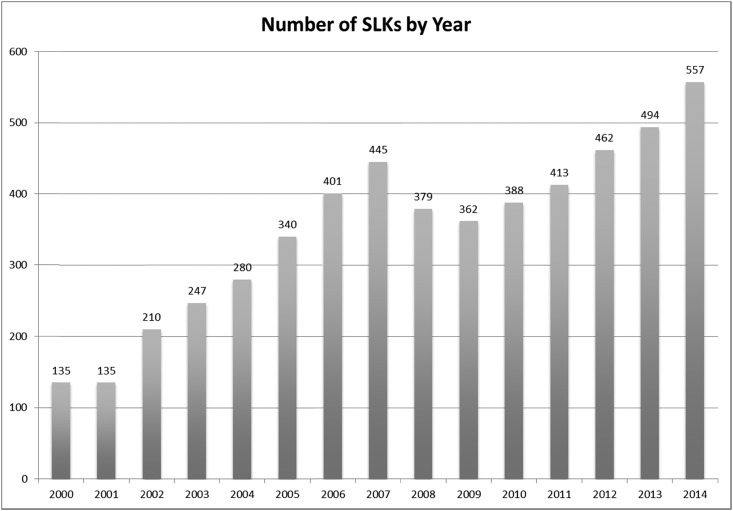
SLKs have been increasing yearly (Figure 1). Before the creation of this policy, kidneys for SLK recipients were used somewhat arbitrarily, with great regional variation in this practice (2). Furthermore, the absence of accepted medical criteria for which recipients would receive an SLK has left the decision up to providers caring for these patients, many of whom are influenced by data suggesting that, in patients with renal dysfunction before liver transplant, renal function and patient survival are better in those who receive a simultaneous kidney (1,3). However, this survival advantage is quite small—about 5% at 1 and 5 years (1,3). Moreover, with our current practice, the number of liver alone (LA) recipients requiring dialysis in the first year after transplant is <10% (4,5). Difficulty in measuring kidney function accurately in patients with cirrhosis and predicting which patients with AKI will not recover after liver transplant have hampered efforts to establish a more uniform practice for SLK. The establishment of medical criteria in allocation of SLK is the first attempt to oversee and regulate this practice.
Figure 1.
Increasing number of simultaneous liver-kidney transplantation (SLK) by year (1).
New Policy
The SLK Committee addressed criteria for assigning a kidney with the liver in cirrhotic patients with AKI, CKD, and metabolic disease (e.g., hyperoxaluria) (Table 1). Under the category of CKD (defined as eGFR≤60 for ≥90 days), the cutoff eGFR of 30 ml/min was chosen, in part, on the basis of data showing a 30% frequency of ESRD over 3 years in liver recipients transplanted with an eGFR below 30 (6). This GFR was also chosen in consideration of that fact that GFR often drops at least 10 ml/min immediately after transplant due to the use of calcineurin inhibitors (7) (bringing eGFR closer to the eGFR required to start waiting time for KA patients). The ability of cirrhotic patients to get a kidney from the pool with a higher GFR (30 ml/min) than that required to start waiting time for a KA patient (20 ml/min) will be perceived as unfair by many, but the inability to accurately access GFR in cirrhotic patients as well as the acuity of illness in these patients at the time of listing justify some pause before making comparisons.
Table 1.
Medical eligibility criteria for simultaneous liver-kidney transplantation
| CKD (must be confirmed by a nephrologist) |
| GFR≤60 for 90 consecutive days and |
| eGFR or CrCl≤30 at or after registration on kidney waiting list or |
| Dialysis (in the setting of ESRD) |
| AKI (must be confirmed by a nephrologist) |
| Dialysis for 6 consecutive weeks |
| eGFR or CrCl≤25 for 6 consecutive weeks |
| Combination of above two criteria |
| Metabolic disease (must be confirmed by a nephrologist) |
| Atypical HUS from mutations factor H or factor I |
| Hyperoxaluria |
| Familial non–neuropathic systemic amyloidosis |
| Methylmalonic aciduria |
CrCl, creatinine clearance; HUS, hemolytic uremic syndrome.
Perhaps even more controversial are the criteria for listing cirrhotic patients with AKI for a kidney. These patients are about 40% of those receiving SLKs. It has been argued that many patients with AKI recover renal function after liver transplant, and few require dialysis in the first year (5,8). Although various durations of dialysis have been used as cutoffs in the past, the committee chose 6 weeks, quoting data that recovery of renal function is not common after dialysis extending beyond 4 weeks (1). There are data to refute these claims, but the committee chose to start with these criteria.
There is little controversy over the need for SLK in certain metabolic diseases. Hence, we will not discuss this category any further.
In addition to criteria for SLK, the committee developed a safety net policy, which allows patients who received an LA but manifest kidney failure after liver transplant to receive priority on the kidney waiting list. Patients who received a liver transplant and are registered on the kidney waiting list between 60 and 365 days after liver transplant and either on chronic hemodialysis or have an eGFR≤20 ml/min will qualify for this increased priority. This policy was created to affect physician decision making to potentially bypass receiving a kidney with the liver knowing that the patient would get priority after listed within the first year after LA transplant. Data collected and analyzed by the SLK Committee for the 10 years before the creation of this policy showed that patient survival in LA patients was not affected if they were on the list and received a kidney within 1 year, but it was adversely affected if they were on the waiting list 3 years or longer (1). Hence, priority for listing was given to these LA recipients and formed the basis for who would be covered by the safety net. As shown in figure 6 of ref. 1, the safety net candidates would never get a kidney over a highly sensitized or zero mismatched KA candidate or a good quality kidney over pediatric candidates (1).
This policy does not address a cohort of patients that some hepatologists call “renal cripples,” referring to liver transplant recipients with poor renal function that is not poor enough (i.e., GFR≤20 ml/min) to merit listing for a new kidney with the safety net policy. These patients are challenging to manage with regard to fluid balance and tacrolimus levels.
Is the Principle of Ethical Justice Being Violated?
Patients with ESRD and their providers are voicing the complaint that the approved UNOS SLK allocation system violates the ethical principle of justice. In this context, justice refers to “fairness in the pattern of distribution of the benefits and burdens of an organ procurement and allocation program” (9,10). Members of the 1992 and 2010 UNOS Ethics Committees envisioned a future where “allocation schemes routinely consider medical need as well as medical benefits, prioritizing the medically sickest patients even if it is predictable that other patients who are not as sick will have better outcomes” (9,10). Furthermore, they concluded that “factors to be considered in the application of the principle of justice are: (1) medical urgency; (2) likelihood of finding a suitable organ in the future; (3) waiting list time; (4) first versus repeat transplants; (5) age; and (6) geographical fairness” (9,10). In other words, waiting time was not the only measure of fairness (9,10).
What should be appreciated is that the allocation to multiorgan transplant (MOT) recipients taking priority over kidney patients has always been an Organ Procurement and Transplantation Network policy long before this SLK policy was formed. Namely, this means that patients accruing time, 1 day at a time year on year, on the kidney transplant waiting list are bypassed every time that a kidney is allocated to an MOT recipient. Therefore, allocating kidneys to MOT recipients over KA candidates waiting longer on the list has been in practice for a long time.
Examples that we may agree with where waiting time has not been the major criteria for transplantation include children and sensitized recipients on the KA waiting list. We virtually all agree that pediatric recipients should be first in line for transplantation. Children have benefited from up to four additional points toward their allocation score (in a system where each point is equal to 1 year of time on the kidney transplant waiting list) determined by their age at the time of listing. In addition, in both the old and new Kidney Allocation Systems (KASs), highly sensitized recipients have also received additional waiting time points. In the new KAS, highly sensitized recipients receive more points than in previous systems. Most if not all kidney transplant centers perceived an increased rate of transplantation in highly sensitized recipients after KAS went into effect, and short-term assessment of KAS on the national level indicates that the objective was achieve (11).
However, just like when we wait in line, we have an understanding that there are some special populations that we will allow to move to the front or to whom we will give up our seat—the elderly, pregnant woman, and the disabled (in no particular order). We should think of the SLK in this light and shift the emphasis away from how to best use a kidney to how to provide the best treatment possible to this unique group of patients. The UNOS SLK Committee members and their resultant recommendations view the allocation process from this vantage point. Namely, SLK recipient candidates are sicker, and therefore, it is ethically appropriate to prioritize them for transplantation over KA patients waiting longer. It is a point of view worthy of some reflection. Whether we agree with the medical criteria set up to guide allocation to these patients is another issue.
Benefits of the New SLK Criteria
Although some of the criteria themselves may be controversial, adoption of them will reduce the variability of practice between centers and the individual preferences that have accompanied this variability (12). With improved consistency, we will be able to better benchmark the practice, monitor it from year to year and across regions, and refine the allocation recommendations if the data collected seem unjust. Systems of this scope and magnitude work best through consensus. Transplantation is fundamentally multidisciplinary, and the priorities of all involved need to be heard and given a voice. The transplantation voice has many more tones than the community of nephrologists—all who march to the same beat for their patients to maximize their rate of transplantation in the context of a marked donor kidney shortage. Therefore, we should appreciate that providers with very different views reached consensus on a system that brings an unregulated practice into a system to be followed by all.
It is possible that the new medical criteria could potentially reduce the number of SLKs performed, because 19% of SLKs performed between 2014 and 2015 did not meet these criteria. The safety net provision of the new policy was created to incentivize transplant programs to not take a kidney out of the pool knowing that their liver transplant alone recipients will get priority for a deceased donor kidney transplantation in the following year if one is needed. However, it is also possible that centers will treat the medical criteria as guidelines that justify doing SLK versus LA, leading to more kidneys being taken out of the pool for SLK versus KA (5).
Concerns about the Multiorgan Allocation Policy
We question the long-standing policy of allowing recipients of MOTs to get priority over all patients waiting for a kidney. This policy has allowed the SLK practice to flourish unregulated, which it has until now. Although allocation of a kidney is considered secondary to allocation of a life-saving heart, lung, and liver and comes along for the ride, we can envision circumstances where there are equally pressing priorities. Which patient is more needy: an SLK candidate listed today, a suffering child, the patient on dialysis with 100% calculated panel reactive antibody who may not receive another offer, or the brittle diabetic awaiting simultaneous pancreas-kidney transplantation? These issues should be reflected on and discussed. It is not just the number of kidneys taken out of the pool with this policy but also, their quality. In 48% of SLKs performed in 2014 and 2015, the kidney donor profile index was <35 (1), thus depriving children and young adults of these higher–quality, longer–lasting kidneys.
Concerns about the SLK Policy
The new criteria for listing liver patients with CKD require that CKD be verified by a nephrologist. This is a benefit of the new policy. The more liberal cutoff eGFR of 30 ml/min for SLK compared with the cutoff of 20 ml/min required for a kidney candidate to start accruing time on the KA waiting list may seem unfair. Abuse of this criteria could also occur with the GFR≤30 ml/min being a one-time measurement. We hope that the role of the nephrologist, now required in this decision making, will decrease the amount of abuse possible. It is important to acknowledge that we are far less accurate at predicting the GFR of a patient with end stage liver disease, and the commonly used methods frequently lead to an overestimation (13). This is due to two major limitations regarding serum creatinine concentrations in patients with cirrhosis: (1) creatinine production is decreased in the setting of cirrhosis, and (2) there is a decreased conversion of creatinine to creatinine secondary to the malnutrition associated with cirrhosis (14). These limitations combined with the expected 10-ml/min drop in GFR when tacrolimus is started are the reasons given for the more liberal eGFR required. This does make some sense.
Our bigger concern with the new policy involves the criteria for receiving SLKs in patients with AKI (Table 1). In previous work and summit meetings on this topic, duration of AKI from 4 to 8 weeks has been suggested as time after which SLK should be considered (2,15). With fewer than 10% of LA recipients requiring dialysis in the first year (4,8), the new criteria do not seem justified. Furthermore, in a single-center study using post–SLK radionuclide renal scans, it was calculated that nearly one third of UNOS criteria recipients recovered a native GFR exceeding 20 ml/min (16). Taken together, it is fair to conclude that a significant fraction of SLK recipients will still experience recovery of their native function after transplantation with these new guidelines. Although it is recognized that compromise was required to create these guidelines, we side with many nephrologists who argue that the medical criteria for SLK listing of patients with AKI are too liberal. It might have made more sense to confine SLK in the setting of AKI mainly to candidates on dialysis for 4–8 weeks, because we all know recovery is less likely the longer that one remains on dialysis. Certainly, with the safety net provision of the new policy, it would seem prudent to have made these criteria more stringent.
Another concern of the new policy revolves around the shortened survival of the kidney in an SLK recipient at a time when longevity matching of kidneys is being emphasized (15). Kidney longevity is better achieved when kidneys go to kidney and not liver recipients (1,17).
Going Forward
The practice of SLK will now be monitored to assure that only patients meeting eligibility criteria are chosen. As stated in the policy paper, 19% of SLK recipients in the past 10 years would not have met the eligibility criteria currently being proposed. These new criteria should eliminate the previously arbitrary nature of this practice. Therefore, on the one hand, order will be brought to a system that has been arbitrary. On the other hand, the eligibility criteria may be too lenient, especially for patients with AKI who could recover renal function. If these criteria are used as a mandate to use more kidneys for liver candidates, it could divert too many kidneys away from the already inadequate pool for KA candidates. If the number of kidneys going to liver candidates (as SLK or after transplant in the safety net) significantly increases from 5% of all kidneys (where it is today), then we should be all be alarmed by this consequence and demand that the policy be revisited.
Continued research and utilization of better criteria, perhaps with the use of biomarkers, are needed to predict the incidence ESRD after liver transplant. This is challenging to do, because as pointed out by Israni et al. (4), the event rate is quite low. We also suggest that consideration of the concept of futility be added to the criteria, so that organs are not offered to patients with poor projected survival. Criteria for this have been discussed (18) and require medical decisions that are challenging but important to consider.
Nephrologists need to be aware of this policy. We also call on the nephrology community to push for an examination of the long-standing policy of prioritizing MOTs over all other candidates. This practice deprives children and highly sensitized recipients of the precious resource that they need and should at least be examined and scrutinized. We encourage transplant surgeons, nephrologists, and hepatologists to use the criteria as just that—minimal criteria—and not use the criteria as a mandate that requires that patients with end stage liver disease receive a kidney if they meet criteria. The risk of the new policy (if it is used as a mandate instead of criteria for minimal eligibility) versus its benefit (finally regulating the SLK practice) remains to be seen. The charge to protect our system of equitable organ allocation is very challenging, but it is a challenge that we must embrace.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Formica RN, Aeder M, Boyle G, Kucheryavaya A, Stewart D, Hirose R, Mulligan D: Simultaneous liver-kidney allocation policy: A proposal to optimize appropriate utilization of scarce resources. Am J Transplant 16: 758–766, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Nadim MK, Sung RS, Davis CL, Andreoni KA, Biggins SW, Danovitch GM, Feng S, Friedewald JJ, Hong JC, Kellum JA, Kim WR, Lake JR, Melton LB, Pomfret EA, Saab S, Genyk YS: Simultaneous liver-kidney transplantation summit: Current state and future directions. Am J Transplant 12: 2901–2908, 2012 [DOI] [PubMed] [Google Scholar]
- 3.Fong TL, Khemichian S, Shah T, Hutchinson IV, Cho YW: Combined liver-kidney transplantation is preferable to liver transplant alone for cirrhotic patients with renal failure. Transplantation 94: 411–416, 2012 [DOI] [PubMed] [Google Scholar]
- 4.Israni AK, Xiong H, Liu J, Salkowski N, Trotter JF, Snyder JJ, Kasiske BL: Predicting end-stage renal disease after liver transplant. Am J Transplant 13: 1782–1792, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Wadei HM, Gonwa TA, Taner CB: Simultaneous liver kidney transplant (SLK) allocation policy change proposal: Is it really a smart move? Am J Transplant 16: 2763–2764, 2016 [DOI] [PubMed] [Google Scholar]
- 6.Ruebner R, Goldberg D, Abt PL, Bahirwani R, Levine M, Sawinski D, Bloom RD, Reese PP: Risk of end-stage renal disease among liver transplant recipients with pretransplant renal dysfunction. Am J Transplant 12: 2958–2965, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chapman JR, Griffiths D, Harding NG, Morris PJ: Reversibility of cyclosporin nephrotoxicity after three months’ treatment. Lancet 1: 128–130, 1985 [DOI] [PubMed] [Google Scholar]
- 8.Gonwa TA, Morris CA, Goldstein RM, Husberg BS, Klintmalm GB: Long-term survival and renal function following liver transplantation in patients with and without hepatorenal syndrome--experience in 300 patients. Transplantation 51: 428–430, 1991 [DOI] [PubMed] [Google Scholar]
- 9.Burdick JF, Turcotte JG, Veatch RM: Principles of organ and tissue allocation and donation by living donors. Transplant Proc 24: 2226, 1992 [PubMed] [Google Scholar]
- 10.OPTN: Ethical Principles in the Allocation of Human Organs, 2015. Available at: https://optn.transplant.hrsa.gov/resources/ethics/ethical-principles-in-the-allocation-of-human-organs. Accessed January 8, 2016
- 11.Stewart DE, Kucheryavaya AY, Klassen DK, Turgeon NA, Formica RN, Aeder MI: Changes in deceased donor kidney transplantation one year after KAS implementation. Am J Transplant 16: 1834–1847, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Parajuli S, Foley D, Djamali A, Mandelbrot D: Renal function and transplantation in liver disease. Transplantation 99: 1756–1764, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Beben T, Rifkin DE: GFR estimating equations and liver disease. Adv Chronic Kidney Dis 22: 337–342, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sherman DS, Fish DN, Teitelbaum I: Assessing renal function in cirrhotic patients: Problems and pitfalls. Am J Kidney Dis 41: 269–278, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Chopra B, Sureshkumar KK: Changing organ allocation policy for kidney transplantation in the United States. World J Transplant 5: 38–43, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levitsky J, Baker T, Ahya SN, Levin ML, Friedewald J, Gallon L, Ho B, Skaro A, Krupp J, Wang E, Spies SM, Salomon DR, Abecassis MM: Outcomes and native renal recovery following simultaneous liver-kidney transplantation. Am J Transplant 12: 2949–2957, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Choudhury RA, Reese PP, Goldberg DS, Bloom RD, Sawinski DL, Abt PL: A paired kidney analysis of multiorgan transplantation: Implications for allograft survival [published online ahead of print March 15, 2016]. Transplantation [DOI] [PubMed] [Google Scholar]
- 18.Lunsford KE, Bodzin AS, Markovic D, Zarrinpar A, Kaldas FM, Gritsch HA, Xia V, Farmer DG, Danovitch GM, Hiatt JR, Busuttil RW, Agopian VG: Avoiding futility in simultaneous liver-kidney transplantation: Analysis of 331 consecutive patients listed for dual organ replacement [published online ahead of print May 26, 2015]. Ann Surg [DOI] [PubMed] [Google Scholar]