Abstract
Graft-versus-host disease (GvHD) remains a major cause of transplant-related mortality. Interleukin-2 (IL-2) plus sirolimus (SIR) synergistically reduces acute GvHD in rodents and promotes regulatory T cells. This phase II trial tested the hypothesis that IL-2 would facilitate STAT5 phosphorylation in donor T cells, expand regulatory T cells, and ameliorate GvHD. Between 16th April 2014 and 19th December 2015, 20 patients received IL-2 (200,000 IU/m2 thrice weekly, days 0 to +90) with SIR (5–14 ng/mL) and tacrolimus (TAC) (3–7 ng/mL) after HLA-matched related or unrelated allogeneic hematopoietic cell transplantation (HCT). The study was designed to capture an increase in regulatory T cells from 16.0% to more than 23.2% at day +30. IL-2/SIR/TAC significantly increased regulatory T cells at day +30 compared to our published data with SIR/TAC (23.8% vs. 16.0%, P=0.0016; 0.052 k/uL vs. 0.037 k/uL, P=0.0163), achieving the primary study end point. However, adding IL-2 to SIR/TAC led to a fall in regulatory T cells by day +90 and did not reduce acute or chronic GvHD. Patients who discontinued IL-2 before day +100 showed a suggested trend toward less grade II-IV acute GvHD (16.7% vs. 50%, P=0.1475). We surmise that the reported accumulation of IL-2 receptors in circulation over time may neutralize IL-2, lead to progressive loss of regulatory T cells, and offset its clinical efficacy. The amount of phospho-STAT3+ CD4+ T cells correlated with donor T-cell activation and acute GvHD incidence despite early T-cell STAT5 phosphorylation by IL-2. Optimizing IL-2 dosing and overcoming cytokine sequestration by soluble IL-2 receptor may sustain lasting regulatory T cells after transplantation. However, an approach to target STAT3 is needed to enhance GvHD prevention. (clinicaltrials.gov identifier: 01927120).
Introduction
Allogeneic hematopoietic cell transplantation (HCT) can cure high-risk hematologic malignancies and blood disorders. Graft-versus-host disease (GvHD) remains a major cause of transplant-related mortality. Sirolimus (SIR) is an immune suppressant that inhibits mTOR (mammalian target of rapamycin) and protein S6 phosphorylation.1 SIR permits interleukin-2 (IL-2) receptor activation of JAK1/JAK3 and STAT5, favoring the differentiation of regulatory T cells (Treg).1 Tregs express high affinity IL-2 receptors2 and respond to minimal levels of cytokine.3 Tregs are implicated in ameliorating GvHD,1 while IL-6 activates JAK2/STAT3 in T cells and fuels GvHD.4 A deficiency of Tregs relative to effector T cells is also associated with increased risk of chronic GvHD.5 A randomized phase II trial using matched related and unrelated donors at the Moffitt Cancer Center (MCC) demonstrated that SIR/tacrolimus (SIR/TAC) was superior to methotrexate (MTX)/TAC in preventing grade II-IV acute GvHD, while facilitating Treg recovery.6 Later, a multicenter, phase III Blood and Marrow Transplant Clinical Trials Network trial reported equivalent rates of acute GvHD between the two regimens among recipients of matched related allografts, though SIR/TAC was associated with significantly less mucositis.7 Adding IL-2 to SIR/TAC is a rational strategy to improve Treg recovery after allogenic HCT given the beneficial immune reconstitution effects of the regimen.6
Interleukin-2 was initially shown to induce FOXP3 expression among peripheral blood mononuclear cells (PBMCs) in patients receiving donor lymphocyte infusions.8 IL-2 (1,000,000 IU/m2/day for 8 weeks) induces significant clinical responses as therapy for steroid-refractory chronic GvHD, in part by expanding Tregs in vivo.9,10 As GvHD prophylaxis, IL-2 (1–200,000 IU/m2 3× week for 6–12 weeks) combined with MTX/TAC facilitates Treg recovery after 30 days of therapy.11 This pediatric clinical trial reported no cases of grade II-IV acute GvHD and few viral infections occurred after allogeneic HCT, supporting the view that IL-2 reduced alloreactivity while sparing T-effector function.11
We hypothesized that replacing broadly suppressive MTX for SIR would increase Treg reconstitution in response to IL-2. It is known that TAC also impairs Treg recovery and function.12,13 Pre-clinical evidence in mice has shown IL-2 and SIR synergistically reduce GvHD,14 but further validation of this TAC-free approach is lacking in robust non-human primate models. We cautiously retained TAC in the IL-2/SIR/TAC regimen, given that others have shown that SIR without a calcineurin inhibitor is not sufficient to prevent acute GvHD.15 Moreover, our prior randomized trial proved that significant Treg expansion could be achieved with SIR even when combined with TAC.6 We had previously demonstrated that the ratio of pSTAT5 to pSTAT3 in pooled CD4+ T cells provides a functional immune rheostat.16 As such, high pSTAT5 would favor Tregs whereas high pSTAT3 would promote alloreactive conventional T cells. Here we report our results from a prospective, phase II trial of IL-2/SIR/TAC, with the intent to maximize donor T-cell STAT5 phosphorylation and Treg reconstitution.
Methods
Study design and end points
Interleukin-2 was combined with SIR/TAC in this phase II, University of South Florida Institutional Review Board approved trial (clinicaltrials.gov identifier: 01927120). The primary end point was %Tregs at day +30. The study was designed to capture a 1.45-fold increase in Tregs from 16.0%, as published with SIR/TAC,6 to more than 23.2% with IL-2/SIR/TAC (upper interquartile range of Treg reconstitution after a year of SIR/TAC6). With 20 evaluable patients, a one-sided t-test or appropriate nonparametric test would achieve 86% power at a significance level of 0.05. A 10% patient replacement for non-evaluable patients was allowed. Thrombotic microangiopathy (TMA)17 and veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS)18 were diagnosed according to standard criteria. Acute GvHD was assessed weekly until day +100.19 Chronic GvHD was evaluated according to NIH consensus criteria.20
Patients
From 16th April 2014 and 19th December 2015, 25 patients were assessed for eligibility. Five patients failed screening and were not enrolled. Eligibility criteria are detailed in the Online Supplementary Methods.
Treatment protocol
Patients received an allogeneic HCT from HLA-matched (HLA-A, -B, -C, and -DRB1)-related or -unrelated donors using PBSC.6 Conditioning included intravenous busulfan (Bu) [area-under-the-curve targeting 3500 (reduced-intensity) or 5300 (myeloablative) mM/L*min/day, respectively, of Bu 3500/Flu or Bu 5300/Flu] or melphalan (140 mg/m2) with fludarabine (Flu/Mel, reduced-intensity).
Graft-versus-host disease prophylaxis
Sirolimus (5–14 ng/mL) and TAC (3–7 ng/ml) were monitored per ARCHITECT. IL-2 (aldesleukin, provided by Prometheus Labs, San Diego, CA, USA) was administered at 200,000 IU/m2 subcutaneously thrice weekly days 0 to +90 (+7). IL-2 was held for fever (>100.4°F), hypotension, creatinine more than 2 mg/dL, or weight gain over 10% of baseline. IL-2 was discontinued for grade II-IV acute GvHD, with the exception of isolated upper gastrointestinal (GI) stage 1 disease.
Regulatory T-cell reconstitution and suppressive function
CD4+ Tregs were identified by surface expression of CD25 and lack of CD1272,21 as gated in our prior SIR/TAC trial (Online Supplementary Figure S1A).6 Activated CD4+, conventional T cells (Tconv) were characterized by co-expression of CD25 and CD127 (Online Supplementary Figure S1A).22–24 Day +30 Treg suppressive potency was verified (n=3). Tregs and Tconv were purified by a cell sorter (BD Biosciences). Tregs were added to 5×103 Tconv, and stimulated with CD3/CD28 beads (Invitrogen, Carlsbad, CA, USA). Total T-cell proliferation was measured on day +3 [CellTiter 96 Aqueous One Solution Cell Proliferation Assay (MTS), Promega, Madison, WI, USA].4
CD4+ T-cell STAT3, STAT5, and S6 protein phosphorylation
T-cell phospho-proteins were measured as described in the Online Supplementary Methods.4,16,25
Statistical analysis
For comparisons of independent data sets, the Mann-Whitney test was used. The paired t-test was used for paired comparisons. ANOVA was used for group comparisons. The Gray method was used to evaluate the difference in acute GvHD incidence rates.26 Survival, cumulative incidence of relapse, and chronic GvHD were analyzed. SAS 9.3 (SAS Institute, Cary, NC, USA) and Prism software v.5.04 (GraphPad Software, San Diego, CA, USA) were used to conduct analyses. P<0.05 was considered significant. Given the sole interest in capturing a significant increase (not a decrease) in Tregs caused by IL-2 at day +30 after allogeneic HCT, a one-tailed test was used to assess the primary end point. A two-tailed test was used for all others.
Results
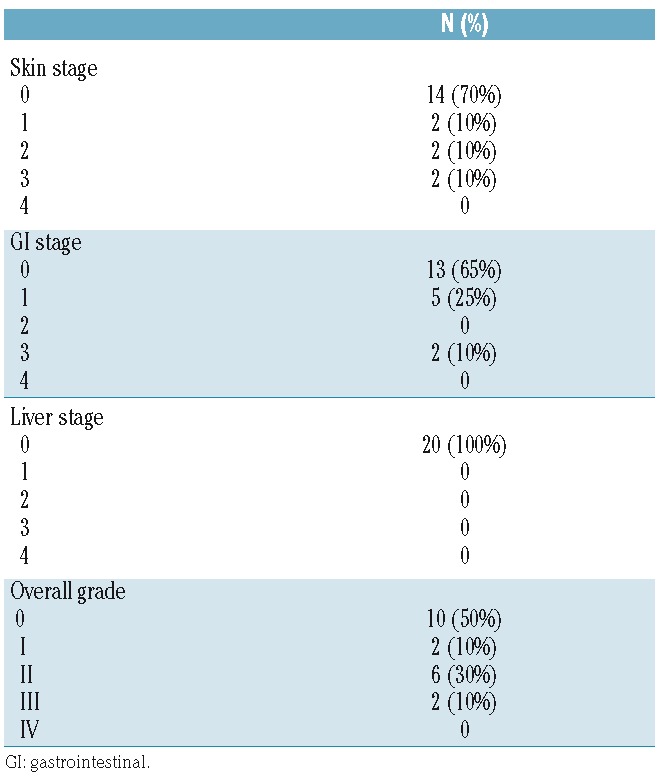
Patients’ characteristics and therapy adherence
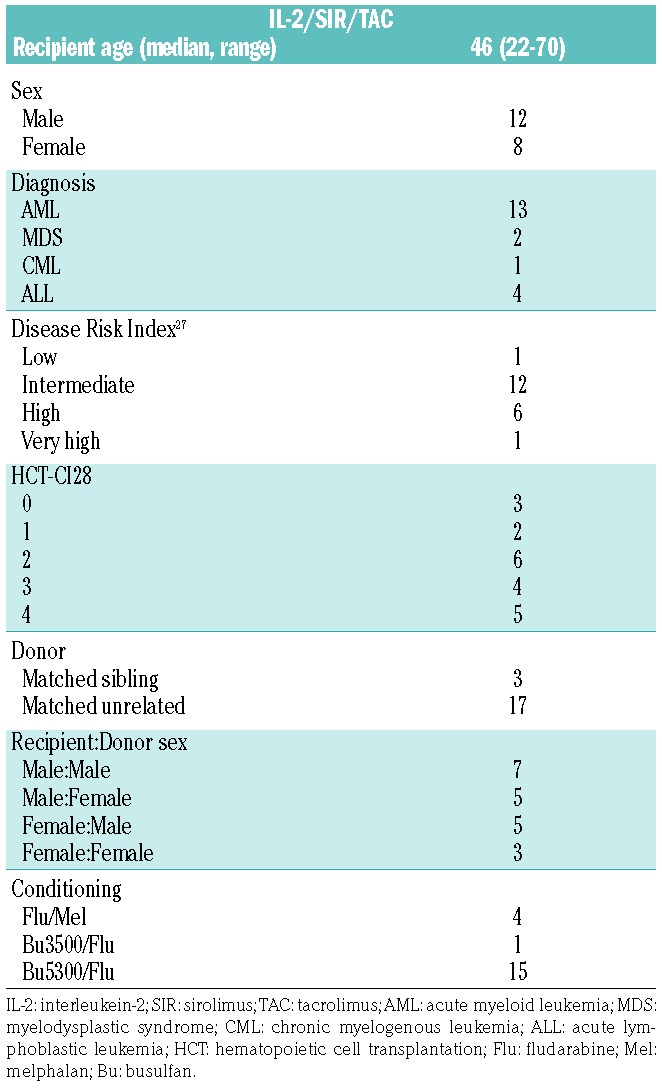
A total of 20 patients received IL-2/SIR/TAC. Patients’ characteristics are described in Table 1. The IL-2/SIR/TAC and SIR/TAC patients were similar according to age, sex, diagnosis, and conditioning regimens.6,27,28 The use of matched-unrelated donors was more prevalent among IL-2/SIR/TAC versus SIR/TAC recipients (P=0.02).6 Among the 20 treated patients, 10 fully completed IL-2 therapy between days 0 to +90 (+7 days). Six patients prematurely discontinued IL-2 injections due to: patient preference (n=3), VOD/SOS (n=2), and angioedema (n=1). A total of 8 patients developed grade II-IV acute GvHD on IL-2/SIR/TAC. IL-2 injections were stopped for 4 of these patients as per protocol. Those with isolated stage 1 upper GI disease were allowed to continue IL-2. The mean exposure of IL-2 was 69 days (range 6–90 days) for protocol-adherent patients, including those who stopped IL-2 for acute GvHD. For those who discontinued cytokine therapy for reasons other than GvHD, the mean exposure of IL-2 was 34 days (range 10–62 days). All patients remained on SIR/TAC after stopping IL-2 therapy, with only one patient discontinuing SIR at day +252 due to relapsed malignancy.
Table 1.
Baseline characteristics of study sample (n=20).
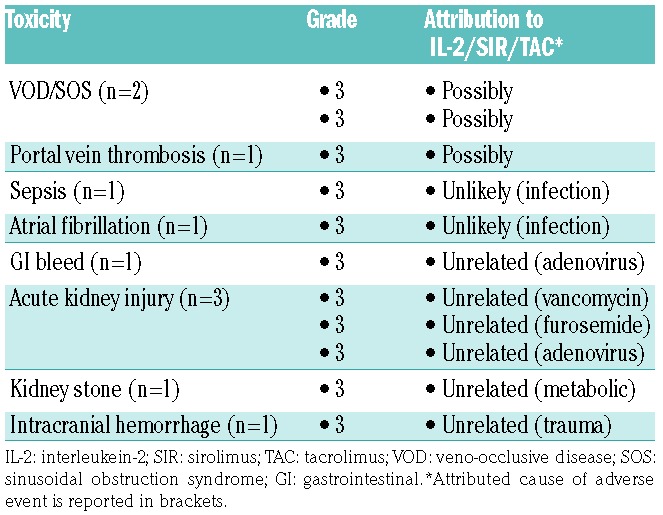
IL-2/SIR/TAC toxicity
Grade 3–5 unexpected or serious adverse events (AE) [according to Common Terminology Criteria for Adverse Events (CTCAE) v.4.03)] were captured up to day +130 or 30 days after the last dose of IL-2 (Table 2). A total of 3 patients experienced VOD/SOS and/or portal vein thrombosis, and the toxicities were attributed toward IL-2/SIR/TAC as being probable (Table 2). One subject developed grade 2 arthralgia on day +84, coinciding with the last dose of planned injections. The 30% intolerance rate of low-dose IL-2 on this trial was unanticipated, as it is typically well tolerated in other post-transplant applications.11,29 No grade 4 or 5 AEs were attributed to IL-2/SIR/TAC. No patients developed capillary leak syndrome or TMA while receiving IL-2/SIR/TAC. At a median follow up of 470 days (121–882 days), one patient died from a bowel obstruction related to a pre-HCT surgical site, 2 patients died from relapsed acute myeloid leukemia and 17 patients survive.
Table 2.
IL-2/SIR/TAC adverse events.
IL-2/SIR/TAC promotes an early wave of Treg expansion
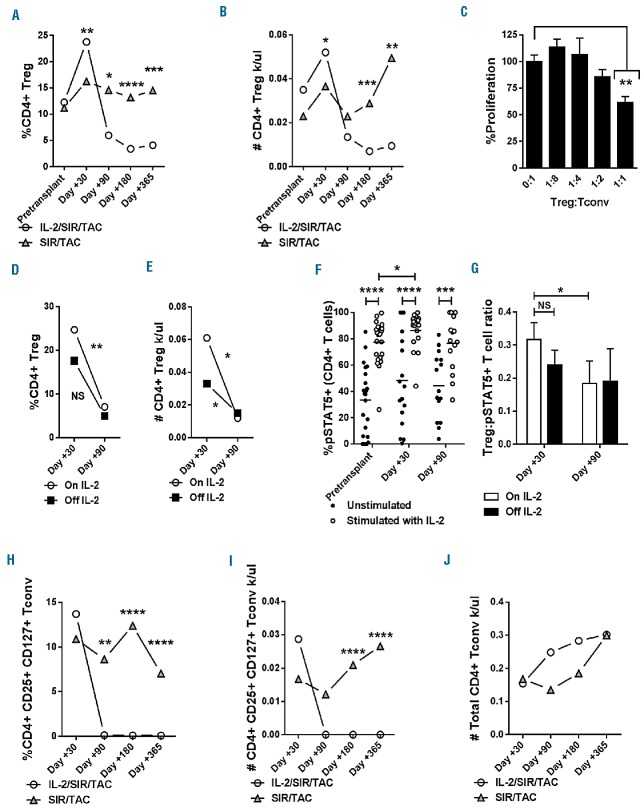
Among a total of 18 evaluable patient samples, IL-2/SIR/TAC significantly increased Treg reconstitution at day +30 compared with published data from SIR/TAC alone and met the primary study end point6 (Figure 1A and B). IL-2/SIR/TAC Tregs obtained on day +30 were suppressive in vitro, albeit at a Treg to Tconv ratio of 1:1 (Figure 1C). The frequency of Tregs on IL-2/SIR/TAC decreased significantly by day +90, compared to day +30 values or SIR/TAC controls (Figure 1A and B). Marked contraction of Treg numbers was observed by day +180, and persisted one year after allogeneic HCT (Figure 1A and B). The sharp decline in Tregs was also observed among the subset of patients who successfully completed all planned doses of IL-2 (Figure 1D and E). Among CD4+ T cells, Tregs exhibit preferential STAT5 activation in response to IL-2 compared to Tconv.1,10,16 Therefore, we measured the amount of resting and IL-2-stimulated pSTAT5+ CD4+ T cells to determine whether the loss of Tregs was due to impaired IL-2 receptor signal transduction. The proportion of IL-2-stimulated pSTAT5+ CD4+ T cells was significantly increased at all tested time points compared to unstimulated controls, indicating IL-2/SIR/TAC T cells maintained IL-2 responsiveness in vitro (Figure 1F). Conversely, the ratio of Treg to pSTAT5+ CD4+T cells decreased significantly from day +30 to day +90 even among the subset of patients actively receiving IL-2 (Figure 1G) and suggests reduced cytokine efficiency over time. While reduced Tregs among those no longer receiving IL-2 was not surprising, the severe decline in Tregs among IL-2-treated patients was unexpected.
Figure 1.
Interleukin-2/sirolimus/tacrolimus (IL-2/SIR/TAC) promotes an early wave of Treg expansion, as well as conventional T cells (Tconv) contraction after allogeneic hematopoietic stem cell transplantation (HCT). (A and B) Kinetics of CD4+, CD25+, CD127− Tregs (median % and absolute #) from day +30 to +365 among IL-2/SIR/TAC (circle) and versus published data from SIR/TAC (triangle) alone (Mann-Whitney test). IL-2 was given from day 0 to day +90, thrice weekly. For IL-2/SIR/TAC: n=18, 16, 12, and 6, at days +30, +90, +180, and +365, respectively. For SIR/TAC: n=36, 32, 21, and 17, at days +30, +90, +180, and +365, respectively. (C) Treg suppression of allo Tconv was verified among 3 independent IL-2/SIR/TAC patients at day +30. A representative experiment is shown. (D and E) Mean CD4+ Tregs (% and absolute #, ±SE) for those actively receiving IL-2 or off cytokine at days +30 and +90 (Mann-Whitney test). (F) Mean %phospho-STAT5+ CD4+ T cells [without (closed circle) or with (open circle) pulse of IL-2 for 15 minutes] at pre-transplant, day +30, and day +90 after allogeneic HCT (ANOVA). (G) Mean ratio of Tregs to IL-2-stimulated pSTAT5+ CD4+ T cells (±SE) for those actively receiving IL-2 or off cytokine at days +30 and +90 (Mann-Whitney test). (H and I) Kinetics of CD4+, CD25+, CD127+ activated Tconv (median % and absolute #) from day +30 to +365 among IL-2/SIR/TAC (circle) and versus published data from SIR/TAC (triangle) alone (Mann-Whitney test). (J) Median values for total CD4+ Tconv from day +30 to +365 among IL-2/SIR/TAC (circle) and versus SIR/TAC (triangle) (Mann-Whitney test). *P<0.05, **P=0.001–0.01, ***P=0.0001–0.001, ****P<0.0001. NS: not significant.
IL-2/SIR/TAC induces contraction of activated Tconv
Interleukin-2 with SIR is known to reduce Tconv in murine allogeneic transplantation.14 IL-2/SIR/TAC led to a late but significant loss of activated CD4+ Tconv by day +180 (Figure 1H and I) identified by expression of the high affinity IL-2 receptor, CD25, and the IL-7 receptor, CD127, recognized markers of activation.22–24 Similar to the observed decline in Tregs, the loss of activated Tconv occurred among all patients treated with IL-2/SIR/TAC irrespective of cytokine treatment adherence (Online Supplementary Figure S2A and B). Distinct from observations made in rodent transplantation, IL-2/SIR/TAC was not entirely lymphodepleting and the total CD4+ T-cell count was preserved (Figure 1J).
The addition of prolonged IL-2 therapy with SIR/TAC does not further reduce acute or chronic GvHD
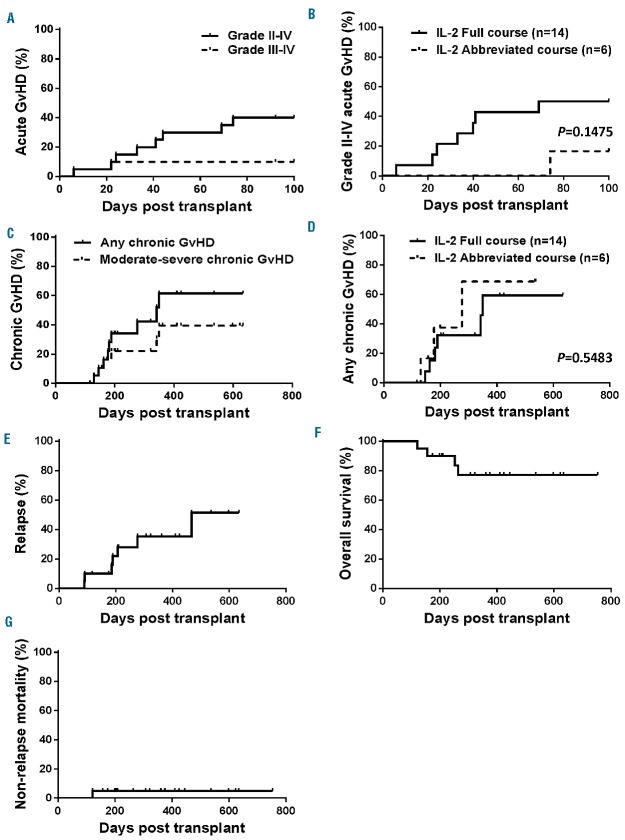
The cumulative incidence of grade II-IV by day +100 was 40% [95% confidence interval (CI): 15.8–63.4] with IL-2/SIR/TAC (Figure 2A) and comparable to our published experience with SIR/TAC alone at 43% (95%CI: 27%–59%).6 The distribution and organ stage of acute GvHD for the 8 IL-2/SIR/TAC patients are detailed in Table 3. No participants died of acute GvHD and none developed steroid refractory disease. Of the 6 patients who discontinued IL-2 for reasons other than acute GvHD and received an abbreviated course of IL-2, only one developed grade II acute GvHD after stopping cytokine therapy (Figure 2B). This supports the view that lack of clinical efficacy was not negatively influenced by attrition of IL-2 treated patients. Conversely, this suggests an abbreviated course of IL-2 with SIR/TAC may be clinically beneficial. The cumulative incidence of all chronic GvHD and moderate-severe disease was respectively 61.5% (95%CI: 36.1–79.3) and 39.4% (95%CI: 11.2–67.2) at a median follow up of 470 days (Figure 2C). Compared to published data with SIR/TAC alone (moderate-severe chronic GvHD: 24% (95%CI: 7%–47%),6 the addition of IL-2 does not appear to reduce chronic GvHD. Receiving a full versus an abbreviated course of IL-2 therapy did not significantly impact the onset of chronic GvHD [59.4% (95%CI: 27.7–81.0) vs. 68.8% (95%CI: 24.5–90.6), P=0.5483] (Figure 2D). Two (10%) patients developed late acute GvHD, one in skin, and one in the GI tract (GI stage 1) on days +113 and +117.
Figure 2.
The addition of prolonged IL-2 therapy with sirolimus/tacrolimus (SIR/TAC) does not further reduce acute or chronic graft-versus-host disease. (A) Cumulative incidence of acute graft-versus-host disease (GvHD) is shown for grade II-IV disease (solid) and severe grade III-IV acute GvHD (dashed line) by day +100 (P=0.1177). The incidence of grade II-IV acute GvHD with IL-2/SIR/TAC [40% (95%CI: 15.8%–63.4%)] is not significantly different from our published data with SIR/TAC [43% (95%CI: 27%–59%)] alone. The acute GvHD characteristics, including organ stage, are detailed in Table 2. (B) Cumulative incidence of grade II-IV acute GvHD among patients who received a full course of IL-2 versus those who prematurely stopped (abbreviated course) IL-2 [16.7% (95%CI: 0.001–77.7) vs. 50% (95%CI: 27.8–77.1), P=0.1475]. (C) Cumulative incidence of chronic GvHD (median follow up of 470 days) based on any chronic GvHD (solid black line) or moderate to severe disease (dashed line) (P=0.2698). (D) Cumulative incidence of chronic GvHD among patients who received a full course of IL-2 versus those who prematurely stopped (abbreviated course) IL-2 [59.4% (95%CI: 27.7–81.0) versus 68.8% (95%CI: 24.5–90.6), P=0.5483]. (E-G) Cumulative incidence of relapse, overall survival, and non-relapse mortality among those treated with IL-2/SIR/TAC.
Table 3.
Acute graft-versus-host disease characteristics.
Relapse and survival
At a median follow up of 470 days, the cumulative incidence of relapse was 35.2% (95%CI: 9.2–63.3) at one year (Figure 2E). Those on IL-2/SIR/TAC were permitted to receive either reduced intensity or myeloablative conditioning. The treated patients also represent a wide range of hematologic malignancies and risk by the Disease Risk Index.27 Of the relapse events, 86% had a pre-HCT Disease Risk Index of intermediate to high with only one low-risk patient. The 1-year overall survival was 77.1% (95%CI: 49.3–90.7) (Figure 2F), with a non-relapse mortality of 5.0% (95%CI: 0.0–65.6) (Figure 2G).
Activated CD25+ CD127+ Tconv are increased among IL-2/SIR/TAC recipients who develop grade II-IV acute GvHD
Interleukin-2/SIR/TAC exerts significant suppression over activated Tconv late after allogeneic HCT. Conversely, the frequency and absolute numbers of CD4+ CD25+ CD127+ Tconv with IL-2/SIR/TAC were similar to SIR/TAC alone at day +30. However, the amount of CD4+ CD25+ CD127+ Tconv at day +30 was significantly increased among IL-2/SIR/TAC patients who developed grade II-IV acute GvHD (Figure 3A and B). The amount of circulating Treg at day +30 was comparable among those with or without acute GvHD by day +100 (Figure 3C and D). As such, the Treg:Tconv ratio at day +30 was significantly higher among IL-2/SIR/TAC patients who never acquired acute GvHD (Figure 3E). Acute GvHD did not correlate with the number of activated Tconv or the Treg:Tconv ratio among the cohort of SIR/TAC controls (Online Supplementary Figure S2C and D).
Figure 3.
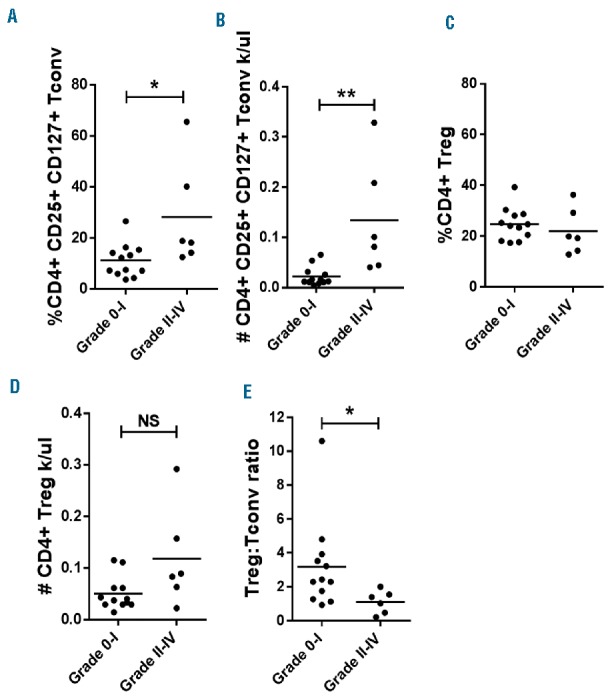
CD4+ CD25+ CD127+ conventional T cells (Tconv) are increased among interleukin-2/sirolimus/tacrolimus (IL-2/SIR/TAC) recipients who develop grade II-IV acute graft-versus-host disease (GvHD). (A and B) Mean amount of CD4+ CD25+ CD127+ Tconv (% and absolute #, ±SE) at day +30 among those who did or did not develop grade II-IV acute GvHD by day +100 (Mann-Whitney test). (C and D) Mean amount of CD4+ CD25+ CD127− Treg (% and absolute #) at day +30 among those who did or did not develop grade II-IV acute GvHD by day +100 (Mann-Whitney test). (E) Mean ratio (±SE) of regulatory T cell (Treg):Tconv at day +30 among those who did or did not develop grade II-IV acute GvHD by day +100 (Mann-Whitney test). *P<0.05, **P=0.001–0.01. NS: not significant.
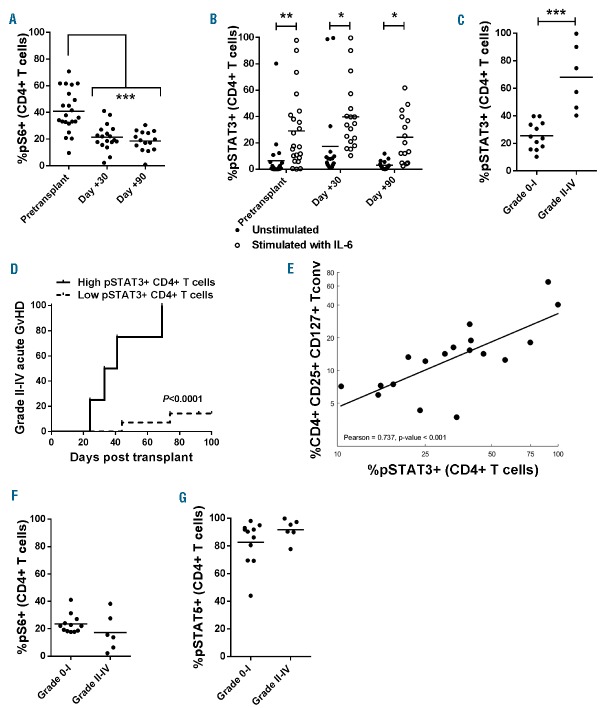
STAT3 phosphorylation correlates with Tconv reconstitution and is not controlled by IL-2/SIR/TAC
As expected, IL-2/SIR/TAC suppressed mTOR signal transduction30 as indicated by a decrease in downstream protein S6 phosphorylation among CD4+ T cells (Figure 4A). Work from our lab and others suggested that STAT5 polarization of donor T cells with IL-2 could reciprocally impair STAT3 phosphorylation which is implicated in acute GvHD pathogenesis.16,31 Conversely, IL-2/SIR/TAC failed to abolish IL-6 signal transduction in circulating CD4+ T cells (Figure 4B). Moreover, the frequency of pSTAT3+ CD4+ T cells at day +30 was significantly increased among IL-2/SIR/TAC patients who developed acute GvHD (Figure 4C). This confirms our earlier observation among patients who predominately received SIR/TAC prophylaxis, which showed that increased pSTAT3+ CD4+ T cells was significantly associated with acute GvHD risk.4 Therefore, adding IL-2 to SIR/TAC did not mitigate this liability as hypothesized. The incidence of grade II-IV acute GvHD was significantly greater among IL-2/SIR/TAC patients with increased pSTAT3+ CD4+ T cells (>48% pSTAT3+) at day +30 (Figure 4D), substantiating our previously determined cut-off point. Patients with elevated pSTAT3+ T cells also developed acute GvHD 20 days earlier than those with less (Figure 4D). Here we observed a strong positive correlation between the amount of pSTAT3+ CD4+ T cells and CD4+ CD25+ CD127+ Tconv reconstitution at day +30 (Figure 3E). The proportion of pS6+ or pSTAT5+ CD4+ T cells at day +30 was similar among those who did and those that did not develop acute GvHD (Figure 4F and G).
Figure 4.
Interleukin-2/sirolimus/tacrolimus (IL-2/SIR/TAC) suppresses mTOR signal transduction but not STAT3 phosphorylation in CD4+ T cells. (A and B) Mean %pS6+ (mTOR signal transduction) and %pSTAT3+ CD4+ T cells [without (closed circle) or with (open circle) pulse of IL-6 for 15 minutes)] at pre-transplant, day +30, and day +90 after allogeneic hematopoietic stem cell transplantation (HCT) (ANOVA). (C) Mean amount of %pSTAT3+ CD4+ T cells on day +30 is significantly increased among patients who develop grade II-IV acute graft-versus-host disease (GvHD) by day +100 (Mann-Whitney test). (D) The previously identified cut-off point of 48% pSTAT3+ CD4+ T cells on day +30 significantly stratified acute GvHD incidence among those on IL-2/SIR/TAC (P<0.0001). The Gray method was used to evaluate the difference in incidence rates between the 2 groups. (E) Correlation between %pSTAT3+ CD4+ T cells versus %CD4+ CD25+ CD127+ conventional T cells. Log-Transformation was performed per the normality assumption test. Pearson correlation coefficient and P-value are shown. (F and G) Percentage of pS6+ and pSTAT5+ CD4+ T cells at day +30 was similar regardless of acute GvHD diagnosis by day +100. *P<0.05, **P=0.001–0.01, ***P=0.0001–0.001.
Discussion
This phase II trial demonstrates that low-dose IL-2 added to SIR/TAC significantly increases Treg reconstitution early after allogenic HCT compared to SIR/TAC alone6 (Day +30% Treg 23.8% vs. 16.0%, P=0.0016; #Treg 0.052 k/uL vs. 0.037 k/uL, P=0.0163). Replacing broadly suppressive MTX with SIR appears to be beneficial in this regard as the reported Treg frequency at day +30 with IL-2/MTX/TAC was 11.1%.11 Despite early Treg reconstitution, the cumulative incidence of grade II-IV acute GvHD with IL-2/SIR/TAC was similar to our published data with SIR/TAC alone.6 Activated CD4+ Tconv from IL-2/SIR/TAC patients were significantly increased at day +30 among those who developed acute GvHD. The proportion of Treg to activated Tconv at day +30 was also significantly higher among patients who remained free of acute GvHD. Adding IL-2 to SIR/TAC largely eliminated the activated Tconv by day +90 compared to SIR/TAC, though this presumably occurred too late to be useful in controlling acute GvHD.
Confirming our prior findings,4 the frequency of IL-6 activated pSTAT3+ CD4+ T cells at day +30 were significantly increased among those who developed grade II-IV acute GvHD. Moreover, we identified a strong correlation between the amount of pSTAT3+ CD4+ T cells and activated Tconv early after HCT. We previously demonstrated that polarizing the ratio of pSTAT5 to pSTAT3 in CD4+ T cells favored Treg expansion and reciprocally impaired Tconv.16 In that work, a direct STAT3 inhibitor was used to shift STAT5 over STAT3 activity.16 Here we tested the alternative hypothesis that supplementing IL-2 could similarly promote STAT5 phosphorylation in donor T cells, with STAT3 otherwise unchecked. Though IL-2 increases the amount of pSTAT5+ CD4+ T cells and Tregs at day +30, IL-2 does not reciprocally prevent T-cell STAT3 phosphorylation.
The early Treg expansion with IL-2/SIR/TAC was short-lived and significantly declined by day +90 despite ongoing cytokine therapy. Understanding that this trial was not powered for acute GvHD prevention, we postulate that the lack of clinical efficacy was greatly influenced by the profound loss of Tregs. In the setting of chronic GvHD treatment, Treg proliferation peaked after four weeks of IL-2 then waned while cytokine therapy continued.9,10,29 While Treg expansion in response to extended IL-2 therapy for chronic GvHD diminished over time, the amount of Tregs after two years of treatment was still greater than baseline, pre-IL-2 levels.29 Conversely, the contraction of Tregs early after allogeneic HCT with low-dose IL-2 and SIR/TAC observed during our study was unexpected and long lasting. It has recently been shown that soluble IL-2 receptor is released into the periphery when low-dose IL-2 is administered for GvHD therapy.29 Moreover, the decline in Tregs while receiving IL-2 coincides with the rise of soluble IL-2 receptor in the plasma of these patients.29 The binding affinity of soluble IL-2 receptor is approximately 10-fold less than its membrane-bound form,32 but it can significantly reduce the biological activity of IL-2.33 While we did not measure soluble IL-2 receptor levels during this trial, we hypothesize that IL-2 sequestration by soluble IL-2 receptor greatly contributed to progressive Treg loss. This is further supported by data showing soluble IL-2 receptor is spontaneously shed immediately after allogeneic HCT.34 Therefore, administering low-dose IL-2 early after transplant may intensify plasma concentrations of soluble IL-2 receptor and exacerbate Treg contraction. Therefore, the lack of quantified soluble IL-2 receptor levels during our clinical trial is an important limitation.
Though we surmise that the loss of Tregs on IL-2/SIR/TAC was mediated by soluble IL-2 receptor, alternative mechanisms were considered and investigated. We verified that T-cell IL-2 receptor signal transduction remained intact even while the Treg numbers declined. We also confirmed that IL-2 protects Tregs from apoptosis (Online Supplementary Figure S3A),10 and therefore activation-induced cell death is an unlikely explanation for the reduced Tregs. While anti-aldesleukin antibodies can occur among IL-2-treated patients, the incidence of neutralizing antibodies is rare.35,36 Moreover, the STAT5 phosphorylation experiments were performed in serum-free culture conditions in vitro and this approach mitigates any potential interference from neutralizing antibodies. The primary mechanism driving contraction of Tregs with IL-2/SIR/TAC remains to be determined; we speculate that the reduced Treg responsiveness to IL-2 may be explained by peripheral cytokine sequestration via soluble receptor rather than impaired receptor signal transduction, antibody-mediated neutralization, or Treg apoptosis.
While fleeting, it is noteworthy that IL-2/SIR/TAC provided early and robust Treg engraftment. Strategies to overcome inhibitory effects by soluble IL-2 receptor are feasible, and may facilitate durable Treg responses to IL-2. In non-human primates, an IgG-IL-2 fusion protein demonstrated enhanced half-life and bioavailability compared to recombinant IL-2.37 The fusion protein also induced sustained Treg proliferation, 4-fold greater than recombinant IL-2.37 Chimeric IL-2/caspase-3 molecules also eliminate alloreactive T cells,38 a source of soluble IL-2 receptor,39 and promotes Treg expansion in vivo.38 The duration and frequency of IL-2 dosing may be optimized to mitigate soluble IL-2 receptor shedding after allogeneic HCT.29 For example, the approach successfully demonstrated in rodents used a very brief course of IL-2 (essentially 3–7 days) with SIR.14 This restricted exposure of IL-2 with SIR significantly expanded a durable population of Tregs and reduced GvHD mortality.14 Though limited by small numbers, IL-2/SIR/TAC patients who prematurely discontinued IL-2 before day +90 (n=6) actually showed a trend toward less acute GvHD compared to protocol-adherent individuals (n=14) [16.7% (95%CI:0.001–77.7) vs. 50% (95%CI: 27.8–77.1), P=0.1475]. We chose to use intermittent injections of low-dose IL-2 based on the favorable pediatric experience in GvHD prophylaxis.11 Besides the concern for IL-2 sequestration, Treg longevity in adult allogeneic HCT recipients may conversely benefit from more consistent IL-2 dosing strategies, such as daily administration or continuous infusion of the cytokine.
Our data support the view that IL-2/SIR/TAC can transiently increase IL-2 signal transduction and early Treg reconstitution after allogeneic HCT. However, IL-2/SIR/TAC does not mitigate STAT3-mediated GvHD and its impact on Treg expansion is not durable. We propose that strategies to optimize IL-2 dosing and allay cytokine neutralization by soluble IL-2 receptor may facilitate lasting Treg recovery. However, such an approach must still consider the impact of unconstrained STAT3 activity even when IL-2 is replenished post transplant.
Supplementary Material
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/5/948
Funding
This work was supported by the Miles for Moffitt Milestone Award (BCB). We thank Prometheus Labs for providing aldesleukin. The Moffitt Cancer Center Flow Cytometry and Biostatistics Cores (P30-CA076292) contributed to the completion of this trial.
References
- 1.Zeiser R, Leveson-Gower DB, Zambricki EA, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111(1):453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203(7):1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zorn E, Nelson EA, Mohseni M, et al. IL-2 regulates FOXP3 expression in human CD4(+)CD25(+) regulatory T cells through a STAT-dependent mechanism and induces the expansion of these cells in vivo. Blood. 2006;108(5):1571–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Betts BC, Sagatys EM, Veerapathran A, et al. CD4+ T cell STAT3 phosphorylation precedes acute GVHD, and subsequent Th17 tissue invasion correlates with GVHD severity and therapeutic response. J Leukoc Biol. 2015;97(4):807–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alho AC, Kim HT, Chammas MJ, et al. Unbalanced recovery of regulatory and effector T cells after allogeneic stem cell transplantation contributes to chronic GVHD. Blood. 2016;127(5):646–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pidala J, Kim J, Jim H, et al. A randomized phase II study to evaluate tacrolimus in combination with sirolimus or methotrexate after allogeneic hematopoietic cell transplantation. Haematologica. 2012;97(12):1882–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs. tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124(8):1372–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zorn E, Mohseni M, Kim H, et al. Combined CD4+ donor lymphocyte infusion and low-dose recombinant IL-2 expand FOXP3+ regulatory T cells following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2009;15(3):382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koreth J, Matsuoka K, Kim HT, et al. Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med. 2011;365(22):2055–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuoka K, Koreth J, Kim HT, et al. Low-dose interleukin-2 therapy restores regulatory T cell homeostasis in patients with chronic graft-versus-host disease. Sci Transl Med. 2013;5(179):179ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kennedy-Nasser AA, Ku S, Castillo-Caro P, et al. Ultra low-dose IL-2 for GVHD prophylaxis after allogeneic hematopoietic stem cell transplantation mediates expansion of regulatory T cells without diminishing antiviral and antileukemic activity. Clin Cancer Res. 2014;20(8):2215–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scotta C, Fanelli G, Hoong SJ, et al. Impact of immunosuppressive drugs on the therapeutic efficacy of ex vivo expanded human regulatory T cells. Haematologica. 2016; 101(1):91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim DG, Koo SK, Park YH, et al. Impact of immunosuppressants on the therapeutic efficacy of in vitro-expanded CD4+CD25+Foxp3+ regulatory T cells in allotransplantation. Transplantation. 2010; 89(8):928–936. [DOI] [PubMed] [Google Scholar]
- 14.Shin HJ, Baker J, Leveson-Gower DB, Smith AT, Sega EI, Negrin RS. Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4+CD25+Foxp3+ regulatory T cells. Blood. 2011;118(8):2342–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnston L, Florek M, Armstrong R, et al. Sirolimus and mycophenolate mofetil as GVHD prophylaxis in myeloablative, matched-related donor hematopoietic cell transplantation. Bone Marrow Transplant. 2012;47(4):581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Betts BC, Veerapathran A, Pidala J, Yu XZ, Anasetti C. STAT5 polarization promotes iTregs and suppresses human T-cell alloresponses while preserving CTL capacity. J Leukoc Biol. 2014;95(2):205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho VT, Cutler C, Carter S, et al. Blood and marrow transplant clinical trials network toxicity committee consensus summary: thrombotic microangiopathy after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2005;11(8):571–575. [DOI] [PubMed] [Google Scholar]
- 18.McDonald GB, Hinds MS, Fisher LD, et al. Veno-occlusive disease of the liver and multiorgan failure after bone marrow transplantation: a cohort study of 355 patients. Ann Intern Med. 1993;118(4):255–267. [DOI] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 20.Pavletic SZ, Lee SJ, Socie G, Vogelsang G. Chronic graft-versus-host disease: implications of the National Institutes of Health consensus development project on criteria for clinical trials. Bone Marrow Transplant. 2006;38(10):645–651. [DOI] [PubMed] [Google Scholar]
- 21.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203(7):1701–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heninger AK, Theil A, Wilhelm C, et al. IL-7 abrogates suppressive activity of human CD4+CD25+FOXP3+ regulatory T cells and allows expansion of alloreactive and autore-active T cells. J Immunol. 2012;189(12): 5649–5658. [DOI] [PubMed] [Google Scholar]
- 23.Touil S, Rosenzwajg M, Landau DA, et al. Depletion of T regulatory cells through selection of CD127-positive cells results in a population enriched in memory T cells: implications for anti-tumor cell therapy. Haematologica. 2012;97(11):1678–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samarasinghe S, Mancao C, Pule M, et al. Functional characterization of alloreactive T cells identifies CD25 and CD71 as optimal targets for a clinically applicable allodepletion strategy. Blood. 2010;115(2):396–407. [DOI] [PubMed] [Google Scholar]
- 25.Krutzik PO, Hale MB, Nolan GP. Characterization of the murine immunological signaling network with phosphospecific flow cytometry. J Immunol. 2005;175(4): 2366–2373. [DOI] [PubMed] [Google Scholar]
- 26.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 27.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123(23):3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorror M, Maris M, Baron F, et al. A modified hematopoietic cell transplantation (HCT)-specific-comorbidity index. Blood. 2004;104(11):324a–325a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koreth J, Kim HT, Jones KT, et al. Efficacy, durability, and response predictors of low-dose interleukin-2 therapy for chronic graft-versus-host disease. Blood. 2016;128(1):130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. J Biol Chem. 1998;273(23): 14484–14494. [DOI] [PubMed] [Google Scholar]
- 31.Yang XP, Ghoreschi K, Steward-Tharp SM, et al. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12(3):247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baran D, Korner M, Theze J. Characterization of the soluble murine IL-2R and estimation of its affinity for IL-2. J Immunol. 1988;141(2):539–546. [PubMed] [Google Scholar]
- 33.Jacques Y, Le Mauff B, Boeffard F, Godard A, Soulillou JP. A soluble interleukin 2 receptor produced by a normal alloreactive human T cell clone binds interleukin 2 with low affinity. J Immunol. 1987;139(7): 2308–2316. [PubMed] [Google Scholar]
- 34.Foley R, Couban S, Walker I, et al. Monitoring soluble interleukin-2 receptor levels in related and unrelated donor allogenic bone marrow transplantation. Bone Marrow Transplant. 1998;21(8):769–773. [DOI] [PubMed] [Google Scholar]
- 35.Allegretta M, Atkins MB, Dempsey RA, et al. The development of anti-interleukin-2 antibodies in patients treated with recombinant human interleukin-2 (IL-2). J Clin Immunol. 1986;6(6):481–490. [DOI] [PubMed] [Google Scholar]
- 36.Granelli-Piperno A, Andrus L, Reich E. Antibodies to interleukin 2. Effects on immune responses in vitro and in vivo. J Exp Med. 1984;160(3):738–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bell CJ, Sun Y, Nowak UM, et al. Sustained in vivo signaling by long-lived IL-2 induces prolonged increases of regulatory T cells. J Autoimmun. 2015;5666–5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yarkoni S, Prigozhina TB, Slavin S, Askenasy N. IL-2-targeted therapy ameliorates the severity of graft-versus-host disease: ex vivo selective depletion of host-reactive T cells and in vivo therapy. Biol Blood Marrow Transplant. 2012;18(4):523–535. [DOI] [PubMed] [Google Scholar]
- 39.Rubin LA, Kurman CC, Fritz ME, et al. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985;135(5):3172–3177. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.