Abstract
Background and objectives
CKD is an important risk factor for cardiovascular disease (CVD) and death. We investigated whether select urine kidney injury biomarkers were associated with higher risk of heart failure (HF), CVD, and death in persons with CKD enrolled in the Chronic Renal Insufficiency Cohort (CRIC) Study.
Design, setting, participants, & measurements
Urine kidney injury molecule-1 (KIM-1), neutrophil gelatinase-associated lipocalin, liver fatty acid-binding protein, and N-acetyl-β-d-glucosaminidase were measured in urine of a subset of CRIC participants (n=2466). We used Cox proportional hazards regression to examine associations between these biomarkers indexed to urinary creatinine (Cr) and (1) HF, (2) a composite of atherosclerotic CVD events (myocardial infarction, ischemic stroke, or peripheral artery disease), and (3) all-cause death.
Results
At baseline, mean age of study participants was 59.5±10.8 years, 46% were women, and 34% had a self-reported history of any CVD. Median follow-up was 6.5 (interquartile range, 5.6–6.8) years. A total of 333 HF events, 282 atherosclerotic CVD events, and 440 deaths were observed during a median follow-up of 6.5 (interquartile range, 5.6–6.8) years. Those in the highest two quintiles of KIM-1/Cr levels had a higher risk of HF relative to the lowest quintile (quintile 5 versus quintile 1 adjusted hazard ratio [aHR] of 1.73 [95% confidence interval, 1.05 to 2.85]). N-acetyl-β-d-glucosaminidase/Cr was associated with HF in continuous analyses (aHR per log SD higher 1.18 [95% confidence interval, 1.01 to 1.38]). Only KIM-1/Cr was independently associated with atherosclerotic CVD events (aHR per log SD higher 1.21 [95% confidence interval, 1.02 to 1.41]), whereas both KIM-1/Cr (quintile 5 versus quintile 1 aHR of 1.56 [95% confidence interval, 1.06 to 2.31]) and neutrophil gelatinase-associated lipocalin/Cr (quintile 5 versus quintile 1 aHR of 1.82 [95% confidence interval, 1.19 to 2.8]) were associated with all-cause death.
Conclusions
Selected urine kidney injury biomarkers were independently associated with higher risk of HF, CVD events, and death in CRIC. Among the biomarkers examined, only KIM-1/Cr was associated with each outcome. Further work is needed to determine the utility of these biomarkers to improve risk prediction for these adverse outcomes.
Keywords: chronic kidney disease; cardiovascular disease; mortality risk; Acetylglucosaminidase; Aged; Atherosclerosis; Biomarkers; creatinine; Fatty Acid-Binding Proteins; Female; Follow-Up Studies; heart failure; Humans; Lipocalin-2; Middle Aged; Myocardial Infarction; Peripheral Arterial Disease; Renal Insufficiency, Chronic; risk factors; Self Report; Stroke; FABP1 protein, human
Introduction
CKD is an important risk factor for cardiovascular disease (CVD) events and death (1). However, mechanisms of CVD onset in CKD are not well established. For example, the pathways by which reductions in estimated eGFR and increases in albuminuria lead to worse CVD outcomes are uncertain.
Urine biomarkers of kidney tubular injury, such as neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), N-acetyl-β-d-glucosaminidase (NAG), and liver fatty acid-binding protein (L-FABP), originally identified as markers of AKI, have also been associated with both the incidence and progression of CKD (2–4). Importantly, CKD is strongly associated with CVD, and AKI has been reported to be associated with CVD events (5,6). These kidney injury biomarkers have also been shown to be associated with overall mortality in HIV (7) and in kidney transplant recipients (8). These markers may represent different injury pathways. NGAL and KIM-1 are upregulated after AKI events during the late-repair phase (as late as 4 weeks after the injury), along with a number of other gene products (9), whereas NAG is shed from injured tubule brush borders (10). L-FABP is thought to play an important antioxidant role under normal conditions and is upregulated by hypoxia (11). Understanding which biologic pathways are associated with CVD may allow for the design of future studies to explore the mechanistic link between these pathways and to evaluate the efficacy of interventions designed to reduce the burden of CVD in these patients.
Previous studies evaluating associations between these biomarkers and CVD events have not examined multiple biomarkers in an exclusively CKD population. We investigated whether NGAL, KIM-1, NAG, and L-FABP were associated with higher risk of heart failure (HF), atherosclerotic CVD events, and all-cause mortality among people with CKD enrolled in a prospective, multicenter study—the Chronic Renal Insufficiency Cohort (CRIC) Study.
Materials and Methods
Study Population
The design of the CRIC Study and baseline characteristics of the study participants have been previously described (12–14). Briefly, adults with an eGFR of 20–70 ml/min per 1.73 m2 were enrolled at 13 sites (clinical centers) in the United States. Important exclusion criteria included polycystic kidney disease, multiple myeloma, or GN on active immunosuppression. A total of 2466 participants who did not experience ESRD before the biomarker urine collection and who had valid measurements of all four biomarkers were analyzed. This was a joint study between the CRIC Study and the CKD Biomarkers Consortium. The CKD Biomarkers Consortium is a collaborative effort involving numerous investigators from multiple institutions working together to pursue the development and validation of novel biomarkers for CKD by assaying biologic specimens and utilizing data from the nation’s largest epidemiologic studies of kidney disease (http://ckdbiomarkersconsortium.org/).
Sample Collection and Laboratory and Clinical Measurements
We used urine samples designed specifically for biomarker studies collected as part of the parent CRIC protocol starting in 2005. These samples are not from study entry, but from the first study visit at which a special proteomic/biomarker urine sample was collected (for each participant, this study visit is the baseline visit for this analysis) (15). Biomarkers at this single time point were measured. Freshly voided urine samples were placed on ice immediately upon collection and, within 1 hour, were spun at 2000×g for 5 minutes in a refrigerated centrifuge; the supernatants were immediately transferred to new tubes and frozen at −80°C. For this analysis, a large screw-top cryovial tube of urine was thawed, realiquoted, and subaliquots were sent to the three performance laboratories for biomarker measurements. All urine aliquots used in this analysis had undergone only one previous freeze–thaw cycle. Each urinary biomarker was assayed at a single laboratory (16,17). Urinary albumin was quantified using an immunoturbidometric method and creatinine was measured by a kinetic colorimetric assay on a COBAS c501 automated analyzer (Roche, Indianapolis, IN), and L-FABP was measured by a two-site sandwich ELISA assay (CMIC, Tokyo, Japan). KIM-1 was measured by a microbead-based sandwich ELISA on a Bioplex-200 platform (Bio-Rad, Hercules, CA), and NAG was measured using an enzymatic assay (Roche). NGAL was measured by a noncompetitive sandwich assay with chemiluminescent signal detection on an ARCHITECT platform (Abbott Diagnostics, Abbott Park, IL). Blind replicate samples and proficiency samples were included for quality assurance and to detect assay drift over time, respectively (18).
To account for differences in urine concentration, our primary analysis was on the basis of biomarker levels normalized to urine creatinine concentration (the primary analysis for all CKD Biomarkers Consortium I studies of urinary biomarkers) (16,17). We performed sensitivity analyses of absolute urine biomarker concentrations. We also examined associations between the inverse of urine creatinine and all outcomes.
Outcomes
Our three outcomes of interest were time to first HF event, atherosclerotic CVD event (encompassing probable or definite myocardial infarction [MI], probable or definite ischemic stroke, or peripheral arterial disease events), and all-cause death, as previously described (5,12,14). Clinical cardiovascular outcomes (including acute MI, HF, arrhythmia, stroke, and peripheral arterial disease) were ascertained during follow-up by central adjudication of relevant medical records. Follow-up time started for each participant at the 2005 CRIC study visit at which the biomarker urine specimen was collected and continued until March 30, 2013. Death and loss to follow-up were considered as censoring events. Ascertainment of death was supplemented by crosslinkage with the Social Security Death Master File.
Statistical Analyses
All analyses were performed using SAS, version 9.3 (SAS Institute Inc., Cary, NC). We used Cox proportional hazards regression to examine the association of each biomarker with time to first HF event, atherosclerotic CVD event, or death. Because of the skewness of the distributions, biomarker levels were log-transformed and analyzed both as quintiles and continuously per SD. For L-FABP, a significant proportion of the cohort had biomarker levels that were below the lower limit of detection; consequently, we divided the cohort into five mutually exclusive groups: those with undetectable levels (n=388), those with detectable levels below the lower limit of detection established by the performance laboratory (n=472), and divided the remaining participants into tertiles of normalized biomarker levels. There was no evidence of nonlinearity in associations between eGFR or urine albumin-to-creatinine ratio (ACR) with CVD events or death. Given the discrepancy in the KIM-1/urinary creatinine (Cr) association with HF and in NAG/Cr with all three outcomes between the quintile and log-transformed analyses, we explored nonlinear relationships using spline analyses.
In addition to unadjusted analyses, we conducted a series of multivariable models to better understand the relationships between these injury biomarkers, established risk factors (19), and outcomes of interest. We first adjusted for demographic characteristics (age, sex, and race/ethnicity) (model 1); then conventional measures of kidney function, including eGFR (estimated using an internally derived CRIC Study equation on the basis of age, sex, race, and standardized serum creatinine and cystatin C measurements [20]), and ACR (model 2); and finally, established CVD risk factors, including diabetes mellitus, smoking status (never, former, or current), prior MI, prior coronary revascularization, known HF, prior ischemic stroke, diagnosed peripheral artery disease, systolic and diastolic BP, body mass index, LDL cholesterol level, HDL cholesterol level, and use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, aldosterone receptor antagonists, statins, and antiplatelet agents (clopidogrel and aspirin) (model 3). All comorbid conditions were determined concurrent with the primary biomarker measurement study visit. We also adjusted for CRIC Clinical Center in all analyses and verified that there was no violation of the proportional hazards assumption, using Schoenfeld residual plots.
Finally, in order to determine whether risk prediction models were improved by addition of the urinary biomarkers, we calculated the Harrell C-index for the demographic, eGFR, ACR, and cardiovascular risk factor model for each outcome. The Harrell C-index is a global index for validating the discriminatory ability of a survival model. It is the fraction of pairs in the data, where the observation with the higher survival time has the higher probability of survival predicted by the model. We then added each normalized biomarker to examine the change in C-index.
Results
Baseline Characteristics and Unadjusted Analyses
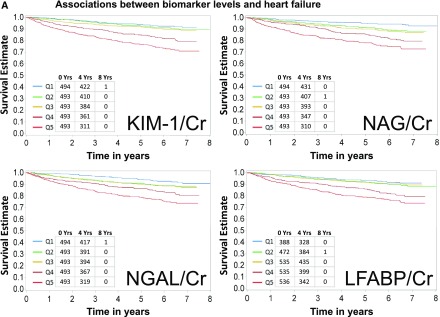
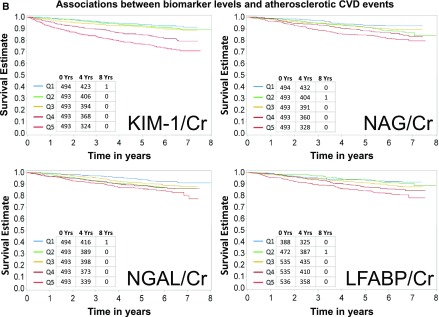
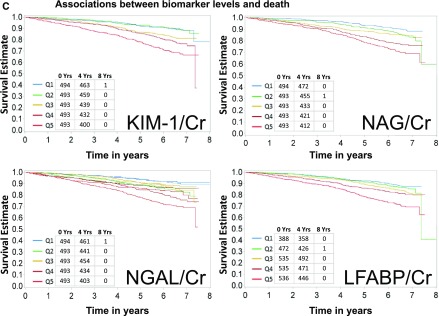
Baseline characteristics of the study population are shown in Table 1. Mean age was 59.5 years, 46% were women, 43% were white, 50% had diabetes mellitus, and 34% had a self-reported history of CVD. Median eGFR and ACR were 44 ml/min per 1.73 m2 and 53 mg/g, respectively. A total of 333 HF events, 282 atherosclerotic CVD events, and 440 deaths were observed during a median follow-up of 6.5 (interquartile range, 5.6–6.8) years. The crude event rates for each outcome were higher with higher levels of each of the four urine biomarkers of interest (Figure 1, A–C). Similarly, in unadjusted models, all four biomarkers were associated with all three outcomes of interest when biomarkers were examined either using quintiles or in a continuous variable (Tables 2–4). L-FABP/Cr was not analyzed as a continuous variable because of the high proportion of L-FABP values that were undetectable (15.7%) or below the lower limit of detection (19.1%). In order to describe the distributions of biomarkers compared with eGFR and ACR, we plotted each biomarker on a histogram for rapid observation of the predominantly linear relationship between each biomarker and eGFR and ACR (Supplemental Figure 1, A–D).
Table 1.
Baseline characteristics of CRIC Study participants with available biomarker data
| Characteristic | Adults with CKD, n=2466 |
|---|---|
| Age, yr, mean±SD | 59.5±10.8 |
| Women, N (%) | 1131 (46) |
| Race/ethnicity, N (%) | |
| Non-Hispanic white | 1049 (43) |
| Non-Hispanic black | 949 (38) |
| Hispanic | 378 (15) |
| Other | 90 (4) |
| Diabetes mellitus, N (%) | 1226 (50) |
| Cardiovascular disease, N (%) | 849 (34) |
| Urinary albumin-to-creatinine, mg/g, median (IQR) | 53 (6–503) |
| eGFR, ml/min per 1.73 m2, mean±SD | 44±18 |
| Systolic BP, mmHg, mean±SD | 127.1±22.1 |
| Diastolic BP, mmHg, mean±SD | 69.7±12.7 |
| Body mass index, kg/m2, mean±SD | 32.1±7.7 |
| ACEi/ARB | 69.6% |
| Aldosterone antagonist | 3.8% |
| Statins | 58.4% |
| Antiplatelet agents | 47.4% |
| KIM-1/Cr, ng/g, median (IQR) | 1399 (758–2618) |
| NGAL/Cr, mcg/g, median (IQR) | 12.75 (3.89–46.19) |
| L-FABP/Cr, mcg/g, median (IQR) | 7.31 (1.88–28.11) |
| NAG/Cr, U/g, median (IQR) | 4.03 (2.41–7.29) |
CRIC, Chronic Renal Insufficiency Cohort; IQR, interquartile range; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; KIM-1, kidney injury molecule-1; Cr, urinary creatinine; NGAL, neutrophil gelatinase-associated lipocalin; L-FABP, liver fatty acid-binding protein; NAG, N-acetyl-β-d-glucosaminidase.
Figure 1.
Higher levels of biomarkers are associated with higher rates of heart failure, atherosclerotic CVD events, and death. (A) Associations between biomarker levels and heart failure. Ranges of quintiles are as follows: KIM-1/Cr: Q1≤661 ng/g; Q2>661–1112 ng/g; Q3>1112–1831 ng/g; Q4>1831–2990 ng/g; Q5>2990 ng/g. NGAL/Cr: Q1≤2.63 mcg/g; Q2>2.63–8.40 mcg/g; Q3>8.4–20.2 mcg/g; Q4>20.2–64.07mcg/g; Q5>64.07 mcg/g. L-FABP/Cr: Q1, undetectable; Q2, detectable but below lower limit of detection; Q3<9.68 mcg/g; Q4≥9.68 to <33.8 mcg/g; Q5≥33.8 mcg/g. NAG/Cr: Q1≤2.08 U/g; Q2>2.08–3.33 U/g; Q3>3.33–4.96 U/g; Q4>4.96–8.36 U/g; Q5>8.36 U/g. (B) Associations between biomarker levels and atherosclerotic cardiovascular disease (CVD) events. Ranges of quintiles are as follows: KIM-1/Cr: Q1≤661 ng/g; Q2>661–1112 ng/g; Q3>1112–1831 ng/g; Q4>1831–2990 ng/g; Q5>2990 ng/g. NGAL/Cr: Q1≤2.63 mcg/g; Q2>2.63–8.40 mcg/g; Q3>8.4–20.2 mcg/g; Q4>20.2–64.07 mcg/g; Q5>64.07 mcg/g. L-FABP/Cr: Q1, undetectable; Q2, detectable but below lower limit of detection; Q3<9.68 mcg/g; Q4≥9.68 to <33.8 mcg/g; Q5≥33.8 mcg/g. NAG/Cr: Q1≤2.08 U/g; Q2>2.08–3.33 U/g; Q3>3.33–4.96 U/g; Q4>4.96–8.36 U/g; Q5>8.36 U/g. (C) Associations between biomarker levels and all-cause death. Ranges of quintiles are as follows: KIM-1/Cr: Q1≤661 ng/g; Q2>661–1112 ng/g; Q3>1112–1831 ng/g; Q4>1831–2990 ng/g; Q5>2990 ng/g. NGAL/Cr: Q1≤2.63 mcg/g; Q2>2.63–8.40 mcg/g; Q3>8.4–20.2 mcg/g; Q4>20.2–64.07 mcg/g; Q5>64.07 mcg/g. L-FABP/Cr: Q1, undetectable; Q2, detectable but below lower limit of detection; Q3<9.68 mcg/g; Q4≥9.68 to <33.8 mcg/g; Q5≥33.8 mcg/g. NAG/Cr: Q1≤2.08 U/g; Q2>2.08–3.33 U/g; Q3>3.33–4.96 U/g; Q4>4.96–8.36 U/g; Q5>8.36 U/g. KIM-1 kidney injury molecule-1. Cr, urinary creatinine; KIM-1, kidney injury molecule-1; L-FABP, liver fatty acid-binding protein; NAG, N-acetyl-β-d-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; Q, quintile.
Table 2.
Association between quintiles of normalized biomarker concentrations and the risk of heart failure
| Biomarker | Events | Unadjusted | Model 1: Demographica Adjusted | Model 2: Model 1+ACR+eGFR | Model 3: Model 2+CVD Risk Factorsb |
|---|---|---|---|---|---|
| KIM-1/Cr, ng/g | |||||
| ≤661 | 29 (10) | Ref | Ref | Ref | Ref |
| >661–1112 | 52 (19) | 1.84 (1.17 to 2.90) | 1.88 (1.19 to 2.96) | 1.38 (0.87 to 2.19) | 1.35 (0.84 to 2.18) |
| >1112–1831 | 56 (21) | 2.05 (1.31 to 3.21) | 2.11 (1.34 to 3.31) | 1.30 (0.82 to 2.07) | 1.11 (0.69 to 1.81) |
| >1831–2990 | 85 (33) | 3.26 (2.14 to 4.97) | 3.25 (2.12 to 4.99) | 1.64 (1.04 to 2.57) | 1.63 (1.02 to 2.60) |
| >2990 | 111 (50) | 4.87 (3.24 to 7.34) | 4.80 (3.15 to 7.31) | 1.72 (1.07 to 2.77) | 1.73 (1.05 to 2.85) |
| Per SD | n/a | n/a | n/a | n/a | |
| NGAL/Cr, mcg/g | |||||
| ≤2.63 | 37 (13) | Ref | Ref | Ref | Ref |
| >2.63–8.40 | 52 (19) | 1.47 (0.96 to 2.24) | 1.37 (0.90 to 2.10) | 1.09 (0.71 to 1.66) | 1.11 (0.72 to 1.71) |
| >8.4–20.2 | 55 (20) | 1.56 (1.03 to 2.36) | 1.39 (0.91 to 2.12) | 0.81 (0.52 to 1.25) | 0.81 (0.52 to 1.26) |
| >20.2–64.07 | 80 (32) | 2.41 (1.63 to 3.56) | 2.27 (1.52 to 3.40) | 0.87 (0.56 to 1.36) | 0.91 (0.58 to 1.42) |
| >64.07 | 109 (48) | 3.62 (2.49 to 5.26) | 3.57 (2.41 to 5.30) | 0.87 (0.54 to 1.40) | 0.99 (0.61 to 1.61) |
| Per SD | 1.54 (1.39 to 1.71) | 1.53 (1.37 to 1.71) | 0.91 (0.78 to 1.05) | 0.97 (0.83 to 1.13) | |
| L-FABP/Cr, mcg/g | |||||
| Undetectable | 32 (14) | Ref | Ref | Ref | Ref |
| Below LLD | 47 (18) | 1.27 (0.81 to 1.99) | 1.30 (0.83 to 2.04) | 1.11 (0.71 to 1.74) | 1.15 (0.73 to 1.83) |
| <9.68 | 54 (19) | 1.19 (0.76 to 1.85) | 1.18 (0.75 to 1.85) | 0.97 (0.62 to 1.52) | 0.95 (0.60 to 1.52) |
| ≥9.68–<33.8 | 80 (29) | 2.38 (1.59 to 3.57) | 2.29 (1.52 to 3.45) | 1.17 (0.76 to 1.78) | 1.16 (0.75 to 1.80) |
| ≥33.8 | 120 (48) | 3.33 (2.25 to 4.93) | 3.30 (2.20 to 4.95) | 1.01 (0.64 to 1.59) | 1.03 (0.64 to 1.66) |
| Per SD | n/p | n/p | n/p | n/p | |
| NAG/Cr, U/g | |||||
| ≤2.08 | 37 (13) | Ref | Ref | Ref | Ref |
| >2.08–3.33 | 45 (16) | 1.25 (0.81 to 1.93) | 1.14 (0.74 to 1.77) | 0.80 (0.51 to 1.25) | 0.77 (0.48 to 1.23) |
| >3.33–4.96 | 48 (18) | 1.38 (0.90 to 2.11) | 1.25 (0.81 to 1.93) | 0.64 (0.41 to 1.02) | 0.68 (0.42 to 1.09) |
| >4.96–8.36 | 84 (34) | 2.62 (1.78 to 3.86) | 2.45 (1.65 to 3.64) | 0.96 (0.61 to 1.49) | 1.02 (0.65 to 1.62) |
| >8.36 | 119 (53) | 4.08 (2.82 to 5.90) | 3.80 (2.59 to 5.56) | 1.09 (0.67 to 1.76) | 1.06 (0.64 to 1.75) |
| Per SD | n/a | n/a | n/a | n/a | |
Data are frequency (rate per 1000 person-yr) or HR (95% confidence interval). ACR, albumin-to-creatinine ratio; CVD, cardiovascular disease; KIM-1, kidney injury molecule-1; Cr, urinary creatinine; Ref, reference group; n/a, not applicable; NGAL, neutrophil gelatinase-associated lipocalin; L-FABP, liver fatty acid-binding protein; LLD, lower limit of detection; n/p, not possible; NAG, N-acetyl-β-d-glucosaminidase.
Demographic adjusted: age, sex, race/ethnicity, and clinical center.
CVD risk factors: diabetes mellitus, smoking, baseline CVD, systolic and diastolic BP, body mass index, LDL, HDL, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, statin, antiplatelet agents, and aldosterone antagonists. KIM-1/Cr and NAG/Cr relationships with heart failure events were not well described by the linear analysis and were better described by splines, thus per SD results are not reported. Because of the many undetectable values of L-FABP/Cr, continuous analyses are unable to be computed and are not reported.
Table 4.
Association between quintiles of normalized biomarker concentrations and the risk of all-cause death
| Biomarker | Events | Unadjusted | Model 1: Demographica Adjusted | Model 2: Model 1+ACR+eGFR | Model 3: Model 2+CVD Risk Factorsb |
|---|---|---|---|---|---|
| KIM-1/Cr, ng/g | |||||
| ≤661 | 53 (17) | Ref | Ref | Ref | Ref |
| >661–1112 | 51 (17) | 0.97 (0.66 to 1.42) | 0.99 (0.68 to 1.46) | 0.85 (0.48 to 1.26) | 0.79 (0.53 to 1.17) |
| >1112–1831 | 86 (29) | 1.72 (1.22 to 2.43) | 1.74 (1.23 to 2.45) | 1.31 (0.92 to 1.87) | 1.13 (0.78 to 1.62) |
| >1831–2990 | 106 (37) | 2.17 (1.56 to 3.02) | 2.10 (1.50 to 2.94) | 1.40 (0.98 to 1.99) | 1.20 (0.83 to 1.73) |
| >2990 | 144 (55) | 3.30 (2.41 to 4.52) | 3.28 (2.36 to 4.55) | 1.86 (1.28 to 2.71) | 1.56 (1.06 to 2.31) |
| Per SD | 1.59 (1.44 to 1.76) | 1.60 (1.44 to 1.78) | 1.30 (1.14 to 1.47) | 1.21 (1.06 to 1.39) | |
| NGAL/Cr, mcg/g | |||||
| ≤2.63 | 46 (15) | Ref | Ref | Ref | Ref |
| >2.63–8.40 | 77 (26) | 1.73 (1.20 to 2.50) | 1.61 (1.12 to 2.33) | 1.40 (0.97 to 2.03) | 1.47 (1.01 to 2.15) |
| >8.4–20.2 | 69 (23) | 1.53 (1.05 to 2.22) | 1.42 (0.98 to 2.08) | 1.04 (0.70 to 1.53) | 1.12 (0.75 to 1.66) |
| >20.2–64.07 | 108 (38) | 2.54 (1.80 to 3.59) | 2.56 (1.80 to 3.66) | 1.46 (0.99 to 2.15) | 1.48 (1.00 to 2.21) |
| >64.07 | 140 (52) | 3.56 (2.55 to 4.97) | 3.82 (2.69 to 5.41) | 1.66 (1.10 to 2.52) | 1.82 (1.19 to 2.80) |
| Per SD | 1.54 (1.41 to 1.68) | 1.59 (1.44 to 1.75) | 1.20 (1.06 to 1.36) | 1.22 (1.07 to 1.39) | |
| L-FABP/Cr, mcg/g | |||||
| Undetectable | 48 (19) | Ref | Ref | Ref | Ref |
| Below LLD | 63 (22) | 1.15 (0.79 to 1.68) | 1.15 (0.79 to 1.68) | 1.02 (0.70 to 1.49) | 0.99 (0.67 to 1.46) |
| <9.68 | 86 (27) | 1.42 (1.00 to 2.02) | 1.36 (0.95 to 1.94) | 1.23 (0.86 to 1.75) | 1.18 (0.82 to 1.71) |
| ≥9.68–<33.8 | 94 (31) | 1.67 (1.18 to 2.37) | 1.53 (1.08 to 2.18) | 0.98 (0.68 to 1.42) | 0.94 (0.64 to 1.37) |
| ≥33.8 | 149 (51) | 2.75 (1.99 to 3.81) | 2.71 (1.93 to 3.79) | 1.23 (0.84 to 1.81) | 1.18 (0.79 to 1.76) |
| Per SD | n/p | n/p | n/p | n/p | n/p |
| NAG/Cr, U/g | |||||
| ≤2.08 | 49 (16) | Ref | Ref | Ref | Ref |
| >2.08–3.33 | 72 (24) | 1.5 (1.05 to 2.16) | 1.41 (0.97 to 2.03) | 1.15 (0.79 to 1.66) | 1.13 (0.77 to 1.66) |
| >3.33–4.96 | 72 (25) | 1.58 (1.1 to 2.26) | 1.44 (1.00 to 2.07) | 0.97 (0.66 to 1.43) | 0.91 (0.61 to 1.36) |
| >4.96–8.36 | 108 (38) | 2.47 (1.76 to 3.47) | 2.30 (1.63 to 3.25) | 1.30 (0.88 to 1.92) | 1.26 (0.84 to 1.87) |
| >8.36 | 139 (51) | 3.3 (2.38 to 4.57) | 3.26 (2.33 to 4.56) | 1.64 (1.08 to 2.49) | 1.36 (0.88 to 2.11) |
| Per SD | 1.45 (1.34 to 1.58) | 1.52 (1.39 to 1.67) | 1.28 (1.13 to 1.45) | 1.20 (1.04 to 1.37) | |
Data are frequency (rate per 1000 person-yr) or HR (95% confidence interval). ACR, albumin-to-creatinine ratio; CVD, cardiovascular disease; KIM-1, kidney injury molecule-1; Cr, urinary creatinine; Ref, reference group; n/a, not applicable; NGAL, neutrophil gelatinase-associated lipocalin; L-FABP, liver fatty acid-binding protein; LLD, lower limit of detection; n/p, not possible; NAG, N-acetyl-β-d-glucosaminidase.
Demographic adjusted: age, sex, race/ethnicity, and clinical center.
CVD risk factors: diabetes mellitus, smoking, baseline CVD, systolic and diastolic BP, body mass index, LDL, HDL, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, statin, antiplatelet agents, aldosterone antagonists. Because of the many undetectable values of L-FABP/Cr, continuous analyses are unable to be computed and are not reported.
Associations of Biomarkers with HF Outcomes
Associations between KIM-1/Cr and HF were attenuated after adjustment for eGFR and ACR but remained statistically significant in the final model (Table 2). In the final adjusted model (model 3), those in the highest two quintiles of KIM-1/Cr levels had higher adjusted rates of HF relative to the lowest quintile (quintile 5 versus quintile 1 adjusted hazard ratio [aHR] of 1.73 [95% confidence interval (95% CI), 1.05 to 2.85]; quintile 4 versus quintile 1 aHR of 1.63 [95% CI, 1.02 to 2.60]), but KIM-1/Cr was not independently associated with HF when examined as a continuous variable. Although NGAL/Cr, NAG/Cr, and L-FABP/Cr were associated with HF in unadjusted models, these associations were attenuated in the adjusted models, in particular after taking into account concomitant eGFR and ACR (model 2) (Table 2). We found that the relationships of KIM-1/Cr and NAG/Cr with HF events were not well described by the linear analysis and were better described by splines (Supplemental Figure 2, A and B) or by the quintile (categorical) analyses. Consequently, the HR for the log SD analysis for the relationships of KIM-1/Cr and NAG/Cr with HF events are not reported.
Associations of Biomarkers with Atherosclerotic CVD Outcomes
After adjustment, higher KIM-1/Cr was independently associated with atherosclerotic CVD events in the continuous analysis only (Table 3). Similar to our findings with HF outcomes, the associations of NGAL/Cr, NAG/Cr, and L-FABP/Cr with atherosclerotic CVD events were no longer statistically significant after adjusting for measures of renal function (model 2) or in the final adjusted model (Table 3). The association between NAG/Cr and atherosclerotic CVD events was better described by a spline (Supplemental Figure 2C) or by the quintile (categorical) analysis.
Table 3.
Association between quintiles of normalized biomarker concentrations and the risk of atherosclerotic CVD events
| Biomarker | Events | Unadjusted | Model 1: Demographica Adjusted | Model 2: Model 1+ACR+eGFR | Model 3: Model 2+CVD Risk Factorsb |
|---|---|---|---|---|---|
| KIM-1/Cr, ng/g | |||||
| ≤661 | 29 (10) | Ref | Ref | Ref | Ref |
| >661–1112 | 47 (17) | 1.67 (1.05 to 2.65) | 1.76 (1.11 to 2.80) | 1.47 (0.92 to 2.35) | 1.45 (0.91 to 2.33) |
| >1112–1831 | 45 (17) | 1.64 (1.03 to 2.61) | 1.71 (1.07 to 2.74) | 1.25 (0.77 to 2.02) | 1.06 (0.65 to 1.73) |
| >1831–2990 | 82 (32) | 3.16 (2.07 to 4.83) | 3.23 (2.09 to 4.97) | 2.07 (1.31 to 3.27) | 1.72 (1.08 to 2.74) |
| >2990 | 79 (34) | 3.38 (2.21 to 5.17) | 3.69 (2.38 to 5.72) | 1.88 (1.15 to 3.09) | 1.56 (0.93 to 2.61) |
| Per SD | 1.61 (1.42 to 1.82) | 1.66 (1.46 to 1.89) | 1.32 (1.13 to 1.54) | 1.21 (1.02 to 1.42) | |
| NGAL/Cr, mcg/g | |||||
| ≤2.63 | 37 (13) | Ref | Ref | Ref | Ref |
| >2.63–8.40 | 61 (23) | 1.73 (1.15 to 2.60) | 1.71 (1.13 to 2.59) | 1.43 (0.95 to 2.17) | 1.58 (1.03 to 2.43) |
| >8.4–20.2 | 51 (19) | 1.42 (0.93 to 2.17) | 1.39 (0.90 to 2.15) | 0.94 (0.60 to 1.47) | 1.14 (0.72 to 1.80) |
| >20.2–64.07 | 60 (23) | 1.78 (1.18 to 2.68) | 2.01 (1.32 to 3.07) | 1.04 (0.65 to 1.65) | 1.32 (0.82 to 2.13) |
| >64.07 | 73 (31) | 2.32 (1.56 to 3.45) | 2.92 (1.92 to 4.43) | 1.10 (0.67 to 1.82) | 1.29 (0.76 to 2.18) |
| Per SD | 1.32 (1.17 to 1.48) | 1.43 (1.26 to 1.62) | 1.03 (0.88 to 1.21) | 1.08 (0.92 to 1.28) | |
| L-FABP/Cr, mcg/g | |||||
| Undetectable | 30 (13) | Ref | Ref | Ref | Ref |
| Below LLD | 39 (15) | 1.10 (0.69 to 1.78) | 1.10 (0.68 to 1.78) | 0.98 (0.61 to 1.59) | 1.00 (0.61 to 1.63) |
| <9.68 | 57 (19) | 1.46 (0.94 to 2.28) | 1.44 (0.92 to 2.25) | 1.27 (0.81 to 1.98) | 1.24 (0.78 to 1.97) |
| ≥9.68–<33.8 | 69 (25) | 1.89 (1.23 to 2.91) | 1.91 (1.24 to 2.95) | 1.20 (0.76 to 1.89) | 1.22 (0.76 to 1.95) |
| ≥33.8 | 87 (34) | 2.60 (1.71 to 3.93) | 2.86 (1.87 to 4.38) | 1.29 (0.79 to 2.11) | 1.33 (0.80 to 2.22) |
| Per SD | n/p | n/p | n/p | n/p | |
| NAG/Cr, U/g | |||||
| ≤2.08 | 37 (13) | Ref | Ref | Ref | Ref |
| >2.08–3.33 | 57 (21) | 1.71 (1.12 to 2.61) | 1.65 (1.08 to 2.52) | 1.25 (0.81 to 1.93) | 1.06 (0.68 to 1.64) |
| >3.33–4.96 | 47 (17) | 1.44 (0.93 to 2.24) | 1.39 (0.89 to 2.16) | 0.82 (0.51 to 1.31) | 0.72 (0.44 to 1.15) |
| >4.96–8.36 | 63 (25) | 2.06 (1.36 to 3.11) | 2.02 (1.33 to 3.08) | 0.94 (0.58 to 1.51) | 0.74 (0.45 to 1.21) |
| >8.36 | 80 (34) | 2.80 (1.88 to 4.17) | 3.06 (2.03 to 4.61) | 1.11 (0.66 to 1.86) | 0.77 (0.45 to 1.33) |
| Per SD | n/a | n/a | n/a | n/a | |
Data are frequency (rate per 1000 person-yr) or HR (95% confidence interval). CVD, cardiovascular disease; ACR, albumin-to-creatinine ratio; KIM-1, kidney injury molecule-1; Cr, urinary creatinine; Ref, reference group; n/a, not applicable; NGAL, neutrophil gelatinase-associated lipocalin; L-FABP, liver fatty acid-binding protein; LLD, lower limit of detection; n/p, not possible; NAG, N-acetyl-β-d-glucosaminidase.
Demographic adjusted: age, sex, race/ethnicity, and clinical center.
CVD risk factors: diabetes mellitus, smoking, baseline CVD, systolic and diastolic BP, body mass index, LDL, HDL, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, statin, antiplatelet agents, and aldosterone antagonists. NAG/Cr relationships with atherosclerotic events were not well described by the linear analysis and were better described by splines, thus per SD results are not reported. Because of the many undetectable values of L-FABP/Cr, continuous analyses are unable to be computed and are not reported.
Associations of Biomarkers and All-Cause Death
Both KIM-1/Cr and NGAL/Cr were associated with a higher risk of all-cause death in the final adjusted quintile analysis (Table 4) and in the continuous analysis. NAG/Cr was associated with death in the quintile analysis even after adjustment for eGFR and ACR (model 2), but this association was attenuated and no longer statistically significant in the final adjusted model. However, in the continuous analysis, NAG/Cr remained associated with death (aHR per log SD higher 1.20 [95% CI, 1.04 to 1.37]). L-FABP/Cr was not associated with death after adjustment for potential confounders.
Sensitivity Analyses
In unadjusted analyses, all non-normalized (raw) biomarkers were associated with HF, atherosclerotic CVD events, and death (Supplemental Tables 1–3). In contrast to the normalized biomarker analysis, KIM-1 was not associated with HF. For atherosclerotic CVD events, results were similar for KIM-1 and KIM-1/Cr. We also examined spline relationships for the non-normalized biomarkers; in this case, only the relationship between NAG and CVD was nonlinear (Supplemental Figure 2D). NGAL was associated with death in continuous analyses (aHR per log SD higher 1.14 [95% CI, 1.01 to 1.28]). To ensure that normalizing our biomarkers would not influence our results, we also examined associations between the inverse of urine creatinine and all outcomes. Results were not statistically significant in unadjusted or adjusted models for HF or atherosclerotic CVD events, and were borderline significant in unadjusted models for the death outcome only (Supplemental Table 4).
Change in Risk Prediction
We examined the C-indices for a base model that included the clinical variables (demographics and cardiovascular risk factors) along with eGFR and ACR. Each normalized biomarker was added to the model separately; however, the C-index did not change appreciably with the addition of any of the four novel urinary biomarkers (Table 5).
Table 5.
C-index for all outcomes
| Model | C-Index | ||
|---|---|---|---|
| Heart Failure | Atherosclerotic Events | Death | |
| Model 1=Final model without eGFR and ACR | 0.80 | 0.77 | 0.74 |
| Model 2=Model 1+eGFR and ACR | 0.81 | 0.78 | 0.77 |
| Model 2+KIM-1/Cr | 0.81 | 0.78 | 0.77 |
| Model 2+NAG/Cr | 0.81 | 0.78 | 0.77 |
| Model 2+NGAL/Cr | 0.81 | 0.78 | 0.77 |
| Model 2+LFABP/Cr | 0.81 | 0.78 | 0.77 |
Final model without eGFR and ACR includes demographics (age, sex, race/ethnicity, clinical center) and CVD risk factors (diabetes mellitus, smoking, baseline CVD, systolic and diastolic BP, body mass index, LDL, HDL, angiotensin-converting enzyme inhibitor, angiotensin receptor blocker; statin, antiplatelet agents, aldosterone antagonists). ACR, albumin-to-creatinine ratio; KIM-1, kidney injury molecule-1; Cr, urinary creatinine; NAG, N-acetyl-β-d-glucosaminidase; NGAL, neutrophil gelatinase-associated lipocalin; L-FABP, liver fatty acid-binding protein; CVD, cardiovascular disease.
Discussion
In a large, well characterized cohort of adults with CKD, we found that urine KIM-1/Cr was independently associated with HF events, atherosclerotic CVD events, and all-cause death and that urine NGAL/Cr was independently associated with death, but not with HF or atherosclerotic CVD events. Although CKD is a known risk factor for these adverse outcomes, the predictive value of urine kidney injury biomarkers beyond eGFR and ACR is less well established. We demonstrated that after adjustment for eGFR and ACR, KIM/Cr and NGAL/Cr were associated with adverse outcomes in the setting of CKD. In contrast, L-FABP/Cr was not associated with the outcomes we studied after multivariable adjustment, and NAG/Cr was only associated with death in the continuous analysis.
Our findings of the association of selected urine kidney injury biomarkers with prospectively observed and adjudicated outcomes in a large cohort of adults with CKD are an important addition to previous work linking these biomarkers with adverse clinical events. These biomarkers were chosen by the CKD Biomarkers Consortium at the time this study was designed as the most promising tubular injury markers in development. To our knowledge, this is one of the only studies to examine multiple kidney injury biomarkers in a CKD population. NGAL/Cr has been associated with death but not with CVD events in studies of elderly men (21), and our study extends those findings to a slightly younger cohort with existing CKD. We have shown previously among CRIC Study participants that NGAL/Cr was associated with atherosclerotic CVD events but not with HF or mortality (5). However, in that study, NGAL was measured in urine from a 24-hour collection that could have been stored at study participant’s home for as long as 1 week, with the possibility that intervening protein degradation may have biased findings toward the null or that other sources of NGAL (for example, leukocytes in the urine) may have been measured. In this study, we analyzed urine samples that were rapidly processed after voiding under standardized conditions. Another difference is that individuals from the original CRIC Study who experienced progression to ESRD before these protocolized urine collections were started in CRIC were not included in this analysis, and those individuals may have included some at the highest risk for atherosclerotic CVD events.
Our findings that KIM-1/Cr levels were associated with HF events, atherosclerotic CVD events, and death differ from the results of the Health ABC Study comprising older community-dwelling individuals, in which postadjustment KIM-1/Cr was associated with death but not with CVD events (22). Similarly, in an analysis of prevalent kidney transplant recipients, NGAL/Cr was associated with higher risks of CVD events, death, and graft failure, whereas KIM-1/Cr was associated with higher risk of death only (8). In an HIV-positive population, these biomarkers were associated with higher rates of death in demographic-adjusted models, whereas associations of KIM-1 with mortality were borderline significant after adjustment for kidney function (7). The results from these cohorts may not be directly comparable to ours because they involve populations with different clinical characteristics. Nonetheless, the associations of KIM-1 with adverse outcomes beyond worsening kidney disease (23) are intriguing, particularly in light of the differing patterns of disease in each of these populations (i.e., native kidney disease in older adults [22], kidney transplant patients [8], and tenofovir-induced tubular dysfunction [7]). Importantly, although these biomarkers do not substantially improve risk prediction on the basis of the change in the C-index (which is only one method of assessing incremental discrimination), the associations detected suggest as yet unknown biologic mechanisms that may improve our understanding of increased CVD risk in the CKD population. KIM-1 may reflect ongoing kidney proximal tubule injury that captures additional systemic risk on the pathway toward CVD and death, even though in a recent cross-sectional study in individuals free of CVD at baseline, KIM-1 and NGAL were not associated with subclinical measures of CVD (24). Studies in rodents demonstrate that both NGAL and KIM-1 are transcriptionally upregulated after ischemia (9); similarly, the associations we demonstrated, together with their implications for proximal tubule injury, may reflect kidney response to a systemic influence or a systemic response to ongoing proximal tubule injury. Although direct comparison of novel biomarker measurements is complex, our KIM-1 measurements were performed in a laboratory that routinely measures KIM-1 with rigorous quality control procedures in place to assure that there is not substantial assay drift over time. In a recent study of patients in intensive care units with AKI (25), median KIM-1 levels measured by the same laboratory as in our study were approximately four-fold higher than levels in our CKD population. It is of interest that the median levels of KIM-1 in our study were substantially lower than those reported in AKI but were still significantly associated with clinical outcomes. Further work in both mouse and human studies may help to determine potential mechanisms that support these associations.
Strengths of our work include the prospective design, large sample size, rigorous outcome adjudication process, and broad ethnic and racial diversity of the study participants. In addition, biomarker measurements were performed on samples collected under uniform conditions using well established assays. Our study also has limitations. By design, some underlying CKD causes (including polycystic kidney disease) were not represented. Urine samples were collected at the time of the study visit, regardless of time of day, and it is unknown how much diurnal variation there is in levels of these biomarkers. Cause-specific etiologies of death are unknown at this time. Finally, biomarker concentrations were ascertained only at a single time point, and we were unable to examine the relationship between changes in biomarker levels over time and subsequent outcomes.
In conclusion, increased levels of urine KIM-1/Cr and NGAL/Cr may reflect kidney injury that encompasses mechanisms associated with higher risk of certain CVD events and death in the setting of CKD. Understanding these mechanisms may help us to delineate the pathways by which CKD increases risk of CVD and death and future interventions to reduce these adverse outcomes.
Disclosures
This study was carried out under the auspices of the CKD Biomarkers Consortium, which was established in 2008 by the National Institute of Diabetes and Digestive and Kidney Diseases to advance the field of CKD biomarker discovery and validation (http://grants.nih.gov/grants/guide/rfa-files/RFA-DK-08-015.html) (16,17). J.V.B. appears as coinventor on KIM-1 patents which have been licensed by Partners Healthcare to a number of companies. He has received royalty income from Partners Healthcare. K.D.L. had reagents donated for previous biomarker studies by Abbott and CMIC. C.-y.H. had reagents donated for previous biomarker studies by Abbott. A.S.G. has received relevant research grants through Kaiser Permanente Northern California Division of Research from the National Institute of Diabetes and Digestive and Kidney Diseases (U01 DK06092 and R01 DK098233) and also from CSL Behring.
Supplementary Material
Acknowledgments
We thank Abbott Laboratories for supporting the measurement of urinary NGAL and CMIC for providing control materials for our studies. We also thank E. Cotter at University College Dublin, Dublin, Ireland for performing the urinary NGAL assays.
This work was supported by the CKD Biomarker Consortium funded by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grants U01DK85649, U01DK085660, and U01DK085689, and by the Intramural Research Program of the NIDDK. Funding for the Chronic Renal Insufficiency Cohort (CRIC) Study was obtained under a cooperative agreement from the NIDDK (grants U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by the following: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award National Institutes of Health (NIH)/National Center for Advancing Translational Sciences (NCATS) grant UL1TR000003; Johns Hopkins University grant UL1 TR-000424; University of Maryland General Clinical Research Center grant M01 RR-16500; the Clinical and Translational Science Collaborative of Cleveland, grant UL1TR000439 from the NCATS component of the NIH and NIH roadmap for Medical Research; Michigan Institute for Clinical and Health Research grant UL1TR000433; University of Illinois at Chicago Clinical and Translational Science award UL1RR029879; Tulane University Translational Research in Hypertension and Renal Biology grant P30GM103337; and Kaiser Permanente NIH/National Center for Research Resources University of California, San Francisco Clinical and Translational Science Institute grant UL1 RR-024131. M.P. is supported by NIH NIDDK grant K23 DK099238.
A portion of this work was presented at the American Society of Nephrology Annual Meeting in San Diego, CA, November 5, 2015 [TH-OR121, TH-PO228].
Abbott Laboratories and CMIC had no role in study design, data collection, data analysis, data interpretation or writing of the report. CRIC Study Investigators also include Lawrence J. Appel (Welch Center for Prevention, Epidemiology and Clinical Research, Johns Hopkins University, Baltimore, Maryland), Jiang He (Departments of Medicine and Epidemiology, Tulane University, New Orleans, Louisiana), James P. Lash (Section of Nephrology, Department of Medicine, University of Illinois at Chicago, Chicago, IL), Akinlolu Ojo (Department of Medicine, University of Michigan, Ann Arbor, Michigan), and Raymond R. Townsend (Department of Medicine and Center Center for Clinical Epidemiology and Biostatistics, University of Pennsylvania, Philadelphia, Pennsylvania).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08560816/-/DCSupplemental.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 2.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA: Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol 212: 209–217, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Peralta CA, Katz R, Bonventre JV, Sabbisetti V, Siscovick D, Sarnak M, Shlipak MG: Associations of urinary levels of kidney injury molecule 1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL) with kidney function decline in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 60: 904–911, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolignano D, Lacquaniti A, Coppolino G, Donato V, Campo S, Fazio MR, Nicocia G, Buemi M: Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin J Am Soc Nephrol 4: 337–344, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu KD, Yang W, Go AS, Anderson AH, Feldman HI, Fischer MJ, He J, Kallem RR, Kusek JW, Master SR, Miller ER 3rd, Rosas SE, Steigerwalt S, Tao K, Weir MR, Hsu CY; CRIC Study Investigators : Urine neutrophil gelatinase-associated lipocalin and risk of cardiovascular disease and death in CKD: Results from the chronic renal insufficiency cohort (CRIC) Study. Am J Kidney Dis 65: 267–274, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gammelager H, Christiansen CF, Johansen MB, Tønnesen E, Jespersen B, Sørensen HT: Three-year risk of cardiovascular disease among intensive care patients with acute kidney injury: A population-based cohort study. Crit Care 18: 492, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peralta C, Scherzer R, Grunfeld C, Abraham A, Tien P, Devarajan P, Bennett M, Butch A, Anastos K, Cohen M, Nowicki M, Sharma A, Young M, Sarnak M, Parikh C, Shlipak M: Urinary biomarkers of kidney injury are associated with all-cause mortality in the Women’s Interagency HIV Study (WIHS). HIV Med 15: 291–300, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bansal N, Carpenter MA, Weiner DE, Levey AS, Pfeffer M, Kusek JW, Cai J, Hunsicker LG, Park M, Bennett M, Liu KD, Hsu CY: Urine injury biomarkers and risk of adverse outcomes in recipients of prevalent kidney transplants: The folic acid for vascular outcome reduction in transplantation trial. J Am Soc Nephrol 27: 2109–2021, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko GJ, Grigoryev DN, Linfert D, Jang HR, Watkins T, Cheadle C, Racusen L, Rabb H: Transcriptional analysis of kidneys during repair from AKI reveals possible roles for NGAL and KIM-1 as biomarkers of AKI-to-CKD transition. Am J Physiol Renal Physiol 298: F1472–F1483, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Sinha V, Vence LM, Salahudeen AK. Urinary tubular protein-based biomarkers in the rodent model of cisplatin nephrotoxicity: A comparative analysis of serum creatinine, renal histology, and urinary KIM-1, NGAL, and NAG in the initiation, maintenance, and recovery phases of acute kidney injury. J Investig Med 2013;61(3):564–568. [DOI] [PubMed] [Google Scholar]
- 11.Kamijo A, Kimura K, Sugaya T, Yamanouchi M, Hikawa A, Hirano N, Hirata Y, Goto A, Omata M: Urinary fatty acid-binding protein as a new clinical marker of the progression of chronic renal disease. J Lab Clin Med 143: 23–30, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators : The chronic renal insufficiency cohort (CRIC) study: Design and methods. J Am Soc Nephrol 14[Suppl 2]: S148–S153, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Fischer MJ, Go AS, Lora CM, Ackerson L, Cohan J, Kusek JW, Mercado A, Ojo A, Ricardo AC, Rosen LK, Tao K, Xie D, Feldman HI, Lash JP; CRIC and H-CRIC Study Groups : CKD in Hispanics: Baseline characteristics from the CRIC (chronic renal insufficiency cohort) and Hispanic-CRIC Studies. Am J Kidney Dis 58: 214–227, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic renal insufficiency cohort (CRIC) study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu C-y, Xie D, Waikar SS, Bonventre JV, Zhang X, Sabbisetti V, Mifflin TE, Coresh J, Diamantidis CJ, He J, Lora CM, Miller ER, Nelson RG, Ojo AO, Rahman M, Schelling JR, Wilson FP, Kimmel PL, Feldman HI, Vasan RS, Liu KD; CRIC Study Investigators : CKD Biomarkers Consortium: Urine biomarkers of tubular injury do not improve on the clinical model predicting chronic kidney disease progression. [published online ahead of print October 28, 2016] Kidney Int : 10.1016/j.kint.2016.09.003 [DOI] [Google Scholar]
- 16.Fufaa GD, Weil EJ, Nelson RG, Hanson RL, Bonventre JV, Sabbisetti V, Waikar SS, Mifflin TE, Zhang X, Xie D, Hsu CY, Feldman HI, Coresh J, Vasan RS, Kimmel PL, Liu KD; Chronic Kidney Disease Biomarkers Consortium Investigators : Association of urinary KIM-1, L-FABP, NAG and NGAL with incident end-stage renal disease and mortality in American Indians with type 2 diabetes mellitus. Diabetologia 58: 188–198, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foster MC, Coresh J, Bonventre JV, Sabbisetti VS, Waikar SS, Mifflin TE, Nelson RG, Grams M, Feldman HI, Vasan RS, Kimmel PL, Hsu CY, Liu KD; CKD Biomarkers Consortium : Urinary biomarkers and risk of ESRD in the atherosclerosis risk in communities study. Clin J Am Soc Nephrol 10: 1956–1963, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu CY, Ballard S, Batlle D, Bonventre JV, Böttinger EP, Feldman HI, Klein JB, Coresh J, Eckfeldt JH, Inker LA, Kimmel PL, Kusek JW, Liu KD, Mauer M, Mifflin TE, Molitch ME, Nelsestuen GL, Rebholz CM, Rovin BH, Sabbisetti VS, Van Eyk JE, Vasan RS, Waikar SS, Whitehead KM, Nelson RG; CKD Biomarkers Consortium : Cross-disciplinary biomarkers research: Lessons learned by the CKD biomarkers consortium. Clin J Am Soc Nephrol 10: 894–902, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Townsend RR, Anderson AH, Chen J, Gadebegku CA, Feldman HI, Fink JC, Go AS, Joffe M, Nessel LA, Ojo A, Rader DJ, Reilly MP, Teal V, Teff K, Wright JT, Xie D: Metabolic syndrome, components, and cardiovascular disease prevalence in chronic kidney disease: Findings from the chronic renal insufficiency cohort (CRIC) study. Am J Nephrol 33: 477–484, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson AH, Yang W, Hsu CY, Joffe MM, Leonard MB, Xie D, Chen J, Greene T, Jaar BG, Kao P, Kusek JW, Landis JR, Lash JP, Townsend RR, Weir MR, Feldman HI; CRIC Study Investigators : Estimating GFR among participants in the chronic renal insufficiency cohort (CRIC) Study. Am J Kidney Dis 60: 250–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helmersson-Karlqvist J, Larsson A, Carlsson AC, Venge P, Sundström J, Ingelsson E, Lind L, Arnlöv J: Urinary neutrophil gelatinase-associated lipocalin (NGAL) is associated with mortality in a community-based cohort of older Swedish men. Atherosclerosis 227: 408–413, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Sarnak MJ, Katz R, Newman A, Harris T, Peralta CA, Devarajan P, Bennett MR, Fried L, Ix JH, Satterfield S, Simonsick EM, Parikh CR, Shlipak MG; Health ABC Study : Association of urinary injury biomarkers with mortality and cardiovascular events. J Am Soc Nephrol 25: 1545–1553, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shlipak MG, Scherzer R, Abraham A, Tien PC, Grunfeld C, Peralta CA, Devarajan P, Bennett M, Butch AW, Anastos K, Cohen MH, Nowicki M, Sharma A, Young MA, Sarnak MJ, Parikh CR: Urinary markers of kidney injury and kidney function decline in HIV-infected women. J Acquir Immune Defic Syndr 61: 565–573, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park M, Shlipak MG, Vittinghoff E, Katz R, Siscovick D, Sarnak M, Lima JA, Hsu CY, Peralta CA: Associations of kidney injury markers with subclinical cardiovascular disease: The multi-ethnic study of atherosclerosis. Clin Nephrol 84: 358–363, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabbisetti VS, Waikar SS, Antoine DJ, Smiles A, Wang C, Ravisankar A, Ito K, Sharma S, Ramadesikan S, Lee M, Briskin R, De Jager PL, Ngo TT, Radlinski M, Dear JW, Park KB, Betensky R, Krolewski AS, Bonventre JV: Blood kidney injury molecule-1 is a biomarker of acute and chronic kidney injury and predicts progression to ESRD in type I diabetes. J Am Soc Nephrol 25: 2177–2186, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.