In this past decade, new therapeutic approaches based on the use of rituximab, high-dose cytosine-arabinoside, and autologous stem cell transplantation (ASCT) have changed the paradigm of mantle cell lymphoma (MCL) treatment, significantly improving the quality and duration of response and increasing survival.1,2 Unfortunately, despite these improvements, MCL is still considered an incurable disease, with all patients eventually relapsing and needing rescue therapy.
There is currently no consensus on the best MCL salvage treatment strategy. Considering the favorable clinical activity and safety profiles of both lenalidomide and bendamustine plus rituximab, the combination of rituximab, lenalidomide, and bendamustine (R2B) may offer a potential therapeutic option for patients with relapsed / refractory (R/R) MCL. On these grounds, we evaluated the efficacy and safety of R2B as second-line therapy in MCL patients that had either relapsed following a single prior line of therapy or were refractory to first-line treatment; consolidation followed by ASCT was considered to be part of first-line treatment.
This prospective, non-randomized, multicenter, phase II clinical trial comprised an induction phase, a consolidation phase, a maintenance phase, and a follow-up phase. The total duration was 60 months, which included an 18-month enrollment period, a 24-month treatment period, and 18 months of follow up. During the induction phase, all patients received two cycles of R2B (rituximab 375 mg/m2 on day 8 of cycle 1, and thereafter on day 1; lenalidomide 10 mg daily on days 1–14; and bendamustine 70 mg/m2 on days 2 and 3 every 28 days). Patients without progressive disease (PD) following the first two cycles received a further two cycles of R2B, while those with PD were treated according to best clinical practice. Patients with a complete or partial response (CR or PR) to induction treatment entered the consolidation phase, while those with stable disease (SD) or PD were treated according to best clinical practice. Consolidation treatment comprised two cycles of R2 (rituximab 375 mg/m2 on day 1, plus lenalidomide 15 mg daily on days 1–21 every 28 days). Patients with a CR or PR following consolidation proceeded to the maintenance phase; those with SD or PD were treated according to best clinical practice. Maintenance treatment comprised lenalidomide 15 mg daily on days 1–21 every 28 days until either disease progression or unacceptable toxicity, for up to 18 cycles (18 months). Lenalidomide dose modification was allowed for hematologic and extra-hematologic toxicities.
The primary trial end point was the CR rate in the intention-to-treat (ITT) population after consolidation treatment. Secondary end points included: adverse events (AEs) of severity grade 3 or over, overall response rate (ORR), progression-free survival (PFS), overall survival (OS), minimal residual disease (MRD), and correlations of outcome with cereblon expression. Response and progression were defined according to Cheson criteria.3 MRD was assessed by allele-specific oligonucleotide (ASO) nested polymerase chain reaction (PCR) in bone marrow (BM) and peripheral blood (PB) samples, using the patient-specific immunoglobulin heavy-chain (IGH) gene rearrangement and/or the Bcl-1/IGH rearrangement as a molecular marker. The sensitivity of the nested PCR approach was three neoplastic rearrangements in 106 normal cells (3.00E-06) in repeated dilution assays, while quantitative PCR had a minimum sensitivity of 1.00E-05.4,5 Safety was evaluated according to Common Terminology Criteria for Adverse Events v.4.0.
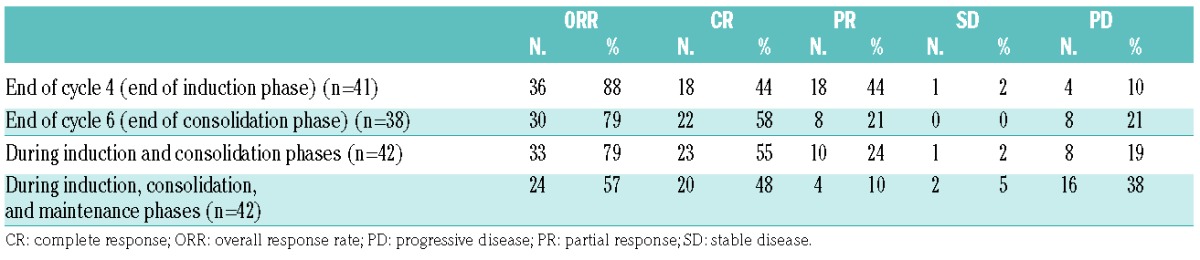
Between April 2012 and June 2013, 42 patients were enrolled at 24 centers in Italy. Baseline patients’ characteristics are summarized in Table 1. All 42 patients enrolled into the trial entered the induction phase, and thus formed the ITT population. Twenty-three of these patients (55%) achieved a CR at the end of the consolidation phase. Response rates by treatment phase are summarized in Table 2.
Table 1.
Baseline patients’ characteristics.
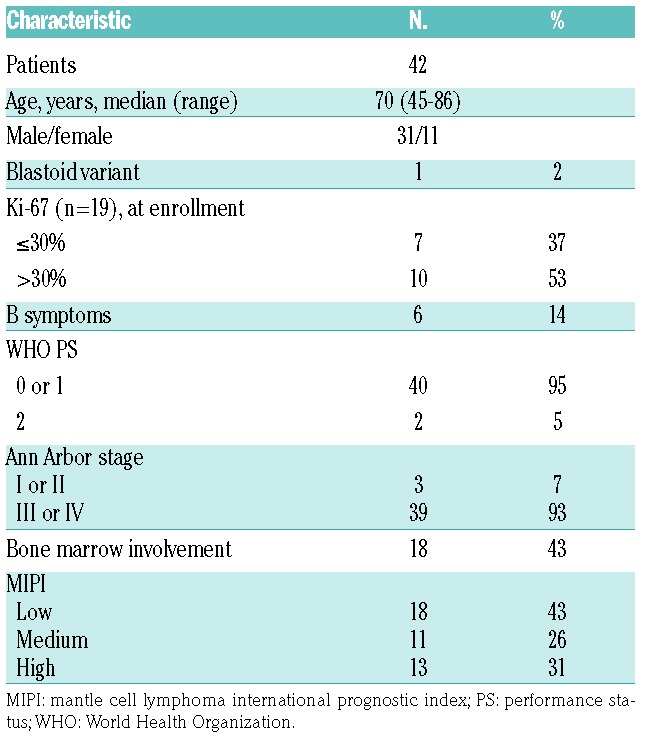
Table 2.
Response rates by treatment phase.
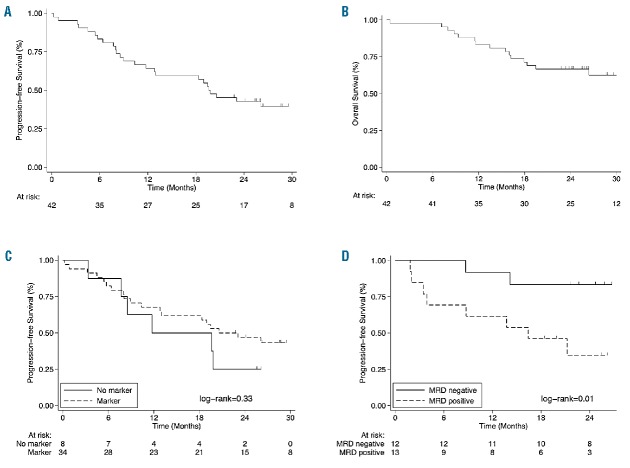
The median duration of follow up in the ITT population was 29 months (IQR, 25–32 months). Median PFS was 20 months (Figure 1A). Twenty-six events (progression or death) occurred during the follow-up period. PFS rates at 12, 18, and 24 months were 64% [95% confidence interval (CI): 0.48–0.77], 59% (95%CI: 0.43–0.72), and 43% (95%CI: 0.28–0.57), respectively. Median OS had not been reached at the time of writing (Figure 1B). OS rates at 12, 18, and 24 months were 83% (95%CI: 0.69–0.92), 71% (95%CI: 0.55–0.83), and 67% (95%CI: 0.50–0.79), respectively.
Figure 1.
Kaplan-Meier curves. (A) Progression-free survival (PFS) in the intention-to-treat (ITT) population (n=42). (B) Overall survival (OS) in the ITT population (n=42). (C) PFS in the ITT population, stratified by availability of a molecular marker of minimal residual disease (MRD). (D) PFS in the subgroup of patients (n=25) who were evaluable for MRD at the end of the induction phase, stratified on the basis of allele-specific oligonucleotide nested polymerase chain reaction MRD evaluations of bone marrow samples.
A molecular marker for MRD analysis (IGH, Bcl-1, or both) was found in 34 (81%) patients. Only responding patients were considered for MRD monitoring. According to nested polymerase chain reaction (PCR), 56% (14 of 25) and 48% (12 of 25) of patients were MRD negative in PB and BM, respectively, at the end of the induction phase; corresponding rates at the end of consolidation treatment were 71% (15 of 21) and 36% (8 of 22), respectively. As expected, there was no difference in median PFS between patients with versus those without a molecular marker (Figure 1C). Median PFS was longer in patients who were MRD negative in BM at the end of the induction phase than in those who were MRD positive (median PFS not reached vs. 16 months; P=0.01) (Figure 1D). By contrast, MRD status in PB had no significant impact on PFS.
The most common grade 3/4 AEs in the induction and consolidation phases were: neutropenia (71%), thrombocytopenia (14%), febrile neutropenia (10%), pulmonary toxicity (7%), anemia (5%), and nephrotoxicity (5%). Notably, significant grade 3/4 neutropenia (72%) was also reported during the maintenance phase. Because of toxicity, the dosage of lenalidomide was reduced or temporarely suspended in 52 out of a total of 577 cycles (9%). Nine patients interrupted treatment: 6 because of treatment-related effects and 3 for other causes. Fifteen deaths occurred during the follow-up period: 11 due to lymphoma, 2 due to toxicities, and 2 due to other causes. No treatment-related deaths occurred during the trial. One patient developed lung cancer during follow up.
Retrospective subanalyses were conducted in an attempt to identify any potential predictors of response (data not shown). Of the factors investigated [these included: age, sex, Ki-67, MCL international prognostic index, use of cytarabine-containing first-line therapy, duration of response (DoR) following first-line therapy, thrombocytopenia grade, neutropenia grade, and cereblon expression], none was found to correlate with response outcomes; cereblon expression did not correlate with PFS during the maintenance phase either. It is possible that our small sample size limited the ability to detect prognostic markers.
In the absence of head-to-head clinical trials, it is difficult to compare the efficacy of R2B versus bendamustine-rituximab (BR), single-agent lenalidomide, or lenalidomide plus dexamethasone or rituximab, because of the heterogeneity of patient populations in different studies. Overall, it appears that the clinical characteristics of patients included in our trial were less favorable than those in previously reported studies of BR in non-Hodgkin lymphokmas.6,7 In view of the apparent less favorable baseline characteristics of our population, the results that we observed (in particular, the 55% CR rate and the 43% PFS at 24 months) appear rather impressive. Our results also compare favorably with those reported in studies that evaluated lenalidomide either as a single agent or as a component of combination regimens.8–12 Notably, the ORR, CR rate, and PFS in our trial also compare favorably with those reported for patients with previously treated, rituximab-resistant or rituximab-refractory MCL, who received R2 in a phase II clinical trial.12 The high activity of the treatment strategy was underlined by a 36% rate of MRD-negativity in BM following induction and consolidation treatment; this was found to be predictive of prolonged PFS (Figure 1D).
We observed a high rate of grade 3/4 neutropenia during R2B induction and R2 consolidation (71%) which compares negatively with the rate previously reported with BR (36%).7,8 The incidence of grade 3/4 neutropenia during maintenance therapy with single-agent lenalidomide was 72%, which is also high when compared with the 40%–44% rate reported in previous studies.9,10 Despite this high rate of severe neutropenia, infectious complications occurred in only 7% of patients, and the incidence of other extra-hematologic toxicities was very low.
Since the initiation of our trial, the Bruton’s tyrosine kinase inhibitor ibrutinib has been introduced for the treatment of R/R MCL. In the first published paper, Wang et al. reported an ORR of 68% (21% CR), an estimated median DoR of 17.5 months, and an estimated median PFS of 13.9 months with this agent, while the median OS had not been reached at the time of publication.13 In a subsequent study, in which ibrutinib was combined with rituximab, the ORR was 88% with 44% CR, and neither median DoR nor median PFS had been reached.14 In both of these studies, ibrutinib was reasonably well tolerated. However, it has recently been demonstrated that MCL refractory to ibrutinib is associated with a very poor prognosis, with an estimated survival of only a few months.15 The question of whether lenalidomide-containing regimens may be effective in patients who are refractory to ibrutinib is worthy of further investigation.
In conclusion, our findings suggest that R2B induction plus R2 consolidation and lenalidomide maintenance may represent an active alternative therapeutic option for patients with R/R MCL. In an era in which new biological agents are rapidly modifying traditional treatment algorithms, the optimal positioning of this treatment strategy has yet to be determined.
Supplementary Material
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Romaguera JE, Fayad L, Rodriguez MA, et al. High rate of durable remissions after treatment of newly diagnosed aggressive mantle-cell lymphoma with rituximab plus hyper-CVAD alternating with rituximab plus high-dose methotrexate and cytarabine. J Clin Oncol. 2005;23(28):7013–7023. [DOI] [PubMed] [Google Scholar]
- 2.Geisler CH, Kolstad A, Laurell A, et al. Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br J Haematol. 2012;158(3):355–362. [DOI] [PubMed] [Google Scholar]
- 3.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–586. [DOI] [PubMed] [Google Scholar]
- 4.Ladetto M, Magni M, Pagliano G, et al. Rituximab induces effective clearance of minimal residual disease in molecular relapses of mantle cell lymphoma. Biol Blood Marrow Transplant. 2006;12(12):1270–1276. [DOI] [PubMed] [Google Scholar]
- 5.Drandi D, Kubiczkova-Besse L, Ferrero S, et al. Minimal residual disease detection by droplet digital PCR in multiple myeloma, mantle cell lymphoma, and follicular lymphoma: a comparison with real-time PCR. J Mol Diagn. 2015;17(6):652–660. [DOI] [PubMed] [Google Scholar]
- 6.Robinson KS, Williams ME, van der Jagt RH, et al. Phase II multicenter study of bendamustine plus rituximab in patients with relapsed indolent B-cell and mantle cell non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(27):4473–4479. [DOI] [PubMed] [Google Scholar]
- 7.Rummel MJ, Al-Batran SE, Kim SZ, et al. Bendamustine plus rituximab is effective and has a favorable toxicity profile in the treatment of mantle cell and low-grade non-Hodgkin’s lymphoma. J Clin Oncol. 2005;23(15):3383–3389. [DOI] [PubMed] [Google Scholar]
- 8.Zinzani PL, Vose JM, Czuczman MS, et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: subset analysis of the NHL-003 study. Ann Oncol. 2013;24(11):2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goy A, Sinha R, Williams ME, et al. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: phase II MCL-001 (EMERGE) study. J Clin Oncol. 2013;31(29):3688–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trněný M, Lamy T, Walewski J, et al. Lenalidomide versus investigator’s choice in relapsed or refractory mantle cell lymphoma (MCL-002; SPRINT): a phase 2, randomised, multicentre trial. Lancet Oncol. 2016;17(3):319–331. [DOI] [PubMed] [Google Scholar]
- 11.Zaja F, De Luca S, Vitolo U, et al. Salvage treatment with lenalidomide and dexamethasone in relapsed/refractory mantle cell lymphoma: clinical results and effects on microenvironment and neoangiogenic biomarkers. Haematologica. 2012;97(3):416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang M, Fayad L, Wagner-Bartak N, et al. Lenalidomide in combination with rituximab for patients with relapsed or refractory mantle-cell lymphoma: a phase 1/2 clinical trial. Lancet Oncol. 2012;13(7):716–723. [DOI] [PubMed] [Google Scholar]
- 13.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang ML, Lee H, Chuang H, et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: a single-centre, open-label, phase 2 trial. Lancet Oncol. 2016;17(1):48–56. [DOI] [PubMed] [Google Scholar]
- 15.Martin P, Maddocks K, Leonard JP, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood. 2016;127(12):1559–1563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.