Abstract
Background:
Postoperative transient hypocalcemia is sequelae of total thyroidectomy (TT), which is observed in up to 50% of patients. Routine oral calcium and Vitamin D supplementation have been proposed to prevent symptomatic hypocalcemia preventing morbidity and facilitating early discharge.
Patients and Methods:
A total of 208 patients with nontoxic benign thyroid disorders, undergoing TT, were serially randomized into four groups: Group A (no supplements were given), Group B (oral calcium – 2 g/day given), Group C (calcium and calcitriol – 1 mcg/day are given), and Group D (calcium, calcitriol, and cholecalciferol – 60,000 IU/day are given). Patients were monitored for clinical and biochemical hypocalcemia (serum calcium, [Sr. Ca] <8 mg/dl), along with serum intact parathormone (Sr. PTH) and magnesium 6 h after surgery and Sr. Ca every 24 h. Intravenous (IV) calcium infusion was started, if any of the above four groups exhibit frank hypocalcemia. Patients are followed up with Sr. Ca and Sr. PTH at 3 and 6 months.
Results:
All groups were age and sex matched. Hypocalcemia was observed in 72/208 (34.61%) cases. Incidence of hypocalcemia was higher in Group A (57.69%) and Group B (50%) compared to Group C (15.38%) and Group D (15.38%). Hypocalcemia necessitating IV calcium occurred in 31/208 (14.90%) patients. IV calcium requirement exceeded in Group A (26.92%) and Group B (23.07%) compared to Group C (5.76%) and Group D (3.84%). There was no statistical difference in basal levels of serum Vitamin D, calcium, magnesium, intact PTH, and 6 h after surgery. Permanent hypoparathyroidism developed in five patients on follow-up.
Conclusion:
Routine postoperative supplementation of oral calcium and Vitamin D will help in the prevention of postthyroidectomy transient hypocalcemia significantly. Preoperative Vitamin D levels do not predict postoperative hypocalcemia.
Keywords: Hypocalcemia, total thyroidectomy, Vitamin D
INTRODUCTION
Postoperative transient hypocalcemia is observed in one-third to one-half of patients undergoing total thyroidectomy (TT).[1] It is now considered sequelae of TT rather than a complication. The incidence of permanent hypoparathyroidism is 3% or less.[2] Majority of these transient hypocalcemia may not warrant long-term calcium and Vitamin D supplementation. However, in a significant proportion of these patients, supplemental calcium and Vitamin D either therapeutically or prophylactically may improve the quality of life, shorten the hospital stay, and promote early discharge. Literature is robust on the role of postoperative intact parathormone (iPTH) level in predicting transient and permanent hypocalcemia.[3,4,5] Various factors contribute to postthyroidectomy hypocalcemia such as hypothermia, Vitamin D deficiency, thyrotoxic osteodystrophy, prolonged surgical time, ischemia of the parathyroids, devascularization, or inadvertent removal of parathyroids.[6,7] Few prospective randomized trials exist in the literature on the benefits of prophylactic and routine supplementation of calcium and Vitamin D in prevention of transient hypocalcemia.[8,9,10] This study is an attempt to fill in the lacunae on the impact of routine supplementation of oral calcium and Vitamin D and its efficacy in preventing the morbidity of temporary hypocalcemia and early safe discharge. In this study, we presume that combination of calcium and calcitriol is vital for preventing postoperative hypocalcemia.
PATIENTS AND METHODS
This prospective randomized controlled study was conducted in the Department of Endocrine Surgery, Madras Medical College. This study was conducted between December 2013 and November 2014. Institutional Ethical Committee approval obtained as per requirements (Approval No. 46062014/ECR/270/Inst./TN/2013).
Patients with nontoxic, benign thyroid disorders undergoing TT were included in the study. Exclusion criteria comprise patients with proven or suspicious of malignancy, hyperthyroidism, reoperative surgery, elevated renal parameters (serum creatinine – >1.2), patients already on supplemental calcium, and Vitamin D.
A total of 208 patients comprising 178 females and 30 males were enrolled in the study. Informed written consent was obtained from all patients. The patients were assigned into four groups using systematic sampling randomization method. Four is used as the sampling interval.
Group A is a control group in which no supplements were given; intervention was started only after the onset of clinical or biochemical hypocalcemia. In Group B, patients were started with supplemental oral calcium alone. Calcium carbonate tablets containing 2000 mg of elemental calcium were given in four daily divided doses. In Group C, patients were supplemented with oral calcium (2000 mg/day) and oral calcitriol (1,25-dihydroxyvitamin D3) 1 mcg/day in four divided doses. In Group D along with oral calcium and calcitriol, cholecalciferol (25-hydroxyvitamin D3 [25(OH) D3]) granules 60000 IU/day for 5 days were given. All these supplemental interventions were started 6 h after surgery.
The preoperative investigations include renal function test (blood urea and serum creatinine), thyroid function test (free T3, free T4 and thyroid-stimulating hormone [TSH]), serum calcium (Sr. Ca), Sr. magnesium (Sr. Mg), Sr. phosphorus, Sr. albumin, serum alkaline phosphatase (SAP), Sr. iPTH (Cobas e411; Roche Diagnostics, Tokyo), and Sr. 25(OH) D (DiaSorin; Liaison, von Hevesy-Strasse, Dietzenbach).
TT was performed in all the cases with a standardized technique as per the institution protocol. The procedures were carried out by one of the authors. An attempt was made to identify and preserve all four parathyroid glands. Parathyroid glands, if inadvertently devascularized or removed, were autotransplanted into sternocleidomastoid muscle immediately.
Sr. PTH, Sr. Ca, and Sr. Mg were measured after 6 h, and then, supplemental interventions were started for Groups B, C, and D as per the protocol. Sr. Ca was measured every 24 h for 5 days.
Patients were monitored for clinical and biochemical hypocalcemia and graded accordingly. Symptoms monitored for mild hypocalcemia are perioral numbness, tingling, paresthesiae of the distal extremities, and occasional muscle cramping. For moderate hypocalcemia, more severe muscle cramps and for severe hypocalcemia, tetanic muscle cramps, carpopedal spasm, seizures, life-threatening laryngospasm, and coma were considered.[11] Standard Chvostek's and Trousseau's sign were monitored in the postoperative period every 6th hourly.
Sr. Ca (corrected) <8.0 mg/dL is considered biochemical hypocalcemia. The cutoff values for mild, moderate, and severe hypocalcemia are 7.50–7.99, 7.00–7.49, and <7.0 mg/dL, respectively. Based on the immediate 6-h postoperative levels, PTH values were classified into above low normal range (>12 pg/ml) and deficient (≤12 pg/ml).
Intravenous (IV) calcium was administered for patients who had moderate and severe symptomatic hypocalcemia irrespective of biochemical calcium values. Patients who had mild symptomatic hypocalcemia were managed with oral calcium and Vitamin D. Patients who were asymptomatic, in spite of biochemical hypocalcemia, were managed as per their group stratification.
Patients were followed up with Sr. Ca and Sr. PTH after 3 and 6 months of TT.
Statistical analysis was done using SPSS software (IBM-SPSS statistics 22.0; SPSS Inc., Chicago, IL, USA). Data were expressed as mean ± standard deviation (minimum – maximum). Multivariate analysis was done using logistic regression analysis. Categorical data analysis was done using Chi-square test and Student's t-test. P <0.05 was considered statistically significant.
RESULTS
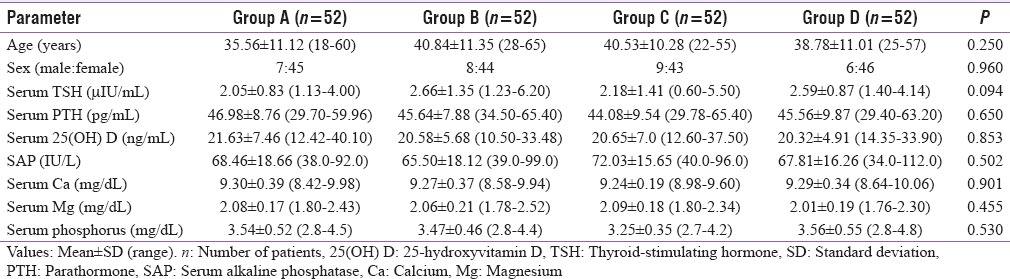
In this present study, a total of 30 (14.4%) males and 178 (85.6%) females were assigned to four study groups using systematic sampling method. The mean age of the study population was 38.93 ± 11.02 (18–65) years, and all the four study groups were age (P = 0.250) and sex matched (P = 0.960). The mean values of various parameters in the preoperative period for the 208 patients include Sr. TSH (2.37 ± 1.16 μIU/ml), Sr. 25(OH) Vitamin D (20.79 ± 6.29 ng/ml), Sr. PTH (45.56 ± 9.00 pg/ml),Sr. Ca (9.27 ± 0.33 mg/dl), SAP (68.45 ± 17.18 U/L), Sr. Mg (2.06 ± 0.19 mg/dl), and Sr. phosphorus (3.45 ± 0.49 mg/dl). There was no statistically significant difference between the mean values among the four study groups. The preoperative investigations of four groups are given in Table 1.
Table 1.
Preoperative levels of various parameters among groups
Intraoperatively, all the four parathyroid glands were identified and preserved with intact vascularity in 148 (71.1%), three glands in 57 (27.4%), and two glands in 3 (1.5%) cases. Autotransplantation of the parathyroid gland was done in 45 (21.6%) cases. The parathyroid gland preservation rate (P = 0.272) and autotransplantation of parathyroid glands (P = 0.191) were comparable among the four groups.
The mean 6-h postoperative values are as follows: Sr. Mg (1.90 ± 0.18 mg/dl), Sr. Ca (8.60 ± 0.57 mg/dl), and Sr. PTH (19.00 ± 9.10 pg/ml). The postoperative PTH values are >12 pg/ml in 109/208 (52.4%) and deficient (≤12 pg/ml) in 99/208 (47.6%). The 6-h postoperative values of Sr. Mg (P = 0.942), Sr. Ca (P = 0.550), and Sr. PTH (P = 0.950) were comparable between study groups.
The mean Sr. Ca values of the 208 study patients from the 1st postoperative day to consecutive 5 postoperative days were 8.57 ± 0.75 mg/dl, 8.72 ± 0.65 mg/dl, 8.93 ± 0.47 mg/dl, 9.12 ± 0.42 mg/dl, and 9.15 ± 0.39 mg/dl, respectively. On the first 3 postoperative days, the Sr. Ca values of Group A and B was significantly lower than Group C and D (P < 0.05), but the calcium levels between Group C and Group D was comparable between them (P > 0.05). The calcium values on day 4 and 5 were comparable between all the groups (P > 0.05). The distribution of postoperative values among the study groups were reported in Table 2 and Chart 1.
Table 2.
Postoperative levels of various parameters among groups
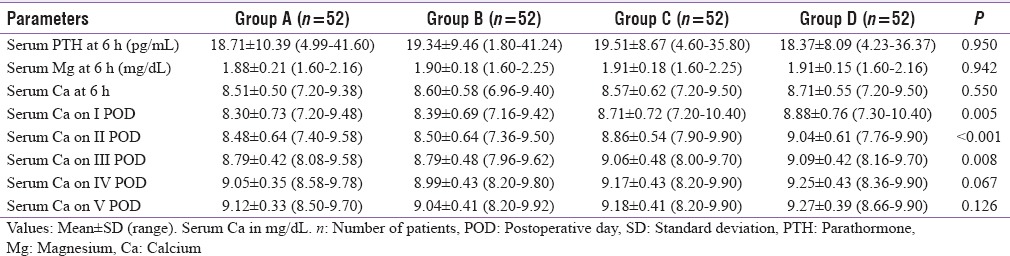
Chart 1.
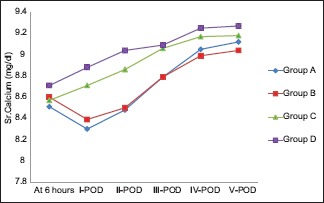
Line diagram showing serum calcium levels among study groups. POD: Postoperative day
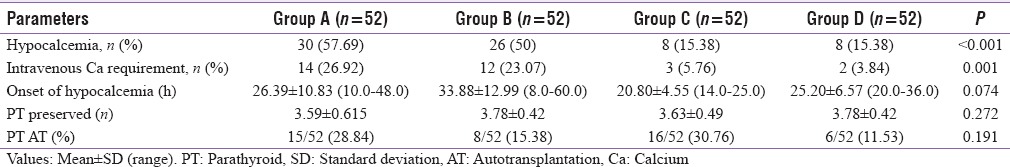
In our study, 72/208 (34.6%) patients developed hypocalcemia, but out of 72 biochemical hypocalcemic patients, only 67 had clinical hypocalcemia. Among 72 biochemical hypocalcemic cases, 41/72 (56.94%) had mild, 28/72 (38.88%) had moderate, and 3/72 (4.16%) had severe hypocalcemia. Out of 67 clinical hypocalcemic cases, 14/67 (20.89%) had mild, 31/67 (46.26%) had moderate, and 22/67 (32.83%) had severe hypocalcemia. The mean duration of onset of hypocalcemia was 27.43 ± 12.34 h. The duration of onset of hypocalcemia among four study groups is comparable (P = 0.271).
Out of the 72/208 hypocalcemic patients, 30/52 (57.69%) were in Group A, 26/52 (50.0%) in Group B, 8/52 (15.38%) in Group C, and 8/52 (15.38%) in Group D. Hypocalcemia was more in Group A and B compared to Group C and D patients (P = 0.000) There was no statistical difference in occurrence of hypocalcemia between individual groups, namely, between A and B as well as between C and D. IV calcium was required in 31/208 (14.9%), 14/52 (26.92%) Group A, 12/52 (23.07%) Group B, 3/52 (5.76%) Group C, and 2/52 (3.84%) Group D cases. Among this subset of hypocalcemic patients, 26/31 belong to Group A and B compared to 5/31 in Group C and D (P = 0.001).
Among the 99/208 patients who had deficient postoperative PTH (<12 pg/ml), only 68/99 had hypocalcemia. In the remaining 31/99 PTH deficient patients who did not have hypocalcemia, 15 were in Group C and 16 in Group D, none from Group A and B (P = 0.001) The rate of hypocalcemia in the four assigned groups is given in Table 3.
Table 3.
Parameters of hypocalcemia among four groups
Parathyroid autotransplantation was done in 45/208 (21.63%) cases comprising 15/52 (28.84%) in Group A, 8/52 (15.38%) in Group B, 16/52 (30.76%) in Group C, and 6/52 (11.53%) in Group D (P = 0.191). Among these 45 cases, 35 (77.77%) developed hypocalcemia, 15/15 (100%) in Group A, 8/8 (100%) in Group B, 8/16 (50%) in Group C, and 4/6 (66.66%) in Group D (P = 0.002). The mean Sr. PTH was lower in parathyroid autotransplanted cases (9.48 ± 3.04 pg/ml) than the remaining patients (21.67 ± 8.42 pg/ml) (P = 0.001) [Table 4].
Table 4.
Comparison parathyroid autotransplanted cases and others
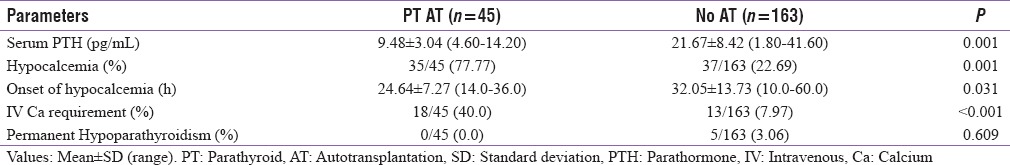
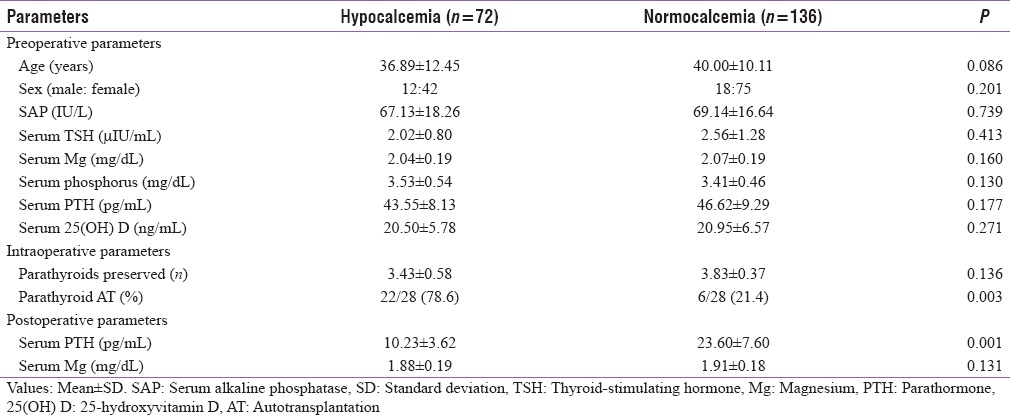
On comparing the hypocalcemic and normocalcemic cohorts, all the preoperative parameters such as age, sex, and biochemical investigations were statistically insignificant. The postoperative Sr. PTH was lower in hypocalcemic (10.23 ± 3.62 pg/ml) than normocalcemic (23.60 ± 7.60 pg/ml) patients (P = 0.001), whereas the rate of parathyroid autotransplantation was higher in hypocalcemic (78.6%) than normocalcemic (21.4%) patients (P = 0.003). The comparisons between both the cohorts are given in Table 5.
Table 5.
Univariate analysis of hypocalcemic and normocalcemic patients
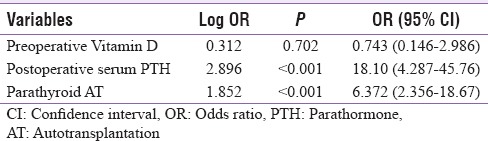
Multivariate analysis performed on factors influencing hypocalcemia found that the preoperative Vitamin D levels did not influence the occurrence of hypocalcemia (P = 0.702). The factors such as parathyroid autotransplantation (odds ratio [OR] = 6.372) and postoperative Sr. PTH levels (OR = 18.10) had a high influence on occurrence of transient hypocalcemia [Table 6].
Table 6.
Multivariate analysis of factors influencing hypocalcemia
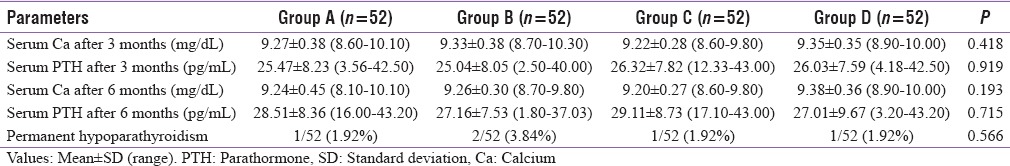
The follow-up levels of Sr. Ca and Sr. PTH after 3 and 6 months are comparable between all four groups. Out of 208 patients, 5 (2.40%) developed permanent hypoparathyroidism on follow-up, but no patients had parathyroid autotransplantation (P = 0.609). The data of follow-up were reported in Table 7.
Table 7.
Follow-up levels of various parameters among groups
DISCUSSION
Postoperative hypocalcemia is important sequelae of TT; it often increases the hospital stay and necessitates multiple blood samplings for serial monitoring of Sr. Ca or PTH levels.[9] Symptomatic hypocalcemia usually develops as late as 24 h to several days after surgery. Postoperative supplementation of oral calcium and Vitamin D may be a useful approach in decreasing or preventing hypocalcemic morbidity and may be a prophylactic tool in the prevention of hypocalcemic crisis.[9] In majority of cases, hypocalcemia resolves spontaneously, but it can remain prolonged in thyrotoxic osteodystrophy and persistent in devascularization and/or inadvertent removal of the parathyroid glands rendering the patient hypoparathyroid or aparathyroid.[10]
Thyrotoxicosis will alter calcium metabolism due to thyrotoxic osteodystrophy. The duration of hyperthyroidism and more importantly the duration of euthyroid state preceding definitive management of hyperthyroidism have a major impact on the postoperative timing and duration of hypocalcemia.[12] Malignancy may warrant lymph node dissection, which would entail extensive dissection with increased probability of vascular compromise to the parathyroid end arteries and inadvertent parathyroid injury. This is more noticeable in patients undergoing central compartment lymph node dissection.[13] The duration of surgery and associated hypothermia might impact on the parathyroid function postoperatively by promoting vasospasm of these parathyroid end arteries. Abnormal renal function will result in altered PTH metabolism, interfering in PTH assay.[14] Completion thyroidectomy or reoperative surgical procedures have been correlated with an increased risk for inadvertent parathyroidectomy because of fibrosis and operative difficulty.[14] For the above said reasons, all these cases are excluded from the study.
In our study, the cohorts were age and sex matched (P = 0.250). Erbil et al. in a study of 130 patients reported that advancing age is a risk factor for hypocalcemia, but age did not have an impact between hypocalcemic and normocalcemic patients in our study.[15]
Our results showed that the preoperative levels of Sr. 25(OH) Vitamin D did not correlate with postoperative Sr. Ca levels and were not a useful predictor of postoperative hypocalcemia which was similar to a study of 103 cases by Chia et al.,[16] but Erbil et al. reported that the preoperative levels of Sr. 25(OH) Vitamin D is a major predictor of postthyroidectomy hypocalcemia.[15]
McLeod et al. showed that postoperative PTH levels <12 pg/ml had 100% sensitivity and 92% specificity for predicting hypocalcemia.[3] Similar results were shown by Sywak et al., and PTH levels predicted postoperative hypocalcemia with a sensitivity of 90% and a specificity of 84%.[4] Grodski and Serpell reiterated this recommendation, reporting that postoperative PTH can be used to accurately predict the development of hypocalcemia and anticipate the need for calcium replacement.[5] However, Lombardi et al. reported that PTH levels <10 pg/ml levels after surgery were unable to accurately predict hypocalcemia in 13.4% of their study population.[17] In our study, 19 patients who received both calcium and Vitamin D supplementation, in spite of deficient PTH (<12 pg/ml), did not develop hypocalcemia.
Bellantone et al. reported hypocalcemia in only 11% compared to 40% of patients without calcium (3 g/day) supplementation.[8] Moore reported that the use of prophylactic calcium (5g/day) alone reduced the risk of hypocalcemic crisis.[18] In our study, the incidence of hypocalcemia in Group B which received only calcium supplementation is similar to Group A which did not receive supplementation (P = 0.189).
On the contrary, the Groups (C and D) which received both calcium and Vitamin D supplements had decreased incidence of hypocalcemia compared to Groups A and B (P = 0.001). Our results are similar to Roh and Park who showed that hypocalcemic symptoms were minimal in calcium and Vitamin D supplemented group but more severe in no intervention group.[19] IV calcium requirement was also increased in the no intervention group, compared to oral calcium and Vitamin D group.
According to a meta-analysis by Alhefdhi et al., there was a significant decrease in the rate of symptomatic hypocalcemia in groups treated with Vitamin D and calcium as compared to no prophylaxis or calcium alone.[20] Similar results were shown by Pisaniello et al., in which early and combined oral administration of both calcium and Vitamin D has great efficacy in preventing postoperative hypocalcemia.[21]
Routine autotransplantation of at least one parathyroid gland has been proposed to prevent permanent hypoparathyroidism but results in an increased risk of postoperative transient hypocalcemia.[22,23] In our study, 78.6% of patients who had parathyroid autotransplantation developed transient hypocalcemia, but none had permanent hypoparathyroidism.
The major limitation of our study is the small sample size. Recruiting more patients and a longer follow-up of 1-year duration may be useful to predict permanent hypoparathyroidism.[24]
CONCLUSION
Supplementation of oral calcium and Vitamin D metabolites reduces the incidence of transient postoperative hypocalcemia and the severity of hypocalcemic symptoms. The requirement of IV calcium infusion is minimized with calcium and Vitamin D supplementation. The addition of cholecalciferol did not provide any added advantage over calcitriol. Preoperative levels of Vitamin D are not a reliable marker to predict the occurrence of hypocalcemia. Immediate autotransplantation of parathyroids results in higher incidence of temporary hypocalcemia but is effective in negating the ill effects of long-term hypocalcemia and permanent hypoparathyroidism.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Wiseman JE, Mossanen M, Ituarte PH, Bath JM, Yeh MW. An algorithm informed by the parathyroid hormone level reduces hypocalcemic complications of thyroidectomy. World J Surg. 2010;34:532–7. doi: 10.1007/s00268-009-0348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reeve T, Thompson NW. Complications of thyroid surgery: How to avoid them, how to manage them, and observations on their possible effect on the whole patient. World J Surg. 2000;24:971–5. doi: 10.1007/s002680010160. [DOI] [PubMed] [Google Scholar]
- 3.McLeod IK, Arciero C, Noordzij JP, Stojadinovic A, Peoples G, Melder PC, et al. The use of rapid parathyroid hormone assay in predicting postoperative hypocalcemia after total or completion thyroidectomy. Thyroid. 2006;16:259–65. doi: 10.1089/thy.2006.16.259. [DOI] [PubMed] [Google Scholar]
- 4.Sywak MS, Palazzo FF, Yeh M, Wilkinson M, Snook K, Sidhu SB, et al. Parathyroid hormone assay predicts hypocalcaemia after total thyroidectomy. ANZ J Surg. 2007;77:667–70. doi: 10.1111/j.1445-2197.2007.04183.x. [DOI] [PubMed] [Google Scholar]
- 5.Grodski S, Serpell J. Evidence for the role of perioperative PTH measurement after total thyroidectomy as a predictor of hypocalcemia. World J Surg. 2008;32:1367–73. doi: 10.1007/s00268-008-9545-5. [DOI] [PubMed] [Google Scholar]
- 6.Thomusch O, Machens A, Sekulla C, Ukkat J, Lippert H, Gastinger I, et al. Multivariate analysis of risk factors for postoperative complications in benign goiter surgery: Prospective multicenter study in Germany. World J Surg. 2000;24:1335–41. doi: 10.1007/s002680010221. [DOI] [PubMed] [Google Scholar]
- 7.Abboud B, Sargi Z, Akkam M, Sleilaty F. Risk factors for postthyroidectomy hypocalcemia. J Am Coll Surg. 2002;195:456–61. doi: 10.1016/s1072-7515(02)01310-8. [DOI] [PubMed] [Google Scholar]
- 8.Bellantone R, Lombardi CP, Raffaelli M, Boscherini M, Alesina PF, De Crea C, et al. Is routine supplementation therapy (calcium and Vitamin D) useful after total thyroidectomy? Surgery. 2002;132:1109–12. doi: 10.1067/msy.2002.128617. [DOI] [PubMed] [Google Scholar]
- 9.Roh JL, Park CI. Routine oral calcium and Vitamin D supplements for prevention of hypocalcemia after total thyroidectomy. Am J Surg. 2006;192:675–8. doi: 10.1016/j.amjsurg.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Kurukahvecioglu O, Karamercan A, Akin M, Tezel E, Ege B, Taneri F, et al. Potential benefit of oral calcium/Vitamin D administration for prevention of symptomatic hypocalcemia after total thyroidectomy. Endocr Regul. 2007;41:35–9. [PubMed] [Google Scholar]
- 11.Bilezikian JE, Marcus R, Levine M. The Parathyroids: Basic and Clinical Concepts. San Diego, California: Academic Press; 2001. Hypoparathyroidism in the differential diagnosis of hypocalcemia; p. 756. [Google Scholar]
- 12.Sasson AR, Pingpank JF, Jr, Wetherington RW, Hanlon AL, Ridge JA. Incidental parathyroidectomy during thyroid surgery does not cause transient symptomatic hypocalcemia. Arch Otolaryngol Head Neck Surg. 2001;127:304–8. doi: 10.1001/archotol.127.3.304. [DOI] [PubMed] [Google Scholar]
- 13.Lin DT, Patel SG, Shaha AR, Singh B, Shah JP. Incidence of inadvertent parathyroid removal during thyroidectomy. Laryngoscope. 2002;112:608–11. doi: 10.1097/00005537-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 14.See AC, Soo KC. Hypocalcaemia following thyroidectomy for thyrotoxicosis. Br J Surg. 1997;84:95–7. [PubMed] [Google Scholar]
- 15.Erbil Y, Bozbora A, Ozbey N, Issever H, Aral F, Ozarmagan S, et al. Predictive value of age and serum parathormone and Vitamin D3 levels for postoperative hypocalcemia after total thyroidectomy for nontoxic multinodular goiter. Arch Surg. 2007;142:1182–7. doi: 10.1001/archsurg.142.12.1182. [DOI] [PubMed] [Google Scholar]
- 16.Chia SH, Weisman RA, Tieu D, Kelly C, Dillmann WH, Orloff LA. Prospective study of perioperative factors predicting hypocalcemia after thyroid and parathyroid surgery. Arch Otolaryngol Head Neck Surg. 2006;132:41–5. doi: 10.1001/archotol.132.1.41. [DOI] [PubMed] [Google Scholar]
- 17.Lombardi CP, Raffaelli M, Princi P, Dobrinja C, Carrozza C, Di Stasio E, et al. Parathyroid hormone levels 4 hours after surgery do not accurately predict post-thyroidectomy hypocalcemia. Surgery. 2006;140:1016–23. doi: 10.1016/j.surg.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Moore FD., Jr Oral calcium supplements to enhance early hospital discharge after bilateral surgical treatment of the thyroid gland or exploration of the parathyroid glands. J Am Coll Surg. 1994;178:11–6. [PubMed] [Google Scholar]
- 19.Roh JL, Park JY, Park CI. Prevention of postoperative hypocalcemia with routine oral calcium and Vitamin D supplements in patients with differentiated papillary thyroid carcinoma undergoing total thyroidectomy plus central neck dissection. Cancer. 2009;115:251–8. doi: 10.1002/cncr.24027. [DOI] [PubMed] [Google Scholar]
- 20.Alhefdhi A, Mazeh H, Chen H. Role of postoperative Vitamin D and/or calcium routine supplementation in preventing hypocalcemia after thyroidectomy: A systematic review and meta-analysis. Oncologist. 2013;18:533–42. doi: 10.1634/theoncologist.2012-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pisaniello D, Parmeggiani D, Piatto A, Avenia N, d'Ajello M, Monacelli M, et al. Which therapy to prevent post-thyroidectomy hypocalcemia? G Chir. 2005;26:357–61. [PubMed] [Google Scholar]
- 22.Zedenius J, Wadstrom C, Delbridge L. Routine autotransplantation of at least one parathyroid gland during total thyroidectomy may reduce permanent hypoparathyroidism to zero. Aust N Z J Surg. 1999;69:794–7. doi: 10.1046/j.1440-1622.1999.01697.x. [DOI] [PubMed] [Google Scholar]
- 23.Lo CY, Lam KY. Routine parathyroid autotransplantation during thyroidectomy. Surgery. 2001;129:318–23. doi: 10.1067/msy.2001.111125. [DOI] [PubMed] [Google Scholar]
- 24.Stack BC, Jr, Bimston DN, Bodenner DL, Brett EM, Dralle H, Orloff LA, et al. American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: Postoperative hypoparathyroidism – Definitions and management. Endocr Pract. 2015;21:674–85. doi: 10.4158/EP14462.DSC. [DOI] [PubMed] [Google Scholar]


