Abstract
Background
The occurrence of brain metastases (at diagnosis or at relapse) in patients with Wilms tumor is very rare.
Methods
We retrospectively reviewed the clinical characteristics of patients with Wilms tumor and relapse to the brain enrolled on the National Wilms Tumor Studies (NWTS) 1–5.
Results
Intracranial relapse was documented in 47 patients (0.5%). Of the 45 patients with adequate data, 26 (58%) patients were male. Thirty-eight (84%) patients had favorable histology Wilms tumor. In 30 patients (67%), the appearance of intracranial disease was preceded by relapse at another site. Ten patients did not have any disease directed therapy. Surgical resection was attempted in 15 patients; gross total resection was achieved in 11 patients. Twenty-nine patients received brain irradiation; the median dose was 3000 cGy (range 1080 – 4000 cGy). Twenty-seven patients received chemotherapy. The five-year overall survival from the time of intracranial relapse was 28.7 % (95% CI: 14.4 – 43.1%). Nine patients (all favorable histology Wilms tumor) were alive with a median follow-up from brain relapse of 140 months (range 35 – 381 months). All nine survivors received radiation therapy, eight received chemotherapy, and four underwent surgery (two gross total resection, two partial resection). The overall survival after brain metastases of the NWTS-5 patients was significantly higher than the overall survival of the NWTS 1–4 patients (p-value=0.029, log rank test).
Conclusions
Patients with Wilms tumor recurrence involving the brain may have durable survival, particularly those treated in recent years. Multimodality therapy including radiation and chemotherapy should be considered for these patients.
Keywords: Wilms tumor, Central nervous system, Radiation, Chemotherapy, Surgery
INTRODUCTION
The remarkable success in the treatment of patients with Wilms tumor has meant that only a small proportion of patients develop disease relapse following initial therapy.[1] The most common site of relapse in children with Wilms tumor is lung followed by abdomen/flank and liver. In contrast to other pediatric renal malignancies, such as clear cell sarcoma of the kidney (CCSK)[2] and rhabdoid tumor of the kidney (RTK)[3], where brain is a frequent metastatic site, recurrence in the brain is very rare in children with Wilms tumor.[4]
Limited information is available on patients with Wilms tumor and brain metastases. Until the mid-1990s, data were available only through case reports or inclusive series describing childhood cancer intracranial metastases.[5–9] Only two series have been published from large cooperative groups describing a total of 21 patients. A retrospective review of the United Kingdom Children’s Cancer Study Group (UKCCSG) trials described seven children with intracranial metastases; all registered on their first three Wilms tumor studies (1980–1996).[4] Three of the seven were alive at the time of publication. In a similar review from the International Society of Paediatric Oncology (SIOP), all 14 of 3040 patients with Wilms tumor diagnosed between 1971 and 2000 who developed subsequent intracranial relapse died.[10] Given its rarity, the optimal treatment of children with Wilms tumor who develop intracranial relapse is not known. To better understand the clinical characteristics, treatment used and outcomes of patients with Wilms tumor and brain metastases, we undertook a retrospective review of patients enrolled on the National Wilms Tumor Studies (NWTS).
MATERIALS AND METHODS
The database of patients enrolled on NWTS 1–5 was queried for patients with favorable or anaplastic Wilms tumor and metastases to the brain, either at diagnosis or at relapse. Patients with CCSK, RTK, renal cell carcinoma, and other renal neoplasms were excluded. Patients were initially treated according to their stage and histology following one of the standard NWTS regimens.[11–16] Clinical details at diagnosis and information on treatment outcome were reported on checklists, narratives and flow sheets submitted by the pediatric oncologist, surgeon and pathologist involved in the actual care of the patient.
Descriptive statistics (frequency and percentage for categorical characteristics; median and range for numerical characteristics) were used. The primary endpoint for evaluation was survival after intracranial relapse. The Kaplan-Meier method was used to construct the survival curve, with the standard error computed using the Peto-Peto method.[17] The overall survival from the time of intracranial relapse between the NWTS-5 and NWTS 1–4 patients was compared using log-rank test.
RESULTS
Patient characteristics
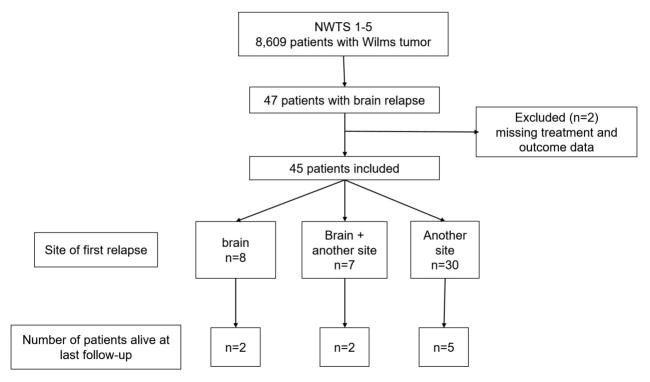
A total of 8609 patients with Wilms tumor were enrolled on the NWTS 1–5 clinical trials between 1969 and 2002. The median follow-up of these patients was 15.1 years (range 0.02–45.6 years). None of the patients had intracranial metastases at diagnosis. Forty-seven patients (0.5 %) developed intracranial metastases during first or subsequent relapse (Figure 1). Treatment and outcome information was not available for two patients and were excluded. Of the 45 patients with adequate data, 26 (58%) were male (Table 1). The median age at initial diagnosis was 3.6 years (range 0.4 – 16 years). Thirty-eight patients (84%) had favorable histology Wilms tumor. The occurrence of intracranial relapse according to the initial disease stage were 7/2552 (0.3%) stage I patients, 12/2161 (0.5%) stage II patients, 14/2180 (0.6%) stage III patients and 10/1184 (0.8%) stage IV patients. The proportion of patients developing brain metastases was approximately 0.5% across NWTS trials except for NWTS-1 trial where it was 1.9%.
Fig. 1.
CONSORT patient flow diagram
Table 1.
Patient and tumor characteristics (n=45)
| NWTS study | |
| 1 | 9 |
| 2 | 4 |
| 3 | 6 |
| 4 | 12 |
| 5 | 14 |
|
| |
| Gender | |
| Male | 26 |
| Female | 19 |
|
| |
| Age at Diagnosis (years) | |
| Median (range) | 3.6 (0.42–16.42) |
|
| |
| Initial Stage | |
| I | 7 |
| II | 12 |
| III | 14 |
| IV | 10 |
| V | 2 |
|
| |
| Pathology | |
| Favorable histology | 38 |
| Diffuse anaplastic | 6 |
| Focal Anaplastic | 1 |
|
| |
| Primary relapse site | |
| Brain | 8 |
| Brain + other | 7 |
| Other | 30 |
|
| |
| Time to 1st relapse (months) | |
| Median (range) | 8.6 (1–139) |
|
| |
| Time to CNS relapse (months) | |
| Median (Range) | 19.5 (3.3–143.7) |
In 30 patients (67%), the appearance of intracranial disease was preceded by relapse at another site (lungs in 21, lungs and bone in 4, lungs and liver in 2, liver only in 1, abdomen in 2). In seven patients, the intracranial relapse occurred simultaneously with relapse at another site and in eight patients, brain was the isolated first site of relapse. Thirty-six patients (80%) had documentation of lung metastasis at diagnosis or relapse, prior to or simultaneously with brain relapse. The median time from diagnosis to intracranial relapse was 19.5 months (range 3 – 144 months).
Treatment
Of the 45 patients with intracranial metastases, 19 patients did not undergo any surgical procedure, and 11 patients underwent a biopsy only (Table 2). Surgical resection was attempted in 15 patients; gross total resection was achieved in 11 patients. Wilms tumor metastases were confirmed by pathological examination in 24 patients; biopsy was non-diagnostic in two patients.
Table 2.
Mulitmodality treatment and clinical outcome
| Surgery | |
| Biopsy only | 11 |
| Gross total resection | 11 |
| Partial resection | 4 |
| No surgery | 19 |
|
| |
| Radiation | |
| Yes | 29 |
| No | 15 |
| Unknown | 1 |
|
| |
| Chemotherapy | |
| Yes | 27 |
| No | 17 |
| Unknown | 1 |
|
| |
| ABMT | |
| Yes | 4 |
| No | 40 |
| unknown | 1 |
|
| |
| Vital Status | |
| Alive | 9 |
| Dead | 36 (3 due to complications of treatment) |
|
| |
| Time to death after brain met (months) | |
| Median (range) | 6.9 (0.03–380.5) |
Twenty-nine patients received brain irradiation; dosing information was available on 24 patients. The median whole brain radiation dose was 3000 cGy (range 1080 – 4000 cGy). Four patients received additional boost to tumor bed. Twenty-seven patients received chemotherapy. The choice of chemotherapy was determined by previous treatment regimens. The chemotherapy agents used included vincristine, doxorubicin, ifosfamide, etoposide, carboplatin, cyclophosphamide, and cisplatin. Four patients underwent high-dose chemotherapy followed by autologous bone marrow transplant. Overall, 10 patients did not have any disease directed therapy (surgery, chemotherapy or irradiation) after the detection of intracranial disease; 1 each in NWTS -1 and -2, 3 each in NWTS-3 and -4, and 2 patients in NWTS-5.
Outcome
Nine patients (20%) were alive at last follow-up (Table 3). The median follow-up (from brain relapse) of surviving patients was 140 months (range 35 – 381 months). The median time to develop intracranial metastases in surviving patients was 17 months (range 3 – 37 months). All survivors had favorable histology Wilms tumor at initial diagnosis. In four patients, brain metastases developed at the time of first relapse; in two of them brain was the only site of relapse. Both of these patients underwent gross total resection of the tumor. All surviving patients received whole brain irradiation with some patients receiving additional boost to tumor sites. One patient with isolated brain metastases underwent gross total resection and brain irradiation, and did not receive chemotherapy. Other patients received regimens consisting of etoposide (n=7), cyclophosphamide (n=5), carboplatin (n=5), ifosfamide (n=2), vincristine (n=2) and cisplatin (n=1). Two patients underwent additional autologous bone marrow transplant with melphalan-based conditioning regimens.
Table 3.
Disease and treatment characteristics of patients who are alive (n=9)
| Initial Stage | Study | First relapse site | Time to first relapse (months) | Time to brain metastases (months) | Surgery | Brain irradiation | Chemotherapy | BMT | Last follow-up from brain relapse (months) |
|---|---|---|---|---|---|---|---|---|---|
| I | 3 | Lung | 8 | 13 | No | 3000 cGy | CDDP/CPM x 2 years | No | 381 |
| I | 4 | Lung | 9 | 25 | PR | 3000 cGy | VP16/carbo X 3 cycles, then VCR/CPMX 2 cycles | No | 237 |
| II | 5 | Lung | 6 | 23 | PR | 2400 cGy + 600cGy boost | VP16/carbo X 9 courses | No | 35 |
| II | 5 | Lung, abdomen | 8 | 17 | Biopsy only | 1080cGy + 1080 c Gy boost | VCR, ifosfamide, carbo, VP16 X 2 cycles/carbo, VP16 X 4 cycles | No | 109 |
| III | 5 | Lung | 15 | 37 | Biopsy only | 2000 cGy | Ifosfamide, VP16 | No | 140 |
| III | 5 | Brain, spinal cord | 28 | 28 | Biopsy only | Craniospinal 4080 cGy | CPM/VP16 alternating with Carbo/VP16 x 5 months | Yes (melphalan/thiotepa) | 199 |
| IV (lung) | 5 | Brain | 8 | 8 | GTR | 1980 cGy + 1000 cGy boost | No | No | 186 |
| IV (lung) | 5 | Brain | 7 | 7 | GTR | 3060 cGy | CPM, VP16 alternating with carbo, VP16 | No | 44 |
| IV (lung) | 5 | Brain, lung, heart | 3 | 3 | Biopsy only | Dose not known | VP16, CPM X 2 cycles | Yes (busulphan/melphalan) | 121 |
PR, partial resection; GTR, gross total resection; CDDP, cisplatin; CPM, cyclophosphamide; VP16, etoposide; VCR, vincristine; Carbo, carboplatin.
Thirty-six patients (80%) were dead at last follow-up. Of these, 33 patients died due to progressive Wilms tumor in the brain or other parts of the body. One patient died due to cardiac arrest during brain biopsy. Two patients died due to likely complications of therapy 341 and 348 months after developing intracranial metastases; one patient died of refractory seizures and the other of glioblastoma multiforme. Both patients were treated with whole brain irradiation (3000cGy, 4000cGy) and chemotherapy for their intracranial relapse. After the development of intracranial disease, 10 patients (22%) died within one month, and 28 patients (62%) died within one year.
The five-year overall survival from the time of intracranial relapse was 28.7% with the 95% confidence interval between 14.4% and 43.1% (Figure 2). Of the 14 patients who developed brain metastases in NWTS-5 study, seven (50%) were alive. Of the 31 patients who developed brain metastases in previous studies (NWTS 1–4), only two were alive. The five-year overall survival after brain metastases of the NWTS-5 patients was significantly higher than that for NWTS 1–4 patients (57% vs. 16%, p=0.029; Figure 3).
Fig. 2.
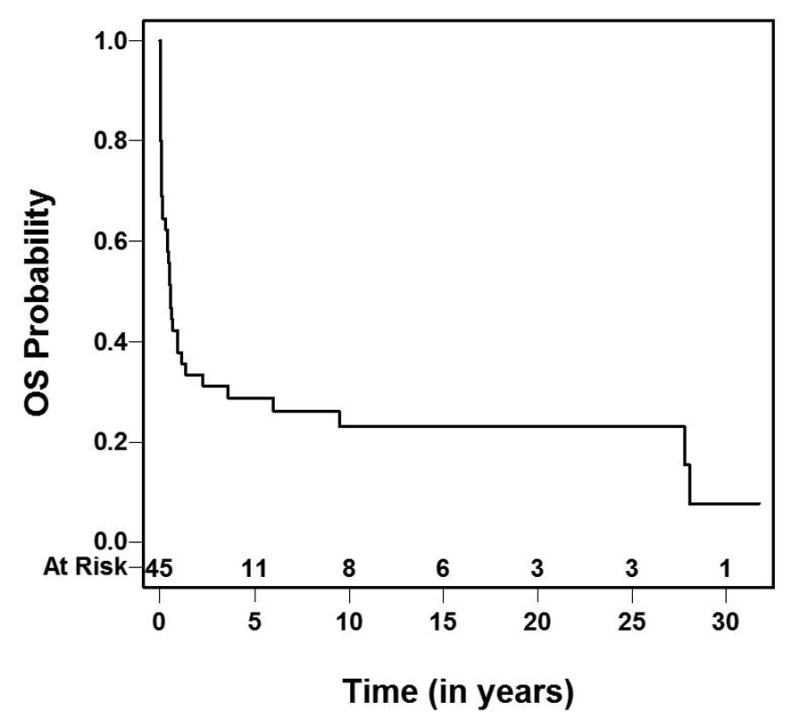
Overall Survival following brain relapse
Fig. 3.
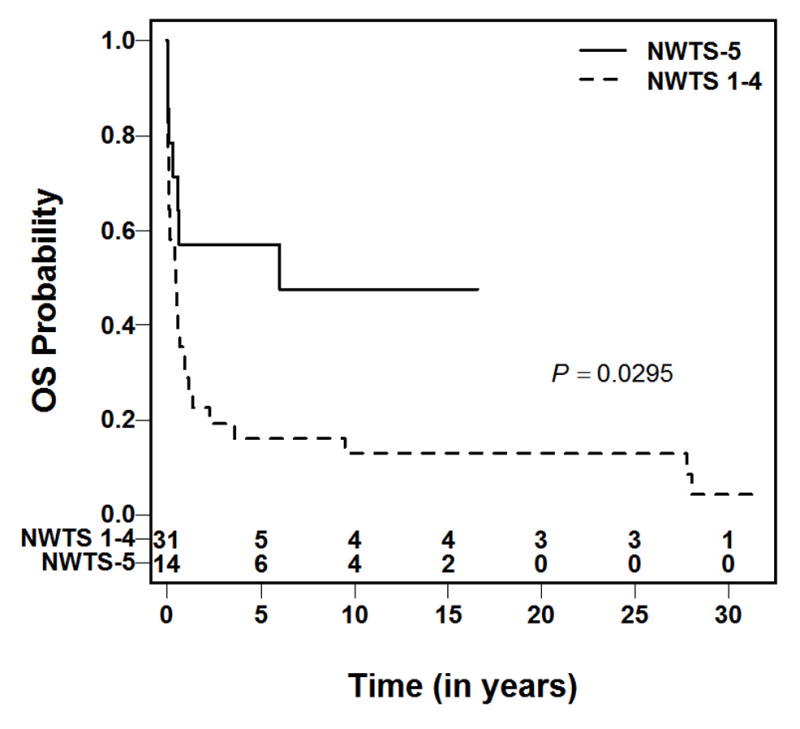
Overall survival following brain relapse of patients enrolled on NWTS-5 and NWTS 1–4.
DISCUSSION
Approximately 0.5% of patients with Wilms tumor enrolled in NWTS 1–5 developed intracranial relapse, confirming the rarity of the brain as a metastatic site. [4, 10] The incidence of intracranial relapse has remained relatively constant over time despite improvements in therapy and overall prognosis. This contrasts with the situation in CCSK, where the prevalence of intracranial metastasis has increased over time.[18, 19] The reason for this is unclear, but it is possible that improved therapy has led to better control of extracranial sites, allowing brain metastases to become manifest in a subgroup of patients with CCSK. This phenomenon does not appear to be the case for Wilms tumor.
Similar to what was observed in the UKCCSG study[4], we found that more boys developed intracranial recurrence than girls. In contrast, the SIOP study reported more central nervous system relapse in girls than boys.[10] Overall, the data published in the literature thus far does not suggest a preponderance of either sex.
Nineteen of the 45 patients did not have histological confirmation that the brain tumor was metastatic Wilms tumor, so we considered the possibility that some had second malignant neoplasms. We believe that second malignant neoplasms are unlikely because 14 of the 19 patients had Wilms tumor progression in an extracranial site prior to the appearance of the brain lesion. In three other patients, brain lesions appeared simultaneously with an extracranial site. In the two remaining patients, one developed progression in the lung after detection of the brain metastasis. In the childhood cancer survivor study, 25-year follow-up of 1254 Wilms tumor survivors revealed only one patient with medulloblastoma as a second malignant neoplasm. [20] Based on the data presented above, while it is possible that some of the tumors could represent second malignant neoplasm, it is highly unlikely.
The survival of patients with recurrent Wilms tumor has improved during the past two decades. With modern treatment regimens, patients who initially received vincristine/dactinomycin have survival estimates of approximately 80% whereas patients who initially received vincristine/dactinomycin/doxorubicin have survival estimates of approximately 50%. [21–23] The lung is by far the most common site of relapse. Some studies suggest that relapse outside the lung is associated with unfavorable prognosis relative to lung-only relapse.[22] In the present series, the 5-year overall survival estimate from the time of brain recurrence was only 28%. While this is inferior to the survival rate for recurrent Wilms tumor in general, the data span a broad time period from NWTS 1–5. The prognosis seems to be improving with advances in multimodality therapy as 50% of patients with intracranial relapse in the most recent NWTSG study (NWTS-5) were alive compared to six percent previously (p=0.03), mirroring the improvement in survival after relapse in general that has occurred over time. The reason for this improvement in survival is likely multifactorial, including the availability of more chemotherapy agents, improvements in surgical and radiation techniques and the recognition that some patients with recurrent disease can be salvaged.
The clinical characteristics of the nine surviving patients were evaluated to identify potential prognostic factors for patients with Wilms tumor and brain metastases (supplementary Table 1). In general, prognostic factors described for relapsed Wilms tumor that convey poor outcome include early relapse, higher initial stage, anaplastic histology, and combined local and distant relapse.[16, 21, 24] Although it is difficult to draw firm conclusions based on the small patient group reported here, histology would seem to be an important factor for outcome because none of the patients with anaplastic Wilms tumor survived. The median time to intracranial relapse for the nine surviving patients was not different from the overall group with intracranial metastases (p=0.59). In addition, length of initial remission, regardless of the first relapse site, was similar for both groups (p=0.36). The smaller number of patients who experienced intracranial relapse and an even smaller number who survived precludes drawing any firm conclusions about initial stage as a prognostic factor in this setting. In fact, a higher proportion of stage IV patients survived in this series than lower stage patients.
The NWTS experience presented does not allow formulating a conclusive therapeutic strategy for intracranial relapse. The therapies administered varied because this study spans a 30-year period in which several therapeutic changes were introduced. Nevertheless, it is noteworthy that only two of the nine salvaged patients had a gross total tumor resection. The UKCCSG investigators also reported survival after incomplete resection as only one of the three surviving patients had undergone a complete resection.[4] Together these data would suggest that excision may not be necessary for salvage. Radiotherapy on the other hand seems indicated as all nine survivors in the present series as well as the three survivors reported by UKCCSG received radiotherapy. However, the optimal dose of radiation is not known. Moreover, there is concern about the long-term effects of radiation therapy in young children as exemplified by the death of one of our patients nearly 30 years after cranial irradiation due to development of glioblastoma multiforme. The current Children’s Oncology Group (COG) Wilms tumor protocols recommend whole brain irradiation to a dose of 3060cGy for older children after surgical resection. In radiosensitive cancers like leukemia and medulloblastoma, a prophylactic whole brain radiation dose of 1800cGy to 2300cGy has been shown to be effective. In order to reduce late toxicity in young children with Wilms tumor, especially those under 3–5 years of age, with a limited number of metastases, it is reasonable to reduce the whole brain dose to 2160cGy followed by a limited volume (tumor or tumor bed only with 0.5cm margin) boost dose of 1080cGy using conformal irradiation or intensity modulated radiation therapy (IMRT). Chemotherapy was administered to eight of the nine survivors though exact role of chemotherapy in this context remains to be ascertained. A majority of the patients received cyclophosphamide, etoposide and carboplatin. The same agents are effective against recurrent Wilms at other sites.[22] The utility of autologous bone marrow transplant in relapsed Wilms tumor is unknown. A meta-analysis of published studies suggested a benefit of high-dose therapy in patients with “very high risk” relapses, a group largely composed of patients who received four or more chemotherapy agents for up-front therapy.[25] Its role in patients with Wilms tumor relapsing to the brain could not be determined in this study. Although two of the four patients who underwent autologous bone marrow transplantation survived, they likely represent a selected population as autologous bone marrow transplantation is often performed in the minimal residual disease setting.
In conclusion, relapse to the brain is a rare occurrence in patients with Wilms tumor. This report is the largest report to date on clinical outcomes of children with Wilms tumor and brain metastases. Unlike intracranial metastases in other pediatric solid tumors, which are typically associated with rapid deterioration, intracranial relapse in Wilms tumor may be curable in patients with favorable histology Wilms tumor. A multimodality treatment approach including radiation therapy, chemotherapy, and surgery, if feasible, is recommended.
Supplementary Material
Supplemental Table S1. Comparison of patient a tumor characteristics of survivors and non-survivors
Acknowledgments
Funding: Research was supported by grants from the National Institutes of Health to the National Wilms Tumor Study Group (CA-42326), the National Wilms Tumor Study Group Late Effects Study (CA-54498), and to the Children’s Oncology Group (U10CA180886, U10CA180899, U10CA098543).
Abbreviations
- NWTS
National Wilms Tumor Study
- CCSK
Clear cell sarcoma of the kidney
- RTK
Rhabdoid tumor of kidney
- UKCCSG
United Kingdom childhood cancer study group
- SIOP
International Society of Paediatric Oncology
- COG
Children’s oncology group
Footnotes
Conflict of Interest statement
The authors have no conflict of interest to report.
References
- 1.Dome JS, Graf N, Geller JI, et al. Advances in Wilms Tumor Treatment and Biology: Progress Through International Collaboration. J Clin Oncol. 2015;33(27):2999–3007. doi: 10.1200/JCO.2015.62.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gooskens SL, Furtwängler R, Spreafico F, et al. Treatment and outcome of patients with relapsed clear cell sarcoma of the kidney: a combined SIOP and AIEOP study. Br J Cancer. 2014 Jun; doi: 10.1038/bjc.2014.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van den Heuvel-Eibrink MM, van Tinteren H, Rehorst H, et al. Malignant rhabdoid tumours of the kidney (MRTKs), registered on recent SIOP protocols from 1993 to 2005: a report of the SIOP renal tumour study group. Pediatr Blood Cancer. 2011;56(5):733–737. doi: 10.1002/pbc.22922. [DOI] [PubMed] [Google Scholar]
- 4.Lowis SP, Foot A, Gerrard MP, et al. Central nervous system metastasis in Wilms’ tumor: a review of three consecutive United Kingdom trials. Cancer. 1998;83(9):2023–2029. doi: 10.1002/(sici)1097-0142(19981101)83:9<2023::aid-cncr20>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 5.Traggis D. Successful treatment of Wilms’ tumor with intracranial metastases. Pediatrics. 1975;56(3):472–473. [PubMed] [Google Scholar]
- 6.Morgan SK, Buse MG. Survival following brain metastases in wilms’ tumor. Pediatrics. 1976;58(1):130–132. [PubMed] [Google Scholar]
- 7.Gandolfi A, Orsoni JG. Occult nephroblastoma (Wilms’ tumor) presenting with symptoms of central nervous system involvement. Acta Neurol (Napoli) 1979;1(5):424–428. [PubMed] [Google Scholar]
- 8.Kortmann RD, Budach W, Niethammer D, Grote EH, Bamberg M. Postoperative limited volume irradiation in a child with a solitary brain metastasis from Wilms tumor: a case report. Med Pediatr Oncol. 1997;29(3):222–223. doi: 10.1002/(sici)1096-911x(199709)29:3<222::aid-mpo11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 9.Bouffet E, Doumi N, Thiesse P, et al. Brain metastases in children with solid tumors. Cancer. 1997;79(2):403–410. doi: 10.1002/(sici)1097-0142(19970115)79:2<403::aid-cncr25>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.van den Heuvel-Eibrink MM, Graf N, Pein F, et al. Intracranial relapse in Wilms tumor patients. Pediatr Blood Cancer. 2004;43(7):737–741. doi: 10.1002/pbc.20150. [DOI] [PubMed] [Google Scholar]
- 11.D’Angio GJ, Breslow N, Beckwith JB, et al. Treatment of Wilms’ tumor. Results of the Third National Wilms’ Tumor Study. Cancer. 1989;64(2):349–360. doi: 10.1002/1097-0142(19890715)64:2<349::aid-cncr2820640202>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 12.D’Angio GJ, Evans A, Breslow N, et al. The treatment of Wilms’ tumor: results of the Second National Wilms’ Tumor Study. Cancer. 1981;47(9):2302–2311. doi: 10.1002/1097-0142(19810501)47:9<2302::aid-cncr2820470933>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 13.D’Angio GJ, Evans AE, Breslow N, et al. The treatment of Wilms’ tumor: Results of the national Wilms’ tumor study. Cancer. 1976;38(2):633–646. doi: 10.1002/1097-0142(197608)38:2<633::aid-cncr2820380203>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Green DM, Beckwith JB, Breslow NE, et al. Treatment of children with stages II to IV anaplastic Wilms’ tumor: a report from the National Wilms’ Tumor Study Group. J Clin Oncol. 1994;12(10):2126–2131. doi: 10.1200/JCO.1994.12.10.2126. [DOI] [PubMed] [Google Scholar]
- 15.Green DM, Breslow NE, Beckwith JB, et al. Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms’ tumor: a report from the National Wilms’ Tumor Study Group. J Clin Oncol. 1998;16(1):237–245. doi: 10.1200/JCO.1998.16.1.237. [DOI] [PubMed] [Google Scholar]
- 16.Dome JS, Cotton CA, Perlman EJ, et al. Treatment of Anaplastic Histology Wilms’ Tumor: Results From the Fifth National Wilms’ Tumor Study. JCO. 2006;24(15):2352–2358. doi: 10.1200/JCO.2005.04.7852. [DOI] [PubMed] [Google Scholar]
- 17.Peto R, Peto J. Asymptotically Efficient Rank Invariant Test Procedures. Journal of the Royal Statistical Society Series A (General) 1972;135(2):185–207. [Google Scholar]
- 18.Radulescu VC, Gerrard M, Moertel C, et al. Treatment of recurrent clear cell sarcoma of the kidney with brain metastasis. Pediatr Blood Cancer. 2008;50(2):246–249. doi: 10.1002/pbc.21131. [DOI] [PubMed] [Google Scholar]
- 19.Gooskens SL, Furtwängler R, Spreafico F, et al. Treatment and outcome of patients with relapsed clear cell sarcoma of the kidney: a combined SIOP and AIEOP study. Br J Cancer. 2014;111(2):227–233. doi: 10.1038/bjc.2014.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Termuhlen AM, Tersak JM, Liu Q, et al. Twenty-Five Year Follow-Up of Childhood Wilms Tumor: A Report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2011;57(7):1210–1216. doi: 10.1002/pbc.23090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinhard H, Schmidt A, Furtwängler R, et al. Outcome of relapses of nephroblastoma in patients registered in the SIOP/GPOH trials and studies. Oncol Rep. 2008;20(2):463–467. [PubMed] [Google Scholar]
- 22.Malogolowkin M, Cotton CA, Green DM, et al. Treatment of Wilms tumor relapsing after initial treatment with vincristine, actinomycin D, and doxorubicin. A report from the National Wilms Tumor Study Group. Pediatr Blood Cancer. 2008;50(2):236–241. doi: 10.1002/pbc.21267. [DOI] [PubMed] [Google Scholar]
- 23.Green DM, Cotton CA, Malogolowkin M, et al. Treatment of Wilms tumor relapsing after initial treatment with vincristine and actinomycin D: a report from the National Wilms Tumor Study Group. Pediatr Blood Cancer. 2007;48(5):493–499. doi: 10.1002/pbc.20822. [DOI] [PubMed] [Google Scholar]
- 24.Grundy P, Breslow N, Green DM, Sharples K, Evans A, D’Angio GJ. Prognostic factors for children with recurrent Wilms’ tumor: results from the Second and Third National Wilms’ Tumor Study. J Clin Oncol. 1989;7(5):638–647. doi: 10.1200/JCO.1989.7.5.638. [DOI] [PubMed] [Google Scholar]
- 25.Ha TC, Spreafico F, Graf N, et al. An international strategy to determine the role of high dose therapy in recurrent Wilms’ tumour. Eur J Cancer. 2012 Sep; doi: 10.1016/j.ejca.2012.07.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. Comparison of patient a tumor characteristics of survivors and non-survivors