Abstract
Insulin signaling regulates various aspects of physiology, such as glucose homeostasis and aging, and is a key determinant of female reproduction in metazoans. That insulin signaling is crucial for female reproductive health is clear from clinical data linking hyperinsulinemic and hypoinsulinemic condition with certain types of ovarian dysfunction, such as altered steroidogenesis, polycystic ovary syndrome, and infertility. Thus, understanding the signaling mechanisms that underlie the control of insulin-mediated ovarian development is important for the accurate diagnosis of and intervention for female infertility. Studies of invertebrate and vertebrate model systems have revealed the molecular determinants that transduce insulin signaling as well as which biological processes are regulated by the insulin-signaling pathway. The molecular determinants of the insulin-signaling pathway, from the insulin receptor to its downstream signaling components, are structurally and functionally conserved across evolution, from worms to mammals – yet, physiological differences in signaling still exist. Insulin signaling acts cooperatively with gonadotropins in mammals and lower vertebrates to mediate various aspects of ovarian development, mainly owing to evolution of the endocrine system in vertebrates. In contrast, insulin signaling in Drosophila and Caenorhabditis elegans directly regulates oocyte growth and maturation. In this review, we compare and contrast insulin-mediated regulation of ovarian functions in mammals, lower vertebrates, C. elegans, and Drosophila, and highlight conserved signaling pathways and regulatory mechanisms in general while illustrating insulin’s unique role in specific reproductive processes.
Keywords: Oocyte development, oogenesis, steroidogenesis, insulin, PI3K/AKT, ERK, PCOS
INTRODUCTION
The successful propagation of a species requires proper oocyte development and ovarian function. Throughout the animal kingdom, oogenesis is regulated by a large number of intra- and extra-ovarian factors. In mammals as well as in other vertebrates, the follicle is the functional unit of the ovary, consisting of three cell types: an outer theca cell layer, an inner granulosa cell mass, and oocytes. Follicular development, meiotic resumption, and ovulation are orchestrated by the highly complex interplay of endocrine, paracrine, and autocrine signals. Three different tiers of regulatory organs—the hypothalamus, anterior pituitary gland, and gonads, the hypothalamic-pituitary-gonadal axis—regulate these events in the life cycle of an oocyte (Nagahama and Yamashita, 2008; Edson et al., 2009).
Insulin, one of the most conserved molecules among animals, also regulates ovarian development and oogenesis. Insulin was initially discovered for its roles in regulating carbohydrate, fat, and protein metabolism in muscle, liver, and adipose tissues (Saltiel and Kahn, 2001; Hua, 2010). Recent studies from the last two decades, however, have rapidly expanded the activities that insulin participates in, including regulation of steroidogenesis in ovarian cells in vitro and in the stromal and follicular compartments of human and murine ovaries (Poretsky et al., 1999; Acevedo et al., 2007). Underscoring these observations and the impact of insulin signaling on ovarian development is the observation that, in humans, hypo- or hyper-insulinemia is associated with significant alterations to ovarian function (Colton et al., 2002; Chang et al., 2005; Kandarakis and Dunaif, 2012). Insulin receptor (IR)-dependent signaling also promotes oocyte maturation in lower vertebrates, such as Xenopus and zebrafish, wherein IR is expressed both in the thecal-granulosa compartment and the oocyte surface (Hainaut et al., 1991; Chuang et al., 1993, 1994; Das et al., 2016a), and regulates oocyte development and maturation in flies and worms (Wu and Brown, 2006; Brown et al., 2008; Lopez et al., 2013). Together, these reports suggest that the role of insulin signaling during oogenesis and ovarian development is conserved from worms to humans, even though physiological and molecular differences exist in their pathways. The mechanisms regulating these diverse insulin-dependent functions are clearly of immense importance, as are their modes of execution throughout phylogeny.
Potential interplay among these signaling events in oocytes undergoing meiotic progression is an area of active research. Keeping these developments in mind, we focus primarily on the recent advancements and the crucial role of insulin-induced signaling pathways in oocyte growth, development, and maturation. Our comparison of worms, flies, teleosts, and mammals highlights the evolutionary conservation of the insulin-signaling pathway in general, while illustrating its unique role(s) in specific reproductive processes.
INSULIN: AN EVOLUTIONARILY CONSERVED MOLECULE
Insulin signaling regulates blood glucose levels and is essential for maintaining energy storage, glucose metabolism, glycogenesis, lipogenesis, cellular growth, survival, and reproduction – additionally it plays a role in aging (Poretsky et al., 1999; Tatar et al., 2001; Taguchi and White, 2008; Hua, 2010; Kenyon, 2010). Physiologically, the cellular function of insulin is primarily mediated via IR, a member of the receptor tyrosine kinase family that is expressed on the cell surface as a heterodimer of two identical α2β2 subunits (reviewed by Lawrence et al., 2007). IR regulates two major cell signaling cascades that affect either metabolic or mitogenic functions: (i) the Phosphatidylinositol-3-kinase (PI3K)/AKT and (ii) RAS/ERK (Extracellular-signal Regulated Kinase) signaling pathways (Figure 1) (described in detail by Cantley et al., 2002; Roux and Blenis, 2004; Taniguchi et al., 2006; Belfiore et al., 2009; Liao and Hung, 2010).
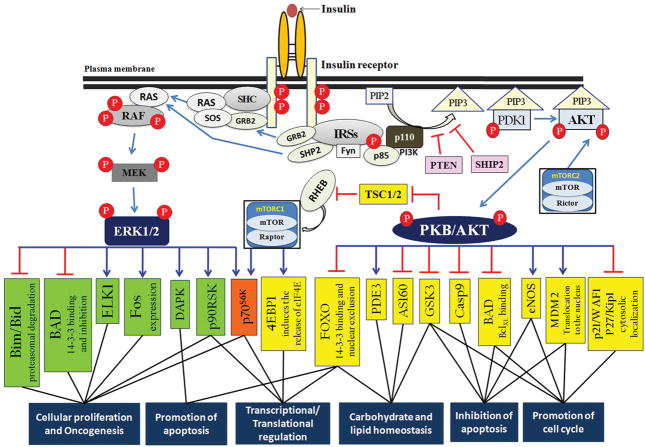
Figure 1.
Insulin-dependent signal transduction pathways. IR undergoes autophosphorylation and activation at the C-terminal domains upon ligand binding. Tyrosine-phosphorylated adaptor proteins recruit signaling proteins via specific protein domains. Active PI3K phosphorylates the membrane phospholipid phosphatidylinositol 4,5-bisphosphate to produce the second messenger PIP3. Phospholipid phosphatases (e.g., phosphatase and tensin homolog deleted on chromosome 10 [PTEN] and SH2 domain-containing inositolphosphatase-2) dephosphorylate and convert PIP3 to phosphatidylinositol 4,5-bisphosphate, and negatively regulate the PI3K pathway. PIP3 recruits phosphoinositide-dependent kinase-1 (PDK1) and AKT to the plasma membrane, where PIP3-bound AKT is phosphorylated at Thr308 by PDK1 and Ser473 by Rictor-mTORC2. Activated AKT phosphorylates and regulates many target proteins (yellow and orange box(es)) either positively (→) or negatively (⊣) to mediate distinct biological events, including (i) maintenance of glucose homeostasis; (ii) cellular survival and growth; (iii) inhibition of apoptosis; (iv) angiogenesis; and (v) regulation of gene transcription and protein synthesis. AKT provides a direct link between insulin signaling and nutrient sensing. The ERK signaling pathway is triggered by activation of the small GTPase protein p21 RAS. Active diphosphorylated ERK shuttles between the cytoplasm and nucleus, and regulates substrate proteins in both compartments (green and orange boxes), to mediate cell growth, survival, and differentiation. 4EBP1, eukaryotic translation initiation factor 4E-binding protein 1; Casp9, Caspase9; DAPK, death-associated protein kinase; eIF4E, eukaryotic translation initiation factor 4E; eNOS, endothelial nitric oxide synthase; GSK3, glycogen synthase kinase 3; MDM2, mouse double minute 2 homolog; p90RSK, ribosomal protein S6 kinase; RHEB, RAS homolog enriched in brain; SHC, SH2 domain-containing; SHIP2, SH2 domain-containing inositolphosphatase-2; SOS, son of sevenless.
The components of insulin signaling, including ligands and receptor proteins, are highly conserved and active among metazoans (Figure 2). For example, Drosophila Insulin-like peptides (ILPs) bind to and activate human IR, and can lower blood glucose levels in rats (Sajid et al., 2011). Reciprocally, bovine and human insulin can bind to Drosophila IR with moderate affinity (Petruzzelli et al., 1985; Sajid et al., 2011), underscoring the structural and functional conservation of insulin signaling in these distant species. Additionally, insulin receptor substrates – PI3K, AKT, and RAS-ERK – are all regulated downstream of insulin signaling in mammals (White, 2003; Taniguchi et al., 2006; Taguchi and White, 2008; Belfiore et al., 2009), Drosophila (Claeys et al., 2002; Badisco et al., 2013; Sim and Denlinger, 2013), and Caenorhabditis elegans (Kenyon, 2010; Murphy and Hu, 2013).
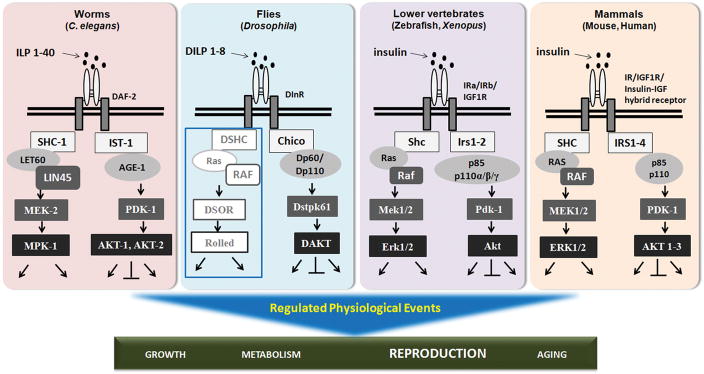
Figure 2.
A conserved insulin-signaling pathway in the ovary. Activation of the IR leads to activation of cellular signaling pathways, whose members have orthologs among all metazoans. The most conserved is the PI3K/AKT/FOXO pathway. The RAS/ERK signaling cascade downstream of insulin signaling is not yet documented in flies (blue box). Included with the well-characterized and conserved function of the insulin signaling pathway in mediating glucose homeostasis, metabolism, growth, is its regulation of reproduction, specifically oogenesis (see text for details).
Members of the human insulin peptide family include insulin, insulin-like growth factor (IGF) 1 and 2, and seven relaxin-related peptides (reviewed by Fernandez and Torres-Alemán, 2012). Despite their similarities at the ligand and receptor levels, insulin regulates only metabolic effects, whereas IGFs act as potent growth and differentiation factors (Belfiore et al., 2009). Insulin is secreted from mammalian pancreatic β cells in response to glucose, while other nutrients, such as free fatty acids and amino acids, can augment glucose-induced insulin secretion (Fu et al., 2013); much less is known about regulation of insulin gene expression or insulin secretion from pancreatic β-cells of lower vertebrates. The transcription factors that regulate the expression of insulin in zebrafish, however, are homologous with those in the mammalian system (Biemar et al., 2001; Kinkel and Prince, 2009), prompting us to hypothesize that the regulation of gene expression in teleosts is similar among vertebrates, but only future research will clarify these mechanisms.
Mature insulin hormone is generated by cleavage of the preprohormone in vertebrates. Conversely, invertebrates genomes encode for small ILPs – although these ILPs do carry the dibasic cleavages sites, and also express several proconvertases, suggesting that the ILPs maybe generated from longer peptides, much like cleavage of preprohormone in vertebrates. The Drosophila genome contains eight ILP-encoding genes (dilp1-8) (Brogiolo et al., 2001; Gronke et al., 2010; Colombani et al., 2012). These dilp-encoded peptides are structurally similar to preproinsulin, of which DILP2 has the highest homology (35% identity) with mammalian insulins (Brogiolo et al., 2001). The C. elegans genome similarly contains genes for ~40 distinct ILPs (daf-28 and ins-1 through ins-39) (reviewed by Li and Kim, 2008). Ninety percent of C. elegans ILPs emanate from neurons that integrate environmental cues to signal to other cell and tissue types (Pierce et al., 2001; Li et al., 2003). In addition, ins-1, ins-7, ins-18, and daf-28 are expressed in non-neuronal tissues, such as the intestine (Pierce et al., 2001; Li et al., 2003; Murphy et al., 2007). Systematic and genetic analysis of all 40 ILP genes in C. elegans as well as genetic analysis of the 8 dilp genes in Drosophila demonstrated that ILPs regulate crucial invertebrate physiology, either alone or in combination, and may possess functional redundancy and/or mediate feedback regulation (Grönke et al., 2010; Cornils et al., 2011; Fernandes de Abreu et al., 2014). Given the varied biological responses generated during the development of worms and flies in response to the different ILPs, important, unanswered questions include if and how environmental or nutritional input specifically regulates the expression of each of the insulin ligand genes to generate the distinct biological responses. Work in Drosophila revealed that distinct ILPs are secreted in distinct manners. For example, DILP2, 3, and 5 are secreted by neurosecretory cells in the fly brain in response to distinct signals in larvae versus adults. Some of these mechanisms include glucose sensing (reviewed in detail by Nassel & Vanden Broeck 2016). However, while IGF-binding peptides been identified in Drosophila (Honegger et al., 2008), whether or not their binding affinity varies with different ILPs in a tissue-specific manner and how they modulate the function of ILPs requires further investigation.
INSULIN REGULATION OF OOGENESIS AND OVARIAN DEVELOPMENT
Oocytes are terminal gametes, so their quality is the principal determinant of early embryonic survival, establishment and maintenance of pregnancy, fetal development, and the acquisition of several birth defects (Gilbert et al., 2015; Zhang and Smith, 2015). The process of oocyte formation, growth, and development establishes the foundation of proper embryogenesis, and key events in oocyte development are shared among phylogenetically distant animal groups. For instance, oocytes originate from primordial germ cells during fetal development in almost all animals and actively migrate to the gonad during early embryonic development from across the gut; an exception is C. elegans, whose primordial germ cells do not need to migrate to the gonad, and instead are carried there during ventral ingression (reviewed by Richardson and Lehmann, 2010). In early growth phases, oocytes actively transcribe many genes whose mRNA are then held in a translationally silent form, owing to shortened polyadenine tails (reviewed by Bachvarova, 1992; Mendez and Richter, 2001). These transcripts are stored for future oocyte development, such as during oocyte maturation (e.g. c-mos, cyclinB), and for post-fertilization processes that are required prior to the maternal-to-zygotic genome transition (reviewd by Langley et al., 2014). Growing oocytes also take up amino acids and several macromolecules from the surrounding follicular cells. De novo synthesis and uptake of macromolecules are both essential for the growth, development, and maturation of the oocyte itself, as well as for the storage of information and materials necessary to support early embryonic development (Picton et al., 1998; Sánchez and Smitz, 2012; Langley et al., 2014). Finally, oocytes of almost all metazoan species arrest at the diplotene (or diakinesis, in C. elegans) stage of prophase-I, known as the primary arrest point (Figure 3); this milestone further aids in the differentiation, growth, and development of oocytes (reviewed by Von Stetina and Orr-Weaver, 2011).
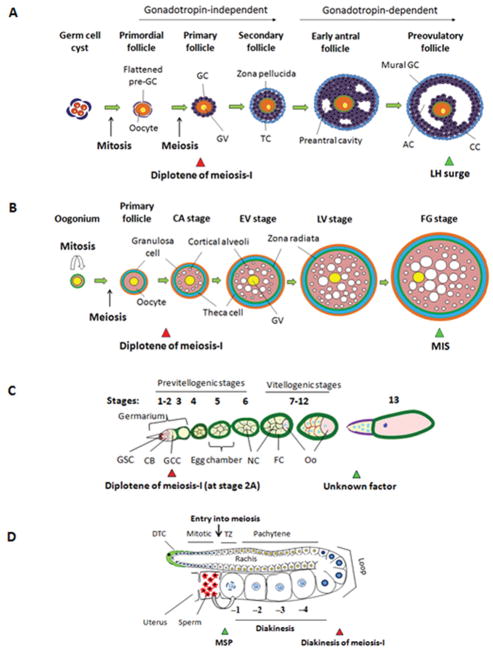
Figure 3.
Stages of oocyte development and meiotic resumption. (a) Formation of primordial follicles varies by species. Primordial follicles develop in utero in humans, versus 1–2 days after birth in mice. A primary follicle, developed from the primordial follicle, consists of a prophase-I–arrested primary oocyte surrounded by somatic granulosa cells (GC). By the secondary stage, the primary oocytes grow, granulosa cells proliferate, and an additional layer of somatic thecal cells (TC) forms outside the basement membrane of the follicle. In both mice and humans, preantral follicle development does not require pituitary gonadotropins. At puberty, FSH secreted by the anterior pituitary promotes further granulosa cell proliferation and survival. As a fluid-filled antrum cavity (AC) begins to form, secondary follicles become early antral follicles. The full-grown primary oocyte is surrounded by proximal cumulus cells (CC) and distal mural granulosa cells in preovulatory follicles. After an LH surge, oocytes undergo meiotic maturation. (b) In fish, the oogonium proliferates and generates primary follicles, at which point the oocytes enter meiosis and arrest at diplotene. The oocytes then begin to enlarge, and form follicles. Next cortical alveoli accumulate within the oocytes, followed by vitellogenesis, which results in increase in the size of the oocyte. Full-grown (FG) follicles initiate oocyte maturation. The germinal vesicle migrates from the center of the oocyte to the periphery, and breaks down in response to maturation-inducing steroid (MIS). (c) In Drosophila, the ovariole is composed of the germarium in the anterior-most part followed by a row of progressively older egg chambers. Within region 1 of the germarium, a cystoblast (CB), derived from a germ-line stem cell, divides four times via mitosis to form a 16-cell germ-line cyst (GCC). Meiosis begins at stage 2A of the germarium, and the oocyte (Oo) arrests at diplotene of prophase-I. The posterior-most germ line cell becomes the oocyte, whereas the remaining 15 cells become nurse cells (NC). The egg chamber is surrounded by a single layer of follicular cells (FC). At stage 13, after an unknown developmental or hormonal signal, the oocyte resumes meiosis and progresses to MI. (d) In C. elegans, adult hermaphrodites have two U-shaped gonads containing germ cells arranged in a distal-to-proximal polarity with respect to the somatic distal tip cell (DTC) at the distal end and the proximal spermatheca/uterus. Germ cells proliferate mitotically at the distal end, known as the “mitotic zone”. At the proximal end of the mitotic region, germ cells switch to meiotic prophase, where they are first in leptotene/zygotene (transition zone [TZ]) and then progress through an extended pachytene followed by diplotene and diakinesis around the loop region. Primary arrest of C. elegans oocytes occurs at diakinesis. In response to sperm and its secreted factor, MSP, the most proximal oocyte (−1) is induced to undergo meiotic maturation. The red and green triangles indicate prophase-I arrest and meiotic resumption, respectively.
Mammalian Oocyte Growth, Development, and Maturation
Gonadotropins (e.g. follicle-stimulating hormone [FSH] and luteinizing hormone [LH]) and sex steroids (e.g. estrogens and progesterone) regulate mammalian folliculogenesis in a stage-dependent manner. Early stages of follicle development – from primordial follicles until the preantral follicle stage – are gonadotropin-independent (Figure 3A) (Edson et al., 2009); instead, they are regulated by locally secreted ovarian factors, such as the KIT ligand and its receptor (Nilsson and Skinner, 2004), nerve growth factor (Dissen et al., 2001), and members of the transforming growth-factor β superfamily, such as anti-Müllerian hormone (Durlinger et al., 1999, 2002), growth differentiation factor 9 (GDF9) (Elvin et al., 1999), and bone morphogenetic protein 4 (BMP4) (Nilsson and Skinner, 2003), BMP7 (Lee et al., 2001), and BMP15 (Yan et al., 2001). Ovarian follicles respond to gonadotropins via their expression of functional FSH and LH receptors, which occurs from the preantral stages of follicular development until formation of a pre-ovulatory, Graafian follicle (Edson et al., 2009). Bidirectional signaling between oocytes and adjacent granulosa cells promote granulosa cell proliferation and differentiation by coordinating with FSH, which in turn drives granulosa cell survival, proliferation, and production of steroid hormones (Diaz et al., 2007). IGF1 has synergistic and complementary functions in the development of the granulosa cells (Edson et al., 2009).
Mammalian oocytes enter meiosis I during fetal development, and subsequently arrest at the diplotene stage of prophase-I until puberty (Sánchez and Smitz, 2012). High concentrations of intra-oocyte cyclic adenosine 3′,5′-monophosphate (cAMP) and activation of cAMP-dependent protein kinase A (PKA) are essential for the maintenance of this prophase-I arrest (Conti et al., 2002; Adhikari and Liu, 2014). Meiotic competence is acquired during later stage of folliculogenesis, and coincides with antrum formation (Figure 3A).
Oocyte maturation induces the release of the oocyte from primary arrest, and enables progression through meiotic metaphase. In mammals, meiotic maturation is triggered by the preovulatory surge in LH via activation of cytosolic Maturation promoting factor (MPF), a heterodimer of catalytic Cycline-dependent kinase 1 (CDK1) and its regulatory component Cyclin B (Nurse, 1990; Solc et al., 2010; Adhikari and Liu, 2014). Active MPF promotes progression from meiotic metaphase I (MI) to metaphase II (MII) through Histone H1 kinase activation, chromosome condensation, spindle formation, dissolution of nuclear envelope (germinal vesicle break down [GVBD]), and release of the first polar body.
Role of Insulin Signaling in Mammalian Oocyte Development
Insulin is routinely used as a supplement for the in vitro culture of preantral follicles (Louhio et al., 2000; Chaves et al., 2012); however, a role for insulin in regulating oocyte growth in vivo has yet to be documented. In vitro studies of cultured mammalian cells showed that insulin stimulation promotes oocyte growth by increasing either the number of gonadotropin receptors or the sensitivity and binding capacity of LH to its cognate receptor (Poretsky and Kalin, 1987). Insulin can also act synergistically with FSH to promote human ovarian thecal-interstitial cell differentiation and proliferation (Duleba et al., 1997), as well as with LH/human chorionic gonadotropin to regulate ovarian function, given that experimentally induced hyperinsulinemia causes increased human chorionic gonadotropin-induced ovarian growth (Poretsky et al., 1992). These data suggest that insulin indirectly regulates folliculogenesis in later stages of oocyte development.
Insulin also regulates ovarian androgen production in humans, suggesting it has an indirect role in early folliculogenesis since ovarian androgen is important for maintaining early follicular development. Indeed, the absence of ovarian androgen receptors in mice causes premature ovarian failure (Hu et al., 2004; Shiina et al., 2006; Gleicher et al., 2011). Thus, the possibility of insulin regulation of early oocyte growth via androgen-androgen receptor system cannot be ruled out.
In vitro culture-based experiments with early stages of human and non-human primate follicles suggest that insulin functions as a survival factor, as increased insulin levels reduce the quantity of atretic follicles, causing an overall increase in the number of viable follicles (Louhio et al., 2000; Xu et al., 2010). In goat preantral follicles, treatment with 10 ng/ml of insulin is associated with FSH-stimulated follicular development and survival (Chaves et al., 2012). Taken together, these data implicate insulin function in multiple aspects of mammalian oocyte growth.
Role of PI3K/AKT and TOR Signaling in Mammalian Oocyte Growth and Development
PI3K/AKT pathways play a major role in the initial recruitment of primordial follicles into the growth phase, although the physiological regulators are not well defined. For example, over-activation of PI3K signaling by oocyte-specific deletion of murine Pten (Phosphatase and tensin homolog deleted on chromosome 10) causes premature activation of primordial follicles, resulting in their rapid depletion in the adult and premature ovarian failure (Reddy et al., 2008). Interestingly, loss of phosphoinositide-dependent kinase-1, a kinase upstream of AKT, in murine oocytes also leads to premature ovarian failure, but in a reciprocal manner (Reddy et al., 2009): Deletion of Pdk1 causes premature ovarian failure as a result of accelerated, direct clearance of primordial follicles from their dormant state. These two types of premature ovarian failure, resulting from loss of Pten or Pdk1, represent distinct etiologies of the human counterpart.
One key downstream mediator of the PI3K/AKT pathway is the transcription factor Forkhead box “Other” 3 (FOXO3). FOXO3 is phosphorylated, and thus inhibited, by activation of the PI3K pathway (Liao and Hung, 2010). Active FOXO3 blocks primordial follicle activation, likely via inhibition of the expression of genes essential to oogenesis and folliculogenesis (John et al., 2007). Mice lacking Foxo3 undergo premature activation of primordial follicles and further depletion of mature follicles (Castrillon et al., 2003). During oocyte development, PI3K signaling seems to function in line with Foxo3 since simultaneous depletion of Pten and Foxo3 in oocytes does not have a synergistic effect on follicle activation (Reddy et al., 2008). A constitutively active form of Foxo3 in murine oocytes, however, produces distinct phenotypes depending on the time and stage of the oocyte. For example, Liu et al. found that active FOXO3, when expressed via Zp3::Cre, causes infertility by blocking oocyte growth, follicular development, and ovulation (Liu et al. 2007). Pelosi et al., however, found that active FOXO3, when expressed via the Kit promoter, maintained an ovarian reserve and increased reproductive capacity in mice (Pelosi et al., 2013). Presumably these differences were due to the expression of FOXO3 at different stages of oocyte development: the Zp3 promoter is active at the primary stage of folliculogenesis and remains active in growing oocytes whereas the Kit promoter is expressed only in primordial and primary follicles (Pelosi et al., 2013). Thus, PI3K-dependent FOXO3 activity has different context-dependent outcomes during oocyte growth and development.
Tuberous sclerosis complex (TSC) and Mammalian target of rapamycin complex 1 (mTORC1) also regulate oocyte growth (reviewed by Adhikari and Liu, 2010). In mice, activation of mTORC1 by loss of either TSC1 or TSC2 activates dormant primordial follicles (Adhikari and Liu, 2010), presumably through elevated activity of Ribosomal protein S6 (rpS6) kinase 1/rpS6 signaling, which promotes protein translation and ribosomal biogenesis in oocytes (Adhikari and Liu, 2010). These observations highlight the role of TSC/mTORC1 signaling in the regulation of female reproduction, although a role for mTOR signaling in mediating oocyte development adds layers of complexity. AKT, for example, regulates TSC/mTORC1 (Huang and Manning, 2009), and AKT-mediated phosphorylation of TSC2 disrupts the TSC1/TSC2 complex that, in turn, inhibits mTORC1, resulting in inhibition of oocyte growth (Huang and Manning, 2009). Inhibition of mTORC1 via deletion of Raptor, however, triggers compensatory activation of PI3K/AKT signaling that maintains normal ovarian follicular development and fertility (Gorre et al., 2014). Thus, the context of mTORC1 inhibition seems to be essential for mediating all the possible outcomes of oocyte growth. Interestingly, the network of AKT signaling gets more complex with the recent observation that mTORC2 activates AKT, and loss of mTORC2 function, via oocyte-specific ablation of Rictor (whose encoded protein activates mTORC2), results in premature ovarian failure, including massive follicular death, excessive loss of functional ovarian follicles, and abnormal gonadal hormone secretion (Chen et al., 2015). These observations suggest that insulin signaling intersects with target of rapamycin (TOR) and PI3K/AKT signaling, and together these pathways play a crucial role in the regulation of oocyte survival, growth, and timely follicular activation in reproductive females. Given the central importance of insulin and TOR signaling to physiology and metabolism, future studies that delineate the exact molecular mechanisms linking the upstream signals to oocyte and ovarian developmental steps are needed.
Insulin Functions as a Rheostat in Mammalian Oocyte to Regulate Oocyte Maturation
Insulin signaling – in addition to contributions by LH, local paracrine factors, IGF, and EGF – regulates meiotic competence and final oocyte maturation in mammals (Eppig et al., 1998, 2000; Acevedo et al., 2007). Preovulatory follicles contain increased insulin abundance compared to the subordinate follicles in vivo, suggesting that insulin affects oocyte maturation (Landau et al., 2000). In vitro, however, insulin negatively impacts embryo development following fertilization, despite morphologically normal MII divisions of the murine oocyte/granulosa cell complexes: Culturing mouse ovarian follicle with high levels (5 μg/ml) of insulin compromises blastocyst development and, often, prevents establishment of normal pregnancy (Eppig et al., 1998). Furthermore, exposure to high levels of insulin during oocyte growth in vivo has detrimental effects on meiotic chromatin remodeling and induces chromosome condensation errors in mice (Acevedo et al., 2007). Thus, high concentrations of insulin clearly impair oocyte growth and induce cellular toxicity. Accordingly, lower concentration of insulin of 10 ng/ml, together with FSH, can initiate meiotic resumption, maintain survival, and stimulate follicular development in goat preantral follicles (Chaves et al., 2012). Thus, while low amounts of insulin promote normal oocyte growth and development, extremely high levels of insulin result in aberrant oocyte development and thus birth defects.
Yet, very low levels of insulin are also harmful, based on fertility phenotypes associated with diabetes. For example, women with insulin-dependent diabetes mellitus suffer from ovarian hypofunction (Poretsky and Kalin, 1987) – a phenotype that is mirrored in mammalian models. Experimentally induced diabetes (type 1) in mice is associated with either a reduction in ovulation rate (Powers et al., 1996) or cessation of ovulatory cycles, which is restored upon insulin treatment (Rogers et al., 1990). Preovulatory oocytes in diabetic mice are markedly smaller and exhibit lower rates of meiotic maturation (Chang et al., 2005). Conversely, exposure of ovarian follicles from diabetic mice to insulin at supraphysiological concentrations stimulates cleavage and maturation of oocytes in vitro (Gong, 2002). Prolonged hyperinsulinemia, however, eventually negatively impacts the developmental competence of oocytes in vivo (Dumesic et al., 2002). Insulin-resistant and hyperinsulinemic mice, for example, display altered estrous cycling and duration independent of hyperglycemia, hyperandrogenism, and increased adipose tissue mass (Nandi et al., 2010). Taken together, these pathophysiological conditions suggest that insulin signaling functions as a tightly controlled rheostat that regulates meiotic competence and oocyte maturation, where too much or too little insulin is harmful to normal oocyte development.
Oocyte Growth, Development and Maturation in Lower Vertebrates
In all oviparous vertebrates, including Xenopus and zebrafish, oogonial proliferation and oocyte recruitment occur throughout the reproductive cycle, and ovarian follicles undergo the same basic pattern of growth followed by final oocyte maturation and ovulation (Wallace, 1985; Tyler and Sumpter, 1996; Kagawa, 2013). Following the primary stage, the secondary growth phase is characterized by oocyte growth due to the synthesis and incorporation of yolk material into the growing oocyte (Figure 3b) (Wallace, 1985). The major event responsible for this extensive growth is known as vitellogenesis, which is characterized by hepatic production of the yolk precursor protein vitellogenin under the direct regulation of 17β-estradiol. Newly synthesized vitellogenin is transported via the blood to the ovary, where it undergoes receptor-mediated endocytosis, resulting in its uptake by growing oocytes (Wallace, 1985; Tyler and Sumpter, 1996).
As in mammals, high cAMP/PKA levels maintain prophase-I arrest in lower vertebrates (Das et al., 2017). In teleosts, prior to ovulation, a surge in pituitary LH triggers follicle cells to synthesize and secrete maturation-inducing steroid – 17α,20β-dihdroxy-4-pregnen-3-one in the majority of fishes, but 17α,20β,21-trihydroxy-4-pregnen-3-one in sciaenids and in marine perciform species (Nagahama and Yamashita, 2008). Binding of maturation-inducing steroid to its membrane progestin receptor α at the oocyte surface activates a pertussis toxin-sensitive inhibitory G protein (Gαi) and subsequently down-regulates activity of adenylyl cyclase, the enzyme responsible for production of cAMP from ATP (Zhu et al., 2003). In addition, the maturation-inducing steroid triggers rapid changes to intracellular phosphorylation–dephosphorylation signaling cascades that ultimately result in the activation of MPF and final oocyte maturation (Nagahama and Yamashita, 2008; Das et al., 2017).
Role of Insulin Signaling in Oocyte Maturation in Lower Vertebrates
The meiotogenic potential of insulin on oocyte maturation was first described in Xenopus over 35 years ago (El-Etr et al., 1979; Hirai et al., 1983). These studies demonstrated that insulin acts directly on the oocyte surface to regulate final oocyte maturation, suggesting that insulin function is independent of progesterone action on meiotic maturation. Xenopus oocytes express both insulin receptor and IGF1 receptors (IGF1Rs), although IGF1Rs are ~15 times more abundant (Hainaut et al., 1991), suggesting the possibility that insulin-induced Xenopus oocyte maturation is mediated, at least partially, by IGF1R (Zhu et al., 1998).
Insulin also induces GVBD in denuded (devoid of surrounding follicular cells) zebrafish oocytes, suggesting that insulin can mediate oocyte maturation via a pathway independent of steroids in teleosts as well (Das et al., 2013). Both insr and igf1r are also expressed by zebrafish and carp oocytes, and expression of insr is lower than that of igf1r (reviewed by Das et al., 2017). Zebrafish mid-vitellogenic follicle-enclosed oocytes (Figure 3b) respond to insulin, but not human chorionic gonadotropin, suggesting that oocytes are only responsive to insulin at an early stage of development (Das et al., 2016a). Insulin likely triggers meiotic resumption by up-regulating membrane abundance of Progesterone receptor α and 20β-hydroxy-steroid dehydrogenase, which is responsible for maturation-inducing steroid synthesis (Das et al., 2016a). In vitro insulin stimulation can also trigger igf3 expression in zebrafish follicle-enclosed oocytes (Das et al., 2016a), suggesting an important role for insulin in modulating the activity of local paracrine factors involved in oocyte maturation (Das et al., 2017).
Insulin-Induced PI3K/AKT in the Regulation of Oocyte Maturation in Lower Vertebrates
The role of the insulin-induced PI3K/AKT signaling pathway in the regulation of oocyte maturation is best characterized in lower vertebrates, owing to the ease of in vitro culture systems, preparation of denuded oocytes divested of follicular cells, and widespread use of cell-permeable pharmacological agents targeting selective intra-oocyte kinases and phosphatases (Chuang et al., 1993; Andersen et al., 1998; Mood et al., 2004; Das et al., 2016b). In Xenopus, activation of PI3K signaling regulates insulin-dependent oocyte maturation (Chuang et al., 1993). For example, constitutively active Akt induces GVBD in Xenopus oocytes to the same extent as that resulting from insulin treatment (Andersen et al., 1998), whereas a dominant-negative Akt mutant blocks insulin-stimulated meiotic resumption (Andersen et al., 2003). While PI3K may regulate insulin-dependent signaling, it does not influence progesterone-mediated meiotic resumption. Specifically, PI3K inhibition either delays (Bagowski et al., 2001) or has no effect on progesterone-stimulated meiotic maturation in Xenopus (Andersen et al., 2003; Mood et al., 2004), suggesting that PI3K-mediated signaling is an auxiliary pathway for progesterone action in this species (Andersen et al., 2003). Downstream of the PKB/AKT pathway, oocyte-specific Phosphodiesterase 3 (Pde3), the enzyme that cleaves cAMP to AMP, mediates insulin/IGF1-induced meiotic resumption (Andersen et al., 1998; Conti et al., 2002).
Insulin also promotes PI3K/AKT signaling pathway in zebrafish oocytes (Das et al., 2013). PI3K is activated within 30 to 60 minutes after insulin stimulation, followed by Akt activation (phosphorylation) within 60 and up to 120 minutes after initial stimulation; oocyte maturation occurs at ~6 to 10 hours. The length of time between insulin stimulation and oocyte maturation emphasizes the possible involvement of intracellular factors downstream of Akt (Das et al., 2013). Inhibition of Pde3 blocks insulin-induced Cdc2 activation and GVBD, but not PI3K/AKT activation, suggesting that Pde3 is a key downstream target of the PI3K/AKT signaling pathway that regulates insulin-dependent meiotic maturation in zebrafish oocytes (Das et al., 2013). Although involvement of PI3K in fish oocyte maturation was documented earlier (Weber and Sullivan, 2001), these results were the first to define the downstream pathways that are modulated by insulin and how they fit the paradigm compared to amphibian and mammalian systems (Das et al., 2013).
Insulin-Induced ERK1/2 in the Regulation of Oocyte Maturation in Lower Vertebrates
In contrast to PI3K/AKT signaling, the kinetics of intra-oocyte ERK activation and their role in oocyte maturation are highly species-specific in lower vertebrates (Kajiura-Kobayashi et al., 2000; Liang et al., 2007; Maitra et al., 2014). In Xenopus, Mos, as well as its downstream targets (Mek, Erk2, and rpS6 kinase), induce meiotic maturation, even in the absence of progesterone, when microinjected into prophase-arrested oocytes (Dupré et al., 2010). While inhibition of Mek activity blocks Mos-mediated meiotic maturation, it fails to block progesterone-induced oocyte maturation in amphibians (Gross et al., 2000). These observations suggest that (i) Mos functions through Erk in oocytes and (ii) apart from Mos/Mek/Erk, an alternative and redundant pathway remains active to resume meiosis. Interestingly, progesterone-induced MPF activation is completely abolished when synthesis of Cyclin B, the regulatory subunit of MPF, and the Mos/Erk pathways are simultaneously impaired (Haccard and Jessus, 2006). And activation of either pathway restores MPF activation, so the two pathways function redundantly during Xenopus oocyte maturation (Haccard and Jessus, 2006).
The Erk pathway also plays an important role in the establishment and maintenance of the spindle assembly checkpoint after microtubule depolymerization to release the cytostatic arrest of MII oocytes in Xenopus (Takenaka et al., 1997; Dupré et al., 2010). Cytostatic factor activity is mediated via the Mos/Mek/Erk2/rpS6 kinase pathway. The Mos/Mek/Erk pathway members are each synthesized and/or activated upon oocyte maturation, and each kinase in the pathway depends only on downstream molecules in the pathway for cytostatic factor-mediated establishment of cell-cycle arrest at MII (Tunquist and Maller, 2003; Dupré et al., 2010).
In Xenopus oocytes, insulin regulates mos mRNA translation via PI3K/AKT-mediated inhibition of glycogen synthase kinase 3β, which facilitates Aurora A-catalyzed Cytoplasmic polyadenylation element binding protein (Cpeb) phosphorylation, cytoplasmic polyadenylation, translation, and oocyte maturation (Sarkissian et al., 2004). Insulin-stimulated Xenopus stage-IV vitellogenic oocytes fail to stimulate Mos synthesis and are unable to activate MPF to undergo maturation (Chesnel et al., 1997). Inhibition of Mos-induced Erk activation in insulin-treated oocytes delays meiotic resumption (Baert et al., 2003), further underscoring the importance of Mos synthesis and the Mos/Erk pathway during insulin-induced oocyte maturation in Xenopus.
A role for the Xenopus GTP-binding protein p21 Ras and serine/threonine kinase Raf-1 has also been demonstrated downstream to insulin signaling and in Erk activation and oocyte maturation (Deshpande and Kung, 1987; Korn et al., 1987; Fabian et al., 1993). Expression of a kinase-defective human RAF-1 protein (KD-RAF) inhibited receptor tyrosine kinase pathway-induced GVBD, suggesting that Xenopus oocyte maturation induced by the receptor tyrosine kinase pathway requires RAF-1 activation (Fabian et al., 1993); similarly, endogenous Mek inhibition significantly delayed insulin-induced oocyte maturation (Baert et al., 2003).
Unlike Xenopus, activation of the Mos/Erk pathway is neither required for steroid–induced oocyte maturation nor sufficient to trigger meiotic resumption in goldfish oocytes (Kajiura-Kobayashi et al., 2000) while pharmacological inhibition of Mek fails to block steroid-induced oocyte maturation in the Atlantic croaker (Pace and Thomas, 2005). Yet, as in mammals and Xenopus, the role of the Mos/Erk pathway as a cytostatic factor that suppresses DNA replication between MI and MII is well-documented in teleosts (Kajiura-Kobayashi et al., 2000; Pace and Thomas, 2005; Liang et al., 2007). In this context, current studies on the role of ERK signaling in insulin-induced oocyte maturation are limited to fishes. Erk1/2 is activated within 30 to 90 minutes of insulin/IGF1 stimulation in the zebrafish and common carp. This time scale is rapid, and precedes MPF activation and GVBD (Paul et al., 2009; Maitra et al., 2014). Interestingly, while Mek inhibition completely blocks insulin/Igf1-induced oocyte maturation in carp (Paul et al., 2009), it only delays insulin-stimulated GVBD in zebrafish (Maitra et al., 2014), suggesting that species-specific differences exist for Erk activation during teleost oocyte maturation. Inhibition of Raf-1 or Mek activity, but not protein synthesis, blocks insulin-induced Erk activation in zebrafish oocytes, suggesting that the membrane-initiated Ras/Raf/Mek pathway, rather than Mos synthesis, regulates Erk1/2 activation in this species (Maitra et al., 2014). Thus, Erk activation is an important component of the insulin-induced signal transduction cascade, leading to activation of MPF and induction of GVBD.
Oocyte Growth, Development, and Maturation in Invertebrates
Drosophila oogenesis (Figure 3c) starts at the anterior end of the ovariole in a microenvironment, the germarium, where both germ line and somatic cells reside and the egg chamber is assembled (Spradling, 1993). Germ-line stem cells self-renew via asymmetric divisions, and give rise to cystoblasts (Shim et al., 2013; Xie, 2013; Ables, 2015). Following four incomplete mitotic divisions, a single germ-line stem cell gives rise to cystoblasts containing 16 individual cells connected by cytoplasmic bridges; one of these cells becomes the egg cell, whereas the others become nurse cells (Spradling, 1993). As the follicle pinches off from the germarium following the formation of the epithelial follicular monolayer, oocytes progress through 14 distinct stages of maturation that are morphologically arranged in a linear fashion according to developmental maturity (Spradling, 1993). Although meiosis begins in region 2A in the germarium, oocytes remain in prophase-I until undergoing meiotic maturation at stage 13 of oogenesis (Spradling, 1993; Xiang et al., 2007; Von Stetina et al., 2008). Two lipophilic hormones – ecdysteroids, which are principally synthesized by the ovary, and juvenile hormone from the corpora allata – stimulate vitellogenin production by the fat body (Brown et al., 2009; Badisco et al., 2013). Unlike in vertebrates, where LH and/or maturation-inducing steroid initiate meiotic resumption, an initiation signal for maturation has yet to be identified in Drosophila; nevertheless, active MPF (Cdk1-cyclin B complex) functions during oocyte maturation (Von Stetina et al., 2008). Additionally, an α-endosulfine homolog, endos, encoding a conserved phosphoprotein, regulates all aspects of Drosophila meiotic maturation, including the timing of GVBD (Von Stetina et al., 2008).
C. elegans exists as either a self-fertilizing XX hermaphrodite, that produces both sperm and oocytes, or a cross-fertilizing XO male. Germ cells, in an adult hermaphroditic animal, at the distal end of the gonad tube, are proliferative, whereas germ cells located more proximally (Figure 3D), near the uterus in the hermaphrodite, are meiotic, first producing sperm at the late L4 stage and then exclusively oocytes in adulthood (reviewed by Hubbard and Greenstein, 2000; Hubbard, 2007; Kimble and Seidel, 2013). Unlike vertebrates and Drosophila, oocyte maturation in C. elegans is controlled by the major sperm protein (MSP), which is secreted by the spermatheca via a vesicle-budding process (Miller et al., 2001; Kosinski et al., 2005). Hermaphrodite adult worms produce a limited number of sperm that secrete MSP, a cytoskeletal actin-like protein that facilitates the amoeboid movement of sperm (Miller et al., 2001). MSP binds to the ephrin receptor protein-tyrosine kinase VAB-1 on oocytes (Miller et al., 2003) and unidentified receptors on the sheath cells (Govindan et al., 2009) to stimulate oocyte meiotic maturation (Kim et al., 2013).
Insulin Regulation of Oocyte Growth in Invertebrates
In Drosophila, insulin signaling regulates germ-line stem cell proliferation (Drummond-Barbosa and Spradling, 2001; Shim et al., 2013) and triggers vitellogenesis from the fat body in response to positive nutritional status (feeding) (Richards et al., 2005; Badisco et al., 2013). Genetic ablation of DILP-expressing neuroendocrine cells in female Drosophila results in reduced fecundity, suggesting that insulin signaling regulates Drosophila reproductive physiology (LaFever and Drummond-Barbosa, 2005; Wu and Brown, 2006).
Nutrient-sensitive TOR and ILP signaling pathways regulate vitellogenesis in flies (reviewed by Hansen et al., 2014). While the functions of TOR seem specific to germ-line stem cells, independent of insulin-like signaling (La Fever et al., 2010), insulin-mediated signaling largely regulates vitellogenesis and oocyte development. In vitro fat body culture experiments using female mosquitoes demonstrated that expression of the vitellogenin gene is induced by a combination of an ecdysteroid and the ILP signaling pathway (Roy et al., 2007). Moreover, ovarian growth is arrested at the previtellogenic stage in flies with mutant Drosophila IR expression (Tatar et al., 2001). Also, flies with mutant Chico expression display reduced proliferation of follicular stem cells, and their egg chambers fail to progress to the vitellogenic stage, even in the presence of abundant nutrients (Bohni et al., 1999; Drummond-Barbosa and Spradling, 2001), suggesting a direct role for insulin in Drosophila vitellogenesis. Interestingly, female sterility due to Chico mutations appears autonomous to the ovary, as transplantation of wild-type, previtellogenic ovaries into Chico-homozygous female flies results in the production of vitellogenic oocytes whereas transplantation of ovaries from Chico- homozygous mutant flies into Chico- homozygous female flies does not restore vitellogenin production (Richard et al., 2005). Thus, disturbed insulin signaling in the ovaries causes a failure of yolk protein uptake.
Reproductive development in C. elegans is also critically regulated by nutrition, and the insulin signaling pathway is highly important to oocyte development (reviewed by Hubbard, 2011). Oocyte growth in C. elegans is directly regulated by vitellogenesis; unlike in Drosophila or zebrafish, where expression of vitellogenin occurs in the fat body and liver, respectively, the yolk proteins are synthesized in the intestine of C. elegans in a hermaphrodite-specific manner (Kimble and Sharrok, 1983; Sharrok, 1983). Indeed, mutations in the insulin-signaling pathway affect vitellogenesis: Reduction of DAF-2/IR signaling results in lower levels of vit-2 and vit-5 gene transcripts as well as yolk proteins (Murphy et al., 2003; DePina et al., 2011), suggesting a direct role for insulin signaling in promoting vitellogenin synthesis and oocyte growth in C. elegans.
Insulin Regulation of Oocyte Maturation in Drosophila
Drosophila germ-line stem cell proliferation, oocyte growth, and development (vitellogenesis) are directly regulated by insulin (described above). Yet, follicles of insulin-signaling-pathway mutants do not reach the oocyte maturation stage (Stage 13) (Drummond-Barbosa and Spradling, 2001; Tatar et al., 2001; LaFever and Drummond-Barbosa, 2005), so determining a role for insulin in meiotic maturation in flies cannot be easily achieved. Despite this conundrum, Seiber and colleagues identified a key role for insulin signaling in the regulation of mitochondrial activity and oocyte maturation (Sieber et al., 2016). They found that reduced insulin signaling lead to premature glycogen accumulation in arrested follicles at Stages 6 and 7, and complete inactivation of insulin signaling triggered mitochondrial respiratory quiescence in late-stage follicles. Quiescence of mitochondrial activity is required for oocyte maturation (Sieber et al., 2016), and inactivation of insulin signaling resulted in premature oocyte maturation. Interestingly, electron transport chain remodeling and glycogen uptake occur in maturing Xenopus oocytes, suggesting that these processes are evolutionarily conserved aspects of oocyte development (Sieber et al., 2016).
Insulin Regulation of Meiotic Progression in C. elegans
Insulin signaling regulates meiotic I progression (pachytene to diplotene) in C. elegans worms (Lopez et al., 2013). DAF-2/insulin-like receptor couples external nutritional conditions with oocyte maturation by activating MPK-1/ERK in pachytene-stage oocytes (Lopez et al., 2013). In presence of food, DAF-2 promotes meiotic progression and produces fertilizable oocytes; in the absence of food, however, DAF-2 remains inactivated and meiotic progression is stalled, halting oocyte production (Lopez et al., 2013). This study by Lopez and colleagues was the first to provide evidence that insulin signaling directly regulates meiotic progression in worms, and suggests the evolutionary conservation of nutritional sensing via the insulin signaling pathway during meiotic progression.
The MPK-1/ERK pathway functions in multiple processes in the C. elegans germ line. Specifically, MPK-1 regulates progression through pachytene, negative regulation of physiological apoptosis in the germ line, control of oocyte growth, and meiotic maturation (Lee et al. 2007; Arur et al. 2009). Of note, MPK-1 is activated in a tight spatial and temporal manner by two distinct receptor-mediated pathways in MI oocytes during C. elegans oogenesis. Proximal oocytes exhibit MPK-1 activation in the presence of sperm (Miller et al. 2001; Lee et al. 2007), and this activation results in the successful transition of diplotene-arrested oocytes to MI, which are fertilized and complete MII. For oocytes in the pachytene phase of MI, however, active MPK-1 is necessary and sufficient for progression of pachytene to diplotene (Lopez et al., 2013). Thus, events of early meiotic progression are regulated by the DAF-2/insulin-like receptor, followed by linear activation of RAS/MEK-2/ERK (Lopez et al., 2013). Further, DAF-18/PTEN attenuates DAF-2–mediated activation of the RAS/MPK-1 pathway (Lopez et al., 2013), indicating the possible interplay between DAF-18 and RAS/ERK signaling in an AGE-1 (AKT-1 homolog)-independent manner. Taken together, these results demonstrate that activation of the RAS/MEK-2/ERK signaling pathway, via the DAF-2 insulin-like receptor, regulates meiotic progression of C. elegans oocytes. Whether DAF-2 signaling is necessary and/or remains active during MSP-induced oocyte maturation must be determined.
POLYCYSTIC OVARY SYNDROME: INSULIN SIGNALING IN A PATHOPHYSIOLOGICAL CONTEXT
Polycystic ovary syndrome (PCOS) is characterized by hyperandrogenism, ovulatory dysfunction, and polycystic ovarian morphology, and affects 5–20% of women of reproductive age worldwide (reviewed by Azziz et al., 2016). Although the etiologies of PCOS vary and different factors are considerably intertwined, correlation of a high insulin level with excess androgen production is a key feature of PCOS (Azziz et al., 2016). This association led to the hypothesis that excess circulatory insulin can stimulate ovarian steroidogenesis in vivo (reviewed by Diamanti-Kandarakis et al., 2008; Diamanti-Kandarakis and Dunaif, 2012). Interestingly, acute insulin infusion (for a few hours) does not result in an increase in ovarian androgen production, suggesting the absence of a direct stimulatory effect of insulin on ovarian androgen production (Stuart et al., 1987; Stuart and Nagamani, 1990). Continuous stimulation of the ovary by insulin over a prolonged period, however, produced changes in ovarian morphology, including hyperthecosis and cyst formation – changes that are commonly observed in women with insulin resistance (Poretsky et al., 1992). Furthermore, exogenous androgens induce cyst formation in the ovary and negatively affect oocyte quality (Manneras et al., 2007; Jimenez et al., 2013). Although the exact mechanisms by which maternal dehydroepiandrosterone exposure alters oocyte competence are not completely understood, abnormal lipid metabolism appears to play an important role (Jimenez et al., 2013). Thus, excess ovarian androgen production by thecal cells and intrinsic insulin resistance, with associated hyperinsulinemia, remain central to PCOS pathophysiology in both lean and obese women (Nestler, 1997; Diamanti-Kandarakis and Dunaif, 2012; Azziz et al., 2016).
Whether insulin or androgen actions are the primary causes of ovarian abnormalities is a subject of major debate – despite increases in gonadotropin-releasing hormone frequency; alterations in the LH-to-FSH ratio; disturbances in coordination and interaction of LH, FSH, IGF1, and anti-Müllerian hormone; and adipose tissue dysfunction (Azziz et al., 2016). Normal insulin levels, in concert with gonadotropin(s), likely stimulate baseline ovarian androgen production, which in turn participates in the regulation of follicle development (Jamnongjit and Hammes, 2006). In contrast, excess insulin promotes increased ovarian androgen production while enhanced LH receptor expression in mural granulosa cells, along with ovarian LH-binding capacity, further increases the abundance of androgen; eventually, this overproduction of androgens could trigger their increased bioavailability by down-regulating hepatic production of steroid hormone-binding globulin (Poretsky and Kalin, 1987; Poretsky et al., 1999; Jamnongjit and Hammes, 2006). A high circulating androgen level may ultimately lead to unregulated follicle growth, with an absence of dominant follicle formation and subsequent ovulation (Jamnongjit and Hammes, 2006).
High-fat-diet-induced infertility and hyperandrogenism was recently corrected by deletion of the IR in ovarian thecal interstitial cells (Wu et al., 2014). Thecal cell-specific Ir-knockout in mice with diet-induced obesity exhibited improved fertility and testosterone levels comparable with those in lean mice (Wu et al., 2014), revealing one causal pathway linking hyperinsulinism with ovarian hyperandrogenism and infertility associated with obesity (Wu et al., 2014). Interestingly, although the molecular mechanisms that drive insulin resistance in patients with PCOS differ from those in other common insulin-resistant states, such as obesity and type 2 diabetes mellitus (Azziz et al., 2016), why the metabolic activity of insulin is lost whereas steroidogenic potential remains at its peak in those with PCOS remains to be determined (Diamanti-Kandarakis and Dunaif, 2012). Might an alternative signaling pathway exist that mediates insulin activity during steroidogenesis in the ovary, but is not involved in metabolic activity (Diamanti-Kandarakis and Dunaif, 2012)?
CONCLUSION
Reproduction changed many times over the course of evolution in response to the needs of species, so species-specific variation to the manner in which reproduction is regulated by extrinsic and intrinsic factors should be expected. Interestingly, the pervasive influence of insulin signaling highlights a connection between nutrition and reproduction in all metazoan species. We describe herein the links between the insulin signaling system and regulation of female gametogenesis.
A commonality of species across the animal kingdom is the presence of the IR on the ovary cell surface and critical signaling nodes (e.g., PI3K, AKT, FOXO, ERK) that regulate several aspects of oogenesis, including germ cell proliferation and maintenance, primordial follicle formation, steroidogenesis, and oocyte maturation. In worms and flies, direct regulatory action of insulin signaling is well evident, whereas in mammals, many of the signaling molecules that are common to the insulin system may be influenced by insulin or other stimulants, so direct action by insulin is not required physiologically. Hence, under the central contribution of insulin signaling under pathophysiological conditions, like PCOS, is not surprising.
Taking into account all of the data presented in this review, we propose that insulin is a key, evolutionarily ancient regulator of female reproduction. As the tight regulatory system (hypothalamic-pituitary-gonadal axis) for reproduction evolved in mammals, the pleiotropic actions of insulin became restricted to metabolism – although the rudimentary segment of the insulin system can still be activated when a metabolic imbalance is present within the reproductive system, which can change blood insulin levels and/or its signaling pathways. Exercise and insulin-sensitizing drugs are presently the only options to reverse reproductive dysfunctions associated with metabolic disorders, such as insulin resistance, diabetes, and obesity. More detailed understanding of insulin’s action in regulating oogenesis from several metazoan species could be instrumental in not only understanding the pathophysiology of these disease states, but in opening the door to important medical applications.
Acknowledgments
Grant sponsor: This study was supported by National Institutes of Health grant GM98200, the Cancer Prevention Research Institute of Texas RP160033, and the American Cancer Society RSG-014-044-DDC.
Abbreviations
- cAMP
cyclic adenosine 3′,5′-monophosphate
- CDK1
Cyclin-dependent kinase 1 (also known as CDC2)
- ERK
Extracellular signal-regulated kinase
- FOXO
Forkhead box “other”
- FSH
follicle-stimulating hormone
- GVBD
germinal vesicle breakdown
- IGF[1R]
Insulin-like growth factor [1 receptor]
- ILP
Insulin-like peptide
- LH
Luteinizing hormone
- IR
Insulin receptor
- MI/II
meiotic division I/II
- MSP
Major sperm protein
- MPF
maturation-promoting factor
- PCOS
polycystic ovary syndrome
- PI3K
Phosphatidylinositol-3-kinase
- PKA
Protein kinase A
- PTEN
Phosphatase and tensin homolog deleted on chromosome 10
- rpS6
Ribosomal protein S6
- [m]TOR
[mammalian] Target of rapamycin
Footnotes
“[We] propose that insulin is a key, evolutionarily ancient regulator of female reproduction.”
References
- Acevedo N, Ding J, Smith GD. Insulin signaling in mouse oocytes. Biol Reprod. 2007;77:872–879. doi: 10.1095/biolreprod.107.060152. [DOI] [PubMed] [Google Scholar]
- Ables ET, Bois KE, Garcia CA, Drummond-Barbosa D. Ecdysone response gene E78 controls ovarian germline stem cell niche formation and follicle survival in Drosophila. Dev Biol. 400(1):33–42. doi: 10.1016/j.ydbio.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari D, Liu K. mTOR signaling in the control of activation of primordial follicles. Cell Cycle. 2010;9:1673–1674. doi: 10.4161/cc.9.9.11626. [DOI] [PubMed] [Google Scholar]
- Adhikari D, Liu K. The regulation of maturation promoting factor during prophase I arrest and meiotic entry in mammalian oocytes. Mol Cell Endocrinol. 2014;382:480–487. doi: 10.1016/j.mce.2013.07.027. [DOI] [PubMed] [Google Scholar]
- Andersen CB, Roth RA, Conti M. Protein kinase B/Akt induces resumption of meiosis in Xenopus oocytes. J Biol Chem. 1998;273:18705–18708. doi: 10.1074/jbc.273.30.18705. [DOI] [PubMed] [Google Scholar]
- Andersen CB, Sakaue H, Nedachi T, Kovasina KS, Clayberger C, Conti M, Roth RA. Protein kinase B/Akt is essential for the insulin but not progesterone stimulated resumption of meiosis in Xenopus oocyte. Biochem J. 2003;369:227–238. doi: 10.1042/BJ20021243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arur S, Ohmachi M, Nayak S, Hayes M, Miranda A, Hay A, Golden A, Schedl T. Multiple ERK substrates execute single biological processes in Caenorhabditis elegans germ-line development. Proc Natl Acad Sci USA. 2009;106:4776–4781. doi: 10.1073/pnas.0812285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Chen ZJ, Dunaif A, Laven JSE, Legro RS, Lizneva D, Natterson-Horowtiz B, Teede HJ, Yildiz BO. Polycytic ovary syndrome. Nat Rev Disease Primer. 2016;2:16057. doi: 10.1038/nrdp.2016.57. [DOI] [PubMed] [Google Scholar]
- Bachvarova RF. A maternal tail of poly(a): The long and the short of it. Cell. 1992;69:895–897. doi: 10.1016/0092-8674(92)90606-d. [DOI] [PubMed] [Google Scholar]
- Badisco L, Wielendaele PV, Vanden Broeck J. Eat to reproduce: a key role for the insulin signaling pathway in adult insects. Front Physiol. 2013;4:202. doi: 10.3389/fphys.2013.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baert F, Bodart JF, Bocquet-Muchembled B, Lescuyer-Rousseau A, Vilain JP. Xp42(Mpk1) activation is not required for germinal vesicle breakdown but for Raf complete phosphorylation in insulin-stimulated Xenopus oocytes. J Biol Chem. 2003;278:49714–49720. doi: 10.1074/jbc.M308067200. [DOI] [PubMed] [Google Scholar]
- Bagowski CP, Myers JW, Ferrell JE., Jr The Classical Progesterone Receptor Associates with p42 MAPK and Is Involved in Phosphatidylinositol 3-Kinase signaling in Xenopus Oocytes. J Biol Chem. 2001;276:37708–37714. doi: 10.1074/jbc.M104582200. [DOI] [PubMed] [Google Scholar]
- Belfiore A, Frasca F, Pandini G, Sciacca L, Vigneri R. Insulin receptor isoforms and insulin receptor/insulin-like growth factor receptor hybrids in physiology and disease. Endocr Rev. 2009;30:586–623. doi: 10.1210/er.2008-0047. [DOI] [PubMed] [Google Scholar]
- Biemar F, Argenton F, Schmidtke R, Epperlein S, Peers B, Driever W. Pancreas development in zebrafish: early dispersed appearance of endocrine hormone expressing cells and their convergence to form the definitive islet. Dev Biol. 2001;230:189–203. doi: 10.1006/dbio.2000.0103. [DOI] [PubMed] [Google Scholar]
- Brogiolo W, Stocker H, Ikeya T, Rintelen F, Fernandez R, Hafen E. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- Brown MR, Clark KD, Gulia M, Zhao Z, Garczynski SF, Crim JW, Suderman RJ, Strand MR. An insulin-like peptide regulates egg maturation and metabolism in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2008;105:5716–5721. doi: 10.1073/pnas.0800478105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Sieglaff DH, Rees HH. Gonadal ecdysteroidogenesis in arthropoda: Occurrence and regulation. Annu Rev Entomol. 2009;54:105–125. doi: 10.1146/annurev.ento.53.103106.093334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley LC. The Phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–218. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- Chang AS, Dale AN, Moley KH. Maternal diabetes adversely affects preovulatory oocyte maturation, development, and granulosa cell apoptosis. Endocrinology. 2005;146:2445–2453. doi: 10.1210/en.2004-1472. [DOI] [PubMed] [Google Scholar]
- Chaves RN, Duarte AB, Rodrigues GQ, Celestino JJ, Silva GM, Lopes CA, Almeida AP, Donato MA, Peixoto CA, Moura AA, Lobo CH, Locatelli Y, Mermillod P, Campello CC, Chen Z, Kang X, Wang L, Dong H, Wang C, Xiong Z, Zhao W, Jia C, Lin J, Zhang W, Yuan W, Zhong M, Du H, Bai X. Rictor/mTORC2 pathway in oocytes regulates folliculogenesis, and its inactivation causes premature ovarian failure. J Biol Chem. 2015;290:6387–6396. doi: 10.1074/jbc.M114.605261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnel F, Bonnec G, Tardivel A, Boujard D. Comparative effects of insulin on the activation of the Raf/Mos-dependent MAP Kinase cascade in vitellogenic versus postvitellogenic Xenopus oocytes. Dev Biol. 1997;188:122–133. doi: 10.1006/dbio.1997.8631. [DOI] [PubMed] [Google Scholar]
- Chuang L-M, Hausdorff SF, Myers MG, Jr, White MF, Birnbaum MJ, Kahn CR. Interactive roles of Ras, insulin receptor substrate-1, and proteins with Src homology-2 domains in insulin signaling in Xenopus oocytes. J Biol Chem. 1994;269:27645–27649. [PubMed] [Google Scholar]
- Chuang L-M, Myers MG, Jr, Backer JM, Shoelson SE, White MF, Birnbaum MJ, Kahin CR. Insulin-stimulated oocyte maturation requires insulin receptor substrate 1 and interaction with the SH2 domains of phosphatidylinositol 3-Kinase. Mol Cell Biol. 1993;13:6653–6660. doi: 10.1128/mcb.13.11.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claeys I, Simonet G, Poels J, Van Loy T, Vercammen L, De Loof A, Vanden Broeck J. Insulin-related peptides and their conserved signal transduction pathway. Peptides. 2002;23:807–816. doi: 10.1016/s0196-9781(01)00666-0. [DOI] [PubMed] [Google Scholar]
- Colombani J, Andersen DS, Leopold P. Secreted peptide Dilp8 coordinates Drosophila tissue growth with developmental timing. Science. 2012;336:582–585. doi: 10.1126/science.1216689. [DOI] [PubMed] [Google Scholar]
- Colton SA, Pieper GM, Downs SM. Altered meiotic regulation in oocytes from diabetic mice. Biol Reprod. 2002;67:220–231. doi: 10.1095/biolreprod67.1.220. [DOI] [PubMed] [Google Scholar]
- Conti M, Andersen CB, Richard F, Mehats C, Chun S-Y, Horner K, Jin C, Tsafriri A. Role of cyclic nucleotide signaling in oocyte maturation. Mol Cell Endocrinol. 2002;187:153–159. doi: 10.1016/s0303-7207(01)00686-4. [DOI] [PubMed] [Google Scholar]
- Cornils A, Gloeck M, Chen Z, Zhang Y, Alcedo J. Specific insulin-like peptides encode sensory information to regulate distinct developmental processes. Development. 2011;138:1183–1193. doi: 10.1242/dev.060905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das D, Khan PP, Maitra S. Participation of PI3-kinase/Akt signaling in insulin stimulation of p34cdc2 activation in zebrafish oocyte: phosphodiesterase 3 as a potential downstream target. Mol Cell Endocrinol. 2013;374:46–55. doi: 10.1016/j.mce.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Das D, Khan PP, Maitra S. Endocrine and paracrine regulation of meiotic cell cycle progression in teleost oocytes: cAMP at the centre of complex intra-oocyte signaling events. Gen Comp Endocrinol. 2017;241:33–40. doi: 10.1016/j.ygcen.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Das D, Nath P, Pal S, Hajra S, Ghosh P, Maitra S. Expression of two insulin receptor subtypes, insra and insrb, in zebrafish (Danio rerio) ovary and involvement of insulin action in ovarian function. Gen Comp Endocrinol. 2016a;239:21–31. doi: 10.1016/j.ygcen.2016.02.005. [DOI] [PubMed] [Google Scholar]
- Das D, Pal S, Maitra S. Releasing prophase arrest in zebrafish oocyte: synergism between maturational steroid and Igf1. Reproduction. 2016b;151:59–72. doi: 10.1530/REP-15-0389. [DOI] [PubMed] [Google Scholar]
- DePina AS, Iser WB, Park S-S, Maudsley S, Wilson MA, Wolkow CA. Regulation of Caenorhabditis elegans vitellogenesis by DAF-2/IIS through separable transcriptional and posttranscriptional mechanisms. BMC Physiol. 2011;11:11. doi: 10.1186/1472-6793-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande AK, Kung HF. Insulin induction of Xenopus laevis oocyte maturation is inhibited by monoclonal antibody against p21 ras proteins. Mol Cell Biol. 1987;7:1285–1288. doi: 10.1128/mcb.7.3.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Dunaif A. Insulin Resistance and the Polycystic Ovary Syndrome Revisited: An Update on Mechanisms and Implications. Endocr Rev. 2012;33:981–1030. doi: 10.1210/er.2011-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakisa E, Argyrakopouloua G, Economoua F, Kandarakia E, Koutsilieris M. Defects in insulin signaling pathways in ovarian steroidogenesis and other tissues in polycystic ovary syndrome (PCOS) J Ster Biochem Mol Biol. 2008;109:242–246. doi: 10.1016/j.jsbmb.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Dissen GA, Romero C, Hirshfield AN, Ojeda SR. Nerve growth factor is required for early follicular development in the mammalian ovary. Endocrinology. 2001;142:2078–2086. doi: 10.1210/endo.142.5.8126. [DOI] [PubMed] [Google Scholar]
- Drummond-Barbosa D, Spradling AC. Stem Cells and Their Progeny Respond to Nutritional Changes during Drosophila Oogenesis. Dev Biol. 2001;231:265–278. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Spaczynski RZ, Olive DL, Behrman HR. Effects of insulin and insulin-like growth factors on proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1997;56:891–897. doi: 10.1095/biolreprod56.4.891. [DOI] [PubMed] [Google Scholar]
- Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenised female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:1111–1119. doi: 10.1210/jcem.87.3.8287. [DOI] [PubMed] [Google Scholar]
- Dupré A, Haccard O, Jessus C. Mos in the oocyte: How to use MAPK independently of growth factors and transcription to control meiotic divisions. J Signal Transduct. 2010;2011:350412. doi: 10.1155/2011/350412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Etr M, Schordert-Slatkine S, Baulieu EE. Meiotic maturation in Xenopus laevis oocytes is initiated by insulin. Science. 1979;205:1397–1399. doi: 10.1126/science.472755. [DOI] [PubMed] [Google Scholar]
- Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13:1035–1048. doi: 10.1210/mend.13.6.0310. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Hosoe M, O’Brien MJ, Pendola FM, Requena A, Watanabe S. Conditions that affect acquisition of developmental competence by mouse oocytes in vitro: FSH, insulin, glucose and ascorbic acid. Mol Cell Endocrinol. 2000;163:109–116. doi: 10.1016/s0303-7207(99)00247-6. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O’Brien MJ, Pendola FL, Watanabe S. Factors affecting the developmental competence of mouse oocytes grown in vitro: follicle-stimulating hormone and insulin. Biol Reprod. 1998;59:1445–1453. doi: 10.1095/biolreprod59.6.1445. [DOI] [PubMed] [Google Scholar]
- Fabian JR, Morrison DK, Daar IO. Requirement for Raf and MAP kinase functioning during the meiotic maturation of Xenopus oocytes. J Cell Biol. 1993;122:645–652. doi: 10.1083/jcb.122.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes de Abreu DA, Caballero A, Fardel P, Stroustrup N, Chen Z, Lee KH, Keyes WD, Nash ZM, López-Moyado IF, Vaggi F, Cornils A, Regenass M, Neagu A, Ostojic I, Liu C, Fernandez AM, Torres-Alemán I. The many faces of insulin-like peptide signaling in the brain. Nat Rev Neurosci. 2012;13:225–239. doi: 10.1038/nrn3209. [DOI] [PubMed] [Google Scholar]
- Fu Z, Gilbert ER, Liu D. Regulation of Insulin Synthesis and Secretion and Pancreatic Beta-Cell Dysfunction in Diabetes. Curr Diabetes Rev. 2013;9:25–53. [PMC free article] [PubMed] [Google Scholar]
- Gilbert I, Macaulay A, Robert C. Oocyte developmental competence and embryo quality: distinction and new perspectives. Anim Reprod. 2015;12:397–407. [Google Scholar]
- Gleicher N, Weghofer A, Barad DH. The role of androgens in follicle maturation and ovulation induction: friend or foe of infertility treatment? Reprod Biol Endocrinol. 2011;9:116. doi: 10.1186/1477-7827-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong JG. Influence of metabolic hormones and nutrition on ovarian follicle development in cattle: practical implications. Domest Anim Endocrinol. 2002;23:229–241. doi: 10.1016/s0739-7240(02)00159-5. [DOI] [PubMed] [Google Scholar]
- Gorre N, Adhikari D, Lindkvist R, Brännström M, Liu K, Shen Y. mTORC1 signaling in Oocytes Is Dispensable for the Survival of Primordial Follicles and for Female Fertility. PLoS One. 2014;9:e110491. doi: 10.1371/journal.pone.0110491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan JA, Nadarajan S, Kim S, Starich TA, Greenstein D. Somatic cAMP signaling regulates MSP-dependent oocyte growth and meiotic maturation in C. elegans. Development. 2009;136:2211–2221. doi: 10.1242/dev.034595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronke S, Clarke DF, Broughton S, Andrews TD, Partridge L. Molecular evolution and functional characterization of Drosophila insulin-like peptides. PLoS Genet. 2010;6:e1000857. doi: 10.1371/journal.pgen.1000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross SD, Schwab MS, Taieb FE, Lewellyn AL, Qian YW, Maller JL. The critical role of the MAP kinase pathway in meiosis II in Xenopus oocytes is mediated by p90rsk. Curr Biol. 2000;10:430–438. doi: 10.1016/s0960-9822(00)00425-5. [DOI] [PubMed] [Google Scholar]
- Haccard O, Jessus C. Redundant pathways for Cdc2 activation in Xenopus oocyte: Either cyclin B or Mos synthesis. EMBO Rep. 2006;7:321–325. doi: 10.1038/sj.embor.7400611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainaut P, Kowalski A, Giorgetti S, Baron V, Van Obberghen E. Insulin and insulin-like growth factor-1 (IGF-1) receptors in Xenopus laevis oocyte. Comparison with insulin receptors from liver and muscle. Biochem J. 1991;273:673–678. doi: 10.1042/bj2730673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen IA, Attardo GM, Rodriguez SD, Drake LL. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front Physiol. 2014;5:103. doi: 10.3389/fphys.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai S, Goascogne CL, Baulieu EE. Induction of germinal vesicle breakdown in Xenopus laevis oocytes: response of denuded oocytes to progesterone and insulin. Dev Biol. 1983;100:214–221. doi: 10.1016/0012-1606(83)90213-0. [DOI] [PubMed] [Google Scholar]
- Honegger B, Galic M, Kohler K, Wittwer F, Brogiolo W, Hafen E, Stocker H. Imp-L2, a putative homolog of vertebrate IGF-binding protein 7, counteracts insulin signaling in Drosophila and is essential for starvation resistance. J Biol. 2008;7:10. doi: 10.1186/jbiol72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Manning BD. A complex interplay between Akt, TSC2 and the two mTOR complexes. Biochem Soc Trans. 2009;37:217–222. doi: 10.1042/BST0370217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard EJA, Greenstein D. The Caenorhabditis elegans Gonad: A Test Tube for Cell and Developmental Biology. Dev Dyn. 2000;218:2–22. doi: 10.1002/(SICI)1097-0177(200005)218:1<2::AID-DVDY2>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Hubbard EJA. Caenorhabditis elegans Germline: A model for stem cell biology. Dev Dyn. 2007;236:3343–3357. doi: 10.1002/dvdy.21335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard EJA. Insulin and germ line proliferation in Caenorhabditis elegans. Vitam Horm. 2011;87:61–77. doi: 10.1016/B978-0-12-386015-6.00024-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamnongjit M, Hammes SR. Ovarian Steroids The Good, the Bad, and the Signals that Raise Them. Cell Cycle. 2006;5:1178–1183. doi: 10.4161/cc.5.11.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez PT, Frolova AI, Chi MM, Grindler NM, Willcockson AR, Reynolds KA, Zhao Q, Moley KH. DHEA-mediated inhibition of the pentose phosphate pathway alters oocyte lipid metabolism in mice. Endocrinology. 2013;154:4835–4844. doi: 10.1210/en.2012-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John GB, Shirley LJ, Gallardo TD, Castrillon DH. Specificity of the requirement for Foxo3 in primordial follicle activation. Reproduction. 2007;133:855–863. doi: 10.1530/REP-06-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagawa H. Oogenesis in teleost fish. Aqua-BioScience Monographs. 2013;6:99–127. [Google Scholar]
- Kajiura-Kobayashi H, Yoshida N, Sagata N, Yamashita M, Nagahama Y. The Mos/MAPK pathway is involved in metaphase II arrest as a cytostatic factor but is neither necessary nor sufficient for initiating oocyte maturation in goldfish. Dev Genes Evol. 2000;210:416–425. doi: 10.1007/s004270000083. [DOI] [PubMed] [Google Scholar]
- Kao S-H, Tseng C-Y, Wan C-L, Su Y-H, Hsieh C-C, Pi H, Hsu H-J. Aging and insulin signaling differentially control normal and tumorous germline stem cells. Aging Cell. 2015;14:25–34. doi: 10.1111/acel.12288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ. The genetics of ageing. Nat Rev. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- Kim S, Spike C, Greenstein D. Control of oocyte growth and meiotic maturation in Caenorhabditis elegans. In: Schedl T, editor. Germ Cell Development in C. elegans, Advances in Experimental Medicine and Biology. Vol. 757. New York: Springer; 2013. pp. 277–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble J, Seidel HC. StemBook, editor. The Stem Cell Research Community. Cambridge (MA): Harvard Stem Cell Institute; 2013. C. elegans germline stem cells and their niche. [PubMed] [Google Scholar]
- Kimble J, Sharrock WJ. Tissue-specific synthesis of yolk proteins in Caenorhabditis elegans. Dev Biol. 1983;96:189–196. doi: 10.1016/0012-1606(83)90322-6. [DOI] [PubMed] [Google Scholar]
- Kinkel MD, Prince VE. On the diabetic menu: Zebrafish as a model for pancreas development and function. Bioessays. 2009;31:139–152. doi: 10.1002/bies.200800123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn LJ, Siebel CW, McCormick F, Roth RA. Ras p21 as a potential mediator of insulin action in Xenopus oocytes. Science. 1987;236:840–843. doi: 10.1126/science.3554510. [DOI] [PubMed] [Google Scholar]
- Kosinski M, McDonald K, Schwartz J, Yamamoto I, Greenstein D. C. elegans sperm bud vesicles to deliver a meiotic maturation signal to distant oocytes. Development. 2005;132:3357–3369. doi: 10.1242/dev.01916. [DOI] [PubMed] [Google Scholar]
- LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309:1071–1073. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- Landau S, Braw-Tal R, Kaim M, Bor A, Bruckental I. Preovulatory follicular status and diet affect the insulin and glucose content of follicles in high-yielding dairy cows. Anim Reprod Sci. 2000;64:181–197. doi: 10.1016/s0378-4320(00)00212-8. [DOI] [PubMed] [Google Scholar]
- Langley AR, Smith JC, Stemple DL, Harvey SA. New insights into the maternal to zygotic transition. Development. 2014;141:3834–3841. doi: 10.1242/dev.102368. [DOI] [PubMed] [Google Scholar]
- Lawrence MC, McKern NM, Ward CW. Insulin receptor structure and its implications for the IGF-1 receptor. Curr Opin Struc Biol. 2007;17:699–705. doi: 10.1016/j.sbi.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Lee MH, Ohmachi M, Arur S, Nayak S, Francis R, Church D, Lambie E, Schedl T. Multiple functions and dynamic activation of MPK-1 extracellular signal-regulated kinase signaling in Caenorhabditis elegans germ-line development. Genetics. 2007;177:2039–2062. doi: 10.1534/genetics.107.081356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WS, Otsuka F, Moore RK, Shimasaki S. Effect of bone morphogenetic protein-7 on folliculogenesis and ovulation in the rat. Biol Reprod. 2001;65:994–999. doi: 10.1095/biolreprod65.4.994. [DOI] [PubMed] [Google Scholar]
- Li C, Kim K. Neuropeptides. The C. elegans Research Community, editor. WormBook. 2008 doi: 10.1895/wormbook.1.142.1. http://www.wormbook.org. [DOI]
- Li W, Kennedy SG, Ruvkun G. daf-28 encodes a C. elegans insulin superfamily member that is regulated by environmental cues and acts in the DAF-2 signaling pathway. Genes Dev. 2003;17:844–858. doi: 10.1101/gad.1066503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang C-G, Su Y-Q, Fan H-Y, Schatten H, Sun Q-Y. Mechanisms regulating oocyte meiotic resumption: roles of mitogen-activated protein kinase. Mol Endocrinol. 2007;21:2037–2055. doi: 10.1210/me.2006-0408. [DOI] [PubMed] [Google Scholar]
- Liao Y, Hung M-C. Physiological regulation of Akt activity and stability. Am J Transl Res. 2010;2:19–42. [PMC free article] [PubMed] [Google Scholar]
- Liu L, Rajareddy S, Reddy P, Du C, Jagarlamudi K, Shen Y, Gunnarsson D, Selstam G, Boman K, Liu K. Infertility caused by retardation of follicular development in mice with oocyte-specific expression of Foxo3a. Development. 2007;134:199–209. doi: 10.1242/dev.02667. [DOI] [PubMed] [Google Scholar]
- Lopez AL, 3rd, Chen J, Joo HJ, Drake M, Shidate M, Kseib C, Arur S. DAF-2 and ERK couple nutrient availability to meiotic progression during Caenorhabditis elegans oogenesis. Dev Cell. 2013;27:227–240. doi: 10.1016/j.devcel.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louhio H, Hovatta O, Sjöberg J, Tuuri T. The effects of insulin, and insulin-like growth factors I and II on human ovarian follicles in long term culture. Mol Hum Reprod. 2000;6:694–698. doi: 10.1093/molehr/6.8.694. [DOI] [PubMed] [Google Scholar]
- Maitra S, Das D, Ghosh P, Hajra S, Roy SS, Bhattacharya S. High cAMP attenuation of insulin-stimulated meiotic G2–M1 transition in zebrafish oocytes: interaction between the cAMP-dependent protein kinase (PKA) and the MAPK3/1 pathways. Mol Cell Endocrinol. 2014;393:109–119. doi: 10.1016/j.mce.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Manneras L, Cajander S, Holmäng A, Seleskovic Z, Lystig T, Lönn M, Stener-Victorin E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology. 2007;148:3781–3791. doi: 10.1210/en.2007-0168. [DOI] [PubMed] [Google Scholar]
- Mendez R, Richter JD. Translational control by CPEB: a means to the end. Nat Rev Mol Cell Biol. 2001;2:521–529. doi: 10.1038/35080081. [DOI] [PubMed] [Google Scholar]
- Miller MA, Nguyen VQ, Lee MH, Kosinski M, Schedl T, Caprioli RM, Greenstein D. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science. 2001;291:2144–2147. doi: 10.1126/science.1057586. [DOI] [PubMed] [Google Scholar]
- Mood K, Bong YS, Lee HS, Ishimura A, Daar IO. Contribution of JNK, Mek, Mos and PI-3K signaling to GVBD in Xenopus oocytes. Cell Signal. 2004;16:631–642. doi: 10.1016/j.cellsig.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Murphy CT, Hu PJ. Insulin/insulin-like growth factor signaling in C. elegans. The C. elegans Research Community, editor. WormBook. 2013 doi: 10.1895/wormbook.1.164.1. http://www.wormbook.org. [DOI] [PMC free article] [PubMed]
- Murphy CT, Lee SJ, Kenyon C. Tissue entrainment by feedback regulation of insulin gene expression in the endoderm of Caenorhabditis elegans. Proc Natl Acad Sci USA. 2007;104:19046–19050. doi: 10.1073/pnas.0709613104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424:277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Nassel DR, Vanden Boreck J. Insulin/IGF signaling in Drosophila and other insects: factors that regulate production, release and post-release action of inslin-like peptides. Cell Mol Life Sci. 2016;73(2):271–290. doi: 10.1007/s00018-015-2063-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagahama Y, Yamashita M. Regulation of oocyte maturation in fish. Dev Growth Differ. 2008;50:S195–S219. doi: 10.1111/j.1440-169X.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- Nandi A, Wang X, Accili D, Wolgemuth DJ. The effect of insulin signaling on female reproductive function independent of adiposity and hyperglycemia. Endocrinology. 2010;151:1863–1871. doi: 10.1210/en.2009-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler JE. Role of hyperinsulinemia in the pathogenesis of the polycystic ovary syndrome, and its clinical implications. Semin Reprod Endocrinol. 1997;15:111–122. doi: 10.1055/s-2007-1016294. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Skinner MK. Kit ligand and basic fibroblast growth factor interactions in the induction of ovarian primordial to primary follicle transition. Mol Cell Endocrinol. 2004;214:19–25. doi: 10.1016/j.mce.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990;344:503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Pace MC, Thomas P. Steroid-induced oocyte maturation in Atlantic croaker (Micropogonias undulatus) is dependent on activation of the phosphatidylinositol 3-kinase/Akt signal transduction pathway. Biol Reprod. 2005;73:988–996. doi: 10.1095/biolreprod.105.041400. [DOI] [PubMed] [Google Scholar]
- Paul S, Pramanick K, Kundu S, Bandyopadhyay A, Mukherjee D. Involvement of PI3 kinase and MAP kinase in IGF-I and insulin-induced oocyte maturation in Cyprinus carpio. Mol Cell Endocrinol. 2009;30:993–100. doi: 10.1016/j.mce.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Pelosi E, Omari S, Michel M, Ding J, Amano T, Forabosco A, Schlessinger D, Ottolenghi C. Constitutively active Foxo3 in oocytes preserves ovarian reserve in mice. Nat Commun. 2013;4:1843. doi: 10.1038/ncomms2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petruzzelli L, Herrera R, Garcia-Arenas R, Rosen OM. Acquisition of insulin-dependent protein tyrosine kinase activity during Drosophila embryogenesis. J Biol Chem. 1985;260:16072–16075. [PubMed] [Google Scholar]
- Picton H, Briggs D, Gosden R. The molecular basis of oocyte growth and development. Mol Cell Endocrinol. 1998;145:27–37. doi: 10.1016/s0303-7207(98)00166-x. [DOI] [PubMed] [Google Scholar]
- Pierce SB, Costa M, Wisotzkey R, Devadhar S, Homburger SA, Buchman AR, Ferguson KC, Heller J, Platt DM, Pasquinelli AA, Liu LX, Doberstein SK, Ruvkun G. Regulation of DAF-2 receptor signaling by human insulin and ins-1, a member of the unusually large and diverse C. elegans insulin gene family. Genes Dev. 2001;15:672–678. doi: 10.1101/gad.867301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999;20:535–582. doi: 10.1210/edrv.20.4.0374. [DOI] [PubMed] [Google Scholar]
- Poretsky L, Clemons J, Bogovich K. Hyperinsulinemia and human chorionic gonadotropin synergistically promote the growth of ovarian follicular cysts in rats. Metabolism. 1992;41:903–910. doi: 10.1016/0026-0495(92)90175-a. [DOI] [PubMed] [Google Scholar]
- Poretsky L, Kalin MF. The gonadotropic function of insulin. Endocr Rev. 1987;8:132–141. doi: 10.1210/edrv-8-2-132. [DOI] [PubMed] [Google Scholar]
- Powers RW, Chambers C, Larsen WJ. Diabetes-mediated decreases in ovarian superoxide dismutase activity are related to blood-follicle barrier and ovulation defects. Endocrinologist. 1996;137:3101–3110. doi: 10.1210/endo.137.7.8770936. [DOI] [PubMed] [Google Scholar]
- Reddy P, Adhikari D, Zheng W, Liang S, Hamalainen T, Tohonen V, Ogawa W, Noda T, Volarevic S, Huhtaniemi I, Liu K. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum Mol Genet. 2009;18:2813–2824. doi: 10.1093/hmg/ddp217. [DOI] [PubMed] [Google Scholar]
- Reddy P, Liu L, Adhikari D, Jagarlamudi K, Rajareddy S, Shen Y, Du C, Tang W, Hamalainen T, Peng SL, Lan Z-J, Cooney AJ, Huhtaniemi I, Liu K. Oocyte-specific deletion of Pten causes premature activation of the primordial follicle pool. Science. 2008;319:611–613. doi: 10.1126/science.1152257. [DOI] [PubMed] [Google Scholar]
- Richard DS, Rybczynski R, Wilson TG, Wang Y, Wayne ML, Zhou Y, Partridge L, Harshman LG. Insulin signaling is necessary for vitellogenesis in Drosophila melanogaster independent of the roles of juvenile hormone and ecdysteroids: female sterility of the chico1 insulin signaling mutation is autonomous to the ovary. J Insect Physiol. 2005;51:455–464. doi: 10.1016/j.jinsphys.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Richardson BE, Lehmann R. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol Cell Biol. 2010;11:37–49. doi: 10.1038/nrm2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers DG, Valdes CT, Elkind-Hirsch KE. The effect of ovarian function on insulin-like growth factor I plasma levels and hepatic IGF-I mRNA. Diabetes Res Clin Pract. 1990;8:235–242. doi: 10.1016/0168-8227(90)90122-a. [DOI] [PubMed] [Google Scholar]
- Roux PP, Blenis J. ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. 2004;68:320–344. doi: 10.1128/MMBR.68.2.320-344.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SG, Hansen IA, Raikhel AS. Effect of insulin and 20-hydroxy ecdysone in the fat body of the yellow fever mosquito, Aedes aegypti. Insect Biochem Mol Biol. 2007;37:1317–1326. doi: 10.1016/j.ibmb.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajid W, Kulahin N, Schluckebier G, Ribel U, Henderson HR, Tatar M, Hansen BF, Svendsen AM, Kiselyov VV, Nørgaard P, Wahlund P-O, Brandt J, Kohanski RA, Andersen AS, Meyts PD. Structural and biological properties of the Drosophila insulin-like peptide 5 show evolutionary conservation. J Biol Chem. 2011;286:661–673. doi: 10.1074/jbc.M110.156018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR. Insulin signaling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- Sánchez F, Smitz J. Molecular control of oogenesis. Biochim Biophys Acta. 2012;1822:1896–1912. doi: 10.1016/j.bbadis.2012.05.013. [DOI] [PubMed] [Google Scholar]
- Sarkissian M, Mendez R, Richter JD. Progesterone and insulin stimulation of CPEB-dependent polyadenylation is regulated by Aurora A and glycogen synthase kinase-3. Genes Dev. 2004;18:48–61. doi: 10.1101/gad.1136004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock WJ. Yolk proteins of Caenorhabditis elegans. Dev Biol. 1983;96:182–188. doi: 10.1016/0012-1606(83)90321-4. [DOI] [PubMed] [Google Scholar]
- Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, Yoshikawa H, Kato S. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci USA. 2006;103:224–229. doi: 10.1073/pnas.0506736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim J, Gururaja-Rao S, Banerjee U. Nutritional regulation of stem and progenitor cells in Drosophila. Development. 2013;140:4647–4656. doi: 10.1242/dev.079087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber MH, Thomsen MB, Spradling AC. Electron transport chain remodeling by GSK3 during oogenesis connects nutrient state to reproduction. Cell. 2016;164:420–432. doi: 10.1016/j.cell.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim C, Denlinger DL. Insulin signaling and the regulation of insect diapause. Front Physiol. 2013;4:189. doi: 10.3389/fphys.2013.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solc P, Schultz RM, Jan Motlik. Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells. Mol Hum Reprod. 2010;16:654–664. doi: 10.1093/molehr/gaq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling AC. Developmental genetics of oogenesis. In: Bate M, Martinez Arias A, editors. The Development of Drosophila melanogaster. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1–70. [Google Scholar]
- Stuart CA, Nagamani M. Insulin infusion acutely augments ovarian androgen production in normal women. Fertil Steril. 1990;54:788–792. [PubMed] [Google Scholar]
- Stuart CA, Prince MJ, Peters EJ, Meyer WJ. Hyperinsulinemia and hyperandrogenemia: in vivo androgen response to insulin infusion. Obstet Gynecol. 1987;69:921–925. [PubMed] [Google Scholar]
- Taguchi A, White MF. Insulin-like signaling, nutrient homeostasis, and life span. Annu Rev Physiol. 2008;70:191–212. doi: 10.1146/annurev.physiol.70.113006.100533. [DOI] [PubMed] [Google Scholar]
- Takenaka K, Gotoh Y, Nishida E. MAPK is required for the spindle assembly checkpoint but is dispensable for the normal M phase entry and exit in Xenopus egg cell cycle extracts. J Cell Biol. 1997;136:1091–1097. doi: 10.1083/jcb.136.5.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi CM, Emanuelli B, Kahn CR. Critical nodes in signaling pathways: insights into insulin action. Nat Rev Mol Cell Biol. 2006;7:85–96. doi: 10.1038/nrm1837. [DOI] [PubMed] [Google Scholar]
- Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110. doi: 10.1126/science.1057987. [DOI] [PubMed] [Google Scholar]
- Tunquist BJ, Maller JL. Under arrest: cytostatic factor (CSF)-mediated metaphase arrest in vertebrate eggs. Genes Dev. 2003;17:683–710. doi: 10.1101/gad.1071303. [DOI] [PubMed] [Google Scholar]
- Tyler CR, Sumpter JP. Oocyte growth and development in teleosts. Rev Fish Biol Fisher. 1996;6:287–318. [Google Scholar]
- Von Stetina JR, Orr-Weaver TL. Developmental control of oocyte maturation and egg activation in metazoan models. Cold Spring Harb Perspect Biol. 2011;3:a005553. doi: 10.1101/cshperspect.a005553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Stetina JR, Tranguch S, Dey SK, Lee LA, Cha B, Drummond-Barbosa D. α-Endosulfine is a conserved protein required for oocyte meiotic maturation in Drosophila. Development. 2008;135:3697–3706. doi: 10.1242/dev.025114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace RA. Vitellogenesis and oocyte growth in non-mammalian vertebrates. In: Browder L, editor. Developmental Biology. I. New York: Pergamon Press; 1985. pp. 127–177. [DOI] [PubMed] [Google Scholar]
- Weber GM, Sullivan CV. In vitro hormone induction of final oocyte maturation in striped bass (Morone saxitallis) follicles is inhibited by blockers of phosphatidylinositol 3-kinase activity. Comp Biochem Physiol Part B. 2001;129:467–473. doi: 10.1016/s1096-4959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1711. doi: 10.1126/science.1092952. [DOI] [PubMed] [Google Scholar]
- Wu Q, Brown MR. signaling and function of insulin-like peptides in insects. Annu Rev Entomol. 2006;51:1–24. doi: 10.1146/annurev.ento.51.110104.151011. [DOI] [PubMed] [Google Scholar]
- Wu S, Divall S, Nwaopara A, Radovick S, Wondisford F, Ko C, Wolfe A. Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes. 2014;63:1270–1282. doi: 10.2337/db13-1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Takeo S, Florens L, Hughes SE, Huo LJ, Gilliland WD, Swanson SK, Teeter K, Schwartz JW, Washburn MP, Jaspersen SL, Hawley RS. The inhibition of polo kinase by matrimony maintains G2 arrest in the meiotic cell cycle. PLoS Biol. 2007;5:e323. doi: 10.1371/journal.pbio.0050323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Bernuci MP, Lawson MS, Yeoman RR, Fisher TE, Zelinski MB, Stouffer RL. Survival, growth, and maturation of secondary follicles from prepubertal, young, and older adult rhesus monkeys during encapsulated three-dimensional culture: effects of gonadotropins and insulin. Reproduction. 2010;140:685–697. doi: 10.1530/REP-10-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C, Wang P, DeMayo J, DeMayo FJ, Elvin JA, Carino C, Prasad SV, Skinner SS, Dunbar BS, Dube JL, Celeste AJ, Matzuk MM. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. doi: 10.1210/mend.15.6.0662. [DOI] [PubMed] [Google Scholar]
- Zhang K, Smith GW. Maternal control of early embryogenesis in mammals. Reprod Fertil Dev. 2015;27:880–896. doi: 10.1071/RD14441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Ohan N, Agazie Y, Cummings C, Farah S, Liu XJ. Molecular cloning and characterization of Xenopus insulin-like growth factor-1 receptor: Its role in mediating insulin-induced Xenopus oocyte maturation and expression during embryogenesis. Endocrinology. 1998;139:949–954. doi: 10.1210/endo.139.3.5824. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Rice CD, Pang Y, Pace M, Thomas P. Cloning, expression, and characterization of a membrane progestin receptor and evidence it is an intermediary in meiotic maturation of fish oocytes. Proc Natl Acad Sci USA. 2003;100:2231–2236. doi: 10.1073/pnas.0336132100. [DOI] [PMC free article] [PubMed] [Google Scholar]