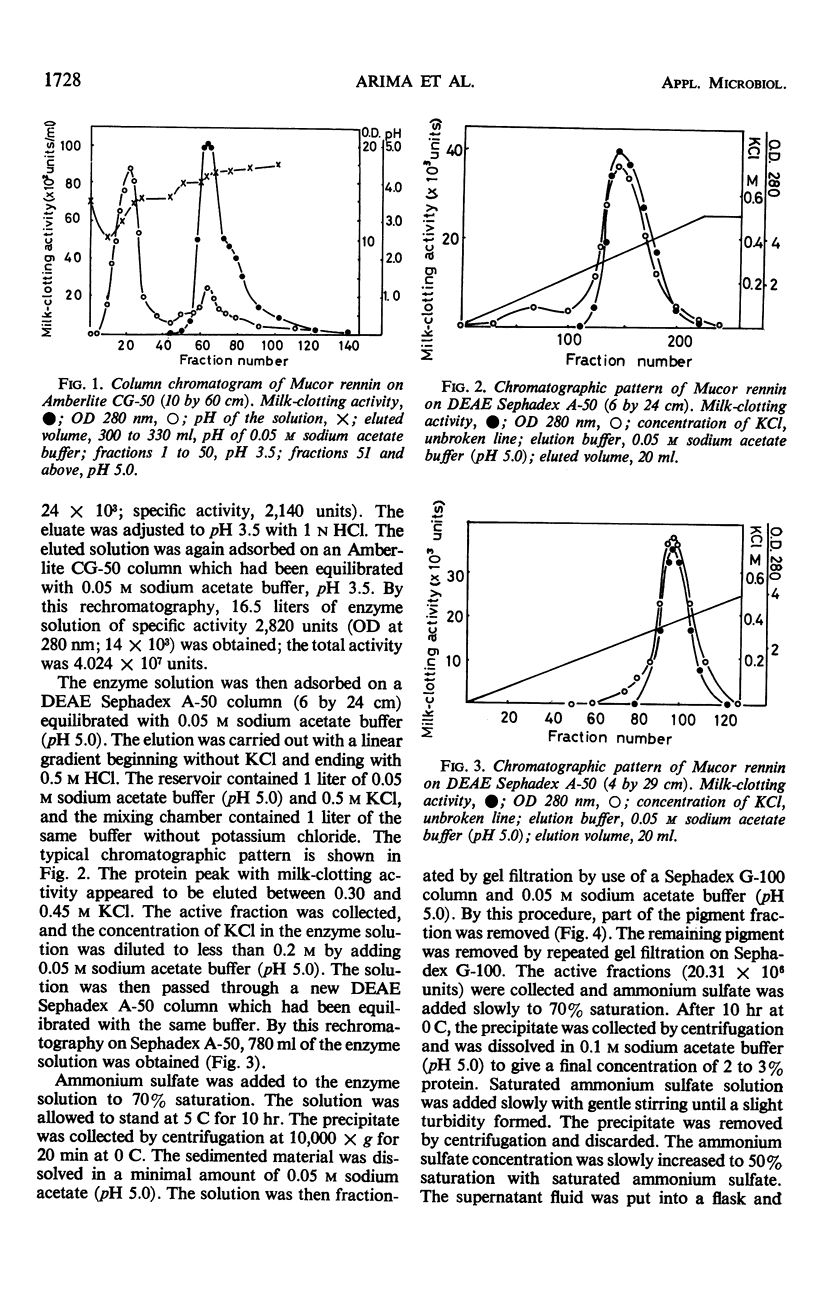
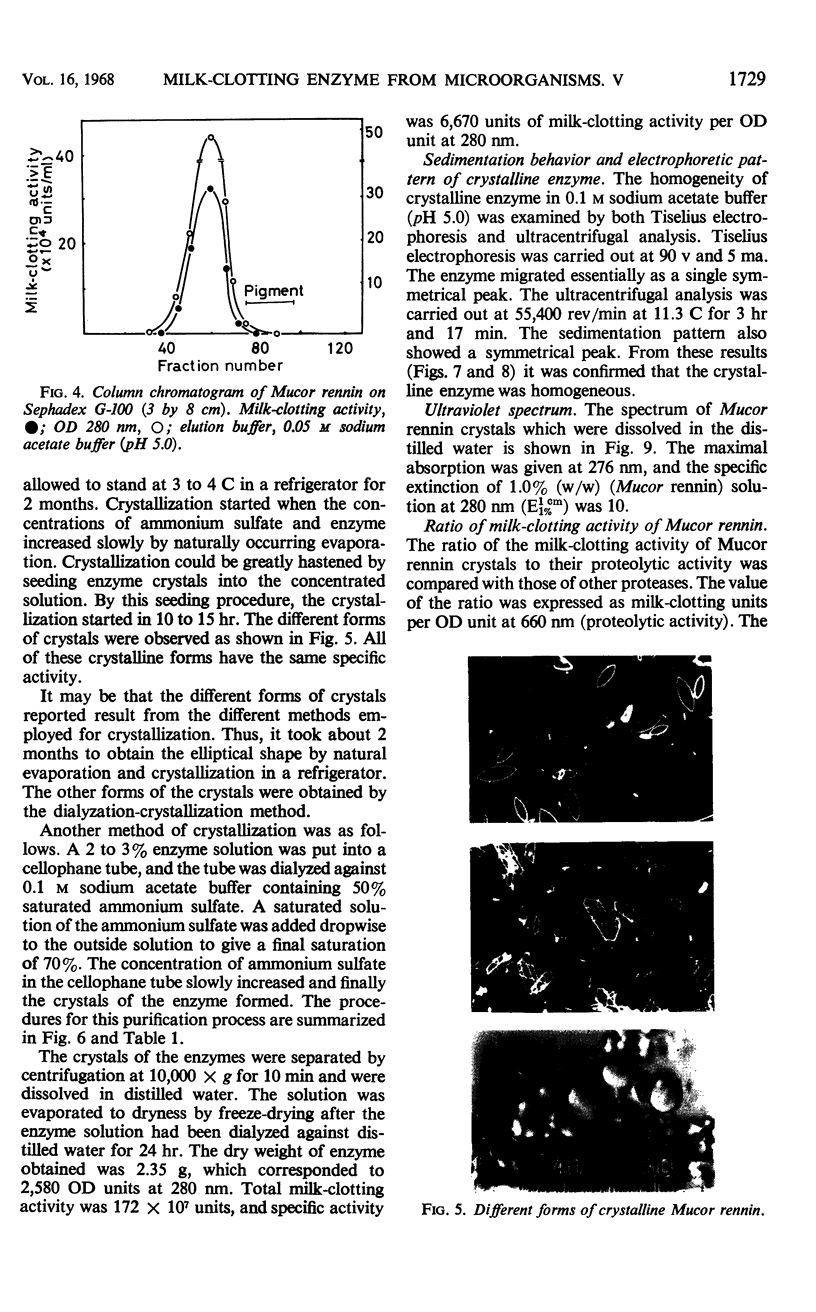
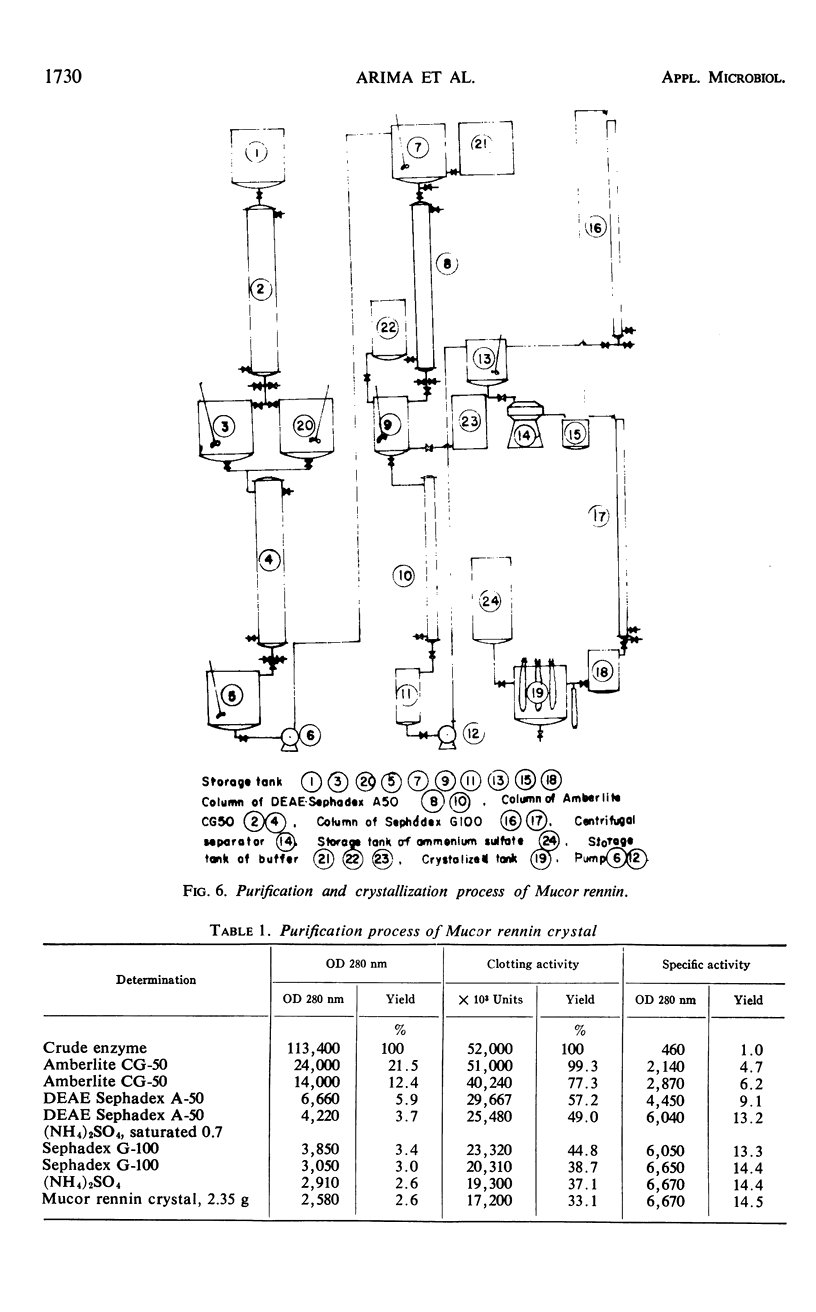
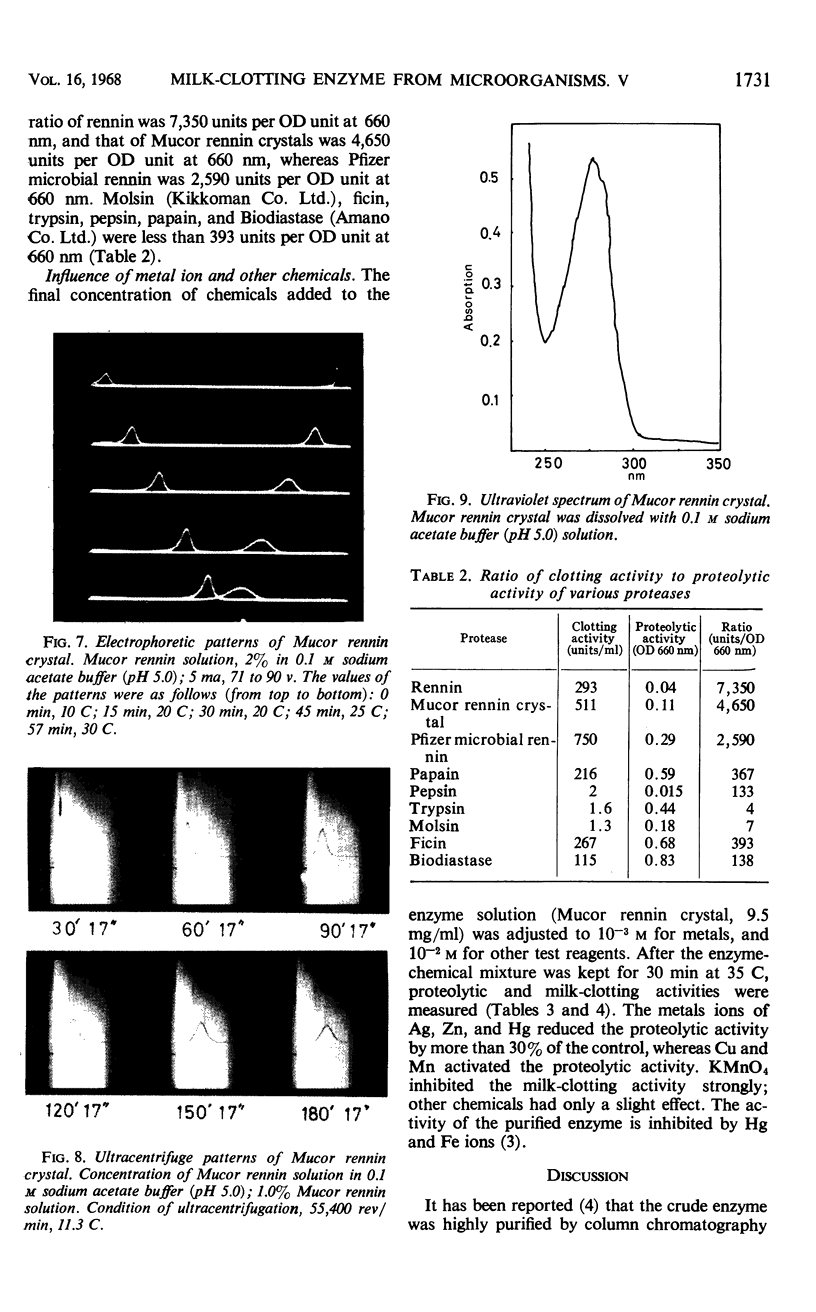
Abstract
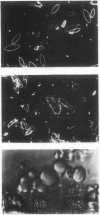
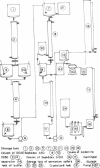
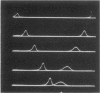
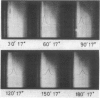
A rennin crystal was obtained from the crude milk-clotting enzyme of Mucor pusillus var. Lindt. The crude enzyme was purified by using columns of Amberlite CG-50, diethylaminoethyl Sephadex A-50, and Sephadex G-100. This purified enzyme was dissolved in 0.1 M sodium acetate (pH 5.0) buffer to a final concentration of 2 to 3%; ammonium sulfate (to 40% saturation) was added, and the resulting solution was placed in cellophane tubes. The enzyme solution was dialyzed against 0.1 M sodium acetate buffer (pH 5) containing ammonium sulfate was added dropwise to the outside solution of the cellophane tube, and the concentration of ammonium sulfate in the cellophane tube increased gradually. The crystals of enzyme were formed in the cellophane tube when the concentration reached approximately 50% saturation. After the enzyme solution was concentrated in the freezer, the crystals were obtained. The activity of the crystalline enzyme was inhibited by Hg2+, Ag+, Zn2+, and KMnO4.
Full text
PDF