Abstract
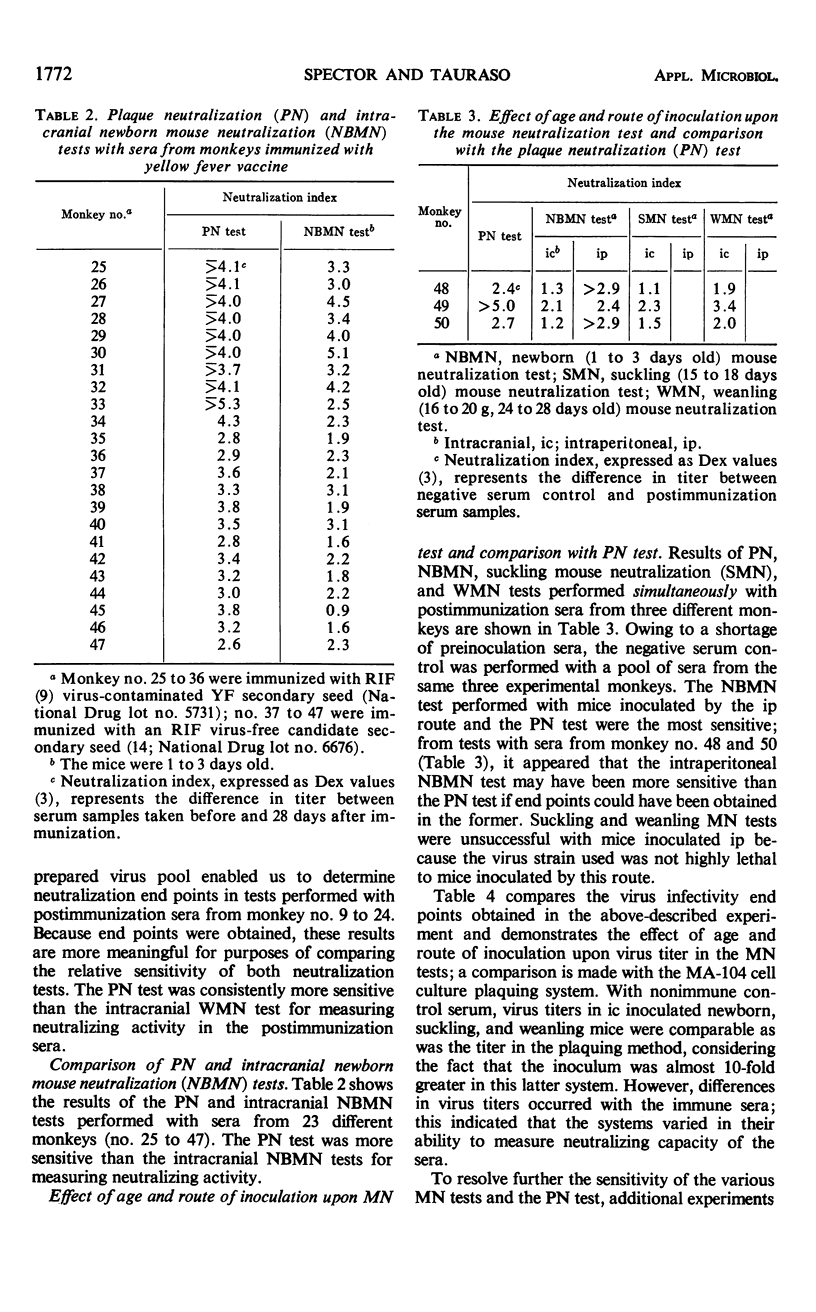
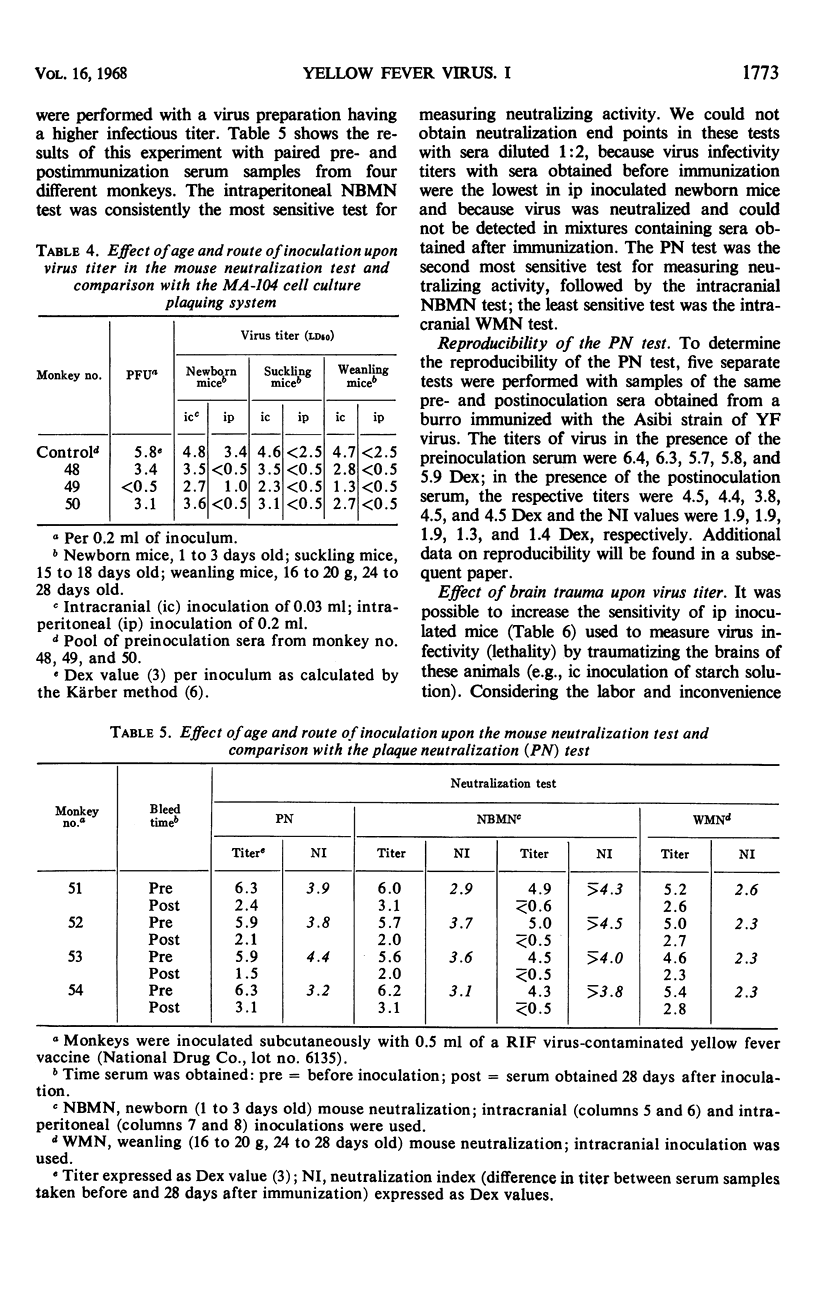
Heretofore, the most reliable way of measuring yellow fever virus antibody was to use the mouse neutralization (MN) test employing either suckling or weanling mice. Certain disadvantages (e.g., expense both of animals and of maintaining a mouse colony, allergic reactions of many laboratory workers, and the relatively long time, 21 days, before end points are reached) are inherent in any program with mice or other laboratory animal species and have discouraged the use of the MN test by many laboratories. A previously reported plaque neutralization (PN) test with primary chick embryo cell cultures could not be consistently reproduced by later investigators. We have developed a convenient and reproducible PN test employing the MA-104 embryonic rhesus monkey kidney cell culture and a single agar-overlay procedure. When compared with MN tests with newborn (1 to 3 days old) and weanling (16 to 20 g, 24 to 28 days old) mice inoculated by the intracranial route, the PN test was the most sensitive for measuring neutralizing antibody; it was also less variable, less costly, and it achieved results in the shortest period of time. End points could be determined in 5 to 6 days for the PN test as compared to 21 days for the MN test.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HENDERSON J. R., TAYLOR R. M. Arthropod-borne virus plaques in agar overlaid tube cultures. Proc Soc Exp Biol Med. 1959 Jun;101(2):257–259. doi: 10.3181/00379727-101-24902. [DOI] [PubMed] [Google Scholar]
- HENDERSON J. R., TAYLOR R. M. Propagation of certain arthropod-borne viruses in avian and primate cell cultures. J Immunol. 1960 Jun;84:590–598. [PubMed] [Google Scholar]
- PORTERFIELD J. S. A plaque technique for the titration of yellow fever virus and antisera. Trans R Soc Trop Med Hyg. 1959 Nov;53:458–466. doi: 10.1016/0035-9203(59)90021-5. [DOI] [PubMed] [Google Scholar]
- PORTERFIELD J. S. Plaque production with yellow fever and related arthropodborne viruses. Nature. 1959 Apr 11;183(4667):1069–1070. doi: 10.1038/1831069b0. [DOI] [PubMed] [Google Scholar]
- Rubin H. A VIRUS IN CHICK EMBRYOS WHICH INDUCES RESISTANCE IN VITRO TO INFECTION WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1105–1119. doi: 10.1073/pnas.46.8.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULZE I. T., SCHLESINGER R. W. Plaque assay of dengue and other group B arthropod-borne viruses under methyl cellulose overlay media. Virology. 1963 Jan;19:40–48. doi: 10.1016/0042-6822(63)90022-9. [DOI] [PubMed] [Google Scholar]
- TAURASO N., SHELOKOV A. PROTECTION AGAINST JUNIN VIRUS BY IMMUNIZATION WITH LIVE TACARIBE VIRUS. Proc Soc Exp Biol Med. 1965 Jul;119:608–611. doi: 10.3181/00379727-119-30251. [DOI] [PubMed] [Google Scholar]
- Tauraso N. M., Spector S. L., Jahnes W. G., Shelokov A. Yellow fever vaccine. I. Development of a vaccine seed free from contaminating avian leukosis viruses. Proc Soc Exp Biol Med. 1968 Apr;127(4):1116–1120. doi: 10.3181/00379727-127-32885. [DOI] [PubMed] [Google Scholar]
- Theiler M. SUSCEPTIBILITY OF WHITE MICE TO THE VIRUS OF YELLOW FEVER. Science. 1930 Apr 4;71(1840):367–367. doi: 10.1126/science.71.1840.367. [DOI] [PubMed] [Google Scholar]


