Abstract
Histone deacetylase inhibitors are members of a class of epigenetic drugs that have proven activity in T-cell malignancies, but little is known about their efficacy in B-cell lymphomas. Abexinostat is an orally available hydroxamate-containing histone deacetylase inhibitor that differs from approved inhibitors; its unique pharmacokinetic profile and oral dosing schedule, twice daily four hours apart, allows for continuous exposure at concentrations required to efficiently kill tumor cells. In this phase II study, patients with relapsed/refractory non-Hodgkin lymphoma or chronic lymphocytic leukemia received oral abexinostat at 80 mg BID for 14 days of a 21-day cycle and continued until progressive disease or unacceptable toxicity. A total of 100 patients with B-cell malignancies and T-cell lymphomas were enrolled between October 2011 and July 2014. All patients received at least one dose of study drug. Primary reasons for discontinuation included progressive disease (56%) and adverse events (25%). Grade 3 or over adverse events and any serious adverse events were reported in 88% and 73% of patients, respectively. The most frequently reported grade 3 or over treatment-emergent related adverse events were thrombocytopenia (80%), neutropenia (27%), and anemia (12%). Among the 87 patients evaluable for efficacy, overall response rate was 28% (complete response 5%), with highest responses observed in patients with follicular lymphoma (overall response rate 56%), T-cell lymphoma (overall response rate 40%), and diffuse large B-cell lymphoma (overall response rate 31%). Further investigation of the safety and efficacy of abexinostat in follicular lymphoma, T-cell lymphoma, and diffuse large B-cell lymphoma implementing a less dose-intense week-on-week-off schedule is warranted. (Trial registered at: EudraCT-2009-013691-47)
Introduction
Histone deacetylases (HDAC) and histone acetyl transferases control acetylation of histone and non-histone proteins, thereby regulating gene transcription, protein function, and protein stability. The aberrant expression of HDAC proteins and mutation in the genes encoding HDACs have been linked to the development of cancers.1–3 The use of HDAC inhibitors causes hyperacetylation of histone and non-histone proteins, leading to the transcriptional activation of tumor suppressor genes, as well as genes involved in cell cycle control, cell division, and apoptosis, resulting in anti-tumor activity.4 More recently, HDAC inhibitors have also been shown to exert immunostimulatory effects on cancer cells. Exposure to HDAC inhibitors was found to up-regulate natural killer cell-activating ligands, major histocompatibility class I and II molecules, proteins involved in antigen presentation, and costimulatory molecules; in colon cancer cells, treatment with an HDAC inhibitor induced immunogenic cell death.5 In addition, HDAC inhibitors can block tumor angiogenesis and indirectly damage DNA by reducing RAD51 protein and inhibiting DNA repair.4
Currently, 4 HDAC inhibitors, vorinostat, romidepsin, belinostat, and panobinostat are approved for the treatment of cutaneous T-cell lymphoma, peripheral T-cell lymphoma, or multiple myeloma based on their potent and specific activity.6–10 These agents, along with other HDAC inhibitors under development, are showing promising results in B-cell malignancies such as non-Hodgkin lymphoma (NHL) and chronic lymphocytic leukemia (CLL),11,12 and solid tumors such as breast cancer.13 The tolerability profiles for different HDAC inhibitors vary. The most commonly reported adverse events include gastrointestinal effects (primarily diarrhea, nausea, and vomiting), constitutional effects (mainly fatigue), hematologic effects (thrombocytopenia and neutropenia), and cardiac toxicities (QTc prolongation); many of these toxicities were found to be dose-limiting.14
Abexinostat, a novel, orally available hydroxamate-containing pan-HDAC inhibitor, differs from approved HDAC inhibitors due to its unique pharmacokinetic profile. Abexinostat was rapidly absorbed following oral administration, and a maximal plasma concentration was achieved within 0.5–1 hour; the terminal elimination half-life was calculated to be approximately four hours.15 Pharmacokinetic modeling was used to determine that the oral dosing schedule for abexinostat, twice daily four hours apart, allows for continuous exposure at concentrations required for efficient tumor cell killing while lowering the peak concentrations associated with once-daily oral administration.16 Abexinostat may, therefore, offer a more active and potentially less toxic treatment option for cancer with a wider therapeutic index than other HDAC inhibitors under development.
In a phase I/II study of 55 patients with relapsed or refractory lymphomas, abexinostat was administered on a 1-week-on / 1-week-off schedule, and the recommended phase II dose was identified as 45 mg/m2 BID (equivalent to 80 mg BID). At this dose, abexinostat was well-tolerated with significant clinical activity and highly durable responses, especially in patients with multiple relapse in follicular lymphoma (FL).17 With this dosing schedule, the incidence of grade 3 or over thrombocytopenia was 20%. Another concurrent phase I study investigated other dosing schedules.15 During phase I of our study, the safety and efficacy of abexinostat using 3 different dosing schedules in 35 patients with relapsed or refractory NHL or CLL were evaluated.15 The recommended phase II dose in this study was also 45 mg/m2 BID (equivalent to 80 mg BID) administered on a more dose-intensive 2-weeks-on / 1-week-off schedule. The use of abexinostat was associated with a manageable toxicity profile and durable responses, and showed itself to be particularly active in patients with relapsed or refractory FL. Here, we report the safety and efficacy results of phase II of the same study.
Methods
This phase II study enrolled patients with relapsed/refractory NHL or CLL aged 18 years or over with histologically confirmed NHL [including FL, T-cell lymphoma (T-CL), diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), or other subtypes] or immunophenotypically confirmed CLL. Patients with NHL other than T-CL had received 2 or more prior lines of standard therapy; patients with T-CL and CLL had received 1 or more prior treatment. Other eligibility criteria included estimated life expectancy more than 12 weeks, Eastern Cooperative Oncology Group performance status 2 or under, adequate hematologic function (absolute neutrophil count >1×109/L, hemoglobin ≥8 g/dL, platelet count >100×109/L or 75×109/L in case of bone marrow involvement), adequate renal function [creatinine <1.5×upper limit of normal (ULN) or creatinine clearance ≥50 mL/min], adequate hepatic function (aspartate amino transferase and alanine aminotransferase <1.5×ULN, total bilirubin <1.5×ULN), and adequate cardiac functions with potassium levels within normal ranges with or without potassium correction. Exclusion criteria included allogeneic bone marrow transplant, major surgery within four weeks before the first abexinostat dose, corticosteroids within ten days or valproic acid within five days before the first abexinostat dose, prior treatment with HDAC inhibitor other than valproic acid, concurrent therapeutic anticoagulation by antivitamin K, immunotherapy, chemotherapy (nitrosoureas within 6 weeks), and radiotherapy within four weeks before the first abexinostat dose.
Written informed consent was obtained from all patients before enrollment. The Institutional Review Board, Research Ethics Board, and Independent Ethics Committee at each site reviewed and approved the protocol prior to initiation of the study.
Patients were given oral abexinostat at 80 mg BID four hours apart for 14 days of a 3-week cycle, and treatment was continued until progressive disease or unacceptable toxicity. The 80 mg BID dose, which corresponds to the recommended phase II dose of 45 mg/m2 BID, had been identified in phase I of the study.15 For patients who could not tolerate abexinostat, treatment was discontinued until resolution of the adverse event; treatment was resumed at a dose of 80 mg BID for five days per week for two weeks of a 3-week cycle in subsequent cycles. A further dose reduction to 60 mg BID for five days per week for two weeks of a 3-week cycle was also permitted, if necessary.
The primary end point was overall response rate (ORR). Secondary end points included duration of response, progression-free survival (PFS), and overall survival. Patients were monitored for response at the end of every cycle. For patients with NHL, response was evaluated based on the recommendations of the International Working Group Revised Response Criteria for Malignant Lymphomas.18 Patients with CLL were evaluated for response according to the guidelines of the International Workshop on Chronic Lymphocytic Leukemia.19 Adverse events were recorded at each visit using the National Cancer Institute CTCAE v.3.0 criteria.
This exploratory study enrolled approximately 16 patients per tumor type. For ORR, 95% exact (Clopper-Pearson) confidence intervals are provided. The Kaplan-Meier method was used for time-to-event analyses.
Results
Patients’ baseline characteristics
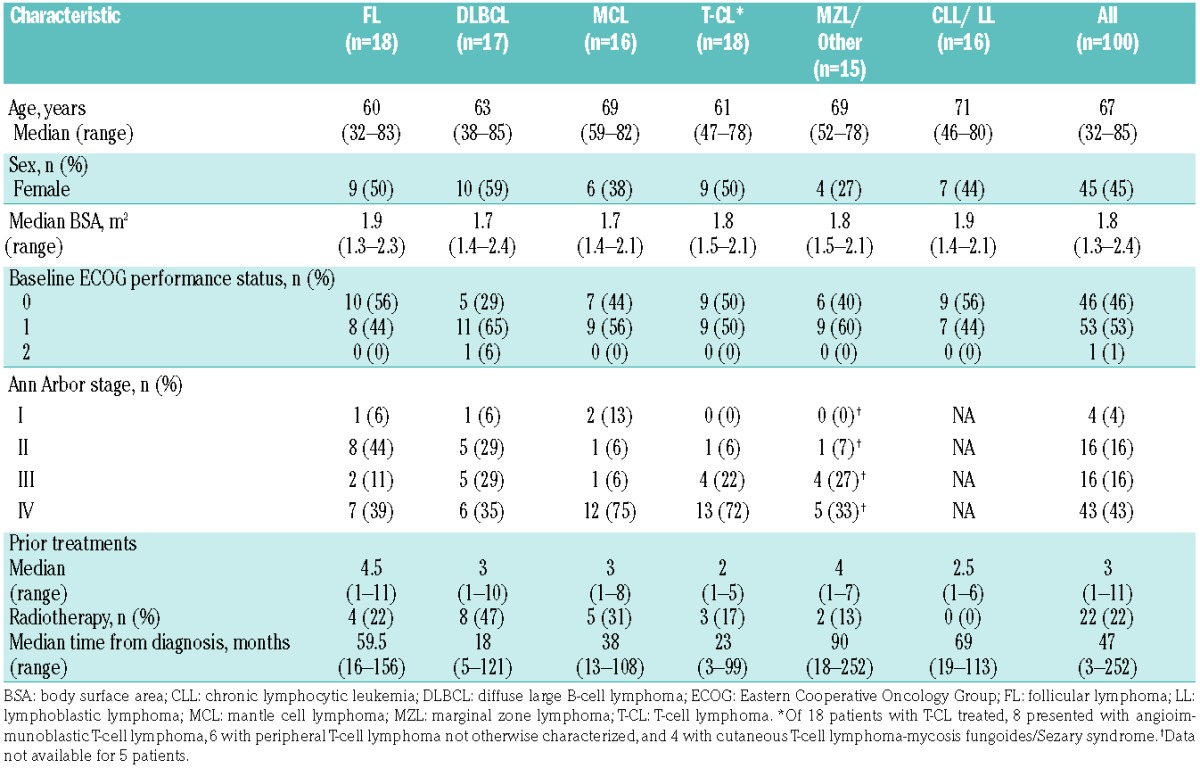
A total of 100 patients with NHL or CLL were enrolled in this phase II study between October 2011 and July 2014. Represented histologies included FL (n=18), DLBCL (n=17), MCL (n=16), T-CL (n=18), MZL and other subtypes (n=15), and CLL/LL (n=16). Patients’ baseline characteristics are presented in Table 1. The median age of the population was 67 years (range 32–85 years). The median number of prior therapies was 3 (range 1–11).
Table 1.
Patients’ baseline characteristics.
Treatment
All patients received at least one dose of study drug. The median duration of treatment was 2.8 months (range 0.7–35.4 months). Patients with FL continued abexinostat treatment longer than other NHL subtypes with a median treatment duration of 5.6 months. The most common primary reasons for withdrawal from the study were progressive disease (56%) and adverse events (25%).
Safety
Treatment-emergent adverse events related to study drug were reported in 98% of patients, with 82% experiencing grade 3 or over events. The most frequently reported grade 3 or over treatment-related AEs were thrombocytopenia (80%), neutropenia (27%), and anemia (12%). The incidence of any grade diarrhea was 42%; however, only 3% of patients reported grade 3 or over diarrhea (Table 2).
Table 2.
Treatment-related adverse events by grade occurring in 5% or more of patients.
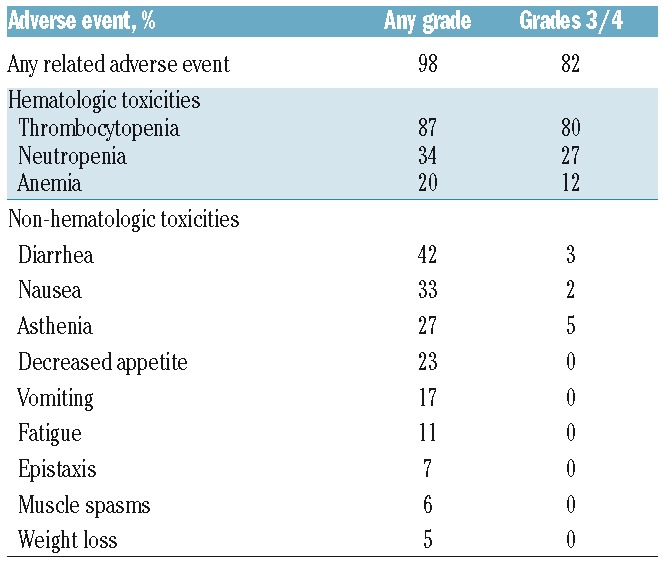
Serious AEs were reported in 73% of patients with 70% reporting grade 3 or over serious AEs. The most commonly reported serious AEs included thrombocytopenia (54%), neutropenia (11%), anemia (7%), and pneumonia (6%). There were 5 deaths on study, although the investigators of the study did not consider any of them to be related to treatment.
Because this trial was conducted at a fixed abexinostat dose, we analyzed whether patients with lower body surface area (BSA), who would have higher drug exposure, experienced greater toxicities. As expected, grade 3 or over AEs were more common in patients with a lower BSA than the median (1.8 m2) when compared with those with BSA at or above the median (98% vs. 78%). Serious AEs were also more common in patients with BSA lower than the median compared with those with BSA at or above the median BSA (90% vs. 56%).
Adverse events led to dose reductions and treatment discontinuations in 21% and 29% of patients, respectively. Thrombocytopenia was the most common toxicity that caused dose reductions, occurring in 17% of patients. Other adverse events leading to dose reductions included asthenia (2 patients) and neutropenia, decreased appetite, hypernatremia, and decreased platelet aggregation (each in 1 patient). Treatment-emergent adverse events that led to treatment discontinuation in at least 2 patients included thrombocytopenia (12 patients), neutropenia (3 patients), anemia (2 patients), and lung infection (2 patients). Gastrointestinal toxicities resulted in no dose reductions and only 3 discontinuations resulting from diarrhea, vomiting, and rectal hemorrhage in one patient each (Online Supplementary Table S1).
Across all tumor types, 4% of patients experienced cardiac treatment-emergent adverse events, with all events being reported as grade 1 or 2. One patient experienced first-degree atrioventricular block and bradycardia. Cardiac failure, defect in intraventricular conduction, and nodal arrhythmia were reported in one patient each; none of these cardiac events were considered serious or related to treatment. Clinically significant abnormalities in electrocardiogram recordings were reported for 8 patients. T-wave inversion, atrial fibrillation, and isolated ventricular premature beat were each reported in 2 patients; localized ST depression and relevant QTc prolongations were each reported in one patient.
In comparison to previous experience with abexinostat administered on a 1-week-on / 1-week-off schedule,17 weekly monitoring for thrombocytopenia showed that platelet counts fell sharply during the 14 days on abexinostat and recovered before the start of the next cycle (Figure 1).
Figure 1.
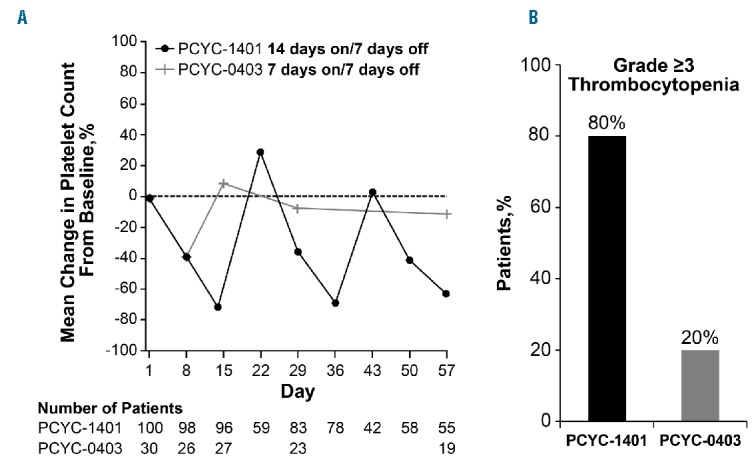
Thrombocytopenia with abexinostat treatment. (A) Platelet counts and (B) incidence of grade 3 or over thromoboctyopenia.
Efficacy
At a median follow up of 18 months, 87 patients were evaluable for efficacy. The 13 patients who did not have baseline or at least one post-baseline tumor evaluation were excluded from the efficacy analysis. The ORR across all tumor types was 28% [95% confidence interval (CI): 19%–38%], with 5% of patients showing complete responses (CR) and a median duration of response of 8.8 months (range 0.0+ to 27.7+ months) (Table 3 and Figure 2). The highest responses were observed in FL, T-CL, and DLBCL with ORRs of 56% (95%CI: 30%–80%), 40% (95%CI: 16%–68%), and 31% (95%CI: 11%–59%), respectively. Median durations of response of 16.0 months [95%CI: 3.3-not available (NA)], 11.5 months (95%CI: 1.4-NA), and 1.9 months (95%CI: 0.7–13.6) were observed in each of these tumor types, respectively. Among patients with T-CL, responses varied by subtypes with higher responses observed in patients with angioimmunoblastic T-cell lymphoma (AITL) with an ORR of 71% (1 CR and 4 PR in 7 evaluable patients) than in patients with peripheral T-cell lymphoma not otherwise characterized (ORR 20%; 1 PR) and cutaneous T-cell lymphoma-mycosis fungoides/Sezary syndrome (ORR 0%). Median PFS was 10.2 months (95%CI: 2.8-NA) for FL, 5.5 months (95%CI: 2.8-NA) for T-CL, and 2.8 months (95%CI: 1.4–3.7) for DLBCL (Figure 3). The PFS for all tumor histologies is shown in Online Supplementary Figure S1. Median overall survival was not reached for FL (95%CI: 18.4-NA), and was 17.8 months (95%CI: 11.2-NA) for T-CL and 10.2 months (95%CI: 4.4-NA) for DLBCL (Figure 4).
Table 3.
Overall response and median duration of response among responders by tumor type.
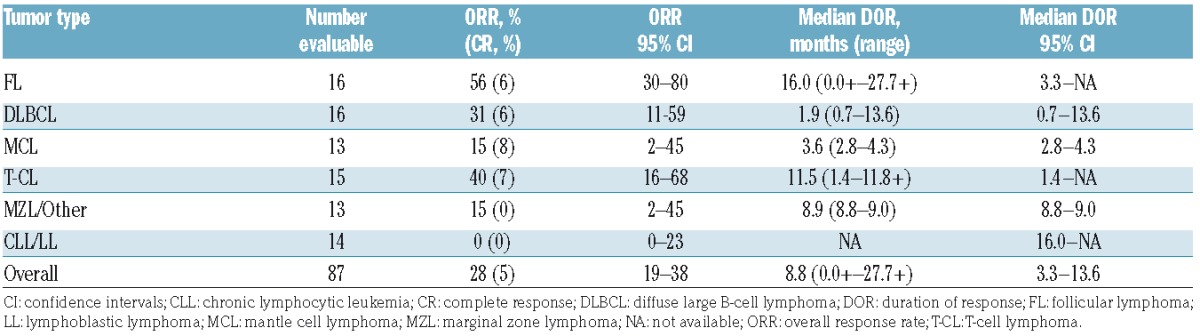
Figure 2.
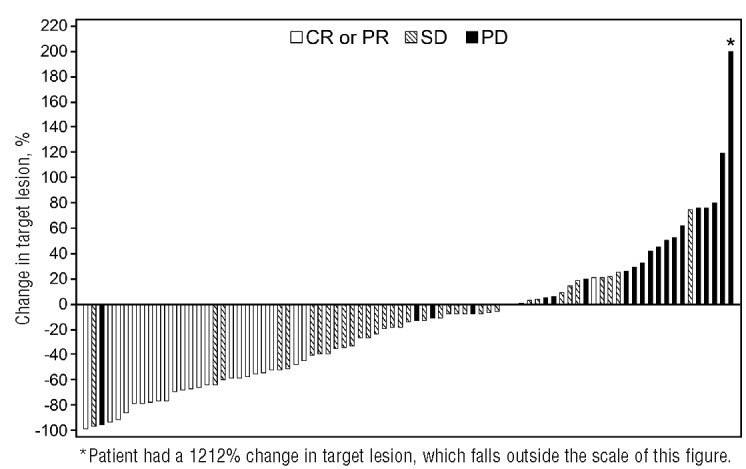
Change in lymph node size. Waterfall plot of maximum change from baseline of the sum of products of the greatest diameters during the treatment phase. CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease.
Figure 3.
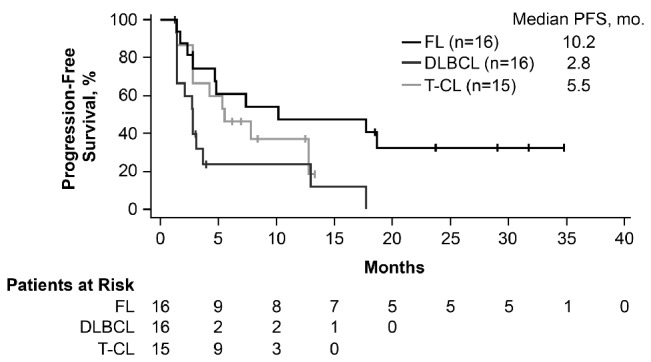
Progression-free survival (PFS) in follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), and T-cell lymphoma (T-CL). mo: months.
Figure 4.
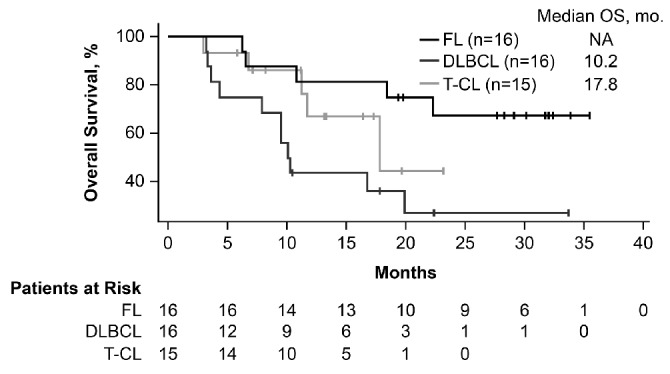
Overall survival (OS) in follicular lymphoma (FL), diffuse large B-cell lymphoma (DLBCL), and T-cell lymphoma (T-CL). mo: months.
Discussion
In this study, we report that the pan-HDAC inhibitor abexinostat showed an ORR across all tumor types of 28%. Promising response rates were observed in patients with FL, T-CL, and DLBCL, with ORRs of 56%, 40%, and 31% in each of these NHL subtypes, respectively. Considering the difficulties involved in cross-trial comparisons, the results for abexinostat in FL are consistent with those of Evens et al., who reported an ORR of 64% with abexinostat in multiply relapsed disease.17 A median PFS of 10.2 months was observed for patients with FL in this study; a median PFS of 20.5 months was reported by Evens et al. Interestingly, oral vorinostat, which has been approved for cutaneous T-cell lymphoma, yielded an ORR of 47% and a median PFS of 15.6 months when tested in patients with relapsed or refractory FL.20
Among patients with DLBCL, the ORR of 31% obtained with abexinostat is similar to the ORR of 28% recently reported for oral panobinostat in relapsed DLBCL.21 In other B-cell malignancies, abexinostat was associated with a modest response rate (15%) among patients with MCL and MZL. No responses to abexinostat were observed among patients with relapsed/refractory CLL, a result that is consistent with reports from other trials of single-agent HDAC inhibitors in this tumor type.22–25 Response to abexinostat in T-CL varied by subtype, with the highest ORR (71%, including 14% CR) in AITL comparing favorably with the ORRs of 30% and 45% reported for romidepsin26 and belinostat.6
Abexinostat showed a manageable toxicity profile in various NHL subtypes in this study. The most frequently occurring non-hematologic events were primarily gastrointestinal, and included diarrhea (42%) and nausea (33%), with most of these events being grade 1 or 2 in severity. Although cardiac toxicities, especially QTc prolongations, are considered a dose-limiting class effect of HDAC inhibitors,14 the use of abexinostat was associated with a low incidence of cardiac events (3%), which resulted in only one patient discontinuing treatment. These toxicities and their rates of occurrence were comparable with those reported in the phase I study15 and substantially lower than the phase I/II study by Evens et al., which reported incidences of nausea and diarrhea of 63% and 50%, respectively, although most of these events were low grade.17 Hematologic adverse events in this study, however, were more frequent and generally more severe than those reported by Evens et al. The incidence of grade 3 or over hematologic adverse events in this study compared to the Evens study were thrombocytopenia (80% vs. 20%) and neutropenia (27% vs. 13%).17
The difference in the toxicity profiles of abexinostat between the 2 studies may largely be attributed to the differences in dosing schedules used. Abexinostat, when administered on a 2-weeks-on / 1-week-off schedule, is associated with higher rates of grade 3 or over hematologic events. In contrast, the 1-week-on / 1-week-off schedule was associated with lower rates of high-grade hematologic toxicities. Platelet counts fell sharply during the 14 days of abexinostat and recovered during the week patients were off treatment (Figure 1). In contrast, fluctuations in platelet counts were less sharp and less prominent with the 1-week-on / 1-week-off schedule. Given that hematologic toxicities, especially thrombocytopenia, were dose-limiting in our study, the 1-week-on / 1-week-off dosing schedule will be explored in future studies.
In conclusion, this phase II study showed that abexinostat is clinically active in patients with relapsed/refractory NHL, especially in patients with FL, T-CL, and DLBCL, who exhibited high response rates and durable tumor control. Abexinostat showed good tolerability during prolonged drug administration; however, using a less dose-intense schedule may mitigate the hematologic toxicity. Abexinostat is currently being tested in a variety of clinical trial settings, and further examination in FL, T-CL, and DLBCL is warranted.
Supplementary Material
Acknowledgments
The authors would like to thank the patients who participated in the study and their supportive families, and the investigators and clinical research staff from the study centers.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/5/903
Funding
The study was originally sponsored by Servier and subsequently by Pharmacyclics LLC, an AbbVie company. Medical writing assistance was provided by Supriya Srinivasan, PhD, and was funded by Pharmacyclics LLC, an AbbVie company.
References
- 1.Barneda-Zahonero B, Parra M. Histone deacetylases and cancer. Mol Oncol. 2012;6(6):579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ropero S, Esteller M. The role of histone deacetylases (HDACs) in human cancer. Mol Oncol. 2007;1(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marchion D, Munster P. Development of histone deacetylase inhibitors for cancer treatment. Expert Rev Anticancer Ther. 2007;7(4):583–598. [DOI] [PubMed] [Google Scholar]
- 4.Mottamal M, Zheng S, Huang TL, Wang G. Histone deacetylase inhibitors in clinical studies as templates for new anticancer agents. Molecules. 2015;20(3):3898–3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.West AC, Smyth MJ, Johnstone RW. The anticancer effects of HDAC inhibitors require the immune system. Oncoimmunology. 2014;3(1):e27414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Connor OA, Horwitz S, Masszi T, et al. Belinostat in patients with relapsed or refractory peripheral T-cell lymphoma: results of the pivotal phase II BELIEF (CLN-19) study. J Clin Oncol. 2015;33(23):2492–2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olsen EA, Kim YH, Kuzel TM, et al. Phase IIb multicenter trial of vorinostat in patients with persistent, progressive, or treatment refractory cutaneous T-cell lymphoma. J Clin Oncol. 2007;25(21):3109–3115. [DOI] [PubMed] [Google Scholar]
- 8.Piekarz RL, Frye R, Turner M, et al. Phase II multi-institutional trial of the histone deacetylase inhibitor romidepsin as monotherapy for patients with cutaneous T-cell lymphoma. J Clin Oncol. 2009; 27(32):5410–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.San-Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014;15(11):1195–1206. [DOI] [PubMed] [Google Scholar]
- 10.Whittaker SJ, Demierre MF, Kim EJ, et al. Final results from a multicenter, international, pivotal study of romidepsin in refractory cutaneous T-cell lymphoma. J Clin Oncol. 2010;28(29):4485–4491. [DOI] [PubMed] [Google Scholar]
- 11.Veliz M, Pinilla-Ibarz J. Treatment of relapsed or refractory chronic lymphocytic leukemia. Cancer Control. 2012;19(1):37–53. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe T. Investigational histone deacetylase inhibitors for non-Hodgkin lymphomas. Expert Opin Investig Drugs. 2010;19(9):1113–1127. [DOI] [PubMed] [Google Scholar]
- 13.Yardley DA, Ismail-Khan RR, Melichar B, et al. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J Clin Oncol. 2013;31(17):2128–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West AC, Johnstone RW. New and emerging HDAC inhibitors for cancer treatment. J Clin Invest. 2014;124(1):30–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morschhauser F, Terriou L, Coiffier B, et al. Phase 1 study of the oral histone deacetylase inhibitor abexinostat in patients with Hodgkin lymphoma, non-Hodgkin lymphoma, or chronic lymphocytic leukaemia. Invest New Drugs. 2015;33(2):423–431. [DOI] [PubMed] [Google Scholar]
- 16.Fouliard S, Robert R, Jacquet-Bescond A, et al. Pharmacokinetic/pharmacodynamic modelling-based optimisation of administration schedule for the histone deacetylase inhibitor abexinostat (S78454/PCI-24781) in phase I. Eur J Cancer. 2013;49(13):2791–2797. [DOI] [PubMed] [Google Scholar]
- 17.Evens AM, Balasubramanian S, Vose JM, et al. A phase I/II multicenter, open-label study of the oral histone deacetylase inhibitor abexinostat in relapsed/refractory lymphoma. Clin Cancer Res. 2016; 22(5):1059–1066. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. [DOI] [PubMed] [Google Scholar]
- 19.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008; 111(12):5446–5456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirschbaum M, Frankel P, Popplewell L, et al. Phase II study of vorinostat for treatment of relapsed or refractory indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. J Clin Oncol. 2011;29(9):1198–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assouline SE, Nielsen TH, Yu S, et al. Phase 2 study of panobinostat with or without rituximab in relapsed diffuse large B-cell lymphoma. Blood. 2016;128(2):185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byrd JC, Marcucci G, Parthun MR, et al. A phase 1 and pharmacodynamic study of depsipeptide (FK228) in chronic lymphocytic leukemia and acute myeloid leukemia. Blood. 2005;105(3):959–967 [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Manero G, Yang H, Bueso-Ramos C, et al. Phase 1 study of the histone deacetylase inhibitor vorinostat (suberoylanilide hydroxamic acid [SAHA]) in patients with advanced leukemias and myelodysplastic syndrome. Blood. 2008; 111(3):1060–1066. [DOI] [PubMed] [Google Scholar]
- 24.Blum KA, Advani A, Fernandez L, et al. Phase II study of the histone deacetylase inhibitor MGCD0103 in patients with previously treated chronic lymphocytic leukemia. Br J Haematol. 2009;147:507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gimsing P, Hansen M, Knudsen LM, et al. A phase I clinical trial of the histone deacetylase inhibitor belinostat in patients with advanced hematological neoplasia. Eur J Haematol. 2008;81(3):170–176. [DOI] [PubMed] [Google Scholar]
- 26.Coiffier B, Pro B, Prince HM, et al. Romidepsin for the treatment of relapsed/refractory peripheral T-cell lymphoma: pivotal study update demonstrates durable responses. J Hematol Oncol. 2014; 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


