Abstract
Oseltamivir is an influenza neuraminidase inhibitor that along with supportive therapy has shown to help critically ill patients infected with H7N9 and H1N1pdm influenza virus strains to recover from disease. The standard of care recommends the administration of oseltamivir via oral route which represents difficulties in patients with gastrointestinal complications. Here we tested the use of aerosol administration of oseltamivir to treat mice infected with influenza A/H7N9 virus or influenza A/H1N1pdm virus and directly compared this approach to the standard of care, oral administration. Using nose only delivery of aerosolized oseltamivir we observed a significant increase in efficacy of the treatment compared to oral administration characterized by reduced body weight loss, increased survival rate and dose sparing. The preclinical data presented here supports the possibility of using this approach in clinical settings.
Influenza A virus infections represent a significant threat to public health, as they occur seasonally and less frequently as pandemics. Influenza viruses rapidly evolve through antigenic drift caused by mutations and antigenic shift as a result of the introduction of novel gene segments from animal strains (Krammer and Palese, 2015). One example of these events is the avian-origin H7N9 influenza virus outbreak currently undergoing in China (Hai et al., 2013). While mild illnesses in human H7N9 cases have been observed, many patients have developed severe respiratory illness (Wang et al., 2016). The virus appears not to be transmitted from person to person, although limited non sustained human-to-human transmission has been observed within some families (Liu et al., 2013).
While vaccination is the most effective means of controlling influenza-associated illness and death, no vaccine is currently approved for prevention of H7N9 infections (Henry Dunand et al., 2016). Timely administration of antivirals to patients severely ill with seasonal, pandemic and avian influenza has been observed to improve clinical outcomes (He et al., 2013; Zhang et al., 2016). Influenza A H7N9 viruses affecting humans are resistant to adamantanes; therefore, the use of neuraminidase inhibitors such as oseltamivir is the treatment of choice for H7N9 influenza virus infections (Hai et al., 2013; Liu et al., 2013). The recommended dose and duration of treatment of oseltamivir is 75 mg twice a day (b.i.d.) for 5 days (in people > 40 kg of body weight) given via oral administration. In some cases, critically ill hospitalized patients with gastrointestinal complications cannot tolerate or absorb oseltamivir administered orally (Thorner, Accessed November 17th 2016); therefore, local administration of oseltamivir to the respiratory tract may be a clinically relevant option for treatment. We compared the efficacy of oseltamivir to treat influenza virus infected mice when administered systemically (via oral or via intraperitoneal administration) or locally (intranasal via droplets or by aerosol). We tested our approach with two different influenza virus strains, influenza A/H7N9 and influenza A/H1N1pdm.
All research studies involving the use of animals were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the Icahn School of Medicine at Mount Sinai. Different groups (n=10 mice per group) of female BALB/c mice (8 to 10 weeks old) purchased from The Jackson Laboratory (Bar Harbor, ME) were infected with an 80% lethal dose (1 LD80) of influenza A Anhui/01/2013 H7N9 in 30 μl of sterile phosphate buffered saline or PBS under ketamine/xylazine (100/10 mg/kg) anesthesia. The virus used is a reassortant virus with the internal genes of influenza A Puerto Rico/08/1934 and the hemagglutinin and neuraminidase genes from the H7N9 virus (rH7N9), allowing the studies to be performed under biosafety level 2 conditions (Henry Dunand et al., 2016). Twelve hours after infection, mice received treatment with 1 or 10 mg/kg/day of oseltamivir (Tamiflu®, Oseltamivir phosphate, Genentech, San Francisco, CA) dissolved in sterile PBS, b.i.d. for 5 days via oral (PO), intraperitoneal (IP), intranasal (IN) or aerosol (AE) administration. These doses are below the recommended dose for PO oseltamivir treatment of 20 mg/kg/day (Tsai et al., 2015) and therefore considered sub-therapeutic. One of our goals was to determine the possibility of dose sparing using local administration of the antiviral. For the PO and IP administration, the corresponding dose was administered in a 100 μl bolus. For the IN treatments mice were slightly anesthetized using isofluorane anesthesia and received the treatment by drop wise instillation of 25 μl containing the corresponding dose.
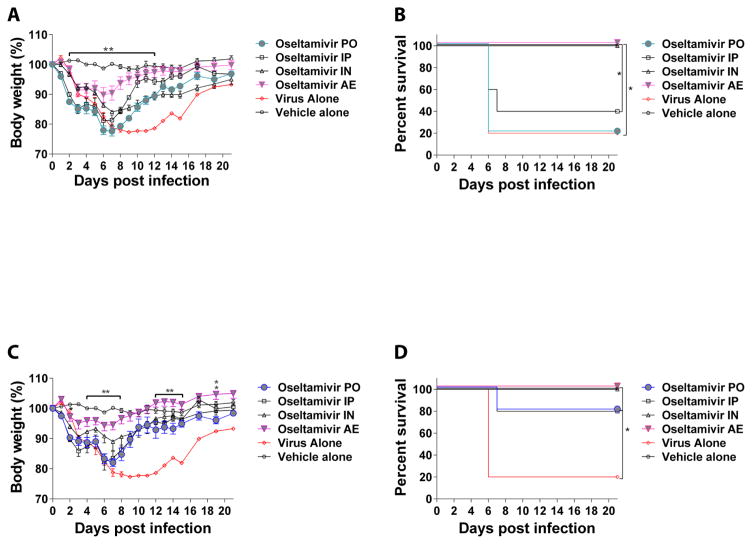
Aerosol administration was done using a nose-only aerosol delivery system (inExpose system; SCIREQ, Montreal, QC, Canada) with an Aerogen Lab nebulizer (Aerogen, Galway, Ireland) as previously described (Leyva-Grado et al., 2015). Mice were monitored daily for clinical signs of infection and body weights were recorded daily for 15 days and then every other day until day 21 post infection. More than 25% loss of the initial body weight was considered the humane endpoint for these studies. The collected data were used to generate body weight curves and Kaplan-Meier survival curves using the GraphPad software (La Jolla, CA). p-values < 0.05 were considered significant. At the lower dose of 1 mg/kg/day, mice that received treatment via AE have a greater reduction in body weight loss compared to the PO treated group (Figure 1A, p<0.01). Mice that received local treatment via AE or IN had a significantly better survival (100% survival in both groups) than the groups treated systemically either via PO (20% survival) or IP (40% survival) (Figure 1B; p<0.05). For mice that received a higher dose of oseltamivir (10 mg/kg/day), we observed a significant difference in body weight gain on days 5–9, 12–15 and 19 post-infection in the group that received treatment via AE compared to the group that received treatment via PO (Figure 1C; p<0.01). No significant differences were observed in survival among the treated groups (Figure 1D). Mice treated via local administration AE or IN, had a 100% survival rate while the groups treated via systemic administration PO or IP, had an 80% survival. All treated groups had a significantly better survival rate compared to the group that only received placebo (Figure 1 D; p<0.05). In a similar study, the use of 20 mg/kg/day b.i.d for 5 days given via PO route conferred similar protection to what we observed here for the PO or IP groups treated with 10/mg/kg/day (Baranovich et al., 2014). In a different study that used a sub-lethal dose of rH7N9 virus infection, a similar regimen (25 mg/kg/day b.i.d. for 5 days via PO route) significantly reduced the body weight loss of the infected mice compared to the placebo group (Marjuki et al., 2014). The results of these studies are similar to what we have observed here for the groups that received treatment via PO or IP routes. However, there are no published data on the use of AE administration of oseltamivir for the treatment of influenza A/H7N9 virus infection.
Figure 1. Body weight and survival of BALB/c mice infected with influenza A/Anhui/1/2013 H7N9 virus and treated with oseltamivir.
Groups of mice were infected with influenza A/Anhui/1/2013 virus and treated with oseltamivir via oral (PO), intraperitoneal (IP), intranasal (IN) or aerosol (AE) routes. Treatment started 12 h post-infection and was administered twice a day for 5 days at 1 mg/kg/day (A and B) or 10 mg/kg/day (C and D) doses. A) Body weight and (B) survival in groups treated with 1 mg/kg/day. (C) Body weight and (D) survival in groups treated with 10 mg/kg/day. (*) indicates a significant difference (In A and C the differences are between the PO groups and the AE groups, filled symbols) (*p<0.05; ** p<0.01). This is representative experiment of 2 repetitions with an n=10 mice per group. Vehicle: Phosphate buffered saline
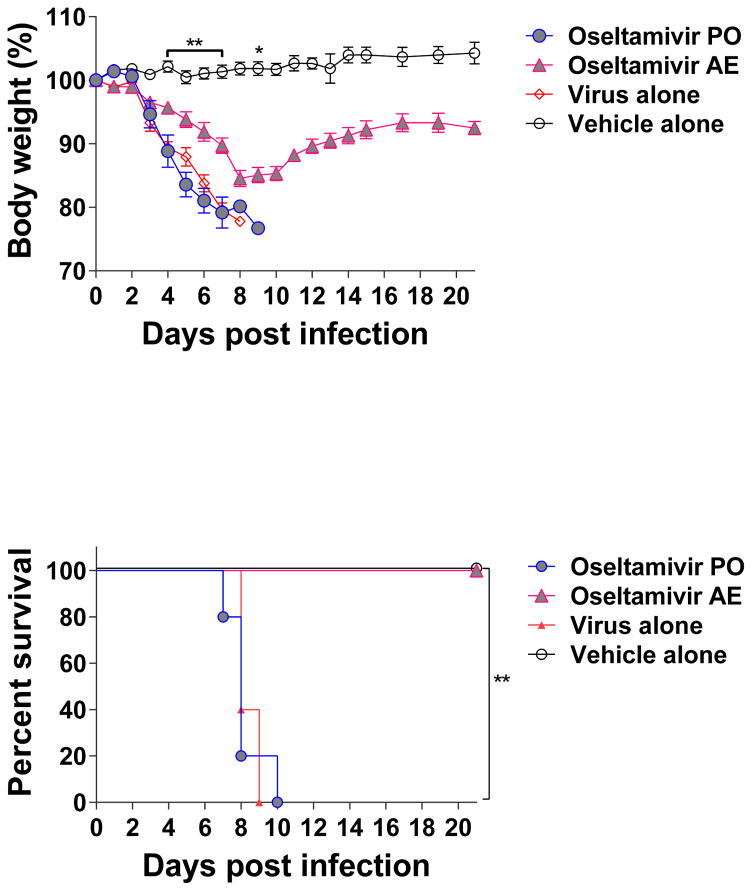
The window of opportunity for oseltamivir treatment is short and the recommendation is to start treatments within 48 h after the onset of clinical signs (CDC, Accesed November 15th 2016), as a result the use of high doses of oseltamivir are necessary to achieve protection. Therefore, the outcomes observed with the low dose in the PO group were expected. In contrast, infected mice treated via AE route with the lower dose of oseltamivir had a reduced morbidity characterized by a significant reduction in body weight loss and a significantly better survival rate compared to mice treated either via PO or IP. A previous study using the IN route to deliver treatment with oseltamivir showed improved survival in mice infected with an influenza A H6N2 virus (Bantia et al., 2001). The virus used for that study expresses a group 1 hemagglutinin and not a group 2 hemagglutinin as the rH7N9 virus tested in our study (Qi et al., 2014). Hence, we decided to test our approach using a mouse model of influenza A H1N1pdm virus infection, a virus currently circulating in the human population that also expresses a group 1 hemagglutinin and a different neuraminidase than the rH7N9 virus(Qi et al., 2014). We used a more stringent approach by increasing the dose of the virus to a lethal dose and we increased the delay of treatment to be started 24 h post-infection. Mice were infected intranasally with a dose equivalent to 3 mLD50 of mouse adapted influenza A/Netherlands/604/2009 H1N1pdm virus in 30 μl of sterile PBS. This virus strain is a prototype of the current pandemic (Herfst et al., 2010) H1N1 viruses with 99 % sequence homology to the virus strain used in the current vaccine preparations. Twenty four hours after infection, different groups of mice (n= 10 per group) received treatment with 10 mg/kg/day of oseltamivir b.i.d. for 5 days via oral (PO) or aerosol (AE) administration as described above for the rH7N9 experiment. Animals were monitored daily and data were recorded and analyzed. Mice that received the treatment via AE administration had a significant reduction in body weight loss compared to the groups treated via PO administration and the untreated control (Figure 2 A; p<0.01). Under these conditions, the PO administration of 10mg/kg/day of oseltamivir was not enough to rescue the mice from infection and all the animals died at a similar rate than the infected-untreated group (Figure 2 B; p<0.01). Despite an average weight loss of about 12% at the nadir, all mice that received treatment via AE survived (Figure 2 A; p<0.01). Preclinical studies using the mouse model of influenza A H1N1pdm virus infection have demonstrated that - to confer protection using oseltamivir via PO in a prophylactic regimen - either the antiviral is administered at high doses (up to 100 mg/kg) (Marjuki et al., 2014) or for extended periods of time (up to 10 days of treatment) (Byrn et al., 2015; Smee et al., 2016). Using a similar regimen as the one we used in this study, treatment of infected mice with 25 mg/kg of oseltamivir via PO three times a day for 5 days only conferred a 20% protection (Nguyen et al., 2012). In contrast, our results show that using oseltamivir via AE route significantly improved the efficacy of the antiviral to rescue mice infected with a lethal dose of an H1N1pdm virus even when treatment started 24 h post-infection.
Figure 2. Body weight and survival of BALB/c mice infected with influenza A/Netherlands/604/2009 H1N1pdm virus and treated with oseltamivir.
Groups of mice were infected with influenza A/Netherlands/604/2009 H1N1pdm virus and treated with oseltamivir via oral (PO) or aerosol (AE) routes. Treatment started 24 h post-infection and was administered twice a day for 5 days at 10 mg/kg/day. A) Body weight and (B) survival in groups (*) indicates a significant difference (*p<0.05; ** p<0.01) between the PO and AE groups (filled symbols). This is representative experiment of 2 repetitions with an n=10 mice per group. Vehicle: Phosphate buffered saline
Despite a small window of opportunity and lack of 100 % efficacy against a number of influenza virus strains; oseltamivir has been proved to be very beneficial, along with supportive therapy, in saving lives of critically ill patients infected with influenza H7N9 virus (He et al., 2013; Zhang et al., 2016) or with the current H1N1pdm virus (Hui et al., 2010). Our results demonstrate that administration of oseltamivir via AE route may improve the efficacy for the treatment of these life threatening infections.
The IN administration of oseltamivir has shown efficacy for the prevention of clinical signs in influenza-infected mice (Bantia et al., 2001; Chand et al., 2005; Sidwell et al., 1998). The studies by Sidwell et al., 1998 showed protection in mice treated via IN route with 0.1 mg/kg/day of oseltamivir acid (the active form of the compound), b.i.d. for 5 days beginning 4 h before infection with influenza A/NWS/1933 virus. We and others have shown that oseltamivir is very effective even at low doses when used prophylactically to prevent clinical signs and mortality in mice infected with laboratory adapted strains of influenza virus such as A/WSN/1933 and A/Puerto Rico/08/1934 (Byrn et al., 2015; Gerritz et al., 2011; Mendel et al., 1998; Ortigoza et al., 2012). As mentioned above, the studies by the group of Babu et al. (Bantia et al., 2001; Chand et al., 2005) also demonstrated protection against mortality in mice treated via IN route with 0.1 mg/kg/day of oseltamivir, b.i.d. for 5 days beginning 4 h before infection with an influenza A H6N2 recombinant virus. Our goal was to test the AE route of administration in a therapeutic setting; therefore prophylactic studies to determine drug dose range were not performed here. Our results confirm and expand the knowledge that oseltamivir can be administered at the site of infection. The improved efficacy of the AE administration for the treatment of H7N9 and H1N1pdm influenza viruses supports the possibility of using this treatment approach in different clinical scenarios including cases of critically ill hospitalized patients (Thorner, Accessed November 17th 2016). Zanamivir (Relenza®, Glaxo-Smith-Kline, Brentford UK), another neuraminidase inhibitor that is approved for inhalation in patients 7 years and older, has been used as an alternative to oseltamivir treatment (Hui et al., 2010); however, in patients with pre-existing medical conditions such as asthma or known allergies to any ingredient of Relenza® including milk proteins, the use of this medication is not recommended (Relenza, Accessed November 17th 2016). In these particular cases the use of aerosolized oseltamivir can be a good therapeutic alternative. Furthermore, we did not observe any signs of respiratory distress such as bronchospasms or sneezing response in the treated mice and considering that oseltamivir is 100% water soluble, aerosolization can be achieved in a formulation potentially less reactogenic than Relenza. Finally, one additional benefit we observed with the use of the AE route is the possibility for dose sparing not only by reducing the dose necessary for protection (a 10 fold dose reduction still protects mice from succumbing to rH7N9 virus infection in the AE group but not in the PO group), as shown in this study; but also, by reducing the number of doses required for protection, currently under investigation. This may be very important because it has been suggested that in patients who have received oseltamivir for extended periods of time there is an increased risk of emergence of oseltamivir-resistant viruses (Hui et al., 2010).
We previously showed that local administration of anti-influenza monoclonal antibodies improved their efficacy to treat infection in mice when compared to systemic administration (Leyva-Grado et al., 2015); therefore, the improvements observed in oseltamivir therapy after aerosol administration are a proof of concept for administering a variety of antiviral drugs through the inhalation route. Finally, we conclude from our studies that aerosol administration of oseltamivir increases the efficacy to treat mice infected with different strains of influenza viruses such as influenza A rH7N9 or influenza A H1N1pdm. We have also shown that using this route the dose of oseltamivir needed for protection can be significantly reduced, at least 10 times compared to the dose required for protection using the oral route of administration.
Highlights.
Aerosol administration improves efficacy of oseltamivir to treat influenza
Animals treated with oseltamivir via aerosol have a better clinical outcome than those treated via oral administration
A dose sparing of 10 fold was observed in groups treated with oseltamivir via aerosol compared to oral administration
Aerosol administration of oseltamivir may be an alternative to treat critically ill influenza virus-infected patients
Acknowledgments
This work was supported in part by NIH contract HHSN272201000019I Task order HHSN27200005 A86. We thank Lawrence Webster for his excellent assistance during his student rotation. We also thank Chen Wang in the Department of Microbiology for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bantia S, Parker CD, Ananth SL, Horn LL, Andries K, Chand P, Kotian PL, Dehghani A, El-Kattan Y, Lin T, Hutchison TL, Montgomery JA, Kellog DL, Babu YS. Comparison of the anti-influenza virus activity of RWJ-270201 with those of oseltamivir and zanamivir. Antimicrob Agents Chemother. 2001;45:1162–1167. doi: 10.1128/AAC.45.4.1162-1167.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranovich T, Burnham AJ, Marathe BM, Armstrong J, Guan Y, Shu Y, Peiris JM, Webby RJ, Webster RG, Govorkova EA. The neuraminidase inhibitor oseltamivir is effective against A/Anhui/1/2013 (H7N9) influenza virus in a mouse model of acute respiratory distress syndrome. J Infect Dis. 2014;209:1343–1353. doi: 10.1093/infdis/jit554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrn RA, Jones SM, Bennett HB, Bral C, Clark MP, Jacobs MD, Kwong AD, Ledeboer MW, Leeman JR, McNeil CF, Murcko MA, Nezami A, Perola E, Rijnbrand R, Saxena K, Tsai AW, Zhou Y, Charifson PS. Preclinical activity of VX-787, a first-in-class, orally bioavailable inhibitor of the influenza virus polymerase PB2 subunit. Antimicrob Agents Chemother. 2015;59:1569–1582. doi: 10.1128/AAC.04623-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. [Accesed November 15th 2016];Influenza antiviral medications: summary for clinicians [Google Scholar]
- Chand P, Bantia S, Kotian PL, El-Kattan Y, Lin TH, Babu YS. Comparison of the anti-influenza virus activity of cyclopentane derivatives with oseltamivir and zanamivir in vivo. Bioorg Med Chem. 2005;13:4071–4077. doi: 10.1016/j.bmc.2005.03.048. [DOI] [PubMed] [Google Scholar]
- Gerritz SW, Cianci C, Kim S, Pearce BC, Deminie C, Discotto L, McAuliffe B, Minassian BF, Shi S, Zhu S, Zhai W, Pendri A, Li G, Poss MA, Edavettal S, McDonnell PA, Lewis HA, Maskos K, Mortl M, Kiefersauer R, Steinbacher S, Baldwin ET, Metzler W, Bryson J, Healy MD, Philip T, Zoeckler M, Schartman R, Sinz M, Leyva-Grado VH, Hoffmann HH, Langley DR, Meanwell NA, Krystal M. Inhibition of influenza virus replication via small molecules that induce the formation of higher-order nucleoprotein oligomers. Proc Natl Acad Sci U S A. 2011;108:15366–15371. doi: 10.1073/pnas.1107906108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hai R, Schmolke M, Leyva-Grado VH, Thangavel RR, Margine I, Jaffe EL, Krammer F, Solorzano A, Garcia-Sastre A, Palese P, Bouvier NM. Influenza A(H7N9) virus gains neuraminidase inhibitor resistance without loss of in vivo virulence or transmissibility. Nat Commun. 2013;4:2854. doi: 10.1038/ncomms3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Huang Q, Shi J, Mei Z, Jie Z. The first patient recovered from avian influenza A H7N9 viral infection: A case report and review of the literature. Respir Med Case Rep. 2013;10:23–26. doi: 10.1016/j.rmcr.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herfst S, Chutinimitkul S, Ye J, de Wit E, Munster VJ, Schrauwen EJ, Bestebroer TM, Jonges M, Meijer A, Koopmans M, Rimmelzwaan GF, Osterhaus AD, Perez DR, Fouchier RA. Introduction of virulence markers in PB2 of pandemic swine-origin influenza virus does not result in enhanced virulence or transmission. J Virol. 2010;84:3752–3758. doi: 10.1128/JVI.02634-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry Dunand CJ, Leon PE, Huang M, Choi A, Chromikova V, Ho IY, Tan GS, Cruz J, Hirsh A, Zheng NY, Mullarkey CE, Ennis FA, Terajima M, Treanor JJ, Topham DJ, Subbarao K, Palese P, Krammer F, Wilson PC. Both Neutralizing and Non-Neutralizing Human H7N9 Influenza Vaccine-Induced Monoclonal Antibodies Confer Protection. Cell Host Microbe. 2016;19:800–813. doi: 10.1016/j.chom.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui DS, Lee N, Chan PK. Clinical management of pandemic 2009 influenza A(H1N1) infection. Chest. 2010;137:916–925. doi: 10.1378/chest.09-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev Drug Discov. 2015;14:167–182. doi: 10.1038/nrd4529. [DOI] [PubMed] [Google Scholar]
- Leyva-Grado VH, Tan GS, Leon PE, Yondola M, Palese P. Direct administration in the respiratory tract improves efficacy of broadly neutralizing anti-influenza virus monoclonal antibodies. Antimicrob Agents Chemother. 2015;59:4162–4172. doi: 10.1128/AAC.00290-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li T, Zheng Y, Wong KW, Lu S, Lu H. Poor responses to oseltamivir treatment in a patient with influenza A (H7N9) virus infection. Emerg Microbes Infect. 2013;2:e27. doi: 10.1038/emi.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marjuki H, Mishin VP, Chesnokov AP, De La Cruz JA, Fry AM, Villanueva J, Gubareva LV. An investigational antiviral drug, DAS181, effectively inhibits replication of zoonotic influenza A virus subtype H7N9 and protects mice from lethality. J Infect Dis. 2014;210:435–440. doi: 10.1093/infdis/jiu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendel DB, Tai CY, Escarpe PA, Li W, Sidwell RW, Huffman JH, Sweet C, Jakeman KJ, Merson J, Lacy SA, Lew W, Williams MA, Zhang L, Chen MS, Bischofberger N, Kim CU. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection. Antimicrob Agents Chemother. 1998;42:640–646. doi: 10.1128/aac.42.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JT, Smee DF, Barnard DL, Julander JG, Gross M, de Jong MD, Went GT. Efficacy of combined therapy with amantadine, oseltamivir, and ribavirin in vivo against susceptible and amantadine-resistant influenza A viruses. PLoS One. 2012;7:e31006. doi: 10.1371/journal.pone.0031006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortigoza MB, Dibben O, Maamary J, Martinez-Gil L, Leyva-Grado VH, Abreu P, Jr, Ayllon J, Palese P, Shaw ML. A novel small molecule inhibitor of influenza A viruses that targets polymerase function and indirectly induces interferon. PLoS Pathog. 2012;8:e1002668. doi: 10.1371/journal.ppat.1002668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, Pujanauski LM, Davis AS, Schwartzman LM, Chertow DS, Baxter D, Scherler K, Hartshorn KL, Slemons RD, Walters KA, Kash JC, Taubenberger JK. Contemporary avian influenza A virus subtype H1, H6, H7, H10, and H15 hemagglutinin genes encode a mammalian virulence factor similar to the 1918 pandemic virus H1 hemagglutinin. MBio. 2014;5:e02116. doi: 10.1128/mBio.02116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relenza GSK. [Accessed November 17th 2016];Prescribing information Relenza [Google Scholar]
- Sidwell RW, Huffman JH, Barnard DL, Bailey KW, Wong MH, Morrison A, Syndergaard T, Kim CU. Inhibition of influenza virus infections in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor. Antiviral Res. 1998;37:107–120. doi: 10.1016/s0166-3542(97)00065-x. [DOI] [PubMed] [Google Scholar]
- Smee DF, Barnard DL, Jones SM. Activities of JNJ63623872 and oseltamivir against influenza A H1N1pdm and H3N2 virus infections in mice. Antiviral Res. 2016;136:45–50. doi: 10.1016/j.antiviral.2016.10.009. [DOI] [PubMed] [Google Scholar]
- Thorner AR. Avian influenza A H7N9: treatment and prevention. UpToDate; [Accessed November 17th 2016]. [Google Scholar]
- Tsai AW, McNeil CF, Leeman JR, Bennett HB, Nti-Addae K, Huang C, Germann UA, Byrn RA, Berlioz-Seux F, Rijnbrand R, Clark MP, Charifson PS, Jones SM. Novel Ranking System for Identifying Efficacious Anti-Influenza Virus PB2 Inhibitors. Antimicrob Agents Chemother. 2015;59:6007–6016. doi: 10.1128/AAC.00781-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Xiao X, Lu J, Chen Z, Li K, Liu H, Luo L, Wang M, Yang Z. Factors associated with clinical outcome in 25 patients with avian influenza A (H7N9) infection in Guangzhou, China. BMC Infect Dis. 2016;16:534. doi: 10.1186/s12879-016-1840-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Gao H, Liang W, Tang L, Yang Y, Wu X, Yu L, Chen P, Zheng S, Ou H, Li L. Efficacy of oseltamivir-peramivir combination therapy compared to oseltamivir monotherapy for Influenza A (H7N9) infection: a retrospective study. BMC Infect Dis. 2016;16:76. doi: 10.1186/s12879-016-1383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]