Abstract
Background
Excess adipose tissue has been implicated in the pathogenesis of insulin resistance and atherosclerosis, and is a key risk factor for blood pressure (BP) elevation. However, circulating levels of adiponectin, a protein produced by adipose tissue and widely implicated in the pathogenesis of insulin resistance and atherosclerosis, are inversely proportional to adiposity. The relationship between adiponectin and incident hypertension has not been determined in the general U.S. population.
Methods
Normotensive participants (n = 1,233) enrolled in the Dallas Heart Study, a multiethnic, probability-based population sample of Dallas County adults were followed for median of 7 years. Retroperitoneal, intraperitoneal, visceral, and subcutaneous adipose tissue were measured at baseline by magnetic resonance imaging. Liver fat content was measured by 1H-magnetic resonance spectroscopy. Relative risk regression was used to determine the association of adiponectin with incident hypertension after adjustment for age, race, sex, BMI, smoking, diabetes, baseline systolic BP, total cholesterol, as well as regional fat depot.
Results
Of the 1,233 study participants (median age 40, 40% black, 56% women), 391 (32%) had developed hypertension over a median follow up of 7 years. Adiponectin levels were associated with reduced risk of incident hypertension (RR 0.81, 95% CI [0.68 – 0.96]) in the fully adjusted model, which included liver fat. Similar results were observed after adjustment for subcutaneous or visceral fat depots when tested individually or simultaneously in the model.
Conclusion
Our study suggested a protective role of adiponectin against incident hypertension independent of body fat distribution.
Keywords: Hypertension, Adiponectin, Leptin, Visceral Adipose Tissue, Fat, Subcutaneous Adipose Tissue
Introduction
Hypertension is projected to afflict 30% of the global population by the year 2025 [1]. Obesity, particularly visceral obesity, is an increasingly common risk factor for hypertension [2–4]. Adipose tissue secretes a number of proteins and hormones, including adiponectin (APN), leptin, and interleukin-6, which have been implicated in the pathogenesis of insulin resistance and inflammation. Compared with subcutaneous fat tissue (SAT), visceral adipose tissue (VAT) secretes significantly less APN [5–7] and leptin [8, 9] which may explain a greater correlation between these adipokines with SAT than VAT. In preclinical models, APN conveys antihypertensive properties while leptin was shown to have the opposite action [10–12]. High circulating APN is associated with a lower risk of hypertension, while high levels of leptin are associated with increased burden of hypertension in several population-based studies in Europe and Asia[13]. However, none of these studies were conducted in ethnically diverse adults in the United States. Furthermore, the confounding influence of VAT, which may play a direct role in the subsequent development of hypertension or indirectly via altering adipokine secretion, was not determined. Accordingly, we examined the relationship between baseline adiponectin and leptin levels and incident hypertension in participants of the Dallas Heart Study, a multiethnic probability-based population sample of Dallas County adults. We also determined the association between these 2 adipokines and incident hypertension after adjustment for SAT, VAT, and liver fat, using the highly sensitive techniques of magnetic resonance imaging and 1H-magnetic resonance spectroscopy.
Materials and Methods
Study Population
The Dallas Heart Study is a multiethnic, probability-based cohort study of Dallas County adults (ages 18 to 65 years at study entry), with deliberate oversampling of African-American participants[14]. As shown in Figure 1, the current study population was drawn from 2,743 participants who completed DHS phase 1 (DHS-1) from 2000 to 2002, which included blood pressure (BP) measurements, laboratory testing, abdominal magnetic resonance imaging (MRI), and 1H-magnetic resonance spectroscopy. Those with baseline hypertension, defined as systolic blood pressure (SBP) ≥ 140 mm Hg or diastolic blood pressure (DBP) ≥ 90 mm Hg, or on antihypertensive treatment for hypertension, were excluded. Participants with borderline BP elevations at baseline (SBP ≥ 130 or DBP ≥ 85 mm Hg) were also excluded to avoid minimal increases in BP meeting the incident hypertension definition. Participants with baseline known CVD were excluded. After these exclusions, 1774 participants were eligible for follow-up. Of these, 1,233 completed all 3 visits of DHS-1 and returned for DHS phase 2 (DHS-2), which consisted of follow-up studies during a single visit between 2007 and 2009. This comprised the current study population. There were no significant differences in medical history, demographics, or biomarker data between eligible participants who did and did not complete DHS-2[15]. All participants provided written informed consent, and the University of Texas-Southwestern Medical Center institutional review board approved the protocol.
Figure 1.
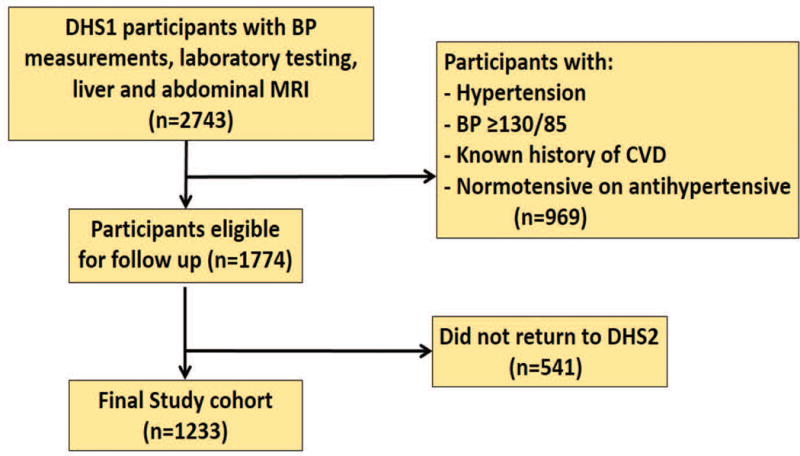
Flow diagram of study cohort
Hypertension Definition
Trained professionals obtained BP measurements after 5 min of rest in the seated position using an automated oscillometric device (Series #52,000, Welch Allyn, Arden, North Carolina). The last three of five total measurements were averaged. In both phases of the DHS, hypertension was defined as SBP ≥140 mm Hg, DBP ≥ 90 mm Hg, or the participant taking any antihypertensive medications.
Abdominal Fat Quantification
All participants in our current cohort (n = 1233) were scanned at their baseline exam by a 1.5-T MRI scanner (Intera, Philips Healthcare, Best, the Netherlands). Retroperitoneal, intraperitoneal, and SAT abdominal fat masses were quantified by a single MRI slice taken at the L2–L3 level as previously described [16]. Areas were converted to mass (kg) as previously described [17]. VAT was defined as the combination of both retroperitoneal and intraperitoneal fat masses to express the total intra-abdominal fat mass[15, 18]. A subset of our subjects (n = 1101) also underwent 1H-magnetic resonance spectroscopy for hepatic triglyceride quantification, as previously described [19].
Biomarker Analysis
Fasting blood samples were collected in tubes containing EDTA and were maintained at 4°C for <4 h. Plasma aliquots were then removed and stored at −80°C until assays were performed. Samples were analyzed for high-sensitivity C-reactive protein (hs-CRP), MMP 9 (matrix metallopeptidase 9), sRAGE (soluble receptor of advanced glycation end products), VEGF (Vascular endothelial growth factor) interleukin (IL)-6, cystatin-C, N-terminal pro–B-type natriuretic peptide (NT-pro-BNP), adiponectin, leptin, fasting glucose, and insulin levels (supplemental appendix).
Statistical Analysis
Baseline demographic, clinical, laboratory, and imaging variables are expressed as mean ± standard deviation, median (25th, 75th percentile) or proportions, as appropriate. Differences in characteristics between participants who remained normotensive and those who developed hypertension were compared using chi-square tests or the Wilcoxon rank sum test. Multivariable relative risk regression models with a log link and binomial error distribution were created to analyze associations between baseline adiponectin as well as leptin and incident hypertension while adjusting for age, sex, race/ethnicity, history of smoking, diabetes mellitus, baseline SBP and individual measures of adiposity. Race and sex-specific adiponectin (supplemental appendix table 2) and leptin levels were used in quintile analysis. Correlation coefficients for select confounding variables are shown in the online appendix. Interactions were also tested in the fully adjusted model to assess for differential relationships between adiponectin and incident hypertension by sex (male vs female), and race (black vs. non-black). Two-sided p values <0.05 were considered significant. All analyses were performed using SAS version 9.4 (SAS Corporation, Cary, North Carolina).
Results
Among 1,233 initially normotensive participants (median age, 40 years at study entry; 56% women; 40% black), 391 (32%) had developed hypertension after a median follow up of 7 years. Baseline characteristics of participants who remained normotensive (NT, n=842) and those who became hypertensive (HT, n=391) are shown in Table 1. Compared with the NT group, participants who developed hypertension at follow up were older, had higher SBP, DBP, BMI, WC, fasting plasma glucose (FPG), total cholesterol (TC), triglycerides (TG), VAT, SAT and Liver fat (Table 1). Median adiponectin among NT subjects was 7.7μg/ml and among those who developed hypertension was 6.7 μg/ml (p < 0.05). Median leptin was 9.5ng/ml among NT subjects and 15.7 ng/ml among those who developed hypertension Table 2 shows baseline characteristics stratified by adiponectin quintiles. Higher levels of baseline adiponectin were associated with lower levels of leptin, BMI, WC, VAT, SAT, RP fat, IP fat Liver fat, TC, TG, FPG, HOMA-IR, and hs-CRP; and higher age, alcohol use, and levels of NT-proBNP (Table 2). Adiponectin levels were inversely correlated with hs-CRP (supplemental appendix table 1, r = −0.22, p< 0.05), MMP-9 (r = −0.15, p < 0.05), and ICAM-1 (−0.07, p <0.05); and were positively correlated with NT-proBNP (r = 0.38, p <0.05) and sRAGE (r = 0.18, p< 0.05). Adiponectin was not correlated with levels of VCAM-1, leptin, IL-6, or VEGF (supplemental appendix table 1)
Table 1.
Baseline Characteristics
| Variable | NT (n = 842) | HT (n= 391) | * p value |
|---|---|---|---|
| Adiponectin, (μg/ml)† | 7.7 (5.0, 10.6) | 6.7 (4.6, 9.2) | <0.01 |
| Leptin, (ng/mL)† | 9.5 (4.2, 19.9) | 15.7 (6.1, 30.0) | <0.01 |
| Age (yrs) | 41±9 | 45 ± 9 | <0.01 |
| Male (%) | 45% | 40% | <0.01 |
| Black (%) | 34% | 51% | <0.01 |
| SBP (mmHg) | 116 ±10 | 127 ±12 | <0.01 |
| DBP (mmHg) | 73± 7 | 80 ± 7 | <0.01 |
| BMI (kg/m2) | 28 ± 6 | 31 ± 7 | <0.01 |
| WC (inches) | 93 ±14 | 100 ±17 | <0.01 |
| VAT (kg) | 1.9 ± 0.9 | 2.2 ±1.0 | <0.01 |
| RPfat (kg) | 0.7 ± 0.4 | 0.9 ±0.5 | <0.01 |
| IP fat(kg) | 1.2 ± 0.6 | 1.4 ±0.8 | <0.01 |
| SAT (kg) | 4.2 ± 2.5 | 5.4 ± 3.2 | <0.01 |
| Liver fat (%) | 4 ± 5 | 6 ± 6 | <0.01 |
| HOMA-IR | 3.1 ±3.5 | 4.2 ± 4.3 | <0.01 |
| Fasting glucose (mg/dl) | 93 ±28 | 99 ± 35 | <0.01 |
| Total cholesterol (mg/dl) | 178 ± 38 | 182 ± 39 | 0.09 |
| HDL (mg/dl) | 51 ± 6 | 50 ± 15 | 0.29 |
| TG, (mg/dL)† | 87 (63, 124) | 98 (68, 149) | <0.01 |
| hs-CRP (mg/dL)† | 1.9 (0.8, 4.4) | 2.6 (1.2, 6.9) | <0.01 |
| NT-proBNP (pg/ml)† | 26.2 (12.7, 49.5) | 25.9 (11.1, 54.4) | 0.84 |
| MCP-1 (pg/ml)† | 164.7 (121.1, 219.4) | 157 (117, 215) | 0.12 |
| IL-6 (pg/ml) | 16.8 (0, 35.8) | 17.0 (0, 32.4) | 0.85 |
| IL-18 (pg/ml) | 477 (338, 713) | 498 (337, 721) | 0.62 |
| Alcohol, g/w† | 6 (0, 39) | 4.3 (0, 39) | 0.54 |
| GFR (ml/min) | 99 ±20 | 101 ± 21 | 0.33 |
p value comparing 2 groups
Median (interquartile range). All other values are mean ± standard deviation or n (%)
BMI = body mass index; DBP = diastolic blood pressure; GFR = glomerular filtration rate; HDL = high-density lipoprotein; HOMA-IR = homeostatic model assessment of insulin resistance; hs-CRP = high-sensitivity C-reactive protein; IHT = incident hypertension; IL-6 = interleukin-6; IL -18 = interleukin 18; IP = intraperitoneal; LDL = low-density lipoprotein; MCP-1 = monocyte chemoattractant protein- 1; NT = normotension; NT-proBNP = N-terminal pro–B-type natriuretic peptide; RP = retroperitoneal; SAT = subcutaneous adipose tissue; SBP = systolic blood pressure, VAT = visceral adipose tissue; WC = waist circumference.
Table 2.
Baseline Characteristics by Age and Sex-Specific Adiponectin Quintiles
| Variable | Q1 | Q2 | Q3 | Q4 | Q5 | * p-trend |
|---|---|---|---|---|---|---|
| Adiponectin, ug/ml† | 3.45 (2.78, 4.14) | 5.3 (4.5, 6.1) | 7.1 (6.2, 8.2) | 9.2 (8.2,10.6) | 13.3 (11.0,17.0) | <0.05 |
| Leptin, ng/mL† | 14.9 (6.3,29.3) | 13.8 (5.4, 25.7) | 11.5 (5.2,3.8) | 9.0 (4.1,18.3) | 7.8 (3.1, 17.3) | <0.05 |
| Age, yrs | 40 ± 9 | 42 ± 8 | 42 ± 8 | 42 ± 9 | 44 ± 9 | <0.05 |
|
| ||||||
| Male, n (%) | 105 (43) | 108 (43) | 108 (44) | 108 (43) | 107 (44) | 0.9 |
| Black, n (%) | 96 (40) | 98 (39) | 97 (39) | 98 (39) | 97 (39) | 0.9 |
| SBP, mm Hg | 120 ± 11 | 120 ± 12 | 119 ± 12 | 119 ± 12 | 120 ± 13 | 0.5 |
| DBP, mm Hg | 76 ±7 | 75 ± 8 | 75 ± 9 | 75 ± 8 | 75 ± 8 | 0.3 |
|
| ||||||
| BMI, kg/m2 | 33 ± 7 | 31 ± 7 | 29 ± 7 | 27 ± 6 | 26 ± 6 | <0.05 |
|
| ||||||
| WC, cm | 102 ± 16 | 99 ± 15 | 96 ± 15 | 91 ± 1.5 | 88 ± 13 | <0.05 |
|
| ||||||
| VAT, kg | 2.4 ± 1.0 | 2.2 ± 0.9 | 2.1 ± 1.0 | 1.8 ± 0.9 | 1.6 ± 0.8 | <0.05 |
| RP fat, kg | 0.9 ± 0.5 | 0.9 ± 0.4 | 0.8 ± 0.4 | 0.7 ± 0.3 | 0.7 ± 0.6 | <0.05 |
| IP fat, kg | 1.4 ± 0.8 | 1.4 ± 0.6 | 1.3 ± 0.6 | 1.1 ± 0.6 | 0.9 ± 0.8 | <0.05 |
| SAT, kg | 5.6 ± 3 | 5.1 ± 2.8 | 4.8± 23.0 | 3.8 ± 2.3 | 3.5 ± 2.2 | <0.05 |
| Liver fat, % | 8 ± 7 | 6 ± 6 | 5 ± 6 | 4 ± 4 | 3 ± 3 | <0.05 |
| HOMA_IR | 5.1 ± 4.4 | 3.8 ±3.1 | 3.4± 3.9 | 2.6 ± 2.7 | 2.5 ± 4.2 | <0.05 |
|
| ||||||
| FPG, mg/dL | 102 ± 43 | 97 ± 31 | 93 ± 17 | 93 ± 35 | 90 ±14 | <0.05 |
|
| ||||||
| TC, mg/dL | 181 ± 41 | 181 ± 39 | 177 ± 36 | 178 ± 37 | 180 ± 38 | 0.9 |
| HDL-C, mg/dL | 44 ± 14 | 46 ± 11 | 50 ± 13 | 56 ± 15 | 59 ± 17 | <0.05 |
| TG, mg/dL† | 111 (70, 79) | 106 (70, 156) | 88(64, 122) | 81(60,116) | 78(56,107) | <0.05 |
| hs-CRP, mg/dL† | 3.7 (1.5, 7.4) | 2.7(1.1,5.9) | 2.2(0.8,5.8) | 1.6(0.7,3.8) | 1.3(0.6,2.8) | <0.05 |
| NT-proBNP, pg/ml† | 17.1 (8.1, 41.3) | 23.3 (10.0, 41.2) | 24.9 (12.6,44.5) | 29.5(14.6,58.9) | 39.3(18,76.5) | <0.05 |
|
| ||||||
| MCP-1, pg/ml† | 159 (124, 219) | 175 (124, 227) | 167 (124, 218) | 156 (111, 211) | 165(119, 214) | 0.4 |
|
| ||||||
| IL-6, pg/ml† | 156 (0, 32.0) | 17.3 (0, 30.7) | 18.8 (0, 41.1) | 16.2(0, 35.8) | 17.3 (0, 35.6) | 0.17 |
| Alcohol, g/w† | 4(0,30) | 3(0, 39) | 8 (1,39) | 8(0,59) | 6(0,59) | <0.05 |
|
| ||||||
| GFR, ml/min | 102 ± 20 | 101 ± 20 | 97 ± 18 | 99 ± 21 | 101 ± 22 | 0.1 |
p trend comparing 5 groups
Median (interquartile range). All other values are mean ± standard deviation or n
BMI = body mass index; DBP = diastolic blood pressure; FPG = fasting plasma glucose; GFR = glomerular filtration rate; HDL = high-density lipoprotein; HOMA-IR = homeostatic model assessment of insulin resistance; hs-CRP = high-sensitivity C-reactive protein; IHT = incident hypertension; IL-6 = interleukin-6; IP = intraperitoneal; LDL = low-density lipoprotein; MCP-1 = monocyte chemoattractant protein- 1; NT = normotension; NT-proBNP = N-terminal pro–B-type natriuretic peptide; RP = retroperitoneal; SAT = subcutaneous adipose tissue; SBP = systolic blood pressure; TC= total cholesterol; VAT = visceral adipose tissue; WC = waist circumference.
Multivariable regression models showed an inverse relationship between baseline adiponectin levels and the risk of incident hypertension after adjusting for potential confounders, including sex, race, baseline age, smoking, diabetes, BMI, total cholesterol, systolic BP, hs-CRP, and NT-pro BNP (model 3, Table 3). This association was also statistically significant in models that added VAT, SAT, IP fat, RP fat, liver fat individually to this baseline model (Table 3 and Fig. 2). When SAT and VAT were simultaneously added to the model 3, the association between adiponectin and incident hypertension remained unchanged (RR 0.84; 95% CI; 0.72 – 0.98). Similarly, when MMP-9, ICAM-1, and sRAGE were simultaneously entered in the model 3 along with VAT, higher levels of adiponectin continued to be associated with lower risk of hypertension (RR 0.79; 95% CI; 0.66 – 0.94). There was a graded reduction in the risk of incident hypertension with increasing race- and sex- specific adiponectin quintiles, which was significant in the fourth and fifth adiponectin quintiles (Fig. 2). In contrast, the association of leptin with incident hypertension seen in the unadjusted model was completely attenuated after adjusting for either BMI or body fat (Table 4 and Fig. 3).
TABLE 3.
Relative risk of developing hypertension per 1 S.D. higher baseline adiponectin
| RR (95%CI) | p- value | |
|---|---|---|
| Unadjusted | 0.77 (0.67 – 0.88) | <0.05 |
| Model 1 | 0.85 (0.73 – 0.99) | <0.05 |
| Model 2 | 0.83 (0.72 – 0.96) | <0.05 |
| Model 3 | 0.81 (0.70 – 0.93) | <0.05 |
| Model 4 | ||
| RP fat (kg) | 0.82 (0.71 – 0.94) | <0.05 |
| IP fat (kg) | 0.82 (0.71– 0.95) | <0.05 |
| VAT fat (kg) | 0.83 (0.72 – 0.96) | <0.05 |
| SAT fat (kg) | 0.81 (0.70 – 0.93) | <0.05 |
| Liver Fat (%) | 0.80 (0.68 – 0.94) | <0.05 |
RR = Relative Risk, CI = Confidence Interval, RP=Retroperitoneal, IP = Intraperitoneal, VAT= Visceral Adipose Tissue, SAT= Subcutaneous Adipose Tissue
Model 1 = age, sex, race, BMI
Model 2 = Model 1 + smoking history, diabetes, baseline systolic BP, total cholesterol
Model 3 = Model 2 + hs-CRP, NT-proBNP
Model 4 = Model 3 + RP fat, IP fat, VAT, SAT, Liver fat added individually
Figure 2.
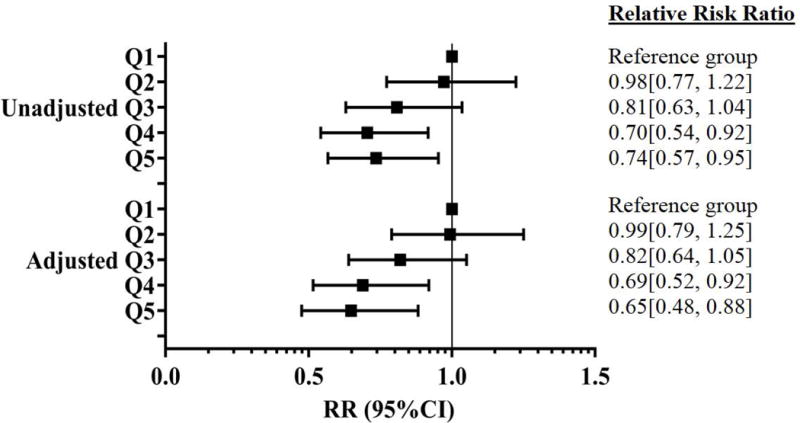
Forest plot of relative risk for incident hypertension according to baseline adiponectin quintiles in the whole population after adjustment for liver fat, clinical, and relevant variables (Age, smoking, diabetes, BMI, total cholesterol, baseline systolic BP, hs-CRP, NT-pro BNP)
TABLE 4.
Relative risk of developing hypertension per 1-S.D. higher baseline leptin
| RR (95%CI) | p- value | |
|---|---|---|
| Unadjusted | 1.25 (1.15 – 1.36) | <0.05 |
| Model 1 | 1.06 (0.94 – 1.19) | 0.33 |
| Model 2 | 1.06 (0.93 – 1.20) | 0.39 |
| Model 3 | 1.06 (0.93 – 1.21) | 0.35 |
| Model 4 | ||
| RP fat (kg) | 1.05 (0.92 – 1.19) | 0.47 |
| IP fat (kg) | 1.04 (0.91 – 1.19) | 0.57 |
| VAT fat (kg) | 1.02 (0.90 – 1.17) | 0.72 |
| SAT fat (kg) | 1.06 (0.93 – 1.21) | 0.39 |
| Liver Fat (%) | 1.03 (0.90 – 1.18) | 0.68 |
RR = Relative Risk, CI = Confidence Interval, RP=Retroperitoneal, IP = Intraperitoneal, VAT= Visceral Adipose Tissue, SAT= Subcutaneous Adipose Tissue
Model 1 = age, sex, race, BMI
Model 2 = Model 1 + smoking history, diabetes, baseline systolic BP, HDL cholesterol, total cholesterol
Model 3 = Model 2 + hs-CRP, NT-proBNP
Model 4 = Model 3 + RP fat, IP fat, VAT, SAT, Liver fat added individually
Figure 3.
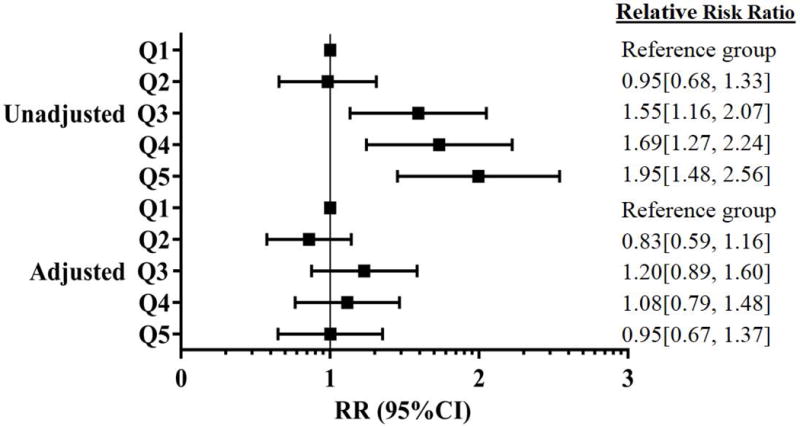
Forest plot for relative risk for incident hypertension according to baseline leptin quintiles in the whole population after adjustment for liver fat, clinical, and relevant variables (age, sex, race, smoking, diabetes, BMI, HDL cholesterol, total cholesterol, baseline systolic BP, hs-CRP, NT-pro BNP)
There were no significant subgroup interactions for the association between adiponectin and incident hypertension in subgroups defined by age, sex, race, diabetes, and BMI (All interaction p-values > 0.1 except for age where interaction p-value = 0.09).
Discussion
The major findings from this study are three fold. First, circulating adiponectin levels is associated with reduced risk of future development of hypertension in a multiethnic, probability-based population sample of Dallas county adults. Second, this association persisted after further adjustment for visceral or subcutaneous fat depots, suggesting an adiposity-independent antihypertensive property of adiponectin. Third, no association with hypertension was seen with leptin, another major peptide produced by adipose.
Adiponectin is secreted by adipose tissue and has been implicated in the pathogenesis of insulin resistance and hypertension[13, 20–23]. Studies in genetically obese KKAy mice demonstrated lower plasma adiponectin levels and higher BP than the control mice. Hypertension in the obese KKAy mice was ameliorated by delivery of adenovirus expressing adiponectin[10]. Adiponectin-deficient mice developed hypertension despite having normal body weight and insulin sensitivity, suggesting direct antihypertensive action of adiponectin independent of obesity[10]. Mechanisms underlying BP lowering effects of adiponectin is unknown but expression of vascular endothelial nitric oxide (NO) synthase (eNOS) and prostaglandin I2 synthase may play a role[10]. More recently, adiponectin knockout mice were shown to display both elevated resting heart rate and 24-hour urinary epinephrine excretion, suggesting sympatho-inhibitory action of adiponectin[11]. Adiponectin may also protect against incident hypertension through its anti-inflammatory effects [24]. C-reactive protein (CRP), an acute phase reactant, has been shown to increase BP in mice[25, 26] and has been linked to increased risk of incident hypertension in humans[27–30]. Consistent with the anti-inflammatory properties of adiponectin, we found that hs-CRP was inversely correlated with adiponectin in our study (r = −0.22, p <0.01).
Despite multiple studies showing a BP lowering property of adiponectin in rodents, the evidence in humans is less consistent. While prospective studies in Chinese and Japanese populations indicated that higher levels of adiponectin were associated with a lower risk of hypertension [31, 32], studies from Denmark [33, 34] and Turkey [35] failed to confirm these findings. The only study conducted in the U.S., the Women’s Health Initiative-Observational Study (WHI-OS), showed an inverse relationship between baseline adiponectin level quartiles and incident hypertension in black but not white postmenopausal women after adjusting for body mass index and relevant risk factors[36]. However, none of these previous studies were conducted in an ethnically diverse US adult men and women. Furthermore, none of these previous studies specifically addressed the relationship between adiponectin and BP after accounting for regional fat accumulation. This is knowledge gap is particularly important as a recent analysis indicated that visceral adiposity, but not total or subcutaneous adiposity, was associated with subsequent development of hypertension[37].
Leptin is another major adipokine involved in energy homeostasis. In contrast to APN, leptin levels were increased in human obesity and reduced after weight loss[24, 38]. Leptin promotes hypertension in rodents via stimulation of sympathetic nervous system through activation of leptin receptors in the hypothalamic neurons[11, 12]. Leptin promotes neointimal hyperplasia via vascular leptin receptors, which may further contribute to elevated BP [39, 40]. Like APN, leptin stimulates NO production in the vascular cells by increasing eNOS phosphorylation and activation[41], which may negate its hypertensive action via sympathetic stimulation. Leptin is a significant predictor of incident hypertension in several population studies from Italy and Denmark[33, 34, 42]. Similarly, one study in older Caucasian Americans demonstrated increased odds of incident hypertension after adjusting for age, BMI, systolic BP, total cholesterol, medications, and cardiovascular disease [43]. However, the results of these studies have been challenged by a recent small study in patients with lipodystrophy showing no significant change in BP after short-term treatment with recombinant human leptin [44]. Our study extends this previous observation in lipodystrophy to a larger and more diverse general population sample, including 40% African Americans, the population with the highest burden of hypertension which has not been examined in prior prospective observational studies.
The strengths of our study include the measurement of adipokines in a large, multiethnic cohort, which significantly improves generalizability to the US population compared with prior studies. The precise measurement of several visceral fat compartments using magnetic resonance imaging and 1H-magnetic resonance spectroscopy allows us to explore the role of these adipokines on incident hypertension beyond measurement of BMI alone. Our study is also limited by several factors. Although adiponectin exists as high molecular weight, low molecular weight, and trimer forms[45–48], only total adiponectin was measured in this study. The observational design precludes any conclusions about causality. Furthermore, adiponectin and leptin were measured only once, limiting any ability to draw conclusions about how longitudinal changes in adiponectin affects incident hypertension. Although our study was not designed with statistical power to formally test for potential interaction by race/ethnicity, the point estimates for association between adiponectin with incident hypertension were similar for Black vs Non-Black participants. Furthermore, the interaction p value for incident hypertension of blacks vs. non-blacks subgroup is not significant (p > 0.1). Thus, we believe that the relationship of adiponectin and incident hypertension is independent of race/ethnicity but needs to be further confirmed in the larger cohorts.
In conclusion, our study suggests a protective role of adiponectin against the future development of hypertension, independent of body fat distribution. Further studies are needed to corroborate the findings from this study and to determine whether the association noted is evident as well for incident hypertension defined on the basis of ambulatory blood pressure measurements.
Supplementary Material
Acknowledgments
We want to thank all patients and medical staff members who participated in the Dallas Heart Study
Sources of Funding: Dr. Vongpatanasin is supported by the UT Southwestern O’Brien Kidney Center and the Kaplan Chair in Hypertension Research. The Dallas Heart Study was funded by the Donald W. Reynolds Foundation and was partially supported award by Award #UL1TR001105 from the National Center for Advancing Translational Sciences of the National Institutes of Health.
Footnotes
Disclosure Statement: The authors have nothing to disclose
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365(9455):217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Grootveld LR, Van Valkengoed IG, Peters RJ, Ujcic-Voortman JK, Brewster LM, Stronks K, Snijder MB. The role of body weight, fat distribution and weight change in ethnic differences in the 9-year incidence of hypertension. Journal of hypertension. 2014;32(5):990–6. doi: 10.1097/HJH.0000000000000135. discussion 996-7. [DOI] [PubMed] [Google Scholar]
- 3.Cassano PA, Segal MR, Vokonas PS, Weiss ST. Body fat distribution, blood pressure, and hypertension. A prospective cohort study of men in the normative aging study. Annals of epidemiology. 1990;1(1):33–48. doi: 10.1016/1047-2797(90)90017-m. [DOI] [PubMed] [Google Scholar]
- 4.Villarreal-Molina MT, Antuna-Puente B. Adiponectin: anti-inflammatory and cardioprotective effects. Biochimie. 2012;94(10):2143–9. doi: 10.1016/j.biochi.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Lihn AS, Bruun JM, He G, Pedersen SB, Jensen PF, Richelsen B. Lower expression of adiponectin mRNA in visceral adipose tissue in lean and obese subjects. Molecular and cellular endocrinology. 2004;219(1–2):9–15. doi: 10.1016/j.mce.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Meyer LK, Ciaraldi TP, Henry RR, Wittgrove AC, Phillips SA. Adipose tissue depot and cell size dependency of adiponectin synthesis and secretion in human obesity. Adipocyte. 2013;2(4):217–26. doi: 10.4161/adip.24953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drolet R, Belanger C, Fortier M, Huot C, Mailloux J, Legare D, Tchernof A. Fat depot-specific impact of visceral obesity on adipocyte adiponectin release in women. Obesity (Silver Spring, Md) 2009;17(3):424–30. doi: 10.1038/oby.2008.555. [DOI] [PubMed] [Google Scholar]
- 8.Minocci A, Savia G, Lucantoni R, Berselli ME, Tagliaferri M, Calo G, Petroni ML, de Medici C, Viberti GC, Liuzzi A. Leptin plasma concentrations are dependent on body fat distribution in obese patients. Int J Obes Relat Metab Disord. 2000;24(9):1139–44. doi: 10.1038/sj.ijo.0801385. [DOI] [PubMed] [Google Scholar]
- 9.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–3. doi: 10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 10.Ohashi K, Kihara S, Ouchi N, Kumada M, Fujita K, Hiuge A, Hibuse T, Ryo M, Nishizawa H, Maeda N, Maeda K, Shibata R, Walsh K, Funahashi T, Shimomura I. Adiponectin replenishment ameliorates obesity-related hypertension. Hypertension. 2006;47(6):1108–16. doi: 10.1161/01.HYP.0000222368.43759.a1. [DOI] [PubMed] [Google Scholar]
- 11.Kajimura D, Lee HW, Riley KJ, Arteaga-Solis E, Ferron M, Zhou B, Clarke CJ, Hannun YA, DePinho RA, Guo XE, Mann JJ, Karsenty G. Adiponectin regulates bone mass via opposite central and peripheral mechanisms through FoxO1. Cell metabolism. 2013;17(6):901–15. doi: 10.1016/j.cmet.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33(1 Pt 2):542–7. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- 13.Kim DH, Kim C, Ding EL, Townsend MK, Lipsitz LA. Adiponectin levels and the risk of hypertension: a systematic review and meta-analysis. Hypertension. 2013;62(1):27–32. doi: 10.1161/HYPERTENSIONAHA.113.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. The American journal of cardiology. 2004;93(12):1473–80. doi: 10.1016/j.amjcard.2004.02.058. [DOI] [PubMed] [Google Scholar]
- 15.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. Jama. 2012;308(11):1150–9. doi: 10.1001/2012.jama.11132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abate N, Burns D, Peshock RM, Garg A, Grundy SM. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. Journal of lipid research. 1994;35(8):1490–6. [PubMed] [Google Scholar]
- 17.Abate N, Garg A, Coleman R, Grundy SM, Peshock RM. Prediction of total subcutaneous abdominal, intraperitoneal, and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. The American journal of clinical nutrition. 1997;65(2):403–8. doi: 10.1093/ajcn/65.2.403. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi T, Boyko EJ, Leonetti DL, McNeely MJ, Newell-Morris L, Kahn SE, Fujimoto WY. Visceral adiposity is an independent predictor of incident hypertension in Japanese Americans. Annals of internal medicine. 2004;140(12):992–1000. doi: 10.7326/0003-4819-140-12-200406150-00008. [DOI] [PubMed] [Google Scholar]
- 19.Szczepaniak LS, Babcock EE, Schick F, Dobbins RL, Garg A, Burns DK, McGarry JD, Stein DT. Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. The American journal of physiology. 1999;276(5 Pt 1):E977–89. doi: 10.1152/ajpendo.1999.276.5.E977. [DOI] [PubMed] [Google Scholar]
- 20.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. The Journal of biological chemistry. 1995;270(45):26746–9. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature medicine. 2002;8(11):1288–95. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 22.Ehsan M, Singh KK, Lovren F, Pan Y, Quan A, Mantella LE, Sandhu P, Teoh H, Al-Omran M, Verma S. Adiponectin limits monocytic microparticle-induced endothelial activation by modulation of the AMPK, Akt and NFkappaB signaling pathways. Atherosclerosis. 2015;245:1–11. doi: 10.1016/j.atherosclerosis.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 23.van Stijn CM, Kim J, Barish GD, Tietge UJ, Tangirala RK. Adiponectin expression protects against angiotensin II-mediated inflammation and accelerated atherosclerosis. PloS one. 2014;9(1):e86404. doi: 10.1371/journal.pone.0086404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang ZV, Scherer PE. Adiponectin, cardiovascular function, and hypertension. Hypertension. 2008;51(1):8–14. doi: 10.1161/HYPERTENSIONAHA.107.099424. [DOI] [PubMed] [Google Scholar]
- 25.Vongpatanasin W, Thomas GD, Schwartz R, Cassis LA, Osborne-Lawrence S, Hahner L, Gibson LL, Black S, Samols D, Shaul PW. C-reactive protein causes downregulation of vascular angiotensin subtype 2 receptors and systolic hypertension in mice. Circulation. 2007;115(8):1020–8. doi: 10.1161/CIRCULATIONAHA.106.664854. [DOI] [PubMed] [Google Scholar]
- 26.Sundgren NC, Vongpatanasin W, Boggan BM, Tanigaki K, Yuhanna IS, Chambliss KL, Mineo C, Shaul PW. IgG receptor FcgammaRIIB plays a key role in obesity-induced hypertension. Hypertension. 2015;65(2):456–62. doi: 10.1161/HYPERTENSIONAHA.114.04670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Yang DH, Park HS, Cho Y, Lee WK, Chun BY, Chae SC. Incremental predictive value of high-sensitivity C-reactive protein for incident hypertension: the Hypertension-Diabetes Daegu Initiative study. Clinical and experimental hypertension (New York, NY : 1993) 2014;36(5):302–8. doi: 10.3109/10641963.2013.810236. [DOI] [PubMed] [Google Scholar]
- 28.Chuang SY, Hsu PF, Chang HY, Bai CH, Yeh WT, Pan HW. C-reactive protein predicts systolic blood pressure and pulse pressure but not diastolic blood pressure: the Cardiovascular Disease Risk Factors Two-Township Study. American journal of hypertension. 2013;26(5):657–64. doi: 10.1093/ajh/hps095. [DOI] [PubMed] [Google Scholar]
- 29.Kong H, Qian YS, Tang XF, Zhang J, Gao PJ, Zhang Y, Zhu DL. C-reactive protein (CRP) gene polymorphisms, CRP levels and risk of incident essential hypertension: findings from an observational cohort of Han Chinese. Hypertension research : official journal of the Japanese Society of Hypertension. 2012;35(10):1019–23. doi: 10.1038/hr.2012.89. [DOI] [PubMed] [Google Scholar]
- 30.Cheung BM, Ong KL, Tso AW, Leung RY, Xu A, Cherny SS, Sham PC, Lam TH, Lam KS. C-reactive protein as a predictor of hypertension in the Hong Kong Cardiovascular Risk Factor Prevalence Study (CRISPS) cohort. Journal of human hypertension. 2012;26(2):108–16. doi: 10.1038/jhh.2010.125. [DOI] [PubMed] [Google Scholar]
- 31.Chow WS, Cheung BM, Tso AW, Xu A, Wat NM, Fong CH, Ong LH, Tam S, Tan KC, Janus ED, Lam TH, Lam KS. Hypoadiponectinemia as a predictor for the development of hypertension: a 5-year prospective study. Hypertension. 2007;49(6):1455–61. doi: 10.1161/HYPERTENSIONAHA.107.086835. [DOI] [PubMed] [Google Scholar]
- 32.Imatoh T, Miyazaki M, Momose Y, Tanihara S, Une H. Adiponectin levels associated with the development of hypertension: a prospective study. Hypertension research : official journal of the Japanese Society of Hypertension. 2008;31(2):229–33. doi: 10.1291/hypres.31.229. [DOI] [PubMed] [Google Scholar]
- 33.Seven E, Husemoen LL, Wachtell K, Ibsen H, Linneberg A, Jeppesen JL. Overweight, adipocytokines and hypertension: a prospective population-based study. Journal of hypertension. 2014;32(7):1488–94. doi: 10.1097/HJH.0000000000000207. discussion 1494. [DOI] [PubMed] [Google Scholar]
- 34.Asferg C, Mogelvang R, Flyvbjerg A, Frystyk J, Jensen JS, Marott JL, Appleyard M, Jensen GB, Jeppesen J. Leptin, not adiponectin, predicts hypertension in the Copenhagen City Heart Study. American journal of hypertension. 2010;23(3):327–33. doi: 10.1038/ajh.2009.244. [DOI] [PubMed] [Google Scholar]
- 35.Onat A, Aydin M, Can G, Koroglu B, Karagoz A, Altay S. High adiponectin levels fail to protect against the risk of hypertension and, in women, against coronary disease: involvement in autoimmunity? World journal of diabetes. 2013;4(5):219–25. doi: 10.4239/wjd.v4.i5.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Manson JE, Gaziano JM, Liu S, Cochrane B, Cook NR, Ridker PM, Rifai N, Sesso HD. Plasma adiponectin and the risk of hypertension in white and black postmenopausal women. Clinical chemistry. 2012;58(10):1438–45. doi: 10.1373/clinchem.2012.191080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chandra A, Neeland IJ, Berry JD, Ayers CR, Rohatgi A, Das SR, Khera A, McGuire DK, de Lemos JA, Turer AT. The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. Journal of the American College of Cardiology. 2014;64(10):997–1002. doi: 10.1016/j.jacc.2014.05.057. [DOI] [PubMed] [Google Scholar]
- 38.Abbas A, Szczepaniak LS, Tuncel M, McGavock JM, Huet B, Fadel PJ, Wang Z, Arbique D, Victor R, Vongpatanasin W. Adiposity-independent sympathetic activity in black men. Journal of applied physiology. 2010;108(6):1613–8. doi: 10.1152/japplphysiol.00058.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schroeter MR, Schneiderman J, Schumann B, Gluckermann R, Grimmas P, Buchwald AB, Tirilomis T, Schondube FA, Konstantinides SV, Schafer K. Expression of the leptin receptor in different types of vascular lesions. Histochemistry and cell biology. 2007;128(4):323–33. doi: 10.1007/s00418-007-0319-1. [DOI] [PubMed] [Google Scholar]
- 40.Schafer K, Halle M, Goeschen C, Dellas C, Pynn M, Loskutoff DJ, Konstantinides S. Leptin promotes vascular remodeling and neointimal growth in mice. Arteriosclerosis, thrombosis, and vascular biology. 2004;24(1):112–7. doi: 10.1161/01.ATV.0000105904.02142.e7. [DOI] [PubMed] [Google Scholar]
- 41.Procopio C, Andreozzi F, Laratta E, Cassese A, Beguinot F, Arturi F, Hribal ML, Perticone F, Sesti G. Leptin-stimulated endothelial nitric-oxide synthase via an adenosine 5′-monophosphate-activated protein kinase/Akt signaling pathway is attenuated by interaction with C-reactive protein. Endocrinology. 2009;150(8):3584–93. doi: 10.1210/en.2008-0921. [DOI] [PubMed] [Google Scholar]
- 42.Galletti F, D’Elia L, Barba G, Siani A, Cappuccio FP, Farinaro E, Iacone R, Russo O, De Palma D, Ippolito R, Strazzullo P. High-circulating leptin levels are associated with greater risk of hypertension in men independently of body mass and insulin resistance: results of an eight-year follow-up study. The Journal of clinical endocrinology and metabolism. 2008;93(10):3922–6. doi: 10.1210/jc.2008-1280. [DOI] [PubMed] [Google Scholar]
- 43.Kramer CK, von Muhlen D, Barrett-Connor E. Does leptin predict incident hypertension in older adults? Clinical endocrinology. 2010;73(2):201–5. doi: 10.1111/j.1365-2265.2010.03781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown RJ, Meehan CA, Gorden P. Leptin Does Not Mediate Hypertension Associated With Human Obesity. Cell. 2015;162(3):465–6. doi: 10.1016/j.cell.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. The Journal of biological chemistry. 2003;278(41):40352–63. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 46.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. The Journal of biological chemistry. 2004;279(13):12152–62. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 47.Tonelli J, Li W, Kishore P, Pajvani UB, Kwon E, Weaver C, Scherer PE, Hawkins M. Mechanisms of early insulin-sensitizing effects of thiazolidinediones in type 2 diabetes. Diabetes. 2004;53(6):1621–9. doi: 10.2337/diabetes.53.6.1621. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circulation research. 2004;94(4):e27–31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


