Abstract
Purpose
Despite advances in orbital radiotherapy (XRT), a significant proportion of patients develop ophthalmic complication like dry eye syndrome (DES). The study evaluates the prevalence of aqueous deficient DES (ADDE) and lacrimal gland (LG) changes through histologic evaluation and ex-vivo expansion potential postorbital XRT.
Methods
With the approval of the institutional review board, medical records of patients who underwent orbital XRT as management protocol were reviewed for evidence of ADDE using DEWS (Dry Eye Workshop) 2007 criteria (n = 51). HuLG was harvested from three of these patients who underwent subsequent orbital exenteration and used for histological studies/ex-vivo culture.
Results
ADDE was noted in 47.07% of the patients, status postorbital XRT, with a prediction of nearly 50% developing it within 0.5 to 2.9 years. ADDE severity was grade 2 (18%), grade 3 (14%), and grade 4 (17%). Other comorbidities were radiation retinopathy (33.4%), radiation-induced cataract (24.9%), and radiation keratopathy (20.8%). Multivariate and univariate analysis showed that fraction of radiation and dose of radiation/fraction were significant risk factors; male gender and young age were protective factors. The post-XRT exenterated HuLG showed near-total effacement of histoarchitecture with intra/periductal and intra/interlobular fibrosis, loss of acini, and reduced secretory activity. The potential of the LG to expand and grow in culture was impaired with loss of stem cells as compared to normal HuLG.
Conclusion
This study documents that orbital-XRT is associated with morphological and functional loss of lacrimal function in nearly 50% of the patients with a prediction of two-third developing ADDE by the end of 5 years.
Translational Relevance
The study provides objective clinical evidence for DES development due to architectural/functional damage to the LG postorbital XRT. Based on recent findings that the LG can be cultured in-vitro, with preservation of stem cells and secretory potential, it would be logical to harvest a portion of LG before radiation, and expand and transplant it to rescue the damaged gland if indicated.
Keywords: orbital radiotherapy/EBRT, lacrimal gland, dry eye syndrome
Introduction
External beam radiation therapy (EBRT) to the head and neck or the orbit is a part of the established protocol to treat oral and ocular malignancies. The damaging effects of radiation on the ocular tissues occur due to direct damage to the surrounding tissues or if the tissues lie in the path of radiation beam. Even though there are effective shielding techniques available, the fact remains that a large proportion of these patients develop radiation-induced side effects like cataract, retinopathy, and dry eye.1–4
Orbital radiotherapy can cause transient erythema, madarosis, telangiectasia, lymphedema, inflammation, and depletion of goblet cells.4–8 Lacrimal and Meibomian glands also suffer extensive damage leading to alterations in the composition of the tear film. This in turn destabilizes the ocular surface setting up a vicious inflammatory loop causing tear hyperosmolarity and tear film instability, which worsens the condition.9–12
Even though dry eye syndrome (DES) is a known side effect of EBRT, very few studies have documented this association. Parsons et al.13 showed that “the incidence of radiation injury increases the rate of dry eye from 0% reported after doses ≤30 Gy to 100% after doses ≥57 Gy.” The study by Gregoire et al.14 showed that even though the technique of intensity modulated radiation therapy (IMRT) reduces the incidence of DES in patients significantly, it cannot completely alleviate it as 5.1% of the patients still present with severe grade 3 dry eye. Similar results were reported by Goyal et al.15
Bhandare et al.16 reported the incidence of dry eye and lacrimal damage in patients who underwent EBRT for extracranial head and neck tumors. Their results indicate that orbital radiotherapy mainly injures the lacrimal gland, conjunctival goblet cells, accessory lacrimal glands, and Meibomian gland structures with nearly 51% of the patients showing DES in their case series.
Radiotherapy has undergone many advancements and refinements over time with improved shielding techniques to protect the neighboring tissues. Despite these advances, our clinical observation is similar to that made by several others wherein high prevalence of DES is a frequent morbidity seen in patients, status postradiation to head and neck region. The present study is an attempt to fill the existing lacuna by analyzing the clinical features of aqueous deficient DES (ADDE) observed in patients who undergo orbital radiotherapy for ocular malignancies by a retrospective analysis of their medical records. As some of the patients included in the study were advised and underwent exenteration of the orbit following radiotherapy, the study provided a unique opportunity to study the histological changes in the lacrimal gland and its potential for ex-vivo expansion, which constitutes the prospective arm of the study.
Material and Methods
Institutional Review Board Approval
The present study is in accordance with the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board of LV Prasad Eye Institute (LVPEI). Written informed consent was taken from the patients for use of their lacrimal tissue for research.
Evaluating the Prevalence of DES in Patients Post Orbital Radiotherapy
Review of Medical Records
Medical records of 220 patients between January 2002 to January 2012 who were diagnosed as having ocular malignancies like retinoblastoma, lymphoma, and sebaceous gland carcinoma, and were advised EBRT were reviewed. In accordance with the institutional policy, institutional review board approval was taken for the study involving human tissue use. A comprehensive ocular workup of all patients, including slit lamp microscopy, Schirmer's score evaluation, and Rose Bengal staining, was performed as a standard protocol, unless there were valid conflicting reasons not to do so. The inclusion and exclusion criteria for the study were as follows:
Inclusion criteria:
Advised and underwent EBRT as part of management protocol
Complied with follow-up visits
Normal Schirmer's score (>10) pre-radiotherapy
Exclusion criteria:
Brachytherapy
Pre-existing DES before radiotherapy (Schirmer's score <10)
Patients who underwent enucleation post radiotherapy
After reviewing records and excluding the ones with incomplete parameters, 51 eyes of 47 patients were included in the study. All of these patients had received intensity modulated therapeutic doses of radiation in appropriate fractions from a 6MV LINAC.
For controls, data from medical records of 28 subjects with normal eye and the normal contralateral eye of the postorbital radiotherapy patients were included.
Determining Schirmer's Score
Schirmer's test is an accepted test for evaluating dry eye status in individuals. In the present study, Schirmer's I test with anesthesia was employed.
A drop of 1% paracaine was instilled in the eye 2 minutes before the test. Values less than 10 mm were considered as indicative of dry eye. In accordance with the DEWS 2007 criteria, the study population was categorized into various grades of dry eye: grade 1 (Schirmer's score 10–15 mm), grade 2 (Schirmer's score ≤10 mm), grade 3 (Schirmer's score ≤5mm), and grade 4 (Schirmer's score ≤2mm).9
Data Collection
Data were collected from the medical records and include the following:
Demographics
Common chief presenting complaints
Clinical diagnoses
First line of therapy
Radiation dose
EBRT side effects
Dry eye status post-EBRT
Data Analysis
The collected data were analyzed using R (version 2.12) and SPSS software version 19, and the graphs were plotted using the same software (s). Univariate and multivariate analysis of the data was done by Cox regression analysis and the Kaplan-Meier product-limit method was used to estimate freedom from DES/existence of DES. A multivariate Cox-regression model with stepwise elimination using Akaike information criteria (AIC) was used to assess the effect of factors such as age, sex, total dose, fraction, and dose per fraction on freedom from dry eye.
Evaluation of the Human Lacrimal Gland Obtained from Patients, Status Posttherapeutic Orbital Radiotherapy
Human Tissue Source
During the study period, three patients under follow-up underwent exenteration following orbital radiotherapy as part of the treatment protocol. The lacrimal gland tissues from these patients were harvested and used for the study after histological confirmation of being free from malignant pathology.
Lacrimal glands harvested from therapeutic exenteration of patients without any prior orbital radiotherapy, which is part of another ongoing study, were used as controls.
These three postradiated lacrimal gland samples were compared with three normal samples of lacrimal glands. Both the test and control tissues were subjected to histology (n = 2)/ultrastructure studies by transmission electron microscopy (TEM) (n = 1) and establishing in-vitro cultures (n = 3).
Immunophenotyping and Ultrastructure Studies
Immunohistochemistry Study.
The harvested gland was processed for immunohistochemistry (IHC) and studied for tissue architecture and the expression of markers for functional competence like lysozyme, S-100, and also presence of stem cells (CD117). The fresh gland was fixed with 10% fresh formalin and embedded in paraffin. Thin 3-μm sections were taken on silane coated glass slides and used for immunostaining according to the standard protocol of the laboratory. Briefly, the paraffin embedded sections were deparaffinized at 70°C in hot air oven and xylene series. The sections were rehydrated in alcohol series and then in distilled water followed by 1× phosphate buffered saline (PBS). The endogenous peroxidase activity was blocked using methanol and hydrogen peroxide; the antigen retrieval done using Tris-EDTA buffer (pH 9). The sections were blocked with 2.5% bovine serum albumin (BSA), and then incubated with the primary antibody (lysozyme, S-100, and CD117) in a moist chamber for 2 hours at room temperature followed by secondary antibody (polymer HRP) incubation for 30 minutes at room temperature. DAB (3,3′-diaminobenzidine) substrate was added to the section to allow color development for 10 minutes. The sections were then counterstaining with hematoxylin and then mounted in distyrene plasticizer xylene (DPX). The sections were visualized under a light microscope.17
Ultrastructure Study.
The postexenterated human lacrimal gland tissue was immediately fixed in TEM fixative, and ultrastructure studies were performed on these at the Ruska Labs, Agriculture University, Hyderabad. Briefly, the human lacrimal gland was fixed in 2.5% glutaraldehyde in phosphate buffer (0.1 M, pH 7.2) for 3 hours at room temperature, washed with PBS, and postfixed in 1% osmium tetroxide for 3 hours. Fixed specimens were then washed, dehydrated, infiltrated, and embedded in Araldite resin. Ultra-thin sections (50–70 nm) were made using an ultra-microtome (Leica Ultra cut UCT-GA-D/E-1/00), mounted on copper grids, and stained with uranyl acetate and Reynolds lead citrate. Sections were scanned using a transmission electron microscope (JEM-2100, JEOL Ltd., Japan).
Establishing In-Vitro Cultures from Postradiated Tissues
Fresh human lacrimal gland, obtained from exenterated specimens, was washed with Hank's Balanced Salt Solution (HBBS) to remove red blood cells. The gland was processed according to the previously published protocol.17 Briefly, the fresh human lacrimal gland was washed with HBBS to remove red blood cells. The tissue was enzymatically digested using a cocktail of collagenase and hyaluronidase. The isolated cells were plated on Matrigel coated tissue culture plates in HepatoSTIM media supplemented with 10% fetal calf serum (FCS), 5 ng/mL epidermal growth factor (EGF), and 2 mM l-glutamine. On the third day, serum was replaced with N2 and the concentration of EGF increased to 50 ng/mL.
Results
Prevalence of DES in Patients Postorbital Radiotherapy
Demographics
Medical records of 220 patients who were diagnosed with oculo-adnexal malignancies were reviewed. As per the institute policy of referral, all cases that had to receive radiotherapy were referred to a radiation oncologist. Of these, excluding the ones that did not meet the study criteria, the final study population was 51 eyes of 47 patients (43 unilateral and four bilateral cases).
The normal eyes (n = 28) from normal subjects or normal contralateral eye of patients were evaluated in order to generate control data.
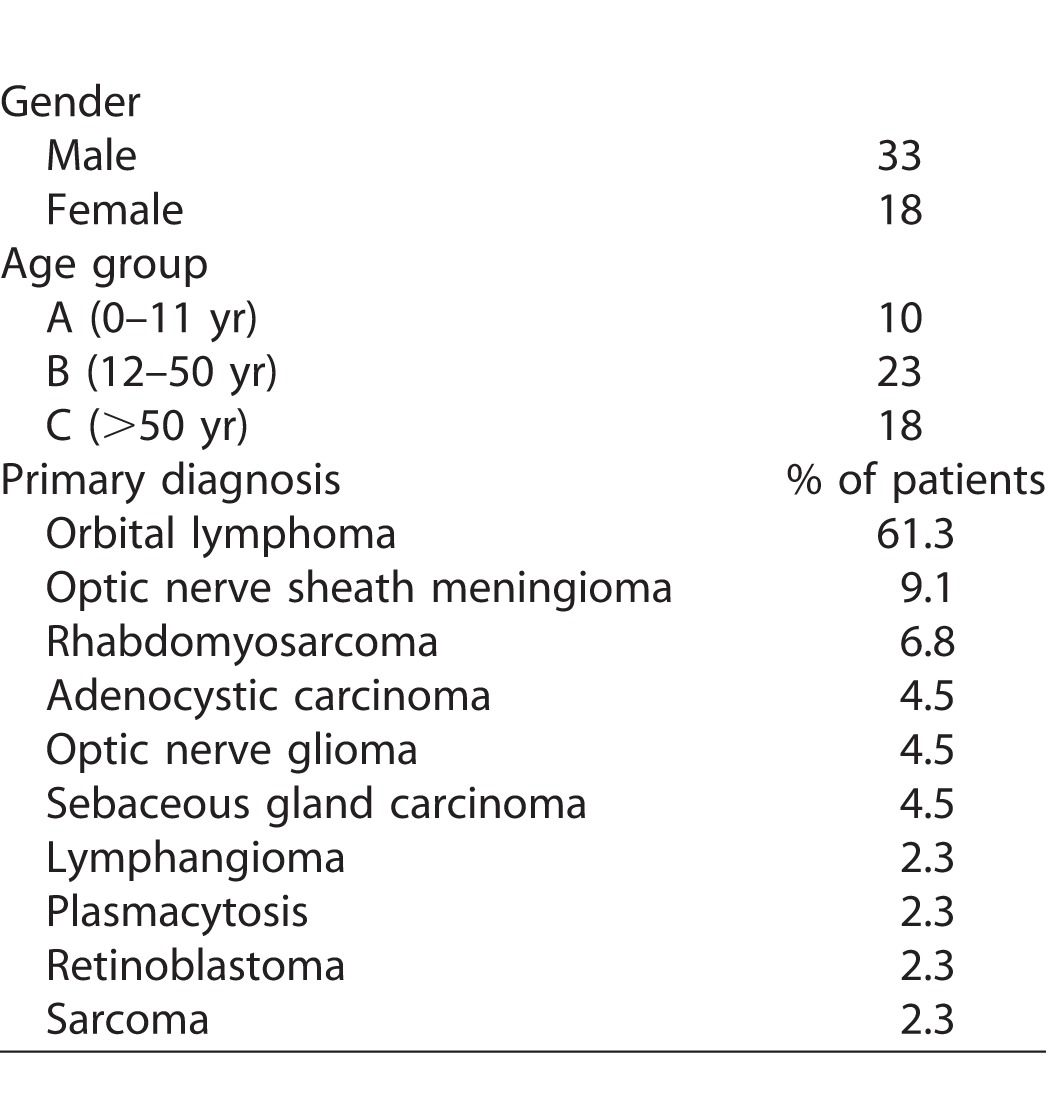
The mean age of the patients was 39.8 years (range, 3–73 years) with a male to female ratio of 33:18 (1.8:1). The patients were divided into three age groups: 0 to 11 years (group A), 12 to 50 years (group B), and >50 years (group C; Table and Supplementary Table S3). The average follow-up time following radiation was 18.46 months (range, 1.17–120.20 months).
Table.
Patients Data: Information Matrix
With regard to their radiotherapy regime, the mean radiation dose was 4899.17 cGy (4000 cGy–6600 cGy); mean fraction being 25.22 (14–37 fractions) while the mean dose per fraction was 196.8 cGy (163 cGy–300 cGy).
The controls (n = 28) were similarly categorized into age groups A, B, and C (Supplementary Table S1). The average age of the control population was 41.39 years (3–73 years) with a male to female ratio of 17:11. The most common ocular malignancies for which the patients underwent EBRT, as well as other patient details, are tabulated in Table.
Dry Eye Status of Controls
The data showed that for the control population, there was 100% survival in group A, 90.9% in the age group B (ADDE in patient at 4.9 months), and 75% in age group C (ADDE in three patients at 6.2 months, 17.2 months, and 47.2 months, respectively) during the study period (Supplementary Figure S1; Supplementary Tables S2 and S3).
Dry Eye Status of Patients
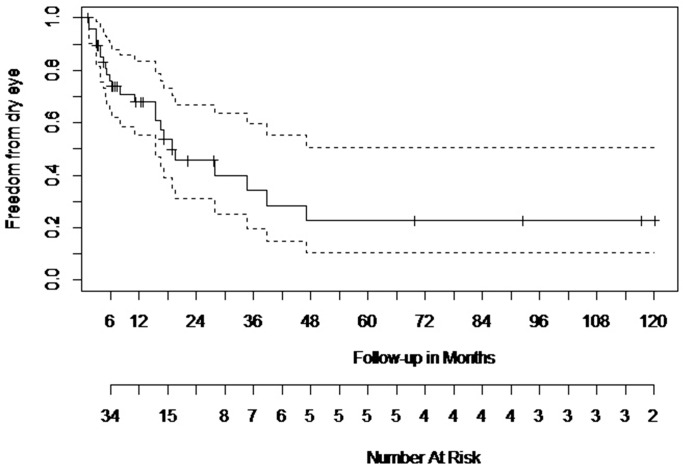
The results of the survival analysis for the patient population indicate that by 6 months post last fraction of radiotherapy, 23.9% of the patients develop ADDE. This percentage increases to 50.2% by the end of 18.8 months and to 77.2% by the end of 47.17 months (3.9 years; Supplementary Table S4; Fig. 1). We have further graded the status of ADDE to obtain a better picture, and the results are in the following section.
Figure 1.
Kaplan Meier Survival Analysis for patient population. The Kaplan Meier Survival Analysis showed that 23.9% of the patients develop ADDE by 6-month postcompletion of radiotherapy, 50.2% by the end of 18.8 months, and 77.2% by the end of 47.17 months. Solid line indicates the data curve; dotted lines represent the confidence intervals.
Dry Eye Grade
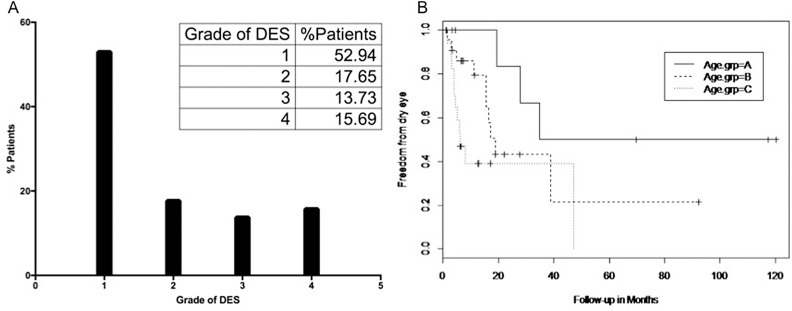
The results indicate that of the 51 eyes of study population, 47.07% develop ADDE postorbital radiotherapy. Of these dry eye patients, 17.65% develop grade 2 ADDE, 13.73% develop grade 3 ADDE, and nearly 15.69 % of the patient population develop grade 4 or severe ADDE (Fig. 2A).
Figure 2.
Dry eye in patient population. (A) Graphical representations of dry eye grading in patient population. (B) Kaplan Meier Survival Analysis curve for age of patient and dry eye development. The analysis showed that it takes 2.9 years for 50% in age group A to develop DES; 1.4 years for those in age group B and 0.5 years for those in age group C to develop ADDE.
Dry Eye and Age of Patients
The effect of therapeutic radiation dose in relation to the age of the patient was also studied. The results indicate that in group A, 50% of the patients develop ADDE by 2.9 years post last session of radiotherapy; in group B, 50% of the patients develop ADDE in 1.4 years, whereas in group C, 50% of the patients develop ADDE by 6 months post the last radiation fraction (Fig. 2B).
Other Ocular Morbidity
The treatment plan for each patient was customized according to tumor volume, location, and other anatomical parameters; however, despite these, side effects were noted. The majority of the side effects noted were radiation retinopathy (33.4%), radiation-induced cataract (24.9%), radiation keratopathy (20.8%), corneal scar (4.2%), and radiation-induced limbal stem cell deficiency (4.2%; Supplementary Table S5).
Univariate and multivariate analysis of the data showed that number of fraction of radiation and dose of radiation per fraction were significant risk factors for developing ADDE (hazard ratio [HR] >10). Total dose, however, was not statistically significant. Male gender was a protective factor with a HR of <0.001. Age group >50 years was a risk factor, even though the present study could not prove it to be statistically so, probably because of the limited sample size. However, the interaction between this age group with the radiation fraction was a significant risk factor.
Morphologic and Culture Characteristics of Human Lacrimal Gland, Status Postradiotherapy
Of the three postradiated lacrimal glands available for the present study, two were evaluated for histology/IHC studies, and one for ultrastructure studies. A fraction of all the three tissues were evaluated for in-vitro culture studies. Three normal lacrimal glands were also used as controls for the study.
Histology and Immunohistochemistry
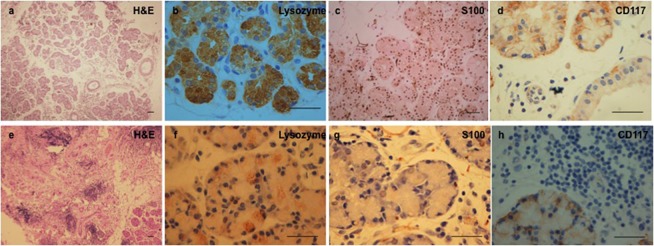
All the three postradiated glands had received an average of 4800 cGy radiation dose in 25 to 30 fractions over a 30- to 45-day period. Morphologically, they showed disorganized and shrunken gland. The lobular architecture was lost with widespread periductal and intralobular fibrosis and lymphocytic inundation. Very few acini were seen preserved, and these showed oval nuclei. Few interlobular ducts that were seen showed bilayered epithelium. Immunophenotyping revealed weak focal positivity for lysozyme, with about 25% of the acinar cells showing mild to moderate positivity; mild to moderate levels of S-100 staining can be seen in 50% of the acinar cells as compared to control tissue (Figs. 3f, 3g). CD117 staining showed a similar pattern of moderate intensity in about 50% of the cells as compared to control tissue (Fig. 3h).
Figure 3.
Comparison of normal (a–d) and postradiated (e–h) human lacrimal gland. The normal lacrimal gland shows intact tissue architecture. The secretory cells stain positive (arrowhead) for lysozyme, myoepithelial cells for S-100 protein and the putative stem cells for CD117. The postradiated tissue shows loss in lobular architecture with widespread periductal and intralobular fibrosis and lymphocytic inundation. Immunophenotyping shows mild to moderate focal positivity for lysozyme, S-100, and CD117, which is reduced both in intensity and number when compared to the control. Scale bar 100 μm.
In contrast, the control tissue showed normal tissue architecture with intact acinar, myoepithelial, and ductal cells. The extracellular matrix (ECM) component appeared normal looking with a few infiltrating lymphocytes (Fig. 3a). The expression of lysozyme was seen in the cytoplasm of the acinar cells and to an extent in the ductal epithelial cells too (Fig. 3b). S-100 protein was seen to be distributed in the myoepithelial cells enveloping the acinar cells (Fig. 3c). CD117 was seen to be localized in the cell membrane of some of the cells in the acinar and ductal compartment (Fig. 3D).
Transmission Electron Microscopy
Ultrastructure of the control lacrimal tissue revealed acinar cells with round nucleus, a number of secretory vesicles, and normal junctional complexes (Figs. 4A–C). In contrast, the postradiated gland showed evidence of extensive tissue damage with breaks in cell membrane and loose cell junctions, disrupted endoplasmic reticulum with dilated cisternae, condensed vesicular and varied shape mitochondria, degenerated large and fragmented nucleus, nuclear membrane breaks, and nucleolus lysis (Figs. 4D–F).
Figure 4.
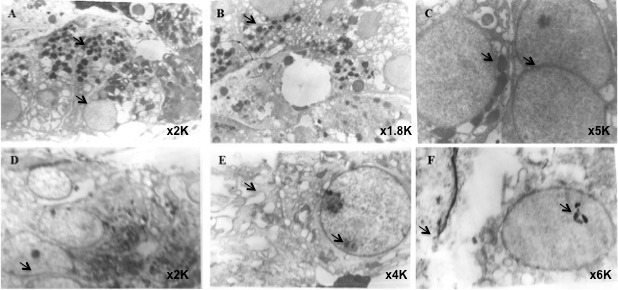
Transmission electron microscopic images of normal (A–C) and postradiated (D–F) human lacrimal gland. Normal human lacrimal gland shows acinar cells with round nucleus, a number of secretory vesicles, and junctional complexes. The postradiated gland showed evidence of tissue damage with breaks in cell membrane and loose cell junctions, disrupted endoplasmic reticulum with dilated cisternae, condensed vesicular and varied shape mitochondria, degenerated large and fragmented nucleus, nucleolus lysis, and nuclear membrane breaks. Print magnifications: A, 3860×; B, 3474×; C, 9650×; D, 3860×; E, 7720×; F, 11,580×.
Establishing In-Vitro Cultures from Postradiated Tissues
The enzymatic digestion of the freshly harvested postradiated lacrimal gland tissue yielded a heterogeneous population of cells with very few epithelial clumps. These cells were seeded on uncoated and collagen I/Matrigel coated tissue culture dishes. The cells remained loose and floating for nearly a week of seeding. A few cells adhered to the flask and showed spindle shape/stellate fibroblast morphology. No growth of epithelial cells was observed even after 3 to 4 days of seeding and 1 to 2 weeks postseeding (Supplementary Fig. S2).
In contrast, the cells isolated from normal HuLG adhere to Matrigel coated plates as discreet islands and start proliferating within 1 to 3 days. By day, 15 to 20 DIV (days in-vitro), these cells form confluent monolayer with thin cytoplasmic borders, polygonal shape, and granular cytoplasm.
Discussion
Radiation-induced damage to the eye and ocular structures occur either during irradiation of ocular/head and neck tumors or when the eye lies in the path of the radiation beam.4,7,8 Despite advances in dose delivery and shielding techniques, a large proportion of patients are seen to develop DES postorbital radiotherapy. The present study was aimed at determining the prevalence of ADDE in patients receiving orbital radiotherapy, and to investigate radiation-induced damage to the lacrimal gland in terms of its histoarchitecture and its potential to proliferate ex-vivo as compared to the normal tissues. The study provides evidence for development of ADDE in more than 50% of patients with evidence of impaired structure and function of the gland.
To address the primary objective, i.e., document the development of ADDE postorbital radiation, the dry eye status was evaluated in all patients receiving orbital radiotherapy (who also met the inclusion criteria of the study) during the study period and an attempt was made to correlate the risk factors. Our results indicate that 47.07% of the patients who undergo orbital EBRT develop ADDE with a prediction of 50% developing it by the end of 1.6 years. When the patient population was further subdivided based on their age into three categories, it was seen that it takes 2.9 years for 50% of the patients belonging to age group A (0–11 years), 1.4 years for 50% of the patients belonging to age group B (12–50), and 0.5 years for 50% of the patients belonging to age group C (>50 years) to develop ADDE postradiotherapy. When their status was categorized into the various grades according to the DEWS 2007 criteria, it was seen that of the 47.07% patients who develop ADDE, 17.65% of them developed grade 2 ADDE, 13.73% developed grade 3 ADDE, while 15.69% developed grade 4/severe ADDE. The most common comorbidities observed were radiation retinopathy (33.4%), radiation induced cataract (24.9%), and radiation keratopathy (20.8%). Multivariate and univariate analysis of the data showed that fraction of radiation and dose of radiation per fraction were significant risk factors for developing DES with a HR of >10 for both the groups. In our analysis, male gender came up as a protective factor with a HR of < 0.001. Apart from these main factors, a significant interaction was also found between factors like age group, Schirmer's value to dose per fraction, total dose to fraction, total dose to dose per fraction, and total dose to gender. Age group C (>50 years) was a risk even though the present study could not prove it to be statistically significant. However, the interaction of radiation fraction to age group C was statistically significant with a HR < 0.001. Overall, the results also indicate age group A (0–11 years) to be a protective factor against development of ADDE.
To the best of our knowledge, the only comparable study is the one published in 2012 by Bhandare et al.16 Even though both the studies have a number of compatible results, there are a few contrasting parameters too. Bhandare et al.16 found that about 51.28% of the patients develop dry eye postradiation leading to visual compromise. A dose–effect association was also reported with 6% of the patients who received 35.0 to 39.99 Gy of radiation developing DES; 50% of those who received 45 to 49.99 Gy and 90% of those who received 60 to 64.99 developed DES. With a mean of 0.9 years (range, 1 month to 3 years), latency of DES was observed to be a function of total dose and dose per fraction. Other factors like age and gender, however, were not found to be statistically significant. Some of these contrast our findings since younger age and male gender come up as protective factors in our analysis. There is no specific explanation we can offer to explain these differences in the results. It could possibly be due to variation in biological parameters, spectrum of cases, and therapy and workup protocol.
The secondary objective of our study was to determine the effect of orbital radiotherapy on the histoarchitecture of human lacrimal gland and its potential to be cultured in-vitro. Being a tertiary care eye hospital with an active ocular oncology services provided us a very rare opportunity to study the lacrimal gland tissue obtained from exenterated specimens of three patients who underwent orbital radiotherapy prior to the current exenteration surgery. Our results indicate that orbital radiotherapy is associated with near total destruction of the histology of human lacrimal gland with negligible number of viable acini, loss of cellular integrity, and gross reduction of secretory function. The ductal cells also appeared to be damaged with extensive periductal and intralobular fibrosis and lymphocyte inundation. Lysozyme, S-100, and CD117 staining showed a pattern of weak focal area positivity (Fig. 3). The ultrastructure studies also corroborate these findings (Fig. 4). Although the biochemical studies for mechanism of cell death were not evaluated here, the ultrastructure features of cell shrinkage, degenerate fragmented nucleus, and nuclear membrane breaks are suggestive of apoptosis. These findings are in concert with those reported in literature.18
A previously published study by our group17 provides preliminary evidence for the existence of stem cells in the human lacrimal gland, which can potentially be recruited to salvage the damaged gland. It is also logical to believe that when the number of stem cells in the gland are damaged beyond repair, then atrophy and destruction of the gland would result, as seen in the case of radiation-induced damage. The histopathology observations of the present study also corroborate these beliefs. With this background, we attempted to culture lacrimal gland cells from postradiated gland. In contrast to the normal human lacrimal gland, which proliferated in cultures in for >30 days, formed 2D and 3D spheres showed presence of differentiated epithelial cells with secretory function and presence of stem cells, and the postradiated gland showed impaired potential to expand in culture.17 One of the possible reasons could be that the numbers of cells that remain viable in the tissue are very low for any kind of in-vitro adjustment or have lost their stem (like) cells that could establish cell cultures. This is also supported by the results of histopathology studies, which showed extensive loss of tissue architecture and loss of CD117 staining. The evidence provided in this study is a rare and novel finding, which shows impairment in structure and function of human lacrimal gland after radiation with loss of stem cells.
The present study also has its set of limitations. One of the major limitations is the lack of subjective patient responses to dry eye questionnaire. Since dry eye has both subjective as well as objective signs, both of them need to be evaluated in order to come to a robust conclusion. It is possible that in patients with ocular malignancies or aggressive benign tumors, the primary focus would be on these rather than the dry eye symptoms; hence, these did not come up as an important presenting symptom in the postoperative checkups and noted in the medical records. Also being a retrospective study, we could only look at the objective signs of dry eye documented in the records. Prospective studies using more than one test to confirm DES could add value to this study. Another limitation of the study is the use of normal contralateral eye of the patients. Since it is difficult to recruit normal age-matched controls for a study, we had to resort to the use of contralateral eye as control in this study. The number of lacrimal gland specimens evaluated in this study for postradiation damage is low, however, considering the nature of the specimen and the rarity of such conditions makes it a valuable contribution.
In summary, the present study documents that orbital radiotherapy, using the current practice patterns and shielding, is associated with development of ADDE in nearly half of the patients with one-sixth of them exhibiting severe ADDE. The clinical and the morphologic data suggest that this irreversible and progressive ADDE is possibly due to the widespread atrophic changes that occur in postradiated lacrimal gland and that lead to loss of regenerative capacity of the lacrimal gland. The high prevalence of ADDE following best practices of orbital radiotherapy warrants exploring possibility of pretherapy banking of tissues, similar to the current practices of tissue bio-banking in patients undergoing radiotherapy for various malignant conditions so as to explore the potential of cell replacement therapy in the future.
Supplementary Material
Acknowledgments
The authors thank Sreedhar Bowenpalli and G. Chenchu Naidu for the technical assistance provided in immunohistochemistry experiments; A. Prof. Laxman Mekala, Ruska Labs, for help with the ultrastructure studies; and Rohini M. Nair for insightful comments on the manuscript.
Supported by International Atomic Energy Agency (IAEA), Hyderabad Eye Research Foundation (HERF), Champalimaud Translational Research Foundation (C-Tracer), Department of Biotechnology (DBT), DST PURSE (Promotion of University Research and Scientific Excellence), University for Potential for Excellence-2, and Council for Scientific and Industrial Research (CSIR).
Disclosure: S. Tiwari, None; A. Bhatt, None; J. Nagamodi, None; M.J. Ali, None; H. Ali, None; M.N. Naik, None; V.A.P. Reddy, None; G.K. Vemuganti, None
References
- 1. Alberti W. Acute and late side effects of radiotherapy for ocular disease: an overview. Front Radiat Ther Oncol. 1997; 30: 281–286. [DOI] [PubMed] [Google Scholar]
- 2. Barabino S,, Raghavan A,, Loeffler J,, Dana R. Radiotherapy-induced ocular surface disease. Cornea. 2005; 24: 909–914. [DOI] [PubMed] [Google Scholar]
- 3. Durkin SR,, Roos D,, Higgs B,, Casson RJ,, Selva D. Ophthalmic and adnexal complications of radiotherapy. Acta Ophthalmol Scand. 2007; 85: 240–250. [DOI] [PubMed] [Google Scholar]
- 4. Gordon KB,, Char DH,, Sagerman RH. Late effects of radiation on the eye and ocular adnexa. Int J Radiation Oncol Biol Physics. 1995; 31: 1123–1139. [DOI] [PubMed] [Google Scholar]
- 5. Fujishima H,, Toda I,, Shimazaki J,, Tsubota K. Allergic conjunctivitis and dry eye. Br J Ophthalmol. 1996; 80: 994–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bessell EM,, Henk JM,, Whitelocke RA,, Wright JE. Ocular morbidity after radiotherapy of orbital and conjunctival lymphoma. Eye. 1987; 1: 90–96. [DOI] [PubMed] [Google Scholar]
- 7. Morita K,, Kawabe Y. Late effects on the eye of conformation radiotherapy for carcinoma of the paranasal sinuses and nasal cavity. Radiology. 1979; 130: 227–232. [DOI] [PubMed] [Google Scholar]
- 8. Nakissa N,, Rubin P,, Strohl R,, Keys H. Ocular and orbital complications following radiation therapy of paranasal sinus malignancies and review of literature. Cancer. 1983; 51: 980–986. [DOI] [PubMed] [Google Scholar]
- 9. The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye Workshop [2007]. Ocul Surf. 2007; 5: 75–92. [DOI] [PubMed] [Google Scholar]
- 10. Zoukhri D. Effect of inflammation on lacrimal gland function. Exp Eye Res. 2006; 82: 885–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jeganathan VS,, Wirth A,, MacManus MP. Ocular risks from orbital and periorbital radiation therapy: a critical review. Int J Radiation Oncol Biol Physics. 2011; 79: 650–659. [DOI] [PubMed] [Google Scholar]
- 12. Karp LA,, Streeten BW,, Cogan DG. Radiation-induced atrophy of the Meibomian gland. Arch Ophthalmology. 1979; 97: 303–305. [DOI] [PubMed] [Google Scholar]
- 13. Parsons JT,, Bova FJ,, Mendenhall WM,, Million RR,, Fitzgerald CR. Response of the normal eye to high dose radiotherapy. Oncology (Williston Park). 1996; 10: 837–847; discussion 847–848, 851–852. [PubMed] [Google Scholar]
- 14. Gregoire V,, De Neve W,, Eisbruch A,, Lee N,, Van den Weyngaert D,, Van Gestel D. Intensity-modulated radiation therapy for head and neck carcinoma. Oncologist. 2007; 12: 555–564. [DOI] [PubMed] [Google Scholar]
- 15. Goyal S,, Cohler A,, Camporeale J,, Narra V,, Yue NJ. Intensity-modulated radiation therapy for orbital lymphoma. Radiation Med. 2008; 26: 573–581. [DOI] [PubMed] [Google Scholar]
- 16. Bhandare N,, Moiseenko V,, Song WY,, Morris CG,, Bhatti MT,, Mendenhall WM. Severe dry eye syndrome after radiotherapy for head-and-neck tumors. Int J Radiation Oncol Biol Physics. 2012; 82: 1501–1058. [DOI] [PubMed] [Google Scholar]
- 17. Tiwari S,, Ali MJ,, Balla MM,, et al. Establishing human lacrimal gland cultures with secretory function. PLoS One. 2012; 7: e29458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stephens LC,, Schultheiss TE,, Peters LJ,, Ang KK,, Gray KN. Acute radiation injury of ocular adnexa. Arch Ophthalmol. 1988; 106: 389–391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.