We investigated whether combining hyper-CVAD and epratuzumab, a humanized monoclonal therapeutic antibody against CD22, in very high-risk younger adults with relapsed/refractory CD22+ precursor B-cell acute lymphocytic leukemia could improve the response of patients. The overall response rate was 50%, including nine complete remissions (30%), one complete response with incomplete platelet recovery (3%), and five partial responses (17%). Four of nine (44%) patients in complete remission, with or without platelet recovery, who were evaluated for minimal residual disease by flow cytometry achieved a negative status. This treatment was, however, associated with short-term survival (median overall and leukemia-free survival of 3 and 4.7 months, respectively). The main grade 3/4 toxicity was pancytopenia; no severe toxicity was related to the epratuzumab infusion.
The prognosis of younger adults with refractory/relapsed acute lymphocytic leukemia (ALL) remains dismal, whether in the presence or absence of the Philadelphia chromosome (Ph+/Ph−).1,2 Hyper-CVAD, developed by the M.D. Anderson group a few years ago, is one of the standard salvage regimens used in this setting.3,4 Recently, targeted therapies using monoclonal antibodies directed against surface antigens such as CD19, CD20 or CD22 have yielded impressive results, improving survival in patients with precursor B-cell (PBC) ALL with blasts expressing these markers.5–8
Our particular interest has been to use epratuzumab, a humanized therapeutic monoclonal antibody against CD22, which acts mainly through antibody-dependent cellular cytotoxicity and signaling properties.9 Phase 1/2 studies combining epratuzumab with chemotherapy, as well as radionuclides such as 90Yttrium, have already shown promising results, both in children and adults with relapsed/refractory CD22+ PBC-ALL.10–14
This was a multicenter prospective phase 2 study aimed at evaluating the efficacy and safety of the “Cheprall” (for Chemotherapy + epratuzumab in ALL patients) salvage regimen, combining epratuzumab 360 mg/m2/day iv on days 1, 8, 15, and 22 plus Hyper-CVAD with cyclophosphamide 300 mg/m2/12 h iv on days 1 to 3, vincristine 2 mg iv on days +4 and +11, doxorubicin 50 mg/m2 iv on day 4, and dexamethasone 40 mg po on days 1 to 4 and 11 to 14. Premedication with corticosteroids and paracetamol was given before epratuzumab administration in order to prevent infusion reactions. Intrathecal injections of methotrexate 15 mg, aracytine 40 mg, and depomedrol 40 mg were given on days 1 and 8. Eligibility criteria were: patients 18–59 years old with refractory PBC-ALL (defined by treatment failure after two successive courses of induction therapy) or relapse less than 6 months after first complete remission (CR), first relapse or beyond, Ph+ B-ALL patients relapsed or refractory to at least one second-generation tyrosine kinase inhibitor. There had to be no involvement of the central nervous system and the Eastern Cooperative Oncology Group performance status score had to be 0–2. Patients had to have >20% of blasts in the bone marrow with CD22+ expression on >30% of the blast population, a creatinine clearance ≥50 mL/min, and serum bilirubin ≤30 mmol/L. The primary objective of the study was the overall response rate defined by CR or CR with incomplete platelets recovery (CRp) or partial response (PR), evaluated between 4 and 6 weeks after day 1. CR was defined as <5% bone marrow blasts, neutrophil count >1×109/L, platelet count >100×109/L and no evidence of extramedullary disease. CRp was defined similarly but without recovery of the platelet count. PR was defined as a decrease of >50% of BM blasts or CR/CRp with persistent extra-medullary disease. Patients in response (CR, CRp or PR) were allowed to receive a second cycle as consolidation. Secondary objectives were overall survival, leukemia-free survival, toxicity and minimal residual disease (MRD), evaluated centrally in a single institution by flow cytometry using a FACS CANTO II (BD, Biosciences, San Jose, CA, USA) flow cytometer. Toxicity was assessed according to the National Cancer Institute Common Toxicity Criteria version 4. Residual leukemic blasts were evaluated using an eight-color panel including antibodies to CD45, CD19, CD10, CD34, CD38, CD58, CD20 and CD22. Two different anti-CD22 antibodies were used. The RFB4 antibody (PE-conjugated, Invitrogen, Camarillo, CA, USA) and epratuzumab bind competitively to CD22, meaning that in the presence of epratuzumab, RFB4 binding is blocked. By contrast, the SHCL-1 antibody (PE- or PerCp-Cy5.5-conjugated, BD Biosciences, San Jose, CA, USA) binds to a non-cross-blocking epitope and can be used to assess the modulation of the CD22 antigen on the blast surface.
The study was approved by Brest University Hospital ethical committee and the clinical trial department of the Agence Française de Sécurité Sanitaire des Produits de Santé (AFSSAPS). Epratuzumab was supplied by Immunomedics, Inc. (Morris Plains, NJ, USA). Written informed consent was obtained from each patient. This trial is registered at ClinicalTrials.gov, with number NCT01219816.
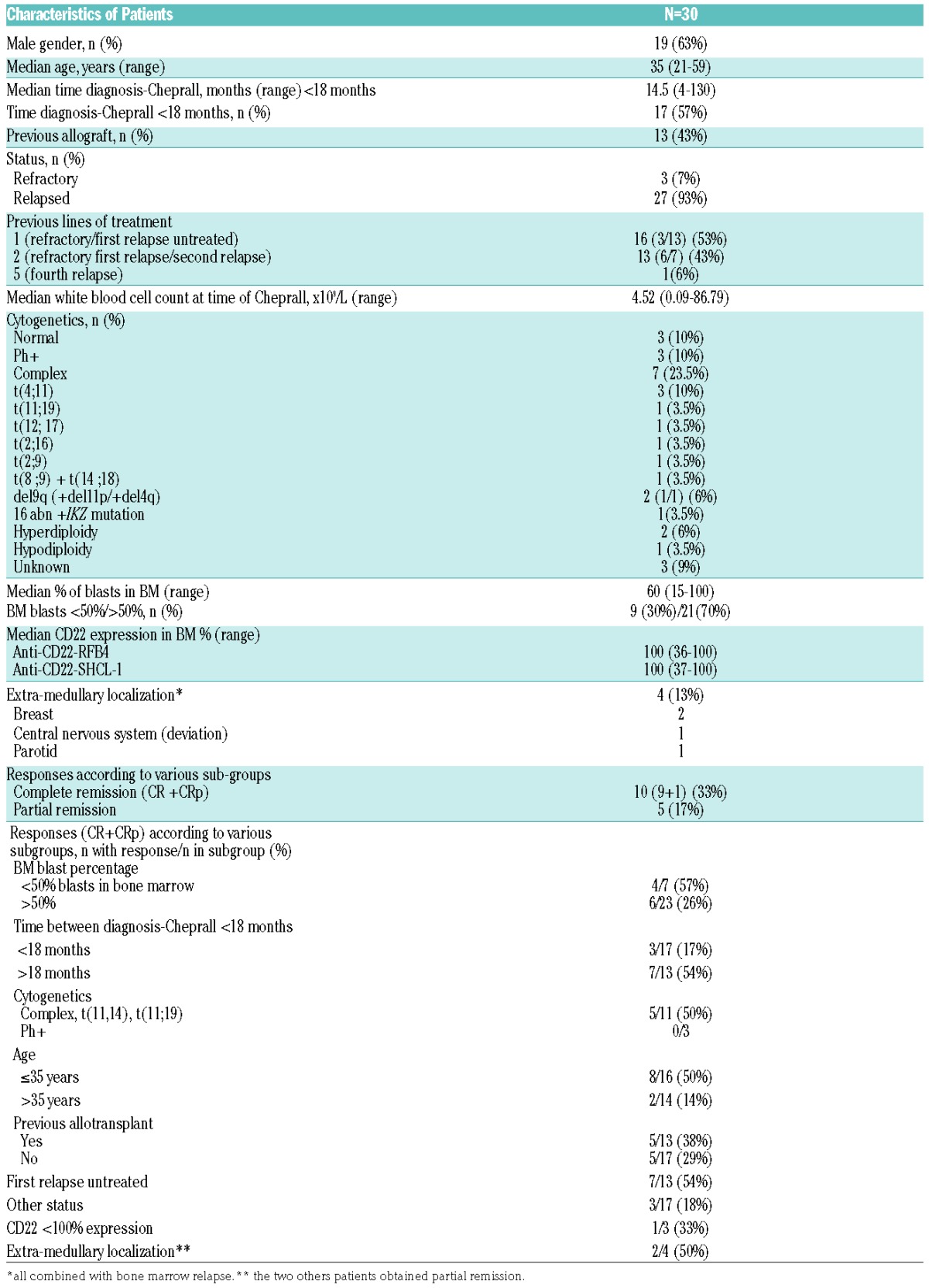
Between January 2011 and April 2016, 31 patients from 11 French centers were enrolled. A combination of epratuzumab + vincristine and dexamethasone (EVD) only was given to one patient who was subsequently excluded from the analysis. Thirty patients were, therefore, assessable (Table 1).
Table 1.
Characteristics and responses of patients with refractory/relapsed CD22+ precursor B-cell acute lymphocytic leukemia.
Cheprall was well-tolerated, with grade 3/4 toxicities being mainly pancytopenia. No severe toxicity was related to the epratuzumab infusion itself. Three patients died during aplasia (1 with sepsis, 1 with a cerebral hemorrhage, and 1 with fusariosis). One grade 3 encephalopathy of unclear etiology was documented after a second cycle of treatment in a responder. Deaths in aplasia and encephalopathy were not found to be related to either epratuzumab itself or the Cheprall regimen overall.
The overall response rate was 50%, including nine CR (33%), one CRp (3%), and five PR (17%). The number of CR/CRp was significantly higher for patients in first untreated relapse (54% versus 18%, P<0.0001), aged below 36 years (50% versus 14%, P<0.001), with ≤50% of bone marrow blasts (57% versus 26%, P<0.0001), and in whom the period between diagnosis and Cheprall treatment was >18 months (54% versus 17%, P<0.0001). MRD was determined in nine out of ten CR/CRp cases. Among them, four (45%) achieved negative MRD (<10−5) demonstrated by an absence of labeling with both anti-CD22 antibodies. None of the non-responders showed CD22 expression as assessed with the RFB4 antibody but all showed full CD22 expression with the SHCL-1 antibody, demonstrating a persistent targeting of epratuzumab on blasts without loss of the CD22/epratuzumab complex from the leukemic cell surface.
All patients in CR/CRp and one patient in PR received consolidation therapy consisting of a second cycle of Cheprall (n=5), EVD (n=5), or blinatumomab (n=1). At the time of analysis, 27 patients had died (3 with aplasia, 23 with progression, 1 with multiple organ failure), while three responders were still alive in CR (at +3.5, +4, and +14 months), including one who was allotransplanted after CR. Six patients received an allogeneic hematopoietic stem cell transplant after Cheprall: four in second CR, one as salvage treatment, and one in third CR. It was the first allotransplant for five patients out of six. Thus, overall, 40% of patients have had successful bridging therapy to allogeneic hematopoietic stem cell transplantation in this study. The median overall survival was 3 months (range, 0.2–34.8) and the median leukemia-free survival for those who achieved CR/CRp was 4.7 months (range, 1.2–12).
In summary, hyper-CVAD + epratuzumab provided objective responses in 50% of this cohort of younger patients with refractory/relapsed CD22+ PBC-ALL and at very high risk for failure. The incidence of CR/CRp was significantly higher among patients in first untreated relapse (54%), a rate which seems better compared than that of a large pooled historic cohort in which this response rate was 40%.2 Moreover, although this may be comparable with rates observed with hyper-CVAD or augmented hyper-CVAD protocols (44% to 60%),3,4 it should be recognized that CR/CRp in these previous studies were achieved sometimes after several cycles and not one, as evaluated here. Disease improvement provided by the Cheprall regimen was, however, short-lived, which could be explained by an insufficient decrease in disease load and/or by failure of the blast cells to respond to epratuzumab. The latter was not due to the absence of fixation of the antibody on leukemic cells, as demonstrated by flow cytometry. The efficacy of epratuzumab could be enhanced by combining it with the radionuclide 90-Yttrium, since this radioimmunotherapy was able to induce CR in some patients.14
Interestingly, almost half the evaluated responders achieved a negative MRD status. That epratuzumab may induce a higher negative MRD rate had already been demonstrated in the pediatric setting.12 However, the positive impact of MRD negativity reported recently for ALL patients in first salvage15 was not observed here.
The partial efficacy of the Cheprall regimen in a population with such a poor prognosis suggests that epratuzumab should be tested in first-line schedules, since it may also contribute to decreasing the level of MRD, especially before allogeneic hematopoietic stem cell transplantation, with the hope of increasing patients’ survival. As epratuzumab has only been investigated in single-arm trials compared with historical controls, randomized trials are now required to determine the potential benefit of the drug prospectively in patients with CD22+ hematologic malignancies.
Supplementary Material
Acknowledgments
We thank local investigators and data managers and the DRC of Nantes (especially Mrs. June Fortun).
Footnotes
Funding: this study was supported by a grant from the French National Cancer Institute (PHRC 2010).
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Cortes JE, Kim DW, Pinilla-Ibarz J, et al. A phase 2 trial of ponatinib in Philadelphia chromosome-positive leukemias. N Engl J Med. 2013;369(19):1783–1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gökbuget N, Dombret H, Ribera JM, et al. International reference analysis of outcomes in adults with B-precursor Ph-negative relapsed/refractory acute lymphoblastic leukemia. Haematologica. 2016;101(12):1524–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koller CA, Kantarjian HM, Thomas D, et al. The hyper-CVAD regimen improves outcome in relapsed acute lymphoblastic leukemia. Leukemia. 1997;11(12):2039–2044. [DOI] [PubMed] [Google Scholar]
- 4.Faderl S, Thomas DA, O’Brien S, et al. Augmented hyper-CVAD based on dose-intensified vincristine, dexamethasone, and asparaginase in adult acute lymphoblastic leukemia salvage therapy. Clin Lymphoma Myeloma Leuk. 2011;11(1):54–59. [DOI] [PubMed] [Google Scholar]
- 5.Frey NV, Luger SM. How I treat adults with relapsed or refractory Philadelphia chromosome-negative acute lymphoblastic leukemia. Blood. 2015;126(5):589–596. [DOI] [PubMed] [Google Scholar]
- 6.Kantarjian HM, DeAngelo DJ, Stelljes M, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maury S, Chevret S, Thomas X, et al. Rituximab in B-lineage adult acute lymphoblastic leukemia. N Engl J Med. 2016;375(11):1044–1053. [DOI] [PubMed] [Google Scholar]
- 8.Topp MS, Stein A, Gokbuget N, et al. Blinatumomab improved overall survival in patients with relapsed or refractory Philadelphia negative B-cell precursor acute lymphoblastic leukemia in a randomized, open-label phase 3 study (TOWER). Haematologica. 2016;101: S149. [Google Scholar]
- 9.Carnahan J, Wang P, Kendall R, et al. Epratuzumab, a humanized monoclonal antibody targeting CD22: characterization of in vitro properties. Clin Cancer Res. 2003;9(10 Pt 2):3982S–3990S. [PubMed] [Google Scholar]
- 10.Raetz EA, Cairo MS, Borowitz MJ, et al. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children’s Oncology Group Pilot Study. J Clin Oncol. 2008;26(22):3756–3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Advani AS, McDonough S, Coutre S, et al. SWOG S0910: a phase 2 trial of clofarabine/cytarabine/epratuzumab for relapsed/refractory acute lymphocytic leukaemia. Br J Haematol. 2014;165(4):504–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raetz EA, Cairo MS, Borowitz MJ, et al. Re-induction chemoimmunotherapy with epratuzumab in relapsed acute lymphoblastic leukemia (ALL): phase II results from Children’s Oncology Group (COG) study ADVL04P2. Pediatr Blood Cancer. 2015;62(7):1171–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chevallier P, Huguet F, Raffoux E, et al. Vincristine, dexamethasone and epratuzumab for older relapsed/refractory CD22+ B-acute lymphoblastic leukemia patients: a phase II study. Haematologica. 2015;100(4):e128–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chevallier P, Eugene T, Robillard N, et al. (90)Y-labelled anti-CD22 epratuzumab tetraxetan in adults with refractory or relapsed CD22-positive B-cell acute lymphoblastic leukaemia: a phase 1 dose-escalation study. Lancet Haematol. 2015;2(3):e108–117. [DOI] [PubMed] [Google Scholar]
- 15.Jabbour E, Short NJ, Jorgensen JL, et al. Differential impact of minimal residual disease negativity according to the salvage status in patients with relapsed/refractory B-cell acute lymphoblastic leukemia. Cancer. 2017;123(2):294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


