Abstract
Background:
Diabetes mellitus (DM) is recognized as an important risk factor to tuberculosis (TB). India has high TB burden, along with rising DM prevalence.
Aim:
This study was conducted to document the coexistence of DM and TB in persons with established TB under the Revised National Tuberculosis Control Program.
Type of Study:
This was a cross-sectional, descriptive observational study conducted at selected Directly Observed Therapy center in Gwalior North Central India.
Materials and Methods:
A total of 550 patients with confirmed diagnosis of TB and on treatment were recruited. The study participants were screened for DM and diagnoses were made on the basis of the World Health Organization criteria. Clinical parameters were compared between persons with DM and those without DM.
Results:
DM/TB co-morbidity was noted in 85 individuals and these made up 15.4% of the study population. The mean age was higher in DM patients with TB (43.4 ± 15.4 vs. 33.1 ± 16.2 years, P = 0.000); the mean duration of symptoms of TB with DM was more (124 ± 16.4 vs. 107.49 ± 10.3 days). Multinomial logistic regression analysis showed that increasing age, positive family history of diabetes, sedentary occupation, and presence of pulmonary TB were significantly associated with diabetes among TB patients.
Conclusions:
Diabetes is an important co-morbid feature to be sought in patients with TB. This study re-echo the need to raise awareness on screening for DM in persons with TB.
Keywords: Comorbidity, diabetes mellitus II, tuberculosis
INTRODUCTION
In the recent decades, with the increasing prevalence of TB, particularly multidrug-resistant tuberculosis (TB), and diabetes mellitus (DM) cases in the world, the relationship is re-emerging as a significant public health problem. The link of DM and TB is more prominent in developing countries where TB is endemic and the prevalence of DM is rising. According to the World Health Organization (WHO), 180 million people are suffering from diabetes worldwide, and it is likely to double in the next 19–20 years, i.e., by the year 2030.[1] About 95% of patients with TB and 70% of patients with DM live in the low- and middle-income countries.[2] The epidemic growth of DM has occurred in developing countries where TB is highly endemic. As a result, DM and TB are increasingly present together, and this calls for renewed interest in this topic.[3] India is facing the dual problem of being the highest TB-burden country having a large number of people with diabetes posing a serious challenge for the health system.[4,5]
Diabetes has long been known to be a risk factor for active TB and reactivation of latent TB. It is also associated with worse TB treatment outcomes. In addition, TB infection in itself can worsen glycemic control. Drug–drug interactions can further complicate the picture, leading to a reduction in the effectiveness of both TB and diabetes treatments, and potential worsening of drug side effects. The presence of DM can cause increase in blood glucose levels, and persistent hyperglycemic levels can have a negative impact on presentation and outcome of TB. According to various previous studies, the chances of treatment failure increase in patients with uncontrolled hyperglycemia and also chances of death during treatment of TB increases in patients with comorbidity than in the ones with normal glycemic status. Recent studies predict that in India, approximately 18.4% of people with pulmonary TB (PTB) cases have diabetes.[6] The increase in diabetes prevalence in India seems to have contributed to the absence of a decrease in TB incidence between 1998 and 2008, despite improvement in TB treatment. Adding the increasing burden of diabetes and TB into the mix will be an extra strain with which many countries will struggle to cope. The fact that the diabetes will worsen the burden of TB like communicable diseases could be the straw that breaks the camel's back for some health systems; on the other hand, this interaction between TB and diabetes could provide the wake-up call that health providers need to kick the National Health Control/Prevention Programmes into action.[7] Since the risk of developing TB is more likely in diabetic patients, this correlation between diabetes and TB could have a negative impact on TB control programs.[8] With following rationale in mind, this study was conducted to estimate the prevalence of impaired glucose tolerance in diagnosed cases of TB and determine various sociodemographic and clinical factors that may be associated with the same retrospectively. All patients included in the study were started with initial intensive phase therapy.
MATERIALS AND METHODS
This study was conducted at the outpatient clinic of the directly observed treatment short-course (DOTS) centers for TB at the Revised National Tuberculosis Control Unit facility in Gwalior district, and the duration was for 6 months. The study was approved by the Research and Ethic Committee of the Gajra Raja Medical College, Gwalior. Informed and written consent were obtained from the study patients. All those who registered for DOTS in these TUs during July 2016 to December 2016 and meeting the selection criteria aged ≥ 18 years. PTB cases confirmed with sputum smear for acid-fast bacilli and X-ray, and extrapulmonary cases confirmed with culture of specimen from the site and or histological evidence were included. Diagnosis of TB was based on standard diagnostic criteria of the Revised National Tuberculosis Control Program.[9] Individuals with type I diabetes were excluded from the study. After identification of sampling frame, cluster random sampling technique was used to recruit the study individuals. The sample size was calculated using the formula N = 4 × pq/L2. The prevalence of diabetes among TB patients (p) was taken 25% in account based on related studies conducted earlier[10,11,12] with allowable error (L) was 15%. Hence, the sample size was 533 which was the minimum sample size and we had taken 550 for rounded off convenience.
Thus, 550 consecutive patients with physician-diagnosed TB (both pulmonary and extrapulmonary) who consented were recruited into the study. Random blood glucose was done in all patients with a glucometer. DM was diagnosed if random blood glucose was > 200 mg/dl in accordance with the WHO guidelines.[13] Random Blood Sugar (RBS) values between 140 and 200 mg/dl were considered as prediabetes. Physical assessment that was done included measurement of weight (kg) and height in meters and also the body mass indices calculated. Patients suffering from TB with no history of diabetes and with normal blood glucose level on screening will be included in normoglycemic TB patients. A pretested questionnaire was administered to collect information regarding sociodemographics and habitual risk factors, namely, smoking, alcohol consumption, and other forms of tobacco use, family history of TB and DM, educational and occupational status, and monthly per capita income. Type of TB, status of TB treatment, and category of treatment were also recorded. Patients already diagnosed with diabetes were interviewed to elicit and record information on duration of diabetes, complications if any, the place where treatment was being undertaken, type of treatment, and adherence to treatment. Socioeconomic status of the study participants was classified according to Agarwal classification based on the latest AICPI of India and per capita income.[14]
Statistical analysis
The study participants were classified into “Dysglycemic TB” and “Normoglycemic TB” group. Further analysis of different variables was done for both groups separately. The analysis was done using EpiInfo version 7, Epical and MS Excel software. The data were analyzed and expressed in the form of mean, standard deviation and 95% confidence intervals (CIs) wherever required. Univariate odds ratio (OR) was calculated as an estimate for relative risk (OR) with 95% CI. Chi-square test and t- test (difference of means) were applied to determine the P value and statistical significance. P <0.05 was considered statistically significant.
RESULTS
The recorded response rate was 95.15%. A total of 550 patients with established TB participated in the study. The sociodemographic information is detailed in Table 1. Gender-wise distribution of study individuals in this study reveals that majority (65.3%) were male. The odds of males developing diabetes with preexisting TB were 2.04 times higher as compared to females, and this was found statistically significant (P = 0.009). The age range of the patients was between 15 and 80 years with the mean age 33.1 ± 16.2 years while the mean age of patients with TB and DM was 43.4 ± 15.4 years and this difference was statistically significant (P = 0.001). DM was documented in all age groups of patients with TB, but patients with DM and TB co-morbidity were significantly older than patients with TB without DM. There were 19 (22.3%) males and 66 (77.6%) females with dysglycemic TB patients. The prevalence of diabetes with TB was highest (36.5%,) in the age group of 31–45 years that was statistically significant (P = 0.005) with OR = 6.4, while highest in normoglycemic was in the age group of 16–30 years (43.9%). The prevalence of TB with diabetes was higher among urban residents than among rural residents (52% vs. 48%), but it was not statistically significant (P = 0.264, OR = 1.3). The majority of the study participants (32.9% dysglycemic and 30.9% normoglycemic TB patients) were illiterate and the chances were more (OR = 1.09) of developing diabetes with TB among illiterates, but it was not statistically significant (P = 0.7). More than half of the dysglycemic and normoglycemic TB patients, 55.3% and 50.9% respectively, were unskilled workers by profession. The odds of developing diabetes among TB patients were highest (OR = 3.68) for people in professional line of work as compared to unemployed people, but the overall difference based their occupation was not statistically significant (P = 0.75). The least proportion of respondents, i.e., 2.3% dysglycemic and 3.1% normoglycemic TB patients respectively, belong to Class I socioeconomic status. The odds of developing diabetes among TB patients increase with decreasing socioeconomic status. However, this difference was found to be statistically insignificant (P = 0.99). The proportion of positive history of diabetes among their parents was significantly higher among dysglycemic TB patients (7.1%) as compared to normoglycemic TB patients (2.6%) (P = 0.33). The percentage of smokers was significantly (P = 0.042, OR = 1.65) higher among dysglycemic TB patients (36.5%) as compared to normoglycemic (25.8%) while proportion of alcoholics also 1.08 times higher among dysglycemic TB patients but was not found statistically significant (P = 0.79). Continuous adherence with DOT were found less (OR = 0.49) in the dysglycemic TB patients then normoglycemic TB patients, but it was not found statistically significant (P = 0.20).
Table 1.
Comparison of sociodemographic variables among tuberculosis patients with diabetes and normoglycemia (n=550)

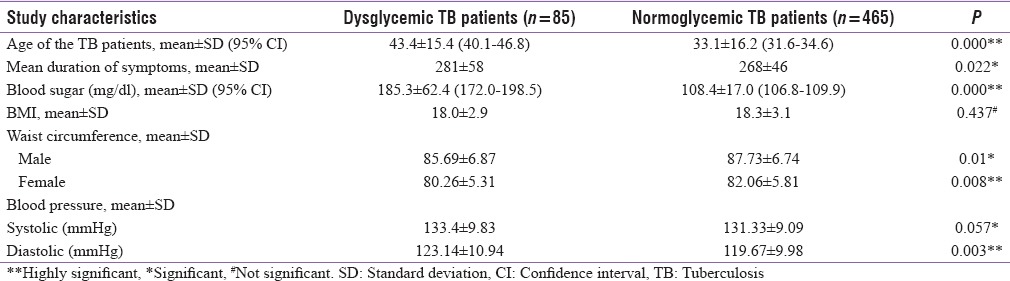
Table 2 shows the comparison of the anthropometric and other study characteristics among patients with normoglycemic and diabetes. The mean duration of symptoms (in days) was 281 (±58) in dysglycemic TB patients while in normoglycemic was 268 (±46) days and this was found statistically significant (P = 0.022). Among 351 TB patients, DM was present in 20 (5.7%). As expected, random plasma glucose levels were statistically significant between both groups (P = 0.001). Mean BMI of the dysglycemic TB patients was less (18.0 ± 2.9) than the normoglycemic, but it was not found significant statistically (P < 0.05). Waist circumference in both genders (male: 87.73 ± 6.74; female 82.06 ± 5.8) and blood pressure [systolic as well as diastolic blood pressure] were found significantly higher in normoglycemic TB patient when compared to dysglycemic TB patients (P < 0.05).
Table 2.
Comparison of anthropometric and other study characteristics in diabetic and nondiabetic tuberculosis patients (n=550)
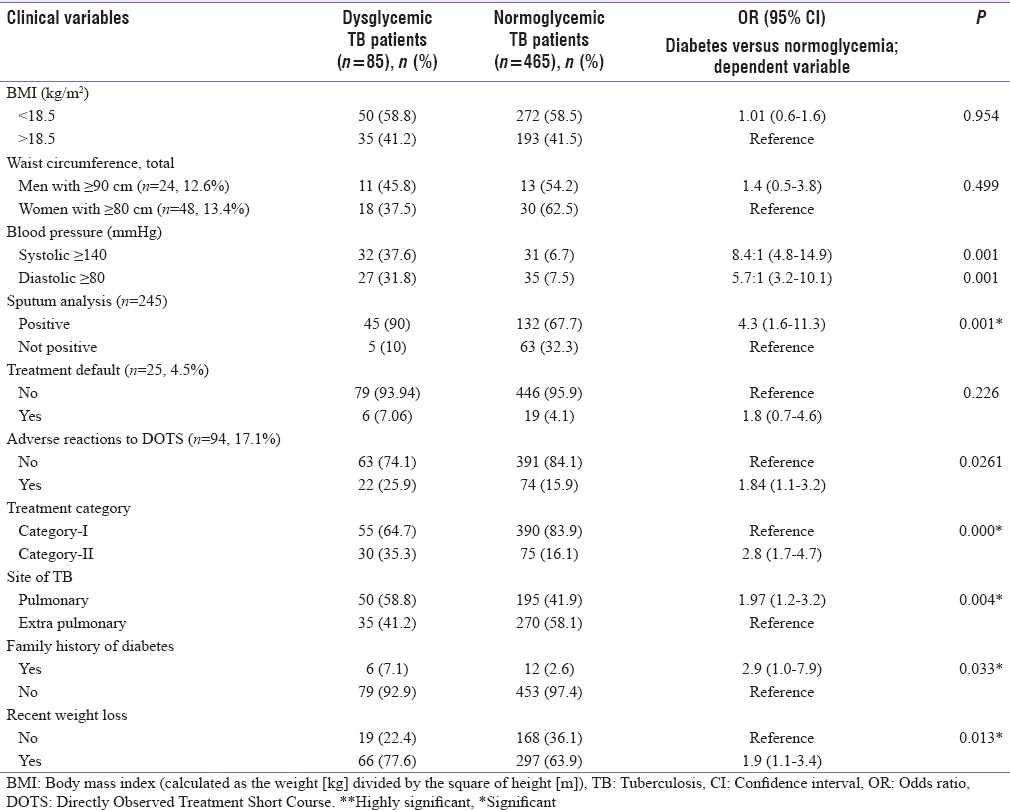
Clinical characteristics of the patients are shown and compared in Table 3. There was no statistically significant difference (P = 0.954) in the prevalence rate according to BMI among those with and without DM (OR = 0.98). A number of TB patients according to waist circumference (men with ≥ 90 cm and Women with ≥ 80 cm) were more found in normoglycemic (OR = 1.4) but not found statistically significant (P = 0.499). The rate of positive sputum smears was higher in patients with diabetes compared to those with normoglycemic. The difference in proportions was statistically significant (Dysglycemic (DTB) 90% vs. Normoglycemic TB (NTB) 67.7%, P = 0.03 and OR = 4.3). TB treatment default was noted in 25 patients, that is, 4.5% of the study population. TB treatment default was similar in patients with comorbidity of diabetes and those without DM (7% vs. 4%) (P = 0.226). Adverse reactions were found in total 94 (17%) patients taking DOTS and were higher in dysglycemic patients (26% vs. 16%) and this difference was found statistically significant (P = 0.026, OR = 1.84). More than 60% and 80% in both groups were receiving Category-I treatment in dysglycemic and normoglycemic TB patients, respectively. The proportion of patients with positive family history and extrapulmonary TB was more in TB patients with diabetes then normoglycemic TB patients (59% vs. 49% and 7% vs. 2.3%), this difference was also found statistically significant (0.004 and 0.033). Similarly, recent weight loss was found more in dysglycemic patients than the normoglycemia (P = 0.013, OR = 1.9).
Table 3.
Comparison of clinical variables among tuberculosis patients with diabetes and normoglycemia
DISCUSSION
The association between DM and TB is well documented and there is substantial evidence to support the fact that diabetes is an important risk factor for TB.[15]
In our study, the DM prevalence among patients with established TB of 15.5% is comparable with the documented prevalence rate of DM in the findings of Restrepo et al.[16] in the United States, Viswanathan et al.[11] in South India and in the study from Taiwan,[17] where they found DM prevalence of 17.8%, 25%, and 16.9%, respectively, with TB. Also in Indonesia, DM is strongly associated with TB with a prevalence of 13.2% (60 of 454 patients with TB had diabetes compared with 3.2% of the control subjects.[18] The high prevalence of DM among TB patients in the study area follows a similar pattern of high prevalence of DM in the general population representing of the situation in India.[19]
All these reports indicate that routine screening for DM among TB patients should be encouraged in areas with high TB burden. This therefore emphasizes the importance of routine screening for DM in all patients with TB. The already fairly well-established TB infrastructure and health personnel could also serve to improve early detection of DM.
Diabetes was more prevalent among men than women (77% vs. 22%, OR = 2.04) confirming the result of Viswanathan et al. study[11] where also diabetes was more prevailing in male TB patients. The higher prevalence of DM among men than women might be an accumulative effect of other risk factors such as smoking, tobacco use and alcohol consumption, which impact both TB and DM. The other reason could be the younger age of women than men since increasing age emerged as a significant risk factor for diabetes. The mean age of the patients with TB and DM was higher than in those with TB alone. This is similar to the study in the United States.[16] This may be related to the fact that type 2 DM is seen more frequently in the older age group, the fact that this study was conducted largely among adults with TB may also be a factor. In our study, majority of the patients were presumed to have type 2 DM with only 25% of the patients studied being 30 years and below.
Global TB control efforts could be adversely affected by a high prevalence of diabetes. Diabetics are not only more prone to contracting TB, but their response to initial intensive phase 4-drug anti-TB treatment is slow as well. This was clearly observed in this cohort of dysglycemic TB patients in our study who stayed longer 14 days in average for intensive phase treatment when compared with their fellow nondiabetic patients (281 [±58] vs. 268 [±46] days). This is similar to the finding of Gupta et al.[20] in India where they found a significantly higher duration of treatment of TB in patient with DM. It is likely owing to altered or impaired immune system response in diabetic patients. None of the diabetic TB patients had multidrug-resistant TB.
With regard to the factors associated with diabetes among Tubercular patients, the present study found that as in general population, increasing age, higher BMI values, and systolic blood pressure were significantly associated whereas waist circumference did not show any significant correlation and these all were similar with a study done by Viswanathan et al.[11] in Tamil Nadu and by Raghuraman et al.[21] BMI, waist circumference, category of treatment, and sputum positivity had no significant association. However, the mean random blood sugar of diabetics was higher than that of nondiabetics (185.3 ± 62.4 mg/dl vs. 108.4 ± 17.0 mg/dl; P = 0.001).
This study has not found any significant association between BMI or waist circumference and diabetes. Similar results were reported by studies by Jain et al.,[22] Raghuraman et al.[21] A study has reported that patients with TB and DM are significantly underweight and have more weight loss.[23] while Alisjahbana et al.[18] have reported a significantly higher median BMI in TB-DM patients. This study has reported a no significant association between alcohol consumption and prevalence of diabetes, compared to nondiabetic TB patients which has not been contradicted to the study of Puducherry[11] and Rewa.[22] Like the current study, most of the studies have demonstrated a higher association of diabetes with sputum positivity.[18,24] We have also noted that the proportion of patients with TB and DM co-morbidity who had a significant family history of DM and smoking was higher than that of patients with TB without DM. Positive family history of diabetes was significantly associated with DM with an OR of 3.08 and association of DM with PTB (OR = 1.97, P < 0.004) particularly for smear-positive cases in this study was similar to that of the findings of Stevenson et al.[25] and Viswanathan et al.[11] In our study, majority of known cases of DM (93.9%) were on regular treatment for diabetes, and it was similar with the result of Viswanathan et al.[11] where it was 92.4%. There was no significant difference in the proportion of treatment default cases between the dysglycemic and normoglycemic TB patients.
TB and DM are two diseases that are individually relatively common and of immense public health significance globally. Their association and consequences are well established.[11,15,16,17] Our study provides new evidence of this association from the 2nd most populous country of world. The results of this study highlight the importance of screening for DM in TB, which hitherto had not been done. Several recent reports indicate the need to consider the increasing trend in prevalence of diabetes in countries like India, which will impact the TB burden as well.[3,16] Considering the growing trend in prevalence of diabetes and huge burden of latent TB infection amongst Indian population, it is necessary to focus on diagnosis of latent TB infection and screening for DM and ensuring good metabolic control amongst those diagnosed with DM. The role of possible chemoprophylaxis for patients with DM and latent TB needs to be carefully considered and evaluated given the magnitude of the burden.[11] With the demographic transition underway globally, increase in life expectancy, improvements in provision of health services, and a subsequent increase in the elderly population, the absolute numbers of cases of diabetes will increase exponentially.[26] The WHO-IUATLD collaborative framework suggests that the type of screening and diagnostic tests for DM in TB patients should be adapted to the context of local health systems and the availability of resources.[27]
Some of the limitations of our study include the fact that the study was conducted within a hospital setting and may therefore not truly represent the true prevalence of the conditions in the community. It is also important to note that this study was a cross-sectional survey and there was no follow-up to determine the response to treatment and relapse rate between the two groups. However, it is our hope that the findings from this preliminary report will serve as a basis for a more prospective large-scale study in the future to determine the association between TB and DM in our population.
CONCLUSIONS
This converging of two epidemics should be a wake-up call for all clinicians and researchers to gear up to meet the challenge of patients afflicted by TB as well as diabetes. It is the time that the “unhealthy partnership” of TB and diabetes receives the attention it deserves. Being forewarned and prepared gives a better chance of reducing the dual burden of disease of DM and TB.[28] The limited resources and infrastructure for diabetes prevention and care at the primary setting make it even more necessary that the health resources and infrastructure for TB control should be further strengthened and serve the purpose of promoting prevention, early detection, and treatment of diabetes among TB patients particularly in India. Although such services can be offered for a limited time, during TB treatment, the intensive contact through DOTS initiative may lay a strong foundation for lifelong good control through self-care training in diabetes while at the same time reduce risk of treatment failure, re-infection, and relapse.[11] Since diabetes is associated with increased risk of developing active TB and also is a leading factor in deaths during TB treatment, failure after treatment, and increased risk of relapse, it becomes increasingly important that early detection and rigorous management of both Diabetes and TB is done for the patients.[18,24,29] After substantial progress in the last two decades, diabetes is estimated to be the cause of 15% of present TB cases, mainly because diabetes impairs host defenses. Patients with concurrent diabetes suffer worse TB treatment outcomes, a higher rate of relapse following TB treatment, and a higher risk of death from TB than patients with TB alone. Treatment of patients affected by both diseases can be challenging, particularly in low-income settings. The rapidly increasing prevalence of diabetes especially in the low- and middle-income countries where TB is endemic is threatening to thwart efforts to tackle TB over the next two decades.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Agarwal AK, Ginisha G, Preeti G, Dwivedi S, Swamai P. The association between diabetes and tuberculosis may be the next challenge for global tuberculosis control worldwide. Indian J Endocrinol Metab. 2016;20:732–3. doi: 10.4103/2230-8210.190565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harries AD, Murray MB, Jeon CY, Ottmani SE, Lonnroth K, Barreto ML, et al. Defining the research agenda to reduce the joint burden of disease from diabetes mellitus and tuberculosis. Trop Med Int Health. 2010;15:659–63. doi: 10.1111/j.1365-3156.2010.02523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruslami R, Aarnoutse RE, Alisjahbana B, van der Ven AJ, van Crevel R. Implications of the global increase of diabetes for tuberculosis control and patient care. Trop Med Int Health. 2010;15:1289–99. doi: 10.1111/j.1365-3156.2010.02625.x. [DOI] [PubMed] [Google Scholar]
- 4.TB India 2012: Annual Status Report, RNTCP. CTD, MOH and FW, New Delhi. 2012. [Last accessed on 2013 Aug 07]. Available from: http://www.tbcindia.nic.in/pdfs/TB%20India%202012.%20Annual%20Report.pdf .
- 5.Mohan V, Sandeep S, Deepa R, Shah B, Varghese C. Epidemiology of type 2 diabetes: Indian scenario. Indian J Med Res. 2007;125:217–30. [PubMed] [Google Scholar]
- 6.United Nations: World Population Prospects 2006 Highlights. United Nations. [Last cited on 2015 Nov 15]. Available from: http://www.un.org/esa/population/publications/wpp2006/WPP2006-Highlights-rev.pdf .
- 7. [Last accessed on 2014 Sep 17];Diabetes and Tuberculosis-A Wake-Up Call. Vol. 2 Available from: http://www.thelancet.com/diabetes-endocrinology . [Google Scholar]
- 8.Dixon B. Diabetes and tuberculosis: An unhealthy partnership. Lancet Infect Dis. 2007;7:444. doi: 10.1016/S1473-3099(07)70144-5. [DOI] [PubMed] [Google Scholar]
- 9.RNTCP at a Glance. [Last accessed on 2017 Feb 02]. Available from: http://www.uttarkashi.nic.in/Dept/Health/RNTCP/RNTCP.pdf .
- 10.Wang Q, Ma A, Han X, Zhao S, Cai J, Ma Y, et al. Prevalence of type 2 diabetes among newly detected pulmonary tuberculosis patients in China: A community based cohort study. PLoS One. 2013;8:e82660. doi: 10.1371/journal.pone.0082660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Viswanathan V, Kumpatla S, Aravindalochanan V, Rajan R, Chinnasamy C, Srinivasan R, et al. Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLoS One. 2012;7:e41367. doi: 10.1371/journal.pone.0041367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balakrishnan S, Vijayan S, Nair S, Subramoniapillai J, Mrithyunjayan S, Wilson N, et al. High diabetes prevalence among tuberculosis cases in Kerala, India. PLoS One. 2012;7:e46502. doi: 10.1371/journal.pone.0046502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Report of WHO Consultation Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization; 1999. Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. [Google Scholar]
- 14.Agarwal A. Social classification: The need to update in the present scenario. Indian J Community Med. 2008;33:50–1. doi: 10.4103/0970-0218.39245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: A systematic review of 13 observational studies. PLoS Med. 2008;5:e152. doi: 10.1371/journal.pmed.0050152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Restrepo BI, Fisher-Hoch SP, Crespo JG, Whitney E, Perez A, Smith B, et al. Type 2 diabetes and tuberculosis in a dynamic bi-national border population. Epidemiol Infect. 2007;135:483–91. doi: 10.1017/S0950268806006935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang PD, Lin RS. Epidemiologic features of diabetics among tuberculosis patients in Taiwan. J Infect Dis Antimicrob Agents. 2000;17:101–5. [Google Scholar]
- 18.Alisjahbana B, van Crevel R, Sahiratmadja E, den Heijer M, Maya A, Istriana E, et al. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int J Tuberc Lung Dis. 2006;10:696–700. [PubMed] [Google Scholar]
- 19.Ramachandran A, Snehalatha C. Current scenario of diabetes in India. J Diabetes. 2009;1:18–28. doi: 10.1111/j.1753-0407.2008.00004.x. [DOI] [PubMed] [Google Scholar]
- 20.Gupta S, Shenoy VP, Bairy I, Srinivasa H, Mukhopadhyay C. Diabetes mellitus and HIV as co-morbidities in tuberculosis patients of rural South India. J Infect Public Health. 2011;4:140–4. doi: 10.1016/j.jiph.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Raghuraman S, Vasudevan KP, Govindarajan S, Chinnakali P, Panigrahi KC. Prevalence of diabetes mellitus among tuberculosis patients in urban Puducherry. N Am J Med Sci. 2014;6:30–4. doi: 10.4103/1947-2714.125863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain MK, Baghel PK, Agrawal R. Study of impaired glucose tolerance in pulmonary tuberculosis. Indian J Community Med. 2006;31:137–9. [Google Scholar]
- 23.Ponce-De-Leon A, Garcia-Garcia Md Mde L, Garcia-Sancho MC, Gomez-Perez FJ, Valdespino-Gomez JL, Olaiz-Fernandez G, et al. Tuberculosis and diabetes in Southern Mexico. Diabetes Care. 2004;27:1584–90. doi: 10.2337/diacare.27.7.1584. [DOI] [PubMed] [Google Scholar]
- 24.Singla R, Khan N, Al-Sharif N, Ai-Sayegh MO, Shaikh MA, Osman MM. Influence of diabetes on manifestations and treatment outcome of pulmonary TB patients. Int J Tuberc Lung Dis. 2006;10:74–9. [PubMed] [Google Scholar]
- 25.Stevenson CR, Forouhi NG, Roglic G, Williams BG, Lauer JA, Dye C, et al. Diabetes and tuberculosis: The impact of the diabetes epidemic on tuberculosis incidence. BMC Public Health. 2007;7:234. doi: 10.1186/1471-2458-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agarwal AK. A challenge for the India to take advantage by demographic transition dividends. Indian J Health Sci. 2016;9:27–30. [Google Scholar]
- 27.Collaborative Framework for Care and Control of Tuberculosis and Diabetes: Report by WHO and IUATLD. Geneva: WHO; [Last accessed on 2013 Sep 15]. Available from: http://www.whqlibdoc.who.int/publications/2011/9789241502252-eng.pdf . [PubMed] [Google Scholar]
- 28.World Health Organization. Tuberculosis: WHO Factsheets 2010. Geneva: WHO; 2010. [Last accessed on 2011 Aug 15]. Available from: http://www.who.int/mediacentre/factsheets/fs104/en/index.html . [Google Scholar]
- 29.Nissapatorn V, Kuppusamy I, Jamaiah I, Fong MY, Rohela M, Anuar AK. Tuberculosis in diabetic patients: A clinical perspective. Southeast Asian J Trop Med Public Health. 2005;36(Suppl 4):213–20. [PubMed] [Google Scholar]


