Abstract
Background and objectives
Despitethe multiple depicted associations of CKD with reduced cardiovascular and overall prognoses, the association of CKD with outcome of patients undergoing transcatheter aortic valve implantation has still not been well described.
Design, setting, participants, & measurements
Data from all hospitalized patients who underwent transcatheter aortic valve implantation procedures between January 1, 2010 and December 31, 2013 in Germany were evaluated regarding influence of CKD, even in the earlier stages, on morbidity, in-hospital outcomes, and costs.
Results
A total of 28,716 patients were treated with transcatheter aortic valve implantation. A total of 11,189 (39.0%) suffered from CKD. Patients with CKD were predominantly women; had higher rates of comorbidities, such as coronary artery disease, heart failure at New York Heart Association 3/4, peripheral artery disease, and diabetes; and had a 1.3-fold higher estimated logistic European System for Cardiac Operative Risk Evaluation value. In-hospital mortality was independently associated with CKD stage ≥3 (up to odds ratio, 1.71; 95% confidence interval, 1.35 to 2.17; P<0.05), bleeding was independently associated with CKD stage ≥4 (up to odds ratio, 1.82; 95% confidence interval, 1.47 to 2.24; P<0.001), and AKI was independently associated with CKD stages 3 (odds ratio, 1.83; 95% confidence interval, 1.62 to 2.06) and 4 (odds ratio, 2.33; 95% confidence interval, 1.92 to 2.83 both P<0.001). The stroke risk, in contrast, was lower for patients with CKD stages 4 (odds ratio, 0.23; 95% confidence interval, 0.16 to 0.33) and 5 (odds ratio, 0.24; 95% confidence interval, 0.15 to 0.39; both P<0.001). Lengths of hospital stay were, on average, 1.2-fold longer, whereas reimbursements were, on average, only 1.03-fold higher in patients who suffered from CKD.
Conclusions
This analysis illustrates for the first time on a nationwide basis the association of CKD with adverse outcomes in patients who underwent transcatheter aortic valve implantation. Thus, classification of CKD stages before transcatheter aortic valve implantation is important for appropriate risk stratification.
Keywords: Transcatheter Aortic Valve Replacement; Aortic Valve Stenosis; chronic renal insufficiency; Treatment Outcome; Economics; Acute Kidney Injury; Comorbidity; Confidence Intervals; coronary artery disease; diabetes mellitus; Female; Germany; heart failure; Hospital Mortality; Humans; Length of Stay; Peripheral Arterial Disease; Prognosis; Renal Insufficiency, Chronic; Risk Assessment; Stroke
Introduction
Severe calcific aortic valve stenosis (AS) is the most common valvular heart disease, affecting up to 8.1% of the general population >85 years old (1). Surgical aortic valve replacement (SAVR) is generally accepted to improve prognosis (2,3). Percutaneous techniques for aortic valve implantation (transcatheter aortic valve implantation [TAVI]) were also able to show an improvement in prognosis, even compared with SAVR (4–9). A 25% increase in survival at 1 year in patients evaluated to be at high risk for surgery is a clear confirmation of this advantage (6).
CKD is associated with higher prevalence and severity of AS (10,11), substantially higher rates of vascular ischemic events (12–14), and increased mortality after SAVR (15–17) and seems to be associated with worse outcome after TAVI (5,8,18) and transcatheter valve degeneration (19). Moreover, it is associated with increased health care and socioeconomic burden (12,20–22). However, the prognostic value of the different stages of CKD on morbidity, in-hospital treatment and complications, and mortality has not been sufficiently investigated.
We aimed to analyze the association of CKD with morbidity, in-hospital complications, short-term outcome, and costs in this nationwide contemporary cohort study of all TAVIs from 2010 to 2013 in Germany.
Materials and Methods
We evaluated anonymized nationwide data from the Research Data Centers of the Federal Bureau of Statistics (Wiesbaden, Germany) on the basis of SAS codes (SAS software, version 9.2; SAS Institute Inc., Cary, NC) as described elsewhere in detail (23). Data of all in-hospital TAVI procedures for the treatment of isolated AS in Germany between January 1, 2010 and December 31, 2013 were included in this analysis (inpatient cardiovascular diagnoses and procedures and codes for coexisting conditions and complications) by using the available diagnostic and procedural codes for acute and chronic conditions (German Procedure Classification [OPS] and International Statistical Classification of Diseases and Related Health Problems, 10th revision, German modification [ICD-10-GM] codes). Patients were classified according to CKD: N18.1 indicates structural abnormalities or genetic traits that point to kidney disease (CKD stage 1), N18.2 indicates normal or mild reduced renal function (CKD stage 2), N18.3 indicates moderate renal insufficiency (CKD stage 3), N18.4 indicates severe renal insufficiency (CKD stage 4), and N18.5 indicates end stage renal failure (CKD stage 5) (Supplemental Appendix, Supplemental Table 1). We calculated the estimated logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) for each patient for estimation of the procedural risk for patients. N17* was defined as AKI (Supplemental Appendix).
Categorical variables are presented as absolute numbers (n) and percentages of the total numbers of the distinct ICD-10-GM and OPS codes for each CKD subgroup. Comparisons of baseline parameters between the six different subgroups were made using the chi-squared test for categorical variables and one-way ANOVA for continuous variables. Continuous variables are presented as means±SD.
All logistic regression analyses (for in-hospital mortality, bleeding, permanent pacemaker implantation [PPI], stroke, and AKI) were carried out (adjusted for CKD stages, approach of aortic valve implantation, sex, age, EuroSCORE, AS, combined aortic valve diseases, chronic heart failure, coronary artery disease [CAD], hypertension, history of myocardial infarction, history of coronary artery bypass graft surgery, history of cardiac surgery, peripheral vascular disease, carotid disease, chronic obstructive lung disease, pulmonary hypertension, atrial fibrillation, and diabetes) using Stata 13 (Stata Corp, College Station, TX). P values of <0.05 were considered as statistically significant.
Results
This cohort contains all 28,716 hospital admissions for TAVI in Germany from 2010 to 2013. Patients were, on average, 80.4±6.1 years old and had a mean preoperative risk (estimated logistic EuroSCORE) of 25%±13%. Of all patients, a total of 11,189 (39.0%) suffered from CKD. Of those, 1.7% (193) had normal kidney function but urine findings (CKD stage 1), 17.6% (1971) had normal or mild reduced renal function (CKD stage 2), 60.4% (6755) had moderate renal insufficiency (CKD stage 3), 12.6% (1405) had severe renal insufficiency (CKD stage 4), and 7.7% (865) had end stage renal failure (CKD stage 5). Baseline characteristics and comorbidities of all patients in relation to CKD stages are presented in Table 1.
Table 1.
Baseline characteristics of patients with transcatheter aortic valve implantation with and without CKD
| Predictors | Without CKD | CKD Stage 1 | CKD Stage 2 | CKD Stage 3 | CKD Stage 4 | CKD Stage 5 | P Value |
|---|---|---|---|---|---|---|---|
| Patients, n (% of all) | 17,527 | 193 | 1971 | 6755 | 1405 | 865 | <0.001 |
| Age, yr, mean±SD | 80.9±6.1 | 80.5±5.7 | 81.1±5.8 | 81.5±5.8 | 81.7±5.9 | 76.4±7.3 | <0.001 |
| Women, n (%) | 10,114 (57.7) | 105 (54.4) | 1007 (51.1) | 3688 (54.6) | 902 (64.2) | 321 (37.1) | <0.001 |
| EuroSCORE, mean (%)±SD. | 20.4±12.2 | 19.4±11.0 | 22.0±12.8 | 23.3±13.7 | 36.1±16.5 | 30.2±17.3 | <0.001 |
| Isolated aortic valve stenosis, n (%) | 12,148 (69.3) | 151 (78.2) | 1328 (67.4) | 4337 (64.2) | 886 (63.1) | 575 (66.5) | <0.001 |
| Combined aortic valve disease, n (%) | 4543 (25.9) | 37 (19.2) | 524 (26.6) | 1993 (29.5) | 432 (30.8) | 236 (27.3) | <0.001 |
| CAD, n (%) | 7764 (44.3) | 93 (48.2) | 943 (47.8) | 3460 (51.2) | 708 (50.4) | 440 (50.9) | <0.001 |
| PAD, n (%) | 1792 (10.2) | 23 (11.9) | 270 (13.7) | 955 (14.1) | 203 (14.5) | 210 (24.3) | <0.001 |
| Carotid disease, n (%) | 1035 (5.9) | 13 (6.7) | 128 (6.5) | 447 (6.6) | 95 (6.8) | 49 (5.7) | 0.29 |
| NYHA 2 | 1450 (8.3) | 13 (6.7) | 193 (9.8) | 527 (7.8) | 106 (7.5) | 64 (7.4) | 0.07 |
| NYHA 3 or 4 | 6312 (36.0) | 80 (41.5) | 887 (45.0) | 3220 (47.7) | 726 (51.7) | 427 (49.4) | <0.001 |
| Previous CABG, n (%) | 2027 (11.6) | 31 (16.1) | 291 (14.8) | 1008 (14.9) | 190 (13.5) | 129 (14.9) | <0.001 |
| Previous cardiac surgery, n (%) | 2980 (17.0) | 34 (17.6) | 386 (19.6) | 1400 (20.7) | 269 (19.2) | 190 (22.0) | <0.001 |
| Previous myocardial infarction, n (%) | 957 (1.8) | 11 (2.9) | 159 (2.7) | 603 (3.0) | 116 (2.8) | 84 (3.2) | <0.001 |
| Atrial fibrillation, n (%) | 7404 (42.2) | 84 (43.5) | 993 (50.4) | 3540 (52.4) | 796 (56.7) | 428 (49.5) | <0.001 |
| COPD, n (%) | 2471 (14.1) | 35 (18.1) | 307 (15.6) | 1176 (17.4) | 228 (16.2) | 152 (17.6) | <0.001 |
| Pulmonary hypertension, n (%) | 3495 (19.9) | 36 (18.7) | 488 (24.8) | 1781 (26.4) | 379 (27.0) | 247 (28.6) | <0.001 |
| Hypertension, n (%) | 11,222 (64.0) | 125 (64.8) | 1178 (59.8) | 4171 (61.8) | 897 (63.8) | 473 (54.7) | <0.001 |
| Diabetes, n (%) | 5038 (28.7) | 82 (42.5) | 710 (36.0) | 2746 (40.7) | 630 (44.8) | 401 (46.4) | <0.001 |
| Dialysis during hospital stay, n (%) | 572 (3.3) | 6 (3.1) | 82 (4.2) | 462 (6.8) | 228 (16.2) | 708 (81.8) | <0.001 |
P values for comparisons of baseline parameters between the five different CKD groups were calculated using the chi-squared test for categorical variables and one-way ANOVA for continuous variables. EuroSCORE, European System for Cardiac Operative Risk Evaluation; CAD, coronary artery disease; PAD, peripheral artery disease; NYHA, New York Heart Association; CABG, coronary artery bypass graft surgery; COPD, chronic obstructive lung disease.
Compared with patients without CKD, those with CKD had increasingly higher EuroSCORE values with higher CKD stages; more frequently had previous coronary artery bypass graft surgery; and more often suffered from more severe chronic heart failure (New York Heart Association 3 and 4), CAD, peripheral artery disease, carotid disease, atrial fibrillation, chronic obstructive pulmonary disease, pulmonary hypertension, and diabetes (Table 1). With higher CKD stage, the proportions of patients with diabetes, CAD, peripheral artery disease, more severe chronic heart failure, atrial fibrillation, and pulmonary hypertension increased markedly.
Procedures, In-Hospital Mortality, Complications, and Complication-Related Mortality
The practice favored the transfemoral approach (transfemoral transcatheter aortic valve implantation [TF-TAVI]): n=20,009 (with CKD: n=7649 [38.3%]; without CKD: n=12,360 [61.8%]). A total of 8707 got a transapical approach (transapical transcatheter aortic valve implantation [TA-TAVI]; with CKD: n=3540 [40.7%]; without CKD: n=5167 [59.0%]).
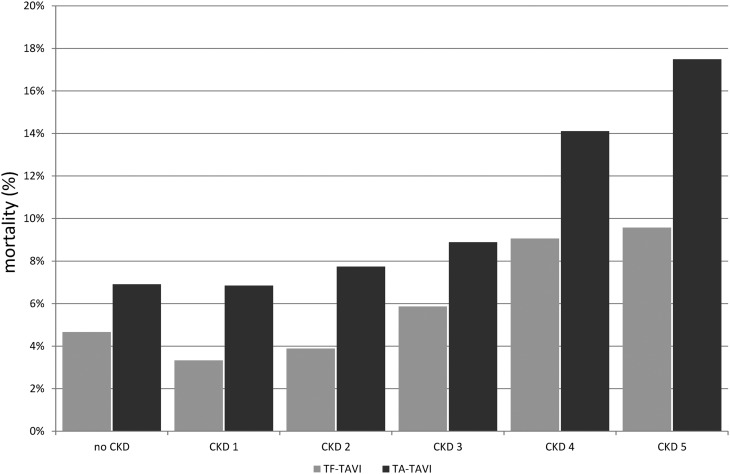
The in-hospital mortality after TAVI was 6.1% (5.2% after TF-TAVI and 8.2% after TA-TAVI). Both rates increased with higher CKD stage and showed the highest rates in CKD stage 5 (9.6% after TF-TAVI and 17.5% after TA-TAVI). Details of in-hospital mortality and procedure-related in-hospital mortality are shown in Figures 1 and 2. In comparison with patients without CKD (mean EuroSCORE: 20.4), patients with CKD stage 1 did not show an increased mortality risk according to their initial EuroSCORE values (EuroSCORE: 19.4; P=0.27), whereas patients with CKD stage 2 or 3 had a moderately increased mortality risk (EuroSCORE: 22.0 and 23.3, respectively; both P<0.001). Patients with CKD stage 4 or 5 had a substantially increased mortality risk (EuroSCORE: 36.1 and 30.2, respectively; both P<0.001). The difference in EuroSCORE values between patients with CKD stages 4 and 5 was also statistically significant (P<0.001). In-hospital mortality remained increased with respect to CKD stage ≥3, even after adjustment for baseline patient characteristics (Table 2).
Figure 1.
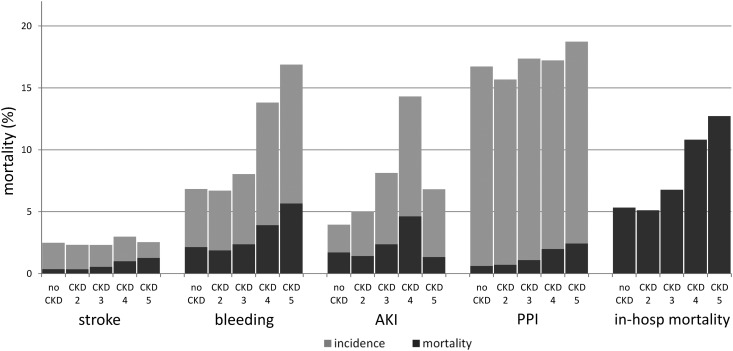
Influence of CKD on In-hospital mortality, complications and complication-related mortality. In-hospital mortality (in-hosp mort), complications, and complication-related mortality without and with known CKD subdivided into the five CKD stages. Because of the small number of patients within the CKD stage 1 group (n=193) and the relative sparsity of in-hospital events, all in-hospital outcomes for the CKD stage 1 group were anonymized and are, therefore, not available. PPI, permanent pacemaker implantation.
Figure 2.
Influence of CKD on Procedure-related in-hospital mortality. Procedure-related in-hospital mortality without and with known CKD subdivided into the five CKD stages. TA-TAVI, transapical transcatheter aortic valve implantation; TF-TAVI, transfemoral transcatheter aortic valve implantation.
Table 2.
Multivariate regression analyses of predictors in-hospital mortality, postinterventional bleeding, and permanent pacemaker implantation (n=28,715)
| Predictors | In-Hospital Mortality | Bleeding | PPI | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | |
| Without renal disease | 1 | 1 | 1 | ||||||
| CKD stage 1 | 0.83 | 0.42 to 1.63 | 0.58 | 0.81 | 0.44 to 1.50 | 0.50 | 0.66 | 0.42 to 1.05 | 0.08 |
| CKD stage 2 | 0.85 | 0.69 to 1.05 | 0.14 | 0.92 | 0.76 to 1.11 | 0.39 | 0.91 | 0.80 to 1.04 | 0.16 |
| CKD stage 3 | 1.13 | 1.00 to 1.27 | 0.04 | 1.10 | 0.99 to 1.23 | 0.08 | 0.99 | 0.92 to 1.07 | 0.81 |
| CKD stage 4 | 1.31 | 1.06 to 1.61 | 0.01 | 1.43 | 1.18 to 1.72 | <0.001 | 0.97 | 0.83 to 1.14 | 0.75 |
| CKD stage 5 | 1.71 | 1.35 to 2.17 | <0.001 | 1.82 | 1.47 to 2.24 | <0.001 | 1.19 | 0.98 to 1.44 | 0.08 |
| TF-TAVI (instead of TA-TAVI) | 0.62 | 0.56 to 0.68 | <0.001 | 0.52 | 0.48 to 0.58 | <0.001 | 2.71 | 2.49 to 2.95 | <0.001 |
| Women | 0.88 | 0.79 to 0.98 | 0.02 | 1.08 | 0.98 to 1.20 | 0.12 | 0.88 | 0.82 to 0.95 | 0.001 |
| Age | 1.00 | 0.99 to 1.01 | 0.58 | 0.98 | 0.97 to 0.98 | <0.001 | 1.01 | 1.00 to 1.02 | 0.03 |
| EuroSCORE | 1.03 | 1.02 to 1.03 | <0.001 | 1.02 | 1.02 to 1.03 | <0.001 | 1.00 | 1.00 to 1.01 | 0.23 |
| Aortic valve stenosis | 0.61 | 0.51 to 0.74 | <0.001 | 0.73 | 0.61 to 0.87 | <0.001 | 0.83 | 0.72 to 0.95 | <0.01 |
| Combined aortic valve diseases | 0.51 | 0.42 to 0.62 | <0.001 | 0.66 | 0.55 to 0.80 | <0.001 | 0.93 | 0.81 to 1.08 | 0.34 |
| NYHA 2 | 0.54 | 0.42 to 0.69 | <0.001 | 0.67 | 0.55 to 0.82 | <0.001 | 1.04 | 0.93 to 1.17 | 0.51 |
| NYHA 3 or 4 | 1.45 | 1.31 to 1.61 | <0.001 | 1.33 | 1.21 to 1.45 | <0.001 | 1.09 | 1.02 to 1.17 | 0.01 |
| CAD | 1.07 | 0.96 to 1.19 | 0.22 | 1.05 | 0.96 to 1.16 | 0.29 | 1.02 | 0.95 to 1.09 | <0.01 |
| Hypertension | 0.70 | 0.63 to 0.77 | <0.001 | 0.78 | 0.71 to 0.86 | <0.001 | 1.03 | 0.96 to 1.10 | 0.37 |
| Previous MI (within 0–4 mo) | 0.67 | 0.45 to 0.99 | 0.04 | 0.79 | 0.57 to 1.11 | 0.18 | 0.79 | 0.60 to 1.03 | 0.08 |
| Previous MI (within 4–12 mo) | 1.01 | 0.59 to 1.72 | 0.98 | 1.07 | 0.66 to 1.73 | 0.78 | 0.91 | 0.63 to 1.32 | 0.63 |
| Previous MI (after 12 mo) | 1.07 | 0.85 to 1.33 | 0.58 | 0.83 | 0.66 to 1.04 | 0.11 | 0.82 | 0.69 to 0.97 | 0.02 |
| Previous CABG | 0.97 | 0.77 to 1.23 | 0.83 | 0.43 | 0.35 to 0.53 | <0.001 | 0.60 | 0.51 to 0.70 | <0.001 |
| Previous cardiac surgery | 0.76 | 0.59 to 0.96 | 0.02 | 1.24 | 1.02 to 1.51 | 0.03 | 1.56 | 1.34 to 1.83 | <0.001 |
| Peripheral vascular disease | 1.00 | 0.85 to 1.16 | 0.96 | 1.03 | 0.90 to 1.19 | 0.64 | 0.97 | 0.95 to 1.09 | 0.63 |
| Carotid disease | 0.77 | 0.63 to 0.95 | 0.01 | 1.07 | 0.90 to 1.28 | 0.41 | 1.01 | 0.87 to 1.17 | 0.86 |
| COPD | 0.88 | 0.76 to 1.02 | 0.09 | 0.88 | 0.77 to 1.00 | 0.05 | 0.99 | 0.90 to 1.09 | 0.83 |
| Pulmonary hypertension | 0.74 | 0.63 to 0.86 | <0.001 | 0.75 | 0.65 to 0.86 | <0.001 | 1.02 | 0.92 to 1.13 | 0.69 |
| Atrial fibrillation | 1.13 | 1.02 to 1.25 | 0.02 | 1.30 | 1.19 to 1.43 | <0.001 | 1.20 | 1.12 to 1.27 | <0.001 |
| Diabetes | 1.04 | 0.93 to 1.15 | 0.49 | 0.99 | 0.90 to 1.09 | 0.82 | 1.08 | 1.01 to 1.15 | 0.03 |
Please note that, in comparison with the data shown in the other tables, one procedure was excluded from the multivariate logistic regression analyses due to incomplete information regarding the covariates. PPI, permanent pacemaker implantation; 95% CI, 95% confidence interval; TF-TAVI, transfemoral transcatheter aortic valve implantation; TA-TAVI, transapical transcatheter aortic valve implantation; EuroSCORE, European System for Cardiac Operative Risk Evaluation; NYHA, New York Heart Association; CAD, coronary artery disease; MI, myocardial infarction; CABG, coronary artery bypass graft surgery; COPD, chronic obstructive lung disease.
The main complications were need for PPI (17% [Figure 1]; results for patients with CKD stage 1 are not available for reasons of data protection) followed by bleeding (7.8%), AKI (5.6%), and stroke (2.5%). Patients with CKD (CKD stage ≥2) had higher frequency of bleeding (9.2% versus 6.8%; P<0.001) and higher frequency of AKI (8.3% versus 4.0%; P<0.001). Regarding the outcome of AKI leading to the need for dialysis, higher CKD stages are associated with higher frequency of dialysis, with a peak in CKD stage 4 (Supplemental Appendix, Supplemental Table 2).
With higher CKD stages, the proportions of patients with bleeding and PPI increased, with the highest frequency in CKD stage 5 (bleeding; 16.9%; PPI: 18.7%). Observed in-hospital complications and complication-related mortality are shown in Figure 2.
With respect to the end point of bleeding events, CKD stages ≥4 were identified as independent risk factors after adjustment for baseline patient characteristics (Table 2). With respect to stroke, however, patients with CKD stage 4 or 5 seem to have a systematically decreased risk (Table 3). With respect to the end point of AKI, patients with CKD stage 3 or 4 are associated with an independent risk (Table 3).
Table 3.
Multivariate regression analyses of predictors for postinterventional stroke and AKI (n=28,715)
| Predictors | Stroke | AKI | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | |
| Without renal disease | 1 | 1 | ||||
| CKD stage 1 | 0.90 | 0.33 to 2.49 | 0.84 | 1.11 | 0.56 to 2.19 | 0.77 |
| CKD stage 2 | 0.86 | 0.63 to 1.19 | 0.37 | 1.10 | 0.88 to 1.37 | 0.40 |
| CKD stage 3 | 0.85 | 0.70 to 1.04 | 0.11 | 1.83 | 1.62 to 2.06 | <0.001 |
| CKD stage 4 | 0.23 | 0.16 to 0.33 | <0.001 | 2.33 | 1.92 to 2.83 | <0.001 |
| CKD stage 5 | 0.24 | 0.15 to 0.39 | <0.001 | 0.87 | 0.65 to 1.18 | 0.38 |
| TF-TAVI (instead of TA-TAVI) | 1.04 | 0.87 to 1.24 | 0.66 | 0.79 | 0.71 to 0.88 | <0.001 |
| Women | 0.63 | 0.53 to 0.75 | <0.001 | 0.58 | 0.52 to 0.65 | <0.001 |
| Age | 0.90 | 0.89 to 0.91 | <0.001 | 0.97 | 0.96 to 0.98 | <0.001 |
| EuroSCORE | 1.12 | 1.03 to 1.04 | <0.001 | 1.03 | 1.03 to 1.04 | <0.001 |
| Aortic valve stenosis | 1.61 | 1.13 to 2.28 | <0.01 | 0.74 | 0.61 to 0.90 | 0.002 |
| Combined aortic valve diseases | 1.68 | 1.16 to 2.43 | <0.01 | 0.53 | 0.43 to 0.66 | <0.001 |
| NYHA 2 | 0.94 | 0.69 to 1.28 | 0.69 | 0.79 | 0.63 to 0.99 | 0.04 |
| NYHA 3 or 4 | 1.18 | 1.00 to 1.39 | 0.05 | 1.66 | 1.49 to 1.85 | <0.001 |
| CAD | 1.00 | 0.84 to 1.17 | 0.96 | 1.06 | 0.95 to 1.19 | 0.28 |
| Hypertension | 0.88 | 0.75 to 1.03 | 0.13 | 0.75 | 0.68 to 0.99 | <0.001 |
| Previous MI (within 0–4 mo) | 0.36 | 0.20 to 0.63 | <0.001 | 0.77 | 0.53 to 1.11 | 0.17 |
| Previous MI (within 4–12 mo) | 1.79 | 0.92 to 3.48 | 0.09 | 1.28 | 0.79 to 2.08 | 0.31 |
| Previous MI (after 12 mo) | 1.08 | 0.75 to 1.56 | 0.69 | 1.16 | 0.93 to 1.44 | 0.20 |
| Previous CABG | 1.00 | 0.67 to 1.51 | 0.99 | 0.60 | 0.47 to 0.75 | <0.001 |
| Previous cardiac surgery | 0.10 | 0.07 to 0.15 | <0.001 | 0.86 | 0.68 to 1.09 | 0.20 |
| Peripheral vascular disease | 0.42 | 0.33 to 0.54 | <0.001 | 0.88 | 0.75 to 1.04 | 0.14 |
| Carotid disease | 0.46 | 0.34 to 0.63 | <0.001 | 0.75 | 0.60 to 0.93 | <0.01 |
| COPD | 0.37 | 0.30 to 0.47 | <0.001 | 0.82 | 0.71 to 0.95 | <0.01 |
| Pulmonary hypertension | 0.19 | 0.15 to 0.24 | <0.001 | 0.69 | 0.59 to 0.81 | <0.001 |
| Atrial fibrillation | 1.19 | 1.02 to 1.39 | 0.03 | 1.55 | 1.40 to 1.73 | <0.001 |
| Diabetes | 1.08 | 0.92 to 1.27 | 0.36 | 1.22 | 1.09 to 1.36 | <0.001 |
Please note that, in comparison with the data shown in the other tables, one procedure was excluded from the multivariate logistic regression analyses due to incomplete information regarding the covariates. 95% CI, 95% confidence interval; TF-TAVI, transfemoral transcatheter aortic valve implantation; TA-TAVI, transapical transcatheter aortic valve implantation; EuroSCORE, European System for Cardiac Operative Risk Evaluation; NYHA, New York Heart Association; CAD, coronary artery disease; MI, myocardial infarction; CABG, coronary artery bypass graft surgery; COPD, chronic obstructive lung disease.
Reimbursement Costs and Resource Utilization
The mean length of in-hospital stay of the TAVI subgroup without CKD (EuroSCORE: 20.4) was 16.4 days. The in-hospital time of patients who suffered from CKD was, on average, 1.2-fold longer (19.7 days). The cost for hospitalization of a patient with TAVI without known CKD was, on average, 34,560 €. The cost for a patient with CKD was, on average, only 1.03-fold higher (35,581 €) (Table 4).
Table 4.
Nonclinical data of patients with transcatheter aortic valve implantation with regard to CKD stages
| Predictors | Without CKD | CKD Stage 1 | CKD Stage 2 | CKD Stage 3 | CKD Stage 4 | CKD Stage 5 |
|---|---|---|---|---|---|---|
| Reimbursement, median (25th–75th percentile), € | 33.758 (33.078–34265) | 34.093 (32.993–34.932) | 33.758 (33.125–34.306) | 33.758 (33.172–34.306) | 33.941 (33.172–35.514) | 34.093 (33.185–35.673) |
| LOS, median (25th–75th percentile), d | 14 (10–20) | 14 (10–21) | 15 (11–21) | 16 (12–23) | 19 (13–28) | 17 (12–28) |
| Discharge destination, n (%) | ||||||
| Home | 8637 (49.3) | 87 (45.1) | 944 (47.9) | 3252 (48.1) | 532 (37.9) | 382 (44.2) |
| Hospital | 3838 (21.9) | 49 (25.4) | 409 (20.8) | 1391 (20.6) | 355 (25.3) | 236 (27.3) |
| Rehabilitation | 4045 (23.1) | 47 (24.4) | 509 (25.8) | 1603 (23.7) | 349 (24.8) | 130 (15.0) |
LOS, length of stay.
Discussion
This analysis contains contemporary in-hospital data of all patients who underwent TAVI in Germany from 2010 to 2013. The findings of this study highlight several new and important aspects of the influence of CKD on patients undergoing TAVI.
First, CKD is common in patients undergoing TAVI, and it is associated with a higher preoperative risk profile. More than one third of all patients undergoing TAVI suffered from CKD.
Second, CKD in patients undergoing TAVI predicts higher rates of in-hospital complications, such as bleeding and AKI. Bleeding, AKI, and PPI are frequent complications after TAVI. Early bleeding complications are described in 8.8%–28.1% (24–27), AKI is described in 0.7%–6.0% (24,26,27), and PPI becomes necessary in 3.4%–34.1% after TAVI (26–29). We observed in this comprehensive cohort a bleeding rate of 7.8%, a frequency of AKI of 5.6%, and the need for PPI rate of 17% after TAVI. When CKD was also present, our data show a baseline risk–independent higher frequency of bleeding in patients with CKD stage 4 or 5 as well as a baseline risk–independent higher frequency of AKI in patients with CKD stage 3 or 4 compared with those patients without CKD. Peri-interventional stroke is one of the rather frequent complications, with a rate of 1.5%–5.5% (25–27,30–32). In good accordance with these reports, we observed a stroke rate of 2.5% after TAVI. Notably, CKD was independently associated with a reduced peri-interventional stroke rate in CKD stages 4 and 5. It is known that CKD and ESRD are associated with a higher risk of stroke (33). However, comparable data concerning peri-interventional rate stroke and reduced renal function are currently still missing.
Third, the most striking finding of this study is the marked influence of CKD on in-hospital procedure– and complication-related mortality: CKD stages 3–5 were associated with substantially higher in-hospital mortality, with a stepwise increase with higher CKD stage compared with in those patients without CKD. The in-hospital mortality of the entire cohort of all TAVIs in Germany between 2010 and 2013 was 6.1%. Comparable data from the Nordic Aortic Valve Intervention Trial, the Placement of Aortic Transcatheter Valves Trial, and the German Aortic Valve Registry showed in-hospital mortality after TAVI of 2.1%–12.6% (26,27,31,33–35). The complication-related mortality after peri-interventional stroke or PPI was associated with CKD, with a stepwise increase with higher stage. This culminates in patients with CKD stage 5. Every second peri-interventional stroke and every third bleeding were fatal. Moreover, despite comparable data, like the Canadian registry data or data from the Heart Center in Leipzig (GARY Registry [34]), TAVI implantation via the transapical approach was associated with 1.7-fold higher in-hospital mortality compared with the procedure via the transfemoral approach. Regarding CKD, in-hospital mortality showed a stepwise increase with higher stage in both procedures. This result is more pronounced than comparative data of the Canadian and GARY registry (34), which demonstrated on average 1.2-fold higher mortality (transfemoral approach: 9.5% versus transapical approach: 11.3%; Canadian registry), despite the significantly higher risk profile in the cohort treated with the transapical approach (36). Hitherto, no randomized study currently exists; as well, the association of CKD was not the subject of comparative studies until now.
Fourth, the lengths of hospital stays were, on average, 1.2-fold longer and reimbursement costs were, on average, 1.03-fold higher in patients who suffered from CKD compared with those without CKD. In general, other studies (37,38) and the study by Eckardt et al. (21) had already showed that CKD is associated with markedly higher in-hospital costs for treating patients with established cardiovascular disease. Our data also confirm these findings in patients undergoing TAVI: CKD is associated with significant additional costs.
Several limitations have to be addressed. First, diagnosis- and procedure-based reports depend substantially on coding behavior. Therefore, we just analyzed diagnoses and procedures, which would be unlikely to be miscoded as described elsewhere in detail (23,39). Factors enhancing the reimbursement, like the end points of our study, are events that were unlikely to be miscoded (diabetes, in-hospital mortality, stroke, AKI, bleeding, and PPI), because they are obligatory for correct reimbursement and therefore, of great importance for the hospitals. Therefore, completeness of these procedures and diagnoses could, therefore, be expected to be comprehensive. On the contrary, diagnoses without any association with reimbursement (hypertension, obesity, dyslipidemia, and smoking) might be underestimated in this analysis. This may also concern CKD stages 1 and 2. Second, the nature of our study is observational. With respect to procedural characteristics, baseline characteristics of patients, and in-hospital complications and mortality, groups with fewer than three procedures were excluded by the Research Data Center to ensure anonymity. These data were not included in summary statistics. The coding rules (ICD-10-GM and OPS) have been in use for >10 years in Germany. They were not modified with regard to the topics of this study.
In summary, our data show the influence of CKD on morbidity, in-hospital complications, short-term outcome, and costs for patients who underwent TAVI. Consequently, CKD classification before TAVI allows potent risk stratification for early outcomes.
Disclosures
H.R. has received speaker honoraria from Sanofi-Aventis, Daiichi-Sankyo, The Medicine Company, Cordis, and Novartis. He has acted as a consultant for BMS and Pluristem. He took part in the conduction of multicenter trials by BARD, BAYER, BIOTRONIK, and Pluristem. J.R. works as proctor for Edwards Lifesciences and a consultant for Medtronic and DirectFlow Medical and has received speaker honoraria from Abbott. He took part in the conduction of trials by BAYER, Medtronic, and Abbott.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10471016/-/DCSupplemental.
References
- 1.Varadarajan P, Kapoor N, Bansal RC, Pai RG: Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 82: 2111–2115, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Vahanian A, Baumgartner H, Bax J, Butchart E, Dion R, Filippatos G, Flachskampf F, Hall R, Iung B, Kasprzak J, Nataf P, Tornos P, Torracca L, Wenink A; Task Force on the Management of Valvular Hearth Disease of the European Society of Cardiology, ESC Committee for Practice Guidelines : Guidelines on the management of valvular heart disease; The task force on the management of valvular heart disease of the European society of cardiology. Eur Heart J 28: 230–268, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Bonow RO, Carabello BA, Kanu C, de Leon AC Jr., Faxon DP, Freed MD, Gaasch WH, Lytle BW, Nishimura RA, O’Gara PT, O’Rourke RA, Otto CM, Shah PM, Shanewise JS, Smith SC Jr., Jacobs AK, Adams CD, Anderson JL, Antman EM, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B; American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons : ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 114: e84–e231, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, Thourani VH, Tuzcu EM, Miller DC, Herrmann HC, Doshi D, Cohen DJ, Pichard AD, Kapadia S, Dewey T, Babaliaros V, Szeto WY, Williams MR, Kereiakes D, Zajarias A, Greason KL, Whisenant BK, Hodson RW, Moses JW, Trento A, Brown DL, Fearon WF, Pibarot P, Hahn RT, Jaber WA, Anderson WN, Alu MC, Webb JG: PARTNER 2 Investigators: Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 374: 1609–1620, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Duncan A, Ludman P, Banya W, Cunningham D, Marlee D, Davies S, Mullen M, Kovac J, Spyt T, Moat N: Long-term outcomes after transcatheter aortic valve replacement in high-risk patients with severe aortic stenosis: The U.K. transcatheter aortic valve implantation registry. JACC Cardiovasc Interv 8: 645–653, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, Gleason TG, Buchbinder M, Hermiller J Jr., Kleiman NS, Chetcuti S, Heiser J, Merhi W, Zorn G, Tadros P, Robinson N, Petrossian G, Hughes GC, Harrison JK, Conte J, Maini B, Mumtaz M, Chenoweth S, Oh JK; U.S. CoreValve Clinical Investigators : Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 370: 1790–1798, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Thomas M, Schymik G, Walther T, Himbert D, Lefèvre T, Treede H, Eggebrecht H, Rubino P, Michev I, Lange R, Anderson WN, Wendler O: Thirty-day results of the SAPIEN aortic Bioprosthesis European Outcome (SOURCE) registry: A European registry of transcatheter aortic valve implantation using the Edwards SAPIEN™ valve. Circulation 122: 62–69, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Webb JG, Altwegg L, Boone RH, Cheung A, Ye J, Lichtenstein S, Lee M, Masson JB, Thompson C, Moss R, Carere R, Munt B, Nietlispach F, Humphries K: Transcatheter aortic valve implantation: Impact on clinical and valve-related outcomes. Circulation 119: 3009–3016, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Grube E, Schuler G, Buellesfeld L, Gerckens U, Linke A, Wenaweser P, Sauren B, Mohr FW, Walther T, Zickmann B, Iversen S, Felderhoff T, Cartier R, Bonan R: Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: Device success and 30-day clinical outcome. J Am Coll Cardiol 50: 69–76, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Varma R, Aronow WS, McClung JA, Garrick R, Vistainer PF, Weiss MB, Belkin RN: Prevalence of valve calcium and association of valve calcium with coronary artery disease, atherosclerotic vascular disease, and all-cause mortality in 137 patients undergoing hemodialysis for chronic renal failure. Am J Cardiol 95: 742–743, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Masuda C, Dohi K, Sakurai Y, Bessho Y, Fukuda H, Fujii S, Sugimoto T, Tanabe M, Onishi K, Shiraki K, Ito M, Nobori T: Impact of chronic kidney disease on the presence and severity of aortic stenosis in patients at high risk for coronary artery disease. Cardiovasc Ultrasound 9: 31, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lüders F, Bunzemeier H, Engelbertz C, Malyar NM, Meyborg M, Roeder N, Berger K, Reinecke H: CKD and acute and long-term outcome of patients with peripheral artery disease and critical limb ischemia. Clin J Am Soc Nephrol 11: 216–222, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drey N, Roderick P, Mullee M, Rogerson M: A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis 42: 677–684, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 15.Brennan JM, Edwards FH, Zhao Y, O’Brien SM, Douglas PS, Peterson ED; Developing Evidence to Inform Decisions About Effectiveness–Aortic Valve Replacement (DEcIDE AVR) Research Team : Long-term survival after aortic valve replacement among high-risk elderly patients in the United States. Circulation 126: 1621–1629, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Thourani VH, Keeling WB, Sarin EL, Guyton RA, Kilgo PD, Dara AB, Puskas JD, Chen EP, Cooper WA, Vega JD, Morris CD, Halkos ME, Lattouf OM: Impact of preoperative renal dysfunction on long-term survival for patients undergoing aortic valve replacement. Ann Thorac Surg 91: 1798–1806, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Grossi EA, Schwartz CF, Yu PJ, Jorde UP, Crooke GA, Grau JB, Ribakove GH, Baumann FG, Ursumanno P, Culliford AT, Colvin SB, Galloway AC: High-risk aortic valve replacement: Are the outcomes as bad as predicted? Ann Thorac Surg 85: 102–106, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto M, Hayashida K, Mouillet G, Hovasse T, Chevalier B, Oguri A, Watanabe Y, Dubois-Randé JL, Morice MC, Lefèvre T, Teiger E: Prognostic value of chronic kidney disease after transcatheter aortic valve implantation. J Am Coll Cardiol 62: 869–877, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Dvir D: First look at long-term durability of transcatheter heart valves: Assessment of valve function up to 10 years after implantation. Presented at the EuroPCR, Paris, May 17, 2016 [Google Scholar]
- 20.Lüders F, Fürstenberg T, Engelbertz C, Gebauer K, Meyborg M, Malyar NM, Reinecke H: The impact of chronic kidney disease on hospitalized patients with peripheral arterial disease and critical limb ischemia. Angiology 68: 145–150, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Eckardt KU, Coresh J, Devuyst O, Johnson RJ, Köttgen A, Levey AS, Levin A: Evolving importance of kidney disease: From subspecialty to global health burden. Lancet 382: 158–169, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G: Chronic kidney disease as a global public health problem: Approaches and initiatives - a position statement from kidney disease improving global outcomes. Kidney Int 72: 247–259, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Reinöhl J, Kaier K, Reinecke H, Schmoor C, Frankenstein L, Vach W, Cribier A, Beyersdorf F, Bode C, Zehender M: Effect of availability of transcatheter aortic-valve replacement on clinical practice. N Engl J Med 373: 2438–2447, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Généreux P, Cohen DJ, Mack M, Rodes-Cabau J, Yadav M, Xu K, Parvataneni R, Hahn R, Kodali SK, Webb JG, Leon MB: Incidence, predictors, and prognostic impact of late bleeding complications after transcatheter aortic valve replacement. J Am Coll Cardiol 64: 2605–2615, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Généreux P, Cohen DJ, Williams MR, Mack M, Kodali SK, Svensson LG, Kirtane AJ, Xu K, McAndrew TC, Makkar R, Smith CR, Leon MB: Bleeding complications after surgical aortic valve replacement compared with transcatheter aortic valve replacement: Insights from the PARTNER I Trial (Placement of Aortic Transcatheter Valve). J Am Coll Cardiol 63: 1100–1109, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Thyregod HG, Steinbrüchel DA, Ihlemann N, Nissen H, Kjeldsen BJ, Petursson P, Chang Y, Franzen OW, Engstrøm T, Clemmensen P, Hansen PB, Andersen LW, Olsen PS, Søndergaard L: Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-year results from the all-comers NOTION randomized clinical trial. J Am Coll Cardiol 65: 2184–2194, 2015 [DOI] [PubMed] [Google Scholar]
- 27.Smith CR, Leon MB, Mack MJ, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Williams M, Dewey T, Kapadia S, Babaliaros V, Thourani VH, Corso P, Pichard AD, Bavaria JE, Herrmann HC, Akin JJ, Anderson WN, Wang D, Pocock SJ; PARTNER Trial Investigators : Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 364: 2187–2198, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Binder RK, Webb JG, Toggweiler S, Freeman M, Barbanti M, Willson AB, Alhassan D, Hague CJ, Wood DA, Leipsic J: Impact of post-implant SAPIEN XT geometry and position on conduction disturbances, hemodynamic performance, and paravalvular regurgitation. JACC Cardiovasc Interv 6: 462–468, 2013 [DOI] [PubMed] [Google Scholar]
- 29.Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S; PARTNER Trial Investigators : Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 363: 1597–1607, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Eggebrecht H, Schmermund A, Voigtländer T, Kahlert P, Erbel R, Mehta RH: Risk of stroke after transcatheter aortic valve implantation (TAVI): A meta-analysis of 10,037 published patients. EuroIntervention 8: 129–138, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Holmes DR Jr., Brennan JM, Rumsfeld JS, Dai D, O’Brien SM, Vemulapalli S, Edwards FH, Carroll J, Shahian D, Grover F, Tuzcu EM, Peterson ED, Brindis RG, Mack MJ; STS/ACC TVT Registry : Clinical outcomes at 1 year following transcatheter aortic valve replacement. JAMA 313: 1019–1028, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Masson P, Webster AC, Hong M, Turner R, Lindley RI, Craig JC: Chronic kidney disease and the risk of stroke: A systematic review and meta-analysis. Nephrol Dial Transplant 30: 1162–1169, 2015 [DOI] [PubMed] [Google Scholar]
- 33.Guyton RA: The placement of aortic transcatheter valve (PARTNER) trial: The surgeon’s perspective: Celebration and concern. Circulation 125: 3237–3239, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Walther T, Hamm CW, Schuler G, Berkowitsch A, Kötting J, Mangner N, Mudra H, Beckmann A, Cremer J, Welz A, Lange R, Kuck KH, Mohr FW, Möllmann H; GARY Executive Board : Perioperative results and complications in 15,964 transcatheter aortic valve replacements: Prospective data from the GARY registry. J Am Coll Cardiol 65: 2173–2180, 2015 [DOI] [PubMed] [Google Scholar]
- 35.Hayashida K, Lefèvre T, Chevalier B, Hovasse T, Romano M, Garot P, Mylotte D, Uribe J, Farge A, Donzeau-Gouge P, Bouvier E, Cormier B, Morice MC: Transfemoral aortic valve implantation new criteria to predict vascular complications. JACC Cardiovasc Interv 4: 851–858, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Rodés-Cabau J, Webb JG, Cheung A, Ye J, Dumont E, Feindel CM, Osten M, Natarajan MK, Velianou JL, Martucci G, DeVarennes B, Chisholm R, Peterson MD, Lichtenstein SV, Nietlispach F, Doyle D, DeLarochellière R, Teoh K, Chu V, Dancea A, Lachapelle K, Cheema A, Latter D, Horlick E: Transcatheter aortic valve implantation for the treatment of severe symptomatic aortic stenosis in patients at very high or prohibitive surgical risk: Acute and late outcomes of the multicenter Canadian experience. J Am Coll Cardiol 55: 1080–1090, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Meyer A, Bunzemeier H, Hausberg M, Walter M, Roeder N, Breithardt G, Reinecke H: Impact of different stages of chronic kidney disease on in-hospital costs in patients with coronary heart disease. Nephrol Dial Transplant 23: 1955–1960, 2008 [DOI] [PubMed] [Google Scholar]
- 38.Laliberté F, Bookhart BK, Vekeman F, Corral M, Duh MS, Bailey RA, Piech CT, Lefebvre P: Direct all-cause health care costs associated with chronic kidney disease in patients with diabetes and hypertension: A managed care perspective. J Manag Care Pharm 15: 312–322, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reinecke H, Unrath M, Freisinger E, Bunzemeier H, Meyborg M, Lüders F, Gebauer K, Roeder N, Berger K, Malyar NM: Peripheral arterial disease and critical limb ischaemia: Still poor outcomes and lack of guideline adherence. Eur Heart J 36: 932–938, 2015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.