Abstract
Background:
Psoriasis has been found to be associated with obesity, metabolic syndrome (MS), diabetes, and cardiovascular risk factors. Metformin treatment showed improvement in cardiovascular risk factors and hyperinsulinemia.
Objective:
To evaluate the efficacy and safety of metformin in psoriasis patients with MS.
Materials and Methods:
This was a single-center, parallel-group, randomized, open-label study with blinded end point assessment of metformin (1000 mg once daily for 12 weeks; n = 20) and placebo (n = 18) in psoriasis patients with MS. Total sample size was 38 participants.
Results:
Statistically significant improvement was observed in mean percentage change in erythema, scaling, and induration (ESI) (P = 0.048) in metformin as compared to placebo while mean percentage change in psoriasis area and severity index (PASI) and physician global assessment (PGA) scores was not significant (PASI – P = 0.215, PGA – P = 0.070). There was a statistically significant difference in percentage of parameters of MS improved following 12 weeks of treatment in metformin (19%) as compared to placebo (8.9%) group (P = 0.046). Statistically significant difference in percentage of patients achieving 75% reduction in ESI scores (P = 0.024). Significant improvement was observed in mean weight, body mass index (BMI), total cholesterol, and low-density lipoprotein (LDL) cholesterol in metformin group as compared to placebo. Improvement in BMI, fasting plasma glucose, serum triglycerides, high-density lipoprotein, LDL, systolic blood pressure, diastolic blood pressure, and total cholesterol was statistically significant in metformin group over the period of 12 weeks. There was no significant difference in adverse events in two groups except weight gain.
Conclusion:
Metformin has shown improvement in psoriasis and parameters of MS, hence can be used for the benefit of psoriasis patients having MS. Large, controlled studies are needed to confirm.
Keywords: Insulin sensitizers, metabolic syndrome, metformin, psoriasis
Trial registration number: Registered in Clinical Trial Registry of India (CTRI) Registration Number: CTRI/2011/12/002252.
INTRODUCTION
Psoriasis has been found to be associated with systemic disorders namely obesity, metabolic syndrome (MS),[1,2] diabetes mellitus,[3] and cardiovascular morbidities.[4,5,6] MS is a cluster of risk factors including central obesity, high triglyceride level, low high-density lipoprotein cholesterol (HDL-C) level, hypertension, and glucose intolerance and is a strong predictor of coronary heart disease, diabetes, and stroke.[4,7,8,9] The pathophysiological features of psoriasis involve inappropriate production of interferon-γ, tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), IL-8, IL-12, IL-17, IL-19, and IL-23, which are the mediators for MS.[10,11,12] Metformin has been shown to improve cardiovascular risk factors. Metformin has shown additional beneficial effects in adults with Type 2 diabetes, including weight reduction, decreased hyperinsulinemia, improved lipid profiles, augmented fibrinolysis, and enhanced endothelial function.[13,14,15,16] Metformin acts as an anti-inflammatory agent by activating adenosine monophosphate-activated protein kinase (AMPK). Activated AMPK induces inhibition of dendritic cell and T-cell activation as well as cell proliferation, which is the hallmark of psoriasis.[17] As metformin has shown to prevent the progression of MS into overt diabetes and can induce weight loss, we planned to evaluate the efficacy and safety of metformin in psoriasis patients with MS. This study is a part of larger study in which we evaluated the prevalence of MS in psoriasis. Psoriasis patients having MS were divided into systemic (moderate-to-severe psoriasis, randomized into metformin and placebo arms) and topical treatment cohort[18] (mild psoriasis, randomized into metformin, pioglitazone, and placebo arms) and were evaluated for the effect of insulin sensitizers on disease parameters and MS. In this paper, we have discussed the results of systemic cohort.
MATERIALS AND METHODS
Clinical trial design
This was a single-center, parallel-group, randomized, open-label study with blinded end point assessment of metformin and placebo in psoriasis patients with MS satisfying inclusion and exclusion criteria. Study was approved by the Institute Ethics Committee of Postgraduate Institute of Medical Education and Research, Chandigarh, India (PGIMER letter number – MS/1145/DM/8229, dated April 1, 2010), and this study was registered on the Clinical Trial Registry of India (CTRI) website (the registration number of the trial is CTRI/2011/12/002252).
All patients visiting psoriasis clinic at our institute were screened for MS and other eligibility criteria. Both men and women, >18 years with plaque psoriasis (moderate [3%–10% body surface area] to severe disease [>10% body surface area][19] disease on treatment [had taken even a single dose of systemic therapy in the past] or treatment naïve [no past history of treatment for their disease]) and having MS and willing to provide written informed consent, were included in the study. MS is defined as the presence of three or more criteria of the modified National Cholesterol Education Program's Adult Treatment Panel III (NCEP ATP III):[20] waist circumference >90 cm in men and >80 cm in women, hypertriglyceridemia ≥150 mg/dl, HDL-C <40 mg/dl in males and <50 mg/dl in females, blood pressure ≥130/85 mmHg, and fasting plasma glucose (FPG) ≥110 mg/dl. Patients with mild disease and systemic therapy other than methotrexate, pregnant or nursing women, significant hepatic impairment (serum bilirubin, aspartate transaminase, alanine transaminase, and alkaline phosphatase >1.5 times the upper limit of normal), renal insufficiency-serum creatinine ≥1.5 mg/dl (men) or ≥1.4 mg/dl (women), and contraindication to metformin were excluded from the study.
Clinical examination including the psoriasis area and severity index (PASI) scores and erythema, scaling, and induration (ESI) scores was done. Clinical photographs of patients were taken at baseline and at the end of treatment. Baseline investigations were done and eligible patients were randomized by simple randomization, in an open-label manner to either placebo or metformin 1000 mg once daily, for a period of 12 weeks after taking written informed consent. All patients were given standard systemic methotrexate and folic acid in addition to study drugs. Methotrexate was started at a dose of 10–30 mg once/week, decreased or increased at the rate of 5 mg fortnightly, depending on the decrease or increase in disease severity, respectively. Although most studies have reported starting methotrexate at low dose and then gradually escalating the dose based on response.[21] However, at some centers, it is started at higher dose as mentioned in the US Food and Drug Administration approved label.[22] This regimen has shown excellent clinical efficacy and good safety profile with overall short duration of therapy with low cumulative dose.[23] Folic acid was administered at the dose of 5 mg twice every week. The randomization codes were computer generated. Randomization codes were concealed in an opaque envelope. The drug dispensation was done by a person who was not involved with the assessment of the study end points. Evaluation for efficacy parameters was done at 0 and 12 weeks. Safety evaluation was also done throughout the study. Hemoglobin and blood counts were done every 2 weeks and liver function tests were done every 4 weeks.
Efficacy evaluation
Blinded end points assessment of the efficacy parameters was done at 12 weeks. Psoriasis lesions were evaluated using PASI (0–72) score[24] and ESI[25] severity (0–3) score. Each component of ESI was graded from 0 to 3; 0 – clear, 1 – mild, 2 – moderate, and 3 – severe. The most severe condition was given 9 points whereas the absence of disease been given 0 points.
All the parameters of MS as defined by modified NCEP ATP III criteria[20] were assessed at baseline and 12 weeks. Serum IL-6 and TNF-α levels was done at 0 and 12 weeks in subgroup (8 patients each in placebo and metformin groups) of patients by Human ELISA Kit (RayBiotech, Inc., Georgia, USA).
The primary efficacy end point was mean percentage change in PASI or ESI scores from baseline after 12 weeks of therapy given along with standard treatment for psoriasis. The secondary efficacy end point was number of parameters of MS improved, change in individual parameters of MS, IL-6, and TNF-α from baseline after 12 weeks of treatment with metformin or placebo. The mean percentage change in physician global assessment (PGA) from baseline and percentage of patients achieving 75% reduction in PASI score in the two treatment groups were other end points.
Sample size calculation
Assuming a standard deviation of 10 in PASI scores and a difference of 10 in PASI score between drug and placebo arm at 12 weeks to be clinically significant at α = 0.05 and with 80% power, a sample size of 16 patients per group has been calculated. Keeping in mind a dropout rate of about 20%, 19 patients will be required to be included in each group.
Statistical analysis
Mean percentage change in PASI, ESI, and PGA scores at 12 weeks from baseline between two groups was carried out using Student's t-test. Chi square test or Fischer's Exact test was used to compare categorical variables. Intragroup comparison of mean changes in individual parameters of MS and lipid profile was carried out by paired t-test and intergroup comparison by Student's t-test. Independent t-test was used for comparison of numerical variables between two treatment groups. Results were analyzed as intention-to-treat (ITT) analysis with last observation carried forward (LOCF). A two-sided P < 0.05 was considered as statistically significant.
RESULTS
A total of 51 psoriasis patients with MS were screened for enrollment in the study from June 2010 to April 2011 [Figure 1]. Out of 51, 13 were excluded from the study. Eighteen and twenty patients were randomized to placebo and metformin treatment groups, respectively. Disposition of patients and reasons for withdrawal were summarized in Figure 1. A total of 17 patients in placebo arm and 18 patients in metformin arm completed the study. All participants as randomized were included in the final analysis as it is ITT analysis with LOCF.
Figure 1.
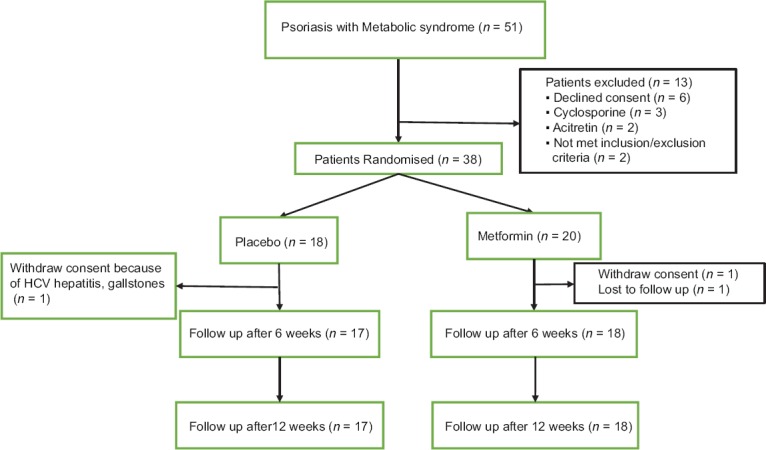
Flowchart of the patients enrolled in the study depicting enrollment, withdrawal, and follow-up of the participants
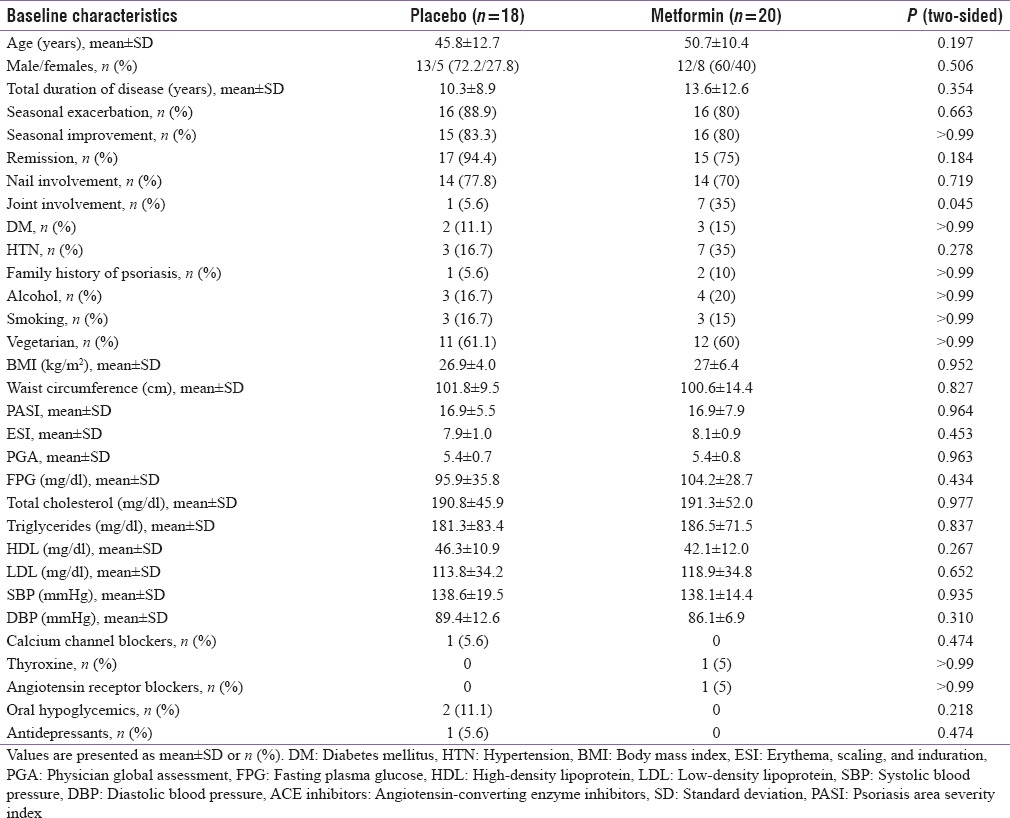
No significant difference was observed in baseline demographics and metabolic characteristics among two treatment groups except joint involvement [Table 1].
Table 1.
Baseline characteristics of two treatment groups
Psoriasis area and severity index, erythema, scaling, and induration, and physician global assessment scores and parameters of metabolic syndrome
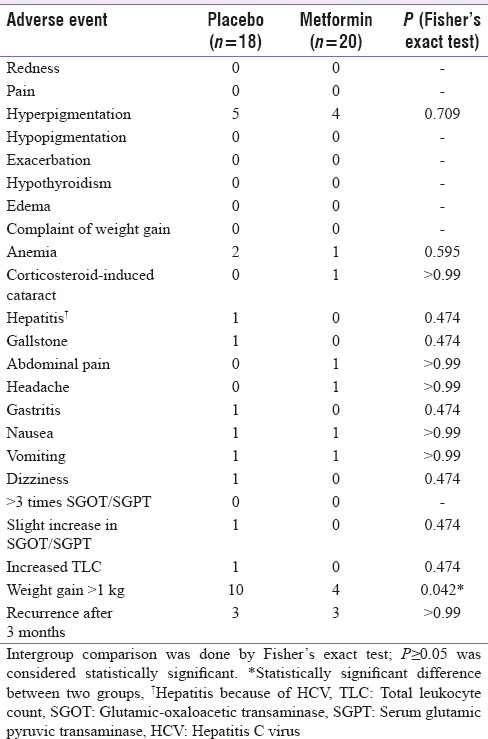
Statistically significant improvement was observed in mean percentage change in ESI (P = 0.048) scores in metformin group as compared to placebo group [Figure 2] while no significant difference was observed in mean percentage change in PASI (P = 0.215) and PGA scores (P = 0.070). There was statistically significant difference in percentage of parameters of MS improved following 12 weeks of treatment in metformin (19%) as compared to placebo (8.9%) group (P = 0.046) [Figure 3]. Statistically significant difference in percentage of patients achieving 75% reduction in ESI scores in metformin (75%) versus placebo (38.9%) (P = 0.024) [Figure 4]. Significant improvement was observed in mean weight, body mass index (BMI), waist circumference (WC), FPG, serum triglycerides, total cholesterol, and low-density lipoprotein cholesterol (LDL-C) [Table 2] in metformin group as compared to placebo. Improvement in BMI, WC, FPG, serum triglycerides, HDL, LDL, systolic blood pressure (SBP), diastolic blood pressure (DBP), and total cholesterol was statistically significant in metformin group over the period of 12 weeks. There was no significant difference in mean change in serum levels of IL-6 (placebo = −65.4 ± 100.1, metformin = −33.6 ± 57.4; P = 0.449) and TNF-α (placebo = 13.9 ± 290.0, metformin = −12.1 ± 82.3; P = 0.810). There was no significant difference in the mean number of adverse events in two groups except weight gain [Table 3].
Figure 2.

Mean percentage change in PASI, ESI, and PGA scores in two treatment groups from baseline (intention to treat analysis). Intergroup comparisons for mean percentage change in PASI, ESI, and PGA scores at 12 weeks from baseline carried out by Student's t test. *Metformin versus placebo, PASI: Psoriasis area severity index, ESI: Erythema, scaling, and induration, PGA: Physician global assessment
Figure 3.
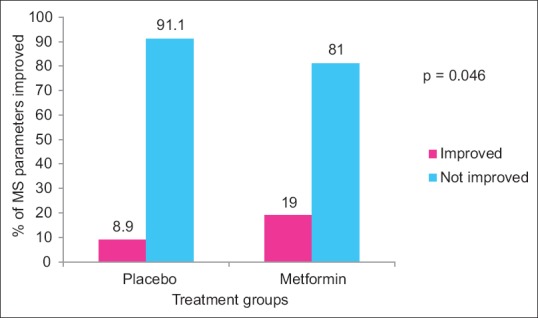
Percentage of parameters of metabolic syndrome improved following 12 weeks of treatment in placebo and metformin groups from baseline (intention to treat analysis). Intergroup comparisons for percentage of parameters of metabolic syndrome improved carried out by Chi-square test, P: Placebo versus metformin
Figure 4.
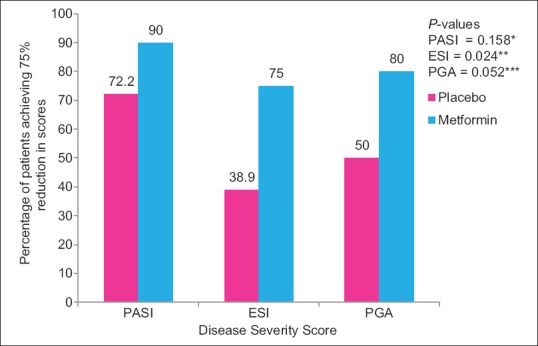
Percentage of patients achieving 75% reduction in PASI, ESI and PGA scores in placebo and metformin groups from baseline (Intention to treat Analysis) Inter-group comparisons for 75% reduction in PASI, ESI and PGA scores between two groups carried out by Chi-square test. **statistically significant difference between groups compared to baseline. *, ***Statistically non-significant difference between groups compared to baseline PASI – Psoriasis Area Severity Index, ESI – Erythema, Scaling and Induration, PGA – Physician Global Assessment
Table 2.
Mean change in weight, body mass index, individual parameters of lipid profile, and metabolic syndrome after 12 weeks of treatment in two treatment groups from baseline (intention to treat analysis)
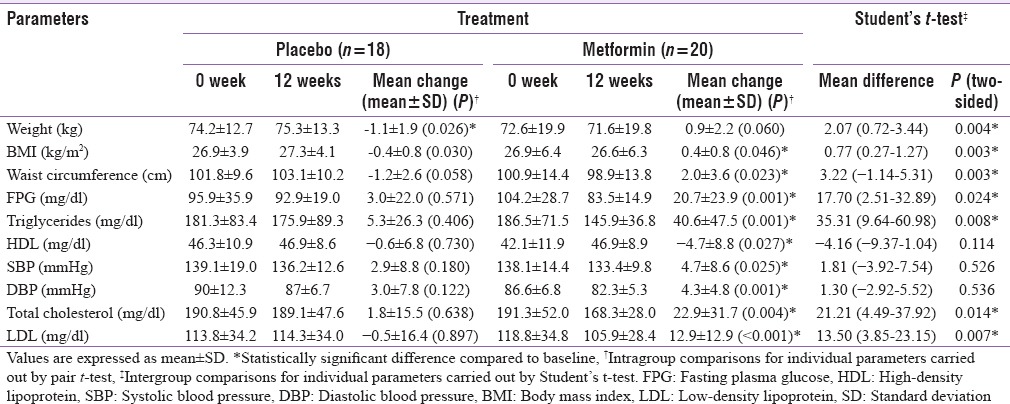
Table 3.
Adverse events observed in placebo and metformin treatment groups in systemic treatment arm
DISCUSSION
Metformin group had greater percentage reduction in mean PASI, ESI, and PGA scores as compared to placebo (% reduction in mean PASI scores – 86.3% vs. 72.0%, % reduction in mean ESI scores – 82.0% vs. 60.4%, and % reduction in mean PGA scores – 79.6% vs. 60.0% in metformin and placebo groups, respectively). Reduction in mean percentage change in PASI score was more in metformin group although not statistically significant. Significance was not achieved probably because of the rapid and almost 100% of patients responded to methotrexate in placebo group as well as an anti-inflammatory response.[17] Methotrexate inhibits AICAR transformylase thereby increasing AICAR concentration which causes activation of AMPK.[17] Second, remitting-relapsing nature of the psoriasis disease might be responsible for the nonsignificant difference between two groups. Metformin acts through activation of AMPK in extracellular signal-related kinase 1/2 signaling pathway leading to cell cycle arrest and therefore inhibition of cell proliferation, hallmark of psoriasis.[26] AMPK activation not only inhibits inducible nitric oxide synthase, dendritic T cell, and monocyte/macrophage activation but also activates IL-10 and TGF-β, thereby exerting its anti-inflammatory action.[17]
Percentage of PASI change in placebo group taking methotrexate was 72% in our study, as compared to the study by Flytström who observed 58% improvement in PASI in methotrexate group.[27] Champion trial[21] and study done by Heydendael et al.[28] reported 75% reduction in PASI in 35.5% and 60% of the patients, respectively. The higher response in our study may be due to the difference in characteristics of psoriasis patients as our patient population had MS. After 12 weeks of treatment with metformin or placebo, group with improvement of MS had greater percentage reduction in mean PASI, ESI, and PGA scores as compared to group with no improvement in MS (PASI – 88.4% vs. 73.7%, ESI – 79.9% vs. 66.5%, and PGA – 78.1% vs. 65.2%, respectively). Second, we used a step-down approach for the use of methotrexate while many clinical studies used a step-up approach for the management of psoriasis. Starting with a higher dose leads to a faster control of disease with rapid response in short duration and low total cumulative dose.[23]
In our study, 45% of the patients had complete improvement in MS in metformin group as compared to 33.3% patients in placebo group (P = 0.52). Greater percentage reduction in mean percentage change in PASI, ESI, and PGA scores with metformin might be because of the effect of metformin on MS parameters. Statistically insignificant difference may be attributed to methotrexate use in both the groups. The use of methotrexate has been shown to reduce cardiovascular mortality and the incidence of myocardial infarction in rheumatoid disease.[29] Mitigation of apoptosis in ischemic myocardium by methotrexate accounts for improved cardiovascular protection.[30]
In addition, metformin group had significantly more improvement in percentage of MS parameters as compared to placebo group. Furthermore, patients taking metformin had statistically significant decrease in weight, BMI, waist circumference, FPG, serum triglycerides, total cholesterol, and LDL-C as compared to patients taking placebo. Mean increase in HDL-C observed in metformin group was 4.7 ± 8.8 mg/dl (P = 0.027), and mean fall in SBP and DBP observed was 4.7 ± 8.6 (P = 0.025) and 4.3 ± 4.8 (P = 0.001) mmHg, respectively. However, difference in mean change in HDL-C, SBP, and DBP was not significantly different from placebo. The baseline characteristics between two groups were similar. Thus, the difference in efficacy between metformin and placebo group observed in our study can be ascribed to the additive effect of methotrexate and metformin as both exert anti-inflammatory effect by activation of AMPK.[17]
BMI is a major risk factor for Type II diabetes.[31] For every 1 kg weight gain, there is nearly a 5% increase in the risk of diabetes.[32] Our study results revealed a mean reduction of 0.9 ± 2.2 kg and 0.4 ± 0.8 kg/m2 in weight and BMI in metformin group, significantly more as compared to placebo group. In the substudy of the UK Prospective Diabetes Study (UKPDS), metformin therapy was associated with a 32% risk reduction for any diabetes-related end point (P = 0.0023), a 42% reduction in diabetes-related death (P = 0.017), 39% fewer myocardial infarctions (P = 0.01), and a 36% reduction in all-cause mortality (P = 0.011) compared with conventional treatment group.[33] Despite the similar levels of HbA1C achieved in metformin and other intensively treated groups (sulfonylureas and insulin), an additional benefit of metformin was observed in reduced risk for any diabetes-related end point, all mortality, and stroke.[34] Similar to our study results, many studies had shown that metformin mildly decreases total and LDL C,[35,37] decreases triglyceride levels,[36,38,39] and may even slightly increase HDL C.[36,38,39] This modest beneficial effect of metformin on lipid profile might partially contribute to benefit on macrovascular disease in the UKPDS study. Metformin prevents the development of MS.[40] It may be concluded that psoriasis patients with MS can benefit from the long-term use of metformin as shown by the improvement in parameters of MS, lipid profile, and weight loss in our study participants and the results of the UKPDS study.
In subgroup analysis, both metformin and placebo group had shown decline in the mean IL-6 and TNF-α levels, but no statistically significant difference was observed. It might be because of the effect of methotrexate on the serum IL-6 and TNF-α level which is given to all the patients. Twenty-five percent (2/8) of patients in both placebo and metformin groups had shown no decline or rather increase in the IL-6 and TNF-α levels, consistent with the relapse in psoriasis within next 6 months. Although number of patients had relapse in the next 6 months were 3 out of 18 in placebo group and 3 out of 20 in metformin group.
The study also has some limitations. Intermediate dose of metformin (1000 mg/day) was used in the study. Second, it was an open-label study although blinded end point assessment was done.
The use of metformin had shown improvement in weight reduction, lipid profiles,[41] and hyperinsulinemia.[15,16] The treatment results of our study have demonstrated the overall superiority of metformin over placebo.
CONCLUSION
Metformin methotrexate combination might improve the clinical outcome in psoriasis patients by improving MS parameters as a result of antiproliferative[26] and anti-inflammatory action of both drugs.[17] However, large, controlled studies are needed to confirm this preliminary finding.
Declarations
Ethical approval
We also declare that the study was assessed and approved by the Institutional Ethics Committee, PGIMER, Chandigarh, and that letter of approval is available with us for examination. Ethics Committee letter number for the approval of study is MS/1145/DM/8229, dated April 1, 2010. From all patients, written informed consent was taken before randomization.
Financial support and sponsorship
This study was carried out in Postgraduate Institute of Medical Sciences, Chandigarh. This study was part of dissertation for degree of Doctrine of Medicine in Clinical Pharmacology. This study was not supported financially by any pharmaceutical company.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Dr. Inderjeet Kaur and Dr. Sunil Dogra for their constant support.
REFERENCES
- 1.Sommer DM, Jenisch S, Suchan M, Christophers E, Weichenthal M. Increased prevalence of the metabolic syndrome in patients with moderate to severe psoriasis. Arch Dermatol Res. 2006;298:321–8. doi: 10.1007/s00403-006-0703-z. [DOI] [PubMed] [Google Scholar]
- 2.Gelfand JM, Yeung H. Metabolic syndrome in patients with psoriatic disease. J Rheumatol Suppl. 2012;89:24–8. doi: 10.3899/jrheum.120237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB, Gelfand JM. Prevalence of cardiovascular risk factors in patients with psoriasis. J Am Acad Dermatol. 2006;55:829–35. doi: 10.1016/j.jaad.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 4.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: A systematic review and meta-analysis of observational studies. J Am Heart Assoc. 2013;2:e000062. doi: 10.1161/JAHA.113.000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel RV, Shelling ML, Prodanovich S, Federman DG, Kirsner RS. Psoriasis and vascular disease-risk factors and outcomes: A systematic review of the literature. J Gen Intern Med. 2011;26:1036–49. doi: 10.1007/s11606-011-1698-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson PW, D'Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–72. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 8.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–28. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 9.Wannamethee SG, Shaper AG, Lennon L, Morris RW. Metabolic syndrome vs. Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–50. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. American Heart Association; National Heart, Lung, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–8. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome. An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Executive summary. Cardiol Rev. 2005;13:322–7. [PubMed] [Google Scholar]
- 12.Alberti KG, Zimmet P, Shaw J. Metabolic syndrome – A new world-wide definition. A consensus statement from the International Diabetes Federation. Diabet Med. 2006;23:469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 13.Cohn G, Valdes G, Capuzzi DM. Pathophysiology and treatment of the dyslipidemia of insulin resistance. Curr Cardiol Rep. 2001;3:416–23. doi: 10.1007/s11886-001-0059-0. [DOI] [PubMed] [Google Scholar]
- 14.Mehnert H. Metformin, the rebirth of a biguanide: Mechanism of action and place in the prevention and treatment of insulin resistance. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S259–64. doi: 10.1055/s-2001-18587. [DOI] [PubMed] [Google Scholar]
- 15.Stadtmauer LA, Wong BC, Oehninger S. Should patients with polycystic ovary syndrome be treated with metformin? Benefits of insulin sensitizing drugs in polycystic ovary syndrome – beyond ovulation induction. Hum Reprod. 2002;17:3016–26. doi: 10.1093/humrep/17.12.3016. [DOI] [PubMed] [Google Scholar]
- 16.Inzucchi S. Metformin or thiazolidinediones as first-line therapy for type 2 diabetes: Focus on cardiovascular protection. Pract Diabetol. 2002;21:7–12. [Google Scholar]
- 17.Glossmann H, Reider N. A marriage of two “Methusalem” drugs for the treatment of psoriasis? Arguments for a pilot trial with metformin as add-on for methotrexate. Dermatoendocrinol. 2013;5:252–63. doi: 10.4161/derm.23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh S, Bhansali A. Randomized placebo control study of insulin sensitizers (Metformin and Pioglitazone) in psoriasis patients with metabolic syndrome (Topical Treatment Cohort) BMC Dermatol. 2016;16:12. doi: 10.1186/s12895-016-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Voorhees AS, Feldman SR, Koo JY, Lebwohl MG, Menter A. The Psoriasis and Psoriatic Arthritis Pocket Guide: Treatment Algorithms and Management Options: National Psoriasis Foundation. [Last accessed on 2015 Sep 17]. Available from: https://www.psoriasis.org/document.doc?id=354 .
- 20.Misra A, Wasir JS, Pandey RM. An evaluation of candidate definitions of the metabolic syndrome in adult Asian Indians. Diabetes Care. 2005;28:398–403. doi: 10.2337/diacare.28.2.398. [DOI] [PubMed] [Google Scholar]
- 21.Saurat JH, Stingl G, Dubertret L, Papp K, Langley RG, Ortonne JP, et al. Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION) Br J Dermatol. 2008;158:558–66. doi: 10.1111/j.1365-2133.2007.08315.x. [DOI] [PubMed] [Google Scholar]
- 22. [Last cited on 2016 Apr 16]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/008085s066lbl.pdf .
- 23.Kumar B, Saraswat A, Kaur I. Short-term methotrexate therapy in psoriasis: A study of 197 patients. Int J Dermatol. 2002;41:444–8. doi: 10.1046/j.1365-4362.2002.01530.x. [DOI] [PubMed] [Google Scholar]
- 24.Langley RG, Ellis CN. Evaluating psoriasis with psoriasis area and severity index, psoriasis global assessment, and lattice system physician's global assessment. J Am Acad Dermatol. 2004;51:563–9. doi: 10.1016/j.jaad.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 25.Sharma V, Kaur I, Kumar B. Calcipotriol versus coal tar: A prospective randomized study in stable plaque psoriasis. Int J Dermatol. 2003;42:834–8. doi: 10.1046/j.1365-4362.2003.01974.x. [DOI] [PubMed] [Google Scholar]
- 26.Li W, Ma W, Zhong H, Liu W, Sun Q. Metformin inhibits proliferation of human keratinocytes through a mechanism associated with activation of the MAPK signaling pathway. Exp Ther Med. 2014;7:389–92. doi: 10.3892/etm.2013.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flytström I, Stenberg B, Svensson A, Bergbrant IM. Methotrexate vs. ciclosporin in psoriasis: Effectiveness, quality of life and safety. A randomized controlled trial. Br J Dermatol. 2008;158:116–21. doi: 10.1111/j.1365-2133.2007.08284.x. [DOI] [PubMed] [Google Scholar]
- 28.Heydendael VM, Spuls PI, Opmeer BC, de Borgie CA, Reitsma JB, Goldschmidt WF, et al. Methotrexate versus cyclosporine in moderate-to-severe chronic plaque psoriasis. N Engl J Med. 2003;349:658–65. doi: 10.1056/NEJMoa021359. [DOI] [PubMed] [Google Scholar]
- 29.Prodanovich S, Ma F, Taylor JR, Pezon C, Fasihi T, Kirsner RS. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262–7. doi: 10.1016/j.jaad.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Elmadhun NY, Sabe AA, Lassaletta AD, Chu LM, Sellke FW. Metformin mitigates apoptosis in ischemic myocardium. J Surg Res. 2014;192:50–8. doi: 10.1016/j.jss.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pi-Sunyer FX. Medical hazards of obesity. Ann Intern Med. 1993;119((7 Pt 2)):655–60. doi: 10.7326/0003-4819-119-7_part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- 32.Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: Findings from a national cohort of US adults. Am J Epidemiol. 1997;146:214–22. doi: 10.1093/oxfordjournals.aje.a009256. [DOI] [PubMed] [Google Scholar]
- 33.Effect of intensive blood-glucose control with metformin on complications in overweight patients with type diabetes (UKPDS). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:854–65. [PubMed] [Google Scholar]
- 34.Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): Prospective observational study. BMJ. 2000;321:405–12. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–9. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med. 1995;333:541–9. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- 37.Robinson AC, Burke J, Robinson S, Johnston DG, Elkeles RS. The effects of metformin on glycemic control and serum lipids in insulin-treated NIDDM patients with suboptimal metabolic control. Diabetes Care. 1998;21:701–5. doi: 10.2337/diacare.21.5.701. [DOI] [PubMed] [Google Scholar]
- 38.Reaven GM, Johnston P, Hollenbeck CB, Skowronski R, Zhang JC, Goldfine ID, et al. Combined metformin-sulfonylurea treatment of patients with noninsulin-dependent diabetes in fair to poor glycemic control. J Clin Endocrinol Metab. 1992;74:1020–6. doi: 10.1210/jcem.74.5.1569149. [DOI] [PubMed] [Google Scholar]
- 39.Wu MS, Johnston P, Sheu WH, Hollenbeck CB, Jeng CY, Goldfine ID, et al. Effect of metformin on carbohydrate and lipoprotein metabolism in NIDDM patients. Diabetes Care. 1990;13:1–8. doi: 10.2337/diacare.13.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, et al. The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: The Diabetes Prevention Program randomized trial. Ann Intern Med. 2005;142:611–9. doi: 10.7326/0003-4819-142-8-200504190-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai X, Wang H, Jing Z, Fu P. The effect of a dual combination of noninsulin antidiabetic drugs on lipids: A systematic review and network meta-analysis. Curr Med Res Opin. 2014;30:1777–86. doi: 10.1185/03007995.2014.921608. [DOI] [PubMed] [Google Scholar]


