Abstract
A cute graft-versus-host disease remains a major threat to a successful outcome after allogeneic hematopoietic cell transplantation. While improvements in treatment and supportive care have occurred, it is unknown whether these advances have resulted in improved outcome specifically among those diagnosed with acute graft-versus-host disease. We examined outcome following diagnosis of grade II-IV acute graft-versus-host disease according to time period, and explored effects according to original graft-versus-host disease prophylaxis regimen and maximum overall grade of acute graft-versus-host disease. Between 1999 and 2012, 2,905 patients with acute myeloid leukemia (56%), acute lymphoblastic leukemia (30%) or myelodysplastic syndromes (14%) received a sibling (24%) or unrelated donor (76%) blood (66%) or marrow (34%) transplant and developed grade II-IV acute graft-versus-host disease (n=497 for 1999–2001, n=962 for 2002–2005, n=1,446 for 2006–2010). The median (range) follow-up was 144 (4–174), 97 (4–147) and 60 (8–99) months for 1999–2001, 2002–2005, and 2006–2010, respectively. Among the cohort with grade II-IV acute graft-versus-host disease, there was a decrease in the proportion of grade III-IV disease over time with 56%, 47%, and 37% for 1999–2001, 2002–2005, and 2006–2012, respectively (P<0.001). Considering the total study population, univariate analysis demonstrated significant improvements in overall survival and treatment-related mortality over time, and deaths from organ failure and infection declined. On multivariate analysis, significant improvements in overall survival (P=0.003) and treatment-related mortality (P=0.008) were only noted among those originally treated with tacrolimus-based graft-versus-host disease prophylaxis, and these effects were most apparent among those with overall grade II acute graft-versus-host disease. In conclusion, survival has improved over time for tacrolimus-treated transplant recipients with acute graft-versus-host disease.
Introduction
Graft-versus-host disease (GvHD) remains one of the most significant barriers to the success of allogeneic hematopoietic stem cell transplantation (HCT). Current pharmacological approaches fail to prevent acute GvHD completely.1–3 Furthermore, durable resolution of acute GvHD following primary steroid-based therapy is infrequent,4–6 and long-term success of systemic immunosuppressive therapies given after steroid therapy is elusive.7 Consequently, acute GvHD and its allied infectious complications and organ failure contribute greatly to early mortality after HCT. However, advances in supportive care over time, in particular improvements in the coverage and potency of antimicrobial agents (e.g. antibacterial, antiviral, and antifungal drugs), hold promise to improve survival for those patients affected by acute GvHD.
Previous large analyses have demonstrated improvement in survival outcome over time for HCT recipients, despite evolution in overall practices including increasing HCT recipient age, use of unrelated donors, and peripheral blood stem cells as the predominant graft type.8 More recent transplants have been shown to have improved overall mortality, as well as reducing grade III-IV acute GvHD, major organ injury, and life-threatening infections through the early post-HCT period.9 Further insight is needed, however, as studies have not examined change in survival outcome over time exclusively in an acute GvHD-affected population, survival outcome according to maximal acute GvHD grade, or effects according to specific GvHD prophylaxis. Additionally, the studies have not examined outcomes of HCT procedures conducted after 2007.
Accordingly, we conducted a large registry analysis to determine whether survival outcome after a diagnosis of acute GvHD has improved significantly over time.
Methods
Data source
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a voluntary working group of more than 450 transplant centers worldwide that contribute detailed data on consecutive allogeneic HCT to the Statistical Center at the Medical College of Wisconsin in Milwaukee or the National Marrow Donor Program (NMDP) Coordinating Center in Minneapolis. Approximately two-thirds of all active transplantation centers worldwide report data to the registry. The registry database includes information on 40–45% of all patients who have received an allotransplant since 1970, with annual updates. Compliance is assessed by periodic audits and accuracy of data is ensured by computerized record checks, physician review of submitted data and on-site audits. Observational studies conducted by the CIBMTR are done with a waiver of informed consent and in compliance with Health Insurance Portability and Accountability Act regulations as determined by the Institutional Review Board and Privacy Officer of the Medical College of Wisconsin.
Selection of patients and definitions
The CIBMTR population of patients consisted of first post-myeloablative sibling or unrelated donor, blood or marrow allogeneic HCT recipients with acute myeloid leukemia, acute lymphoblastic leukemia or myelodysplastic syndromes transplanted between 1999 and 2012 who developed grade II-IV acute GvHD within 100 days after HCT. Only those with acute GvHD onset within 100 days after HCT were included, as this analysis is focused on classic acute GvHD, not late acute GvHD. The maximal severity of acute GvHD for each subject was used in all analyses.10 A total of 2,905 eligible cases with complete research data available were identified. This final study population was generated from the total (n=17,244) number of first US allogeneic HCT procedures for acute myeloid leukemia, acute lymphoblastic leukemia and myelodysplastic syndromes from 1999–2012 after exclusion of umbilical cord blood transplants (n=2,748), donor types other than matched sibling or unrelated donors (n=654), in-vivo or ex-vivo T-cell-depleted grafts (n=4,149), reduced intensity or non-myeloablative conditioning regimens (n=2,133), those with grade 0–1 acute GvHD or missing GvHD information (n=4,002), those surviving patients with <100 days follow up (n=18), relapse prior to acute GvHD diagnosis (n=176), development of acute GvHD more than 100 days after HCT (n=88) or within 7 days of HCT (n=113), or other consent or research form related exclusions (n=258). Overall, molecular typing was available for 1,810 cases (83%) with >99.9% concordance between the 8/8 and well-matched and ≤7/8 and partially or mismatched groups.
Study endpoints
The primary endpoint was overall survival (OS), defined as death from any cause. Secondary endpoints considered were the following: disease-free survival (DFS), defined as survival in continuous complete remission; transplant-related mortality (TRM), defined as death in continued remission; malignancy relapse, defined as recurrence of the malignancy for which HCT was performed; and chronic GvHD.11 All endpoints were estimated from the date of acute GvHD onset, as the exact date of maximal grade II-IV acute GvHD was not collected.
Statistical analysis
Included patients were divided into three cohorts based on the year of transplant (1999–2001, 2002–2005, 2006–2012). Univariate analysis compared the outcomes of OS, DFS, TRM, relapse and chronic GvHD incidence between these three cohorts. Multivariate analyses were performed using Cox proportional hazards regression models. All the clinical variables were tested for the affirmation of the proportional hazards assumption. Factors violating the proportional hazards assumption were adjusted through stratification. A stepwise model selection procedure was then used to identify clinical variables that were associated with each particular outcome with a threshold of 0.05 for both entry and stay in the model. Interactions between the main variable ‘Year of transplant’ and the adjusted covariates were tested, and no significant interactions between the ‘Year of transplant’ and the adjusted covariates were detected at the significance level of 0.01 in any of the models. A significant center effect was detected for the outcomes of OS and TRM for patients with grade II-IV acute GvHD only among those treated with cyclosporine A, and adjustment for this effect was performed. Potential covariates included patient’s age (by decades), sex and race (Caucasian, African American, Other), Karnofsky performance status (<90%, ≥90%), time from diagnosis to HCT (<2 weeks, 2 weeks – 1 month, 2 months – 100 days), disease (acute myeloid leukemia, acute lymphoblastic leukemia, myelodysplastic syndromes), disease status at time of HCT (early, intermediate, advanced), donor type and age (HLA-identical sibling, unrelated well-matched 18–32 years, unrelated well-matched 33–49 years, unrelated well-matched 50+ years, unrelated partially or mismatched 18–32 years, unrelated partially or mismatched 33–49 years, unrelated partially or mismatched 50+ years),12 donor-recipient sex match, donor-recipient cytomegalovirus serological status, use of total body irradiation (TBI) in the regimen, graft source (bone marrow, peripheral blood mobilized stem cells), and GvHD prophylaxis (cyclosporine ± others, and tacrolimus ± others). The product limit estimator proposed by Kaplan Meier was used to estimate the median and range of the follow-up time. The probabilities of OS and DFS for all patients were calculated using the Kaplan Meier estimator, with the variance estimated by the Greenwood formula. Cumulative incidence estimates were calculated for other endpoints to account for competing risks. Non-relapse death was a competing risk in the estimation of malignancy relapse, death without chronic GvHD was a competing risk for estimation of chronic GvHD, and relapse was a competing risk for estimation of TRM. SAS version 9.4 (SAS Institute, Cary, NC, USA) was used for all the analyses.
Results
Patients
Between 1999 and 2012, 2,905 un-manipulated blood (66%) or marrow (34%) sibling (24%) or unrelated donor (76%) allograft recipients with acute myeloid leukemia (56%), acute lymphoblastic leukemia (30%) or myelodysplastic syndromes (14%) developed grade II-IV acute GvHD. Conditioning was myeloablative in all cases. Among the 1,759 patients who received TBI-based conditioning, 1326 (75%) received cyclophosphamide/TBI, 210 (12%) received cyclophosphamide/TBI/others, and 153 (9%) received TBI/etoposide. Among the 1,146 patients who did not receive TBI as a part of their conditioning regimen, 691 (60%) received busulfan/cyclophosphamide, 114 (10%) received busulfan/cyclophosphamide/others, and 295 (26%) received busulfan/fludarabine as their regimen. Donor, recipient, and transplant characteristics are summarized in Table 1. The median follow-up for surviving patients was 144 (4–174), 97 (4–147) and 60 (8–99) months for those transplanted in 1999–2001, 2002–2005, and 2006–2012, respectively. Of the 1,077 patients who received cyclosporine-based GvHD prophylaxis, 922 (86%) also received methotrexate while 56 (5%) received mycophenolate mofetil (MMF); 28 of these received both methotrexate and mycophenolate mofetil. The remainder mostly received cyclosporine alone ± steroids (n=87, or 8%). Of the 1,767 patients who received tacrolimus-based GvHD prophylaxis, 1,376 (78%) also received methotrexate while 229 (13%) received mycophenolate mofetil; 63 of these received both methotrexate and mycophenolate mofetil. The remainder mostly received tacrolimus with sirolimus (n=99, or 6%). Other infrequent approaches were seen in both groups, in the order of <1–2% of the total each. Trends in GvHD prophylaxis, in this patient population that developed acute GvHD, favored predominant use of tacrolimus-based approaches in the later time period. When compared to cyclosporine-based GvHD prophylaxis, recipients of tacrolimus-based GvHD prophylaxis were comparable, but were more likely to have had a well-matched unrelated donor across all time periods (P<0.001), and to have received peripheral blood stem cells in recent years (P<0.001) (data not shown).
Table 1.
Transplantation (patient, donor, and transplant) and graft-versus-host disease characteristics.
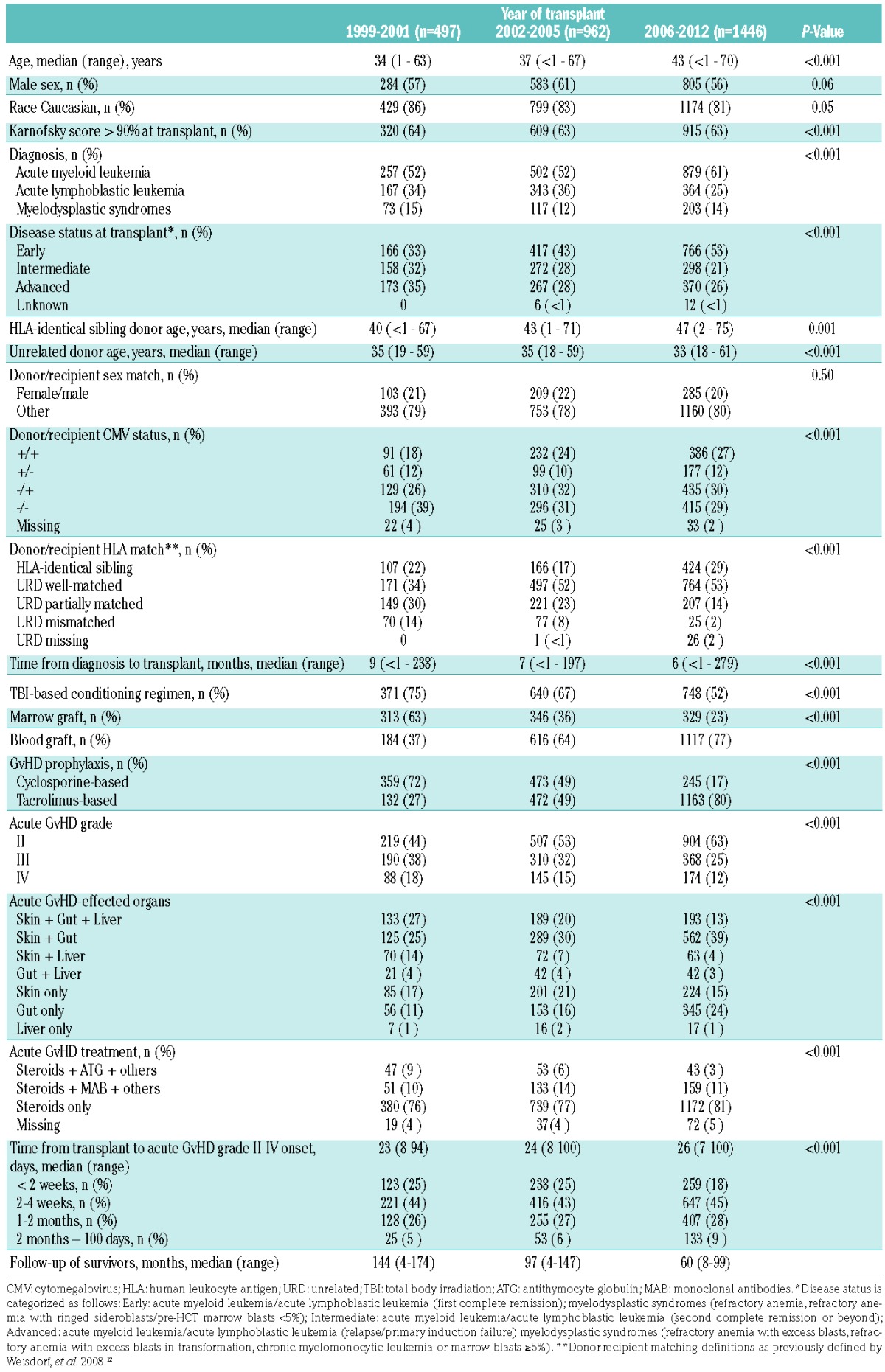
Characteristics of the acute graft-versus-host disease
Overall, the proportion of grade III-IV acute GvHD decreased over time, being 56%, 47%, and 37% for the periods 1999–2001, 2002–2005, and 2006–2012, respectively (P<0.001). Time of onset of acute GvHD remained unchanged over time. The proportion of patients with skin, gut and liver (concurrent 3-organ involvement) acute GvHD decreased (P=0.0014), while gut acute GvHD with or without skin involvement increased with time (P<0.0001 and P<0.0001, respectively). The majority of patients in all time cohorts were treated with steroids only as primary acute GvHD therapy. Approximately 15–20% received antithymocyte globulin or other monoclonal antibodies in addition to corticosteroids (Table 1).
Outcomes
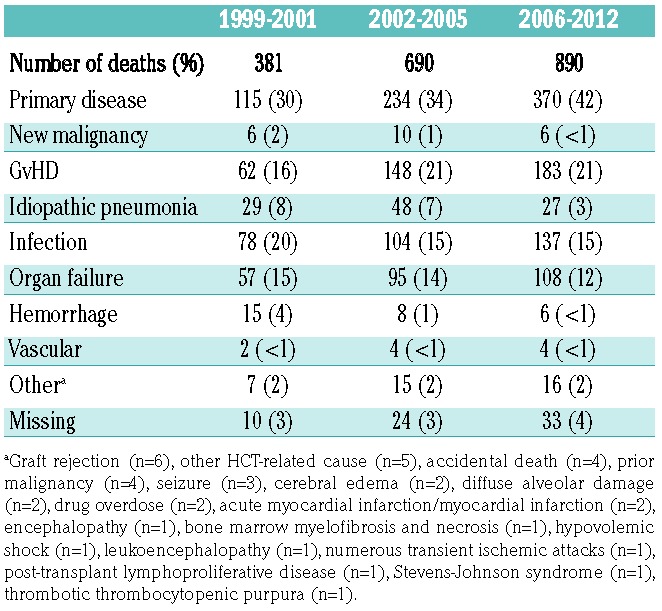
Univariate analyses summarized in Table 2 showed improvements in OS as well as a reduction in TRM at 100 days, 6 months, and 1, 2, and 3 years for patients who developed grade II or grade III-IV acute GvHD. Proportionally, relapse of the primary disease, GvHD/infection and organ failure were the causes of the majority of deaths overall; however, trends over time supported reductions in idiopathic pneumonia, organ failure, hemorrhage, and infectious mortality (Table 3). No significant differences in relapse or chronic GvHD were observed across time period cohorts for those with overall maximal grade II acute GvHD. Alongside a marked reduction in TRM for those with overall maximal grade III-IV acute GvHD over time, relapse incidence increased.
Table 2.
Univariate probabilities of transplant outcomes, among allogeneic transplant recipients who developed acute graft-versus-host disease grade II or grade III-IV.
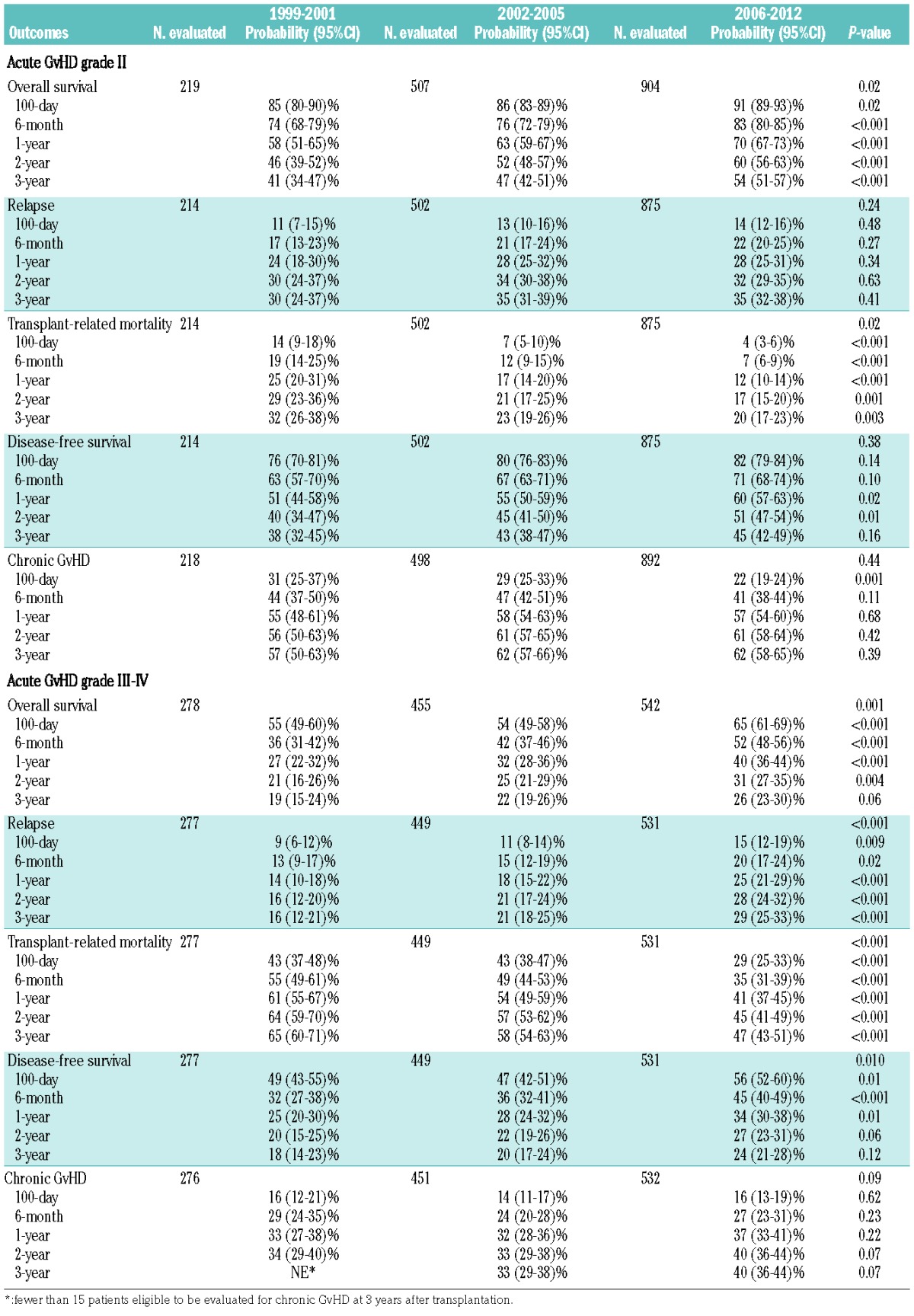
Table 3.
Causes of death.
Prognostic factors
Two major multivariate modeling approaches were implemented to examine the effect of time period on OS and TRM following the diagnosis of acute GvHD. First, the effect of time period on outcome among cases of grade II-IV acute GvHD was examined separately for the tacrolimus-based GvHD prophylaxis group and cyclosporine-based prophylaxis group (Table 4). Reasons for this analysis include a recent trend to use tacrolimus rather than cyclosporine-based acute GvHD prophylaxis. These analyses demonstrated a significant improvement in OS and reduction in TRM with more recent HCT recipients among those who received tacrolimus-based GvHD prophylaxis. In contrast, these effects were not observed among those who received cyclosporine-based GvHD prophylaxis. These conclusions remained in secondary analyses that: (i) re-categorized time period cohorts (1999–2003, 2004–2008, 2009–2012), and (ii) excluded patients with upper gastrointestinal tract acute GvHD.
Table 4.
Multivariate analysis results: effect of time cohort on overall survival and transplant-related mortality.
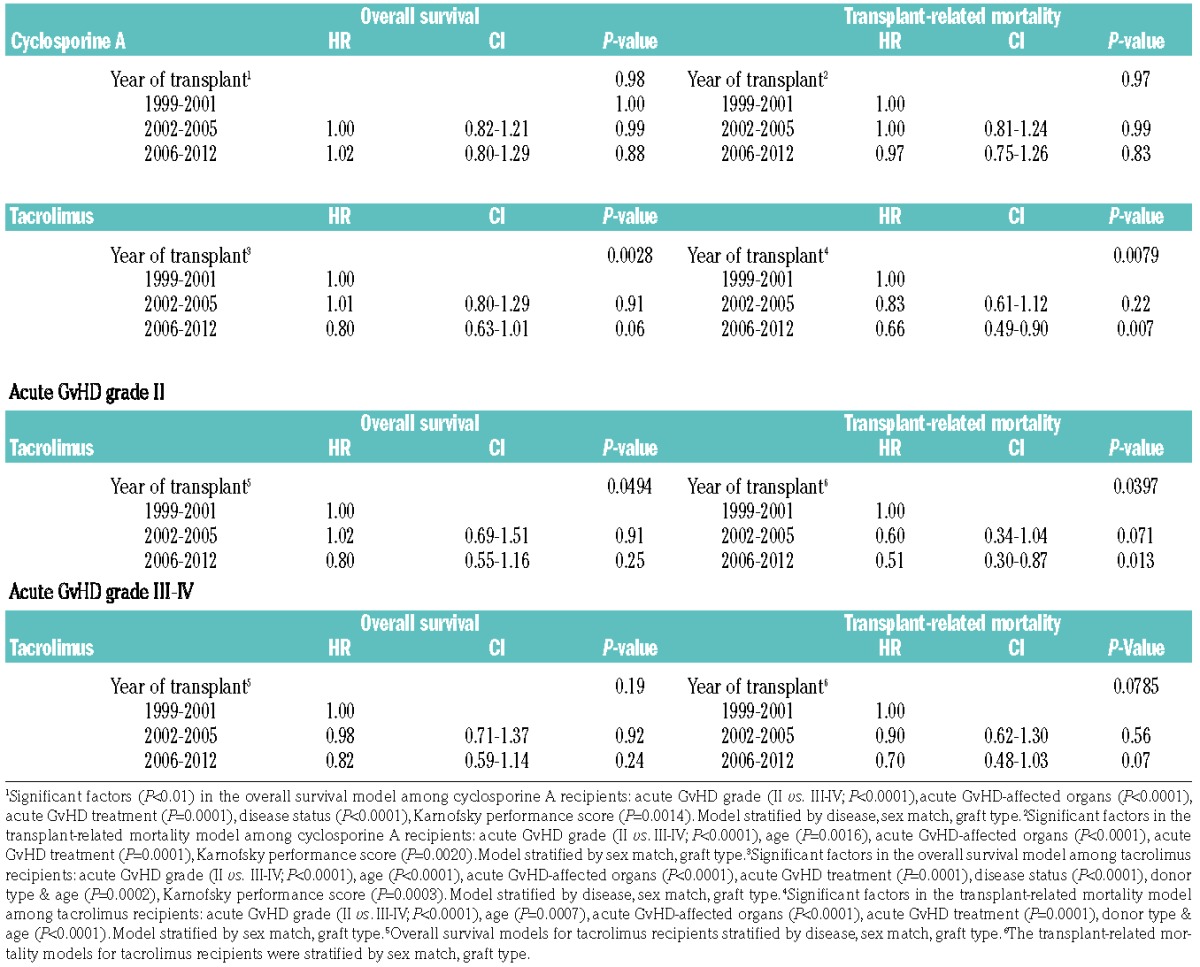
Given the significant impact of overall acute GvHD grade on OS and TRM, a second modeling approach separately examined outcomes for the subgroups with either overall grade II or grade III-IV acute GvHD (Table 4). Similar reductions in hazards for OS and TRM were observed for the tacrolimus-based prophylaxis group in both overall grade II and grade III-IV acute GvHD, however these effects were only significant in the overall grade II group. Adjusted OS and TRM plots for the cyclosporine- and tacrolimus-based GvHD prophylaxis groups separately are presented in Figure 1.
Figure 1.
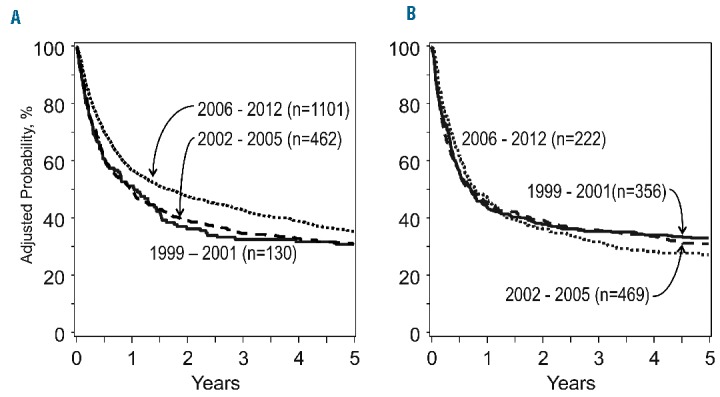
The adjusted probability of overall survival following a diagnosis of grade II-IV acute graft-versus-host disease. Patients treated with tacrolimus-based GVHD prophylaxis are detailed in (A) and those with cyclosporine-based GVHD prophylaxis are detailed in (B).
Figure 2.
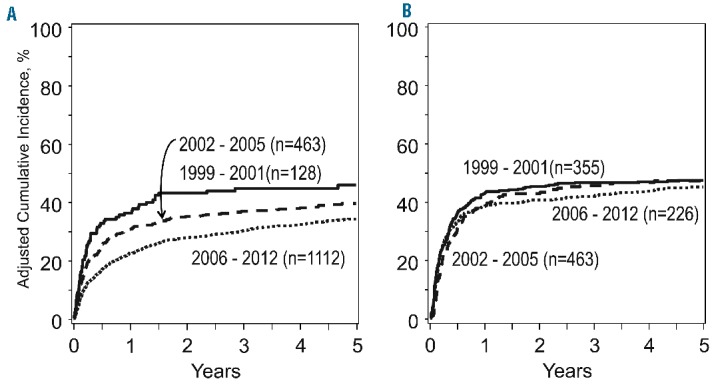
The adjusted probability of transplant-related mortality following a diagnosis of grade II-IV acute graft-versus-host disease. Patients treated with tacrolimus-based GVHD prophylaxis are detailed in (A) and those with cyclosporine-based GVHD prophylaxis are detailed in (B).
Discussion
This analysis provides new insight into trends in acute GvHD severity over time and determinants of mortality after the diagnosis of acute GvHD, and clarifies the relative impact of advances in GvHD outcomes according to overall acute GvHD grade and GvHD prophylaxis groups. We report several key findings. These data demonstrate a shift in maximal grade of acute GvHD over time, such that the relative contribution of grade III-IV disease among those affected by acute GvHD in the period of 2006–2012 is 20% lower than that in 1999–2001 and 10% lower than that in 2002–2005. This reduction in grade III-IV disease is associated with a marked shift in GvHD pro phylaxis delivered over time, with tacrolimus-based approaches comprising 80% of cases in the period 2006–2012. With that, mortality has decreased over time, and deaths from organ failure and infection have decreased. These data provide current benchmarks for further analyses and counseling of patients for expected current HCT outcomes using these approaches.
Multivariate analyses demonstrated significant improvements in OS and TRM over time but, importantly, refined these conclusions further. The effects appear to be limited to those treated with tacrolimus-based GvHD prophylaxis, and most apparent in those with overall grade II acute GvHD. Given these findings, we further examined GvHD characteristics, GvHD therapy, and causes of death among the cyclosporine and tacrolimus groups restricted to overall grade II disease (Online Supplementary Tables S1 and S2). Among grade II cases, the cyclosporine group had greater and more persistent representation of three-organ involvement (skin+liver+gastrointestinal) across the time period cohorts compared to the tacrolimus group. This GvHD phenotype (skin+liver+gastrointestinal) was associated with greater mortality on multivariate analysis. Additionally, re-categorization of the acute GvHD subjects in the cyclosporine and tacrolimus groups according to the refined Minnesota risk classification demonstrated that a greater and relatively fixed proportion of overall grade II acute GvHD subjects in the cyclosporine group had high-risk disease over the respective time period cohorts (Online Supplementary Table S3).13 High-risk patients by this classification have been shown to have inferior treatment response and increased TRM.13,14 Otherwise, we found no major differences in acute GvHD therapy between the cyclosporine and tacrolimus-based prophylaxis groups to explain differential survival outcome. Analysis of cause of death information suggested a decrease in deaths related to infections and organ failure in the tacrolimus group, but not in the cyclosporine group. We acknowledge that prior major randomized trials examining tacrolimus versus cyclosporine (both in combination with methotrexate) have demonstrated no major difference in survival outcome.2,3 This current retrospective analysis differs in that it is exclusively focused on survival estimation from time of acute GvHD onset among those with acute GvHD, and includes patients transplanted in the decade following these major trials.
While these effects were most apparent in overall grade II acute GvHD, we note that similar effects were seen in the grade III-IV GvHD group that had received tacrolimus-based prophylaxis. These findings are further supported by a recent analysis that examined mortality after grade III-IV acute GvHD among 427 patients, in whom significant improvements in OS and TRM were observed in the comparison of a 2007–2012 group versus a 1997–2006 group.15 These findings were predominantly identified in the grade IV acute GvHD subgroup in this analysis. While important differences between the two study populations limit direct comparison, these findings support the concept that HCT outcome has improved for patients with grade III-IV acute GvHD also in the current era.
We note the following limitations to this analysis. First, we conducted analyses based on the reported maximal acute GvHD severity, but survival estimation was done from the time of the onset of the acute GvHD. Next, limitations in data on cyclosporine and tacrolimus therapeutic drug levels, prednisone dose information, and type/extent of second-line therapy make detailed analyses of treatment intensity on outcome not possible. Additionally, detailed acute GvHD treatment response data are not available, so we cannot characterize the burden of steroid-refractory disease across groups. Furthermore, based on the stated inclusion criteria for the analysis, these findings cannot be extended to recipients of reduced intensity conditioning. Finally, insufficient data on antimicrobial agents limit analysis of changes in these practices over time as a potential determinant of improved outcome. In conclusion, this analysis has shown that survival has improved over time in tacrolimus-based acute GvHD prophylaxis HCT recipients who develop acute GvHD.
Supplementary Material
Acknowledgments
CIBMTR Support List
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-15-1-0848 and N00014-16-1-2020 from the Office of Naval Research; and grants from Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas Pharma US; AstraZeneca; Be the Match Foundation; *Bluebird Bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cellular Dynamics International, Inc.; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; *Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Merck & Co, Inc.; Mesoblast; MesoScale Diagnostics, Inc.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; Perkin Elmer, Inc.; Pfizer, Inc; *Sanofi US; *Seattle Genetics; *Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; University of Minnesota; and *Wellpoint, Inc.
The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/102/5/958
Corporate Members
References
- 1.Cutler C, Logan B, Nakamura R, et al. Tacrolimus/sirolimus vs tacrolimus/methotrexate as GVHD prophylaxis after matched, related donor allogeneic HCT. Blood. 2014;124(8):1372–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nash RA, Antin JH, Karanes C, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062–2068. [PubMed] [Google Scholar]
- 3.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92(7):2303–2314. [PubMed] [Google Scholar]
- 4.Alousi AM, Weisdorf DJ, Logan BR, et al. Etanercept, mycophenolate, denileukin, or pentostatin plus corticosteroids for acute graft-versus-host disease: a randomized phase 2 trial from the Blood and Marrow Transplant Clinical Trials Network. Blood. 2009;114(3):511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine JE, Logan B, Wu J, et al. Graft-versus-host disease treatment: predictors of survival. Biol Blood Marrow Transplant. 2010;16(12):1693–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bolanos-Meade J, Logan BR, Alousi AM, et al. Phase 3 clinical trial of steroids/mycophenolate mofetil vs steroids/placebo as therapy for acute GVHD: BMT CTN 0802. Blood. 2014;124(22):3221–3227;quiz 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pidala J, Anasetti C. Glucocorticoid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant. 2010;16(11): 1504–1518. [DOI] [PubMed] [Google Scholar]
- 8.Hahn T, McCarthy PL, Jr, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013;31(19):2437–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363(22):2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15(6):825–828. [PubMed] [Google Scholar]
- 11.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. [DOI] [PubMed] [Google Scholar]
- 12.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14(7):748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacMillan ML, Robin M, Harris AC, et al. A refined risk score for acute graft-versus-host disease that predicts response to initial therapy, survival, and transplant-related mortality. Biol Blood Marrow Transplant. 2015;21(4):761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacMillan ML, DeFor TE, Weisdorf DJ. What predicts high risk acute graft-versus-host disease (GVHD) at onset?: identification of those at highest risk by a novel acute GVHD risk score. Br J Haematol. 2012;157(6):732–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Jawahri A, Li S, Antin JH, et al. Improved treatment-related mortality and overall survival of patients with grade IV acute GVHD in the modern years. Biol Blood Marrow Transplant. 2016;22(5):910–918. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


