Abstract
A short review is given of recent work showing that the flagellar rotary motor of the bacterium Escherichia coli remodels to match its operating point (the fraction of time that it spins clockwise) to the requirements of the chemotaxis signaling network, and to provide the torque necessary to operate at different viscous loads.
Keywords: chemotaxis, motility, E. coli; adaptation
Introduction
The bacterial flagellar motor is a remarkably dynamic machine: not only does it spin at rates of about 300 Hz when viscous loads are low, it runs either clockwise (CW) or counter‐clockwise (CCW), depending upon the output of the chemotaxis signaling network, represented by the concentration of a small phosphorylated cytoplasmic protein, CheY‐P. The activity of the kinase that phosphorylates this signaling molecule (CheA) is regulated by receptors that span the cytoplasmic membrane and monitor the concentrations of a variety of small molecules able to pass through porins of the outer cell membrane. The receptors form large clusters, primarily near the cell poles, while the motors, about four in number for E. coli, are scattered along the sides of the cell. The coupling between the receptor kinases and the several motors is affected by diffusion of CheY‐P. When the motors spin CCW, they are phase locked by physical interactions between their helical flagellar filaments, which form a coherent bundle, driving the cell steadily forward, in a so‐called run. When one or more motors spin CW, the bundle comes apart, and the cell moves erratically in place, in what is called a tumble. Cells can swim up spatial gradients of chemical attractants by suppressing tumbles as the concentration of chemical attractants increase, that is, by swimming more smoothly as life gets better. A cell does this by lowering the concentration of CheY‐P.
The motor is shown schematically in Figure 1, with components described in the figure legend. These are proteins named for the genes that encode them, identified originally by the phenotypes of their null mutations. The components starting with F were all called Fla, because the flagella were missing. A crisis developed when the number of components exceeded 26 and as workers on Salmonella, a close cousin of E. coli, used a different notation. This problem was resolved by agreement between bacterial geneticists, whence fla became flg, flh, fli, or flj, depending upon where the gene was found on the E. coli genetic map.1 Additional parts lists are given in the appendix of Ref. 2.
Figure 1.
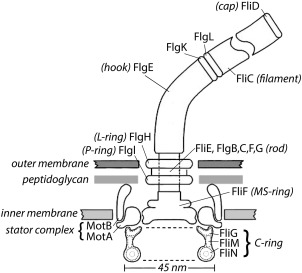
A scale drawing of the base of the E. coli flagellum, embedded in three layers of the cell wall. The outer and inner layers are fluid, but the intermediate layer, the peptidoglycan, is rigid, which gives the cell the shape of a rod with semi‐spherical end caps. The external components of the flagellum include the filament with distal cap and the hook, and the internal components include the rod, the L‐ and P‐rings, the MS‐ring, the C‐ring, and the stator complexes. The filament (a polymer of the protein FliC, also called flagellin) is shown broken, since it is several μm long. Polymerization occurs under the distal cap (FliD). Two adapter proteins (hook‐associated proteins, FlgK and FlgL) enable the hook (FlgE) to flex and the filament to rotate rigidly. The filament is a propeller that exhibits different polymorphic forms depending upon direction of rotation and torsional load, while the hook is a flexible coupling (or universal joint). A flexible coupling is required because the hooks project from the sides of the cell while the bundle of filaments (∼4 in number) that pushes the cell forward tends to align with the long axis of the cell. The rod (or drive shaft: FliE, FlgB,C,F, and G) is connected to the hook at its distal end and to the MS‐ring at its proximal end. The rod passes through the L‐ and P‐rings (FlgH and FlgI), which are mounted in the lipopolysaccharide and peptidoglycan layers, respectively, and are thought to serve as bushings. Torque is generated when protons flow from the outside to the inside of the cell through two channels in a stator complex, bounded by 4 MotA and 2 MotB. Each stator complex – there are as few as 1 or as many as 11 – is linked to the peptidoglycan by the C‐terminus of MotB and interacts electrostatically via a cytoplasmic domain of MotA with a ridge in FliG farthest from the axis of rotation. Other components of the C‐ring, FliM and FliN, interact with the signaling molecule of the chemotaxis network, CheY‐P, to control the direction of rotation. At room temperature, the direction of rotation in the absence of CheY‐P is counterclockwise (the direction of rotation of the rod when viewed from outside of the cell, i.e., from the top in this figure). Each motor comprises 26 copies of FliF and FliG, 34‐45 copies of FliM, and 34‐45 tetramers of FliN. The FliN tetramers appear as donuts in the cross‐sectional view of the C‐ring shown here. Not shown: CheY‐P; FliH, an export component known to interact with FliN; FliL, a component that enhances torque and interacts with the stator complex and with the MS‐ring; and the flagellar export apparatus that coordinates the export of axial flagellar components and is mounted at the center of the cytoplasmic face of the MS‐ring. This figure is Figure 1 from Ref. 19.
The C‐ring is involved in force generation and switching, by which the motor chooses its direction of rotation. A component of the stator (MotA) pushes on charged ridges at the periphery of the ring of FliG subunits, driving the rotation. CheY‐P binds first to FliM and then to FliN,3 triggering conformational changes in FliG that favor CW rotation.
Mot mutants are those for which flagella exist but are paralyzed; they involve two proteins, MotA and MotB. These appear in clusters of 4 MotA and 2 MotB, the latter linking each complex to the peptidoglycan, the rigid component of the cell wall. Each MotA/MotB complex includes two proton channels that span the cytoplasmic membrane. Rotation is driven by passage of ions through these channels.4 In marine bacteria, these proteins are called PomA and PomB, and the ions are sodium ions.
Several years ago, Philippe Cluzel and Michael Surette, working with Stan Leibler5 made strains in which all of the CheY was phosphorylated, varied the level of expression of CheY, used 0.5‐μm latex beads to visualize free rotating flagella, and measured their CW bias, the fraction of time that they spun CW, as a function of the concentration of CheY‐P. They found a steep function (with Hill coefficient 10.3 ± 1.1) centered at 3.1 μM. Given the steep response, the cell cannot do chemotaxis unless the CheY‐P concentration remains near 3.1 μM; otherwise, the cell would spend nearly all of its time only running or only tumbling. Therefore, Cluzel et al. suggested that the cell likely has an additional molecular mechanism to keep [CheY‐P] within a narrow operating range.
One mechanism that might work would involve feedback that adjusts the receptor kinase activity up or down as the CW bias moves down or up. We looked for such a mechanism using a FRET technique. CheY is phosphorylated by the receptor kinase CheA, and CheY‐P is dephosphorylated by the phosphatase CheZ, which reduces the CheY‐P lifetime to a few tenths of a second. With the FRET technique, energy is transferred from CheZ‐CFP to CheY‐YFP. This provides a measure, in vivo, of the concentration of CheY‐P.6 We used a variation of this technique in which energy is transferred from a bioluminescent derivative of CheZ to CheY‐P, working with a suspension of swimming cells, which enabled us to jam their flagellar bundles by addition of anti‐filament antibody. This jamming, which would be expected to perturb any motor‐to‐kinase feedback mechanism, had no effect on kinase activity.7
The matter stood at this point for several years, until we found that the motor can shift its operating point to match the output of the chemotaxis signaling pathway. It does so by recruiting FliM and FliN when the CW bias is low.8, 9 This increases the sensitivity of the motor to CheY‐P. It was known that FliM exhibits signal‐dependent turnover,10 but the reasons for this were not clear. The behavioral experiments were done by following the rotation of 1.0‐μm latex beads by laser dark‐field microscopy,11 and concentrations of FliM‐YFP or FliN‐YFPint were assessed by TIRF microscopy.
There is one FliN tetramer (shaped like a doughnut) per FliM monomer, with adjacent tetramers separated by a C‐terminal domain of FliM. The number of subunits that are bound to the motor and their rates of exchange proved to depend upon the direction of motor rotation, not upon CheY‐P binding per se.12 A smaller number of FliM monomers or FliN tetramers (34) is found in clockwise‐spinning motors and a larger number (45) in counterclockwise‐spinning motors. The number of FliF or FliG subunits remain fixed at 26.13, 14 We wonder where the additional copies of FliM and FliN might be accommodated?
Since motors change the numbers of FliM and FliN subunits as they adapt and these numbers depend upon CW bias, the response curve of Cluzel et al.5 represents motors with different numbers of FliM and FliN. What does the response curve look like for motors with fixed numbers of FliM and FliN, for example, for motors with an adapted CW bias of 0.5 ± 0.1? We did that experiment by adding and removing a nonmetabolizable attractant and found a Hill coefficient of 20.7 ± 1.6. This ultrasensitivity is easily the highest found among known allosteric protein complexes.15
If the motor remodels components of the switch, might it also remodel other things, for example, components of the power train? We found this to be the case by measuring the rotation rates of 1 μm‐diameter latex beads added suddenly to sticky‐filament stubs, or by measuring the rotation rates of cells tethered suddenly by sticky‐filament stubs.16 Thus, motors spinning for some time at very low loads were suddenly exposed to very high loads. The initial rate of rotation revealed the number of force‐generating units driving the sticky‐filament stubs (usually 1, sometimes 2), while the subsequent behavior revealed the number of elements added later (as many as 10, over a period of about 5 min). Thus, motors required to produce larger torques do so by adding more force‐generating units (more copies of the MotA/MotB complex). This was confirmed by TIRF microscopy with motors containing YFP‐MotB. Load‐dependent addition of force‐generating units also has been studied by Tipping et al.17 We know that motors running near zero load (driving hooks without filaments) spin about 300 Hz.18 Assuming that this process is driven by 2 protons moving through 1 MotA/MotB complex 26 times per revolution, the flux is 52 x 300 = 15,600 protons/s. Since the motor is not doing any work, this is power wasted. So it makes sense to add more force generating units only upon demand.
References
- 1. Iino T, Komeda Y, Kutsukake K, Macnab RM, Matsumura P, Parkinson JS, Simon MI, Yamaguchi S (1988) New unified nomenclature for the flagellar genes of Escherichia coli and Salmonella typhimurium . Microbiol Rev 52:533–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Berg HC (2004) E. coli in Motion. Springer: New York. [Google Scholar]
- 3. Sarkar MK, Paul K, Blair D (2010) Chemotaxis signaling protein CheY binds to the rotor protein FliN to control the direction of flagellar rotation in Escherichia coli . Proc Natl Acad Sci USA 107:9370–9375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Blair DF (2003) Flagellar movement driven by proton translocation. FEBS Lett 545:86–95. [DOI] [PubMed] [Google Scholar]
- 5. Cluzel P, Surette M, Leibler S (2000) An ultrasensitive bacterial motor revealed by monitoring signaling proteins in single cells. Nature 287:1652–1655. [DOI] [PubMed] [Google Scholar]
- 6. Sourjik V, Vaknin A, Shimizu TS, Berg HC (2007) In vivo measurement by FRET of pathway activity in bacterial chemotaxis. Meth Enzymol 423:365–391. [DOI] [PubMed] [Google Scholar]
- 7. Shimizu TS, Delalez N, Pichler K, Berg HC (2006) Monitoring bacterial chemotaxis by using bioluminescence resonance energy transfer: absence of feedback from the flagellar motors. Proc Natl Acad Sci USA 103:2093–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yuan J, Branch RW, Hosu BG, Berg HC (2012) Adaptation at the output of the chemotaxis signalling pathway. Nature 484:233–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Branch RW, Sayegh MN, Shen C, Nathan VSJ, Berg HC (2014) Adaptive remodelling by FliN in the bacterial rotary motor. J Mol Biol 426:3314–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Delalez NJ, Wadhams GH, Rosser G, Xue Q, Brown MT, Dobbie IM, Berry RM, Leake MC, Armitage JP (2010) Signal‐dependent turnover of the bacterial flagellar switch protein FliM. Proc Natl Acad Sci USA 107:11347–11351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yuan J, Fahrner KA, Berg HC (2009) Switching of the bacterial flagellar motor near zero load. J Mol Biol 390:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lele PP, Branch RW, Nathan VSJ, Berg HC (2012) Mechanism for adaptive remodeling of the bacterial flagellar switch. Proc Natl Acad Sci USA 109:20018–20022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sowa Y, Rowe AD, Leake MC, Yakushi T, Homma M, Ishijima A, Berry RM (2005) Direct observation of steps in rotation of the bacterial flagellar motor. Nature 437:916–919. [DOI] [PubMed] [Google Scholar]
- 14. Suzuki H, Yonekura K, Namba K (2004) Structure of the rotor of the bacterial flagellar motor revealed by electron cryomicroscopy and single‐particle image analysis. J Mol Biol 337:105–113. [DOI] [PubMed] [Google Scholar]
- 15. Yuan J, Berg HC (2013) Ultrasensitivity of an adaptive bacterial motor. J Mol Biol 425:1760–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lele PP, Hosu BG, Berg HC (2013) Dynamics of mechanosensing in the bacterial flagellar motor. Proc Natl Acad Sci USA 110:11839–11844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tipping MJ, Delalez NJ, Lim R, Berry RM, Armitage JP (2013) Load‐dependent assembly of the bacterial flagellar motor. mBio 4:e0051–e0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuan J, Berg HC (2008) Resurrection of the flagellar rotary motor near zero load. Proc Natl Acad Sci USA 105:1182–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hosu BG, Nathan VSJ, Berg HC (2016) Internal and external components of the bacterial flagellar motor rotate as a unit. Proc Natl Acad Sci USA 113:4783–4787. [DOI] [PMC free article] [PubMed] [Google Scholar]


