Abstract
Purpose
To review the efficacy and optimal use of parathyroid hormone and parathyroid hormone-related protein analogs in osteoporosis treatment.
Recent findings
The parathyroid hormone analog teriparatide, a potent stimulator of bone remodeling, increases hip and spine bone mineral density and reduces the risk of vertebral and non-vertebral fractures in postmenopausal osteoporotic women. The parathyroid hormone-related protein analog, abaloparatide, also reduces fracture incidence but has pharmacological effects that differ from teriparatide, particularly in cortical bone. These analogs provide maximal benefit when their use is followed by a potent antiresorptive medication. Moreover, studies have shown that the combination of teriparatide and the RANK-ligand inhibitor, denosumab, increase bone density and estimated strength more than monotherapy and more than any currently available regimen.
Summary
Parathyroid hormone and parathyroid hormone-related protein analogs, whether as monotherapy, in combination with antiresorptive agents, or in sequence with antiresorptive agents, will likely play an expanding role in osteoporosis management.
Keywords: parathyroid hormone, teriparatide, abaloparatide, osteoporosis, anabolic agents, combination therapy, bone mineral density
Introduction
The introduction of teriparatide, a parathyroid hormone (PTH) analog that is comprised of the first 34 amino acids of the endogenous hormone, was an important advance in osteoporosis therapy. And more than a decade later, it remains the only approved osteoporosis drug whose primary mechanism of action involves the stimulation of new bone formation. The full-length PTH protein, PTH 1-84, did not receive regulatory approval to treat osteoporosis in the United States although it has been approved for the treatment of hypoparathyroidism. An analog of PTH-related protein (PTHrP) is currently under regulatory review and may be available in 2017. All of these agents are currently administered by subcutaneous injection though efforts are currently underway to develop less invasive modes of delivery (1). In contrast to the primarily catabolic effects of chronic infusions of teriparatide (and other PTH analogs), intermittent subcutaneous administration of the drug or related compounds have a net positive effect on calcium balance and increase bone mass, particularly in trabecular bone (2, 3). These agents stimulate the proliferation and activity of osteoblasts with a peak effect on bone formation in humans after 6–12 months of exposure (4–8). But while PTH and PTHrP analogs are correctly classified as “anabolic”, it is important to recognize that they are not purely so as they concomitantly stimulate osteoclastic bone resorption, a property that may be necessary to mediate a portion of the corresponding positive anabolic effects of the drugs (5, 7). Conversely, the pro-resorptive effect of these analogs may also be among the factors that ultimately limit their therapeutic potential, particularly at anatomic sites comprised of significant cortical bone where they progressively increase cortical porosity (9–11). Therapeutic approaches that limit the pro-resorptive properties of these agents while preserving the anabolic effects underlie the current development of novel agents as well as combination therapy approaches. In this review, we will assess the effects of these analogs on bone metabolism, bone mineral density, and fracture risk, either alone or when used concomitantly with antiresorptive medications. Moreover, as many patients with established disease will likely be treated with both anabolic and antiresorptive drugs during their course of treatment, we will also review the effects of the use of PTH and PTHrP analogs in sequence with the more commonly used antiresorptive medications.
Mechanism of Action
PTH and PTHrP analogs exert their effects through binding to the PTH/PTHrP receptor, which is expressed in many tissues including on the surface of osteoblasts, osteocytes, and renal tubule cells (12). The anabolic pharmacologic efficacy of PTH and PTHrP appears to require intermittent administration as prolonged receptor stimulation enhances the effects on bone resorption whereas intermittent stimulation preferentially enhances bone formation (13). The molecular mechanisms that underlie the net anabolic activity of PTH and PTHrP analogs, however, are still being defined. Specifically, one of the principle questions that remains unanswered is what portion of the anabolic effects of these drugs are mediated through the initial stimulation of bone resorption (through the release of growth factors from the skeletal matrix) as opposed to direct effects on osteoblasts (decreased apoptosis), osteocytes (decreased sclerostin production), and lining cells (restored anabolic activity) (14). Whatever the relative importance of these mechanisms, however, the net effect of PTH and PTHrP analogs on the human skeleton is increased trabecular bone mass and improved trabecular microarchitecture with a concomitant increase in cortical bone porosity and a possible increase in cortical thickness and bone size (15–18). Cumulatively, these effects are therapeutically beneficial in terms of increases in estimated bone strength of the spine, hip and peripheral anatomic sites as well as decreased fractures (6, 8, 10, 11, 19–22).
Teriparatide Monotherapy
Effects on Bone Metabolism, Density, and Microarchitecture
Teriparatide administration stimulates bone resorption and formation in a dose-responsive fashion. Specifically, serum bone formation markers (and biopsy-documented bone formation rates) increase within days to weeks of the initiation of PTH analog therapy while the increase in resorption markers are delayed (5–7, 23–26). These observations led to the hypothesis that an “anabolic window” exists in the first several months of teriparatide therapy during which the anabolic effect predominates. Notably, the teriparatide-induced stimulation of bone formation peaks after 6–12 months of therapy, after which serum markers of both osteoblast and osteoclast activity slowly revert toward baseline levels (23). This waning of osteoblast stimulation may be one of the limiting factors in the clinical use of PTH analogs for longer periods. Moreover, studies investigating the effect of a “second course” of teriparatide therapy in men and postmenopausal women have suggested that the anabolic potential of teriparatide remains reduced even after a full year “drug holiday” (27). The mechanisms underlying this osteoblast “fatigue” have not been defined.
Teriparatide, as well as PTH 1-84, induce their largest increases in BMD at the spine, an anatomic site rich in trabecular bone (6, 8, 23). Conversely, changes in total hip and femoral neck BMD are minimal in the first year but increase modestly thereafter (6, 8, 28–30) (table 1). In studies that have directly compared the increases in BMD between PTH and PTHrP analogs and oral or parenteral bisphosphonates, spine BMD has been shown to increase more after 18–24 months of teriparatide or PTH 1-84 than with bisphosphonates whereas the increases in hip and femoral neck BMD are similar or slightly lower (7, 30–32). At the 1/3 distal radius, a site composed of predominantly cortical bone; teriparatide decreases DXA-derived areal BMD (6, 8). Whether this decrease represents true bone loss or rather a combination of bone loss, under-mineralized new bone, and subtle changes in bone size is not clear. The effects of PTH analogs on skeletal microarchitecture have been investigated primarily via bone biopsy of the iliac crest or high resolution QCT (HR-pQCT) of the peripheral skeleton. In the iliac crest, teriparatide significantly increases trabecular bone volume and connectivity, as well as cortical thickness (16, 33, 34). At the distal radius and tibia, teriparatide increases trabecular volumetric BMD and FEA-estimated strength but progressively decreases cortical volumetric BMD and increases cortical porosity for up to 24-months (11).
Table 1.
Mean changes in areal BMD of the lumbar spine, total hip, femoral neck, and distal radius in postmenopausal osteoporotic women treated with PTH and PTHrP analogs alone or in combination with antiresorptive medications (range given when multiple studies are referenced) (6, 8, 22, 28–31).
| Lumbar Spine | Total Hip | Femoral Neck | Distal Radius | |
|---|---|---|---|---|
| 12-month change in BMD | ||||
| teriparatide 20 mcg daily | +6–8% | +0–1% | +0–1% | −1.5% |
| parathyroid hormone 1-84 100 mcg daily | +6% | +0–1% | +0–1% | −3% |
| abaloparatide 80 mcg daily | +8–9% | +2.8% | +2.2% | −1% |
| teriparatide plus zoledronic acid | +7.5% | +2.3% | +2.2% | -- |
| parathyroid hormone 1-84 plus alendronate | +6.3% | +1.9% | +1.8% | −1.1% |
| teriparatide plus denosumab | +9.1% | +4.9% | +4.2% | +2.6% |
| 24-month change in BMD | ||||
| teriparatide 20 mcg daily | +9.5 | +2% | +2.6% | −1.7% |
| teriparatide plus denosumab | +12.9 | +6.3 | +6.8% | +2.2% |
Effects on Fracture Reduction
The anti-fracture efficacy of teriparatide was first demonstrated in a double-blind placebo-controlled study of 1637 postmenopausal osteoporotic women randomized to receive once-daily injections of placebo or teriparatide at a dose of 20 μg or 40 μg daily (8). The median duration of treatment was only 18 months as the trial was stopped early due to the concomitant finding of an increased incidence of osteosarcoma in rats receiving high doses of the drug (discussed in more detail below) (35, 36). Both doses of teriparatide reduced the risk of a new vertebral fracture by two thirds and non-vertebral fragility fractures were by slightly over 50%. While hip and spine BMD increased more with 40-μg of teriparatide than with 20-μg, there was no significant difference in anti-fracture efficacy between the 2 doses (8). Once weekly teriparatide, at a dose of 56.5 μg was also shown to reduce the risk of vertebral fracture but this dose is not available in most parts of the word (37). Once daily PTH 1-84 (100-μg) was investigated in a randomized controlled trial of 2532 osteoporotic postmenopausal women and it also reduced the rate of new or worsened vertebral fracture but did not significantly reduce the rate of non-vertebral fractures and did not receive US regulatory approval (6).
Effects of PTH analogs in Men and in Glucocorticoid-Induced Osteoporosis
The efficacy of teriparatide was studied in a trial of 437 osteoporotic men and the changes in BMD through 11-months of follow-up were similar to those described in women (8, 38). Moreover, in an 30-month follow-up to this study, men who had been treated with teriparatide experienced 83% fewer vertebral fractures than those treated with placebo (39). Several smaller studies have also confirmed the anabolic effects of PTH in men (40, 41).
Glucocorticoid induced osteoporosis (GIO), unlike postmenopausal osteoporosis, is characterized by bone turnover suppression and reduced osteoblast function (42). Thus, it was hypothesized that an anabolic agent such as teriparatide would be more effective than antiresorptive medications in this population. This hypothesis was confirmed in an 18-month trial and subsequent 18 month extension in which 428 women and men with GIO were randomized to alendronate 10-mg daily or teriparatide 20-μg daily and those treated with teriparatide experienced larger increases in spine and hip BMD as well as reduced rates of new vertebral fractures (43, 44). In a separate GIO study confined to men, teriparatide (20-μg daily) also increased BMD, improved trabecular and cortical microarchitecture of the radius and tibia, and increased FEA-estimated vertebral strength more than risedronate (35 mg weekly) (45). Benefits of teriparatide in GIO have also been demonstrated in trials with weekly teriparatide in observational cohorts, and in women on concomitant hormone replacement therapy (46–48).
Effects of PTH analogs in Combination with Antiresorptive Medications
Unlike most common medical conditions, the treatment of osteoporosis is distinctive in that there is no accepted role for using more than one drug at a time. Over the past decade, however, several studies have explored the skeletal effects of using PTH analogs along with antiresorptive drugs.
PTH analogs and Estrogens/Selective Estrogen Receptor Modulators
While many of earliest studies of combination therapy focused on the co-administration of estrogen and PTH analogs, the consistent lack of a monotherapy control group limits their interpretation (49–54). In a relatively short-term 6-month controlled trial, 137 postmenopausal women with osteoporosis were randomized to receive teriparatide (20-μg daily) plus the selective estrogen-receptor modulator, raloxifene (60-mg daily) or teriparatide plus placebo (no raloxifene monotherapy arm) (55). Bone formation (as assessed by changes in N-terminal propeptide of type 1 collagen, PINP), increased similarly in both groups while bone resorption (as assessed by changes in C-telopeptide, CTX) increased less in women treated with combination therapy. Despite these seemingly preferential changes in bone turnover, however, total hip BMD (but not BMD at other anatomic sites) increased more in the teriparatide monotherapy group than the combination group. Conversely, in a separate study of 125 postmenopausal women, lumbar spine BMD (but not total hip or femoral neck BMD) increased more in women in whom 9-months of raloxifene was added to on-going teriparatide therapy than in women treated with continued teriparatide monotherapy (56). Taken together, it remains unclear if either estrogens or raloxifene provide benefit when combined with PTH but longer studies and those utilizing a teriparatide-alone control group are needed to fully define the potential of these approaches.
PTH analogs and Bisphosphonates
The nitrogen-containing bisphosphonates act by binding to hydroxyapatite and inhibiting the enzyme farnesyl diphosphate synthase in the cholesterol biosynthetic pathway, suppressing protein geranylgeranylation, and hence osteoclastic bone resorption (57). These drugs persist in the bone matrix for prolonged periods but there are differences in the potency and persistence of the biologic activity among these agents that may account for their distinct effects when combined with anabolic agents (58). For example, in a randomized controlled trial of postmenopausal osteoporotic women assigned to receive PTH 1-84 (100-μg daily), alendronate 10-mg daily, or both for 12-months, lumbar spine BMD increased similarly in all groups while hip BMD increased similarly in the alendronate and combinations groups but, as noted above, did not increase with only 12 months of PTH 1-84 monotherapy (30). When volumetric trabecular BMD of the vertebral bodies were measured by quantitative CT in this study, a different pattern emerged as BMD increased 2-fold more in the PTH 1-84 monotherapy group than in the combination group. In a 30-month clinical trial in which postmenopausal women were randomized to receive either alendronate 10-mg daily, teriparatide 40-μg daily, or both medications, both DXA-derived spine and hip BMD increased more in women treated with teriparatide alone than those treated with either alendronate alone or both agents (23). In both of these studies, biochemical markers of bone turnover were less completely suppressed in subjects receiving combination therapy than in subjects receiving alendronate alone, demonstrating a partial bisphosphonate-induced blunting of the anabolic effects of the PTH analog. This lack of an additive effect on BMD was also apparent in a 12-month trial of 412 postmenopausal women randomized to a single infusion of zoledronic acid 5 mg, teriparatide 20-μg daily, or the combination of both medications. In this study, spine BMD increased similarly in the combination group and the teriparatide monotherapy group and total hip and femoral neck BMD increased similarly in the combination group and the zoledronic acid monotherapy group (59).
In a study aimed to assess the effects of adding teriparatide to ongoing alendronate, hip and spine BMD increased more in postmenopausal women in whom teriparatide was added to alendronate compared to women in whom alendronate was discontinued when teriparatide was started (60). Because there was no group in which alendronate was continued without anabolic therapy, it is unclear whether adding teriparatide is preferable to simply continuing alendronate. In a separate study taking the opposite approach, alendronate was added to ongoing teriparatide and spine and hip BMD increased more than when teriparatide monotherapy was continued (though no comparison was made to switching from teriparatide to alendronate) (56, 61). Taken together with other smaller combination therapy studies (62, 63), the data suggests that the co-administration of bisphosphonates and PTH analogs does not provide clinical benefits compared to monotherapy, though approaches that involve adding one class of agent to patients already being treated with the other class of agents deserve further exploration.
Teriparatide Combined with Denosumab
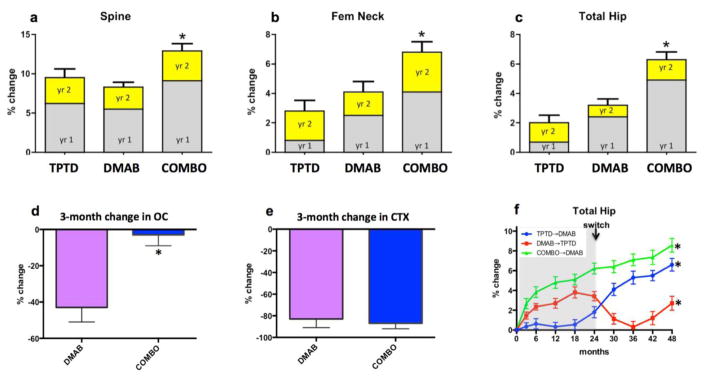
Denosumab is a monoclonal antibody that blocks the binding of receptor activator of NFκB (RANK)-ligand to its osteoclast-derived receptor, RANK, thus inhibiting osteoclast formation, activation and survival and hence bone resorption (64, 65). In the Denosumab and Teriparatide Administration (DATA) studies, 94 postmenopausal women with osteoporosis were randomly assigned to receive teriparatide (20-μg daily), denosumab (60-mg every 6-months), or both medications for 24-month and BMD at the spine and hip increased most in those treated with both drugs (figure, panels a–c) (28, 29). Moreover, most of the benefit of combination therapy was manifest during the first 12-months of treatment, during which lumbar spine BMD increased by 9.1% in the combination group versus 6.2% and 5.5% in the teriparatide or denosumab groups, respectively. Moreover, the 1-year changes at the total hip exceeded a simple additive effect as hip BMD increased by 4.9% in the combination group compared to 0.7% and 2.5%, in the teriparatide and denosumab groups, respectively. Finally, the concomitant administration of denosumab was able to prevent the loss in distal radius BMD that was observed with teriparatide monotherapy.
Figure.
Year 1 and year 2 change in BMD in patients treated with teriparatide (TPTD), denosumab (DMAB), or both medications (COMBO) in the DATA study (panels a–c). Change in c-telopeptide (CTX) and osteocalcin (OC) 3-months after treatment initiation with denosumab (DMAB) or both medications (COMBO) in the DATA study (panels d and e). Change in total hip BMD over 48 months in patients treated with 2 years of teriparatide followed by 2 years of denosumab, 2 years of denosumab followed by 2 years of teriparatide, or 2 years of combination therapy followed by 2 years of denosumab in the DATA-Switch study. *p<0.05 versus other groups. Adapted from references (28, 29, 72)
The pattern of the changes in bone turnover also differed in patients treated with combined denosumab and teriparatide than in studies in which patients were treated with bisphosphonate-containing combinations. Specifically, bone resorption markers were identical in patients treated with denosumab monotherapy and those treated with combined denosumab and teriparatide whereas markers of bone formation were significantly more suppressed in those treated with denosumab alone, especially at the early time points (figure, panels d and e). Together, these findings suggest that the superior efficacy of combined denosumab and teriparatide may be due, at least in part, to denosumab’s ability to fully block the pro-resorptive effects of teriparatide while still allowing for teriparatide-induced stimulation of modeling-based bone formation, particularly at anatomic sites rich in cortical bone. Consistent with this hypothesis, cortical volumetric BMD and thickness increased more in women treated with combined denosumab plus teriparatide than either monotherapy group (assessed by HR-pQCT of the radius and tibia). Moreover, cortical porosity, which increased progressively in women treated with teriparatide alone remained stable (and similar to denosumab monotherapy) in women treated with both drugs for up to 24-months (11).
Effects of PTH Analogs in Sequence with Antiresorptive Medications
Antiresorptive Therapy after PTH Analogs
When PTH analogs are discontinued, BMD at both the spine and hip progressively decrease with the rate of the decline being faster in estrogen deficient women than in eugonadal men (27, 66). Because the use of these analogs is limited to 18–24 months by regulatory authorities, this becomes an important clinical issue in virtually all patients treated with these drugs. And while some studies suggest that teriparatide continues to protect patients from fractures even after it is stopped (and despite on-going bone loss), the potential advantages of maintaining or further increasing BMD in these patients are clear (66, 67). Importantly, oral bisphosphonates have consistently demonstrated that they reliably prevent bone loss in patients transitioning from anabolic drugs and in most cases further increase BMD at both the hip and spine (66, 68–70). The selective estrogen receptor modulator, raloxifene, also prevents bone loss at the hip and spine but increases BMD at the femoral neck only (71). More recently, it was reported that even larger BMD gains are achieved when teriparatide is followed by denosumab. Specifically, in the DATA-Switch study (figure, panel f), spine and femoral neck BMD increased by an additional 9.4% and 5.8%, respectively, in women transitioning from teriparatide to an additional 2 years of denosumab. Moreover, at the distal radius, where 2 years of teriparatide decreased BMD by 2%, denosumab fully reversed this BMD loss within 6 months (72). Finally, denosumab also further increased BMD in women who had already received 2-years of combined teriparatide and denosumab therapy, resulting in cumulative 4-year BMD gains of 16.0% and 9.1% at the spine and femoral neck, respectively (72).
PTH Analogs After Hormonal or Bisphosphonate Therapy
Because bisphosphonates are present in the skeletal matrix long after their administration has been discontinued, it had been theorized that PTH analogs may have differential effects when used in patients who had been treated with these drugs as compared to treatment-naïve patients (58). Several clinical trials subsequently investigated the effects of teriparatide when used after antiresorptive agents, though their interpretation is limited by non-randomized designs. Nonetheless, a consistent finding in these trials is that teriparatide-induced increases in spine BMD, while significant, are more modest when used after bisphosphonates than when administered de novo. In contrast, BMD at the hip has been reported to either remain stable or decrease slightly when bisphosphonate-treated women are then treated with a PTH analog for 1 year, though after 2 years hip BMD does increase (60, 73–76).
In a non-randomized comparison study contrasting the changes in BMD in postmenopausal women pre-treated with raloxifene or alendronate for 1.5–3 years, teriparatide increased BMD more in patients treated with the raloxifene than alendronate, particularly at early time points (75). Similarly, when the effects of teriparatide were assessed in patients who had been exposed to one of several oral bisphosphonates, BMD increased less in those previously treated with bisphosphonates with higher affinity to hydroxyapatite (such as alendronate) compared to those treated with bisphosphonates with a lower affinity (such as risedronate or etidronate) (58, 73–75). Together, these studies suggest that a residual bisphosphonate presence may blunt the anabolic potential of the PTH analog even after the drug has been discontinued, though how long such an effect persists is not known.
Teriparatide After Denosumab
Because the effects of denosumab do not persist in the skeleton after the drug is discontinued, it was hypothesized that the use of teriparatide after denosumab would avoid the blunting observed when teriparatide follows bisphosphonates (72). This hypothesis was tested, but ultimately contradicted, in the DATA-Switch study in which women who received 2 years of denosumab followed by 2 years of teriparatide experienced significant transient bone loss at the spine and even more extensive and progressive bone loss at the hip and distal radius (figure, panel f). In fact, when the total 4-year changes are compared, women treated with 2 years of teriparatide followed by 2 years of denosumab experienced a 3.9% greater increase in hip BMD compared to those treated with 2 years of denosumab followed by 2 years of teriparatide. Perhaps most strikingly, the transition from denosumab to teriparatide was associated with extremely accelerated bone remodeling as 6-months after the transition median osteocalcin and CTX levels were 275% and 200% above their original baseline, respectively. Moreover, this high level of bone remodeling persisted as these markers remained well above baseline even after 24-months of teriparatide. The mechanism by which teriparatide exerts this prominent stimulatory effect on bone metabolism in patients discontinuing denosumab is unknown but may relate to teriparatide stimulating a large pool of dormant osteoclast precursors in patients exposed to long-term RANKL inhibition. Given the recent reports that even the less-extensive increase in bone remodeling that occurs when denosumab is discontinued without adding teriparatide may be associated with an increased incidence of multiple vertebral fractures (77–81), it is prudent for physicians to avoid switching patients from denosumab to teriparatide until the clinical ramifications are fully defined.
Abaloparatide
Abaloparatide is a synthetic peptide analog of PTH-related protein (rhPTHrP 1-34) that binds to the PTH/PTHrP receptor with a greater selectivity to the RG conformation than does teriparatide thus mediating a more transient response in PTH/PTHrP-receptor-expressing cells (82). It has been hypothesized that this differential selectivity mediates the modest differences in the clinical effects of abaloparatide versus teriparatide, particularly in sites comprised of significant cortical bone (table) (83). In a recently published phase 3 trial in which approximately 2400 women were randomized to receive blinded daily subcutaneous injections of placebo, abaloparatide 80 μg, or open-label teriparatide (20 μg) for 18 months, those treated with abaloparatide experienced an 86% relative risk reduction in new morphometric vertebral fractures compared to placebo while teriparatide reduced the risk by 80% (no significant difference between abaloparatide and teriparatide) (22). Abaloparatide also reduced the risk of non-vertebral fracture by 43%, which differed significantly from placebo but not open label teriparatide (which reduced the risk by a non-significant 28% relative to placebo). Abaloparatide and teriparatide increased spine BMD similarly but hip and femoral neck BMD increased by approximately 1% more in those treated with abaloparatide compared to teriparatide. Interestingly, biochemical markers of both bone resorption and formation increased less with abaloparatide than teriparatide and the incidence of hypercalcemia was also lower with abaloparatide (6.4%) compared to teriparatide (3.4%). The above clinical trial has been extended for an additional 6 months during which subjects who received 18 months of either abaloparatide or placebo then received 6 months of oral alendronate (70 mg daily). The results of this extension are not yet published.
Osteosarcoma
A thorough discussion of the safety issues related to PTH and PTHrP analogs is beyond the scope of this review. Nonetheless it should be noted that toxicology studies investigating the effect these analogs on young rats show dose-dependent osteosclerosis, extra-medullary hematopoiesis, and increased risk of osteosarcoma when administered at doses greater than the human equivalent starting at a young age (35, 36, 84–86). Based on these findings, teriparatide and PTH 1-84 labels include a “Black Box” warning as likely will abaloparatide should it be approved. In 2003, The Osteosarcoma Surveillance Study was initiated to evaluate a potential association between teriparatide and osteosarcoma in humans. After 7 years of the planned 15-year follow-up, no association between teriparatide use and subsequent osteosarcoma was detected (87).
Conclusion
As the only currently approved anabolic agent used in the treatment of osteoporosis in the U.S., teriparatide, despite limitations of high cost and parenteral route of delivery, has proven to be an important therapeutic option in a diverse group of patients at high risk of fracture. In the coming months, abaloparatide may join teriparatide as an additional anabolic option in the management of established postmenopausal osteoporosis. Current studies demonstrate that PTH and PTHrP analogs are most effective when used before, rather than following, antiresorptive medications. Moreover, it is clear that the transition from denosumab to teriparatide is unique in its stimulation of rapid, high-turnover bone loss and should be avoided. Finally, while the combination of bisphosphonates and PTH analogs do not appear to provide a significant advantage compared to monotherapy with PTH analogs alone, combined denosumab and teriparatide additively increase spine and hip BMD and improve skeletal microarchitecture at peripheral sites. The combination of denosumab and teriparatide must now be assessed for its capacity to reduce fracture incidence in patients with severe disease.
Footnotes
Compliance with Ethical Guidelines
Conflict of Interest
Benjamin Leder reports grants and personal fees from Amgen, grants from Lilly, during the conduct of the study; personal fees from Lilly, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Polyzos SA, Makras P, Efstathiadou Z, Anastasilakis AD. Investigational parathyroid hormone receptor analogs for the treatment of osteoporosis. Expert Opin Investig Drugs. 2015;24(2):145–57. doi: 10.1517/13543784.2015.973021. [DOI] [PubMed] [Google Scholar]
- 2.Silva BC, Bilezikian JP. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol. 2015;22:41–50. doi: 10.1016/j.coph.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kousteni S, Bilezikian JP. The cell biology of parathyroid hormone in osteoblasts. Curr Osteoporos Rep. 2008;6(2):72–6. doi: 10.1007/s11914-008-0013-9. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein JS, Leder BZ, Burnett SM, Wyland JJ, Lee H, de la Paz AV, et al. Effects of teriparatide, alendronate, or both on bone turnover in osteoporotic men. J Clin Endocrinol Metab. 2006;91(8):2882–7. doi: 10.1210/jc.2006-0190. [DOI] [PubMed] [Google Scholar]
- 5.Glover SJ, Eastell R, McCloskey EV, Rogers A, Garnero P, Lowery J, et al. Rapid and robust response of biochemical markers of bone formation to teriparatide therapy. Bone. 2009;45(6):1053–8. doi: 10.1016/j.bone.2009.07.091. [DOI] [PubMed] [Google Scholar]
- 6.Greenspan SL, Bone HG, Ettinger MP, Hanley DA, Lindsay R, Zanchetta JR, et al. Effect of recombinant human parathyroid hormone (1-84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med. 2007;146(5):326–39. doi: 10.7326/0003-4819-146-5-200703060-00005. [DOI] [PubMed] [Google Scholar]
- 7.McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, et al. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165(15):1762–8. doi: 10.1001/archinte.165.15.1762. [DOI] [PubMed] [Google Scholar]
- 8.Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- 9.Hansen S, Hauge EM, Jensen JE, Brixen K. Differing effects of PTH 1-34, PTH 1-84 and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis. An 18 month open-labeled observational study using HR-pQCT. J Bone Miner Res. 2012 doi: 10.1002/jbmr.1784. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald HM, Nishiyama KK, Hanley DA, Boyd SK. Changes in trabecular and cortical bone microarchitecture at peripheral sites associated with 18 months of teriparatide therapy in postmenopausal women with osteoporosis. Osteoporos Int. 2011;22(1):357–62. doi: 10.1007/s00198-010-1226-1. [DOI] [PubMed] [Google Scholar]
- •11.Tsai JN, Uihlein AV, Burnett-Bowie SM, Neer RM, Derrico NP, Lee H, et al. Effects of Two Years of Teriparatide, Denosumab, or Both on Bone Microarchitecture and Strength (DATA-HRpQCT study) J Clin Endocrinol Metab. 2016:jc20161160. doi: 10.1210/jc.2016-1160. This study demonstrates that 2 years of combined teriparatide and denosumab improves bone microarchitecture and estimated strength more than the individual treatments. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardella T. Interactions of PTH with Receptors and Signaling. In: Bilezikian J, editor. The Parathyroids. 3. New York, NY: Elsevier; 2015. pp. 65–80. [Google Scholar]
- 13.Frolik CA, Black EC, Cain RL, Satterwhite JH, Brown-Augsburger PL, Sato M, et al. Anabolic and catabolic bone effects of human parathyroid hormone (1-34) are predicted by duration of hormone exposure. Bone. 2003;33(3):372–9. doi: 10.1016/s8756-3282(03)00202-3. [DOI] [PubMed] [Google Scholar]
- 14.Martin TJ. Bone biology and anabolic therapies for bone: current status and future prospects. Journal of bone metabolism. 2014;21(1):8–20. doi: 10.11005/jbm.2014.21.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dempster DW, Cosman F, Zhou H, Nieves JW, Bostrom M, Lindsay R. Effects of Daily or Cyclic Teriparatide on Bone Formation in the Iliac Crest in Women on No Prior Therapy and in Women on Alendronate. J Bone Miner Res. 2016;31(8):1518–26. doi: 10.1002/jbmr.2822. [DOI] [PubMed] [Google Scholar]
- 16.Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18(11):1932–41. doi: 10.1359/jbmr.2003.18.11.1932. [DOI] [PubMed] [Google Scholar]
- 17.Nishiyama KK, Cohen A, Young P, Wang J, Lappe JM, Guo XE, et al. Teriparatide increases strength of the peripheral skeleton in premenopausal women with idiopathic osteoporosis: A pilot HR-pQCT study. J Clin Endocrinol Metab. 2014:jc20141041. doi: 10.1210/jc.2014-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parfitt AM. Parathyroid hormone and periosteal bone expansion. J Bone Miner Res. 2002;17(10):1741–3. doi: 10.1359/jbmr.2002.17.10.1741. [DOI] [PubMed] [Google Scholar]
- 19.Hansen S, Hauge EM, Beck Jensen JE, Brixen K. Differing effects of PTH 1-34, PTH 1-84, and zoledronic acid on bone microarchitecture and estimated strength in postmenopausal women with osteoporosis: an 18-month open-labeled observational study using HR-pQCT. J Bone Miner Res. 2013;28(4):736–45. doi: 10.1002/jbmr.1784. [DOI] [PubMed] [Google Scholar]
- 20.Keaveny TM, Donley DW, Hoffmann PF, Mitlak BH, Glass EV, San Martin JA. Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res. 2007;22(1):149–57. doi: 10.1359/jbmr.061011. [DOI] [PubMed] [Google Scholar]
- 21.Keaveny TM, McClung MR, Wan X, Kopperdahl DL, Mitlak BH, Krohn K. Femoral strength in osteoporotic women treated with teriparatide or alendronate. Bone. 2012;50(1):165–70. doi: 10.1016/j.bone.2011.10.002. [DOI] [PubMed] [Google Scholar]
- ••22.Miller PD, Hattersley G, Riis BJ, Williams GC, Lau E, Russo LA, et al. Effect of Abaloparatide vs Placebo on New Vertebral Fractures in Postmenopausal Women With Osteoporosis: A Randomized Clinical Trial. JAMA. 2016;316(7):722–33. doi: 10.1001/jama.2016.11136. This study reports that the PTHrP analog, abaloparatide, reduces the risk of new vertebral and nonvertebral fractures in postmenopausal osteoporotic women. [DOI] [PubMed] [Google Scholar]
- 23.Finkelstein JS, Wyland JJ, Lee H, Neer RM. Effects of teriparatide, alendronate, or both in women with postmenopausal osteoporosis. J Clin Endocrinol Metab. 2010;95(4):1838–45. doi: 10.1210/jc.2009-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dempster DW, Roschger P, Misof BM, Zhou H, Paschalis EP, Alam J, et al. Differential Effects of Teriparatide and Zoledronic Acid on Bone Mineralization Density Distribution at 6 and 24 Months in the SHOTZ Study. J Bone Miner Res. 2016;31(8):1527–35. doi: 10.1002/jbmr.2825. [DOI] [PubMed] [Google Scholar]
- •25.Cosman F, Dempster DW, Nieves JW, Zhou H, Zion M, Roimisher C, et al. Effect of Teriparatide on Bone Formation in the Human Femoral Neck. J Clin Endocrinol Metab. 2016;101(4):1498–505. doi: 10.1210/jc.2015-3698. This study describes teriparatide’s ability to rapidly stimulate bone formation in cancellous and endocortical envelopes of the femoral neck in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with PTH(1-34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J Bone Miner Res. 2007;22(4):495–502. doi: 10.1359/jbmr.070104. [DOI] [PubMed] [Google Scholar]
- 27.Leder BZ, Neer RM, Wyland JJ, Lee HW, Burnett-Bowie SM, Finkelstein JS. Effects of teriparatide treatment and discontinuation in postmenopausal women and eugonadal men with osteoporosis. J Clin Endocrinol Metab. 2009;94(8):2915–21. doi: 10.1210/jc.2008-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••28.Tsai JN, Uihlein AV, Lee H, Kumbhani R, Siwila-Sackman E, McKay EA, et al. Teriparatide and denosumab, alone or combined, in women with postmenopausal osteoporosis: the DATA study randomised trial. Lancet. 2013;382(9886):50–6. doi: 10.1016/S0140-6736(13)60856-9. This is the first study to demsonstrate that, unlike combinations utilizing bisphosphonates, combined teriparatide and denosumab increases hip and spine BMD in postmenopausal women more than either agent alone and more than has been reported with any approved therapy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •29.Leder BZ, Tsai JN, Uihlein AV, Burnett-Bowie SA, Zhu Y, Foley K, et al. Two years of Denosumab and teriparatide administration in postmenopausal women with osteoporosis (The DATA Extension Study): a randomized controlled trial. J Clin Endocrinol Metab. 2014;99(5):1694–700. doi: 10.1210/jc.2013-4440. This study extends the results of reference 28 and reports that 2 years of concomitant teriparatide and denosumab therapy increases BMD more than either medication alone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Black DM, Greenspan SL, Ensrud KE, Palermo L, McGowan JA, Lang TF, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349(13):1207–15. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 31.Cosman F, Eriksen EF, Recknor C, Miller PD, Guanabens N, Kasperk C, et al. Effects of intravenous zoledronic acid plus subcutaneous teriparatide [rhPTH(1-34)] in postmenopausal osteoporosis. J Bone Miner Res. 2011;26(3):503–11. doi: 10.1002/jbmr.238. [DOI] [PubMed] [Google Scholar]
- 32.Body JJ, Gaich GA, Scheele WH, Kulkarni PM, Miller PD, Peretz A, et al. A randomized double-blind trial to compare the efficacy of teriparatide [recombinant human parathyroid hormone (1-34)] with alendronate in postmenopausal women with osteoporosis. The Journal of clinical endocrinology and metabolism. 2002;87(10):4528–35. doi: 10.1210/jc.2002-020334. [DOI] [PubMed] [Google Scholar]
- 33.Reeve J, Meunier PJ, Parsons JA, Bernat M, Bijvoet OL, Courpron P, et al. Anabolic effect of human parathyroid hormone fragment on trabecular bone in involutional osteoporosis: a multicentre trial. British medical journal. 1980;280(6228):1340–4. doi: 10.1136/bmj.280.6228.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2001;16(10):1846–53. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 35.Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M. Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol Pathol. 2004;32(4):426–38. doi: 10.1080/01926230490462138. [DOI] [PubMed] [Google Scholar]
- 36.Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30(3):312–21. doi: 10.1080/01926230252929882. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura T, Sugimoto T, Nakano T, Kishimoto H, Ito M, Fukunaga M, et al. Randomized Teriparatide [human parathyroid hormone (PTH) 1-34] Once-Weekly Efficacy Research (TOWER) trial for examining the reduction in new vertebral fractures in subjects with primary osteoporosis and high fracture risk. J Clin Endocrinol Metab. 2012;97(9):3097–106. doi: 10.1210/jc.2011-3479. [DOI] [PubMed] [Google Scholar]
- 38.Orwoll ES, Scheele WH, Paul S, Adami S, Syversen U, Diez-Perez A, et al. The effect of teriparatide [human parathyroid hormone (1-34)] therapy on bone density in men with osteoporosis. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2003;18(1):9–17. doi: 10.1359/jbmr.2003.18.1.9. [DOI] [PubMed] [Google Scholar]
- 39.Kaufman JM, Orwoll E, Goemaere S, San Martin J, Hossain A, Dalsky GP, et al. Teriparatide effects on vertebral fractures and bone mineral density in men with osteoporosis: treatment and discontinuation of therapy. Osteoporos Int. 2005;16(5):510–6. doi: 10.1007/s00198-004-1713-3. [DOI] [PubMed] [Google Scholar]
- 40.Finkelstein JS, Hayes A, Hunzelman JL, Wyland JJ, Lee H, Neer RM. The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med. 2003;349(13):1216–26. doi: 10.1056/NEJMoa035725. [DOI] [PubMed] [Google Scholar]
- 41.Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP. Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. The Journal of clinical endocrinology and metabolism. 2000;85(9):3069–76. doi: 10.1210/jcem.85.9.6818. [DOI] [PubMed] [Google Scholar]
- 42.Weinstein RS. Clinical practice. Glucocorticoid-induced bone disease. The New England journal of medicine. 2011;365(1):62–70. doi: 10.1056/NEJMcp1012926. [DOI] [PubMed] [Google Scholar]
- 43.Saag KG, Shane E, Boonen S, Marin F, Donley DW, Taylor KA, et al. Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med. 2007;357(20):2028–39. doi: 10.1056/NEJMoa071408. [DOI] [PubMed] [Google Scholar]
- 44.Saag KG, Zanchetta JR, Devogelaer JP, Adler RA, Eastell R, See K, et al. Effects of teriparatide versus alendronate for treating glucocorticoid-induced osteoporosis: thirty-six-month results of a randomized, double-blind, controlled trial. Arthritis Rheum. 2009;60(11):3346–55. doi: 10.1002/art.24879. [DOI] [PubMed] [Google Scholar]
- 45.Gluer CC, Marin F, Ringe JD, Hawkins F, Moricke R, Papaioannu N, et al. Comparative effects of teriparatide and risedronate in glucocorticoid-induced osteoporosis in men: 18-month results of the EuroGIOPs trial. J Bone Miner Res. 2013;28(6):1355–68. doi: 10.1002/jbmr.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lane NE, Sanchez S, Modin GW, Genant HK, Pierini E, Arnaud CD. Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest. 1998;102(8):1627–33. doi: 10.1172/JCI3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seno T, Yamamoto A, Kukida Y, Hirano A, Kida T, Nakabayashi A, et al. Once-weekly teriparatide improves glucocorticoid-induced osteoporosis in patients with inadequate response to bisphosphonates. SpringerPlus. 2016;5(1):1056. doi: 10.1186/s40064-016-2704-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karras D, Stoykov I, Lems WF, Langdahl BL, Ljunggren O, Barrett A, et al. Effectiveness of teriparatide in postmenopausal women with osteoporosis and glucocorticoid use: 3-year results from the EFOS study. J Rheumatol. 2012;39(3):600–9. doi: 10.3899/jrheum.110947. [DOI] [PubMed] [Google Scholar]
- 49.Reeve J, Davies UM, Hesp R, McNally E, Katz D. Treatment of osteoporosis with human parathyroid peptide and observations on effect of sodium fluoride. Bmj. 1990;301(6747):314–8. doi: 10.1136/bmj.301.6747.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reeve J, Bradbeer JN, Arlot M, Davies UM, Green JR, Hampton L, et al. hPTH 1-34 treatment of osteoporosis with added hormone replacement therapy: biochemical, kinetic and histological responses. Osteoporos Int. 1991;1(3):162–70. doi: 10.1007/BF01625448. [DOI] [PubMed] [Google Scholar]
- 51.Reeve J, Arlot ME, Bradbeer JN, Hesp R, McAlly E, Meunier PJ, et al. Human parathyroid peptide treatment of vertebral osteoporosis. Osteoporos Int. 1993;3(Suppl 1):199–203. doi: 10.1007/BF01621906. [DOI] [PubMed] [Google Scholar]
- 52.Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, et al. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350(9077):550–5. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]
- 53.Cosman F, Nieves J, Woelfert L, Formica C, Gordon S, Shen V, et al. Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res. 2001;16(5):925–31. doi: 10.1359/jbmr.2001.16.5.925. [DOI] [PubMed] [Google Scholar]
- 54.Ste-Marie LG, Schwartz SL, Hossain A, Desaiah D, Gaich GA. Effect of teriparatide [rhPTH(1-34)] on BMD when given to postmenopausal women receiving hormone replacement therapy. J Bone Miner Res. 2006;21(2):283–91. doi: 10.1359/JBMR.051020. [DOI] [PubMed] [Google Scholar]
- 55.Deal C, Omizo M, Schwartz EN, Eriksen EF, Cantor P, Wang J, et al. Combination teriparatide and raloxifene therapy for postmenopausal osteoporosis: results from a 6-month double-blind placebo-controlled trial. J Bone Miner Res. 2005;20(11):1905–11. doi: 10.1359/JBMR.050714. [DOI] [PubMed] [Google Scholar]
- •56.Muschitz C, Kocijan R, Fahrleitner-Pammer A, Lung S, Resch H. Antiresorptives overlapping ongoing teriparatide treatment result in additional increases in bone mineral density. J Bone Miner Res. 2013;28(1):196–205. doi: 10.1002/jbmr.1716. This study reports that alendronate, when added to 9 months of teriparatide in postmenopausal women increases BMD more than continuing teriparatide monotherapy. [DOI] [PubMed] [Google Scholar]
- 57.Reszka AA, Rodan GA. Mechanism of action of bisphosphonates. Curr Osteoporos Rep. 2003;1(2):45–52. doi: 10.1007/s11914-003-0008-5. [DOI] [PubMed] [Google Scholar]
- 58.Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, et al. Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone. 2006;38(5):617–27. doi: 10.1016/j.bone.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Delmas PD, Munoz F, Black DM, Cosman F, Boonen S, Watts NB, et al. Effects of yearly zoledronic acid 5 mg on bone turnover markers and relation of PINP with fracture reduction in postmenopausal women with osteoporosis. J Bone Miner Res. 2009;24(9):1544–51. doi: 10.1359/jbmr.090310. [DOI] [PubMed] [Google Scholar]
- 60.Cosman F, Wermers RA, Recknor C, Mauck KF, Xie L, Glass EV, et al. Effects of teriparatide in postmenopausal women with osteoporosis on prior alendronate or raloxifene: differences between stopping and continuing the antiresorptive agent. J Clin Endocrinol Metab. 2009;94(10):3772–80. doi: 10.1210/jc.2008-2719. [DOI] [PubMed] [Google Scholar]
- 61.Muschitz C, Kocijan R, Fahrleitner-Pammer A, Pavo I, Haschka J, Schima W, et al. Overlapping and Continued Alendronate or Raloxifene Administration in Patients on Teriparatide: Effects on Areal and Volumetric Bone Mineral Density The CONFORS Study. J Bone Miner Res. 2014 doi: 10.1002/jbmr.2216. [DOI] [PubMed] [Google Scholar]
- 62.Walker MD, Cusano NE, Sliney J, Jr, Romano M, Zhang C, McMahon DJ, et al. Combination therapy with risedronate and teriparatide in male osteoporosis. Endocrine. 2013;44(1):237–46. doi: 10.1007/s12020-012-9819-4. [DOI] [PubMed] [Google Scholar]
- 63.Schafer AL, Sellmeyer DE, Palermo L, Hietpas J, Eastell R, Shoback DM, et al. Six months of parathyroid Hormone (1-84) administered concurrently versus sequentially with monthly ibandronate over two years: the PTH and ibandronate combination study (PICS) randomized trial. J Clin Endocrinol Metab. 2012;97(10):3522–9. doi: 10.1210/jc.2012-1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756–65. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 65.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 66.Prince R, Sipos A, Hossain A, Syversen U, Ish-Shalom S, Marcinowska E, et al. Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. J Bone Miner Res. 2005;20(9):1507–13. doi: 10.1359/JBMR.050501. [DOI] [PubMed] [Google Scholar]
- 67.Lindsay R, Scheele WH, Neer R, Pohl G, Adami S, Mautalen C, et al. Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med. 2004;164(18):2024–30. doi: 10.1001/archinte.164.18.2024. [DOI] [PubMed] [Google Scholar]
- 68.Rittmaster RS, Bolognese M, Ettinger MP, Hanley DA, Hodsman AB, Kendler DL, et al. Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab. 2000;85(6):2129–34. doi: 10.1210/jcem.85.6.6614. [DOI] [PubMed] [Google Scholar]
- 69.Kurland ES, Heller SL, Diamond B, McMahon DJ, Cosman F, Bilezikian JP. The importance of bisphosphonate therapy in maintaining bone mass in men after therapy with teriparatide [human parathyroid hormone(1-34)] Osteoporos Int. 2004;15(12):992–7. doi: 10.1007/s00198-004-1636-z. [DOI] [PubMed] [Google Scholar]
- 70.Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353(6):555–65. doi: 10.1056/NEJMoa050336. [DOI] [PubMed] [Google Scholar]
- 71.Eastell R, Nickelsen T, Marin F, Barker C, Hadji P, Farrerons J, et al. Sequential treatment of severe postmenopausal osteoporosis after teriparatide: final results of the randomized, controlled European Study of Forsteo (EUROFORS) J Bone Miner Res. 2009;24(4):726–36. doi: 10.1359/jbmr.081215. [DOI] [PubMed] [Google Scholar]
- •72.Leder BZ, Tsai JN, Uihlein AV, Wallace PM, Lee H, Neer RM, et al. Denosumab and teriparatide transitions in postmenopausal osteoporosis (the DATA-Switch study): extension of a randomised controlled trial. Lancet. 2015;386(9999):1147–55. doi: 10.1016/S0140-6736(15)61120-5. This study demonstrates that in postmenopausal women, while switching from teriparatide or combined teriparatide/densoumab to densoumab monotherapy results in additional BMD gains, switching from denosumab to teriparatide results in accelerated bone turnover and signficant bone loss, particularly at the hip and distal radius. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Boonen S, Marin F, Obermayer-Pietsch B, Simoes ME, Barker C, Glass EV, et al. Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2008;93(3):852–60. doi: 10.1210/jc.2007-0711. [DOI] [PubMed] [Google Scholar]
- 74.Miller PD, Delmas PD, Lindsay R, Watts NB, Luckey M, Adachi J, et al. Early responsiveness of women with osteoporosis to teriparatide after therapy with alendronate or risedronate. J Clin Endocrinol Metab. 2008;93(10):3785–93. doi: 10.1210/jc.2008-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ettinger B, San Martin J, Crans G, Pavo I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res. 2004;19(5):745–51. doi: 10.1359/JBMR.040117. [DOI] [PubMed] [Google Scholar]
- 76.Cosman F, Nieves JW, Zion M, Garrett P, Neubort S, Dempster D, et al. Daily or Cyclical Teriparatide Treatment in Women With Osteoporosis on no Prior Therapy and Women on Alendronate. J Clin Endocrinol Metab. 2015;100(7):2769–76. doi: 10.1210/jc.2015-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Recknor C, Beck Jensen JE, Gilchrist N, Wang A, Pannacciuli N, Smith S, et al. Discontinuation of Denosumab and Associated Fracture Incidence: Analysis From FREEDOM and its Extension. Annual Meeting of the American Society of Bone and Mineral Research; Houston, TX. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Popp AW, Zysset PK, Lippuner K. Rebound-associated vertebral fractures after discontinuation of denosumab-from clinic and biomechanics. Osteoporos Int. 2016;27(5):1917–21. doi: 10.1007/s00198-015-3458-6. [DOI] [PubMed] [Google Scholar]
- 79.Lamy O, Gonzalez-Rodriguez E, Stoll D, Hans D, Aubry-Rozier B. Severe rebound-associated vertebral fractures after denosumab discontinuation: nine clinical cases report. J Clin Endocrinol Metab. 2016:jc20163170. doi: 10.1210/jc.2016-3170. [DOI] [PubMed] [Google Scholar]
- 80.Aubry-Rozier B, Gonzalez-Rodriguez E, Stoll D, Lamy O. Severe spontaneous vertebral fractures after denosumab discontinuation: three case reports. Osteoporos Int. 2016;27(5):1923–5. doi: 10.1007/s00198-015-3380-y. [DOI] [PubMed] [Google Scholar]
- 81.Anastasilakis AD, Makras P. Multiple clinical vertebral fractures following denosumab discontinuation. Osteoporos Int. 2016;27(5):1929–30. doi: 10.1007/s00198-015-3459-5. [DOI] [PubMed] [Google Scholar]
- 82.Hattersley G, Dean T, Corbin BA, Bahar H, Gardella TJ. Binding Selectivity of Abaloparatide for PTH-Type-1-Receptor Conformations and Effects on Downstream Signaling. Endocrinology. 2016;157(1):141–9. doi: 10.1210/en.2015-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Leder BZ, O’Dea LS, Zanchetta JR, Kumar P, Banks K, McKay K, et al. Effects of abaloparatide, a human parathyroid hormone-related peptide analog, on bone mineral density in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2015;100(2):697–706. doi: 10.1210/jc.2014-3718. [DOI] [PubMed] [Google Scholar]
- 84.Sato M, Vahle J, Schmidt A, Westmore M, Smith S, Rowley E, et al. Abnormal bone architecture and biomechanical properties with near-lifetime treatment of rats with PTH. Endocrinology. 2002;143(9):3230–42. doi: 10.1210/en.2002-220149. [DOI] [PubMed] [Google Scholar]
- 85.Jolette J, Wilker CE, Smith SY, Doyle N, Hardisty JF, Metcalfe AJ, et al. Defining a noncarcinogenic dose of recombinant human parathyroid hormone 1-84 in a 2-year study in Fischer 344 rats. Toxicol Pathol. 2006;34(7):929–40. doi: 10.1080/01926230601072301. [DOI] [PubMed] [Google Scholar]
- 86.Hattersley G, Attalla B, Varela A, Smith S. Comparison of osteosarcoma incidence between abaloparatide (BA058) and PTH (1--34) in long term rat studies. European Calcified Tissue Society Congress; Oct 17; Prague, Czech Republic. 2014. [Google Scholar]
- 87.Andrews EB, Gilsenan AW, Midkiff K, Sherrill B, Wu Y, Mann BH, et al. The US postmarketing surveillance study of adult osteosarcoma and teriparatide: study design and findings from the first 7 years. J Bone Miner Res. 2012;27(12):2429–37. doi: 10.1002/jbmr.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]