Abstract
Bone marrow microenvironment is fundamental for hematopoietic homeostasis. Numerous efforts have been made to reproduce or manipulate its activity to facilitate engraftment after hematopoietic stem cell transplantation but clinical results remain unconvincing. This probably reflects the complexity of the hematopoietic niche. Recent data have demonstrated the fundamental role of stromal and myeloid cells in regulating hematopoietic stem cell self-renewal and mobilization in the bone marrow. In this study we unveil a novel interaction by which bone marrow mesenchymal stromal cells induce the rapid differentiation of CD11b+ myeloid cells from bone marrow progenitors. Such an activity requires the expression of nitric oxide synthase-2. Importantly, the administration of these mesenchymal stromal cell-educated CD11b+ cells accelerates hematopoietic reconstitution in bone marrow transplant recipients. We conclude that the liaison between mesenchymal stromal cells and myeloid cells is fundamental in hematopoietic homeostasis and suggests that it can be harnessed in clinical transplantation.
Introduction
Mesenchymal stromal cells (MSC) play a crucial role in tissue homeostasis whereby they control inflammation and regulate stem cell renewal and differentiation. Their immunomodulatory properties, which target both adaptive and innate immune responses, have been extensively documented in vitro and in vivo.1–7 Although the underlying mechanisms are only partially known, it is widely accepted that the combination of soluble factors and contact-dependent interactions plays a fundamental role. Upregulation of inducible nitric oxide synthase (iNOS or NOS2), one of the key MSC transcriptional responses resulting in the secretion of a short-lived molecule with potent immunomodulatory effects, nitric oxide (NO),8,9 is not sufficient in itself to explain MSC the immunosuppressive activities. In fact, one of the main MSC effector mechanisms is the recruitment and functional modulation of myeloid cells, such as inflammatory monocytes and tissue macrophages.6,10–14
MSC also contribute to the hematopoietic stem cell (HSC) niche in which they regulate hematopoietic cell number and differentiation.15,16 These properties have been harnessed therapeutically to promote hematopoietic regeneration. However, early in vivo animal studies have not been unequivocally confirmed by clinical investigations.18,19 Although the mechanisms by which MSC regulate HSC are still unknown, it is arguable that, resembling what has been described for their immunosuppressive actions, MSC require other cells to execute their functions.20 In particular, a few studies have described that the interaction between MSC and bone marrow (BM) macrophages contributes to the retention of HSC in the BM21 and prevents their exhaustion.20–24 The nature of this interaction has not, however, been elucidated.
In this work, we have tested the hypothesis that MSC may skew the differentiation and expansion of BM myeloid progenitors with the ability to accelerate hematopoietic reconstitution. We have observed that MSC selectively promote the expansion and differentiation of CD11b+ cells from the BM and that this function is largely dependent on NOS2. Ex-vivo generated MSC-induced CD11b+ cells exhibit the ability to accelerate hematopoietic engraftment and reconstitution.
Methods
Cell cultures and media
Murine BM MSC were generated from crushed femora and tibiae of wild type (WT) C57Bl/6 or Nos2−/− mice (for further information, see the Online Supplementary Appendix). Human BM MSC were generated from human BM mononuclear cells (MNC). All samples were collected after informed consent had been obtained in accordance with Ethics Committee approval from the Province of Monza-Brianza (Italy) (approval date: 16 Oct 2014, approval file name: BM-NICHE). Further details on the methods used to generate murine and human MSC, and on the generation of MSC-induced myeloid cells, are presented in the Online Supplementary Appendix.
Flow cytometry
Unspecific binding sites were blocked with phosphate-buffered saline supplemented with 1% fetal bovine serum and either Fc blocker (CD16/32, eBioscience) or whole mouse IgG (Sigma Aldrich) before cells were incubated with the respective monoclonal antibody at 4°C for 30 min. After incubation, cells were washed twice with phosphate-buffered saline and analyzed by flow cytometry with a BD FACSCalibur, BD FACS CantoII, BD LSRII or BD Fortessa (BD Biosciences, NJ, USA). Data were subsequently analyzed using FlowJo software (Oregon, USA). A complete list of antibodies used is given in the Online Supplementary Appendix.
In vivo experiments
For the adoptive transfer of MSC, sublethally irradiated (split dose of 800 cGy) WT CD45.1 C57Bl/6 recipients were transplanted by tail vein injection with 2×106 BM cells and 0.2×106 WT or Nos2−/− MSC 4 h after the second dose of irradiation. Mice were sacrificed after 13 days and spleens and BM analyzed by FACS.
For the adoptive transfer of CD11b+ cells, sublethally irradiated (split dose of 800 cGy) WT CD45.1 C57Bl/6 recipients were transplanted with 5×104 BM cells alone or with 2×106 CD45.2+ MSC-induced CD11b+ cells 4 h after the second dose of irradiation. Peripheral blood samples were taken every 2 weeks after the transplant up to 4 months. Peripheral blood was lysed with lysis buffer (150 mM NH4Cl, 1 mM KHCO3, 0.1 mM EDTA, pH 7.3), and cells were stained for flow cytometry.
Statistical analysis
Data were analyzed using GraphPad Prism Software. An unpaired two-tailed Student t-test was run with a confidence interval of 95%, and expressed as mean ± standard error of the mean (SEM) or standard deviation (SD). For analysis of three groups of data, one-way analysis of variance with Bonferroni’s multiple comparison test was used, and expressed as mean ± SEM.
Results
Mesenchymal stromal cells induce the expansion and differentiation of CD11b+ cells from bone marrow myeloid progenitors
Unfractionated BM MNC were cultured in the presence or absence of MSC. After 4 days, cultures were analyzed for the expression of the myeloid markers CD11b and Gr-125 (Figure 1A). Whilst in the control cultures the vast majority of BM cells consisted of CD11b+Gr-1high, in those with MSC there was a marked skew towards the formation of a large proportion of CD11b+Gr-1low-neg (50.6% ± 3.9%) (Figure 1B). Analysis of absolute cell numbers revealed that the presence of MSC could not only maintain the survival of total CD11b+ cells as compared to BM MNC cultured alone (5.7×106 ± 1.8×106 compared to 1.7×106 ±1.4×106) (Figure 1C), but also drive a selective retention and/or expansion of the Gr-1low-neg population.
Figure 1.
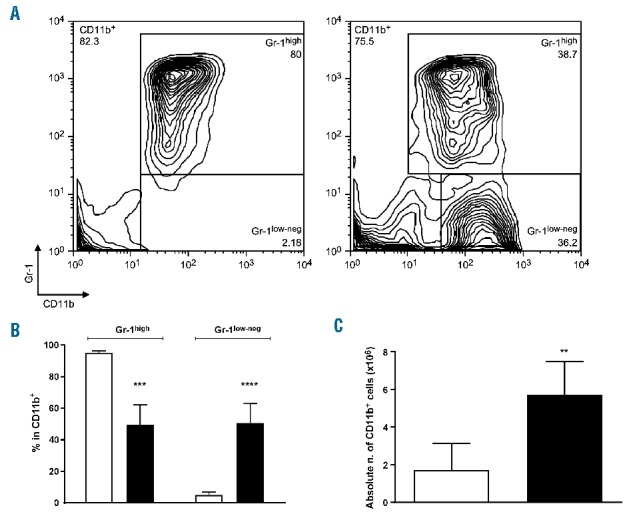
Mesenchymal stromal cells induce the differentiation of bone marrow mononuclear cells into myeloid cells. BM MNC were cultured alone or with MSC (ratio 5:1) for 4 days. (A) Proportion of CD11b+Gr-1high and CD11b+Gr-1low-neg cells in the live gate. A typical result of ten independently performed experiments is shown. (B) Percentage of Gr-1high and Gr-1low-neg cells in CD11b+ cells. Mean of ten independent experiments, ± SEM. *** P<0.001 **** P<0.0001 unpaired t test. (C) Absolute number of CD11b+ cells recovered from initial seeding from BM cultured alone (white bars) or with MSC (black bars) for 4 days. Mean of ten independent experiments, ± SEM **P<0.005, unpaired t test.
At morphological analysis the MSC-induced CD11b+ myeloid cells consisted of a fairly homogeneous population of large cells with reniform nuclei and abundant pale vacuolated cytoplasm with granules (Figure 2A). The immunophenotype of CD11b+ sorted cells revealed a 6-fold increase in F4/80+ (36.5%±10.3%), a 3-fold increase in IL4Rα+ (18.2%±7.5%), and a 2-fold increase in CD169+ (2.3%±0.6%) cells when compared to BM MNC cultured alone (Figure 2B, left panel). BM MNC cultured with MSC also expressed CD115 (48.6%±12.4%), CD206 (20.6%±2%) and CD68 (16.5%±4.9%) (Figure 2B, left panel). These macrophage markers were expressed only in the Gr-1low-neg subset (Figure 2B, right panel), whilst CD115 was detected both in the Gr-1high and the Gr-1low-neg subsets.
Figure 2.
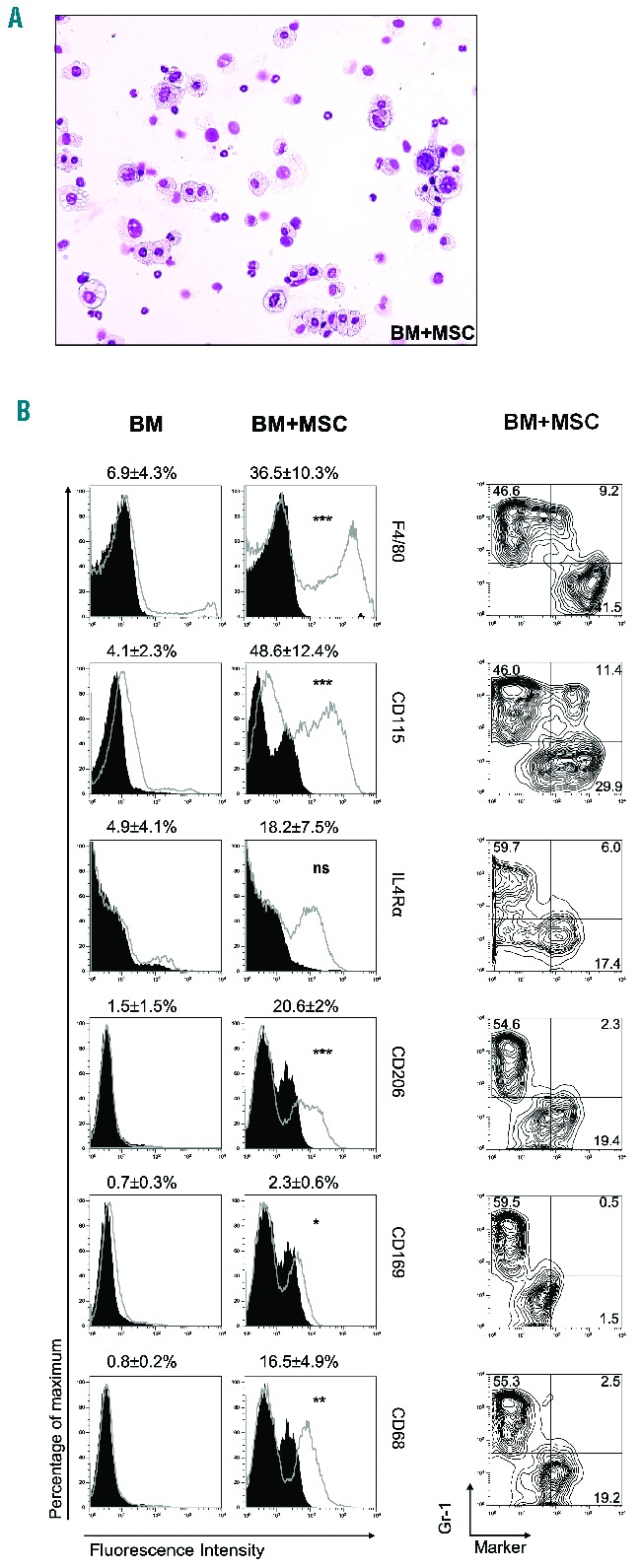
Mesenchymal stromal cell-induced CD11b+ cells consist of a large proportion of M0 macrophages. (A) May-Grünwald Giemsa staining of cytospin preparations of CD11b+ cells isolated from BM MNC cultured with MSC for 4 days. (B) BM MNC cultured alone or with MSC for 4 days were evaluated for the expression of macrophage surface markers within the CD11b+ gated population (open histograms) against their matched isotype controls (filled histograms). Contour plots within the CD11b+ gated population show the expression of each surface marker versus Gr-1 expression in BM MNC cultured with MSC. Contour and histograms plots from one out of six independent experiments, and mean fluorescence intensity values presented as mean ± SD of six independent experiments. *P<0.05, **P<0.01, ***P<0.001, unpaired t test, all comparisons between ‘BM’ versus ‘BM+MSC’.
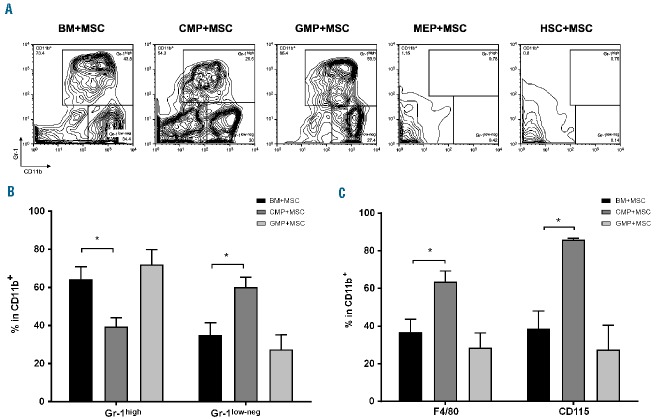
To understand the target cells of MSC-induced myeloid differentiation, FACS-sorted HSC, common myeloid progenitors (CMP) or granulocyte/macrophage progenitors (GMP) were cultured with MSC. Megakaryocyte/erythroid progenitors (MEP) and unfractionated BM MNC were used as negative or positive control of differentiation, respectively. MSC induced the differentiation of only CMP and GMP into CD11b+Gr-1high and CD11b+Gr-1low-neg cells, with no effect on HSC or MEP (Figure 3A). The proportion of Gr-1low-neg cells from CMP cultures was higher than in the cultures with unfractionated BM (Gr-1low-neg: 60.1% ± 8.9% versus 35% ± 12.8% in unfractionated BM+MSC) (Figure 3B), and, accordingly, a 2-fold increase in the percentage of CD11b+F4/80+ cells (63.6% ± 9.8% versus 36.8% ± 13.7% in BM+MSC) and a higher percentage of CD11b+CD115+ cells (85.8% ± 1.3% versus 38.6% ± 18.9% in BM+MSC) (Figure 3C).
Figure 3.
Mesenchymal stromal cell-induced CD11b+ differentiation targets committed myeloid progenitors but not hematopoietic stem cells. Unfractionated BM MNC (BM+MSC) or sorted CMP (CMP+MSC), GMP (GMP+MSC), MEP (MEP+MSC) and HSC (HSC+MSC) were cultured with MSC for 4 days. (A) Proportion of CD11b+Gr-1high and CD11b+Gr-1low-neg cells in the live gate. A typical result of four independently performed experiments is shown. (B) Percentage of Gr-1high and Gr-1low-neg cells within CD11b+ cells from BM, CMP or GMP cultures with MSC. Mean of four independent experiments, ± SEM. *P<0.05 unpaired t test. (C) Percentage of F4/80+ and CD115+ cells within the CD11b+ gate. Mean of four independent experiments, ± SEM *P<0.05, unpaired t test.
Mesenchymal stromal cell-induced CD11b+ Gr-1low-neg formation is Nos2-dependent
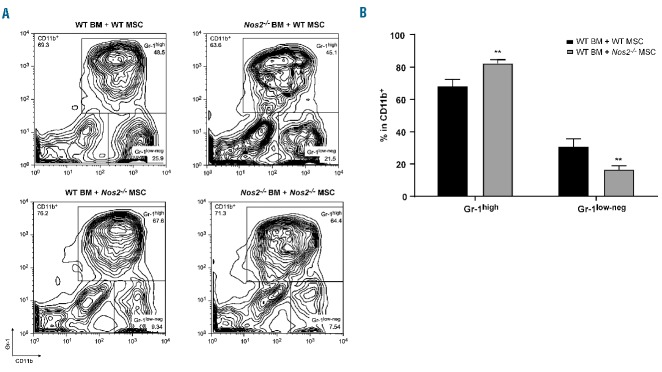
Since NOS2 is one of the key effector molecules in MSC immunomodulatory properties,8,9 we tested the hypothesis that it could also be fundamental for the generation of Gr-1low-neg cells. The differentiation of CD11b+Gr-1low-neg cells was significantly impaired in cultures with Nos2−/− MSC (16.6% ± 1.2% versus 30.8% ± 2.5% in BM+MSC WT), which correlated with an increased proportion in CD11b+Gr-1high cells (82.3% ± 1.2% versus 68.2% ± 2.2% in BM+MSC WT) (Figure 4A,B). Nos2−/− BM cells did not affect the ability of MSC to induce the generation of CD11b+Gr-1low-neg cells (Figure 4A).
Figure 4.
Mesenchymal stromal cell-induced myeloid cell formation is Nos2-dependent. (A, B) BM MNC isolated from WT or Nos2−/− mice were cultured with MSC isolated from WT or Nos2−/− mice for 4 days. (A) Contour plots represent CD11b+Gr-1high and CD11b+Gr-1low-neg proportions in the live gate. A typical result of three independently performed experiments is shown. (B) Percentages of Gr-1high and Gr-1low-neg within the CD11b+ gate. Mean of three independent experiments ±SEM, **P<0.01, unpaired t test.
Mesenchymal stromal cells increase macrophage formation during hematopoietic reconstitution
The ability of MSC to drive the expansion and differentiation of CD11b+ cells was then studied in vivo. Sublethally irradiated mice were transplanted with BM cells from a congenic (CD45.1+) donor either alone or with WT or Nos2−/− MSC. The proportion of the donor myeloid populations was analyzed in the BM and spleens of recipient mice 13 days after the transplant. At this time-point there was no difference in CD45.1+ engraftment (Figure 5A). However, the proportion and absolute numbers of Gr-1negF4/80+SSClow macrophages were increased in mice receiving BM and WT MSC compared to the control group (3.2%±0.3% versus 1.44%±0.18%; absolute number: 0.46×106±0.12×106 versus 0.16×106±0.06×106) (Figure 5B,C). An increase in the proportion (Figure 5D) but not in the absolute number (Online Supplementary Figure S4E) of Ly6G−Ly6C+ monocytes was found in the WT MSC-treated group (23.5%±1.9% versus 16.9%±1.5%; absolute number: 1.06×106±0.14×106 versus 0.96×106±0.25×106).
Figure 5.
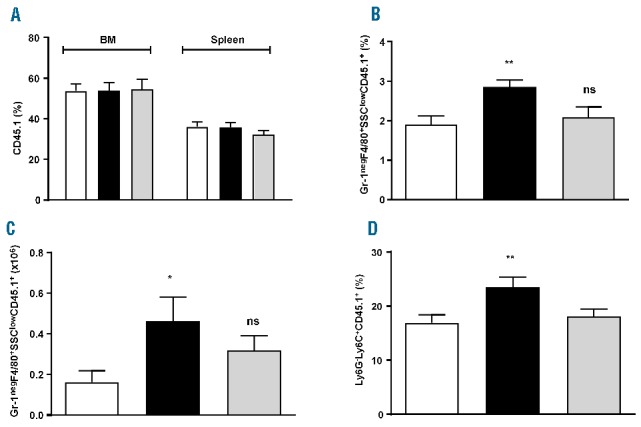
Adoptive transfer of mesenchymal stromal cells increases macrophage formation during hematopoietic reconstitution. Sublethally irradiated (800 cGy) CD45.2 WT recipients were injected with 2×106 CD45.1 WT BM cells, either alone (BM – white bars) or in combination with 0.2×106 CD45.2 WT MSC (BM+WT MSC – black bars) or 0.2×106 CD45.2 Nos2−/− MSC (BM+Nos2−/− MSC – gray bars). 13 days after the transfer, BM and spleen were analyzed by FACS. (A) Percentage of donor engraftment in BM and spleen. (B, C) Percentage (B) and absolute number (C) of Gr-1negF4/80+SSClow macrophages within donor hematopoiesis (CD45.1+ cells) in the BM. Mean of three independent experiments ±SEM, *P<0.05, **P<0.01 One-way analysis of variance (ANOVA) with the Bonferroni multiple comparison test. (D) Percentage of Ly6G−Ly6C+ monocytes within donor hematopoiesis (CD45.1+ cells) in the BM. Mean of three independent experiments ±SEM, **P<0.01 One-way ANOVA with the Bonferroni multiple comparison test.
Confirming in vitro results, the adoptive transfer of Nos2−/− MSC failed to increase the proportion and absolute number of CD11b+Gr-1negF4/80+SSClow macrophages and the proportion of Ly6G−Ly6C+ monocytes within the donor CD45.1+ engraftment (Gr-1negF4/80+SSClow macrophages: 2.1%±0.26% BM+Nos2−/− MSC; absolute number: 0.32×106±0.07×106 BM+Nos2−/− MSC. Ly6G−Ly6C+ monocytes: 18.1%±1.3% BM+Nos2−/− MSC) (Figure 5B–D).
There was no difference in the proportion or absolute number of neutrophils or eosinophils in the donor engraftment in all treated groups (Online Supplementary Figure S4C,D). The adoptive transfer of MSC and BM did not affect hematopoietic generation of donor myeloid cells in the spleen of recipient mice (data not shown).
Ex-vivo mesenchymal stromal cell-induced CD11b+ cells accelerate hematopoietic reconstitution
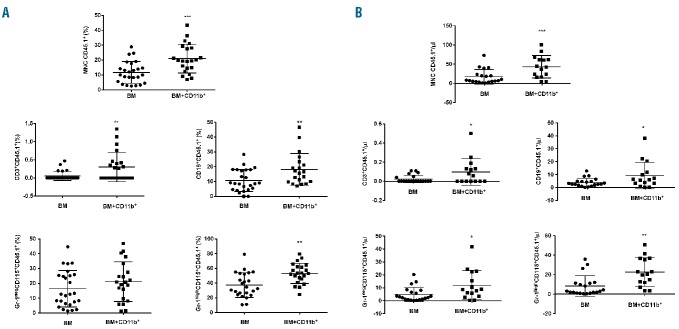
In order to further characterize the role of MSC-induced CD11b+ cells in vivo, we investigated their effect on hematopoietic reconstitution. For this purpose, sublethally irradiated mice received a BM transplant from a congenic donor with or without CD11b+ cells purified from the BM-MSC co-cultures. The engraftment of donor cells was monitored in the peripheral blood every 2 weeks. The group given CD11b+ cells showed a higher proportion (11.7%±1.5% versus 20.9%±2.1%) and absolute number (17.0±4.2 versus 43.3±7.5 CD45.1+ leukocytes/μL) of donor cells as compared to the control group already at 2 weeks. Such an increment was also observed in each leukocyte compartment (neutrophils: 37.4%±3.4% versus 53.3%±2.9%, and 8.4±2.4 versus 22.8±3.8 CD45.1+ neutrophils/μL; monocytes: 16.4%±2.4 versus 21.2%±2.9%, and 4.9±1.2 versus 12.1±2.9 CD45.1+ monocytes/μL, P=0.0195; B lymphocytes: 10.7%±1.5% versus 18.2%±2.4%, and 3.9±0.7 versus 9.3±2.7 CD45.1+ B lymphocytes/μL; T lymphocytes: 0.06%±0.03% versus 0.3%±0.09%, and 0.02±0.01 versus 0.1±0.04 CD45.1+ T lymphocytes/μL) (Figure 6A,B).
Figure 6.
Adoptive transfer of mesenchymal stromal cell-induced CD11b+ cells accelerates engraftment. Sublethally irradiated (800 cGy) CD45.2 WT recipients were injected with 5×104 CD45.1 WT BM cells, either alone (BM) or in combination with 2×106 MSC-induced CD11b+ cells (BM+CD11b+). Peripheral blood samples were taken 14 days after the transplant, and the frequency of donor hematopoiesis analyzed by FACS as described in the Methods. (A) Frequency of donor hematopoiesis in MNC, T cells (CD3+), B cells (CD19+), monocytes (Gr-1negCD115+) and neutrophils (Gr-1highCD115+). Mean of four independent experiments ± SEM, **P<0.01, ***P<0.001, unpaired t test. (B) Absolute number of donor hematopoietic cells in MNC, T cells (CD3+), B cells (CD19+), monocytes (Gr-1negCD115+) and neutrophils (Gr-1highCD115+). Mean of four independent experiments ±SEM, *P<0.05, **P<0.01, unpaired t test.
Analysis of the absolute numbers of leukocyte populations in peripheral blood showed that CD11b+ cells not only expanded donor engraftment but they also enhanced the recovery of total hematopoiesis. At 2 weeks, the group of mice given CD11b+ cells showed higher absolute counts of neutrophils (15.2±3.4 versus 42.1±5.9 neutrophils/μL), B lymphocytes (27.8±4.8 versus 51.5±7.7 B lymphocytes/μL) and monocytes (22.6±3.7 versus 37.9±4.1 monocytes/μL) (Online Supplementary Figure S5A).
Monitoring of the hematopoietic engraftment at later stages revealed that the CD11b+-driven hematopoietic reconstitution was still evident 4 weeks after the transplant (Online Supplementary Figures S5B and S6A,B). At 6 weeks the enhancement effect was selectively observed within the B and T lymphocytes compartments, whilst no difference in the quality and quantity of engraftment was found from 8 weeks onwards (Online Supplementary Figures S5B and S6A,B).
Human mesenchymal stromal cells induce the differentiation of CD14+ cells from bone marrow mononuclear cells
Finally, we asked whether the newly discovered ability of murine MSC to induce macrophage differentiation is also a property of human MSC. For this purpose, human BM MNC were cultured alone or with human MSC for 7 days. We decided to analyze the proportions of CD14+, CD14+HLA-DRlow-neg and CD14+CD16+ cells, which best represent total myeloid population, macrophages and non-classical monocytes, respectively. The presence of human MSC induced an increment in CD14+ cells with a selective effect on non-classical monocytes (CD14+: 15.5%±2.2% versus 25.8%±3.6%, in human BM and human BM cultured with human MSC, respectively; CD14+CD16+: 2.2%±0.6% versus 7.5%±1.6%, in human BM and human BM cultured with human MSC, respectively) (Figure 7A). The analysis of the absolute numbers within the CD45+ population confirmed the statistically significant increase in non-classical monocytes (CD14+CD16+: 0.01×106±0.003×106 versus 0.08×106±0.03×106 in human BM and human BM cultured with human MSC, respectively) (Figure 7B). In contrast to what we observed in the murine system, the addition of the NOS2 inhibitor 1400W to cultures did not affect myeloid differentiation (data not shown).
Figure 7.
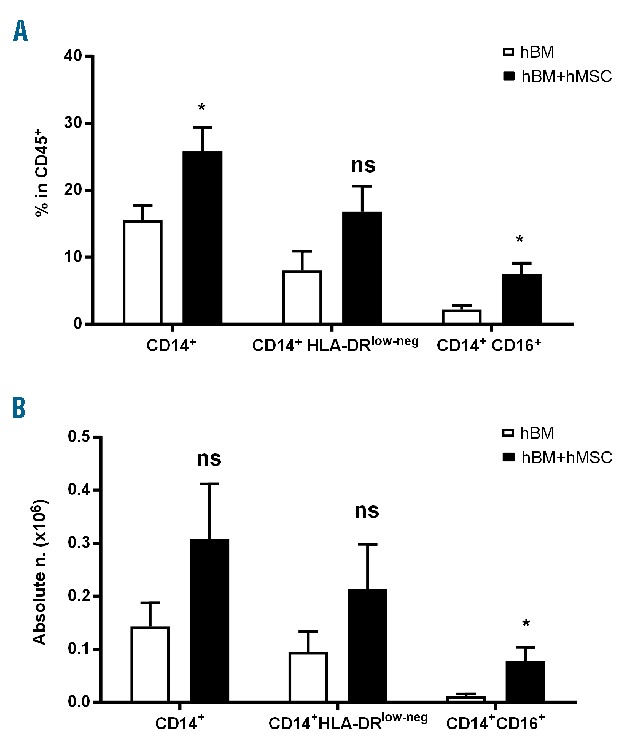
Human mesenchymal stromal cells induce the differentiation of human bone marrow mononuclear cells into myeloid cells. Human BM MNC were cultured alone or with MSC (ratio 10:1). After 7 days, the percentages (A) and absolute numbers (B) of CD14+, CD14+HLA-DRlow-neg and CD14+CD16+ in the CD45+ population were analyzed. Mean of six independent experiments, ± SEM. *P<0.05, unpaired t test.
Discussion
Our study unveils a previously unknown property of BM MSC consisting in the ability to differentiate and expand in vitro and in vivo myeloid cells from BM progenitors. Importantly, the administration of these myeloid cells accelerates hematopoietic reconstitution in BM transplant recipients.
The mechanism by which MSC promote myeloid expansion is largely dependent on NOS2. Although NOS2 has been extensively identified as the main effector mechanism accounting for MSC immunosuppressive activity and similarly implicated in myeloid immunobiology, this is the first study that attributes NOS2 the ability to differentiate and expand myeloid cells and macrophages. In contrast to the MSC-mediated immunosuppressive activity,8 we observed that the generation and expansion of myeloid cells was not influenced by inflammatory molecules such as tumor necrosis factor-α and interferon-γ (data not shown). This suggests that, despite using similar mechanisms, MSC-induced myeloid differentiation occurs independently of inflammation.
The potent ability of myeloid cells expanded ex-vivo by MSC to accelerate hematopoietic regeneration has at least two implications. The first is that the role of MSC in regulating HSC differentiation is not exclusively a direct one but can also be exerted by recruiting accessory cells. This has already been documented in the landscape of MSC immunomodulation.26 Furthermore, the regenerative properties on the hematopoietic system may apply to other tissues. The molecular and functional profile of MSC-educated myeloid cells recapitulates that of the resident macrophages which have been described as involved in tissue repair activity in other organs.27,28 Therefore, our data might lend support to the notion that stroma/fibroblasts orchestrate tissue homeostasis.
The second set of implications affects the clinical approach to the use of MSC. MSC have long been studied for their ability to promote hematopoietic engraftment based on the evidence that they are a crucial component of the stem cell niche. After the initial investigation in xenogeneic models showing that MSC co-transplanted with umbilical cord blood CD34+ cells resulted in an increase in BM engraftment,17 subsequent studies indicated that such engraftment is only transient.29,30 Similar confounding factors can be found in clinical studies in pediatric31,32 and adult19,33–35 cohorts with small and heterogeneous groups of patients. If MSC graft facilitating activity is the consequence of inducing BM progenitors to differentiate into regenerative myeloid cells, the variability in clinical outcome may result from the content of progenitors in the original donor inoculum rather than the actual direct activity of MSC on HSC differentiation.
Our data highlight the complexity of the MSC ‘clinical niche effect’. Although the adoptive transfer of MSC at the time of BM transplantation significantly increased the percentage and number of donor macrophages in the BM (Figure 5B,C), we could not observe a consequent increment in the overall donor hematopoietic engraftment (Figure 5A). This discrepancy could be explained by the low number of macrophages produced by the MSC infusion. In fact, when BM cells were infused with a high number of ex-vivo MSC-induced myeloid cells, the hematopoietic engraftment was greatly enhanced (Figure 6).
The crucial role of myeloid cells in facilitating engraftment is also supported by recent clinical evidence in which MSC have been used to condition cord blood HSC ex-vivo in order to accelerate hematopoietic recovery. Our data shed light on the interpretation of these successful clinical results.36 In that study, the MSC-conditioned cord blood unit did not engraft, indicating it mainly played a facilitating effect on the engraftment of the co-transplanted unmanipulated unit. Our study suggests that the graft facilitating effect might have been mediated by MSC-expanded monocytes, as confirmed by the phenotypic analysis of their MSC-educated cord blood HSC. Therefore, we propose the intriguing possibility that ex-vivo MSC induced myeloid cells could be harnessed in the clinical setting to expedite hematopoietic recovery and immune reconstitution in high-risk transplantation procedures.37
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/5/818
Funding
This work was supported by Bloodwise 15029 and Stellar FP7 EU grant.
References
- 1.Di Nicola M, Carlo-Stella C, Magni M, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–3843. [DOI] [PubMed] [Google Scholar]
- 2.Corcione A, Benvenuto F, Ferretti E, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–372. [DOI] [PubMed] [Google Scholar]
- 3.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naive and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101(9):3722–3729. [DOI] [PubMed] [Google Scholar]
- 4.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–2827. [DOI] [PubMed] [Google Scholar]
- 5.Ramasamy R, Fazekasova H, Lam EW, et al. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83(1):71–76. [DOI] [PubMed] [Google Scholar]
- 6.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15(1):42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta N, Su X, Popov B, et al. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. J Immunol. 2007;179(3):1855–1863. [DOI] [PubMed] [Google Scholar]
- 8.Ren G, Zhang L, Zhao X, et al. Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell. 2008;2(2):141–150. [DOI] [PubMed] [Google Scholar]
- 9.Sato K, Ozaki K, Oh I, et al. Nitric oxide plays a critical role in suppression of T-cell proliferation by mesenchymal stem cells. Blood. 2007;109(1):228–234. [DOI] [PubMed] [Google Scholar]
- 10.Melief SM, Geutskens SB, Fibbe WE, Roelofs H. Multipotent stromal cells skew monocytes towards an anti-inflammatory interleukin-10-producing phenotype by production of interleukin-6. Haematologica. 2013;98(6):888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maggini J, Mirkin G, Bognanni I, et al. Mouse bone marrow-derived mesenchymal stromal cells turn activated macrophages into a regulatory-like profile. PLoS One. 5(2):e9252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francois M, Romieu-Mourez R, Li M, Galipeau J. Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther. 2012;20(1):187–195. [DOI] [PubMed] [Google Scholar]
- 13.Choi H, Lee RH, Bazhanov N, Oh JY, Prockop DJ. Anti-inflammatory protein TSG-6 secreted by activated MSCs attenuates zymosan-induced mouse peritonitis by decreasing TLR2/NF-kappaB signaling in resident macrophages. Blood. 2011;118(2): 330–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortiz LA, Dutreil M, Fattman C, et al. Interleukin 1 receptor antagonist mediates the antiinflammatory and antifibrotic effect of mesenchymal stem cells during lung injury. Proc Natl Acad Sci USA. 2007;104(26):11002–11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20(8):833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noort WA, Kruisselbrink AB, in’t Anker PS, et al. Mesenchymal stem cells promote engraftment of human umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2002;30(8):870–878. [DOI] [PubMed] [Google Scholar]
- 18.Koc ON, Gerson SL, Cooper BW, et al. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18(2):307–316. [DOI] [PubMed] [Google Scholar]
- 19.Lazarus HM, Koc ON, Devine SM, et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol Blood Marrow Transplant. 2005;11(5):389–398. [DOI] [PubMed] [Google Scholar]
- 20.Lymperi S, Ersek A, Ferraro F, Dazzi F, Horwood NJ. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood. 2011;117(5):1540–1549. [DOI] [PubMed] [Google Scholar]
- 21.Chow A, Lucas D, Hidalgo A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208(2):261–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludin A, Itkin T, Gur-Cohen S, et al. Monocytes-macrophages that express alpha-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat Immunol. 2012;13(11):1072–1082. [DOI] [PubMed] [Google Scholar]
- 23.Kollet O, Dar A, Shivtiel S, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12(6):657–664. [DOI] [PubMed] [Google Scholar]
- 24.Mansour A, Abou-Ezzi G, Sitnicka E, et al. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J Exp Med. 2012;209(3):537–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peranzoni E, Zilio S, Marigo I, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 22(2):238–244. [DOI] [PubMed] [Google Scholar]
- 26.Marigo I, Dazzi F. The immunomodulatory properties of mesenchymal stem cells. Semin Immunopathol. 2011;33(6):593–602. [DOI] [PubMed] [Google Scholar]
- 27.de Couto G, Liu W, Tseliou E, et al. Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest. 2015;125(8):3147–3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu C, Wu C, Yang Q, et al. Macrophages mediate the repair of brain vascular rupture through direct physical adhesion and mechanical traction. Immunity. 2016;44(5): 1162–1176. [DOI] [PubMed] [Google Scholar]
- 29.Jaganathan BG, Tisato V, Vulliamy T, et al. Effects of MSC co-injection on the reconstitution of aplastic anemia patient following hematopoietic stem cell transplantation. Leukemia. 2010;24(10):1791–1795. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez-Garcia M, Yanez RM, Sanchez-Dominguez R, et al. Mesenchymal stromal cells enhance the engraftment of hematopoietic stem cells in an autologous mouse transplantation model. Stem Cell Res Ther. 2015;6:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ball LM, Bernardo ME, Roelofs H, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110(7):2764–2767. [DOI] [PubMed] [Google Scholar]
- 32.Bernardo ME, Ball LM, Cometa AM, et al. Co-infusion of ex vivo-expanded, parental MSCs prevents life-threatening acute GVHD, but does not reduce the risk of graft failure in pediatric patients undergoing allogeneic umbilical cord blood transplantation. Bone Marrow Transplant. 2011;46(2):200–207. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalo-Daganzo R, Regidor C, Martin-Donaire T, et al. Results of a pilot study on the use of third-party donor mesenchymal stromal cells in cord blood transplantation in adults. Cytotherapy. 2009;11(3):278–288. [DOI] [PubMed] [Google Scholar]
- 34.Wu Y, Wang Z, Cao Y, et al. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells with a myeloablative regimen for refractory/relapsed hematologic malignancy. Ann Hematol. 2013;92(12): 1675–1684. [DOI] [PubMed] [Google Scholar]
- 35.Wu Y, Cao Y, Li X, et al. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells for severe aplastic anemia: successful engraftment and mild GVHD. Stem Cell Res. 2014;12(1):132–138. [DOI] [PubMed] [Google Scholar]
- 36.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367(24):2305–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ballen KK, Koreth J, Chen YB, Dey BR, Spitzer TR. Selection of optimal alternative graft source: mismatched unrelated donor, umbilical cord blood, or haploidentical transplant. Blood. 2012;119(9):1972–1980. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.