Abstract
OBJECTIVE
The objective of the study was to determine incontinence pessaries’ mechanism of action by measuring changes on urodynamic studies (UDS) and dynamic magnetic resonance imaging (MRI) with and without pessaries in place.
STUDY DESIGN
Women with stress incontinence had UDS and MRI performed with and without incontinence dish pessaries.
RESULTS
Fifteen women were evaluated. Pessary insertion resulted in increased urethral resistance; detrusor pressures increased (33–45 cm H2O) and maximal flow rates decreased (30 to 19 mL/second). With Valsalva on MRI, pessaries were associated with decreased posterior urethrovesical angles (175–130°), bladder neck elevation (0.3 below to 0.8 cm above the pubococcygeal line) and increased urethral lengths (2.4 to 2.97 cm). Bladder neck funneling with cough occurred in 14 patients without pessaries and 3 with pessaries.
CONCLUSION
On UDS and MRI following pessary placement, continence restoration was associated with decreased posterior urethrovesical angles, bladder neck descent and funneling, and increased urethral lengths and resistance to urine flow.
Keywords: magnetic resonance imaging, pessary, urinary incontinence, urodynamic studies
Stress urinary incontinence (SUI), involuntary urine loss that occurs with increased intraabdominal pressure,1 affects many women. A number of theories postulate why SUI occurs. Pessaries are among the few nonsurgical treatments of SUI. Pessary treatment is simple and can therefore serve as starting point in understanding the mechanisms underlying the restoration of continence.
Current theories of continence include Enhorning’s pressure transmission theory, the hammock hypothesis, and the integral theory.2–4 Enhorning’s theory postulates that the proximal urethra must be subject to intraabdominal pressure to close during cough or Valsalva, maintaining continence.2 The hammock hypothesis proposes that structures posterior to the urethra form a back-stop. When intraabdominal pressure increases, the urethra is compressed against this supportive tissue and continence is maintained.3
Supportive structures surrounding the urethra are also pivotal in preserving continence in the integral theory.4 This theory proposes that musculoelastic properties of the vagina not only contribute to formation of a backstop posterior to the urethra but also result in lengthening of urethra, both components of continence. These theories are not mutually exclusive, and parts of 1 or more the theories may explain successful treatment of SUI.
Pessaries offer a unique approach to understanding continence mechanisms because they provide a readily reversible method of restoring continence. They allow the study of patients during periods of continence and incontinence that are separated in time by only minutes. Using both magnetic resonance imaging (MRI) and urodynamic studies (UDS), we quantified the acute anatomic and functional changes that followed placement of pessaries and restored continence. We performed this pilot study to both reveal pessaries’ mechanism of action and improve our understanding of mechanisms of continence in general.
Materials and Methods
Women using pessaries to treat urinary continence were recruited for this study from October 2005 through August 2006. Women were excluded if they were pregnant, younger than 18 years old, had a urinary tract infection, or had greater than stage II prolapse on their Pelvic Organ Prolapse Quantitation examination.5 We excluded women with greater than stage II prolapse because of concerns that more advanced stages of prolapse could be accompanied by distortion of the pelvic floor that could obscure findings specific for urinary incontinence. Women were eligible to participate in this study if they had a history of SUI, urethral hypermobility on examination, and were able to undergo MRI. We required evidence of stress incontinence on physical examination or urodynamic evaluation prior to performance of MRI. The University of New Mexico Institutional Review Board approved this study, and participants gave written informed consent prior to testing.
A standardized history and physical examination form was used for all subjects. The Urogenital Distress Inventory-6 (UDI-6)6 was administered and urinalysis obtained prior to urodynamic testing. The Q-tip test was performed before and after pessary insertion. Urethral hypermobility was defined as Q-tip excursion greater than 30 degrees with Valsalva.
Complex urodynamic testing was performed before and after pessary placement according to International Continence Society guidelines.7 Nurses experienced in UDS performed the tests. A seven French double lumen fluid filled catheter was placed in the bladder and urethra. A single lumen catheter was placed in the vagina to measure intraabdominal pressure. Postvoid residuals and intravesical and urethral pressures were recorded. Duet Sensic (Mediwatch U.K. Ltd, Ruby, United Kingdom) urodynamic computer software was used on a Mediwatch Duet Sensic urodynamic machine (Mediwatch U.K. Ltd).
Bladders were filled with liquid at 50 mL/min with patients lying at a 45° incline. Sensory symptoms and presence of detrusor contractions were noted. Patients performed cough and Valsalva at 200 mL and again at maximal capacity. The maneuvers were repeated with patients standing if leakage did not occur with the previously mentioned provocative maneuvers. At maximal bladder capacity, static urethral pressure profilometry was performed, withdrawing the catheter through the urethra using an electronic puller at 1 mm/sec. Patients then voided with the catheters in place. The procedures were repeated following pessary placement.
Subjects used the same silicone incontinence dish pessaries for both UDS and MRI. Incontinence dish pessaries were used because they are MRI compatible. If patients used other pessaries prior to the study, they were fit with incontinence dish pessaries similar in size to their usual pessaries, which also restored continence.
MRI was performed on a 1.5 Tesla Siemens Symphony MRI (Siemens AG Medical Solutions, Erlangen, Germany) with a phased array pelvic coil to maximize spatial resolution. All sequences were performed with the subjects reclining. Subjects did not void for at least 30 minutes prior to MRI. Initial MRI scanning was performed without a pessary in place. Imaging was performed using T2W fast spin echo sequence in axial and sagittal planes to identify a midline sagittal imaging plane and establish anatomic landmarks using optimal spatial resolution. Subsequently T2 single-shot, fast-spin echo sequences were performed in the midline sagittal plane with the patient at rest and during Valsalva maneuver. Each image acquisition lasted approximately 20 seconds.
These sequences were followed by cinematic MRI in the sagittal plane using a true fast imaging with steady-state precession (FISP) (steady state gradient echo) with T2/T1 (ratio of spin relaxation time to spin lattice relaxation time) weighting. This technique allowed for exquisite temporal resolution with limited spatial resolution. Using this MRI sequence, we acquired the 20–30 subsecond images needed to accurately observe the change in morphology and location of the bladder neck over time. These cinematic sequences were performed during Valsalva and cough. These imaging sequences were completed in 10–15 minutes.
After the true FISP images were obtained, an incontinence dish pessary was placed while the patient remained on the MRI table. The true FISP cinematic imaging sequence and the aforementioned T2W single-shot sequence were then repeated in the same manner as performed without the pessary in place. Patients voided at the end of the session and urine volume was measured.
Measurements performed on single-shot, spin-echo MRI images included the following: the distance between the bladder neck and the pubococcygeal line,8 the distance between the bladder neck and posterior pubis,8 the midurethra and posterior pubis, and measurement of the posterior urethrovesical angle.9 The posterior urethrovesical angle was the angle that intersected the axis of the proximal urethra and a posterior line representing at least one third of the bladder base.9 Measurements made on cinematic images included distance of the bladder neck to the pubococcygeal line and posterior pubis during Valsalva and cough, presence or absence of bladder neck funneling during cough, and the posterior urethrovesical angle. Location of the pessary relative to the bladder neck was also noted.
MRI measurements were performed by 2 board-certified radiologists experienced in cross-sectional body imaging. Values for the measurements above were reached by consensus. Radiologists were unaware of UDS results and nurses performing UDS were unaware of MRI results.
Statistics
Our power analysis was based on prior work comparing changes of the bladder neck location before and after pessary placement.9 Assuming 80% power, alpha = 0.05, and 50% change in bladder neck position, 14–16 patients were needed for this study. Continuous variables comparing within subject change were evaluated using 2-tailed paired t tests and the Wilcoxon test. Comparisons between subjects were evaluated using unpaired t tests. Categorical variables were analyzed by Fisher’s exact test and McNemar’s test of symmetry. Statistical analysis was performed using STATA software (version 10, Statacorp, PLC, College Station, TX).
Results
Eighteen women were recruited for this study. Of these, 1 declined further participation prior to her MRI, and 2 were ineligible for MRI: 1 subject lacked urethral hypermobility, and the other lacked objective urine leakage. The 15 women who had both UDS and MRI data for analysis form the basis of this study.
The median age of subjects was 52 years (range, 31–65), median parity was 2 (range, 0–6), and median body mass index was 26 kg/m2 (range, 20–49). Two women had stage I prolapse, and the remainder had stage II. Although 4 women (27%) had previously undergone hysterectomy, only 1 (7%) had a prior incontinence procedure, and none had prior prolapse surgery.
Prior to the study, 7 women (47%) used incontinence ring pessaries, the remaining 8 (53%) used incontinence dish pessaries. The median UDI-6 score with pessary use for these women was 3 (range, 1–7), with possible scores ranging from 0 to 18, with higher scores representing worse symptoms.
Before pessary placement, all 15 women had urethral hypermobility. Following pessary placement, 7 (7 of 14, 50%) continued to have hypermobility. One subject did not tolerate urodynamic testing and refused to have urodynamic and Q-tip testing repeated with the pessary in place.
Three significant changes occurred on UDS following pessary placement; maximal flow rates decreased, detrusor pressures increased, and functional urethral lengths increased (Table 1). Static mean maximal urethral closure pressures did not change with pessary placement, and there were no differences in first sensation and maximal bladder capacity before and after pessary placement (Table 1). The mean leak point pressure was 87.5 ± 31.2 cm water prior to pessary placement. Leak point pressures with and without pessaries could not be compared because of the small number of women (3 of 15, 20%) who leaked following pessary placement. All subjects had postvoid residuals less than 50 mL prior to and at the end of urodynamic testing. None of the patients had uninhibited detrusor contractions during testing.
TABLE 1.
UDS table
| Mean UDS parameters | Without pessary (± SD) | With pessary (± SD) | P value |
|---|---|---|---|
| First sensation (mL) | 52 (52) | 45 (48) | .61a |
| Maximal bladder capacity (mL) | 330 (88) | 319(104) | .57a |
| Maximal flow rate (mL/sec) | 30 (12) | 19 (8) | < .001a |
| Detrusor pressure (cm H2O) | 33 (26) | 45 (8) | < .05b |
| Functional urethral length (cm) | 3.4 (0.7) | 3 9 (0 8) | < .05a |
| Maximal urethral closure pressure (cm H2O) | 62.8 (26.1) | 63 5 (23 5) | .86a |
Two-sided t test.
Wilcoxon signed-rank test.
Komesu. Restoration of continence by pessaries. Am J Obstet Gynecol 2008.
Four significant changes occurred on MRI following pessary placement (Table 2). On imaging done during Valsalva (T2 spin relaxation time, single-shot, spinecho) or cough (true FISP cine) the bladder neck was elevated farther above the pubococcygeal line. In addition, the number of women with funneling decreased (Table 2). Images obtained during Valsalva (T2 single-shot, spin-echo) also showed that the posterior urethrovesical angle was more acute and urethral length greater after pessary placement (Table 2). These later measurements could not be accurately obtained during cough with true FISP imaging.
TABLE 2.
MRI findings
| MRI parameters (mean) |
Without pessary (± SD) (n = 15) |
With pessary (± SD) (n = 15) |
P value |
|---|---|---|---|
| Distance bladder neck above the pubococcygeal line with Valsalva (cm) | −0.31 (1. 03) | 0.79 (1.19) | < .0001a |
| Posterior urethrovesical angle with Valsalva (degrees) | 175.3 (31.2) | 130.1 (23.5) | < .001a |
| Urethral length with Valsalva (cm) | 2.39 (0.84) | 2.97 (0.52) | < .05a |
| Funneling occurred with cough (%) | 14 of 15(93) | 3 of 15 (20) | .001b |
| Distance midurethra and posterior pubic bone with Valsalva (cm) | 1.35 (0.37) | 1.17 (0.49) | .18a |
| Distance bladder neck and posterior pubic bone with Valsalva (cm) | 1.72 (0.47) | 1.69 (0.73) | .81a |
Two-sided t test.
McNemar’s test of symmetry.
Komesu. Restoration of continence by pessaries. Am J Obstet Gynecol 2008.
Pessaries varied in location relative to the bladder neck. The pessary knob was at the bladder neck in most women (9 of 15, 60%), distal to the bladder neck in 3 (20%) women, and cranial to the bladder neck in 3 (20%) women. Funneling was present on MRI with cough in 14 women (14 of 15, 93%) prior to pessary placement. Funneling resolved in the 11 women with pessaries located at or distal to the bladder neck. In the 3 women whose pessaries were cranial to the bladder neck, funneling did not resolve.
Voided volumes following MRI were recorded in 10 subjects. The median volume voided at the end of the MRI was 300 mL (range, 100–800 mL).
Whereas all 15 women had objective leakage with cough and/or Valsalva prior to pessary placement, only 3 leaked with cough or Valsalva following pessary placement. There were no differences following pessary placement between women who leaked and who did not in terms of functional urethral lengths (3.36 cm vs 3.98 cm, respectively, P = .27), maximal urethral closure pressures (41 cm H20 vs66, P = .1), maximal flow rates (19.2 mL/sec vs 18.5, P = .9), or maximal detrusor pressures (28 cm H2O vs 46.5, P = .34). One woman in the leak group and 2 in the no-leak group had funneling on MRI. There were no differences on MRI comparing the leak and no-leak groups in bladder neck distance above the pubococcygeal line (−0.13 cm vs 1.025 cm, P = .14), posterior urethrovesical angle (142° vs 127°, P = .3), and urethral length (2.6 cm vs 3.06 cm, P = .2).
Comment
We observed several anatomic and urodynamic changes following pessary placement that may contribute to the restoration of continence. With pessary placement, the urethra remained relatively fixed, compared with the bladder base. Relative fixation of the urethra despite bladder base descent during Valsalva or cough resulted in a more acute posterior urethrovesical angle. The maintenance of an acute posterior urethrovesical angle therefore represents stabilization of the urethra and indicates that pessary placement restores a key component of continence according to both the hammock hypothesis and the integral theory of continence.3,4
In these theories stabilization of the urethra during Valsalva or cough allows forces applied from above to compress the urethra. The importance of urethral stabilization is supported by clinical studies showing that a widened posterior urethrovesical angle is associated with incontinence.10–12 If these theories are correct, however, limiting the change in the posterior urethral angle caused by cough or Valsalva may be more indicative of urethral stabilization than is maintenance of an absolute value for the angle.
The observed decrease in bladder neck funneling following pessary insertion may also be a function of stabilization of the proximal urethra. Funneling resolved in women in whom the pessary knob was at or below the bladder neck, possibly because stabilization of the posterior urethra allows forces applied superiorly and anteriorly to maintain apposition of the urethral walls. This is consistent with prior work showing resolution of funneling following both Burch and midurethral sling procedures.13,14 A pessary located at the bladder neck may mimic a Burch colposuspension, whereas pessary located distal to the bladder neck may be more akin to a midurethral sling.
Further findings in our study, however, suggest that pessary placement may restore continence by mechanisms in addition to posterior urethral support. We found decreased maximal urine flow rates and increased detrusor pressures following pessary placement. These findings indicate increased urethral resistance.
Two mechanisms may explain this increased urethral resistance. Besides providing a backstop against which force directed from above can be applied, the knobs on incontinence dishes may directly compress the urethra at rest. This compression could increase urethral resistance and result in increased detrusor pressure and decreased flow rates. Alternatively, the observed increase in urethral length rather than compression by the pessary knob could be the cause of increased urethral resistance. Pessary placement increased urethral length on MRI and increased functional urethral length on UDS. The latter finding is consistent with that of Bhatia et al.15
Previous investigators have stressed the importance of urethral length in continence.16–18 In vitro animal studies have also shown increasing anatomic urethral length results in increased urethral resistance.16 Poiseuille’s law indicates that resistance to flow increases linearly with increase in a tube’s length and is proportional to the radius to the fourth power. If lengthening of the urethra results in even minor decrements of the lumen, this would further enhance the effect of urethral lengthening on urethral resistance to flow.
Lastly, the elevation of the bladder neck demonstrated on our MRI images suggests a third possible mechanism for restoration of continence following pessary placement. Enhorning’s theory proposes that elevation of the bladder neck and proximal urethra is central to the maintenance of continence. In theory, elevation of the bladder neck and proximal urethra allows intraabdominal pressure to be applied extrinsically on these structures during cough or Valsalva. If this mechanism of continence is important following pessary placement, elevation of dynamic urethral closing pressures would be expected. Bhatia et al15 found elevated dynamic urethral closure pressures in women following pessary insertion. Dynamic closure pressures, closure pressures measured with cough, were not performed in our study, and therefore, the contribution of bladder neck elevation to continence with the use of continence dish pessaries remains uncertain.
This study is a pilot study performed to determine feasibility of obtaining high-resolution MRI images, including cine imaging, in patients following pessary placement. A weakness of this study was that because of cost, we were unable to include a control group. Three patients did leak despite pessary placement. Trends in these patients suggested more obtuse posterior urethral angles, shorter urethral lengths, lower maximal urethral closure pressures, and lower detrusor pressures, but the size of this subgroup is not sufficient for meaningful analysis. (Figures 1 and 2).
FIGURE 1. MRI without and with pessary.
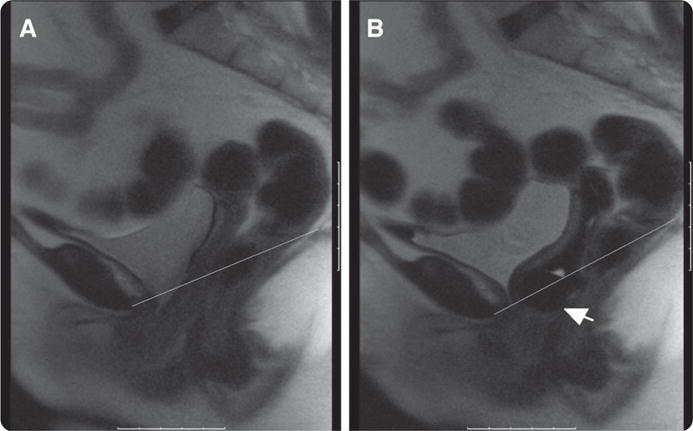
A, Valsalva without pessary. Line represents pubococcygeal line. B, Valsalva with pessary. Line represents pubococcygeal line. Arrow points to pessary knob
Komesu. Restoration of continence by pessaries. Am J Obstet Gynecol 2008.
FIGURE 2. Fast MRI (valsalva) with and without pessary.
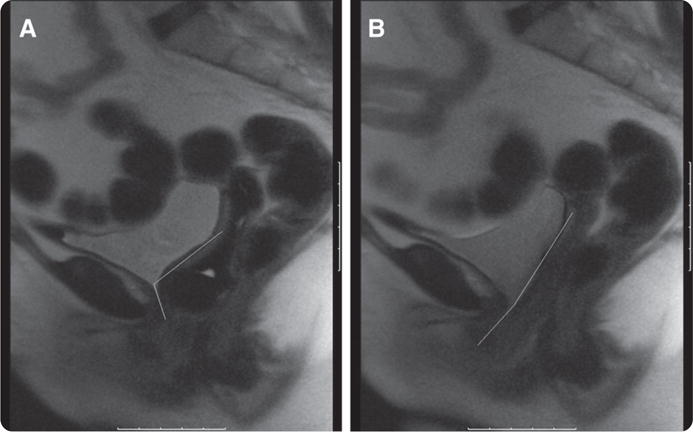
A, Valsalva with pessary. Lines illustrate posterior urethro-vesical angle. B, Valsalva without pessary. Lines illustrate posterior urethro-vesical angle.
Komesu. Restoration of continence by pessaries. Am J Obstet Gynecol 2008.
This study suggests that a number of mechanisms are responsible for continence restoration by incontinence dish pessaries. In the future, we plan to perform a larger study comparing imaging and UDS findings between patients in whom pessary placement restores continence and those in whom it does not. In that study we also plan to measure dynamic closing pressures to better determine whether bladder neck elevation plays an important role in the restoration of continence, compared with posterior urethral stabilization.
The major strength of this study is its unique approach to evaluating continence mechanisms of pessaries. One prior study evaluated urodynamic changes with pessary placement but did not obtain correlative imaging, whereas 1 other study evaluated postpessary placement MRIs but did not obtain UDS data.9,15 We utilized both of these tools in the same subjects to evaluate pessaries’ mechanism of action in restoring continence. Concurrent use of UDS and MRI to evaluate the changes that occur with pessary placement in patients with SUI is a novel method investigating the roles of multiple proposed mechanisms of continence restoration. Some or all of these mechanisms may explain how pessaries reverse incontinence.
Acknowledgments
This study was supported by the University of New Mexico Research Allocation Committee Grant, Tobacco Settlement Fund #C-2275-T. 0002-9378/$34.00
Footnotes
This study was presented at the 28th Annual Scientific Meeting of the American Urogynecologic Society, Hollywood, FL, Sept. 27–29, 2007.
Reprints not available from the authors
References
- 1.Abrams P, Cardozo L, Fall M, et al. The standardization of terminology of lower urinary tract function: Report from the Standardization subcommittee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 2.Enhorning GE. A concept of urinary continence. Urol Int. 1976;31:3–5. doi: 10.1159/000280024. [DOI] [PubMed] [Google Scholar]
- 3.Delancey JO. Structural support of the urethra as it relates to stress urinary incontinence: The hammock hypothesis. Am J Obstet and Gynecol. 1994;170:1713–20. doi: 10.1016/s0002-9378(94)70346-9. [DOI] [PubMed] [Google Scholar]
- 4.Petros PEP, Ulmsten UI. An integral theory of female urinary incontinence. Acta Obstet Gynecol Scand. 1990;69(Suppl 153):7–31. doi: 10.1111/j.1600-0412.1990.tb08027.x. [DOI] [PubMed] [Google Scholar]
- 5.Bump RC, Mattiasson A, Brubaker LP, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175:10–7. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 6.Uebersax JS, Wyman JF, Shumaker SA, et al. Short forms to asses life quality and symptom distress for urinary incontinence in women: The Incontinence Impact Questionnaire and the Urogenital Distress Inventory. Neurourol Urodyn. 1995;14:131–9. doi: 10.1002/nau.1930140206. [DOI] [PubMed] [Google Scholar]
- 7.Schäfer W, Abrams P, Liao L, et al. Good urodynamic practices; uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn. 2002;21:261–74. doi: 10.1002/nau.10066. [DOI] [PubMed] [Google Scholar]
- 8.Maubon AJ, Boncoeur-Martel MP, Juhan V, et al. Static and dynamic MRI of a urinary control intra-vaginal device. Eur Radiol. 2000;10:879–84. doi: 10.1007/s003300051028. [DOI] [PubMed] [Google Scholar]
- 9.Fantl JA, Hurt WG, Bump RC. Urethral axis ad sphincteric function. Am J Obstet Gynecol. 1986;144:554–8. doi: 10.1016/0002-9378(86)90278-4. [DOI] [PubMed] [Google Scholar]
- 10.Roberts H. Cystourethrography in women. Br J Radiol. 1952;25:253–9. doi: 10.1259/0007-1285-25-293-253. [DOI] [PubMed] [Google Scholar]
- 11.Jeffocoate TNA, Roberts H. Observations on stress incontinence of urine. Am J Obstet Gynecol. 1952;64:721–38. doi: 10.1016/s0002-9378(16)38792-0. [DOI] [PubMed] [Google Scholar]
- 12.Green TH. Development of a plan for the diagnosis and treatment of urinary stress incontinence. Am J Obstet Gynecol. 1962;83:632. doi: 10.1016/s0002-9378(16)35894-x. [DOI] [PubMed] [Google Scholar]
- 13.Koelle D, Windisch J, Doerfler D, Marth C, Kroopshofer S. Effect of tension-free vaginal tape operation on urethral closure function. Urology. 2006;67:524–8. doi: 10.1016/j.urology.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 14.Skala C, Emons G, Krauss T, et al. Postoperative funneling after anti-incontinence surgery—a prognostic indicator? Part 1: Colposuspension. Neurourol Urodyn. 2004;23:636–42. doi: 10.1002/nau.20056. [DOI] [PubMed] [Google Scholar]
- 15.Bhatia NN, Bergman A, Gunning JE. Urodynamic effects of a vaginal pessary in women with stress urinary incontinence. Am J Obstet Gynecol. 1983;147:876–84. doi: 10.1016/0002-9378(83)90239-9. [DOI] [PubMed] [Google Scholar]
- 16.Bruschini H, Schmidt RA, Tanagho EA. Effect of urethral stretch on urethral pressure profile. Invest Urol. 1977;15:107–11. [PubMed] [Google Scholar]
- 17.Lapides J, Ajemian EP, Stewart BH, Lichtwardt JR, Breakey BA. Physiopathology of stress incontinence. Surg Gynecol Obstet. 1960;3:224–31. [PubMed] [Google Scholar]
- 18.Petros P. Changes in bladder neck geometry and closure pressure after midurethral anchoring suggest a musculoelastic mechanism activates closure. Neurourol Urodyn. 2003;22:191–7. doi: 10.1002/nau.10085. [DOI] [PubMed] [Google Scholar]


