Abstract
Background
and objectives Patients with CKD are at risk of hospital-acquired complications (HACs). We sought to determine the association of preventable HACs with mortality, length of stay (LOS), and readmission.
Design, setting, participants, & measurements
All adults hospitalized from April of 2003 to March of 2008 in Alberta were characterized by kidney function and occurrence of preventable HACs. CKD was defined by eGFR<60 ml/min per 1.73 m2 and/or albumin-to-creatinine ratio >3–30 mg/mmol for >3 months in the time frame from 365 to 90 days before admission. Regression models examined the association of HACs with outcomes.
Results
Of 536,549 hospitalizations, 8.5% (n=45,733) had CKD and 9.8% of patients with CKD had one or more potentially preventable HAC. In patients with potentially preventable HACs, proportions of death within index hospitalization and from discharge to 90 days were 17.7% and 6.8%, respectively. In patients with CKD, comparing with those hospitalizations without potentially preventable HACs, the adjusted odds ratio (OR) of mortality during index hospitalization and from hospital discharge to 90 days in patients with one or more preventable HAC was 4.67 (95% confidence interval [95% CI], 4.17 to 5.22) and 1.08 (95% CI, 0.94 to 1.25), respectively. Median incremental LOS in patients with one or more preventable HAC was 9.86 days (95% CI, 9.25 to 10.48). The OR for readmission with preventable HAC was 1.24 (95% CI, 1.15 to 1.34). In a cohort with and without CKD, the adjusted ORs of mortality during index hospitalization in patients with CKD and no preventable HACs, patients without CKD and with preventable HACs, and patients with CKD and preventable HACs were 2.22 (95% CI, 1.69 to 2.94), 5.26 (95% CI, 4.98 to 5.55), and 9.56 (95% CI, 7.23 to 12.56), respectively (referenced to patients without CKD or preventable HACs).
Conclusions
Preventable HACs are associated with higher mortality, incremental LOS, and greater risk of readmission, especially in people with CKD. Targeted strategies to reduce complications should be a high priority.
Keywords: chronic kidney disease; Epidemiology and outcomes; Adult; Alberta; Albumins; creatinine; hospitalization; Humans; Length of Stay; Odds Ratio; Patient Discharge; Patient Readmission; Receptor, Epidermal Growth Factor; Renal Insufficiency, Chronic; Risk; EGFR protein, human
Introduction
Hospital-acquired complications (HACs) are undesirable and unintended clinical conditions, distinct from the admitting diagnosis and other conditions present at admission, that may occur during hospitalization. HACs are common and occur in 2.9%–23% of all hospitalizations (1–3), and in a general population are associated with poor outcomes, including higher mortality, greater readmission, and longer length of stay (LOS) in hospital, compared with those without complications (4–7). Hospitalization is common among patients with CKD, and may confer susceptibility to HACs because of increased risk of bleeding, electrolyte abnormalities, and adverse drug effects, among others. Studies conducted in the United States and Canada have demonstrated that the presence of CKD is associated with a greater risk of potentially preventable HAC than patients with normal kidney function (8,9). Targeting prevention efforts on patients at high risk of HAC, such as those with CKD, readily identified through commonly conducted laboratory tests, may be warranted.
In hospitalized patients with CKD, the association of mortality, LOS, and readmission with preventable HACs has not been determined to our knowledge. The clinical outcomes associated with preventable HACs are important to understand to frame the potential benefit of strategies aimed at reducing complications. Understanding the consequences of these complications on outcomes will inform prioritization and the scope of investment in prevention efforts.
Using a population-based cohort from Alberta, Canada, we sought to determine the association of preventable HACs on clinical outcomes of mortality, LOS, and readmission, in hospitalized patients including those with CKD.
Materials and Methods
Study Population
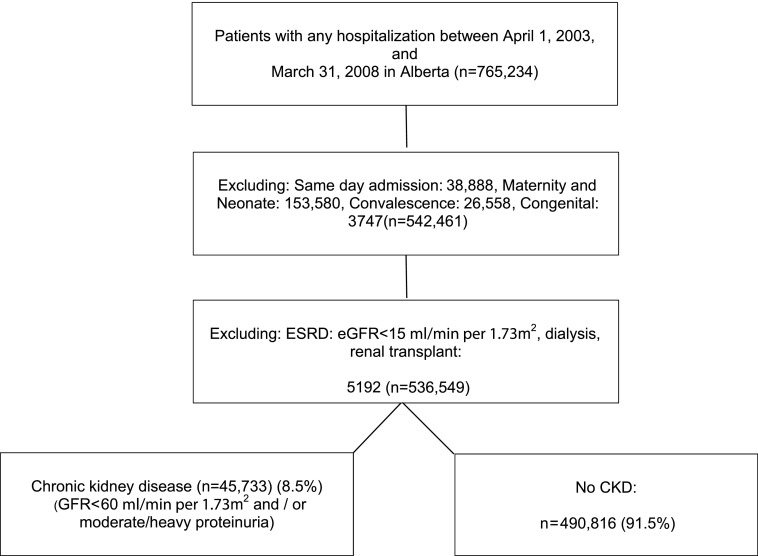
The study cohort comprised all adults (aged ≥18 years) in Alberta hospitalized from April 1, 2003 to March 31, 2008 (Figure 1) from the population-based Alberta Kidney Disease Network cohort (10). The first (index) hospitalization was considered for each patient. All medical and surgical admissions with the exception of maternity/neonatal, congenital malformation, convalescence, and same day admission, were included. We used inpatient administrative data to stratify admissions into medical or surgical, where possible (11).
Figure 1.
Study flowchart to construct cohort of patients with and without CKD.
Assessment of Patient Characteristics
CKD was defined by eGFR<60 ml/min per 1.73 m2 (estimated using the Modification of Diet in Renal Disease equation) and/or moderate to high albuminuria defined as an albumin-to-creatinine ratio >3–30 mg/mmol. The average of all outpatient eGFR measurements from 365 to 90 days before admission were used; we excluded eGFR measurements within 3 months of admission to ensure that AKI did not affect CKD determination. Patients with ESRD (defined as dialysis, renal transplant, or eGFR<15 ml/min per 1.73 m2) were excluded. Further categorization using the Kidney Disease Improving Global Outcomes (KDIGO) 2012 Clinical Practice Guidelines (12) was used to categorize CKD as moderate, high, or very high risk; the remaining patients were classified as non-CKD.
Comorbid conditions, including cancer, cerebrovascular disease, congestive heart failure, chronic obstructive pulmonary disease, dementia, diabetes with complications and without complications, HIV/AIDS, metastatic solid tumor, myocardial infarction, mild liver disease, moderate/severe liver disease, para/hemiplegia, peptic ulcer disease, peripheral vascular diseases, and rheumatologic disease were identified using validated algorithms applied to hospitalization discharge abstracts and physician claims data (9,13). The reason for hospitalization was categorized into 16 homogeneous groups using the International Classification of Disease version 10 Canadian Modifications (ICD 10 CA) (Supplemental Appendix A).
Hospital administrative data includes “diagnoses type 2,” which captures all HACs. These >4000 ICD 10 CA diagnostic codes were mapped into 16 groups and 76 subgroups according to clinical similarity (Supplemental Appendix B). In the United States, 3M Health Information Systems released a list of hospital complications deemed to be potentially preventable. Briefly, panels of clinicians (two general internists and one pediatrician supplemented by a surgical or obstetric specialist as needed) reviewed each of approximately 14,400 diagnosis values in the International Classification of Disease Ninth Revision Clinical Modification (ICD 9 CM) coding scheme and classified 1562 as potentially preventable in-hospital complications (14,15). Pulmonary embolism, deep vein thrombosis, major gastro-intestinal complications with significant bleeding, and decubitus ulcer are examples of potentially preventable complications. That information was used to identify 63 potentially preventable complications by manually remapping ICD-9-CM diagnostic codes to ICD 10 CA. (Supplemental Appendix C).
Hospitalization and vital statistics data were used to determine study outcomes, including LOS of the index hospitalization, mortality during hospitalization and up to 90 days after discharge, and all cause readmission 90 days after discharge. Patients who migrated out of province (n=22) within 90 days after discharge were excluded from analyses of postdischarge events.
Statistical Analyses
In patients with CKD we compared outcomes in patients with and without preventable HACs, and a second analysis included all patients with and without CKD. Mean, SD, 25th and 75th percentiles, and median were used to describe continuous variables. Categorical variables were described as proportions of the cohort with or without a condition. Multivariable regression, logistic regression, Poisson, and quantile analyses (where appropriate) were used in this study. The independent association of one or more potentially preventable HACs with risk of mortality within index hospitalization, mortality from discharge to 90 days, and readmission within 90 days were analyzed using a logistic regression model; a multivariable regression model was used to analyze incremental LOS associated with one or more potentially preventable HACs. Poisson regression was used to calculate population-attributable risk. Quantile regression was used to determine association of outcomes with one or more potentially preventable HACs within quantiles of LOS. Quantile regression models allow one to assess how any quantile of a conditional distribution changes with patient characteristics, for example, LOS (0%–25%, 25%–50%) (16). All models were accounted for potential confounders. We used purposeful selection model building, and interaction between CKD and potentially preventable HACs was tested. The fully adjusted models included reason for admission, age, sex, admission type (categorical; urgent versus elective defined in hospital administrative data), LOS, severity of CKD (where appropriate), and 16 comorbid conditions. All analyses were also adjusted for complications deemed not to be preventable. The attributable risk percentage was calculated to determine the proportion of mortality and readmission that may be attributable to preventable HACs in patients with CKD, and this formula was used: Population Attributable Risk percent (PAR%) =Pe(RRe-1)/(1+Pe[RRe-1]), where RRe is relative risk and Pe is proportion of outcomes in cohort of patients with and without HACs (17). In additional analyses, we categorized the number of preventable HACs as one, two to three, four to five, and more than five preventable HACs. We stratified patients with CKD to examine moderate risk, high risk, and very high risk CKD as defined by KDIGO, and determined outcomes in these subgroups in a sensitivity analysis. Analyses were conducted among patients with CKD only, as well as the larger cohort with and without CKD. In additional sensitivity analyses, we considered all HACs (preventable and nonpreventable) as the exposure variable. Longer LOS may increase exposure of patients to complications; as such, a quantile regression model was used to determine association of outcomes of interest and potentially preventable HACs by LOS quantile. In other sensitivity analyses, the association of mortality with preventable HACs was determined considering the time frame from hospital admission to 90 days after hospital discharge in patients with CKD. The analysis was undertaken using Stata, version 13. The Health Research Ethics Board of the University of Alberta and University of Calgary approved the study.
Results
Patient Characteristics
During the study period, 765,234 patients were admitted to hospitals in Alberta, Canada, and 536,549 hospitalizations met the inclusion criteria (Figure 1). In the full cohort, 6.7% and 2.6% of patients had an eGFR<60 ml/min per 1.73 m2 and moderate to heavy proteinuria, respectively, and 45,733 patients (8.5% of cohort) had CKD. In patients with CKD, the proportion of hospitalizations with one or more preventable HAC was 9.8%. (Table 1) Patients with preventable HACs experienced a high proportion of nonpreventable complications (32.8%); only 2.1%–3.6% of patients without preventable HACs had nonpreventable complications. The mean age of patients with CKD with preventable HACs was greater than patients without preventable HAC cases. In patients with CKD and without potentially preventable HACs, the median LOS in hospitalizations without a preventable HAC was 5 days (25th–75th percentile; 2–9 days) compared with 13 days (25th–75th percentile; 7–29 days) in those hospitalizations with preventable HACs. Postprocedural complications (cardiovascular, respiratory, and other complications of surgical and medical care) were the most common preventable HACs, followed by anemia and acid-base balance, fluid electrolyte balance, or metabolic disorders (9) in patients with CKD (Supplemental Appendix D).
Table 1.
Cohort characteristics
| Cohort Characteristics | With Potentially Preventable HACsa | Without Potentially Preventable HACsa | ||
|---|---|---|---|---|
| Demographics for All Patients | ||||
| No. of patients (%) | With CKD | 45,733 (100) | 4494 (9.8) | 41,239 (90.2) |
| No CKD | 490,816 (100) | 26,374 (5.4) | 464,442 (94.6) | |
| Age, yr, mean (SD) | With CKD | 72 (14) | 74 (13) | 71 (15) |
| No CKD | 50 (22) | 61 (20) | 50 (22) | |
| Men, % | With CKD | 43.6 | 45.9 | 43.4 |
| No CKD | 50 | 51 | 49 | |
| Top three most responsible diagnoses (reason for admission) categories in patients with CKD, % | ||||
| Diseases of circulatory system | With CKD | 21 | 29 | 20 |
| No CKD | 12 | 23 | 11 | |
| Neoplasm | With CKD | 11 | 15 | 11 |
| No CKD | 9 | 17 | 9 | |
| Diseases of the digestive system | With CKD | 11 | 10 | 11 |
| No CKD | 14 | 13 | 14 | |
| Admission type, % (urgent) | With CKD | 70 | 67 | 71 |
| No CKD | 71 | 65 | 72 | |
| Medical admissionb | With CKD | 19,524 (54) | 1581 (45) | 17,943 (55) |
| No CKD | 208,926(57) | 9640 (46) | 199,286 (57) | |
| Surgical admissionb | With CKD | 16,275 (45) | 1928 (54) | 14,347 (44) |
| No CKD | 156,361 (42) | 10,966 (53) | 145,395 (42) | |
| Preventable HACs | ||||
| None (%) | With CKD | — | — | 41,239 (90.2) |
| No CKD | — | — | 464,442 (94.6) | |
| 1 | With CKD | — | 2861 (6.3) | — |
| No CKD | — | 18,083 (3.7) | — | |
| 2–3 | With CKD | — | 1231 (2.7) | — |
| No CKD | — | 6488 (1.3) | — | |
| 4–5 | With CKD | — | 284 (0.6) | — |
| No CKD | — | 1244 (0.2) | — | |
| >5 | With CKD | — | 118 (0.3) | — |
| No CKD | — | 599 (0.1) | — | |
| Nonpreventable HACs, % | With CKD | 2952 (6.5) | 1475 (32.8) | 1477 (3.6) |
| No CKD | 16,830 (3.4) | 7139 (27.0) | 9691 (2.1) | |
| CKD category, %c (only CKD cohort) | Moderate risk | 26,930 (58.9) | 2249 (54.1) | 24,501 (59.4) |
| High risk | 12,364 (27.0) | 1287 (28.7) | 11,077 (26.8) | |
| Very high risk | 6439 (14.1) | 778 (17.3) | 5661 (13.7) | |
| LOS mean (SD) [25th–75th percentile] | With CKD | 10 (20) [2–11] | 23 (30) [7–29] | 9 (17) [2–9] |
| No CKD | 7 (21) [2–6] | 20 (35) [6–23] | 6 (19) [1–6] | |
| LOS median | With CKD | 5 | 13 | 5 |
| No CKD | 3 | 11 | 3 | |
| Mortality in index hospitalization, % | With CKD | 2315 (5.1) | 790 (17.7) | 1525 (3.7) |
| No CKD | 8858 (1.8) | 2884 (11.0) | 5974 (1.3) | |
| Mortality from discharge to 90 d, % | With CKD | 2228 (5.0) | 304 (6.8) | 1978 (4.8) |
| No CKD | 10,034 (2.0) | 1085 (4.1) | 8049 (1.9) | |
| All-cause readmission within 90 d | With CKD | 11,281 (24.7) | 1326 (29.5) | 9955 (24.1) |
| No CKD | 85,816 (17.5) | 7027 (26.4) | 78,710 (17.0) | |
HAC, hospital-acquired complication; —, not applicable; LOS, length of stay.
P value <0.001 in all characteristics between patients with and without potentially preventable HACs.
Some admissions could not be classified as medical/surgical.
Using the Kidney Disease Improving Global Outcomes risk classification.
Patients without CKD were younger and experienced a shorter LOS. Cardiovascular disease comprised the highest proportion of “most responsible” (principal) diagnosis in non-CKD patients with any potentially preventable HACs, accounting for 23% of admissions. In patients without CKD, the proportion of hospitalizations with one or more preventable HAC was 5.4% (Table 1). In patients without CKD who developed potentially preventable HACs, 27% of hospitalizations experienced non-preventable complications..
Risk of Mortality in Index Hospitalization among Patients with CKD
The unadjusted probability of hospital mortality in patients with and without preventable HACs was 17.6% and 3.7%, respectively, and was similar when stratified by medical or surgical admission. In fully adjusted analyses, the odds ratio (OR) of death during index hospitalization in patients with one or more preventable hospital complications was 4.67 (95% confidence interval [95% CI], 4.17 to 5.22). The OR of mortality was higher among patients with more potentially preventable HACs (Table 2). The population-attributable risk percentage of in-hospital mortality that may be because of a preventable HAC was 27%.
Table 2.
Association of outcomes in patients with CKD with potentially preventable HACs
| Potentially Preventable HACs | Mortality: Index Hospitalizationa (95% CI) | Mortality: Discharge to 90 da (95% CI) | Incremental LOS (d),b median (95% CI) | Readmission Discharge to 90 da (95% CI) |
|---|---|---|---|---|
| ≥1 | 4.67 (4.17 to 5.22) | 1.08 (0.94 to 1.25)c | 9.86 (9.25 to 10.47) | 1.24 (1.15 to 1.34) |
| 1 | 3.56 (3.11 to 4.07) | 1.17(0.99 to 1.38)c | 7.20 (6.54 to 7.96) | 1.21(1.11 to 1.32) |
| 2–3 | 6.52 (5.50 to 7.73) | 0.91 (0.71 to 1.18)c | 12.09 (11.81 to 13.98) | 1.28 (1.12 to 1.46) |
| 4–5 | 10.47 (7.78 to 14.08) | 0.88 (0.54 to 1.44)c | 17.14 (14.94 to 19.34) | 1.35 (1.04 to 1.75) |
| >5 | 18.89 (12.12 to 29.44) | 1.29 (0.67 to 2.47)c | 39.92 (36.54 to 43.30) | 1.48 (1.00 to 219) |
HAC, hospital-acquired complication; 95% CI, 95% confidence interval; LOS, length of stay.
Fully adjusted for age, admission type (elective versus urgent), sex, LOS, severity of CKD, nonpreventable complications, and 16 comorbid conditions.
Fully adjusted for age, admission type (elective versus urgent), sex, severity of CKD, nonpreventable complications, and 16 comorbid conditions. Comorbid conditions: cancer, cerebrovascular disease, congestive heart failure, chronic obstructive pulmonary disease, dementia, diabetes with complications, diabetes with no complications, HIV/AIDS, metastatic solid tumor, myocardial infarction, mild liver disease, moderate/severe liver disease, para/hemiplegia, peptic ulcer disease, peripheral vascular diseases, rheumatologic diseases; reference: admissions without HAC.
Nonsignificant.
Risk of Mortality from Discharge to 90 Days in Patients with CKD
Among patients surviving the index admission, 6.8% with any preventable HAC died within 90 days postdischarge, compared with 4.8% in patients without preventable HACs. The fully adjusted OR of mortality from discharge to 90 days in patients with one or more preventable HAC was 1.08 (95% CI, 0.94 to 1.25) (Table 2).
Incremental LOS in Index Hospitalization in Patients with CKD
After controlling for all variables, incremental LOS was 9.86 days (95% CI, 9.25 to 10.47) in patients with one or more preventable HAC, and higher LOS was observed with more potentially preventable HACs, with an incremental 17.14 days (95% CI, 14.94 to 19.34) in patients with four to five preventable HACs (Table 2).
Risks of Readmission from Discharge to 90 Days in Patients with CKD
Among surviving patients, 29.5% of those with one or more preventable HACs were readmitted within 90 days of discharge, compared with 24.1% in those without. In fully adjusted analyses, patients with one or more preventable HACs were more likely to be readmitted to hospital with an OR of 1.24 (95% CI, 1.15 to 1.34). Patients with one preventable HAC were rehospitalized 21% more often than those without HACs (OR, 1.23; 95% CI, 1.17 to 1.35) and the OR was 1.48 (95% CI, 1.08 to 2.34) in patients with CKD and more than five HACs. (Table 2). Population-attributable risk of readmission because of preventable HACs was 2.6%
Population-Attributable Risk Percentage
PAR estimates are useful for providing a measure of the proportion of outcomes that can be attributed to individual or multiple causal factors. Given the proportion of patients with CKD and potentially preventable complications (Figure 1, Table 1), hospital admissions with one or more (mean, 1.7) potentially preventable HACs may lead to an additional 45,000 in-hospital patient deaths, 21,000 readmissions within 90 days, and 4.5 million additional hospital days when extrapolated to the population of North America.
Outcomes in Entire Cohort
Further analyses were done to determine the effect of the presence or absence of both potentially preventable HACs as well as CKD. A new cohort that included patients with and without CKD was assembled. The presence of both CKD and potentially preventable HACs were associated with all outcomes of interest (Table 3). A significant interaction between CKD and potentially preventable HACs was found for all outcomes. A graded increase in the risk of mortality in index hospitalization, mortality from discharge to 90 days, LOS stay, and readmission within 90 days were noted with the presence of CKD and preventable complications. For example, the association of index mortality and incremental LOS were 9.56 (95% CI, 7.23 to 12.65) and 11.42 (95% CI, 9.93 to 12.91), respectively, in patients with CKD and potentially preventable HACs compared with patients without CKD and without potentially preventable HACs.
Table 3.
Association of outcomes in patients with and without CKD with potentially preventable HACs
| Cohort | Mortality: Index Hospitalizationa (95% CI) | Discharge to 90-d Mortalitya (95% CI) | Incremental LOS (d),b Median (95% CI) | Readmission Discharge to 90 da (95% CI) |
|---|---|---|---|---|
| With CKD and no preventable HACs | 2.22 (1.69 to 2.94) | 1.49 (1.11 to 2.00) | 1.67 (0.29 to 3.06) | 1.45 (1.25 to 1.69) |
| Non-CKD and with preventable HACs | 5.26 (4.98 to 5.55) | 1.20 (1.12 to 1.29) | 9.73 (9.4 to 9.98) | 1.41 (1.36 to 1.45) |
| With CKD and preventable HACs | 9.56 (7.23 to 12.65) | 1.68 (1.23 to 2.29) | 11.42 (9.93 to 12.91) | 1.67 (1.42 to 1.96) |
HAC, hospital-acquired complication; 95% CI, 95% confidence interval; LOS, length of stay. Reference group: patients without CKD and without potentially preventable HACs.
Fully adjusted for age, admission type (elective versus urgent), sex, LOS, nonpreventable complications, and 16 comorbid conditions.
Fully adjusted for age, admission type (elective versus urgent), sex, nonpreventable complications, and 16 comorbid conditions.
Sensitivity Analyses
The association of higher mortality with preventable HACs persisted when patients with CKD were subdivided into LOS quantiles. The OR of in-hospital mortality in patients who developed preventable HACs was numerically largest in the 0%–25% quantile (all patients LOS ≤7 days) with an OR of 20.6 (95% CI, 16.44 to 25.8), and was lower but was still significant in the subsequent quantiles (Table 4). The absolute risk of mortality was low in patients without potentially preventable HACs, which is a contributor to the large value of the OR in first quantile. When mortality was assessed over a time frame from admission to 90 days after discharge, the OR associated with one or more preventable HACs was 3.06 (95% CI, 2.77 to 3.37).
Table 4.
ORs of index hospitalization mortality, by LOS quantile, in a CKD cohort with potentially preventable HACs (referenced to the CKD cohort without potentially preventable HACs)
| Quantile | 0%–25% | 25%–50% | 50%–70% | 75%–100% |
|---|---|---|---|---|
| LOS | ≤7 | 8 to ≤23 | 24 to ≤29 | >29 |
| OR (95% CI) | 20.60 (16.44 to 25.80) | 3.6 (3.02 to 4.29) | 2.20 (1.45 to 3.34) | 2.37 (1.89 to 2.97) |
| Absolute no. of deaths (%) | 224 (25) | 277 (33) | 62 (41) | 227 (50) |
Fully adjusted for age, admission type (elective versus urgent), sex, severity of CKD, nonpreventable complications, and 16 comorbid conditions. OR, odds ratio; LOS, length of stay; HAC, hospital-acquired complication; 95% CI, 95% confidence interval.
Discussion
In this large population-based cohort of hospitalized patients, we found that the risk of mortality, LOS in hospital, and 90-day readmission was higher in patients who experienced one or more potentially preventable complication during a hospitalization; this risk was even greater among patients with CKD. The risk of these clinically important outcomes was higher in patients with more potentially preventable complications. The magnitude of this association is large, with a five-fold higher risk of mortality in index hospitalization, and almost 30% higher risk of rehospitalization within 90 days after discharge in patients with CKD and one or more preventable HAC. As an example of the potential implications, we extrapolated our findings to North America. It is estimated that in 2013, approximately 3.25 million patients with CKD (8.5%) were admitted in North America (18,19). Although there are acknowledged limitations and potential biases for using the population-attributable risk percentage, if the association of preventable complications and adverse outcomes is causal, they lead to an additional 45,000 in-hospital patient deaths, 21,000 readmissions within 90 days, and 4.5 million additional hospital days—although this is speculative, particularly in the United States where the health care system differs from Canada. Considerable gains in health outcomes would be achieved if even a proportion of these attributable consequences can be reduced.
HACs can be reduced, although the approach can be complex. In a general hospitalized population, various strategies have been implemented, including environmental efforts to control hospital infections and procedures for management of patients with Foley catheters, leading to a relatively high preventable HAC rate (48.73 and 58.17 per 1000 discharge) being reduced to 32.36 and 48.15, respectively (20). Preventive strategies targeted at patients with greater risk of preventable HACs may result in a proportionately greater reduction of poor clinical outcomes.
Patients with CKD are predisposed to complications during hospitalization (21), possibly because of factors such as impaired coagulation, altered renal handling of medications requiring drug dosing changes, predisposition to drug toxicity, and susceptibility to infection, among others. An analysis of data from the Veterans Health Administration for 2004–2005, demonstrated that patients with CKD had a higher risk for several HACs compared with patients with normal kidney function (adjusted incidence rate ratio, 1.19; 95% CI, 1.13 to 1.25) (8). Similarly, our previous work examining a population-based cohort found that patients with CKD had an OR of preventable HACs of 1.20 (95% CI, 1.16 to 1.24) (9). As CKD is readily identifiable using routine and commonly performed laboratory tests, targeting patients with CKD to prevent complications may be feasible. Implementing preventive strategies in the general population including patients with CKD may improve quality of care; however, patients with CKD may benefit more from such interventions.
Other findings from our study merit consideration. First, patients with preventable HACs also had more nonpreventable complications, compared with those with no preventable HACs. It is not clear why complications cluster in certain patients, but we speculate that this may occur in more complex patients, may accrue as patients stay in hospital longer, or be a follow-on effect (for example, management of a complication may predispose to other complications). The effect of any preventative strategy on this clustering of complications is unknown. Second, although in general greater severity of CKD is associated with poorer clinical outcomes in a graded fashion (22), this was not observed in our study. (Supplemental Appendix F) Although CKD is a risk factor for the occurrence of preventable HACs, we speculate that over this very short time frame of observation, preventable HACs appear to have primacy with respect to adverse outcomes, and the severity of CKD may not exert its influence.
To our knowledge no study has investigated the association of clinical outcomes with preventable HACs in patients with CKD. Previous studies on hospital complications and subsequent clinical outcomes in general hospitalized patients have reported significant consequences for patients and health care networks, including 6 days longer LOS, four more deaths in 1000 hospitalizations, and $US 40,000 incremental cost (15). Although the results of our study examining the combined CKD and non-CKD patients are similar to other studies, the magnitude in the CKD population who develop preventable HACs appears larger, which is perhaps a reflection of this sicker patient population.
Strengths of this study include the use of a population-based cohort and inclusion of community, teaching, and specialized hospitals, which may improve generalizability. Outpatient laboratory data including eGFR and eGFR and proteinuria level values were used before hospitalization to define patients with CKD. We adjusted our model for LOS, age, sex, admission type (urgent versus elective), severity of CKD, and comorbid conditions, as well as for hospital complications that are not considered to be preventable, the latter of which has not been performed in other studies to the best of our knowledge.
Our findings are subject to a number of limitations. First, we do not have access to certain clinical variables, such as BP control, lifestyle factors (smoking, exercise, and diet), and severity of admitting disease and comorbid conditions. Second, hospital-level factors including volume, location, and hospital type were not available. Third, the nature and behavior of preventable HACs is not precisely defined given the use of administrative data to identify these complications; details including circumstances leading to a complication, the extent to which complications alter the process of disease and care, and how complications and comorbid conditions interact are unknown. This limitation could only be overcome by conducting a chart review or prospective study, which would lack the power of a population-based analysis. Fourth, misclassification of preventable complications may take place, however this should not invalidate results as our sensitivity analyses examining all HACs showed similar results. (Supplemental Appendix E). Fifth, there are limitations and potential biases in using the population-attributable risk percentage, which may alter the results of PAR percentage. Finally, the association of outcomes of interest with preventable HACs may mediate through other pathways, such as greater exposure to develop HACs by longer LOS or burden of illness, although our analyses adjusted for available data on comorbidity as well as days in hospital. In sensitivity analyses, this association was persistent in all quantiles of LOS, which may indicate that preventable HACs independent from LOS lead to higher risk of mortality. Although absolute mortality was smaller in the 0%–25% quantile of LOS (<7 days), the magnitude of association of preventable HACs and mortality was greater. The explanation for this has yet to be fully explored; we speculate that HACs that occur earlier in a hospital admission when patients may be more unwell may have a greater effect.
Preventable HACs are associated with a dramatic increase in risk of in-hospital mortality, as well as longer LOS, mortality at 90 days after discharge, and readmission at 90 days. The magnitude of this association is larger in patients with CKD compared with those without. Our findings may inform prioritization of prevention efforts and reduce the rate of preventable complications with attributable clinical outcomes, eventually improving health care in this patient population. Further investigations are needed to examine evidence-based preventive strategies on the risk of potentially preventable HACs, with the goal of improving quality of care and outcomes for hospitalized patients with CKD.
Disclosures
None.
Supplementary Material
Acknowledgments
The Interdisciplinary Chronic Disease Collaboration is funded by Alberta Innovates-Health Solution - Collaborative Research and Innovation Opportunities Team Grants program. This work was supported by the Kidney Health Research Chair (held by S.K.), and by the Division of Nephrology at the University of Alberta.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Avoiding Preventable Complications in Hospitalized Patients with CKD,” on pages 713–714.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.09410916/-/DCSupplemental.
References
- 1.Baker GR, Norton PG: Adverse events and patient safety in Canadian health care. CMAJ 170: 353–354, 2004 [PMC free article] [PubMed] [Google Scholar]
- 2.Baker GR, Norton PG, Flintoft V, Blais R, Brown A, Cox J, Etchells E, Ghali WA, Hébert P, Majumdar SR, O’Beirne M, Palacios-Derflingher L, Reid RJ, Sheps S, Tamblyn R: The Canadian adverse events study: The incidence of adverse events among hospital patients in Canada. CMAJ 170: 1678–1686, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jackson T, Fong A, Liu M, Murray K, Walz L, Houston C, Walker K, Dean S: Incremental costs of hospital-acquired complications in Alberta, Canada. BMC Health Service Research 11[Suppl 1]: A15, 2011 [Google Scholar]
- 4.Baker GR, Norton PG, Flintoft V, Blais R, Brown A, Cox J, Etchells E, Ghali WA, Hébert P, Majumdar SR, O’Beirne M, Palacios-Derflingher L, Reid RJ, Sheps S, Tamblyn R: The Canadian adverse events study: The incidence of adverse events among hospital patients in Canada. CMAJ 170: 1678–1686, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan NA, Quan H, Bugar JM, Lemaire JB, Brant R, Ghali WA: Association of postoperative complications with hospital costs and length of stay in a tertiary care center. J Gen Intern Med 21: 177–180, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paradis AR, Stewart VT, Bayley KB, Brown A, Bennett AJ: Excess cost and length of stay associated with voluntary patient safety event reports in hospitals. Am J Med Qual 24: 53–60, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Zhan C, Miller MR: Excess length of stay, charges, and mortality attributable to medical injuries during hospitalization. JAMA 290: 1868–1874, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Seliger SL, Zhan M, Hsu VD, Walker LD, Fink JC: Chronic kidney disease adversely influences patient safety. J Am Soc Nephrol 19: 2414–2419, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohlouli B, Tonelli M, Jackson T, Hemmelgam B, Klarenbach S: Risk of hospital-acquired complications in patients with chronic kidney disease. Clin J Am Soc Nephrol 11: 956–963, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hemmelgarn BR, Clement F, Manns BJ, Klarenbach S, James MT, Ravani P, Pannu N, Ahmed SB, MacRae J, Scott-Douglas N, Jindal K, Quinn R, Culleton BF, Wiebe N, Krause R, Thorlacius L, Tonelli M: Overview of the alberta kidney disease network. BMC Nephrol 10: 30, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Canadian Institute for Health Information: CMG+. Available at: https://www.cihi.ca/en/data-and-standards/standards/case-mix/cmg. Accessed May 25, 2016
- 12.Stevens PE, Levin A: Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members: Evaluation and management of chronic kidney disease: Synopsis of the kidney disease: Improving global outcomes 2012 clinical practice guideline. Ann Intern Med 158: 825–830, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Tonelli M, Wiebe N, Fortin M, Guthrie B, Hemmelgarn BR, James MT, Klarenbach SW, Lewanczuk R, Manns BJ, Ronksley P, Sargious P, Straus S, Quan H: Alberta Kidney Disease Network: Methods for identifying 30 chronic conditions: Application to administrative data. BMC Med Inform Decis Mak 15: 31, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Texas Health and Human Services: Potentially Preventable Complications in the Texas Medicaid Population State Fiscal Year: 2012. Available at: https://hhs.texas.gov/reports/2013/11/potentially-preventable-complications-texas-medicaid-population-state-fiscal-year. Accessed December 8, 2016
- 15.Hughes JS, Averill RF, Goldfield NI, Gay JC, Muldoon J, McCullough E, Xiang J: Identifying potentially preventable complications using a present on admission indicator. Health Care Financ Rev 27: 63–82, 2006 [PMC free article] [PubMed] [Google Scholar]
- 16.Koenker R, Bassett G Jr: Regression quantiles. Econometrica: journal of the econometric society 40: 33–50, 1978 [Google Scholar]
- 17.Northridge ME: Public health methods--Attributable risk as a link between causality and public health action. Am J Public Health 85: 1202–1204, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.CIHI eReporting: DAD/HMDB: Inpatient Hospitalizations Volumes, Length of Stay, and Standardized Rates - Detailed Report A (Volumes & LOS), 2016. Available at: https://apps.cihi.ca/mstrapp/asp/Main.aspx. Accessed May 21, 2016
- 19.HCUPnet: A Tool for Identifying, Tracking, and Analyzing National Hospital Statistics, 2016. Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp?Id=F0754D388B963512&Form=DispTab&JS=Y&Action=Accept. Accessed May 21, 2016
- 20.Lagoe RJ, Westert GP, Czyz AM, Johnson PE: Reducing potentially preventable complications at the multi hospital level. BMC Res Notes 4: 271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX: Chronic kidney disease and mortality risk: A systematic review. J Am Soc Nephrol 17: 2034–2047, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.