Abstract
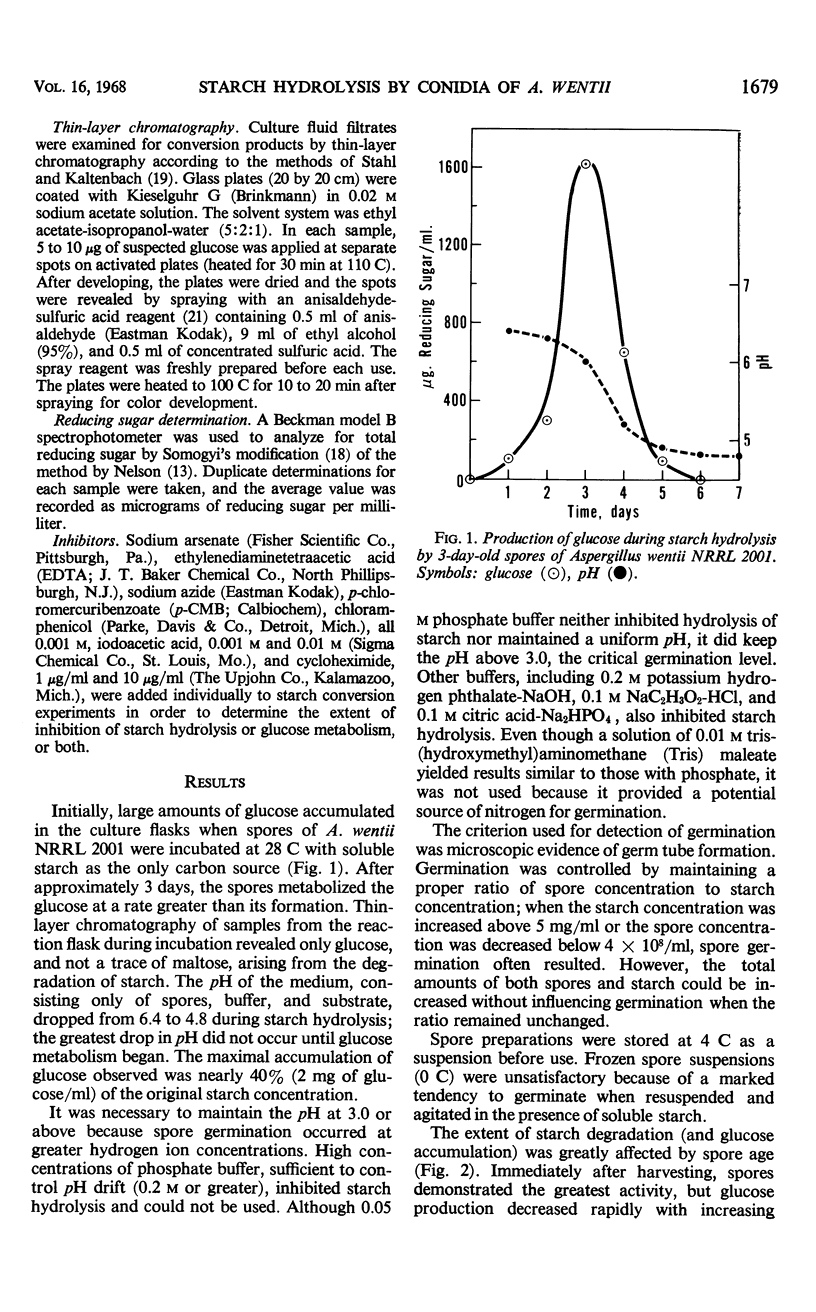
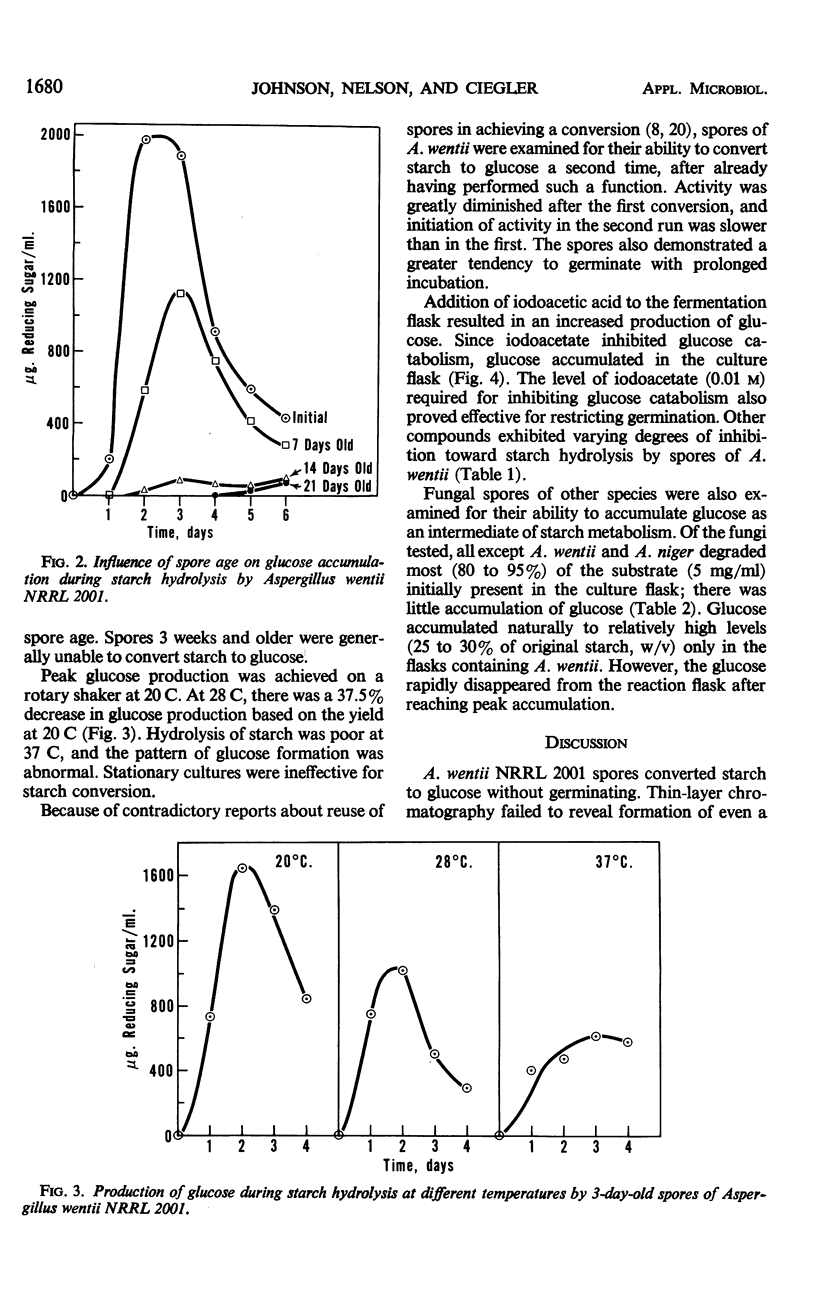
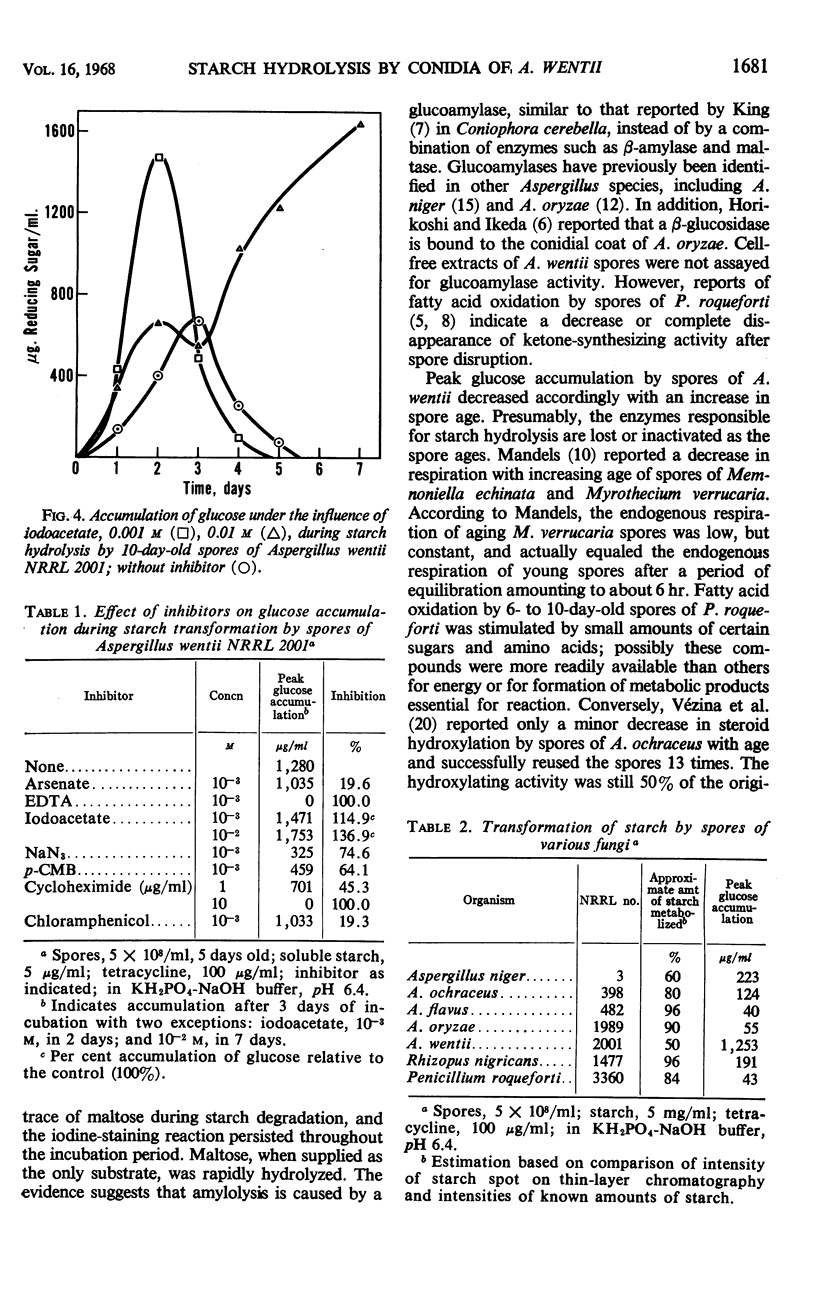
Soluble starch was hydrolyzed to glucose by conidia of Aspergillus wentii NRRL 2001. Peak yields of glucose were achieved in 3 days. A glucoamylase-like enzyme was assumed to be responsible since maltose was not detected during the conversion. Spore age, storage conditions, and temperature affected the level of glucose accumulated. Iodoacetate inhibited catabolism of the glucose formed and this inhibition increased product yield. Spores of other fungi also hydrolyzed starch but none accumulated glucose naturally as did A. wentii spores.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GEHRIG R. F., KNIGHT S. G. Fatty acid oxidation by spores of Penicillium roqueforti. Appl Microbiol. 1963 Mar;11:166–170. doi: 10.1128/am.11.2.166-170.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEHRIG R. F., KNIGHT S. G. Formation of ketones from fatty acids by spores of Penicillium roqueforti. Nature. 1958 Nov 1;182(4644):1237–1237. doi: 10.1038/1821237a0. [DOI] [PubMed] [Google Scholar]
- Horikoshi K., Ikeda Y. Studies on the spore coats of aspergillus oryzae. II. Conidia coat-bound beta-glucosidase. Biochim Biophys Acta. 1965 Nov 1;101(3):352–357. doi: 10.1016/0926-6534(65)90014-x. [DOI] [PubMed] [Google Scholar]
- King N. J. The glucoamylase of Coniophora cerebella. Biochem J. 1967 Nov;105(2):577–583. doi: 10.1042/bj1050577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R. C. The metabolism of triglycerides by spores of Penicillium roqueforti. J Gen Microbiol. 1967 Jan;46(1):65–70. doi: 10.1099/00221287-46-1-65. [DOI] [PubMed] [Google Scholar]
- Lawrence R. C. The oxidation of fatty acids by spores of penicillium roqueforti. J Gen Microbiol. 1966 Sep;44(3):393–405. doi: 10.1099/00221287-44-3-393. [DOI] [PubMed] [Google Scholar]
- Mandels G. R., Vitols R. Constitutive and induced trehalose transport mechanisms in spores of the fungus Myrothecium verrucaria. J Bacteriol. 1967 Jan;93(1):159–167. doi: 10.1128/jb.93.1.159-167.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAZUR J. H., ANDO T. The action of an amyloglucosidase of Aspergillus niger on starch and malto-oligosaccharides. J Biol Chem. 1959 Aug;234(8):1966–1970. [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Singh K., Rakhit S. Mechanism of side-chain degradation of C21 steroids by spores of Septomyxa affinis. Biochim Biophys Acta. 1967 Aug 8;144(1):139–144. doi: 10.1016/0005-2760(67)90086-0. [DOI] [PubMed] [Google Scholar]
- Singh K., Sehgal S. N., Vézina C. Transformation of steroids by Mucor griseo-cyanus. Can J Microbiol. 1967 Sep;13(9):1271–1281. doi: 10.1139/m67-172. [DOI] [PubMed] [Google Scholar]
- VEZINA C., SEHGAL S. N., SINGH K. Transformation of steroids by spores of microorganisms. I. Hydroxylation of progesterone by conidia of Aspergillus ochraceus. Appl Microbiol. 1963 Jan;11:50–57. doi: 10.1128/am.11.1.50-57.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]



