Sickle cell disease (SCD) is associated with pulmonary hypertension (PH) which results in high morbidity and mortality.1 There are well-established therapies for pulmonary arterial hypertension (PAH), but few reports about their use in SCDPH.2 We report the clinical course of 11 SCDPH patients on maximal supportive therapy including other pulmonary vasodilators, who received compassionate therapy with prostacyclin-analogs at four PH treatment centers. Inclusion criteria for this retrospective study included PH diagnosis by standard right heart catheterization (RHC), and prostacyclin-analog therapy via any route of delivery for at least four weeks. Data were collected subject to availability in the medical record, including type of prostacyclin-analog therapy, maximal dose and duration, and follow-up data at least 100 days after initiating therapy. Statistical analyses were performed using GraphPad Prism version 5.0 (La Jolla, CA, USA), using paired t-test and Pearson product-moment correlation. P<0.05 was considered significant.
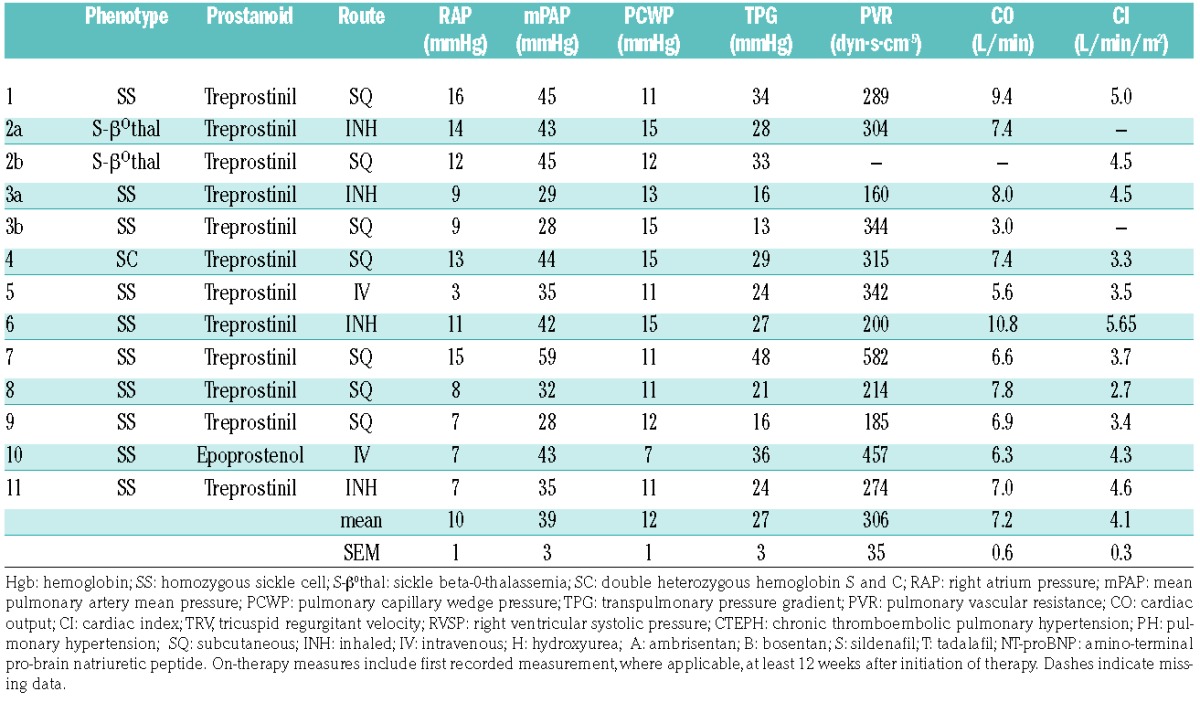
Age, sex, and distribution of hemoglobinopathies of our patients are similar to those of other published cohorts.3,4 The hemodynamic profile reveals elevated right atrial pressures and mean pulmonary arterial pressure (mPAP), with relatively higher cardiac outputs and lesser elevation in pulmonary vascular resistance (PVR), as typically seen in SCDPH. However, notably, the average cardiac output was lower than that of other cohorts, with or without PH.5 All patients in our cohort met risk criteria identified in our recent report (PVR ≥115 dyn·s·cm−5, mPAP ≥28 mmHg and transpulmonary gradient ≥12 mmHg) which are each strongly correlated with increased mortality in SCDPH.6 All patients were on background therapy (oral pulmonary vasodilator) which they had tolerated with clinical improvement. The physicians treating these patients offered them a trial of prostacyclin-analog therapy due to worsening of their clinical condition and RHC evidence of progressive PH.
The results are detailed in Table 1. Two patients received inhaled prostacyclin-analog therapy initially, later transitioning to subcutaneous infusion prostacyclin-analog, yielding 13 courses of prostacyclin therapy in 11 patients. All patients had transpulmonary gradients considerably above 12 mmHg. Most had pulmonary vascular resistance (PVR) values typical of high cardiac output disorders, such as those with severe anemia or large ventriculoseptal defects, who have very low baseline PVR that rises into the “normal” range as mPAP rises. Seven patients were diagnosed with chronic thromboembolic pulmonary hypertension (CTEPH) by computed tomography angiography and/or nuclear medicine ventilation perfusion scans. The remainder had no identifiable risk factor for PH besides SCD. All parenteral therapies were initiated in-patient, with gradual dose escalations (most of the patients received treprostinil 0.5 – 1.25 ng/kg/min every 12 – 24 hours up to an approximate dose of 15 ng/kg/min, and then escalated every 3–6 days by 1.25 ng/kg/min). Maximal doses were identified at a median of 19.2 weeks after initiation of prostacyclin-analog therapy. Median duration of follow up in living patients was 13 months after the initiation of prostacyclin-analog therapy.
Table 1A.
Clinical features prior to initiation of prostacyclin therapy and on therapy.
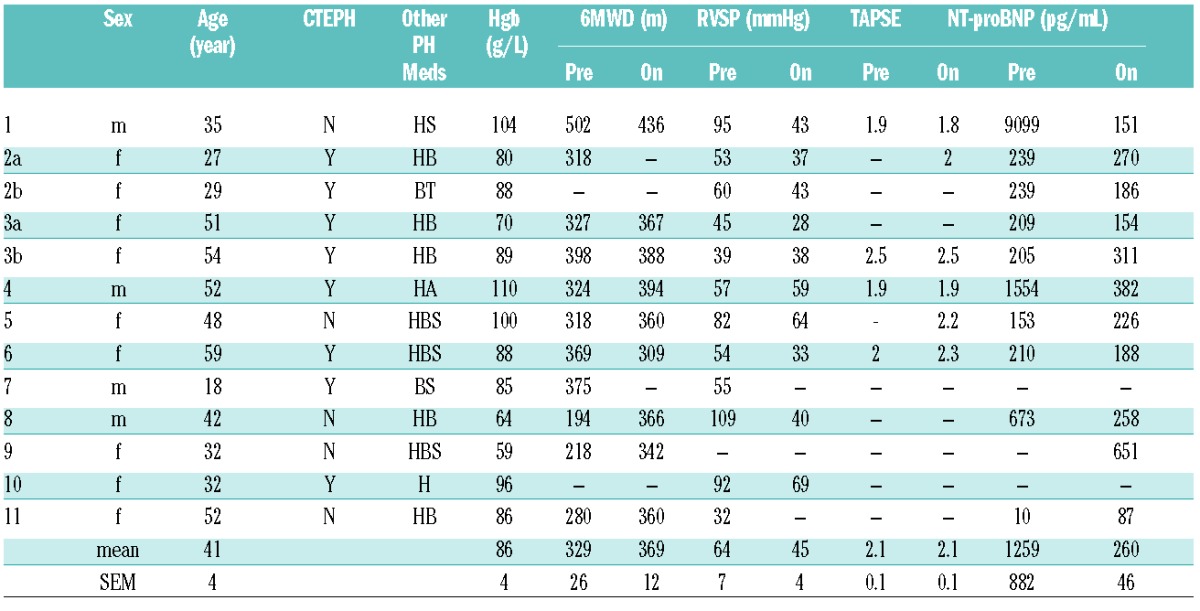
Table 1B.
Cohort hemodynamics prior to initiation of prostacyclin therapy.
In 10 cases in which both pre- and on-therapy data were available, right ventricular systolic pressure (RVSP) decreased significantly from baseline measurements over a median of 20.4 weeks of prostacyclin therapy (mean ± standard error, 69 ± 24 vs. 45 ± 14 mmHg, P= 0.008) (Figure 1A), an average decrease of 35%. In 9 cases in which sufficient data were available, six minute walk test distance (6MWD) trended upward by an average of 20% (39m) over a median of 28.3 weeks (331 ± 93 m vs. 370 ± 35 m, n = 9, P= 0.14) (Figure 1B). There was no discernible difference in 6MWD and RVSP response to prostacyclin-analogs between the PH and CTEPH subgroups.
Figure 1.
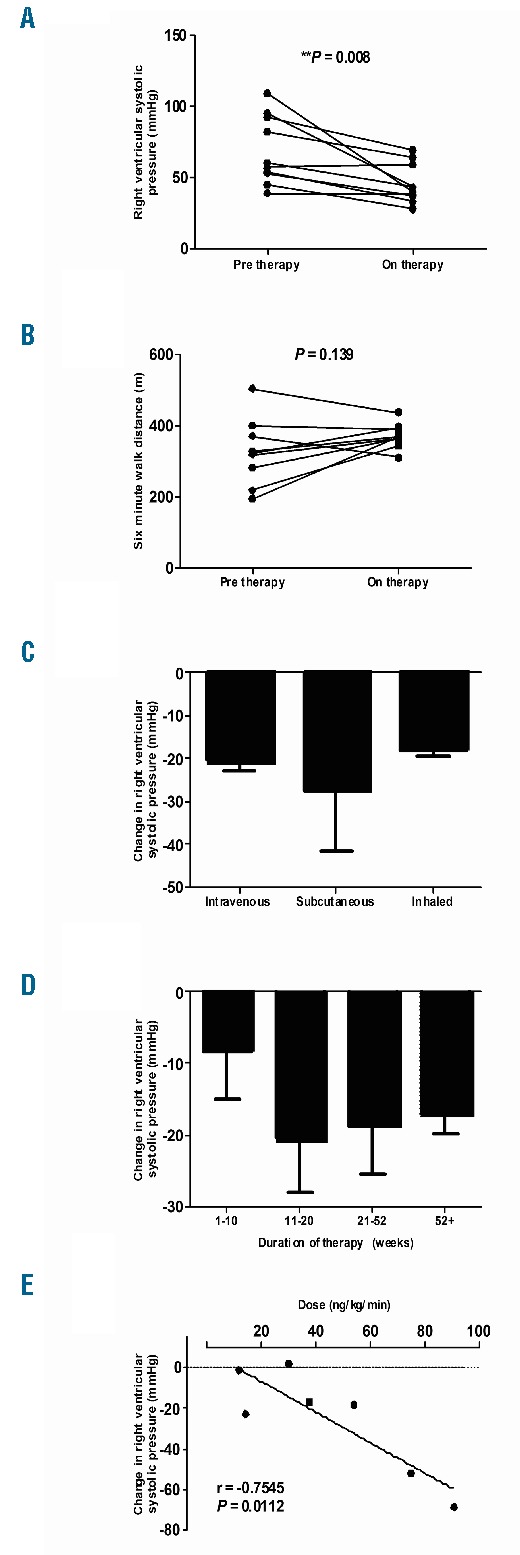
Right ventricular systolic pressures (RVSP) as estimated by echocardiogram (A) and 6-minute walk distances (B) at baseline and on therapy. On therapy, RVSP and 6-minute walk distances include first recorded measurement, where applicable, at least 12 weeks after initiation of therapy (median 20.4 and 28.4 weeks, respectively). Changes in RVSP from baseline are presented by route of drug delivery: intravenous, n=2; subcutaneous, n=5; and inhaled, n=3 (C), by weeks after start of prostanoid therapy (D), and by dose of intravenously and subcutaneously administered therapy (E). Sequential changes in right ventricular systolic pressures by weeks include all recorded values for all patients in our cohort after initiation of therapy, 1–10 weeks, n=5; 11–20 weeks, n=6; 21–52 weeks, n=11; 52+ weeks, n=4. Prostanoid doses collected by closest temporal proximity to first recorded post-therapy measurement, where applicable, at least 12 weeks after start of therapy. *Significance assessed by paired analysis.
Changes in RVSP were most pronounced in patients receiving subcutaneous infusion of prostacyclin-analog, with an average decline of 27 mmHg (n=5), compared with decreases of 21 mmHg (n=2) with intravenous and 18 mmHg (n=3) with inhaled administration (Figure 1C). For all treated patients, RVSP improved modestly in the first ten weeks, and the largest observed response 11–20 weeks after initiating therapy (Figure 1D). RVSP change was less pronounced in patients on therapy for 21–52 weeks or longer. Reduction of RVSP was highly proportional to dose of drug administered by intravenous or SQ routes (r = −0.7545, P=0.01) (Figure 1E).
The 13 courses of prostacyclin-analog initiations were evaluated for complications. Ten patients reported the following: inguinal swelling, headaches, dizziness, pharyngeal symptoms, jaw pain, painful injection sites, bacteremia and line sepsis. Two deaths occurred in the cohort. The first death was of unknown etiology in a patient receiving intravenous prostacyclin-analog therapy for four years. The second death was due to right ventricular failure in a patient receiving subcutaneous therapy for six months.
This is the first report of prostacyclin therapy ever reported in an SCD cohort of this size and addresses an area of controversy concerning management of SCDPH.7,8 Use of prostacyclin-analog therapy is well established in idiopathic PAH, but not necessarily in other forms of PH.9 The modes of delivery and the prostacyclin-analogs were non-homogeneous; nonetheless, this report is helpful for its description of the response and tolerability of this previously undocumented intervention. There is precedence for similar reports.10–12 To date, prostacyclin-analog therapy has not been recommended for SCDPH due to apparent fear of high-cardiac output pulmonary edema, line sepsis, and line thrombosis. The only relevant publication is a brief report describing tolerance of continuous intravenous epoprostenol in one patient for 1.5 years.3
Despite our small number of subjects, we found a significant response in the RVSP. Although follow-up RHC results would be preferable for a literature report,13 we found insufficient follow-up RHC data in our clinical chart review to report. The greatest RVSP response was in the subcutaneous prostacyclin-analog group, although the number of patients is too small to draw definitive conclusions. Improvement in RVSP appeared to peak after the first two months of therapy. RVSP decreased after four months, but was still higher after one year than the initial value. Improvement in RVSP was highly proportionate to the dose delivered, suggesting an important dose-response relationship. This implies a true pharmacological effect in SCDPH and emphasizes the clinical importance of aggressive dose titration, similar to other forms of PH.14
We observed apparent signs of functional improvement. The increment in 6MWD was a statistical trend in this small number of patients, but its magnitude of change despite background therapy seems striking. Our observed median difference of 39m is in line with criteria of the minimal clinically important difference required for prospective controlled trials in PH, established as 33–40m.
Complications were common with prostacyclin-analog therapy, but no greater than expected.6 There were no episodes of high cardiac output pulmonary edema. Two deaths occurred, one due to right ventricular failure. Finally, while the mortality rate in this cohort is high, the mortality among SCDPH patients is already quite high, making it difficult to determine outside a controlled study as to whether the intervention is able to impact the high baseline mortality reported previously. The limitations of this retrospective study are the small number of patients, the heterogeneity of disease and treatment modalities, and the varying time of enrollment.
In this cohort of 11 SCD patients, parenteral, especially subcutaneous, prostacyclin-analog therapy was well tolerated and appeared to be effective. Although more experience is needed, there is no evidence that clinicians should avoid prostacyclin therapy when clinically indicated in patients with SCD and PH.
Supplementary Material
Footnotes
Funding: funding was provided by the National Heart, Lung and Blood Institute Division of Intramural Research (1 ZIA HL006014-04).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Manci EA, Culberson DE, Yang YM, et al. Causes of death in sickle cell disease: an autopsy study. Br J Haematol. 2003;123(2):359–365. [DOI] [PubMed] [Google Scholar]
- 2.Benza RL, Barst RJ, Galie N, et al. Sitaxsentan for the treatment of pulmonary arterial hypertension: a 1-year, prospective, open-label observation of outcome and survival. Chest. 2008;134(4):775–782. [DOI] [PubMed] [Google Scholar]
- 3.Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101(4):1257–1261. [DOI] [PubMed] [Google Scholar]
- 4.Parent F, Bachir D, Inamo J, et al. A hemodynamic study of pulmonary hypertension in sickle cell disease. N Engl J Med. 2011;365(1):44–53. [DOI] [PubMed] [Google Scholar]
- 5.Fonseca GH, Souza R, Salemi VM, Jardim CV, Gualandro SF. Pulmonary hypertension diagnosed by right heart catheterisation in sickle cell disease. Eur Respir J. 2012;39(1):112–118. [DOI] [PubMed] [Google Scholar]
- 6.Mehari A, Alam S, Tian X, et al. Hemodynamic predictors of mortality in adults with sickle cell disease. Am J Respir Crit Care Med. 2013;187(8):840–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simonneau G, Parent F. Pulmonary hypertension in patients with sickle cell disease: not so frequent but so different. Eur Respir J. 2012;39(1):3–4. [DOI] [PubMed] [Google Scholar]
- 8.Smith KA, Kawut SM. Is resistance futile? Am J Respir Crit Care Med. 2013;187(8):790–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barst RJ. Diagnosis and treatment of pulmonary artery hypertension. Curr Opin Pediatr. 1996;8(5):512–519. [DOI] [PubMed] [Google Scholar]
- 10.Jones DK, Higenbottam TW, Wallwork J. Treatment of primary pulmonary hypertension intravenous epoprostenol (prostacyclin). Br Heart J. 1987;57(3):270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnett CF, Bonura EJ, Nathan SD, et al. Treatment of sarcoidosis-associated pulmonary hypertension. A two-center experience. Chest. 2009;135(6):1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Apostolopoulou SC, Manginas A, Cokkinos DV, Rammos S. Effect of the oral endothelin antagonist bosentan on the clinical, exercise, and haemodynamic status of patients with pulmonary arterial hypertension related to congenital heart disease. Heart. 2005;91(11):1447–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher MR, Forfia PR, Chamera E, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179(7):615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Homma S. Dose-dependent reduction in pulmonary vascular resistance with epoprostenol in pulmonary arterial hypertension. Circ J. 2010;74(10):2062–2063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


