Abstract
Background and Objective:
Hyperprolactinemia affects the reproductive endocrine axis; however, the degree of dysfunction may vary depending on etiology. The aim of the present study was to analyze menstrual cyclicity in patients with prolactinoma and drug-induced hyperprolactinemia (DIH).
Methodology:
Patients with prolactinoma and DIH were retrospectively analyzed for menstrual cyclicity at presentation and response to therapy.
Results:
Of 128 females with hyperprolactinemia, 58 had prolactinoma (41 microadenoma and 17 macroadenoma) and 39 had DIH. Patients with prolactinoma had higher prolactin levels and increased frequency of oligomenorrhea (77.5% vs. 46%) as compared to DIH. Patients with macroprolactinoma had more severe menstrual disturbances compared to microprolactinoma. A higher percentage of patients with microprolactinoma and DIH achieved regular menstrual cycles compared to macroprolactinoma postcabergoline treatment (85% and 90% vs. 65%). There was no correlation between time to regularization of menstrual cycles with age, menstrual cycle length, duration of menstrual irregularity, or initial prolactin level in patients with prolactinoma. Linear regression analysis showed a significant association between time to regularization of menstrual cycles with time to normalization of prolactin levels (P = 0.001).
Conclusion:
There is a prompt restoration of menstrual cycles in patients with microprolactinoma and DIH. Patients with macroprolactinoma have more severe menstrual disturbances and lesser frequency of cycle restoration postcabergoline treatment compared to microprolactinoma and DIH.
Keywords: Drug-induced hyperprolactinemia, hyperprolactinemia, menstrual cycles, periods cyclicity, prolactinoma
INTRODUCTION
Hyperprolactinemia affects the reproductive endocrine axis at multiple levels. Prolactinoma and drug-induced hyperprolactinemia (DIH) are the most common causes of hyperprolactinemia. Menstrual cyclicity may be variably affected in patients with DIH and prolactinoma since drugs and pituitary tumor itself may affect the reproductive endocrine axis.[1,2,3] Common drugs implicated for hyperprolactinemia such as antipsychotic agents alter other neurotransmitters such as histamine, gamma-aminobutyric acid (GABA) and opiates that may affect the hypothalamic-gonadal axis.[4,5,6] On the other hand, patients with prolactinoma may have gonadotrope dysfunction due to mass effect of the tumor. Furthermore, associated thyroid hormone and corticotroph deficiency may also affect menstrual cyclicity. Thus, due to the different etiologic profile, menstrual cyclicity and response to therapy may be different in patients with prolactinoma and DIH. There is paucity of comparative data related to the menstrual cyclicity in patients with prolactinoma and DIH. In the present study, we aimed to analyze the menstrual cycles and response to treatment in patients with prolactinoma and DIH.
METHODOLOGY
Patients with hyperprolactinemia who presented to the pituitary clinic of the Endocrine Department of this hospital from 2010 to 2014 were subjects for this study. Hyperprolactinemia was defined as prolactin levels > 25 ng/ml documented on two separate occasions with at least one sample estimated in pooled sera. Males with hyperprolactinemia were excluded from the study. Data related to presenting complaints, clinical examination, biochemical and hormonal parameters, radiologic imaging, and relevant history including drug history, etiologic diagnosis, and treatment were noted from the clinical records. Patients were subgrouped based on the clinical diagnosis. A note of clinical symptoms and menstrual cyclicity at presentation and posttreatment during follow-up was made. Regular menstrual cycles were considered when menstrual cyclicity was at interval of 21–35 days. Missing data were recorded from patients during subsequent clinic visits or telephonically. Prolactin, thyroid function tests, luteinizing hormone (LH), follicle-stimulating hormone (FSH), and estradiol levels were performed by chemiluminescence (vitros ECIQ, Johnson and Johnson).
Statistical analysis
All the data were entered into Microsoft Excel and converted into SPSS version 11 (Chicago, Illinois, USA). The data are expressed as mean ± standard deviation. Patients were subdivided into various groups according to etiologic diagnosis. Independent t-test was used to compare the parametric variables between patients with prolactinoma and DIH. Chi-square test was used for the comparison of nonparametric variables. Mann–Whitney U-tests and Kruskal–Wallis tests were performed to calculate the P value where the data were not normally distributed. P value was considered statistically significant at values <0.05. Pearson's correlation between linear variables was performed where appropriate. Linear regression analysis was performed with duration to normalization of regular cycles as the dependent variable.
RESULTS
One hundred and twenty-eight females with hyperprolactinemia with average age of 32.2 ± 9.4 years (15–64 years) presented during the study period. Of 128, 39 patients (30.5%) had DIH, 58 (44.5%) had prolactinoma, 8 (6.3%) had nonsecretory adenoma, 4 (3.1%) had acromegaly, and 4 had primary hypothyroidism whereas there was a single patient each with pituitary abscess and partial empty sella. Thirteen patients (10.2%) were classified as idiopathic hyperprolactinemia since no diagnosis could be made.
Of 128 patients, 32 (25%) had regular periods, 89 (69.5%) had irregular periods whereas 7 (5.4%) patients were postmenopausal. Out of 58 patients with prolactinoma, 41 patients had microadenoma whereas 17 had macroadenoma. DIH was attributed to prokinetics in 24, antipsychotics in 13, and oral contraceptives in 2 patients. Table 1 gives the comparative frequency of different medications causing hyperprolactinemia. Prokinetic agents were the most common drugs causing hyperprolactinemia followed by antipsychotic agents. Patients with prolactinoma had a greater frequency of headache (77.5% vs. 30.7%) and visual complaints (27.5% vs. 2.56%) compared to those with DIH.
Table 1.
Frequency of drugs causing hyperprolactinemia
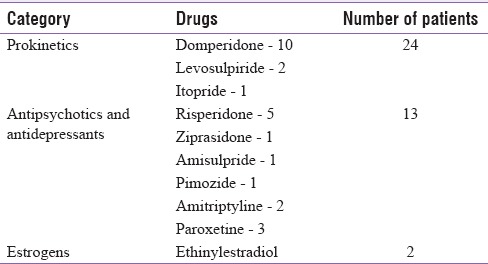
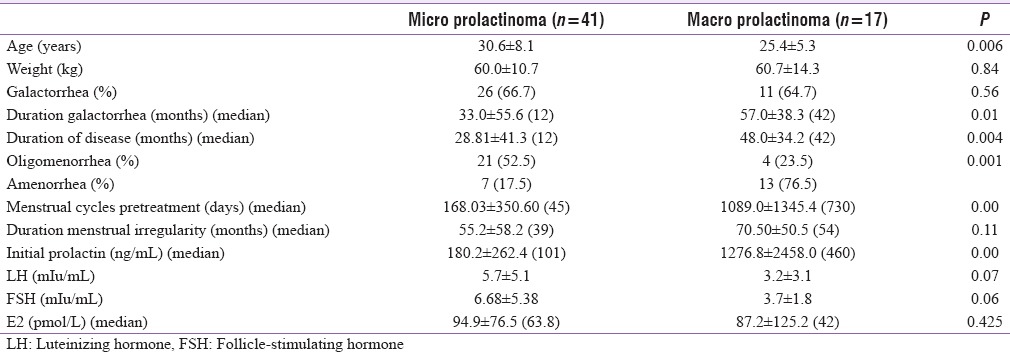
Table 2 gives the comparative clinical and hormonal data between patients with DIH and prolactinoma. Patients with DIH were older and more obese as compared to patients with prolactinoma. Initial prolactin levels were higher in patients with prolactinoma (P = 0.046); however LH, FSH, and estradiol levels were comparable in the two groups. Thyroid and adrenal function dysfunction was also comparable in the two groups (32.0% and 39.0% and 0.0% and 7.5%, respectively). Patients with DIH had a higher frequency of galactorrhea. Patients with prolactinoma had a greater degree of menstrual abnormalities (77.5% vs. 46.1%), along with greater duration of menstrual abnormalities as compared to patients with DIH [Figure 1]. Table 3 gives the comparative clinical and biochemical parameters in patients with microprolactinoma versus macroprolactinoma. Patients with macroprolactinoma were younger, had greater degree of menstrual irregularities, and had higher prolactin levels as compared to patients with microprolactinoma.
Table 2.
Clinical and hormonal parameters between drug-induced hyperprolactinemia and prolactinoma
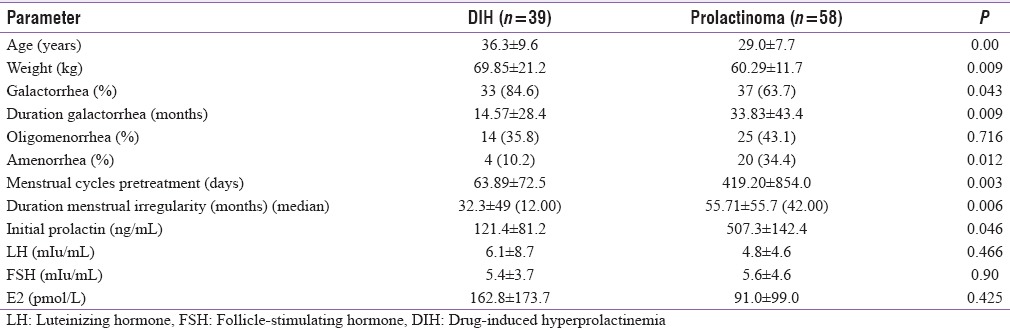
Figure 1.
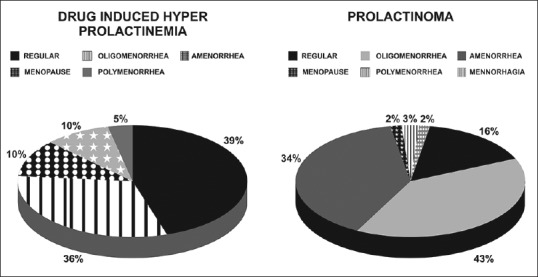
Comparative menstrual patterns in patients with drug-induced hyperprolactinemia and prolactinoma
Table 3.
Clinical and biochemical characteristics in patients with microprolactinoma versus macroprolactinoma
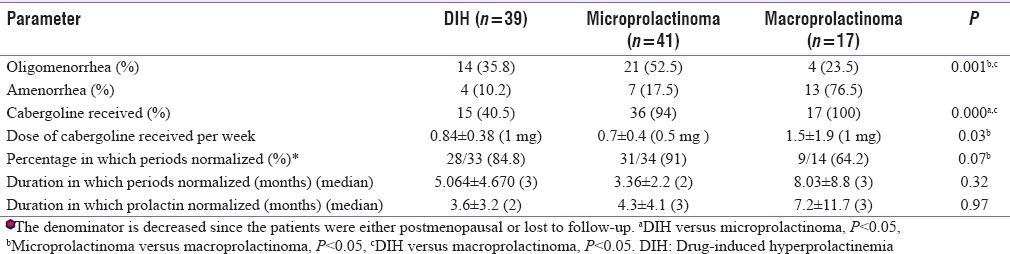
The management for DIH included either stopping the causative agent or change to atypical antipsychotics or cabergoline where causative agent could not be changed. A greater number of patients with prolactinoma received cabergoline in comparison to patients with DIH [Table 4]. In spite of a greater degree of menstrual cycle irregularity in patients with microprolactinoma, the patients who attained regular periods were comparable between patients with DIH and microprolactinoma. The duration to achieve regular periods was also comparable in the two groups. A higher number of patients with microprolactinoma achieved regular menstrual cycles as compared to those with macroprolactinoma (91% vs. 64%). Eight patients conceived, of which there were four normal deliveries, three MTP, and one abortion. Patients with macroprolactinoma who continued to have irregular periods either had panhypopituitarism, coexisting polycystic ovaries, hypothyroidism or were the ones who did not regularly follow the treatment. One patient who did not achieve regularity of periods with cabergoline given 5 mg/week and still has her prolactin levels ranging from 148 to 442 ng/ml has been asked to get operated second time.
Table 4.
Response to treatment in patients with drug-induced hyperprolactinemia and microprolactinoma and macroprolactinoma
The mean duration of irregular periods in patients with prolactinoma before initiation of treatment was around 42 months. The duration to normalization of periods in these patients with prolactinoma was around 3–5 months. Patients with amenorrhea had higher prolactin levels as compared to those with oligomenorrhea (410.9 ± 486.48 ng/ml vs. 162.76 ± 162.94 ng/ml, P = 0.005). There was no correlation between time to regularization of menstrual cycles with age (P = 0.32), menstrual cycle length pretreatment (P = 0.78), duration of menstrual irregularity (P = 0.8), initial prolactin level (P = 0.91), LH, FSH, or estradiol levels (P = NS). There was a significant correlation between time to regularization of menstrual cycles and time to normalization of prolactin levels (P = 0.00). Linear regression analysis also showed association between these two parameters (P = 0.001). There was no difference in time to regularization of periods in patients with oligomenorrhea versus amenorrhea (4.32 ± 3.94 months vs. 5.2 ± 7.03 months, respectively, P = 0.087). Four patients with amenorrhea of 3–4 years duration had regular periods within 1–4 months. One patient with macroprolactinoma and amenorrhea of 9 years achieved regular menstrual cycles within 2 months of cabergoline treatment. She subsequently had two pregnancies.
DISCUSSION
Hyperprolactinemia commonly affects the hypothalamic-gonadal axis of females at multiple levels. The most common abnormality is inhibition of normal pulsatile secretion of gonadotropin-releasing hormone of the hypothalamus.[7,8] Prolactin also has direct effects on the ovary. In vitro perfusion studies of human ovaries show that prolactin directly suppresses progesterone and estrogen secretion.[9] Prolactin inhibits estrogen formation by antagonizing the stimulatory effects of FSH on aromatase activity and directly inhibiting aromatase activity.[10,11] Polycystic changes in the ovaries have also been described in patients with hyperprolactinemia.
In the present study, we observed that prolactin-secreting tumors followed by drugs were the most common causes of hyperprolactinemia. Patients with DIH were older and more obese as compared to those with prolactinoma. Prolactin levels were lower; however, frequency of galactorrhea was higher in patients with Drug induced hyperprolactinemia compared to prolactinoma. Previous studies have also shown that frequency of galactorrhea is independent of prolactin levels.[1,3] Patients with prolactinoma had greater incidence of amenorrhea and lower estrogen levels. Yazigi et al. speculated that in hypoestrogenemic states, high levels of prolactin fail to stimulate milk production, which together with an individual response in breasts, may partly explain the lack of galactorrhea in some patients.[2] This could explain a lower frequency of galactorrhea in patients with prolactinoma compared to DIH (84% vs. 66%) in the present study in spite of higher prolactin levels. Berinder et al. also observed that galactorrhea was present in around 50% of patients with prolactinoma.[3]
We observed a high frequency of menstrual disorders (around 80%) in patients with prolactinoma. Apart from effects of high prolactin on the hypothalamaic gonadal axis, menstrual disorders in patients with prolactinoma could also be caused by structural damage to the gonadotropes by large tumors. In the present study, around 44% and 35% of patients with prolactinoma had oligomenorrhea and amenorrhea, respectively. Berinder et al. in a study on 271 women with hyperprolactinemia (71% of whom had prolactinomas) observed that 26% had oligomenorrhea and 61% had amenorrhea.[3] Touraine et al. similarly observed in their cohort of 122 patients with prolactinoma that around 66% patients had amenorrhea and 26% oligomenorrhea.[12] The frequency of amenorrhea was much higher in these two studies compared to our study. This could be because both these studies included patients with idiopathic hyperprolactinemia. Furthermore, the frequency of macroadenomas was higher in the study by Touraine (36%) compared to our study (29%).
Around 52% of patients with DIH had menstrual abnormalities in the present study. Prokinetics were the most common cause of hyperprolactinemia followed by antipsychotics. Prokinetic drugs, commonly used in gastrointestinal disorders, induce hyperprolactinemia through a dopamine antagonistic mechanisms.[13] The prevalence rates of menstrual abnormalities with antipsychotic agents range between 15 and 50%.[6] In female patients with psychosis, apart from effects of antipsychotic agents, menstrual irregularities may be partly due to dysfunction of the hypothalamic–pituitary–gonadal axis and partly attributed to abnormalities of neurotransmitters or their receptors including D1, D2, histamine, serotonin, GABA, and opiates.[4] Antidepressant drugs with serotoninergic activity, including selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, and some tricyclics, can cause modest and generally asymptomatic hyperprolactinemia.[13] Two patients in the present study were taking estrogens. In the studies documenting hyperprolactinemia, the incidence among women on oral contraceptives containing estrogens is variously reported from 12% to 30%.[13,14]
There were more severe menstrual abnormalities in patients with prolactinoma compared to DIH. Frequency of amenorrhea and duration of menstrual irregularities were higher and estrogen levels lower among patients with prolactinoma. Patients with macroprolactinoma were younger, had greater degree of menstrual irregularities, and had higher prolactin levels as compared to patients with microprolactinoma. In spite of a greater degree of menstrual dysfunction among patients with microprolactinoma compared to DIH, there was a prompt restoration of menstrual cyclicity in around 90% patients with cabergoline treatment. Cabergoline is a selective and long-lasting dopamine agonist, which inhibits prolactin secretion with a duration of action up to 21 days after single oral doses of 0.3–1 mg.[15] Colao et al. observed that 82% of 145 patients with hyperprolactinemia resumed periods within 6 months.[16] Webster observed that 72% of patients treated with cabergoline had regular menstrual cycles, but excluded were the patients with prolactinoma.[17] Berinder et al. observed that around 94% of their patients achieved regular menstrual cycles with cabergoline treatment. However, the time required to achieve regular menstrual cycles was not given in this study.[3]
Patients with macroprolactinoma had a greater degree of menstrual dysfunction compared to DIH and microprolactinoma (75% amenorrhea vs. 10% and 17%, respectively). Verhelst et al. also reported more frequent menstrual disturbances in macroprolactinoma versus microprolactinoma (76% vs. 100%).[18] Patients with macroprolactinoma also required a higher cabergoline dose compared to patients with microprolactinoma (1 mg per week vs. 0.5 mg per week) in the present study. Verhelst also similarly observed that patients with a macroprolactinoma needed a higher median cabergoline dose, compared with those with idiopathic hyperprolactinemia or a microprolactinoma: 1.0 mg/week versus 0.5 mg/week. Patients attaining regular menstrual cycles were also significantly lesser in patients with macroprolactinoma compared to DIH and microprolactinoma (65% vs. 84% and 91% respectively). While resumption of menstrual cyclicity has been variably reported from 72% to 90% in various series, we could not find data related to comparative menstrual cyclicity between microprolactinoma and macroprolactinoma postcabergoline treatment.[3,12,18]
The median duration of restoration of regular menstrual cycles among patients with prolactinoma in the present study was around 3–5 months. The duration to regularization of menstrual cycles correlated with time required for normalization of prolactin levels. There was no difference in the time required to achieve regular menstrual cycles among patients with amenorrhea versus oligomenorrhea. Patients with amenorrhea had higher prolactin levels as described previously.[3,12] This indicates that neither the prolactin levels nor the duration or severity of menstrual abnormalities affected the restoration to regular periods in patients with prolactinoma. Such a prompt restoration of gonadal function was also reported among 17 males with prolactinoma by De Rosa et al.[19] They observed improvement of libido within 2 months and sperm counts within 3 months of cabergoline treatment. They speculated that prolactin not only affects sexual steroid secretion but also plays a pivotal role in spermatogenesis. Prolactin receptors are present in all stages of the cycle of the seminiferous epithelium, the surface of Leydig and Sertoli cells, all phases of spermatogonia and spermatocytes in male rats.[20]
One patient with macroprolactinoma and amenorrhea of 9 years achieved regular menstrual cycles within 2 months of cabergoline treatment. Author has seen an 11-year-old amenorrheic patient with prolactinoma who became pregnant within 1–2 months of cabergoline initiation. This is surprising since even hypogonadol females with such a long duration of amenorrhea require estrogen priming before progesterone replacement for endometrial shedding.
A limitation in our study is that it was retrospective in nature and performed in a tertiary hospital which might not be representative of community phenotypic presentations of hyperprolactinemia. However, it provides a follow-up on outcomes, especially related to menstrual cyclicity in DIH and prolactinoma, the two most common causes of hyperprolactinemia. In spite of a greater severity of menstrual abnormalities in patients with microprolactinoma compared to DIH, there is a prompt restoration of regular menstrual cycles with cabergoline treatment in around 90% patients. Patients with macroprolactinoma have more severe menstrual disturbances and lesser frequency of cycle restoration compared to microprolactinoma postcabergoline treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Schlechte J, Sherman B, Halmi N, VanGilder J, Chapler F, Dolan K, et al. Prolactin-secreting pituitary tumors in amenorrheic women: A comprehensive study. Endocr Rev. 1980;1:295–308. doi: 10.1210/edrv-1-3-295. [DOI] [PubMed] [Google Scholar]
- 2.Yazigi RA, Quintero CH, Salameh WA. Prolactin disorders. Fertil Steril. 1997;67:215–25. doi: 10.1016/S0015-0282(97)81900-0. [DOI] [PubMed] [Google Scholar]
- 3.Berinder K, Stackenäs I, Akre O, Hirschberg AL, Hulting AL. Hyperprolactinaemia in 271 women: Up to three decades of clinical follow-up. Clin Endocrinol (Oxf) 2005;63:450–5. doi: 10.1111/j.1365-2265.2005.02364.x. [DOI] [PubMed] [Google Scholar]
- 4.Haddad PM, Wieck A. Antipsychotic-induced hyperprolactinaemia: Mechanisms, clinical features and management. Drugs. 2004;64:2291–314. doi: 10.2165/00003495-200464200-00003. [DOI] [PubMed] [Google Scholar]
- 5.Madhusoodanan S, Parida S, Jimenez C. Hyperprolactinemia associated with psychotropics – A review. Hum Psychopharmacol. 2010;25:281–97. doi: 10.1002/hup.1116. [DOI] [PubMed] [Google Scholar]
- 6.Santoni JP, Saubadu S. Adverse events associated with neuroleptic drugs: Focus on neuroendocrine reactions. Acta Ther. 1995;21:193–204. [Google Scholar]
- 7.Sauder SE, Frager M, Case GD, Kelch RP, Marshall JC. Abnormal patterns of pulsatile luteinizing hormone secretion in women with hyperprolactinemia and amenorrhea: Responses to bromocriptine. J Clin Endocrinol Metab. 1984;59:941–8. doi: 10.1210/jcem-59-5-941. [DOI] [PubMed] [Google Scholar]
- 8.Winters SJ, Troen P. Altered pulsatile secretion of luteinizing hormone in hypogonadal men with hyperprolactinaemia. Clin Endocrinol (Oxf) 1984;21:257–63. doi: 10.1111/j.1365-2265.1984.tb03467.x. [DOI] [PubMed] [Google Scholar]
- 9.Demura R, Ono M, Demura H, Shizume K, Oouchi H. Prolactin directly inhibits basal as well as gonadotropin-stimulated secretion of progesterone and 17 beta-estradiol in the human ovary. J Clin Endocrinol Metab. 1982;54:1246–50. doi: 10.1210/jcem-54-6-1246. [DOI] [PubMed] [Google Scholar]
- 10.Dorrington JH, Gore-Langton RE. Antigonadal action of prolactin: Further studies on the mechanism of inhibition of follicle-stimulating hormone-induced aromatase activity in rat granulosa cell cultures. Endocrinology. 1982;110:1701–7. doi: 10.1210/endo-110-5-1701. [DOI] [PubMed] [Google Scholar]
- 11.Krasnow JS, Hickey GJ, Richards JS. Regulation of aromatase mRNA and estradiol biosynthesis in rat ovarian granulosa and luteal cells by prolactin. Mol Endocrinol. 1990;4:13–12. doi: 10.1210/mend-4-1-13. [DOI] [PubMed] [Google Scholar]
- 12.Touraine P, Plu-Bureau G, Beji C, Mauvais-Jarvis P, Kuttenn F. Long-term follow-up of 246 hyperprolactinemic patients. Acta Obstet Gynecol Scand. 2001;80:162–8. doi: 10.1034/j.1600-0412.2001.080002162.x. [DOI] [PubMed] [Google Scholar]
- 13.Torre DL, Falorni A. Pharmacological causes of hyperprolactinemia. Ther Clin Risk Manag. 2007;3:929–51. [PMC free article] [PubMed] [Google Scholar]
- 14.Luciano AA, Sherman BM, Chapler FK, Hauser KS, Wallace RB. Hyperprolactinemia and contraception: A prospective study. Obstet Gynecol. 1985;65:506–10. [PubMed] [Google Scholar]
- 15.Ferrari C, Crosignani PG. Medical treatment of hyperprolactinaemic disorders. Hum Reprod. 1986;1:507–14. doi: 10.1093/oxfordjournals.humrep.a136464. [DOI] [PubMed] [Google Scholar]
- 16.Colao A, Sarno AD, Cappabianca P, Briganti F, Pivonello R, Somma CD, et al. Gender differences in the prevalence, clinical features and response to cabergoline in hyperprolactinemia. Eur J Endocrinol. 2003;148:325–31. doi: 10.1530/eje.0.1480325. [DOI] [PubMed] [Google Scholar]
- 17.Webster J, Piscitelli G, Polli A, Ferrari CI, Ismail I, Scanlon MF. A comparison of cabergoline and bromocriptine in the treatment of hyperprolactinemic amenorrhea. Cabergoline Comparative Study Group. N Engl J Med. 1994;331:904–9. doi: 10.1056/NEJM199410063311403. [DOI] [PubMed] [Google Scholar]
- 18.Verhelst J, Abs R, Maiter D, van den Bruel A, Vandeweghe M, Velkeniers B, et al. Cabergoline in the treatment of hyperprolactinemia: A study in 455 patients. J Clin Endocrinol Metab. 1999;84:2518–22. doi: 10.1210/jcem.84.7.5810. [DOI] [PubMed] [Google Scholar]
- 19.De Rosa M, Colao A, Di Sarno A, Ferone D, Landi ML, Zarrilli S, et al. Cabergoline treatment rapidly improves gonadal function in hyperprolactinemic males: A comparison with bromocriptine. Eur J Endocrinol. 1998;138:286–93. doi: 10.1530/eje.0.1380286. [DOI] [PubMed] [Google Scholar]
- 20.Hondo E, Kurohmaru M, Sakai S, Ogawa K, Hayashi Y. Prolactin receptor expression in rat spermatogenic cells. Biol Reprod. 1995;52:1284–90. doi: 10.1095/biolreprod52.6.1284. [DOI] [PubMed] [Google Scholar]


