Abstract
Lipid–drug conjugates (LDCs) are drug molecules that have been covalently modified with lipids. The conjugation of lipids to drug molecules increases lipophilicity and also changes other properties of drugs. The conjugates demonstrate several advantages including improved oral bioavailability, improved targeting to the lymphatic system, enhanced tumor targeting, and reduced toxicity. Based on the chemical nature of drugs and lipids, various conjugation strategies and chemical linkers can be utilized to synthesize LDCs. Linkers and/or conjugation methods determine how drugs are released from LDCs and are critical for the optimal performance of LDCs. In this review, different lipids used for preparing LDCs and various conjugation strategies are summarized. Although LDCs can be administered without a delivery carrier, most of them are loaded into appropriate delivery systems. The lipid moiety in the conjugates can significantly enhance drug loading into hydrophobic components of delivery carriers and thus generate formulations with high drug loading and superior stability. Different delivery carriers such as emulsions, liposomes, micelles, lipid nanoparticles, and polymer nanoparticles are also discussed in this review.
Keywords: lipid, drug delivery, prodrug, conjugation, chemical bonds
Graphical abstract
1. INTRODUCTION
Lipid–drug conjugates (LDCs) are drug molecules that have been covalently modified with lipids. LDCs have demonstrated several advantages including improved oral bioavailability, enhanced tumor targeting, reduced toxicity, and enhanced drug loading into delivery carriers. Based on the chemical structures of drugs and lipids, various conjugation strategies and chemical linkers can be utilized to synthesize LDCs. This review is intended to extensively discuss the use of LDCs as a strategy for enhancing drug delivery. Different lipids used for synthesizing conjugates and various conjugation approaches will be summarized. The advantages of using LDCs will also be introduced. Finally, LDC delivery carriers including carrier-free systems, emulsions, liposomes, micelles, lipid nanoparticles, polymer nanoparticles, and others will be reviewed.
2. CONJUGATION STRATEGIES
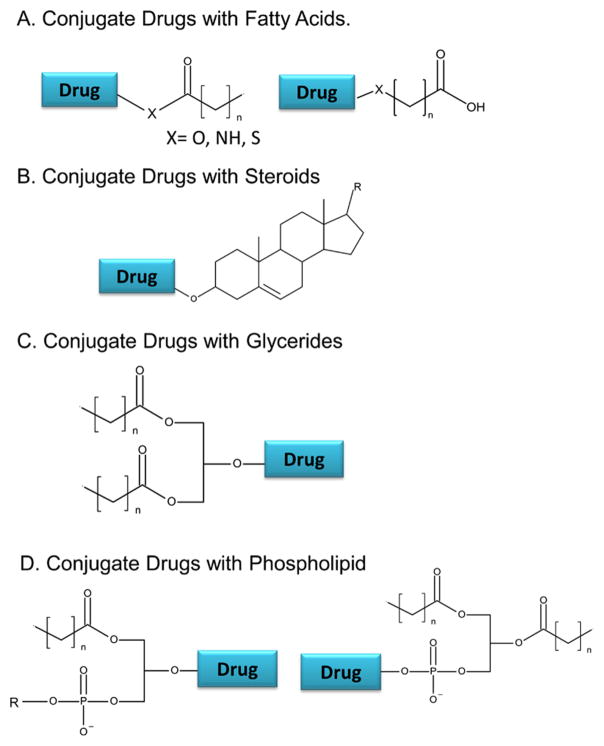
2.1. Conjugate Drugs with Fatty Acids
Fatty acids contain a hydrocarbon chain and a reactive carboxylic acid. The commonly used strategy is to conjugate the lipid’s carboxyl end with a hydroxyl or amine group of the drug to form a stable ester or amide linkage (Figure 1). Numerous fatty acids and their derivatives have been used for the conjugation. Docosahexaenoic acid (DHA), squalenoic acid (SQ), stearic acid (SA), and palmitic acid (PA) are a few examples in this category.1–11 A list of various lipids used for LDCs is compiled in Table 1.
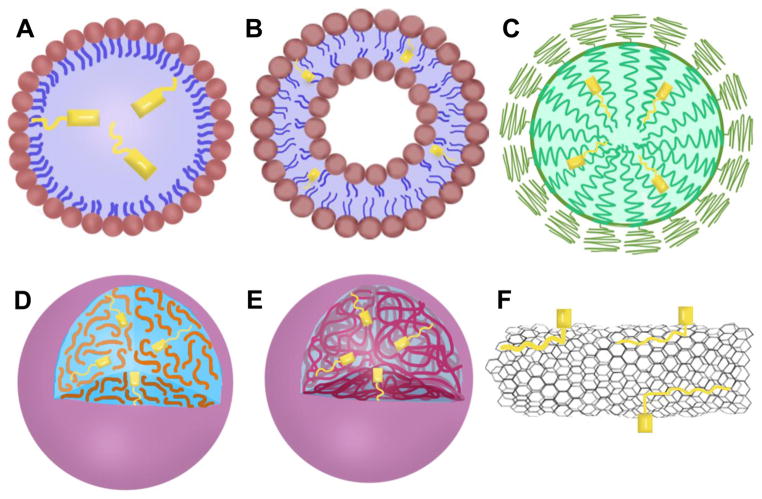
Figure 1.
Conjugation strategies for synthesizing LDCs. (A) Drugs can be conjugated with fatty acids at the carboxylic acid end or an ω-carbon. (B) Drugs can be conjugated with steroids at the hydroxyl group of the steroidal ring system. (C) Drugs can be conjugated to glycerides at the sn2 hydroxyl. (D) Drugs can be conjugated to phospholipids via the sn2 hydroxyl group or via phosphate group to form a phosphoester.
Table 1.
Lipid–Drug Conjugates and Their Delivery Systems
| lipids | drugs | delivery systems |
|---|---|---|
| fatty acids | ||
| squalene | acyclovir | no carrier140 |
| gemcitabine | LDC nanoparticle,93,95–97 nanocomposite,132 liposome141 | |
| nucleoside analogue | LDC nanoparticle142 | |
| paclitaxel | lipid vesicle,116 self-assembled nanoparticle143 | |
| curcumin | nanoassembly144 | |
| siRNA | self-assembled nanoparticle73,145 | |
| adenosine | self-assembled nanoparticle146 | |
| doxorubicin | nanoassembly99 | |
| stearic acid | gemcitabine | polymer nanoparticle,53 lipid nanoparticle,2–4,7,125,147 micelle1,5 |
| 5-fluorouracil | lipid nanoparticle81 | |
| oleic acid | docetaxel | nanostructured lipid carrier84 |
| paclitaxel | emulsion104 | |
| palmitic acid | dexamethasone | emulsion39 |
| paclitaxel | immunoemulsion106 | |
| doxorubicin | micelle56 | |
| TGX-221 | micelle122 | |
| siRNA | polymer nanoparticle75 | |
| capecitibane | lipid nanoparticle43 | |
| DHA | doxorubicin | no carrier43 |
| 10-hydroxycamptothecin | no carrier69 | |
| paclitaxel | no carrier9,11 | |
| linoleic acid | paclitaxel | liposome119 |
| octadecanoic acid | gemcitabine | nanoassembly90,91 |
| lauric acid | cytarabine | self-assembled nanofiber68 |
| α-tocopherol | siRNA | no carrier64 |
| oligonucleotide | no carrier78 | |
| SN-38 | polymer nanoparticle48 | |
| steroids | ||
| cholesterol | 5-fluorouracil | no carrier13 |
| paclitaxel | lipid nanoparticle,16 lipophilic nanoparticle15 | |
| siRNA | no carrier,17,58,74 high-density lipoprotein14 | |
| ursodeoxycholic acid | zidovudine | polymer nanoparticle19,127 |
| lithocholic acid | tamoxifen | no carrier21 |
| glycerides | ||
| melphalan | liposome23,32 | |
| mycophenolic acid | lipid-based formulation24–26 | |
| mitomycin C | liposomes27–30 | |
| methotrexate | liposome23 | |
| testosterone | no carrier33 | |
| paclitaxel | polymer nanoparticle15 | |
| phospholipids | ||
| gemcitibane | liposome,36 no carrier34 | |
| chlorambucil | liposome35 | |
2.2. Conjugate Drugs with Steroids
Steroids are molecules grouped by their common 4-ringed structure (Figure 1). Cholesterol and cholic acid derivatives are steroids that have been conjugated to drug molecules. The hydroxyl group attached to the ring of steroids is the primary location of conjugation in most studies. Conjugation of drugs with cholesterol provides the benefits of decreased side effects, targeted tumor delivery, and efficient cellular uptake.12–14 Cancerous cells overexpress low-density lipoprotein (LDL) receptors and require large amounts of cholesterol for their rapid growth.13 As a result, cholesterol drug conjugates facilitate the loading of anticancer agents into lipoproteins. In addition, as endogenous carriers, lipoprotein loaded drugs efficiently target LDL receptors on malignant cells. For example, cholesterol conjugated 5-fluorouracil (5-FU) exhibited better anticancer effects than those of free unconjugated 5-FU. The conjugation strategy used the hydroxyl group on cholesterol to form a carbonyl link with fluorouracil.13 Cholesterol conjugates have also been formed with paclitaxel15,16 and small interfering RNA (siRNA).14,17,18
Cholic acid is another steroid that has been used in lipid–drug conjugates in the form of ursodeoxycholic acid (UDCA) and lithocholic acid. UDCA, unlike cholesterol, has three hydroxyl groups available for conjugation. The OH functional group farthest from the steroidal ring system has been used as the conjugation site.19,20 Bile acid conjugated zidovudine (AZT) can potentially enhance its antiviral efficacy on HIV due to its reduced rate of hydrolysis in human plasma, enhanced CNS penetration, and reduced transport-mediated resistance.19 Lithocholic acid (LCA) has also been used in lipid–drug conjugates for tamoxifen through the covalent attachment of LCA molecule to its amine group. Due to positive charge and the presence of a lipophilic group, cationic LCA conjugated tamoxifen showed better anticancer efficacy than that of tamoxifen. Lipids in the conjugates enhance drug interaction with cells.21
2.3. Conjugate Drugs with Glycerides
Triglycerides (TG) are formed by combining glycerol with three fatty acid molecules via ester linkage (Figure 1). Researchers have developed a strategy to replace one of these fatty acyl groups, usually at position 2, with a drug molecule in order to take the advantage of TG metabolism pathways known as the triglyceride deacylation–reacylation pathway.22–24 In this pathway, TG will be hydrolyzed in the gastrointestinal (GI) lumen to form 2-monoglyceride (2-MG) and free fatty acids. The monoglyceride will then be absorbed into enterocytes, where it will be reacylated to form a triglyceride. Triglyceride will then be incorporated into lipoprotein, followed by accumulation in the lymphatic system. The glyceride conjugated drugs take the advantage of the lymphatic transport pathway to improve drug absorption and enhance lymphatic targeting.
Mycophenolic acid (MPA) was conjugated with glyceride to generate a triglyceride mimetic prodrug.24–26 The glyceride MPA conjugate contains MPA at the 2-position and two palmitoyl moieties at the 1- and 3-positions (2-MPA–TG) of glyceride. MPA reacts with the glycerol OH at its reactive carboxyl end. In the GI tract, 2-MPA–TG will be converted into 2-MPA–MG and absorbed. Then, absorbed 2-MPA–MG will be reacylated into 2-MPA–TG in the enterocytes. 2-MPA–TG conjugates assemble themselves into lipoprotein carriers and then distribute to the lymphatic system. Other examples of glyceride conjugated drugs include mitomycin C (MMC),27–30 methotrexate,31,32 testosterone,33 and paclitaxel.15
2.4. Conjugate Drugs with Phospholipids
There are two strategies to conjugate a drug to a phospholipid: linkage at the phosphate group or attachment on position 2 of the glycerol backbone (Figure 1).22 A conjugate prepared from these two strategies can form liposomes or enhance the incorporation of drugs into phospholipid/lipid-based delivery systems. The first method was reported in a study led by Alexander et al.,34 who used the hydroxyl on gemcitabine as a conjugation site to link with a phosphate group of a phospholipid.34 The second method has been used to create a secretory phospholipase A2 sensitive (sPLA2) prodrug. sPLA2 is an enzyme that specifically cleaves phospholipid esters at the sn2 position.35,36 An example in this category is the chlorambucil prodrug.35
3. CHEMICAL BONDS
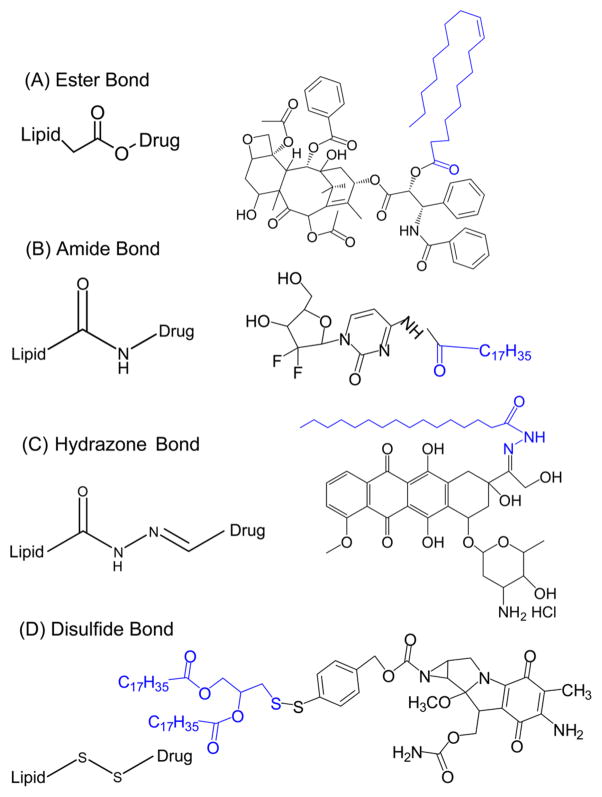
3.1. Ester Bonds
Ester bonds are among the most commonly used bonds to form lipid–drug conjugates. They are usually formed by reaction between the hydroxyl group of the parent drugs and the carboxylic acid group of the lipids as displayed in Figure 2A. Drugs with carboxylic groups may also conjugate with lipids having hydroxyl groups. Sometimes, a linker or spacer such as a succinic acid is used to facilitate the conjugation.37
Figure 2.
Various chemical bonds used for preparing LDCs. (A) Ester bond: Paclitaxel conjugated with oleic acid via ester bond.104 (B) Amide bond: Gemcitabine conjugated with stearic acid via amide bond.1 (C) Hydrazone bond: Doxorubicin conjugated with palmitic acid via a hydrazone bond.56 (D) Disulfide bond: Mitomycin C conjugated with lipid through a disulfide bond.28
A typical ester bond can be degraded gradually through hydrolysis with the aid of enzymes (such as esterase) to release active drugs. Ester bonds have been successfully applied in the preparation of lipid conjugates of multiple drugs including paclitaxel,10 nucleoside triphosphates,38 zidovudine,20 metho-trexate,32 acyclovir,39 and many others. When conjugated with a lipase insensitive ester bond, the LDC releases the drug at a slow rate, which may negatively affect the therapeutic efficacy.40 On the other hand, the formation of a lipase sensitive ester bond can facilitate drug release.36,41–43
The chemical structure of adjacent groups and spacers may also affect the cleavage of ester bonds. For example, a bromide next to the ester bond facilitates the cleavage of the ester bond and the release of conjugate drugs.40,44–46 Similarly, an adjacent disulfide bond may also facilitate cleavage.47 Some spacers or linkers are also used in the preparation of conjugates. Those spacers or linkers not only facilitate the formation of conjugates between two molecules without compatible functional groups but also affect ester hydrolysis.37,48–51
3.2. Amide Bonds
Amide bonds are used to form the lipid–drug conjugates similarly to ester bonds. LDCs are usually formed by the chemical reaction between a carboxylic end of the lipid and an amine group on a drug. This process is known as carbodiimide coupling (Figure 2B). Lipophilic prodrugs with amide bonds are inactive until the amide bond is cleaved to free the active drug. Amide bonds were used for synthesizing lipid conjugates of several drugs such as doxorubicin52 and gemcitabine.53 Amide bonds tend to have a slower rate of hydrolysis compared to that of ester bonds due to their higher stability,52 which may negatively affect the therapeutic efficacy. In a study, nanoparticles were prepared with N-DOX–TOS (lipid conjugated doxorubicin with an amide bond). N-DOX–TOS showed less cytotoxic effects on cancer cells compared to free doxorubicin formulations due to delayed or incomplete hydrolysis of the prodrug.52
3.3. Hydrazone Bonds
Hydrazones are used in the formation of lipid–drug conjugates for their pH-sensitive characteristics. At neutral pH, hydrazones show little to no decomposition, while at a lower pH the bond decomposes efficiently.8 Therefore, hydrazone-linked molecules can be cleaved in acidic environments such as endosomes or lysosomes (pH 4.0 to 6.0) and tumor tissues (pH 6.0 to 6.8).54 Hydrazone bonds have been successfully used to synthesize pH-sensitive lipid conjugated doxorubicin (Figure 2C).55–57
3.4. Disulfide Bonds
Disulfide lipid conjugates are stable in the extracellular oxidative environments but will be cleaved after cellular uptake in response to the reductive intracellular environment. The unique property of disulfide bonds has been utilized to prepare environmentally responsive prodrugs (Figure 2D). For example, a lipophilic prodrug of mitomycin C (MMC) was synthesized and used for the treatment of drug resistant human ovarian carcinoma. The disulfide bond in the molecule allowed the drug and lipid moiety to be cleaved effectively at the tumor site.27 Thiolytic microenvironments in tumors present the advantage of using disulfide bonds for lipid–drug conjugates. Disulfide bonds have also been used to prepare drug conjugate with siRNA and oligonucleotide (ODN) to enhance the drug’s intracellular delivery and release.58,59
3.5. Other Bonds
Several other linkers have also been used successfully in the preparation of lipid–drug conjugates. Thioethers, unlike disulfide bonds, consist of only one sulfur atom that is connected to 2 carbons (C–S–C). Squalene conjugated siRNA was designed by linking squalene to the 3′ end of the sense strand using maleimide–sulfhydryl chemistry.60 Carbamate bonds are similar to amide bonds, with the exception of including oxygen (ROCONR2) that may be viewed as an ester–amide. 5-Fluorouracil (5-FU), a chemo-therapeutic agent for solid tumors, was modified at its 4-N with alkyl chains to form a carbamate linkage.43,61 Another study linked a palmitoyl moiety to 5-FCPal, a derivative of 5-FU, using the same carbamate linkage.43 Phosphodiester bonds have been used in the preparation of lipid conjugates of oligonucleotides62,63 and siRNA.17,64
4. ADVANTAGES OF LIPID–DRUG CONJUGATES
4.1. Improve the Performance of Orally Administered Drugs
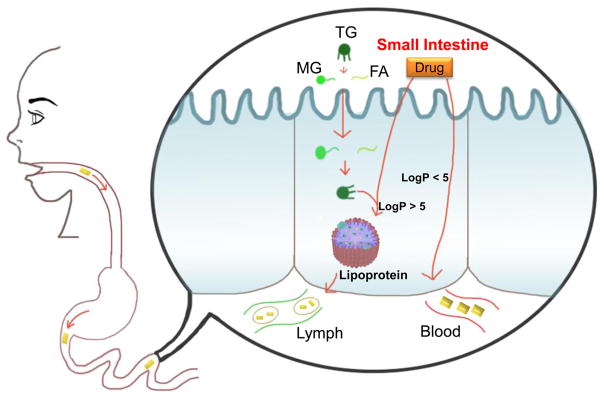
Oral administration of many drugs faces clinical challenges due to its poor absorption and GI tract irritation. Barriers such as the first-pass effect and other metabolic factors limit the effectiveness of delivering these drugs. Linking lipid moieties to drugs has been investigated as a method to overcome these challenges. Through increased lipophilicity and the use of lipid metabolism pathways, drugs in LDCs can avoid premature hydrolysis and exhibit increased interactions with cell membranes.65–67 Lymphatic system targeting is used to improve the effectiveness of orally administered drugs. The lymphatic system functions in the transport of dietary lipids (i.e., triglycerides, phospholipids, etc.) from the intestine to lymphatic capillaries (Figure 3). Lipoprotein assembly occurs if the drug formulation or drug conjugates contain a substantial amount of lipid to favorably partition or to biochemically integrate into enterocytes.65 Lipid–drug conjugates use this pathway to distribute throughout the body using lymphatic capillaries. Lymphatic targeting enhances drug bioavailability through avoidance of the first-pass metabolism. Porter’s groups reported that mycophenolic acid triglyceride conjugate showed significant enhancement in lymphatic drug transport and demonstrated a great potential for lymphatic targeting.24–26 In addition, cancer metastasis begins in the lymph nodes of the tissues. Lymphatic targeted delivery of antitumor agents has the potential to treat metastatic malignancies.65,66
Figure 3.
LDC enhanced drug targeting into lymph system. Triglyceride (TG) mimetic prodrugs are converted to monoglyceride (MG) and fatty acid (FA) in the GI tract through hydrolysis. The absorbed MG and FA are resynthesized to TG in enterocytes. TG will be incorporated into lipoproteins and be transported into the lymph system. Similarly, other lipophilic LDC (log P > 5) will also be transferred into lymph system.
Apomorphine is a drug usually subcutaneously administered for Parkinson’s disease. Borkar et al. developed diester prodrugs of apomorphine which can be delivered orally by a self-emulsifying drug delivery system (SEDDS).67 The fatty acids, either dipalmitoyl or dilauroyl, and digestible lipids of the SEDDS promote lymphatic delivery by increasing the LogP value of apomorphine from LogP 2.0 to 12.9 and 17.1 respectively. It is believed that a value of LogP above 5 is required for effective lymphatic traveling. In vitro studies proved that the apomorphine prodrug in SEDDS is a substrate of lipase. The lipid excipients in the system were an important factor in decreasing its degradation and improving oral delivery. The lipids attached to apomorphine decrease its degradation while traveling through the GI tract. Therefore, apomorphine in the conjugates will be finally released in the intestine in the presence of pancreatic lipases and esterases. In another study cytarabine (Ara-C) was conjugated with a lauric acid moiety.68 Ara-C shows poor oral bioavailability due to low lipophilicity, weak ability to permeate membranes, and metabolic degradation. The lipid–drug conjugate exhibited a great stability in plasma that led to a 32.8-fold increase in Ara-C bioavailability when administered orally to rats. An antitumor agent, SN38, was modified to improve its oral delivery by attaching undecanoate (C20).66 The formation of lipophilic prodrug led to the increase in the uptake of drugs in enterocytes, resulting in more than doubled increase in drug permeation through the intestinal epithelium of rats.
4.2. Improve the Delivery of Anticancer Drugs
4.2.1. Enhance Tumor Targeting and Reduce Toxicity
Many tumor cells show a high uptake and metabolism of lipids to compensate for their rapid proliferation. Lipophilic prodrugs enable rapid drug distribution into tumors and the release of active drugs in response to the tumor microenvironment. Both the lipid moiety and the chemical bond of conjugates are critical for the tumor targeting.
Tumor targeting can be enhanced through the conjugation of drugs with specific lipids such as DHA and cholesterol. Tumor cells aggressively take up natural fatty acids for their source of energy and the supply of biochemical precursors in various processes.8,31,69 DHA receptors on the membranes of tumor cells facilitate the binding of lipid–drug conjugate.8 For example, DHA–10-hydroxycamptothecin, a DHA drug conjugate, showed a greater tumor growth inhibition in the treatment of human colorectal cancer and lung cancer than that of the established therapies (cisplatin).69 DHA provided preferential uptake of the prodrug in the malignant cells and thus showed a better inhibition of tumor growth than that of cisplatin. Cholesterol drug conjugates have also been used to enhance the delivery of chemotherapy drug into tumors. Cholesterol drug conjugates are more selective in tumor targeting than that of free drugs due to higher uptake of cholesterol in tumors.12,68 Cholesterol and other lipids also enable the LDC transportation via lipoprotein receptors which are overexpressed on tumor cells.40 Tumor microenvironment may also be explored to facilitate the release of active drugs in tumor and further enhance antitumor activities. For example, hydrazones and disulfide bonds, as mentioned in Chemical Bonds, were used to take advantage of the acidic and thiolytic tumor tissue environment for the enhancement of antitumor activities.
Lipophilic prodrugs could increase tumor targeting and reduce toxicities of anticancer drugs. The exposure of normal tissues to harmful chemicals is minimized because the chemotherapy drugs are kept as inactive prodrugs prior to the delivery to tumor sites.9,27,37,69 In tumor cells, prodrugs will be metabolically converted into active drugs to kill tumor cells. For example, DHA–paclitaxel (DHA–PTX) was prepared to improve the drug’s tumor targeting ability and to reduce its toxicity to normal tissues.8,9 Pharmacokinetics study showed that DHA–PTX was distributed mainly in the intravascular plasma after intravenous injection. DHA–PTX maintained high drug concentrations in plasma and tumor for longer time than that of free paclitaxel.8,9 Due to the reduced toxicity, DHA–PTX dose could be increased by 4.4-fold with no increase in toxicity.9
4.2.2. Overcome Drug Resistance
Multidrug resistance (MDR) is a major cause for the failure of many cancer treatments. MDR transporters are overexpressed on cancer cells and cause the efflux of drugs from the cells. Lipid–drug conjugates have demonstrated their potential for overcoming drug resistance in chemical therapy.27,37,57
Lipid conjugated MMC, an antitumor agent, was encapsulated in polymer-grafted liposomes. Compared with the treatment with free MMC or liposomal doxorubicin, lipid conjugated MMC revealed a significant suppression of p-gp mediated drug resistance in ovarian cancer cells.27,37 An N-tetradecylamido derivative in lipid conjugated doxorubicin increases the lipophilicity, decreases toxicity, and creates a sustained release of doxorubicin. Compared to free doxorubicin, the derivative was not so readily pumped out during in vitro testing with ovarian cancer cells. It was found that cytotoxicity of lipid doxorubicin conjugates was maintained for 3 days. In another study, lipid conjugated doxorubicin was tested for their anticancer activities in drug resistant cells. Doxorubicin conjugated with N-heptadecanoyl hydrazine was more effective than unsaturated C18 lipid conjugated doxorubicin and free doxorubicin in their anticancer activities. The (menthoxycarbonyl)undecanoyl hydrazone conjugated doxorubicin was more active than free doxorubicin in the treatment resistant cancers.57
4.3. Promote Penetration into the Brain
The blood brain barrier (BBB) is a selectively permeable barrier that separates circulating blood from the cranial fluid. The barrier allows the passage of small, uncharged, and lipid-soluble molecules. BBB may limit the use of drugs with low lipophilicity to effectively treat CNS diseases.70 The covalent attachment of a lipid to a drug can enhance its brain delivery by increasing lipophilicity or by targeting receptors that facilitate the transport of the lipid through the BBB.71
A few studies showed that lipid–drug conjugates improved brain targeting. Glycerides have been linked to various drugs aiming to enhance their CNS delivery. Therapeutic agents such as GABA, L-Dopa, and phenytoin have been linked with glycerides or formulated as pseudoglycerides to improve CNS drug targeting and to increase CNS drug activities. Pseudoglycerides replace a fatty acid of a triglyceride with a drug molecule. The brain penetration index of glyceride-linked GABA showed a 127-fold increase compared to free GABA, establishing substantial evidence that glyceride prodrugs increase brain targeting.72
4.4. Enhance Delivery of Gene Medicines
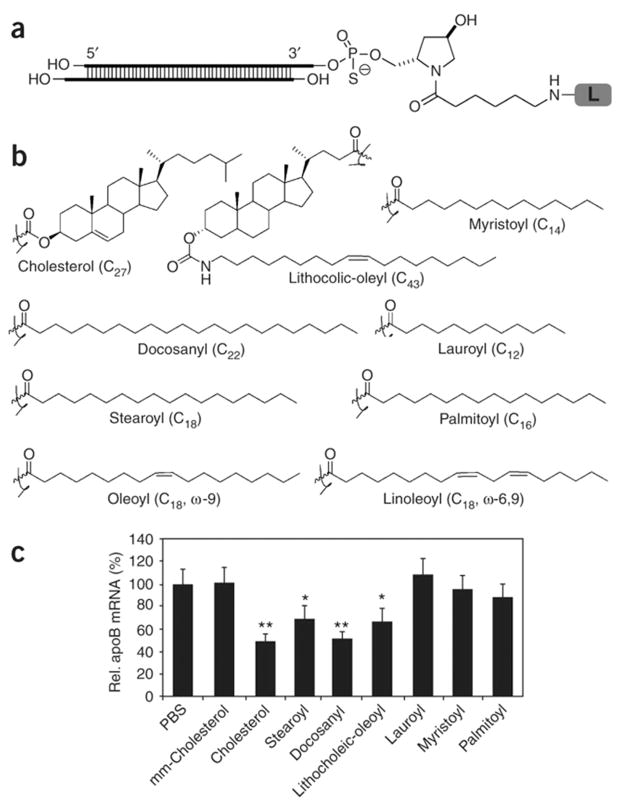
Nucleic acid based gene medicines (e.g., siRNA, ODN) are novel promising therapeutic agents. However, their clinical use is limited due to their poor stability, large molecular size, and negative charge. Therefore, there is a great need for the development of delivery strategies for these molecules. LDCs have been used in multiple studies to facilitate the delivery of gene medicines.73–75 Lipid–siRNA conjugates showed the improvement of in vivo performance. Cholesterol,14,17,58,74,76 squalene,73,77 palmitic acid,75 and α-tocopherol64 are lipids that have been used to synthesize siRNA–lipid conjugates. Conjugating siRNA with lipids would result in decreased degradation, enhanced cellular uptake, and ultimately improved gene silencing activity. For example, one study showed a significant gene silencing activity of cholesteryl–siRNA.14 In this study, the cholesterol–siRNA conjugates were efficiently loaded into lipoprotein carriers, which can enhance their cellular uptake and lead to effective silencing of target oncogene. In a separate study, cholesterol conjugated siRNA demonstrated enhanced binding to serum proteins and improved the in vivo pharmacokinetic profiles.76 In another study, Wolfrum et al. conjugated siRNA with a variety of lipholiphic molecules including bile acids and fatty acids. Their studies showed that conjugation with lipophilic molecules improved siRNA cellular uptake and enhanced gene silencing in vivo (Figure 4).17 In addition, their research results indicated that the interactions between siRNA–lipid conjugates and lipoproteins, lipoprotein receptors, and transmembrane proteins played an important role on the cellular uptake of conjugates. Conjugation of siRNA with lipids such as cholesterol, bile acids, and long-chain fatty acids enhances their conjugate binding to lipoproteins, including both high-density lipoprotein (HDL) and low-density lipoprotein (LDL), and facilitates the cellular uptake.17 In contrast, short- and medium-chain fatty acid siRNA conjugates showed a low binding affinity to lipoproteins. The binding efficiencies of lipid–siRNA conjugates to lipoproteins correlated with their abilities to reduce target mRNA levels in vivo.17 Another study indicated that conjugation of siRNA to lipophilic palmitic acid enhanced the loading efficiency of siRNA in a nanoparticle based delivery carrier. A 35-fold increase in cellular internalization was exhibited by the use of palmitic acid conjugated siRNA in comparison to that of unconjugated siRNA.75 Use of lipid conjugate of oligonucleotides for the enhancement of gene delivery can be found in many publications.59,62,63,78
Figure 4.
Lipophilic siRNA conjugates have different in vivo activities. (a) Structure of lipid conjugated siRNAs. The desired lipid (L) is conjugated to the 3′-end of the sense strand of the apoB siRNA4 (siRNA-apoB1) via a trans-4-hydroxyprolinol linker (Hyp). Sense 5′-GUCAUCACACUGAAUACCAAU*Hyp-L-3′ and antisense 5′-AUU-GGUAUUCAGUGUGAUGAc*a*C-3′. Lowercase letters represent 2′-O-methyl-sugar-modified nucleotides, and asterisks stand for phosphorothioate backbone. (b) Chemical structure of different lipids used for synthesizing lipid conjugated siRNAs. (c) In vivo silencing of apoB mRNA by lipid conjugated siRNAs. Liver apoB mRNA levels were normalized to GAPDH mRNA 24 h after three daily intravenous injections of saline or 50 mg/kg stearoyl-siRNA-apoB1, dodecyl-siRNA-apoB1, lithocholic-oleyl-siRNA-apoB1, or docosanyl-siRNA-apoB1 (n = 5 per group). Data show apoB mRNA levels as a percentage of the saline treatment group and are expressed as mean ± SD. Data marked with asterisks are statistically significant relative to the saline treatment group as calculated by ANOVA without replication, alpha value 0.05 (*, P < 0.05; **, P < 0.005). Reprinted with permission from ref 17. Copyright 2007 Nature Publishing Group.
4.5. Facilitate Drug Loading into Delivery Carriers
Lipophilic drugs usually have superior drug loading in delivery carriers containing a lipophilic components. In contrast, hydrophilic drugs suffer from low loading efficiency and drug leakage in these delivery systems. Conjugation of lipid with hydrophilic drugs has been used to increase the lipophilicity of hydrophilic drugs and to enhance their compatibility with the lipophilic components of drug delivery carriers. This conjugation approach can enhance the affinity of drugs with carriers and reduce drug leakage.56,79–82 The phospholipid bilayer of liposomes, the lipophilic core of micelles, and the internal oil phase of emulsions are a few examples of components that serve as LDC reservoirs. For example, a fatty acid derivative of diminazene diaceturate was loaded into solid lipid nanoparticles with high efficiency.70 In another study, 2-behenoyl–paclitaxel, a fatty acid derivative of paclitaxel, increased drug loading in lipid-based nanoparticles from 10% to 47%.83 Similarly, encapsulation efficiency in polymeric nano-particles reached to approximately 68% when 4-(N)-stearoyl gemcitabine was incorporated at 1:10 drug/polymer ratio.53 Multiple other studies also showed that LDCs have better carrier loading than that of parent drugs.56,80–82,84
4.6. Achieve Extended Drug Release
Lipophilic prodrugs have also been used to achieve extended drug release. This strategy has been successfully used to develop several FDA approved extended release formulations of antipsychotics such as haloperidol decanoate, fluphenazine decanoate, and fluphenazine enanthate.85 The conjugation of drugs with lipids produced lipophilic prodrugs with low solubility in water and high solubility in oils. Those conjugates are formulated in injectable oils and administered via intramuscular or subcutaneous injection. The extended release profile of these formulations made it possible to treat patients with one injection every 4–6 weeks, which greatly improved patient compliance. In addition to the oil solution, lipophilic prodrug could also be formulated as aqueous suspensions. For example, paliperidone palmitate extended-release injectable suspension was approved by the FDA in 2009.86 This prodrug was prepared through conjugation of paliperidone, an active metabolite of risperidone, with palmitic acid. Paliperidone palmitate was formulated as a ready-to-use aqueous nanocrystal suspension and administered monthly through intramuscular injection.86 Recently, aripiprazole lauroxil was approved as a long-acting injectable formulation for treating schizophrenia.87 It is an aqueous suspension of lipophilic prodrug administered via intramuscular injection. By using different lipids to synthesize LDCs, we can precisely control their physical chemical properties including melting point, partition coefficient, and solubility of LDCs. The physical chemical properties of LDCs will, in turn, affect drug release profiles.85 Inspired by the applications of LDCs in long-acting injectable formulations for treating antipsychotics, several other studies have been performed to explore their applications in developing extended release formulations for other drugs. For example, a lipophilic prodrug approach was used to develop a one-month sustained release formulation of nalmefene, which is a drug used to reduce alcohol intake.88 In another report, lipophilic prodrug of pioglitazone was studied to develop a once-monthly injectable formulation for the treatment of diabetics.89
5. DELIVERY SYSTEMS FOR LIPID–DRUG CONJUGATE
5.1. Carrier-Free System
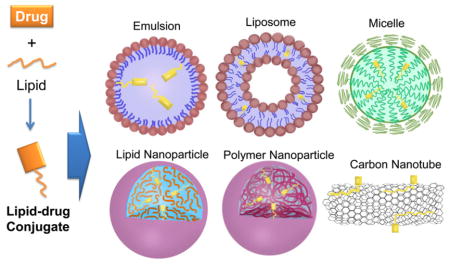
A carrier-free system is one in which an LDC is administered without a delivery carrier. Linking a lipid to a hydrophilic drug leads to the formation of an amphiphilic molecule that can self-assemble into nano-particles without or with minimal use of stabilizer.43,49–51,90–102 Among these LDCs, squalene–drug conjugates have been most extensively studied. Squalenoylation refers to covalently conjugating the terpene lipid to a drug molecule. Squalene–drug conjugates can self-assemble to form nanoparticles in aqueous solution. For example, squalene–gemcitabine conjugate (SQ–GEM) forms nanoassemblies with gemcitabine at the core and squalene at the surface acting as protective shell.92 SQ–GEM nanoparticles exhibited enhanced anticancer activity in various tumor cell lines. In another study, 1,1′,2-trisnorsqualenoyl–gemcitabine conjugate was designed.95 The conjugates form amphiphilic vesicles with increased drug half-life and mean residence time. Other anticancer agents, including paclitaxel50,51,102 and doxorubicin,99 have been conjugated with squalene to form nanoassemblies with enhanced activity when compared with the parent drug. In another study, SQ–GEM was decorated with SQ conjugated targeting peptide CKAAN through coprecipitation.49,103 The nanoassemblies with CKAAN showed a higher specificity in cellular uptake and cytotoxicity in Paca-2 pancreatic cancer cells.103 In a separate study, a technology called lipocore was developed. In this system, particles composed of pure lipid–drug conjugate (e.g., C16 derivative of paclitaxel) was protected by pegylated lipids such as DSPE-PEG.46 Sharma et al. used Tween 80 to stabilize stearic acid conjugate cytarabine NPs.82 Although the carrier-free systems are promising for drug delivery, most LDCs are formulated in various drug delivery carriers including emulsion, liposome, micelle, lipid nano-particle, polymer nanoparticle, and others (Figure 5).
Figure 5.
Different delivery carriers for LDCs: (A) emulsion, (B) liposome, (C) micelle, (D) lipid nanoparticle, (D) polymer nanoparticle, and (F) carbon nanotube.
5.2. Emulsions
O/w emulsions have also been frequently used to deliver LDCs.39,67,104–106 Emulsions are biocompatible carriers that permit the incorporation of LDCs in the oil droplets. Emulsions improve the delivery of an LDC by solubilizing the drug, reducing toxicity, and decreasing drug clearance rates.39,104–106
Studies have shown that the use of o/w emulsions are well-tolerated and they can entrap lipid–drug conjugates with a high efficiency.39,104,105,107 In a study, researchers incorporated paclitaxel–oleate into a nanosized emulsion.104 In comparison to the commercial paclitaxel formulation containing a toxic solvent, Cremophor EL, paclitaxel–oleate nanoemulsion showed lower toxicity. Another study showed higher entrapment efficiency of dexamethasone–palmitate (DXP) than that of unconjugated free dexamethasone.39 DXP was designed to inhibit VEGF-induced vascular leakage in the treatment of ophthalmic diseases. DXP emulsion could retain the drug and control its release. The sustained formulation maintained DXP levels for 9 months which are sufficiently high to inhibit VEGF.
Emulsions can be further modified to enhance their specificity and targeting ability. A study used a cholesterol-rich microemulsion (LDE) to carry paclitaxel–oleate.105 The cholesterol component of the emulsion enhanced the targeting ability by enabling its LDL carrier to bind to its corresponding receptors and facilitate the drug delivery and cellular internalization. LDL receptors are overexpressed in leukemia cells and other solid tumors. LDE paclitaxel–oleate has been tested in clinical studies on patients with breast cancer and gynecologic cancer. LDE formulation showed improved PK profile, enhanced drug accumulation in tumors, and reduced toxicity.108,109 In another study, an immunoemulsion was designed to deliver paclitaxel–palmitate to treat metastatic prostate cancer through targeting the HER-2 receptor.106 By attaching Herceptin, an anti-HER2 monoclonal antibody, the immunoemulsion delivers the prodrug efficiently to cells that overexpress HER-2 receptors.
5.3. Liposomes
Liposomes are drug delivery systems that permit the entrapment of hydrophilic drugs in the aqueous core and the incorporation of lipophilic prodrugs in the lipid membrane. Liposomes prevent the premature metabolism of prodrugs by acting as a second layer of protection for the active agent (the first being the lipid–drug conjugates).23,110–114 The addition of a lipid moiety to a therapeutic agent improved its membrane affinity, drug loading, stability, and retention in the liposomal formulation.113,115–117 Arouri et al. synthesized a phospholipid–drug conjugate (C6-RAR prodrug). This lipophilic prodrug can mix with other lipid components to form stable liposomes. The prodrug can be cleaved by phospholipase A2, which is upregulated in cancer cells.36
Pegylated liposomes are used frequently as delivery carriers for LDCs because of their ability to have longer circulation and better tumor targeting ability than that of LDCs. LDCs delivered by pegylated liposomes include octadecanoyl analogue of 8-chloroadenosine,110 squalenoyl–gemcitabine,118 mitomycin C prodrug,27–30 and diglyceride doxorubicin conjugate.114 Due to its lipophilic nature, the LDC’s encapsulation efficiency in liposomes is usually high. The encapsulation of LDCs in pegylated liposome further enhances drug stability and their circulation time.
In several published studies, liposomes are modified with targeting molecules to further enhance their target specificity and cellular internalization. For example, methotrexate and melphalan were conjugated with 1,2-dioleoylglyceride to form MTX–DOG and Mlph–DOG. The formed LDCs were delivered with liposomes surface modified with the sialyl Lewis X/A (SiaLeX/A) tetrasaccharide ligand.32 This ligand can recognize selectins overexpressed on inflammatory sites and tumor sites.23,32 This liposome formulation demonstrated the ability to target tumor vasculature. In several other studies, LDCs were delivered by liposomes modified with other targeting molecules such as iRGD peptide,119 hyaluronic acid,120 and monoclonal antibody.112
5.4. Micelles
Micelles are composed of amphiphilic macromolecules that self-assemble into core–shell structured nanocarriers. The hydrophobic core can encapsulate hydrophobic drugs through noncovalent interactions. Conjugating drugs with lipids can significantly improve their interaction with micelle cores. This strategy improves the stability of drug-loaded micelle formulation. Several different micelle formulations have been used to delivery LDCs.56
DSPE-PEG micelles have been investigated as the carriers for lipid–drug conjugates in several studies. For example, an acid-sensitive doxorubicin–palmitate conjugate was loaded into DSPE-PEG micelles.56 Due to the increased lipophilicity of the prodrug, loading of prodrug in micelle was highly efficient. This formulation demonstrated excellent drug loading stability, due to enhanced interaction between drug and micelle hydrophobic core. In another study, stearoyl gemcitabine (Gem-C18) was synthesized and was loaded into DSPE-PEG/tocopherol-PEG1000-succinate (TPGS) micelles to prevent inactivation of the drug from rapid deamination.1 The formulation demonstrated better anticancer activities than free drug in a pancreatic cancer tumor model due to the increase in drug systemic circulation and drug concentrations in tumors.
Micelles prepared with other polymers such as PEG-PCL10,44 and PEG-PLA121 have also been used for the delivery of lipophilic prodrugs of geldanamycin, paclitaxel, and gemcitabine. These prodrug micelle formulations showed improved AUC, higher drug concentrations in tumors, and reduced toxicity. Polymeric micelles decorated with targeting molecules can further enhance tumor specific drug delivery. For example, lipid prodrug of TGX-221 was incorporated into PEG-PCL micelles decorated with a prostate-specific membrane aptamer (PSMAa10).122 The modified micelle showed effective inhibition of cell growth in PSMA-positive cells without affecting PSMA-negative cells.
Acidic pH-sensitive micelles have also been used to enhance the delivery of lipophilic prodrug stearoyl gemcitabine (Gem-C18).5,123 In studies conducted by Cui’s group, PEG and lipid were conjugated through an acidic pH cleavable linker to synthesize a material that can be used to prepare Gem-C18 loaded micelles. This micelle can be cleaved in response to the acidic environment in the lysosomal compartments to facilitate the release of drugs. Compared to acid-insensitive micelle formulations, the acid-sensitive micelles exhibited quick release of parent drug (gemcitabine) and showed improved anticancer activities. In addition, this formulation showed the ability to overcome gemcitabine resistance in cells with high expression of ribonucleotide reductase subunit M1.
5.5. Lipid Nanoparticles
Lipid nanoparticles (NPs) can encapsulate LDCs in the lipophilic core. The lipid core serves as a reservoir to effectively load lipophilic drugs. Hydrophilic drugs need to be converted into lipid–drug conjugates to enhance the loading into lipid NPs.70,124 Both solid and liquid lipid NPs were used for the delivery of LDCs.
Lipid–drug conjugates delivered by solid lipid nanoparticles (SLN) showed a high drug loading and enhanced drug delivery to specific organs. For example, a thin-layer ultrasonication method was used to prepare SLN of 3′,5′-dioctanoyl-5-fluoro-2′-deoxyuridine (DO-FUdR).80 This method was able to achieve drug loading capacity of 29.02% and drug entrapment of 96.62%. SLN formulations can also improve a drug’s ability to penetrate the BBB.70,80 Lipophilic derivatives of diminazene diaceturate were loaded in Tween-80 coated SLNs.70 It has demonstrated that the Tween-80 coating enhanced nano-particle’s stability and has the potential to improve the uptake of drugs in brain. Liver targeting was also achieved in an SLN formulation of N-stearyl-5-fluorouracil.81
Cui’s group developed a lipid NP formulation to deliver stearoyl gemcitabine (Gem-C18).125 The lipid NPs were composed of soy lecithin and glycerol monostearate. The NP formulation has been used to effectively treat gemcitabine resistant cancers. In the subsequent studies, the same group further modified NPs with DSPE-PEG to prolong their circulation in the blood and to enhance drug accumulation in tumors.126 The NPs were further decorated with epidermal growth factor (EGF) to specifically target tumor cells overexpressing the epidermal growth factor receptor.7 Gem-C18 nanoparticles decorated with epidermal growth factor can effectively treat drug resistant cancers because this NP protects the prodrug from deamination, increases drug potency, and delivers drug efficiently to intracellular compartments.3,6
In addition to SLN, oil-filled NPs were also used for the delivery of LDCs. The compatibility with carrier lipid core is critical for efficient drug encapsulation. In a study, “core-match technology” was explored to improve loading capability of oleate conjugated docetaxel in oleic acid containing NPs.84 This formulation showed higher drug loading, improved stability, and sustained drug release, when compared with those of lipid NPs loaded with free docetaxel. In another study, 2-bromohexadecanoyl–docetaxel was formulated into lipid NP with an oily core. The formulation demonstrated good drug retention, extended drug release, and long-term storage stability.40 BTM is an abbreviation describing the design of the NP containing Brij 78, vitamin E TPGS, and an oily core composed of Miglyol 808. A recent study examined the advantage of using BTM nanoparticles (BTM NP) to deliver docetaxel conjugates for the treatment of non-small cell lung cancer. Compared to that of Taxotere, treatment with BTM NP increased in vivo progression-free survival by 35 days and mean survival of the nude mice by 27 days.45 BTM NPs were also used to deliver C22 lipid conjugated paclitaxel (C22-PTX).83
5.6. Polymer Nanoparticles
Poly(lactic-co-glycolic acid) (PLGA) nanoparticles have been extensively used in the delivery of LDCs. Dalpiaz et al. prepared PLGA NPs to encapsulate ursodeoxycholic acid (UCDA) conjugated zidovudine (AZT).127 PLGA NPs were also used to deliver 4-(N)-stearoyl gemcitabine.2,53 In other studies, PEG-PLA NPs were used to deliver LDCs. The presence of PEG could stabilize the NP and prolong drug circulation in the blood. SN-38 is a topoisomerase I inhibitor. This inhibitor is toxic, unstable, and incompatible with various delivery systems. The formation of a tocopherol succinate derivative increased SN-38’s affinity for NPs.48 PLGA NPs were also modified on the surface with targeting ligands such as lectin, aptamer, and cetuximab.128–130 These targeting ligands enhance drug tumor cell targeting through the interaction with tumor cell specific receptors.
NPs prepared with other polymers were also investigated for the delivery of LDCs. Sarett et al. used endosomolytic polymer nanoparticles to deliver siRNA–palmitate conjugate.131 Conjugation of hydrophobic palmitate with siRNA improves siRNA’s loading into NPs. As a result, a lower polymer/siRNA ratio is required to efficiently condense siRNA. Due to the improvement in stability and endosome escape of siRNA, high potency and great longevity of gene silencing were achieved.75 A recent study describes the delivery of paclitaxel oleate via poly(beta-amino-ester) (PbAE) nanoparticles.131 Cellular uptake and release of paclitaxel oleate from PbAE NPs was faster than those from poly caprolactone (PCL) NPs probably due to the pH sensitivity of PbAE.
5.7. Others
Several other carriers were also used for the delivery of LDCs. Arias et al. designed squalenoyl–gemcitabine (SQ–GEM) NPs which incorporated magnetite nanocrystals as a theranostic agent.132 This system showed the enhancement in the delivery of drugs into tumor with the aid of a magnetic field. At the same time, it can also be used to enhance tumor imaging.132 In another study, Shao et al. reported that conjugating paclitaxel with lipids enhances the drug’s loading into carbon nanotubes (CNT).79 The enhanced loading was achieved through the hydrophobic interaction between lipid in the conjugate and the surface of CNT. Use of lipoproteins for LDC delivery can be found in other reports.12–14,17,71,76 The functions of lipoproteins include the transportation of cholesterol and dietary triglycerides. These lipoprotein carriers can be used to facilitate the delivery and cellular uptake of the LDC in tumor cells with overexpressed lipoprotein receptors. Other reported delivery carriers and formulations for LDCs include mesoporous silica nanoparticles,133 sesame seed oil injections,88 lipid nanocapsule hydrogels,134 and extended release injectable formulations.89
6. CONCLUSION AND FUTURE PERSPECTIVES
LDCs have been successfully utilized to enhance the delivery of various drugs including both small molecular and macro-molecular drugs. The conjugation of lipids significantly increases the lipophilicity and changes properties of drugs. These changes in the drug properties could potentially facilitate drug delivery into the lymphatic system, improve oral bioavailability, enhance tumor targeting, and many others. Some LDCs can form self-assembled nanoparticles by themselves and can be administered without any delivery carriers. However, most LDCs are being entrapped in carriers prior to the delivery. The lipid “tail” in the LDC could significantly enhance the loading of LDCs into delivery carriers through a hydrophobic interaction. This enhanced hydrophobic interaction can help to improve drug loading, prevent the leakage of drugs, and increase formulation stability. Currently, the design of LDCs and selection of delivery carriers are mostly based on a “trial and error” approach. It will be worthwhile to systemically study the compatibility between LDC and carrier molecules, and their relationship with drug loading capacity. Computational modeling is a valuable tool to simulate the interaction and to identify the best match between LDCs and delivery carriers. The increased availability of various lipid molecules and delivery carriers will be helpful in identifying the best match while the computational modeling can assist in the formulation optimization process.
We have mentioned in the previous part of this review that LDCs have been successfully used to develop several FDA approved extended release injectable formulations for the treatment of antipsychotics. In addition, several other LDCs have been tested in clinical trials for other applications. For example, DHA conjugated paclitaxel, or Taxoprexin, has been evaluated in several clinical trials for the treatment of various cancers including non-small cell lung cancer, melanoma, liver cancer, prostate cancer, pancreatic cancer, kidney cancer, and colorectal cancer.135 Similarly, a liposome formulated lipophilic drug of docetaxel (MNK-010) has also been developed and under clinical trials.136,137 GemSQ is a squalene conjugated gemcitabine developed based on the pioneering work by Patrick Couvreur. GemSQ is currently under phase III clinical trials.138 Another good example in the categories is the development of cholesterol conjugated siRNA. The strategy in the preparation of cholesterol conjugated siRNA was used by Arrowhead Pharmaceuticals in their product development of ARC-520 for treating chronic hepatitis B virus infection.139 The success in the use of LDC strategy in the development of FDA approved drug formulations and several ongoing clinical trials of similar products demonstrated the great potential of LDC strategy in the commercialization.
Several challenges are associated with the development of LDC-based drug formulations. Usually, LDCs are inactive prodrugs. Therefore, the cleavage of linkers to efficiently release the parent drug is critical for achieving optimal therapeutic performance. Many LDCs have slow drug release profiles when lipids are conjugated with drugs through ester or amide bonds. The slow drug release can be an advantage for developing extended release formulations. However, it can be a major concern for the applications requiring a quick release of active drugs to achieve therapeutic effects. For example, most anticancer treatments need to have sufficient drug concentrations at the tumor site to effectively treat cancers. Many studies showed that lipid conjugated anticancer drugs with noncleavable or slowly cleavable linkers were usually less potent than parent drugs. Therefore, linkers with better drug release profiles are preferred in the synthesis of LDCs for anticancer applications. For example, a pH-sensitive hydrazine linker has been utilized to synthesize LDCs which can efficiently release drugs in response to an acidic pH environment in tumor cells.56 The selection of linker is restricted by the chemical structure and the availability of functional groups in both drugs and lipids. In the future, more efforts are needed to design linkers which can be triggered to release the drugs from LDCs in response to various environment changes. In addition, the discovery of linkers with different and tunable release profiles is critical for developing optimal LDC formulations for specific clinical applications.
Acknowledgments
We thank the providers of the following grants for support: NIH-SC3 grant (SC3GM109332), NSF-PREM grant, and DOD-Collaborative Undergraduate HBCU Student Summer Training Program Award. We also thank Kejera Janee Roberts for assistance in graphics.
Footnotes
Notes
The authors declare no competing financial interest.
References
- 1.Wang Y, Fan W, Dai X, Katragadda U, McKinley D, Teng Q, Tan C. Enhanced tumor delivery of gemcitabine via PEG-DSPE/TPGS mixed micelles. Mol Pharmaceutics. 2014;11:1140–50. doi: 10.1021/mp4005904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu S, Li X, Lansakara-P DSP, Kumar A, Cui Z. A nanoparticle depot formulation of 4-(N)-stearoyl gemcitabine shows a strong anti-tumour activity. J Pharm Pharmacol. 2013;65:236–42. doi: 10.1111/j.2042-7158.2012.01599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Angel RE, Blando JM, Hogan MG, Sandoval MA, Lansakara-P DSP, Dunlap SM, Hursting SD, Cui Z. Stearoyl gemcitabine nanoparticles overcome obesity-induced cancer cell resistance to gemcitabine in a mouse postmenopausal breast cancer model. Cancer Biol Ther. 2013;14:357–64. doi: 10.4161/cbt.23623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wonganan P, Lansakara-P DSP, Zhu S, Holzer M, Sandoval MA, Warthaka M, Cui Z. Just getting into cells is not enough: mechanisms underlying 4-(N)-stearoyl gemcitabine solid lipid nanoparticle’s ability to overcome gemcitabine resistance caused by RRM1 overexpression. J Controlled Release. 2013;169:17–27. doi: 10.1016/j.jconrel.2013.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu S, Wonganan P, Lansakara-P DSP, O’Mary HL, Li Y, Cui Z. The effect of the acid-sensitivity of 4-(N)-stearoyl gemcitabine-loaded micelles on drug resistance caused by RRM1 overexpression. Biomaterials. 2013;34:2327–39. doi: 10.1016/j.biomaterials.2012.11.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lansakara-P DSP, Rodriguez BL, Cui Z. Synthesis and in vitro evaluation of novel lipophilic monophosphorylated gemcitabine derivatives and their nanoparticles. Int J Pharm. 2012;429:123–34. doi: 10.1016/j.ijpharm.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandoval MA, Sloat BR, Lansakara-P DSP, Kumar A, Rodriguez BL, Kiguchi K, Digiovanni J, Cui Z. EGFR-targeted stearoyl gemcitabine nanoparticles show enhanced anti-tumor activity. J Controlled Release. 2012;157:287–96. doi: 10.1016/j.jconrel.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Li L, Jiang W, Yang Z, Zhang Z. Synthesis and preliminary antitumor activity evaluation of a DHA and doxorubicin conjugate. Bioorg Med Chem Lett. 2006;16:2974–7. doi: 10.1016/j.bmcl.2006.02.066. [DOI] [PubMed] [Google Scholar]
- 9.Bradley MO, Webb NL, Anthony FH, Devanesan P, Witman PA, Hemamalini S, Chander MC, Baker SD, He L, Horwitz SB, Swindell CS. Tumor targeting by covalent conjugation of a natural fatty acid to paclitaxel. Clin Cancer Res. 2001;7:3229–38. [PubMed] [Google Scholar]
- 10.Forrest ML, Yanez JA, Remsberg CM, Ohgami Y, Kwon GS, Davies NM. Paclitaxel prodrugs with sustained release and high solubility in poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelle nanocarriers: pharmacokinetic disposition, tolerability, and cytotoxicity. Pharm Res. 2008;25:194–206. doi: 10.1007/s11095-007-9451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bradley MO, Swindell CS, Anthony FH, Witman PA, Devanesan P, Webb NL, Baker SD, Wolff AC, Donehower RC. Tumor targeting by conjugation of DHA to paclitaxel. J Controlled Release. 2001;74:233–6. doi: 10.1016/s0168-3659(01)00321-2. [DOI] [PubMed] [Google Scholar]
- 12.Radwan AA, Alanazi FK. Targeting cancer using cholesterol conjugates. Saudi Pharm J. 2014;22:3–16. doi: 10.1016/j.jsps.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radwan AA, Alanazi FK. Design and synthesis of new cholesterol-conjugated 5-Fluorouracil: a novel potential delivery system for cancer treatment. Molecules. 2014;19:13177–87. doi: 10.3390/molecules190913177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding Y, Wang W, Feng M, Wang Y, Zhou J, Ding X, Zhou X, Liu C, Wang R, Zhang Q. A biomimetic nanovector-mediated targeted cholesterol-conjugated siRNA delivery for tumor gene therapy. Biomaterials. 2012;33:8893–905. doi: 10.1016/j.biomaterials.2012.08.057. [DOI] [PubMed] [Google Scholar]
- 15.Ansell SM, Johnstone SA, Tardi PG, Lo L, Xie S, Shu Y, Harasym TO, Harasym NL, Williams L, Bermudes D, Liboiron BD, Saad W, Prud’homme RK, Mayer LD. Modulating the therapeutic activity of nanoparticle delivered paclitaxel by manipulating the hydrophobicity of prodrug conjugates. J Med Chem. 2008;51:3288–96. doi: 10.1021/jm800002y. [DOI] [PubMed] [Google Scholar]
- 16.Stevens PJ, Sekido M, Lee RJ. A folate receptor-targeted lipid nanoparticle formulation for a lipophilic paclitaxel prodrug. Pharm Res. 2004;21:2153–7. doi: 10.1007/s11095-004-7667-5. [DOI] [PubMed] [Google Scholar]
- 17.Wolfrum C, Shi S, Jayaprakash KN, Jayaraman M, Wang G, Pandey RK, Rajeev KG, Nakayama T, Charrise K, Ndungo EM, Zimmermann T, Koteliansky V, Manoharan M, Stoffel M. Mechanisms and optimization of in vivo delivery of lipophilic siRNAs. Nat Biotechnol. 2007;25:1149–57. doi: 10.1038/nbt1339. [DOI] [PubMed] [Google Scholar]
- 18.Ylasenko YV, Alekseeva AS, Vodovozova EL. Synthesis of a fluorescent analog of methotrexate lipophilic prodrug. Bioorg Khim. 2014;40:125–8. [PubMed] [Google Scholar]
- 19.Dalpiaz A, Paganetto G, Pavan B, Fogagnolo M, Medici A, Beggiato S, Perrone D. Zidovudine and ursodeoxycholic acid conjugation: design of a new prodrug potentially able to bypass the active efflux transport systems of the central nervous system. Mol Pharmaceutics. 2012;9:957–68. doi: 10.1021/mp200565g. [DOI] [PubMed] [Google Scholar]
- 20.Dalpiaz A, Contado C, Mari L, Perrone D, Pavan B, Paganetto G, Hanuskova M, Vighi E, Leo E. Development and characterization of PLGA nanoparticles as delivery systems of a prodrug of zidovudine obtained by its conjugation with ursodeoxycholic acid. Drug Delivery. 2014;21:221–32. doi: 10.3109/10717544.2013.844744. [DOI] [PubMed] [Google Scholar]
- 21.Yadav K, Bhargava P, Bansal S, Singh M, Gupta S, Sandhu G, Kumar S, Sreekanth V, Bajaj A. Nature of the charged head group dictates the anticancer potential of lithocholic acid-tamoxifen conjugates for breast cancer therapy. MedChemComm. 2015;6:778–787. [Google Scholar]
- 22.Zaro JL. Lipid-based drug carriers for prodrugs to enhance drug delivery. AAPS J. 2015;17:83–92. doi: 10.1208/s12248-014-9670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuznetsova NR, Stepanova EV, Peretolchina NM, Khochenkov DA, Boldyrev IA, Bovin NV, Vodovozova EL. Targeting liposomes loaded with melphalan prodrug to tumour vasculature via the Sialyl Lewis X selectin ligand. J Drug Targeting. 2014;22:242–250. doi: 10.3109/1061186X.2013.862805. [DOI] [PubMed] [Google Scholar]
- 24.Han S, Quach T, Hu L, Wahab A, Charman WN, Stella VJ, Trevaskis NL, Simpson JS, Porter CJ. Targeted delivery of a model immunomodulator to the lymphatic system: comparison of alkyl ester versus triglyceride mimetic lipid prodrug strategies. J Controlled Release. 2014;177:1–10. doi: 10.1016/j.jconrel.2013.12.031. [DOI] [PubMed] [Google Scholar]
- 25.Han S, Hu L, Quach T, Simpson JS, Trevaskis NL, Porter CJH. Profiling the Role of Deacylation-Reacylation in the Lymphatic Transport of a Triglyceride-Mimetic Prodrug. Pharm Res. 2015;32:1830–1844. doi: 10.1007/s11095-014-1579-9. [DOI] [PubMed] [Google Scholar]
- 26.Han S, Hu L, Gracia, Quach T, Simpson JS, Edwards GA, Trevaskis NL, Porter CJH. Lymphatic Transport and Lymphocyte Targeting of a Triglyceride Mimetic Prodrug Is Enhanced in a Large Animal Model: Studies in Greyhound Dogs. Mol Pharmaceutics. 2016;13:3351–3361. doi: 10.1021/acs.molpharmaceut.6b00195. [DOI] [PubMed] [Google Scholar]
- 27.Zalipsky S, Saad M, Kiwan R, Ber E, Yu N, Minko T. Antitumor activity of new liposomal prodrug of mitomycin C in multidrug resistant solid tumor: insights of the mechanism of action. J Drug Target. 2007;15:518–30. doi: 10.1080/10611860701499946. [DOI] [PubMed] [Google Scholar]
- 28.Amitay Y, Shmeeda H, Patil Y, Gorin J, Tzemach D, Mak L, Ohana P, Gabizon A. Pharmacologic Studies of a Prodrug of Mitomycin C in Pegylated Liposomes (Promitil®): High Stability in Plasma and Rapid Thiolytic Prodrug Activation in Tissues. Pharm Res. 2016;33:686–700. doi: 10.1007/s11095-015-1819-7. [DOI] [PubMed] [Google Scholar]
- 29.Gabizon A, Amitay Y, Tzemach D, Gorin J, Shmeeda H, Zalipsky S. Therapeutic efficacy of a lipid-based prodrug of mitomycin C in pegylated liposomes: studies with human gastro-entero-pancreatic ectopic tumor models. J Controlled Release. 2012;160:245–53. doi: 10.1016/j.jconrel.2011.11.019. [DOI] [PubMed] [Google Scholar]
- 30.Gabizon AA, Tzemach D, Horowitz AT, Shmeeda H, Yeh J, Zalipsky S. Reduced toxicity and superior therapeutic activity of a mitomycin C lipid-based prodrug incorporated in pegylated liposomes. Clin Cancer Res. 2006;12:1913–20. doi: 10.1158/1078-0432.CCR-05-1547. [DOI] [PubMed] [Google Scholar]
- 31.Vlasenko YV, Alekseeva AS, Vodovozova EL. Synthesis of a fluorescent analogue of methotrexate lipophilic prodrug. Russ J Bioorg Chem. 2014;40:114–117. [PubMed] [Google Scholar]
- 32.Kuznetsova NR, Sevrin C, Lespineux D, Bovin NV, Vodovozova EL, Meszaros T, Szebeni J, Grandfils C. Hemocompatibility of liposomes loaded with lipophilic prodrugs of methotrexate and melphalan in the lipid bilayer. J Controlled Release. 2012;160:394–400. doi: 10.1016/j.jconrel.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 33.Hu L, Quach T, Han S, Lim SF, Yadav P, Senyschyn D, Trevaskis NL, Simpson JS, Porter CJH. Glyceride-Mimetic Prodrugs Incorporating Self-Immolative Spacers Promote Lymphatic Transport, Avoid First-Pass Metabolism, and Enhance Oral Bioavailability. Angew Chem, Int Ed. 2016 doi: 10.1002/anie.201604207. [DOI] [PubMed] [Google Scholar]
- 34.Alexander RL, Greene BT, Torti SV, Kucera GL. A novel phospholipid gemcitabine conjugate is able to bypass three drug-resistance mechanisms. Cancer Chemother Pharmacol. 2005;56:15–21. doi: 10.1007/s00280-004-0949-0. [DOI] [PubMed] [Google Scholar]
- 35.Pedersen PJ, Christensen MS, Ruysschaert T, Linderoth L, Andresen TL, Melander F, Mouritsen OG, Madsen R, Clausen MH. Synthesis and biophysical characterization of chlorambucil anticancer ether lipid prodrugs. J Med Chem. 2009;52:3408–15. doi: 10.1021/jm900091h. [DOI] [PubMed] [Google Scholar]
- 36.Arouri A, Mouritsen OG. Anticancer double lipid prodrugs: liposomal preparation and characterization. J Liposome Res. 2011;21:296–305. doi: 10.3109/08982104.2011.563365. [DOI] [PubMed] [Google Scholar]
- 37.Chhikara BS, Mandal D, Parang K. Synthesis, anticancer activities, and cellular uptake studies of lipophilic derivatives of doxorubicin succinate. J Med Chem. 2012;55:1500–10. doi: 10.1021/jm201653u. [DOI] [PubMed] [Google Scholar]
- 38.Gollnest T, de Oliveira TD, Schols D, Balzarini J, Meier C. Lipophilic prodrugs of nucleoside triphosphates as biochemical probes and potential antivirals. Nat Commun. 2015;6:8716. doi: 10.1038/ncomms9716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Daull P, Paterson CA, Kuppermann BD, Garrigue JS. A preliminary evaluation of dexamethasone palmitate emulsion: a novel intravitreal sustained delivery of corticosteroid for treatment of macular edema. J Ocul Pharmacol Ther. 2013;29:258–69. doi: 10.1089/jop.2012.0044. [DOI] [PubMed] [Google Scholar]
- 40.Feng L, Benhabbour SR, Mumper RJ. Oil-filled lipid nanoparticles containing 2′-(2-bromohexadecanoyl)-docetaxel for the treatment of breast cancer. Adv Healthcare Mater. 2013;2:1451–7. doi: 10.1002/adhm.201300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arouri A, Hansen AH, Rasmussen TE, Mouritsen OG. Lipases, liposomes and lipid-prodrugs. Curr Opin Colloid Interface Sci. 2013;18:419–431. [Google Scholar]
- 42.Pan D, Schmieder AH, Wang K, Yang X, Senpan A, Cui G, Killgore K, Kim B, Allen JS, Zhang H, Caruthers SD, Shen B, Wickline SA, Lanza GM. Anti-angiogenesis therapy in the Vx2 rabbit cancer model with a lipase-cleavable Sn 2 taxane phospholipid prodrug using alpha(v)beta(3)-targeted theranostic nanoparticles. Theranostics. 2014;4:565–78. doi: 10.7150/thno.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gong X, Moghaddam MJ, Sagnella SM, Conn CE, Danon SJ, Waddington LJ, Drummond CJ. Lamellar crystalline self-assembly behaviour and solid lipid nanoparticles of a palmityl prodrug analogue of Capecitabine–a chemotherapy agent. Colloids Surf, B. 2011;85:349–59. doi: 10.1016/j.colsurfb.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 44.Xiong MP, Yanez JA, Remsberg CM, Ohgami Y, Kwon GS, Davies NM, Forrest ML. Formulation of a geldanamycin prodrug in mPEG-b-PCL micelles greatly enhances tolerability and pharmacokinetics in rats. J Controlled Release. 2008;129:33–40. doi: 10.1016/j.jconrel.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng L, Feng L, Yuan H, Benhabbour SR, Mumper RJ. Development of a novel orthotopic non-small cell lung cancer model and therapeutic benefit of 2′-(2-bromohexadecanoyl)-docetaxel conjugate nanoparticles. Nanomedicine. 2014;10:1497–1506. doi: 10.1016/j.nano.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perkins WR, Ahmad I, Li X, Hirsh DJ, Masters GR, Fecko CJ, Lee J, Ali S, Nguyen J, Schupsky J, Herbert C, Janoff AS, Mayhew E. Novel therapeutic nano-particles (lipocores): trapping poorly water soluble compounds. Int J Pharm. 2000;200:27–39. doi: 10.1016/s0378-5173(00)00329-x. [DOI] [PubMed] [Google Scholar]
- 47.Borrelli S, Christodoulou MS, Ficarra I, Silvani A, Cappelletti G, Cartelli D, Damia G, Ricci F, Zucchetti M, Dosio F, Passarella D. New class of squalene-based releasable nano-assemblies of paclitaxel, podophyllotoxin, camptothecin and epothilone A. Eur J Med Chem. 2014;85:179–90. doi: 10.1016/j.ejmech.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 48.Alferiev IS, Iyer R, Croucher JL, Adamo RF, Zhang K, Mangino JL, Kolla V, Fishbein I, Brodeur GM, Levy RJ, Chorny M. Nanoparticle-mediated delivery of a rapidly activatable prodrug of SN-38 for neuroblastoma therapy. Biomaterials. 2015;51:22–9. doi: 10.1016/j.biomaterials.2015.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valetti S, Maione F, Mura S, Stella B, Desmaele D, Noiray M, Vergnaud J, Vauthier C, Cattel L, Giraudo E, Couvreur P. Peptide-functionalized nanoparticles for selective targeting of pancreatic tumor. J Controlled Release. 2014;192:29–39. doi: 10.1016/j.jconrel.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 50.Dosio F, Reddy LH, Ferrero A, Stella B, Cattel L, Couvreur P. Novel nanoassemblies composed of squalenoyl-paclitaxel derivatives: synthesis, characterization, and biological evaluation. Bioconjugate Chem. 2010;21:1349–61. doi: 10.1021/bc100154g. [DOI] [PubMed] [Google Scholar]
- 51.Caron J, Maksimenko A, Wack S, Lepeltier E, Bourgaux C, Morvan E, Leblanc K, Couvreur P, Desmaele D. Improving the antitumor activity of squalenoyl-paclitaxel conjugate nanoassemblies by manipulating the linker between paclitaxel and squalene. Adv Healthcare Mater. 2013;2:172–85. doi: 10.1002/adhm.201200099. [DOI] [PubMed] [Google Scholar]
- 52.Duhem N, Danhier F, Pourcelle V, Schumers JM, Bertrand O, Leduff CS, Hoeppener S, Schubert US, Gohy JF, Marchand-Brynaert J, Preat V. Self-assembling doxorubicin-tocopherol succinate prodrug as a new drug delivery system: synthesis, characterization, and in vitro and in vivo anticancer activity. Bioconjugate Chem. 2014;25:72–81. doi: 10.1021/bc400326y. [DOI] [PubMed] [Google Scholar]
- 53.Gupta A, Asthana S, Konwar R, Chourasia MK. An insight into potential of nanoparticles-assisted chemotherapy of cancer using gemcitabine and its fatty acid prodrug:a comparative study. J Biomed Nanotechnol. 2013;9:915–25. doi: 10.1166/jbn.2013.1591. [DOI] [PubMed] [Google Scholar]
- 54.Abu Ajaj K, Graeser R, Kratz F. Zosuquidar and an albumin-binding prodrug of zosuquidar reverse multidrug resistance in breast cancer cells of doxorubicin and an albumin-binding prodrug of doxorubicin. Breast Cancer Res Treat. 2012;134:117–29. doi: 10.1007/s10549-011-1937-9. [DOI] [PubMed] [Google Scholar]
- 55.Liang CH, Ye WL, Zhu CL, Na R, Cheng Y, Cui H, Liu DZ, Yang ZF, Zhou SY. Synthesis of doxorubicin alpha-linolenic acid conjugate and evaluation of its antitumor activity. Mol Pharmaceutics. 2014;11:1378–90. doi: 10.1021/mp4004139. [DOI] [PubMed] [Google Scholar]
- 56.Li F, Snow-Davis C, Du C, Bondarev ML, Saulsbury MD, Heyliger SO. Preparation and Characterization of Lipophilic Doxorubicin Pro-drug Micelles. J Visualized Exp. 2016 doi: 10.3791/54338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Effenberger K, Breyer S, Schobert R. Modulation of doxorubicin activity in cancer cells by conjugation with fatty acyl and terpenyl hydrazones. Eur J Med Chem. 2010;45:1947–54. doi: 10.1016/j.ejmech.2010.01.037. [DOI] [PubMed] [Google Scholar]
- 58.Chen Q, Butler D, Querbes W, Pandey RK, Ge P, Maier MA, Zhang L, Rajeev KG, Nechev L, Kotelianski V, Manoharan M, Sah DW. Lipophilic siRNAs mediate efficient gene silencing in oligodendrocytes with direct CNS delivery. J Controlled Release. 2010;144:227–32. doi: 10.1016/j.jconrel.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 59.Cheng K, Ye Z, Guntaka RV, Mahato RI. Enhanced hepatic uptake and bioactivity of type alpha1(I) collagen gene promoter-specific triplex-forming oligonucleotides after conjugation with cholesterol. J Pharmacol Exp Ther. 2006;317:797–805. doi: 10.1124/jpet.105.100347. [DOI] [PubMed] [Google Scholar]
- 60.Raouane M, Desmaele D, Gilbert-Sirieix M, Gueutin C, Zouhiri F, Bourgaux C, Lepeltier E, Gref R, Ben Salah R, Clayman G, Massaad-Massade L, Couvreur P. Synthesis, Characterization, and in Vivo Delivery of siRNA-Squalene Nanoparticles Targeting Fusion Oncogene in Papillary Thyroid Carcinoma. J Med Chem. 2011;54:4067–4076. doi: 10.1021/jm2000272. [DOI] [PubMed] [Google Scholar]
- 61.Sagnella SM, Gong X, Moghaddam MJ, Conn CE, Kimpton K, Waddington LJ, Krodkiewska I, Drummond CJ. Nanostructured nanoparticles of self-assembled lipid pro-drugs as a route to improved chemotherapeutic agents. Nanoscale. 2011;3:919–24. doi: 10.1039/c0nr00781a. [DOI] [PubMed] [Google Scholar]
- 62.Godeau G, Staedel C, Barthelemy P. Lipid-conjugated oligonucleotides via “click chemistry” efficiently inhibit hepatitis C virus translation. J Med Chem. 2008;51:4374–6. doi: 10.1021/jm800518u. [DOI] [PubMed] [Google Scholar]
- 63.Weber RJ, Liang SI, Selden NS, Desai TA, Gartner ZJ. Efficient targeting of fatty-acid modified oligonucleotides to live cell membranes through stepwise assembly. Biomacromolecules. 2014;15:4621–6. doi: 10.1021/bm501467h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishina K, Unno T, Uno Y, Kubodera T, Kanouchi T, Mizusawa H, Yokota T. Efficient in vivo delivery of siRNA to the liver by conjugation of alpha-tocopherol. Mol Ther. 2008;16:734–40. doi: 10.1038/mt.2008.14. [DOI] [PubMed] [Google Scholar]
- 65.Trevaskis NL, Kaminskas LM, Porter CJH. From sewer to saviour [mdash] targeting the lymphatic system to promote drug exposure and activity. Nat Rev Drug Discovery. 2015;14:781–803. doi: 10.1038/nrd4608. [DOI] [PubMed] [Google Scholar]
- 66.Bala V, Rao S, Li P, Wang S, Prestidge CA. Lipophilic Prodrugs of SN38: Synthesis and in Vitro Characterization toward Oral Chemotherapy. Mol Pharmaceutics. 2016;13:287–94. doi: 10.1021/acs.molpharmaceut.5b00785. [DOI] [PubMed] [Google Scholar]
- 67.Borkar N, Li B, Holm R, Hakansson AE, Mullertz A, Yang M, Mu H. Lipophilic prodrugs of apomorphine I: preparation, characterisation, and in vitro enzymatic hydrolysis in biorelevant media. Eur J Pharm Biopharm. 2015;89:216–23. doi: 10.1016/j.ejpb.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 68.Liu J, Zhao D, Ma N, Luan Y. Highly enhanced leukemia therapy and oral bioavailability from a novel amphiphilic prodrug of cytarabine. RSC Adv. 2016;6:35991–35999. [Google Scholar]
- 69.Wang Y, Li L, Jiang W, Larrick JW. Synthesis and evaluation of a DHA and 10-hydroxycamptothecin conjugate. Bioorg Med Chem. 2005;13:5592–9. doi: 10.1016/j.bmc.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 70.Olbrich C, Gessner A, Kayser O, Müller RH. Lipid-Drug-Conjugate (LDC) Nanoparticles as Novel Carrier System for the Hydrophilic Antitrypanosomal Drug Diminazenediaceturate. J Drug Targeting. 2002;10:387–396. doi: 10.1080/1061186021000001832. [DOI] [PubMed] [Google Scholar]
- 71.Nikanjam M, Gibbs AR, Hunt CA, Budinger TF, Forte TM. Synthetic nano-LDL with paclitaxel oleate as a targeted drug delivery vehicle for glioblastoma multiforme. J Controlled Release. 2007;124:163–71. doi: 10.1016/j.jconrel.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 72.Lambert DM. Rationale and applications of lipids as prodrug carriers. Eur J Pharm Sci. 2000;11(Suppl 2):S15–S27. doi: 10.1016/s0928-0987(00)00161-5. [DOI] [PubMed] [Google Scholar]
- 73.Urbinati G, de Waziers I, Slamic M, Foussigniere T, Ali HM, Desmaele D, Couvreur P, Massaad-Massade L. Knocking Down TMPRSS2-ERG Fusion Oncogene by siRNA Could be an Alternative Treatment to Flutamide. Mol Ther–Nucleic Acids. 2016;5:e301. doi: 10.1038/mtna.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Petrova NS, Chernikov IV, Meschaninova MI, Dovydenko IS, Venyaminova AG, Zenkova MA, Vlassov VV, Chernolovskaya EL. Carrier-free cellular uptake and the gene-silencing activity of the lipophilic siRNAs is strongly affected by the length of the linker between siRNA and lipophilic group. Nucleic Acids Res. 2012;40:2330–44. doi: 10.1093/nar/gkr1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sarett SM, Kilchrist KV, Miteva M, Duvall CL. Conjugation of palmitic acid improves potency and longevity of siRNA delivered via endosomolytic polymer nanoparticles. J Biomed Mater Res, Part A. 2015;103:3107–16. doi: 10.1002/jbm.a.35413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, Elbashir S, Geick A, Hadwiger P, Harborth J, John M, Kesavan V, Lavine G, Pandey RK, Racie T, Rajeev KG, Rohl I, Toudjarska I, Wang G, Wuschko S, Bumcrot D, Koteliansky V, Limmer S, Manoharan M, Vornlocher HP. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–8. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 77.Ali HM, Maksimenko A, Urbinati G, Chapuis H, Raouane M, Desmaele D, Yasuhiro H, Harashima H, Couvreur P, Massaad-Massade L. Effects of silencing the RET/PTC1 oncogene in papillary thyroid carcinoma by siRNA-squalene nanoparticles with and without fusogenic companion GALA-cholesterol. Thyroid. 2014;24:327–38. doi: 10.1089/thy.2012.0544. [DOI] [PubMed] [Google Scholar]
- 78.Nishina T, Numata J, Nishina K, Yoshida-Tanaka K, Nitta K, Piao W, Iwata R, Ito S, Kuwahara H, Wada T, Mizusawa H, Yokota T. Chimeric Antisense Oligonucleotide Conjugated to [alpha]-Tocopherol. Mol Ther–Nucleic Acids. 2015;4:e220. doi: 10.1038/mtna.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shao W, Paul A, Zhao B, Lee C, Rodes L, Prakash S. Carbon nanotube lipid drug approach for targeted delivery of a chemotherapy drug in a human breast cancer xenograft animal model. Biomaterials. 2013;34:10109–19. doi: 10.1016/j.biomaterials.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 80.Wang JX, Sun X, Zhang ZR. Enhanced brain targeting by synthesis of 3′,5′-dioctanoyl-5-fluoro-2′-deoxyuridine and incorporation into solid lipid nanoparticles. Eur J Pharm Biopharm. 2002;54:285–90. doi: 10.1016/s0939-6411(02)00083-8. [DOI] [PubMed] [Google Scholar]
- 81.Yu BT, Sun X, Zhang ZR. Enhanced liver targeting by synthesis of N1-stearyl-5-Fu and incorporation into solid lipid nanoparticles. Arch Pharmacal Res. 2003;26:1096–101. doi: 10.1007/BF02994764. [DOI] [PubMed] [Google Scholar]
- 82.Sharma P, Dube B, Sawant K. Synthesis of cytarabine lipid drug conjugate for treatment of meningeal leukemia: development, characterization and in vitro cell line studies. J Biomed Nanotechnol. 2012;8:928–37. doi: 10.1166/jbn.2012.1464. [DOI] [PubMed] [Google Scholar]
- 83.Ma P, Rahima Benhabbour S, Feng L, Mumper RJ. 2′-Behenoyl-paclitaxel conjugate containing lipid nanoparticles for the treatment of metastatic breast cancer. Cancer Lett. 2013;334:253–62. doi: 10.1016/j.canlet.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sun B, Luo C, Li L, Wang M, Du Y, Di D, Zhang D, Ren G, Pan X, Fu Q, Sun J, He Z. Core-matched encapsulation of an oleate prodrug into nanostructured lipid carriers with high drug loading capability to facilitate the oral delivery of docetaxel. Colloids Surf, B. 2016;143:47–55. doi: 10.1016/j.colsurfb.2016.02.065. [DOI] [PubMed] [Google Scholar]
- 85.Remenar JF. Making the leap from daily oral dosing to long-acting injectables: lessons from the antipsychotics. Mol Pharmaceutics. 2014;11:1739–49. doi: 10.1021/mp500070m. [DOI] [PubMed] [Google Scholar]
- 86.Chue P, Chue J. A review of paliperidone palmitate. Expert Rev Neurother. 2012;12:1383–97. doi: 10.1586/ern.12.137. [DOI] [PubMed] [Google Scholar]
- 87.Raedler LA. Aripiprazole Lauroxil (Aristada): Long-Acting Atypical Antipsychotic Injection Approved for the Treatment of Patients with Schizophrenia. Am Health Drug Benefits. 2016;9:40–3. [PMC free article] [PubMed] [Google Scholar]
- 88.Gaekens T, Guillaume M, Borghys H, De Zwart LL, de Vries R, Embrechts RC, Vermeulen A, Megens AA, Leysen JE, Herdewijn P, Annaert PP, Atack JR. Lipophilic nalmefene prodrugs to achieve a one-month sustained release. J Controlled Release. 2016;232:196–202. doi: 10.1016/j.jconrel.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 89.Sanrame CN, Remenar JF, Blumberg LC, Waters J, Dean RL, Dong N, Kriksciukaite K, Cao P, Almarsson O. Prodrugs of pioglitazone for extended-release (XR) injectable formulations. Mol Pharmaceutics. 2014;11:3617–23. doi: 10.1021/mp500359a. [DOI] [PubMed] [Google Scholar]
- 90.Jin Y, Lian Y, Du L, Wang S, Su C, Gao C. Self-assembled drug delivery systems. Part 6: in vitro/in vivo studies of anticancer N-octadecanoyl gemcitabine nanoassemblies. Int J Pharm. 2012;430:276–81. doi: 10.1016/j.ijpharm.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 91.Jin Y, Lian Y, Du L. Self-assembly of N-acyl derivatives of gemcitabine at the air/water interface and the formation of nanoscale structures in water. Colloids Surf, A. 2012;393:60–65. [Google Scholar]
- 92.Couvreur P, Reddy LH, Mangenot S, Poupaert JH, Desmaele D, Lepetre-Mouelhi S, Pili B, Bourgaux C, Amenitsch H, Ollivon M. Discovery of new hexagonal supramolecular nanostructures formed by squalenoylation of an anticancer nucleoside analogue. Small. 2008;4:247–53. doi: 10.1002/smll.200700731. [DOI] [PubMed] [Google Scholar]
- 93.Reddy LH, Dubernet C, Mouelhi SL, Marque PE, Desmaele D, Couvreur P. A new nanomedicine of gemcitabine displays enhanced anticancer activity in sensitive and resistant leukemia types. J Controlled Release. 2007;124:20–7. doi: 10.1016/j.jconrel.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 94.Rejiba S, Reddy LH, Bigand C, Parmentier C, Couvreur P, Hajri A. Squalenoyl gemcitabine nanomedicine overcomes the low efficacy of gemcitabine therapy in pancreatic cancer. Nanomedicine. 2011;7:841–9. doi: 10.1016/j.nano.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 95.Reddy LH, Khoury H, Paci A, Deroussent A, Ferreira H, Dubernet C, Decleves X, Besnard M, Chacun H, Lepetre-Mouelhi S, Desmaele D, Rousseau B, Laugier C, Cintrat JC, Vassal G, Couvreur P. Squalenoylation favorably modifies the in vivo pharmacokinetics and biodistribution of gemcitabine in mice. Drug Metab Dispos. 2008;36:1570–7. doi: 10.1124/dmd.108.020735. [DOI] [PubMed] [Google Scholar]
- 96.Reddy LH, Marque PE, Dubernet C, Mouelhi SL, Desmaele D, Couvreur P. Preclinical toxicology (subacute and acute) and efficacy of a new squalenoyl gemcitabine anticancer nanomedicine. J Pharmacol Exp Ther. 2008;325:484–90. doi: 10.1124/jpet.107.133751. [DOI] [PubMed] [Google Scholar]
- 97.Reddy LH, Renoir JM, Marsaud V, Lepetre-Mouelhi S, Desmaele D, Couvreur P. Anticancer efficacy of squalenoyl gemcitabine nanomedicine on 60 human tumor cell panel and on experimental tumor. Mol Pharmaceutics. 2009;6:1526–35. doi: 10.1021/mp900099e. [DOI] [PubMed] [Google Scholar]
- 98.Bui DT, Nicolas J, Maksimenko A, Desmaele D, Couvreur P. Multifunctional squalene-based prodrug nanoparticles for targeted cancer therapy. Chem Commun (Cambridge, U K) 2014;50:5336–8. doi: 10.1039/c3cc47427e. [DOI] [PubMed] [Google Scholar]
- 99.Maksimenko A, Dosio F, Mougin J, Ferrero A, Wack S, Reddy LH, Weyn AA, Lepeltier E, Bourgaux C, Stella B, Cattel L, Couvreur P. A unique squalenoylated and nonpegylated doxorubicin nanomedicine with systemic long-circulating properties and anticancer activity. Proc Natl Acad Sci U S A. 2014;111:E217–E226. doi: 10.1073/pnas.1313459110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maksimenko A, Alami M, Zouhiri F, Brion JD, Pruvost A, Mougin J, Hamze A, Boissenot T, Provot O, Desmaele D, Couvreur P. Therapeutic modalities of squalenoyl nanocomposites in colon cancer: an ongoing search for improved efficacy. ACS Nano. 2014;8:2018–32. doi: 10.1021/nn500517a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caron J, Reddy LH, Lepetre-Mouelhi S, Wack S, Clayette P, Rogez-Kreuz C, Yousfi R, Couvreur P, Desmaele D. Squalenoyl nucleoside monophosphate nanoassemblies: new prodrug strategy for the delivery of nucleotide analogues. Bioorg Med Chem Lett. 2010;20:2761–4. doi: 10.1016/j.bmcl.2010.03.070. [DOI] [PubMed] [Google Scholar]
- 102.Mura S, Zouhiri F, Lerondel S, Maksimenko A, Mougin J, Gueutin C, Brambilla D, Caron J, Sliwinski E, LePape A, Desmaele D, Couvreur P. Novel Isoprenoyl Nanoassembled Prodrug for Paclitaxel Delivery. Bioconjugate Chem. 2013;24:1840–1849. doi: 10.1021/bc400210x. [DOI] [PubMed] [Google Scholar]
- 103.Valetti S, Mura S, Noiray M, Arpicco S, Dosio F, Vergnaud J, Desmaele D, Stella B, Couvreur P. Peptide conjugation: before or after nanoparticle formation? Bioconjugate Chem. 2014;25:1971–83. doi: 10.1021/bc5003423. [DOI] [PubMed] [Google Scholar]
- 104.Lundberg BB, Risovic V, Ramaswamy M, Wasan KM. A lipophilic paclitaxel derivative incorporated in a lipid emulsion for parenteral administration. J Controlled Release. 2003;86:93–100. doi: 10.1016/s0168-3659(02)00323-1. [DOI] [PubMed] [Google Scholar]
- 105.Rodrigues DG, Maria DA, Fernandes DC, Valduga CJ, Couto RD, Ibanez OC, Maranhao RC. Improvement of paclitaxel therapeutic index by derivatization and association to a cholesterol-rich microemulsion: in vitro and in vivo studies. Cancer Chemother Pharmacol. 2005;55:565–76. doi: 10.1007/s00280-004-0930-y. [DOI] [PubMed] [Google Scholar]
- 106.Goldstein D, Gofrit O, Nyska A, Benita S. Anti-HER2 cationic immunoemulsion as a potential targeted drug delivery system for the treatment of prostate cancer. Cancer Res. 2007;67:269–75. doi: 10.1158/0008-5472.CAN-06-2731. [DOI] [PubMed] [Google Scholar]
- 107.Huynh L, Leroux JC, Allen C. Enhancement of docetaxel solubility via conjugation of formulation-compatible moieties. Org Biomol Chem. 2009;7:3437–46. doi: 10.1039/b906862g. [DOI] [PubMed] [Google Scholar]
- 108.Dias ML, Carvalho JP, Rodrigues DG, Graziani SR, Maranhao RC. Pharmacokinetics and tumor uptake of a derivatized form of paclitaxel associated to a cholesterol-rich nanoemulsion (LDE) in patients with gynecologic cancers. Cancer Chemother Pharmacol. 2007;59:105–11. doi: 10.1007/s00280-006-0252-3. [DOI] [PubMed] [Google Scholar]
- 109.Pires LA, Hegg R, Valduga CJ, Graziani SR, Rodrigues DG, Maranhao RC. Use of cholesterol-rich nanoparticles that bind to lipoprotein receptors as a vehicle to paclitaxel in the treatment of breast cancer: pharmacokinetics, tumor uptake and a pilot clinical study. Cancer Chemother Pharmacol. 2009;63:281–7. doi: 10.1007/s00280-008-0738-2. [DOI] [PubMed] [Google Scholar]
- 110.Jiao YY, Wang XQ, Lu WL, Yang ZJ, Zhang Q. A novel approach to improve the pharmacokinetic properties of 8-chloro-adenosine by the dual combination of lipophilic derivatisation and liposome formulation. Eur J Pharm Sci. 2013;48:249–58. doi: 10.1016/j.ejps.2012.10.026. [DOI] [PubMed] [Google Scholar]
- 111.Cupri S, Graziano ACE, Cardile V, Skwarczynski M, Toth I, Pignatello R. A study on the encapsulation of an occludin lipophilic derivative in liposomal carriers. J Liposome Res. 2015;25:287–293. doi: 10.3109/08982104.2014.992025. [DOI] [PubMed] [Google Scholar]
- 112.Crosasso P, Brusa P, Dosio F, Arpicco S, Pacchioni D, Schuberî F, Cattel L. Antitumoral activity of liposomes and immunoliposomes containing 5-fluorouridine prodrugs. J Pharm Sci. 1997;86:832–9. doi: 10.1021/js9604467. [DOI] [PubMed] [Google Scholar]
- 113.Sarpietro MG, Micieli D, Rocco F, Ceruti M, Castelli F. Conjugation of squalene to acyclovir improves the affinity for biomembrane models. Int J Pharm. 2009;382:73–9. doi: 10.1016/j.ijpharm.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 114.Kuznetsova NR, Svirshchevskaya EV, Skripnik IV, Zarudnaya EN, Benke AN, Gaenko GP, Molotkovskii YG, Vodovozova EL. Interaction of liposomes bearing a lipophilic doxorubicin prodrug with tumor cells. Biochemistry (Moscow) Supplement Series A: Membrane and Cell Biology. 2013;7:12–20. [Google Scholar]
- 115.Sarpietro MG, Accolla ML, Santoro N, Mansfeld FM, Pignatello R, Toth I, Castelli F. Calorimetry and Langmuir-Blodgett studies on the interaction of a lipophilic prodrug of LHRH with biomembrane models. J Colloid Interface Sci. 2014;421:122–31. doi: 10.1016/j.jcis.2014.01.040. [DOI] [PubMed] [Google Scholar]
- 116.Sarpietro MG, Ottimo S, Paolino D, Ferrero A, Dosio F, Castelli F. Squalenoyl prodrug of paclitaxel: synthesis and evaluation of its incorporation in phospholipid bilayers. Int J Pharm. 2012;436:135–40. doi: 10.1016/j.ijpharm.2012.06.034. [DOI] [PubMed] [Google Scholar]
- 117.Giuffrida MC, Dosio F, Castelli F, Sarpietro MG. Lipophilic prodrug of paclitaxel: interaction with a dimyristoylphosphatidylcholine monolayer. Int J Pharm. 2014;475:624–31. doi: 10.1016/j.ijpharm.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 118.Pili B, Reddy LH, Bourgaux C, Lepetre-Mouelhi S, Desmaele D, Couvreur P. Liposomal squalenoyl-gemcitabine: formulation, characterization and anticancer activity evaluation. Nanoscale. 2010;2:1521–1526. doi: 10.1039/c0nr00132e. [DOI] [PubMed] [Google Scholar]
- 119.Du R, Zhong T, Zhang WQ, Song P, Song WD, Zhao Y, Wang C, Tang YQ, Zhang X, Zhang Q. Antitumor effect of iRGD-modified liposomes containing conjugated linoleic acid-paclitaxel (CLA-PTX) on B16–F10 melanoma. Int J Nanomed. 2014;9:3091–105. doi: 10.2147/IJN.S65664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Arpicco S, Lerda C, Dalla Pozza E, Costanzo C, Tsapis N, Stella B, Donadelli M, Dando I, Fattal E, Cattel L, Palmieri M. Hyaluronic acid-coated liposomes for active targeting of gemcitabine. Eur J Pharm Biopharm. 2013;85:373–80. doi: 10.1016/j.ejpb.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 121.Daman Z, Ostad S, Amini M, Gilani K. Preparation, optimization and in vitro characterization of stearoyl-gemcitabine polymeric micelles: a comparison with its self-assembled nanoparticles. Int J Pharm. 2014;468:142–51. doi: 10.1016/j.ijpharm.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 122.Zhao Y, Duan S, Zeng X, Liu C, Davies NM, Li B, Forrest ML. Prodrug strategy for PSMA-targeted delivery of TGX-221 to prostate cancer cells. Mol Pharmaceutics. 2012;9:1705–16. doi: 10.1021/mp3000309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhu S, Lansakara-P DSP, Li X, Cui Z. Lysosomal delivery of a lipophilic gemcitabine prodrug using novel acid-sensitive micelles improved its antitumor activity. Bioconjugate Chem. 2012;23:966–80. doi: 10.1021/bc2005945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Peira E, Chirio D, Battaglia L, Barge A, Chegaev K, Gigliotti CL, Ferrara B, Dianzani C, Gallarate M. Solid lipid nanoparticles carrying lipophilic derivatives of doxorubicin: preparation, characterization, and in vitro cytotoxicity studies. J Micro-encapsulation. 2016;33:381–390. doi: 10.1080/02652048.2016.1202342. [DOI] [PubMed] [Google Scholar]
- 125.Chung WG, Sandoval MA, Sloat BR, Lansakara-P DSP, Cui Z. Stearoyl gemcitabine nanoparticles overcome resistance related to the over-expression of ribonucleotide reductase subunit M1. J Controlled Release. 2012;157:132–40. doi: 10.1016/j.jconrel.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sloat BR, Sandoval MA, Li D, Chung WG, Lansakara-P DSP, Proteau PJ, Kiguchi K, DiGiovanni J, Cui Z. In vitro and in vivo anti-tumor activities of a gemcitabine derivative carried by nanoparticles. Int J Pharm. 2011;409:278–88. doi: 10.1016/j.ijpharm.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dalpiaz A, Contado C, Mari L, Perrone D, Pavan B, Paganetto G, Hanuskovà M, Vighi E, Leo E. Development and characterization of PLGA nanoparticles as delivery systems of a prodrug of zidovudine obtained by its conjugation with ursodeoxycholic acid. Drug Delivery. 2014;21:221–232. doi: 10.3109/10717544.2013.844744. [DOI] [PubMed] [Google Scholar]
- 128.Karra N, Nassar T, Ripin AN, Schwob O, Borlak J, Benita S. Antibody Conjugated PLGA Nanoparticles for Targeted Delivery of Paclitaxel Palmitate: Efficacy and Biofate in a Lung Cancer Mouse Model. Small. 2013;9:4221–4236. doi: 10.1002/smll.201301417. [DOI] [PubMed] [Google Scholar]
- 129.Neutsch L, Wirth EM, Spijker S, Pichl C, Kahlig H, Gabor F, Wirth M. Synergistic targeting/prodrug strategies for intravesical drug delivery–lectin-modified PLGA microparticles enhance cytotoxicity of stearoyl gemcitabine by contact-dependent transfer. J Controlled Release. 2013;169:62–72. doi: 10.1016/j.jconrel.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 130.Dhar S, Kolishetti N, Lippard SJ, Farokhzad OC. Targeted delivery of a cisplatin prodrug for safer and more effective prostate cancer therapy in vivo. Proc Natl Acad Sci U S A. 2011;108:1850–5. doi: 10.1073/pnas.1011379108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lundberg BB. Preparation and characterization of polymeric pH-sensitive STEALTH(R) nanoparticles for tumor delivery of a lipophilic prodrug of paclitaxel. Int J Pharm. 2011;408:208–12. doi: 10.1016/j.ijpharm.2011.01.061. [DOI] [PubMed] [Google Scholar]
- 132.Arias JL, Reddy LH, Othman M, Gillet B, Desmaele D, Zouhiri F, Dosio F, Gref R, Couvreur P. Squalene based nanocomposites: a new platform for the design of multifunctional pharmaceutical theragnostics. ACS Nano. 2011;5:1513–21. doi: 10.1021/nn1034197. [DOI] [PubMed] [Google Scholar]
- 133.Malfanti A, Miletto I, Bottinelli E, Zonari D, Blandino G, Berlier G, Arpicco S. Delivery of Gemcitabine Prodrugs Employing Mesoporous Silica Nanoparticles. Molecules. 2016;21:522. doi: 10.3390/molecules21040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bastiancich C, Vanvarenberg K, Ucakar B, Pitorre M, Bastiat G, Lagarce F, Preat V, Danhier F. Lauroyl-gemcitabine-loaded lipid nanocapsule hydrogel for the treatment of glioblastoma. J Controlled Release. 2016;225:283–93. doi: 10.1016/j.jconrel.2016.01.054. [DOI] [PubMed] [Google Scholar]
- 135.https://clinicaltrials.gov.
- 136.Fitch RM, Wojdyla JK, Blackledge JA, McGhee WD. Abstract 4400: Anti-tumor activity of liposomal docetaxel prodrug MNK-010 on PC3 human prostate xenografts in mice. Cancer Res. 2015;75:4400–4400. [Google Scholar]
- 137.Rochon LS, Devarakonda K, Martinez J, Williams K, Hamilton EP, Shapiro G, Sukari A. Abstract CT230: A phase I first in human dose escalation trial of MNK-010 in subjects with advanced solid tumors. Cancer Res. 2015;75:CT230–CT230. [Google Scholar]
- 138.https://www.epo.org/learningevents/europeannventor/finalists/2013/couvreur/feature.htm.
- 139.http://www.natap.org/2014/AASLD/AASLD_153.htm. Phase II, dose ranging study of ARC-520, a siRNA-based therapeutic, in patients with chronic hepatitis B virus infection.
- 140.Stella B, Arpicco S, Rocco F, Burgalassi S, Nicosia N, Tampucci S, Chetoni P, Cattel L. Nonpolymeric nanoassemblies for ocular administration of acyclovir: pharmacokinetic evaluation in rabbits. Eur J Pharm Biopharm. 2012;80:39–45. doi: 10.1016/j.ejpb.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 141.Pili B, Reddy LH, Bourgaux C, Lepetre-Mouelhi S, Desmaele D, Couvreur P. Liposomal squalenoyl-gemcitabine: formulation, characterization and anticancer activity evaluation. Nanoscale. 2010;2:1521–6. doi: 10.1039/c0nr00132e. [DOI] [PubMed] [Google Scholar]
- 142.Couvreur P, Stella B, Reddy LH, Hillaireau H, Dubernet C, Desmaele D, Lepetre-Mouelhi S, Rocco F, Dereuddre-Bosquet N, Clayette P, Rosilio V, Marsaud V, Renoir JM, Cattel L. Squalenoyl nanomedicines as potential therapeutics. Nano Lett. 2006;6:2544–8. doi: 10.1021/nl061942q. [DOI] [PubMed] [Google Scholar]
- 143.Borrelli S, Christodoulou MS, Ficarra I, Silvani A, Cappelletti G, Cartelli D, Damia G, Ricci F, Zucchetti M, Dosio F, Passarella D. New class of squalene-based releasable nano-assemblies of paclitaxel, podophyllotoxin, camptothecin and epothilone A. Eur J Med Chem. 2014;85:179–190. doi: 10.1016/j.ejmech.2014.07.035. [DOI] [PubMed] [Google Scholar]
- 144.Cheikh-Ali Z, Caron J, Cojean S, Bories C, Couvreur P, Loiseau PM, Desmaële D, Poupon E, Champy P. Squalenoyl-curcumin” Nanoassemblies as Water-Dispersible Drug Candidates with Antileishmanial Activity. ChemMedChem. 2015;10:411–418. doi: 10.1002/cmdc.201402449. [DOI] [PubMed] [Google Scholar]
- 145.Raouane M, Desmaele D, Gilbert-Sirieix M, Gueutin C, Zouhiri F, Bourgaux C, Lepeltier E, Gref R, Ben Salah R, Clayman G, Massaad-Massade L, Couvreur P. Synthesis, characterization, and in vivo delivery of siRNA-squalene nanoparticles targeting fusion oncogene in papillary thyroid carcinoma. J Med Chem. 2011;54:4067–76. doi: 10.1021/jm2000272. [DOI] [PubMed] [Google Scholar]
- 146.Lepeltier E, Bourgaux C, Amenitsch H, Rosilio V, Lepetre-Mouelhi S, Zouhiri F, Desmaele D, Couvreur P. Influence of the nanoprecipitation conditions on the supramolecular structure of squalenoyled nanoparticles. Eur J Pharm Biopharm. 2015;96:89–95. doi: 10.1016/j.ejpb.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 147.Lansakara-P DSP, Rodriguez BL, Cui Z. Synthesis and in vitro evaluation of novel lipophilic monophosphorylated gemcitabine derivatives and their nanoparticles. Int J Pharm. 2012;429:123–134. doi: 10.1016/j.ijpharm.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]