Abstract
Background and objectives
Higher serum uric acid levels, even within the reference range, are strongly associated with increased activity of the renin-angiotensin system (RAS) and risk of incident hypertension. However, the effect of lowering serum uric acid on RAS activity in humans is unknown, although the data that lowering serum uric acid can reduce BP are conflicting.
Design, setting, participants, & measurements
In a double-blind placebo-controlled trial conducted from 2011 to 2015, we randomly assigned 149 overweight or obese adults with serum uric acid ≥5.0 mg/dl to uric acid lowering with either probenecid or allopurinol, or to placebo. The primary endpoints were kidney-specific and systemic RAS activity. Secondary endpoints included mean 24-hour systolic BP, mean awake and asleep BP, and nocturnal dipping.
Results
Allopurinol and probenecid markedly lowered serum uric acid after 4 and 8 weeks compared with placebo (mean serum uric acid in allopurinol, probenecid, and placebo at 8 weeks was 2.9, 3.5, and 5.6 mg/dl, respectively). The change in kidney-specific RAS activity, measured as change in the median (interquartile range) renal plasma flow response to captopril (in ml/min per 1.73 m2) from baseline to 8 weeks, was −4 (−25 to 32) in the probenecid group (P=0.83), −4 (−16 to 9) in the allopurinol group (P=0.32), and 1 (−21 to 17) in the placebo group (P=0.96), with no significant treatment effect (P=0.77). Similarly, plasma renin activity and plasma angiotensin II levels did not significantly change with treatment. The change in mean (±SD) 24-hour systolic BPs from baseline to 8 weeks was −1.6±10.1 with probenecid (P=0.43), −0.4±6.1 with allopurinol (P=0.76), and 0.5±6.0 with placebo (P=0.65); there was no significant treatment effect (P=0.58). Adverse events occurred in 9%, 12%, and 2% of those given probenecid, allopurinol, or placebo, respectively.
Conclusions
In contrast to animal experiments and observational studies, this randomized, placebo-controlled trial found that uric acid lowering had no effect on kidney-specific or systemic RAS activity after 8 weeks or on mean systolic BP. These data do not support the hypothesis that higher levels of uric acid are a reversible risk factor for increased BP.
Keywords: Uric acid, BP, allopurinol, probenecid, renin angiotensin system, randomized controlled trials, urate, urate lowering therapy, placebo controlled
Introduction
Higher serum uric acid levels are strongly and independently associated with an increased risk of incident hypertension in multiple prospective observational studies. In one meta-analysis of these studies, for example, a 1 mg/dl higher serum uric acid was associated with a higher risk of developing hypertension by 13%, an association that persisted across the normal range of uric acid levels (1,2). We and others have shown in cross-sectional analyses that nonhypertensive individuals with higher serum uric acid levels have increased activation of their systemic and kidney-specific renin-angiotensin system (RAS) (3,4). These observations support animal studies in which experimentally raising the serum uric acid increased RAS activity and elevated systolic BP (SBP), responses that could be reversed with administration of uric acid–lowering medication such as the xanthine oxidase inhibitor, allopurinol (5–13). These animals also developed arteriolopathy, which was ameliorated only by RAS inhibition (11,14).
To date, whether uric acid lowering can attenuate systemic and kidney-specific RAS activation in humans has not been elucidated. Also, clinical trials of uric acid lowering have demonstrated mixed results on BP lowering (15,16). Only a few of these trials were placebo-controlled and double-blinded and, in addition, few sought to evaluate the potential of uric acid lowering as a preventative measure for hypertension. The two most rigorous such trials published thus far were conducted in overweight adolescents with prehypertension or newly diagnosed stage 1 hypertension (17,18). In these two studies, uric acid lowering (with either allopurinol or probenecid) produced very large reductions in BP. Rigorous studies are lacking, however, in adults. In this randomized double-blind, placebo-controlled trial, we examined the effect of uric acid lowering for 8 weeks on kidney-specific and systemic RAS activity and ambulatory BP in nonhypertensive overweight or obese adults. Overweight and obese individuals were selected because they represent a clinically important population in which to study the effect of serum uric acid lowering on BP. These individuals constitute approximately two-thirds of the adult United States population, are at increased risk for the development of hypertension, and are more likely to have elevated uric acid levels relative to lean individuals.
Materials and Methods
Study Population
Participants were recruited from the greater Boston area from 2011 through 2014 using several mechanisms. First, we contacted participants through a Partners Healthcare database that included individuals who had previously expressed an interest in participating in clinical trials. Second, we recruited through local media outlets, such as billboards placed in nearby colleges and universities, local newspapers, and through online advertisement (such as Craigslist). Inclusion criteria were overweight or obesity (i.e., a body mass index [BMI] ≥25 kg/m2), a serum uric acid level ≥5 mg/dl, and no known history of hypertension (i.e., SBP≥140 mmHg, diastolic BP [DBP] ≥90 mmHg, or use of BP-lowering medications). Potentially eligible participants were excluded if they were pregnant or had a history of diabetes, coronary heart disease, CKD (defined as an eGFR<60 ml/min per 1.73 m2 using the Modification of Diet in Renal Disease estimating equation) (19), known active malignancy (except nonmelanoma skin cancer), or a history of chronic liver disease (or baseline elevations of either alanine aminotransferase or aspartate aminotransferase). The study was approved by the Institutional Review Board at Brigham and Women’s Hospital (BWH). All participants provided written informed consent before entry into the study. The trial was registered with ClinicalTrials.gov (Identifier NCT01320722) in March 2011 before recruitment of participants.
Study Design
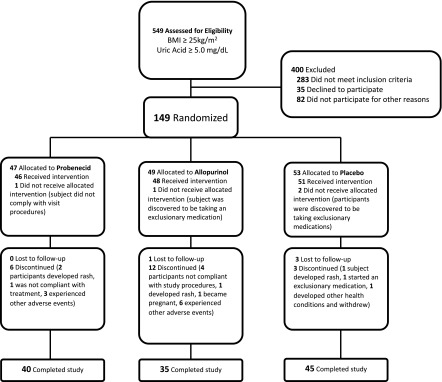
The study design is shown in Figure 1. After an initial phone screen, 549 individuals were asked to come in for a screening visit where a study physician explained the study and performed a detailed history and physical exam. Information about past medical history including medication use was obtained, as were measurements of BP, serum uric acid, serum creatinine, liver function tests, and electrolytes. One hundred and forty-nine individuals who met eligibility criteria and provided informed consent then underwent concealed stratified randomization (1:1:1; and on the basis of race and sex) using a computer-based algorithm to be treated with probenecid, allopurinol, or placebo for 8 weeks. Investigators, participants, data collectors, and data analyzers were all blinded to allocation. The research pharmacist, who supervised the randomization and provided participants with study medication, was not blinded.
Figure 1.
Consort diagram displaying how individuals were recruited, randomized and studied during the trial. BMI, body mass index.
After the screening visit, willing and eligible participants were scheduled for three more visits, including a baseline visit (after which study medication was provided), a check-up visit at 4 weeks (including a pill count, symptom check, and laboratory studies to evaluate for adverse effects, such as liver function tests and serum creatinine), and a final visit at 8 weeks (after which study medication was discontinued). Participants were also contacted by research staff each week they were taking study medication in order to ascertain compliance and screen for potential adverse effects.
Study Measurements
At the baseline and 8-weeks visits, participants were admitted to the hospital and underwent measurement of kidney-specific and systemic RAS activity; after discharge, 24-hour ambulatory BP was assessed.
Kidney-Specific RAS.
Participants underwent salt loading 3 days before RAS measurement at baseline and at 8 weeks of treatment. Participants were instructed to consume three packets of chicken or vegetarian bouillon daily (150 mmol of sodium/d) starting 2 days before and on the day of admission to the Center for Clinical Investigation (CCI) at BWH. Successful salt loading was confirmed with an overnight 12-hour urine collection performed in the CCI (urine sodium content ≥75 mmol/12 h). After an overnight 8-hour fast, participants remained supine for the duration of the study. Two intravenous catheters were inserted, one for infusion and one for blood collection. An 8 mg/kg loading dose of para-aminohippurate (PAH) was administered 60 minutes before the administration of 25 mg of oral captopril. This loading dose was followed by a continuous infusion of PAH at 12 mg/min to achieve plasma PAH concentration in the middle of the range at which tubular secretion dominates excretion. At this concentration of PAH, clearance is independent of plasma PAH levels, and effective renal plasma flow (RPF) is defined as the rate of PAH clearance calculated from steady-state plasma PAH concentrations, as described previously (20). Effective RPF is normalized to body surface area of 1.73 m2. Three precaptopril measurements and three postcaptopril measurements of RPF were made. The change in RPF in response to captopril is a measure of the vasodilator effect from inhibiting angiotensin II (AngII)–mediated vascular tone and therefore the degree of kidney-specific RAS activity. Although necessarily indirect, the bio-assay of renal vasodilator responsiveness to inhibition of the RAS has been demonstrated to provide reproducible measures of renal specific RAS activity in multiple studies that employed various agents and in different patient subsets (21,22).
Systemic RAS.
During the first morning of each inpatient CCI visit, participants remained supine for >60 minutes before blood collection for measurement of plasma renin activity (PRA) and serum AngII levels. PRA was measured by radioimmunoassay (Diasorin, Stillwater, MN). AngII was measured using a double-antibody radioimmunoassay (ALPCO, Salem, NH). Both PRA and AngII assays were performed by the Brigham Research Assay Core, with intra-assay coefficient of variation (CV) of 4.6%–10% and interassay CV of 5.6%–7.6% for PRA, and intra-assay CV of 7.1%–11.3% and interassay CV of 5.8%–18.1% for AngII.
Ambulatory BP Measurement.
After discharge from the CCI (after the baseline and 8-weeks inpatient visits), all participants were fitted with an ambulatory BP monitor (Space-Labs 90207 Ambulatory BP Device; Redmond, WA) that was to be worn for 24 hours. The monitors were preset to measure SBP and DBP every 30 minutes from 6:00 a.m. to 22:00 p.m. and every 60 minutes from 22:00 p.m. to 6:00 a.m. Each participant recorded in a sleep diary the time that they went to bed in the evening and the time that they rose in the morning. A 24-hour ambulatory measurement was considered complete if ≥70% of the programmed BP measurements were successfully recorded, as has previously been described (23). We assessed the change in mean 24-hour SBP, taken as the 24-hour SBP at 8 weeks minus the corresponding BP from baseline. In each case, mean 24-hour SBP was the average of all SBP readings recorded during the 24-hour period after discharge from the CCI. Mean DBP, mean asleep SBP, and percent nocturnal dipping were secondary outcomes. Mean awake and asleep SBPs were defined as the average of SBPs during the periods of wakefulness and sleeping per participant sleep diary. Nocturnal dipping was defined as the difference in mean awake SBP and mean asleep SBP divided by mean awake BP.
Other Measurements.
Demographic, clinical, and laboratory data were obtained at the outpatient screening visit. Serum uric acid was measured at screening visit, baseline inpatient visit, 4-weeks check-up visit, and at the 8-weeks follow-up inpatient visit. All serum uric acid measurements were performed using the Roche Cobas c analyzer using a colorimetric uricase method (Roche Diagnostics, Indianapolis, IN), with an intra-assay CV of 0.6% and an interassay CV of 1.3%.
Study Treatments
Participants were treated initially with either probenecid 500 mg once daily, allopurinol 300 mg once daily, or placebo once daily, depending upon randomized allocation. A 4-week supply of study drugs was provided at the baseline visit by the research pharmacist with instructions to take one pill daily of the assigned drug, to be started after completion of the baseline measurements. All medications were formulated to be physically indistinguishable (i.e., by sight, smell, and weight).
Participants returned for a check-up visit at 4 weeks, at which time serum uric acid was remeasured. The research pharmacist (who was unblinded) viewed the results of the serum uric acid, and provided the subject with the subsequent 4-week supply of medication. The second 4-week supply of medication included instructions to take two pills once per day (one from each of two bottles); if the serum uric acid was <3 mg/dl, the research pharmacist provided those in the allopurinol group with one bottle of allopurinol (300 mg) and one bottle of placebo, whereas if the serum uric acid was ≥3 mg/dl, the research pharmacist provided the subject with two bottles that each contained allopurinol 300 mg (so that the total daily dose was 600 mg). A similar strategy was used for participants in the probenecid arm (who took either 500 or 1000 mg daily during the second 4 weeks of the study, depending upon the serum uric acid at the 4-weeks check-up visit). Participants in the placebo arm were provided with two bottles of placebo. Study treatments were discontinued after the ABP measurement at 8 weeks was completed.
Statistical Analyses
The distribution of baseline characteristics in each treatment group was assessed to describe the population recruited and to confirm effective randomization of participants using ANOVA. The effect of treatment assignment on serum uric acid levels at baseline, 4, and 8 weeks of treatment was measured using t tests to compare those assigned treatment with allopurinol or probenecid with those assigned placebo. We compared median kidney-specific RAS (RPF response to captopril), systemic RAS (levels of PRA and AngII), and mean 24-hour SBP from baseline to 8 weeks within treatment groups using Wilcoxon signed rank sum test and paired t tests. We tested for the effect of treatment assignment (probenecid, allopurinol, or placebo) on changes in these endpoints using repeated measures analysis (Kruskal–Wallis test for median change in kidney-specific RAS from baseline to 8 weeks and repeated measures using fixed effects models for mean BP).
In sensitivity analyses, we compared the baseline characteristics of participants who completed the study with those who failed to complete the 8-week study. In addition, we evaluated the association of the 8-week change in serum uric acid with corresponding changes in kidney-specific RAS (RPF response to captopril), systemic RAS (levels of PRA and AngII), and mean 24-hour SBP using Pearson correlation coefficients. Statistical significance was set for P<0.05; P values were two-tailed. All analyses were performed using the SAS statistical package (version 9.4; SAS Institute Inc., Cary, NC).
We assumed an SD for change in RPF from captopril of 24 ml/min per 1.73 m2: therefore, with 45 participants per group, we had 90% power to detect a decrease in change in RPF from captopril (i.e., a “Δ-Δ”) of 18 ml/min per 1.73 m2 (24). Sample-size calculations for BP were performed assuming SD of the change in SBP to be 8 mmHg, on the basis of previous studies (25). Therefore, with 45 participants per group we expected to have 80% power to detect a 5.0-mmHg fall in SBP with treatment. All power calculations were on the basis of a 10% rate of drop-out, and a type 1 error rate of 5%.
Results
We screened 549 overweight or obese, nonhypertensive individuals; of these, 149 were willing and eligible to participate in the 8-week study (Figure 1) and were randomized (47 to probenecid, 49 to allopurinol, and 53 to placebo). The randomized population had a mean age of 41±14 years and a mean BMI of 34.0±6.3 kg/m2. The population was 62% white and 50% men. The mean uric acid level at baseline was 6.1±0.9 mg/dl, and the mean eGFR was 101±17 ml/min per 1.73 m2. There were no significant differences in any of the baseline characteristics between the probenecid, allopurinol, and placebo groups (Table 1), including baseline uric acid and SBP levels.
Table 1.
Baseline characteristics of all randomized participants
| Characteristic | Probenecid (n=47) | Allopurinol (n=49) | Placebo (n=53) |
|---|---|---|---|
| Mean age (SD), yr | 37(14) | 43(13) | 41(14) |
| Men, n (%) | 24 (51.1) | 25 (51.0) | 25 (47.2) |
| White, n (%) | 27 (57.5) | 32 (65.3) | 33 (62.3) |
| Clinical measures | |||
| Mean serum uric acid (SD), mg/dl | 6.1 (1.1) | 6.1 (0.9) | 6.1 (0.8) |
| Mean body mass index (SD), kg/m2 | 33.4(6.6) | 35.7(6.3) | 33(5.9) |
| Mean eGFRa (SD), ml/min per 1.73 m2 | 102 (18) | 99 (17) | 102 (18) |
| Mean serum creatinine (SD), mg/dl | 0.9 (0.2) | 0.9 (0.1) | 0.9 (0.1) |
| Mean serum ALT (SD), IU/L | 20.7 (9.5) | 18.5 (8.0) | 19.0 (7.8) |
| Mean serum AST (SD), IU/L | 18.9 (5.5) | 17.8 (6.0) | 16.8 (3.4) |
| Mean systolic BP (SD), mmHg | 119 (11) | 119 (12) | 119 (10) |
| Mean diastolic BP (SD), mmHg | 77 (7) | 78 (8) | 78 (7) |
ALT, Alanine Transaminase; AST, Aspartate Transaminase.
eGFR calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.
Of those allocated to probenecid, five were withdrawn due to adverse events, two were noncompliant with the study protocol, and 40 completed the study. Of those allocated to allopurinol, seven were withdrawn due to adverse events, four were noncompliant with the study protocol, one became pregnant, two took an exclusionary medication, one was lost to follow-up, and 35 completed the study. Of those allocated to placebo, three were withdrawn due to adverse events, three were lost to follow-up, two were started on exclusionary medications, and 45 completed the study. Baseline characteristics were not different between those who completed the 8-week study (n=120) and those who failed to complete the study (n=29).
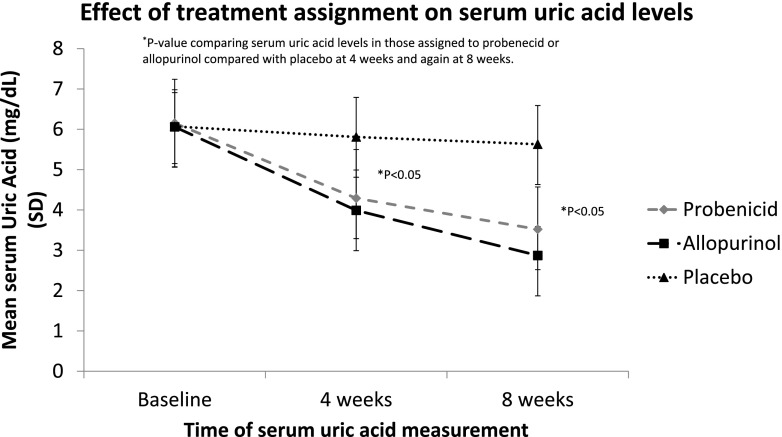
Serum uric acid decreased significantly from baseline to 4 weeks and from 4 to 8 weeks in those assigned to treatment with either probenecid or allopurinol, as compared with placebo (Figure 2). Specifically, mean serum uric acid fell from 6.1 mg/dl at baseline in the probenecid group to 4.3 mg/dl at 4 weeks and 3.5 mg/dl at 8 weeks (P <0.001). Mean serum uric acid in the allopurinol group decreased from 6.1 mg/dl at baseline to 4.0 mg/dl at 4 weeks and 2.9 mg/dl at 8 weeks (P <0.001). In the placebo group, the mean serum uric acid was 6.1 mg/dl at baseline, 5.8 mg/dl at 4 weeks, and 5.6 mg/dl at 8 weeks (P=0.48).
Figure 2.
The effect of treatment assignment on serum uric acid level during trial. (*P value comparing serum uric acid levels in those assigned to probenecid or allopurinol compared with placebo at 4 weeks and again at 8 weeks).
There were no clinically significant or statistically significant differences in the primary outcome, change in kidney-specific RAS activity, in any treatment group. The response of RPF to captopril was 38 (interquartile range, 14–67) ml/min per 1.73 m2 at baseline and 33 (12–72) ml/min per 1.73 m2 at 8 weeks in the probenecid group (P=0.83); 41 (16–65) ml/min per 1.73 m2 at baseline and 36 (17–55) ml/min per 1.73 m2 at 8 weeks in the allopurinol group (P=0.32); and 30 (7–44) ml/min per 1.73 m2 at baseline and 30 (−1 to 48) ml/min per 1.73 m2 at 8 weeks in the placebo group (P=0.96). There was no difference in the 8-week change in RPF response with either probenecid or allopurinol and the corresponding 8-week change with placebo (Ps 0.77 and 0.58, respectively), nor was there any effect of treatment assignment (P=0.77, Table 2). Systemic RAS activity measured by PRA increased nonsignificantly after 8 weeks of treatment in all three groups (from 0.3 [0.1–0.6] to 0.4 [0.2–0.7] ng/ml per hour with probenecid [P=0.13]; from 0.3 [0.1–0.5] to 0.3 [0.2–0.5] ng/ml per hour with allopurinol [P=0.54]; and from 0.2 [0.1–0.4] to 0.2 [0.1–0.6] ng/ml per hour with placebo [P=0.13]). Similarly, serum AngII levels did not change significantly after 8 weeks of treatment with probenecid, allopurinol, or placebo.
Table 2.
Effects of uric acid lowering on renal and systemic renin-angiotensin system
| Parameter | Probenecid (n=40) | Allopurinol (n=35) | Placebo (n=45) | Probenecid Versus Placebo Pb | Allopurinol Versus Placebo Pb | Treatment Effect Pc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 8-Wk | Change from Baseline | Pa | Baseline | 8-Wk | Change from Baseline | Pa | Baseline | 8-Wk | Change from Baseline | Pa | ||||
| Precaptopril RPF | |||||||||||||||
| No. of participants | 40 | 40 | 35 | 34 | 45 | 45 | |||||||||
| Median (IQR), ml/min per 1.73 m2 | 480 (421–568) | 474 (428–556) | −3 (−27 to 19) | 0.40 | 489 (409–550) | 467 (404–540) | 5 (−10 to 28) | 0.28 | 534 (457–571) | 505 (456–547) | −4 (−35 to 10) | 0.29 | 0.81 | 0.12 | 0.25 |
| Postcaptopril RPF | |||||||||||||||
| No. of participants | 40 | 40 | 35 | 34 | 45 | 45 | |||||||||
| Median (IQR), ml/min per 1.73 m2 | 531 (447–643) | 503 (464–608) | −6 (−31 to 36) | 0.73 | 517 (442–567) | 495 (44–571) | 5 (−30 to 28) | 0.97 | 544 (480–613) | 535 (476–593) | −11 (−30 to 19) | 0.18 | 0.54 | 0.34 | 0.63 |
| Change in RPF | |||||||||||||||
| No. of participants | 40 | 40 | 35 | 34 | 45 | 45 | |||||||||
| Median (IQR), ml/min per 1.73 m2 | 38 (14–67) | 33 (12–72) | −4 (−24 to 32) | 0.83 | 41 (16–65) | 36 (17–55) | −4 (−16 to 9) | 0.32 | 30 (7–44) | 30 (−1 to 48) | 1 (−21 to 17) | 0.96 | 0.77 | 0.58 | 0.77 |
| PRA | |||||||||||||||
| No. of participants | 39 | 40 | 35 | 33 | 43 | 44 | |||||||||
| Median (IQR), ng/ml per h | 0.3 (0.1–0.6) | 0.4 (0.2–0.7) | 0.1 (−0.2 to 0.4) | 0.13 | 0.3 (0.1–0.5) | 0.3 (0.2–0.5) | 0.0 (−0.2 to 0.2) | 0.54 | 0.2 (0.1–0.4) | 0.2 (0.1–0.6) | 0.1 (−0.1 to 0.2) | 0.13 | 0.60 | 0.74 | 0.81 |
| Ang II | |||||||||||||||
| No. of participants | 40 | 39 | 34 | 34 | 45 | 45 | |||||||||
| Median (IQR), pg/ml | 18.8 (15.3–22.3) | 20.3 (18.5–22.7) | 1.7 (0.3.0–4.7) | 0.18 | 19.1 (17.0–23.3) | 21.4 (18.2–24.4) | 1.6 (−1.6 to 5.1) | 0.04 | 19.0 (16.4–20.9) | 19.1 (17.0–21.5) | −0.8 (−2.2 to 3.3) | 0.93 | 0.28 | 0.14 | 0.30 |
RPF, renal plasma flow; IQR, interquartile range; PRA, plasma renin activity; AngII, angiotensin II.
P value from the Wilcoxon signed rank test of change in parameter within an individual from baseline to 8 wk.
P value from the Kruskal–Wallis test of change in parameter between baseline and 8 wk within individuals across treatment groups.
Of the 120 participants who completed the study (40 in the probenecid group, 35 in the allopurinol group, and 45 in the placebo group), 82 had >70% of the programmed BP measurements successfully recorded at both the baseline and 8-weeks visits (25, 27, and 30 in the probenecid, allopurinol, and placebo groups, respectively). There were no clinically significant or statistically significant differences in change in mean 24-hour SBP in any of the treatment groups (Table 3). In addition, there were no significant effects of treatment assignment on change in mean 24-hour SBP from baseline to 8 weeks comparing probenecid with placebo (P=0.34), allopurinol with placebo (P=0.59), or any uric acid lowering with placebo (P=0.39). When we analyzed all of the individuals who completed the study (including those who had <70% of programmed ABP measurements successfully recorded), there remained no clinically significant changes in 24-hour SBP in any treatment group (Supplemental Table 1).
Table 3.
Ambulatory BP results from individuals who completed both visits and had complete 24-h BP recordings
| ABP Parameter | Probenecid (n=25) | Allopurinol (n=27) | Placebo (n=30) | Probenecid Versus Placebo Pb | Allopurinol Versus Placebo Pb | Treatment Effect Pc | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 8-Wk | Change from Baseline | Pa | Baseline | 8-Wk | Change from Baseline | Pa | Baseline | 8-Wk | Change from Baseline | Pa | ||||
| Mean overall SBP (SD), mmHg | 126.9 (10.2) | 125.2 (9.1) | −1.6 (10.1) | 0.43 | 124.1 (8.8) | 123.7 (9.4) | −0.4 (6.1) | 0.76 | 122.4 (9.7) | 122.9 (9.5) | 0.5 (6.0) | 0.65 | 0.34 | 0.59 | 0.58 |
| Mean overall DBP (SD), mmHg | 73.3 (7.8) | 73.8 (6.9) | 0.6 (6.2) | 0.66 | 72.1 (7.1) | 72.0 (6.5) | −0.2 (4.9) | 0.82 | 71.9 (7.4) | 72.6 (6.2) | 0.6 (5.2) | 0.51 | 0.96 | 0.53 | 0.81 |
| Mean awake SBP (SD), mmHg | 130.0 (9.2) | 128.1 (9.3) | −1.8 (9.8) | 0.36 | 126.7 (8.4) | 125.1 (9.6) | −1.6 (6.4) | 0.21 | 125.5 (9.0) | 124.9 (10.1) | −0.6 (6.1) | 0.60 | 0.57 | 0.55 | 0.81 |
| Mean asleep SBP (SD), mmHg | 119.2 (12.4) | 116.2 (9.6) | −2.9 (11.8) | 0.23 | 117.1 (10.0) | 118.6 (11.7) | 1.4 (8.3) | 0.37 | 113.9 (12.2) | 116.2 (10.5) | 1.9 (9.1) | 0.26 | 0.08 | 0.80 | 0.14 |
| Mean nocturnal dipping (SD), % | 8.4 (6.8) | 9.2 (6.1) | 0.8 (6.1) | 0.51 | 7.5 (5.3) | 5.2 (7.1) | −2.4 (6.7) | 0.08 | 9.4 (6.2) | 6.9 (6.2) | −2.4 (6.8) | 0.06 | 0.07 | 0.97 | 0.13 |
ABP, ambulatory BP; SBP, systolic BP; DBP, diastolic BP.
P value from paired t test between ABP measurement at baseline and at 8 wk within each individual.
P value from Repeated Measures analysis assuming fixed effects, for the effect of treatment assignment on the change in ABP measurement from baseline to 8 wk.
As with 24-hour SBP, there were no significant (clinical or statistical) effects of any treatment on any of the other BP endpoints, including mean 24-hour DBP, asleep SBP, awake SBP, or nocturnal SBP dipping (Supplemental Table 1, Table 3). In sensitivity analyses, changes in uric acid levels between baseline and 8 weeks were not correlated with any measurable change in either kidney-specific or systemic RAS activity, or ABP parameters during the same period (Supplemental Tables 2 and 3). No significant interaction was found between treatment assignment and race or sex for the major outcomes (renal specific RAS activity and mean 24-hour SBP).
Adverse events did not differ significantly among treatment arms (Table 4). The most common adverse event was abdominal discomfort, which occurred in one, six, and five participants in the probenecid, allopurinol, and placebo arms, respectively. Serious adverse events occurred in four participants who received probenecid, in six participants who received allopurinol (including two with an elevation in creatinine and three with an elevation in liver enzymes), and in two participants who received placebo.
Table 4.
Adverse events by treatment assignment among those that received study medication
| Adverse Events | Probenecid (n=46), n | Allopurinol (n=48), n | Placebo (n=51), n |
|---|---|---|---|
| Serious adverse events | |||
| All | 4 | 6 | 2 |
| Rash | 2 | 1 | 1 |
| Elevated creatinine | 1 | 2 | 0 |
| Elevated liver enzymes | 0 | 3 | 1 |
| Decreased hematocrit | 1 | 0 | 0 |
| Minor adverse events | |||
| All | 16 | 36 | 25 |
| Headache | 3 | 2 | 2 |
| Abdominal discomfort | 1 | 6 | 5 |
| Fatigue/sleepiness | 4 | 4 | 2 |
| Dizziness/lightheaded | 1 | 2 | 3 |
| Nausea | 2 | 3 | 3 |
| Diarrhea | 2 | 1 | 0 |
| Flushing | 1 | 0 | 1 |
| Tingling in extremities | 0 | 2 | 0 |
| Decreased appetite | 1 | 4 | 4 |
| Altered taste | 0 | 3 | 2 |
| Joint pain | 0 | 3 | 2 |
| Other/miscellaneous | 1 | 6 | 3 |
Discussion
Considerable observational data collected in multiple populations have demonstrated a robust association between higher serum uric acid levels and an increased risk of incident hypertension, with evidence that uric acid–mediated RAS activation is a mechanism for such an association. As an example, in vitro studies with human vascular endothelial cells and adipocytes found that incubating cells with high levels of uric acid upregulates elements of the RAS within cells and increases AngII secretion. Also, rats fed oxonic acid, a uricase inhibitor, had a doubling of their serum uric acid levels, increased production of renin in the juxtaglomerular apparatus, plus renal vasoconstriction and a marked increase in SBP and DBP. In the case of the rat experiments, the changes were all prevented or reversed with the administration of allopurinol, a xanthine oxidase inhibitor, which reduces serum uric acid (11,14,26,27). Further work using this rat model demonstrated that prolonged elevations in serum uric acid produced small vessel disease in the kidney with thickening of the afferent arterioles. Because the changes were prevented by pretreatment with RAS blockade but not with other antihypertensive medications, it is possible that arteriolopathy was induced by hyperuricemia leading to RAS activation (14). These findings led Feig et al. (28) to propose a two-step model to explain the mechanism, through which high uric acid levels lead to hypertension. The first step is a uric acid–dependent activation of RAS leading to reversible vasoconstriction, and the second step is irreversible uric acid–mediated arteriosclerosis that results in salt-sensitive hypertension.
Several observational studies in humans have investigated the association between uric acid levels and RAS activity in humans. Higher serum uric acid levels were associated with a lower basal RPF and a blunted renal vascular response to AngII infusion in a study of 249 participants, consistent with increased kidney-specific RAS activation in the setting of hyperuricemia (4). By contrast, a smaller study of 52 women observed that higher serum uric acid was associated with a larger BP increase with AngII infusion, possibly reflecting reduced (rather than increased) systemic RAS activity and basal AngII levels in the setting of hyperuricemia (29). A possible explanation for these potentially conflicting results is that uric acid may lead to lower systemic RAS activity but higher kidney-specific RAS activity. However, a small open-label trial of 60 hypertensive participants with serum uric acid levels >7.0 mg/dl found that treatment with febuxostat (a xanthine oxidase inhibitor) for 6 months produced a 29% reduction in serum uric acid levels, a 33% reduction in PRA, and a 14% reduction in the plasma aldosterone concentration, suggesting that reducing uric acid reduced systemic RAS activity (30). Several elements of the design of that study warrant cautious interpretation of those results, including an open-label design, lack of control for dietary sodium intake before measurement of RAS, and failure to control for RAS-blocking medications.
We found that reducing serum uric acid by either 30% (probenecid) or 43% (allopurinol), in the context of a blinded randomized study design, had no effect on measures of kidney-specific RAS activity (measured by response of RPF to captopril) or systemic RAS activity (PRA and AngII levels). These results are not consistent with the finding of the febuxostat trial. Several differences between the trials may explain the conflicting outcomes. First, the open-label trial recruited hypertensive participants with serum uric acid levels ≥7.0 mg/dl, whereas our trial recruited nonhypertensive participants with serum uric acids levels ≥5.0 mg/dl. However, it would be expected that normotensive and prehypertensive individuals (as in our study) with moderate hyperuricemia would fit “step one” of Feig’s two-step model of hyperuricemia-induced hypertension, and therefore be more (rather than less) responsive to lowering uric acid levels with respect to RAS activity than those with preexisting hypertension and severe hyperuricemia, a group expected to fit into the unresponsive “step two” (28). Second, the treatment time was shorter (8 weeks) in our trial compared with the febuxostat trial (6 months), and it may be that the effects of uric acid lowering on RAS activity in humans require a prolonged period of treatment (in contrast to the more rapid effects noted in animal or human in vitro models).
Two randomized controlled trials performed by Feig and colleagues involving adolescents (11–17 years), including one with 30 individuals who had stage 1 hypertension and serum uric acid ≥6 mg/dl and one with 60 obese nonhypertensive individuals who had serum uric acid ≥5 mg/dl, found consistent and dramatic reductions in BP (measured using ABP monitors) after 4–8 weeks of uric acid–lowering therapy with either allopurinol or probenecid (17,18). In one of these trials, allopurinol reduced mean 24-hour systolic pressure by 6.3 mmHg and, in the other, allopurinol or probenecid reduced mean 24-hour systolic pressure by 8.9–9.2 mmHg (17,18), with the greater BP reduction detected among those without hypertension and milder hyperuricemia. Interestingly, the BP-lowering effects were most significant among those with serum uric acid levels ≥5.0 mg/dl and without baseline hypertension. This suggests that, in adolescents at least, individuals with more moderate uric acid and normal BP levels are more susceptible to BP lowering from uric acid reduction. In contrast to the observational data and the two trials in adolescents, we found no effect of uric acid lowering on any BP parameter in overweight or obese adults with elevated baseline serum uric acid levels.
Studies performed in adults that investigated the effects of allopurinol and, less frequently, probenecid on BP have been conflicting and often of lower methodologic quality (e.g., unblinded, BP as a secondary endpoint, with an unspecified measurement protocol) (31). Of these previous adult trials, one, which was performed to assess carotid intimal media thickness after stroke, found a significant reduction in BP among those assigned to allopurinol treatment (32,33). Of the five trials which failed to detect a significant effect on SBP with uric acid lowering (34–38), only one included normotensive participants and was limited in that investigators were not blinded to treatment assignment, and clinic BP rather than ambulatory BP was measured (35). In a recent meta-analysis of trials involving patients with CKD, use of allopurinol did not significantly reduce SBP compared with either placebo or no treatment (15). Additional efforts to assess for causality between hyperuricemia and development of hypertension include a recent Mendelian randomization study performed in a Danish population cohort of 58,072 participants. In this analysis, a genetic score of uric acid variability was not associated with BP, thereby providing evidence against a causal association of uric acid with increased BP (39). Other Mendelian randomization studies have come to similar conclusions, whereas smaller genetic studies with <600 participants have shown conflicting results (40–42).
The findings of our trial are consistent with many of the previous smaller interventional studies in adults, showing no effect of uric acid lowering on BP. An important question is why lowering serum uric acid levels appeared to have such a profound effect on BP in adolescents, with reductions in SBP ranging from 7 to 10 mmHg with 4–8 weeks of treatment, in stark contrast to our findings in adults with a similar degree of comorbidity. One obvious issue to consider is whether the pathophysiology of hyperuricemia in younger individuals differs from that in adults. Animal models of hyperuricemia-induced hypertension suggest that hyperuricemia has an acute effect on BP through activation of the RAS and decreased endothelial nitric oxide synthase, with the effect becoming chronic as a result of microvascular disease (11,14,43). If hyperuricemia has similar early and late effects in humans, with the late effect (i.e., microvascular damage) being more prevalent in adults than adolescents, then lowering uric acid in adults may not result in the same BP reduction as it does in children.
Our study has several limitations that deserve mention. First, we recruited individuals with serum uric acid levels ≥5.0 mg/dl and BP<140/90 mmHg. It is possible that a population with higher baseline serum uric acid or hypertension could have had a RAS activity and BP-reducing benefit from uric acid–lowering therapy. Yet in vitro studies suggest that changes in uric acid levels nearly identical to those found in our trial (Figure 2, raising uric acid from 3 to 6 mg/dl) lead to a 4–5-fold increase in RAS activity, suggesting an appropriate population was selected (26). Also, epidemiologic evidence suggests that association of higher uric acid levels with increased hypertension risk is continuous and is present at levels of uric acid well within the normal range (below the cutoff we used for eligibility). In addition, our cutoff for uric acid of ≥5.0 mg/dl was identical to that used in one of the adolescent studies that reported a profound effect on BP (18). Also, our findings are consistent with several smaller prior trials of adults with hypertension or other cardiovascular comorbidity, suggesting that the lack of effect we found was not due to our nonhypertensive population (38). Furthermore, if hyperuricemia leads to hypertension via the aforementioned two-step process, then selecting an adult population before the onset of hypertension would be expected to produce a larger BP-lowering effect than if a hypertensive population were examined (17,18). Second, our study duration was limited to 8 weeks of treatment, which may have been too short to identify effects of uric acid lowering on RAS activity and BP; it is conceivable that elevated uric acid levels produce endothelial dysfunction which increases RAS activity and these aberrancies requires a longer period than 8 weeks to recover. However, several previous studies showing improved endothelial function with treatment of hyperuricemia used similar durations of treatment as in our study (34,44–46). Moreover, the adolescent studies found effects on BP with the same or even shorter durations of uric acid lowering (17,18). Third, several participants who completed our study had incomplete ABP measurements, potentially reducing the power of our study. However, on the basis of calculations using participants who had complete ABP measurements, we still had 80% power to detect clinically meaningful treatment effects. Additionally, there were no discernible reductions in BP in the treatment arms, and therefore it does not appear that a lack of statistical power limited our ability to detect an important BP reduction.
In summary, in this randomized, double-blind, placebo-controlled trial of overweight or obese individuals with high uric acid levels, lowering serum uric acid with either probenecid or allopurinol did not improve either kidney-specific or systemic RAS activity, in contrast to animal experiments and observational studies. Consistent with these findings, BP measured using 24-hour ABP monitoring did not show any benefit from uric acid lowering from either probenecid or allopurinol. This finding is consistent with smaller and less rigorous interventional studies and large Mendelian randomization studies, suggesting that the strong observational association of higher uric acid with elevation in BP may not be causal in adults.
Disclosures
G.C.C. is a consultant for Allena Pharmaceuticals and Astra Zeneca and receives royalties from UptoDate.
Supplementary Material
Acknowledgments
This study was funded by a grant from the National Institutes of Health/National Heart, Lung, and Blood Institute (1R01HL105440-01).
Because G.C.C. was the Editor-in-Chief of CJASN during the time this manuscript underwent peer review, he was not involved in the peer-review process for this manuscript. Another editor oversaw the peer-review and decision-making process for this manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.10771016/-/DCSupplemental.
References
- 1.Grayson PC, Kim SY, LaValley M, Choi HK: Hyperuricemia and incident hypertension: A systematic review and meta-analysis. Arthritis Care Res (Hoboken) 63: 102–110, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forman JP, Choi H, Curhan GC: Uric acid and insulin sensitivity and risk of incident hypertension. Arch Intern Med 169: 155–162, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abiko H, Konta T, Hao Z, Takasaki S, Suzuki K, Ichikawa K, Ikeda A, Shibata Y, Takeishi Y, Kawata S, Kato T, Kubota I: Factors correlated with plasma renin activity in general Japanese population. Clin Exp Nephrol 13: 130–137, 2009 [DOI] [PubMed] [Google Scholar]
- 4.Perlstein TS, Gumieniak O, Hopkins PN, Murphey LJ, Brown NJ, Williams GH, Hollenberg NK, Fisher ND: Uric acid and the state of the intrarenal renin-angiotensin system in humans. Kidney Int 66: 1465–1470, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Feig DI: Uric acid: A novel mediator and marker of risk in chronic kidney disease? Curr Opin Nephrol Hypertens 18: 526–530, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, Johnson RJ: Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 41: 1287–1293, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Kang DH, Han L, Ouyang X, Kahn AM, Kanellis J, Li P, Feng L, Nakagawa T, Watanabe S, Hosoyamada M, Endou H, Lipkowitz M, Abramson R, Mu W, Johnson RJ: Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. Am J Nephrol 25: 425–433, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Kang DH, Nakagawa T, Feng L, Johnson RJ: Nitric oxide modulates vascular disease in the remnant kidney model. Am J Pathol 161: 239–248, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ: A role for uric acid in the progression of renal disease. J Am Soc Nephrol 13: 2888–2897, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ: Hyperuricemia induces endothelial dysfunction. Kidney Int 67: 1739–1742, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ: Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38: 1101–1106, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Corry DB, Eslami P, Yamamoto K, Nyby MD, Makino H, Tuck ML: Uric acid stimulates vascular smooth muscle cell proliferation and oxidative stress via the vascular renin-angiotensin system. J Hypertens 26: 269–275, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Eräranta A, Kurra V, Tahvanainen AM, Vehmas TI, Kööbi P, Lakkisto P, Tikkanen I, Niemelä OJ, Mustonen JT, Pörsti IH: Oxonic acid-induced hyperuricemia elevates plasma aldosterone in experimental renal insufficiency. J Hypertens 26: 1661–1668, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, Kang DH, Gordon KL, Watanabe S, Nakagawa T, Lan HY, Johnson RJ: Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol 282: F991–F997, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Bose B, Badve SV, Hiremath SS, Boudville N, Brown FG, Cass A, de Zoysa JR, Fassett RG, Faull R, Harris DC, Hawley CM, Kanellis J, Palmer SC, Perkovic V, Pascoe EM, Rangan GK, Walker RJ, Walters G, Johnson DW: Effects of uric acid-lowering therapy on renal outcomes: A systematic review and meta-analysis. Nephrol Dial Transplant 29: 406–413, 2014 [DOI] [PubMed] [Google Scholar]
- 16.Agarwal V, Hans N, Messerli FH: Effect of allopurinol on blood pressure: A systematic review and meta-analysis. J Clin Hypertens (Greenwich) 15: 435–442, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feig DI, Soletsky B, Johnson RJ: Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: A randomized trial. JAMA 300: 924–932, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soletsky B, Feig DI: Uric acid reduction rectifies prehypertension in obese adolescents. Hypertension 60: 1148–1156, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Price DA, Porter LE, Gordon M, Fisher ND, De’Oliveira JM, Laffel LM, Passan DR, Williams GH, Hollenberg NK: The paradox of the low-renin state in diabetic nephropathy. J Am Soc Nephrol 10: 2382–2391, 1999 [DOI] [PubMed] [Google Scholar]
- 21.Fisher ND, Jan Danser AH, Nussberger J, Dole WP, Hollenberg NK: Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation 117: 3199–3205, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Hollenberg NK, Fisher ND: Renal circulation and blockade of the renin-angiotensin system. Is angiotensin-converting enzyme inhibition the last word? Hypertension 26: 602–609, 1995 [DOI] [PubMed] [Google Scholar]
- 23.Parati G, Stergiou G, O’Brien E, Asmar R, Beilin L, Bilo G, Clement D, de la Sierra A, de Leeuw P, Dolan E, Fagard R, Graves J, Head GA, Imai Y, Kario K, Lurbe E, Mallion JM, Mancia G, Mengden T, Myers M, Ogedegbe G, Ohkubo T, Omboni S, Palatini P, Redon J, Ruilope LM, Shennan A, Staessen JA, vanMontfrans G, Verdecchia P, Waeber B, Wang J, Zanchetti A, Zhang Y; European Society of Hypertension Working Group on Blood Pressure Monitoring and Cardiovascular Variability : European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens 32: 1359–1366, 2014 [DOI] [PubMed] [Google Scholar]
- 24.Forman JP, Price DA, Stevanovic R, Fisher ND: Racial differences in renal vascular response to angiotensin blockade with captopril or candesartan. J Hypertens 25: 877–882, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Ard JD, Coffman CJ, Lin PH, Svetkey LP: One-year follow-up study of blood pressure and dietary patterns in dietary approaches to stop hypertension (DASH)-sodium participants. Am J Hypertens 17: 1156–1162, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Yu MA, Sánchez-Lozada LG, Johnson RJ, Kang DH: Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J Hypertens 28: 1234–1242, 2010 [PubMed] [Google Scholar]
- 27.Zhang JX, Zhang YP, Wu QN, Chen B: Uric acid induces oxidative stress via an activation of the renin-angiotensin system in 3T3-L1 adipocytes. Endocrine 48: 135–142, 2015 [DOI] [PubMed] [Google Scholar]
- 28.Feig DI: The role of uric acid in the pathogenesis of hypertension in the young. J Clin Hypertens (Greenwich) 14: 346–352, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Samimi A, Ramesh S, Turin TC, MacRae JM, Sarna MA, Reimer RA, Hemmelgarn BR, Sola DY, Ahmed SB: Serum uric acid level, blood pressure, and vascular angiotensin II responsiveness in healthy men and women. Physiol Rep 2: e12235, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tani S, Nagao K, Hirayama A: Effect of febuxostat, a xanthine oxidase inhibitor, on cardiovascular risk in hyperuricemic patients with hypertension: A prospective, open-label, pilot study. Clin Drug Investig 35: 823–831, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Gois PH, Souza ER: Pharmacotherapy for hyperuricemia in hypertensive patients. Cochrane Database Syst Rev 1: CD008652, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Siu YP, Leung KT, Tong MK, Kwan TH: Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 47: 51–59, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Higgins P, Walters MR, Murray HM, McArthur K, McConnachie A, Lees KR, Dawson J: Allopurinol reduces brachial and central blood pressure, and carotid intima-media thickness progression after ischaemic stroke and transient ischaemic attack: A randomised controlled trial. Heart 100: 1085–1092, 2014 [DOI] [PubMed] [Google Scholar]
- 34.George J, Carr E, Davies J, Belch JJ, Struthers A: High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 114: 2508–2516, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Dogan A, Yarlioglues M, Kaya MG, Karadag Z, Dogan S, Ardic I, Dogdu O, Kilinc Y, Zencir C, Akpek M, Ozdogru I, Oguzhan A, Kalay N: Effect of long-term and high-dose allopurinol therapy on endothelial function in normotensive diabetic patients. Blood Press 20: 182–187, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Khan F, George J, Wong K, McSwiggan S, Struthers AD, Belch JJ: Allopurinol treatment reduces arterial wave reflection in stroke survivors. Cardiovasc Ther 26: 247–252, 2008 [DOI] [PubMed] [Google Scholar]
- 37.Momeni A, Shahidi S, Seirafian S, Taheri S, Kheiri S: Effect of allopurinol in decreasing proteinuria in type 2 diabetic patients. Iran J Kidney Dis 4: 128–132, 2010 [PubMed] [Google Scholar]
- 38.Segal MS, Srinivas TR, Mohandas R, Shuster JJ, Wen X, Whidden E, Tantravahi J, Johnson RJ: The effect of the addition of allopurinol on blood pressure control in African Americans treated with a thiazide-like diuretic. J Am Soc Hypertens 9: 610–619.e611, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palmer TM, Nordestgaard BG, Benn M, Tybjærg-Hansen A, Davey Smith G, Lawlor DA, Timpson NJ: Association of plasma uric acid with ischaemic heart disease and blood pressure: Mendelian randomisation analysis of two large cohorts. BMJ 347: f4262, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sedaghat S, Pazoki R, Uitterlinden AG, Hofman A, Stricker BH, Ikram MA, Franco OH, Dehghan A: Association of uric acid genetic risk score with blood pressure: The Rotterdam study. Hypertension 64: 1061–1066, 2014 [DOI] [PubMed] [Google Scholar]
- 41.Mallamaci F, Testa A, Leonardis D, Tripepi R, Pisano A, Spoto B, Sanguedolce MC, Parlongo RM, Tripepi G, Zoccali C: A polymorphism in the major gene regulating serum uric acid associates with clinic SBP and the white-coat effect in a family-based study. J Hypertens 32: 1621–1628, discussion 1628, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Parsa A, Brown E, Weir MR, Fink JC, Shuldiner AR, Mitchell BD, McArdle PF: Genotype-based changes in serum uric acid affect blood pressure. Kidney Int 81: 502–507, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson RJ, Segal MS, Srinivas T, Ejaz A, Mu W, Roncal C, Sánchez-Lozada LG, Gersch M, Rodriguez-Iturbe B, Kang DH, Acosta JH: Essential hypertension, progressive renal disease, and uric acid: A pathogenetic link? J Am Soc Nephrol 16: 1909–1919, 2005 [DOI] [PubMed] [Google Scholar]
- 44.Yelken B, Caliskan Y, Gorgulu N, Altun I, Yilmaz A, Yazici H, Oflaz H, Yildiz A: Reduction of uric acid levels with allopurinol treatment improves endothelial function in patients with chronic kidney disease. Clin Nephrol 77: 275–282, 2012 [DOI] [PubMed] [Google Scholar]
- 45.Meléndez-Ramírez G, Pérez-Méndez O, López-Osorio C, Kuri-Alfaro J, Espinola-Zavaleta N: Effect of the treatment with allopurinol on the endothelial function in patients with hyperuricemia. Endocr Res 37: 1–6, 2012 [DOI] [PubMed] [Google Scholar]
- 46.Doehner W, Schoene N, Rauchhaus M, Leyva-Leon F, Pavitt DV, Reaveley DA, Schuler G, Coats AJ, Anker SD, Hambrecht R: Effects of xanthine oxidase inhibition with allopurinol on endothelial function and peripheral blood flow in hyperuricemic patients with chronic heart failure: Results from 2 placebo-controlled studies. Circulation 105: 2619–2624, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.