Abstract
Background
Electrocardiography (ECG), predictive of adverse outcomes in the general population, has not been studied in cancer survivors. We evaluated the prevalence of ECG abnormalities and associations with mortality among childhood cancer survivors.
Methods
Major and minor abnormalities were coded per the Minnesota Classification system for participants in the St. Jude Lifetime Cohort Study (n=2,715) and community controls (n=268). Odds ratios (OR) and 95% confidence intervals (CI) were calculated using multivariable logistic regression and hazard ratios (HR) using Cox proportional hazards regression.
Results
Survivors were a median age of 31.3 (range: 18.4–63.8) years at evaluation and 7.4 (range: 0–24.8) years at diagnosis. Prior therapies included cardiac-directed radiation (29.5%), anthracycline (57.9%) and alkylating (60%) chemotherapies. The prevalence of minor ECG abnormalities was similar among survivors and controls (65.2% versus 67.5%, p=0.6). Major ECG abnormalities were identified in 10.7% of survivors and 4.9% of controls (p<0.001). Among survivors, the most common major abnormalities were isolated ST/T wave abnormalities (7.2%), evidence of myocardial infarction (3.7%), and left ventricular hypertrophy with strain pattern (2.8%). Anthracyclines ≥300 mg/m2 (OR 1.7 95% CI 1.1–2.5) and cardiac radiation (OR 2.1 95% CI 1.5–2.9 [1–1999 cGy], 2.6 95% CI 1.6–3.9 [2000–2999 cGy], 10.5 95% CI 6.5–16.9 [≥3000 cGy]) were associated with major abnormalities. Thirteen participants had a cardiac-related death. Major abnormalities were predictive of all-cause mortality (HR 4.0 95% CI 2.1–7.8).
Conclusions
Major ECG abnormalities are common among childhood cancer survivors, associated with increasing doses of anthracyclines and cardiac radiation, and predictive of both cardiac and all-cause mortality.
Keywords: Electrocardiogram, Cancer, Childhood Cancer Survivors
Introduction
Cardiovascular complications of cancer therapy can hinder active treatment and contribute to late morbidity and mortality among survivors. Traditional cytotoxic agents as well as newer immune-based therapies have acute and chronic cardiac effects.1 Anthracycline-induced heart failure has been the most studied and resulted in recommendations for echocardiographic surveillance from both cardiology and oncology professional societies.2 However, cardiac effects of cancer therapy may also include coronary artery disease, valvular dysfunction, pericardial complications, or rhythm and conduction disturbances,3, 4 which may not be adequately evaluated by traditional echocardiography.
While evidence for routine electrocardiographic (ECG) screening in the asymptomatic general population is mixed,5 cardiovascular monitoring frequently begins with an ECG. Numerous population based studies suggest that ECG abnormalities are prevalent and predictive of incident cardiovascular events.6 Cardiovascular surveillance has become increasingly important for cancer survivors and efforts to improve early identification of cardiotoxicity advocate for newer, more advanced imaging modalities such as cardiac MRI and tissue Doppler imaging.7–9 However, no studies have systematically assessed ECG abnormalities among cancer survivors, leaving the role of ECG monitoring in this population poorly defined.
Cardiovascular disease is the leading non-cancer cause of death among children cured of cancer,10 and follow-up guidelines recommend baseline ECG testing followed by echocardiography based upon treatment and age at exposure.11 Arrhythmias have been reported in small cohorts12, 13 or single diagnostic groups14, but a detailed investigation of rhythm disturbances is lacking. Some have suggested nominal yield from ECG testing, but these evaluations were limited to cardiotoxic-exposed survivors, potentially missing abnormalities among those with other exposures.15 Using the St. Jude Lifetime Cohort (SJLIFE),16, 17 an ongoing study evaluating health outcomes among adults previously treated for a pediatric malignancy, we systematically assessed ECG findings, irrespective of treatment exposures. The aim of this investigation was to determine the occurrence of and risk factors for ECG abnormalities and associations between abnormalities and mortality among adult survivors of childhood cancer.
Methods
Participants
Among 4,153 eligible cancer survivors (≥18 years old and ≥10 years from diagnosis), treated for a malignancy at St. Jude Children’s Research Hospital, 2,715 (65%) returned for a comprehensive health evaluation, including a standard 12-lead resting ECG between November 1, 2007 and June 30, 2014. Two hundred twenty-one were lost to follow-up, 251 elected to participate by survey only, 375 declined, 458 were pending contact/scheduling, and 133 were missing either ECG (n=47) or radiation dosimetry (n=86) (Supplement Figure 1). A control group, frequency matched on age, sex, and race/ethnicity, was randomly and concurrently recruited from friends and non-first degree relatives of current and former St. Jude patients (i.e. individuals who might be accompanying or visiting a patient), minimizing the chance of close relatives of SJLIFE participants being a control participant. Documents and procedures were approved by the institutional review board; all participants provided informed consent.
Major and minor ECG abnormalities
Primary outcomes were major and minor ECG abnormalities classified by the Minnesota Code.18 Recordings were obtained (MAC1200, GE Healthcare, Milwaukee, WI) following a 5-minute rest, electronically stored, and digitally transferred to the Epidemiological Cardiology Research Center (Wake Forest University School of Medicine, Winston-Salem, NC) for central review and coding. Major ECG abnormalities included major ventricular conduction defects (complete left or right bundle branch block, major ventricular conduction delay with QRS>120 ms), definite myocardial infarction (defined as the presence of major Q wave abnormalities), possible myocardial infarction (defined as the presence of minor Q/QS wave plus major ST/T abnormalities), isolated major ST/T wave abnormalities, left ventricular hypertrophy with strain pattern, advanced atrioventricular conduction abnormalities (complete or second degree AV block), pacemaker, atrial fibrillation/flutter, and others. The QT interval was calculated with the QT index, which accounts for the distribution of QTc in the general population.19 Minor ECG abnormalities included minor isolated Q/QS wave abnormalities, minor isolated ST/T wave abnormalities, high R waves/increased QRS voltage denoting left or right ventricular hypertrophy without strain pattern, non-ischemic ST segment elevation, incomplete (left or right) bundle branch block, short PR interval, left axis deviation, right axis deviation, atrial and ventricular premature beats, atrial enlargement, and others. Participants with only minor ECG abnormalities were classified as having “any minor abnormalities,” and participants with major ECG abnormalities with or without coexisting minor ECG abnormalities were classified as having “any major ECG abnormalities”.
Mortality
Mortality was ascertained using a combination of the National Death Index (NDI) search (through December 31, 2013) and the St. Jude Children’s Research Hospital Cancer Registry (continuous process). Cause of death was identified using ICD 9 and 10 codes from the NDI or direct review of death certificates, medical records, or next-of-kin interviews conducted by registry staff. (Causes of death are shown in Supplemental Table I.)
Other variables
Sociodemographic information, including age, sex, race, and smoking status was obtained from questionnaires. Smoking was classified as current, past, or never, with those who reported smoking at least 100 cigarettes, but not in the last month, categorized as past smokers. Body mass index was calculated by dividing weight by height squared (kg/m2) and classified as underweight (<18.5 kg/m2), normal (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥30.0 kg/m2). Dyslipidemia was defined as a fasting low-density lipoprotein cholesterol ≥160 mg/dl, high-density lipoprotein cholesterol <40 mg/dl (men) or <50 mg/dl (women), triglycerides >150 mg/dl, or on treatment for a lipid abnormality. Diabetes was defined as a fasting blood glucose ≥126 mg/dl, HgbA1C ≥6.5%, or on an oral anti-hyperglycemic agent or insulin. Hypertension was defined as a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg, or taking an antihypertensive medication.
For cancer survivors, diagnosis and treatment information were obtained from medical records. Mean radiation dose to the heart in centigray (cGy) (including scatter doses) was determined by radiation physicists at MD Anderson Cancer Center (Houston, TX) using energy source, tumor dose, and field location.20 Cumulative anthracycline (doxorubicin equivalents) and alkylating agent (cyclophosphamide equivalents) doses were calculated in milligrams per square meter (mg/m2). 21, 22
Statistics
Descriptive statistics were used to characterize participants, non-participants, and controls and compared using Chi-squared statistics or two-sample t-tests as appropriate. The prevalence (percentages and 95% confidence intervals (CIs)) of major and minor ECG abnormalities were calculated overall and by cancer diagnosis and treatment exposures. We had >90% power to detect a difference between the two proportions using a two-sided, two-sample binomial test with an alpha level of 0.05. Multivariable logistic regression was used to evaluate associations between demographic characteristics, lifestyle variables, and treatment exposures and major or minor ECG abnormalities. Variables with p-values ≤0.2 on univariate analysis were retained in final models. Cox proportional hazards regression was used to evaluate associations between ECG abnormalities and risk of death and cardiovascular events. SAS version 9.3 was used for all analyses and p-values <0.05 were considered statistically significant.
The SJLIFE Study is funded by the National Cancer Institute Grants U01 CA195547 and CA21765 and the American Lebanese Syrian Associated Charities (ALSAC). The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper, and its final contents.
Results
Participating survivors differed from non-participants by sex (p <0.01) (Supplemental Table II) and were slightly younger than controls (median age 31.1 years (18.4–63.8) vs. 34.7 years (18.3–70.2), p<0.001) (Table I). Nearly a third (29.5%) of survivors had received cardiac-directed radiation, and 57.9% and 60% were exposed to anthracycline and/or alkylating chemotherapies, respectively. Survivors and controls did not differ on traditional cardiovascular risk factors such as body habitus, smoking, hypertension, diabetes, or dyslipidemia. Pertinent chronic health conditions, graded per a modified version of the National Cancer Institute’s Common Terminology Criteria for Adverse Events,17 and the distribution of cardiovascular medications used in the cohort are provided in Supplemental Tables III and IV.
Table I.
Demographic, Diagnostic, Treatment, and Lifestyle Characteristics of Study Participants and Controls
| Participant Survivors | Controls | p-value | |||
|---|---|---|---|---|---|
| N=2715 | (%) | N=268 | (%) | ||
| Gender | |||||
| Female | 1321 | (48.7) | 140 | (52.2) | 0.3 |
| Male | 1394 | (51.3) | 128 | (47.8) | |
| Race | |||||
| White | 2281 | (84.0) | 236 | (88.1) | 0.1 |
| Non-white | 434 | (14.0) | 32 | (11.9) | |
| Age at Diagnosis (years) | |||||
| 0 – 4 | 1019 | (37.5) | N/A | ||
| 5 – 9 | 627 | (23.1) | |||
| 10 – 14 | 634 | (23.4) | |||
| ≥ 15 | 434 | (16.0) | |||
| Age at Evaluation (years) | |||||
| 18 – 29 | 1195 | (44.0) | 87 | (32.5) | |
| 30 – 39 | 1014 | (37.3) | 100 | (37.3) | <0.001 |
| 40 – 49 | 427 | (15.7) | 57 | (21.2) | |
| ≥ 50 | 79 | (3.0) | 24 | (9.0) | |
| Time Since Diagnosis (years) | |||||
| 10 – 20 | 1106 | (40.7) | N/A | ||
| 20 – 30 | 1038 | (38.2) | |||
| > 30 | 571 | (21.1) | |||
| Diagnosis Group | |||||
| Leukemia | 1042 | (38.3) | N/A | ||
| Sarcomas | 363 | (13.4) | |||
| Hodgkin Lymphoma | 320 | (11.8) | |||
| Non-Hodgkin Lymphoma | 200 | (7.4) | |||
| Central Nervous System (CNS) | 263 | (9.7) | |||
| Neuroblastoma | 121 | (4.5) | |||
| Wilms Tumor | 174 | (6.4) | |||
| Other | 232 | (8.5) | |||
| Cardiac Radiation (cGy) | |||||
| None | 1915 | (70.5) | N/A | ||
| 1 – 1999 | 469 | (17.3) | |||
| 2000 – 2999 | 220 | (8.1) | |||
| ≥ 3000 | 111 | (4.1) | |||
| Anthracyclines (mg/m2) | |||||
| None | 1142 | (42.1) | N/A | ||
| 1 – 200 | 1037 | (38.2) | |||
| 201 – 400 | 448 | (16.5) | |||
| > 400 | 88 | (3.2) | |||
| Alkylating agents (mg/m2) | |||||
| None | 1087 | (40.0) | N/A | ||
| 1 – 9000 | 846 | (31.2) | |||
| > 9000 | 782 | (28.8) | |||
| Body Mass Index (kg/m2) | |||||
| Normal/underweight (< 25) | 997 | (36.7) | 95 | (35.4) | 0.7 |
| Overweight (25 – 29) | 757 | (27.9) | 71 | (26.5) | |
| Obese (≥ 30) | 961 | (35.4) | 102 | (38.1) | |
| Smoker | |||||
| Past | 312 | (11.5) | 43 | (16.5) | 0.1 |
| Current | 643 | (23.7) | 54 | (20.7) | |
| Never | 1760 | (64.8) | 164 | (62.8) | |
| Hypertension | |||||
| No | 1933 | (71.2) | 190 | (70.9) | 0.9 |
| Yes | 782 | (28.8) | 78 | (29.1) | |
| Diabetes | |||||
| No | 2533 | (93.3) | 254 | (94.8) | 0.4 |
| Yes | 182 | (6.7) | 14 | (5.2) | |
| Dyslipidemia | |||||
| No | 1399 | (51.5) | 154 | (57.5) | 0.1 |
| Yes | 1316 | (48.5) | 114 | (42.5) | |
A major ECG abnormality was identified in 10.7% (290 of 2,715) of survivors compared to 4.9% (13 of 268) of controls (p<0.001, Table II). The most common abnormalities among survivors were isolated major ST/T wave abnormalities (7.2%), evidence of definite or probable myocardial infarction (3.7%), and left ventricular hypertrophy with strain pattern (2.8%). These findings were asymptomatic, with the exception of 4 of 99 survivors with pathologic Q waves who reported knowledge of a prior myocardial infarction. The prevalence of minor ECG abnormalities was similar between survivors (65.2%) and controls (67.5%).
Table II.
Major and Minor ECG Abnormalities among Childhood Cancer Survivors
| Major ECG Abnormalities | Survivors | Controls | ||||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | |||
|
|
|
|||||
| Major isolated ST/T wave abnormalities | 194 | (7.2) | 10 | (3.7) | ||
| Evidence of myocardial infarction (pathologic Q waves) | 99 | (3.7) | 2 | (0.7) | ||
| Left ventricular hypertrophy with strain pattern | 75 | (2.8) | 3 | (1.1) | ||
| Ventricular conduction defects | 30 | (1.1) | 0 | (0.0) | ||
|
|
||||||
| Complete left bundle branch block | 5 | 0 | ||||
| Complete right bundle branch block | 13 | 0 | ||||
| Non-specific major intraventricular block | 8 | 0 | ||||
| Bi-fascicular block | 4 | 0 | ||||
|
|
||||||
| Major QT prolongation | 8 | (0.3) | 1 | (0.4) | ||
| Pacemaker† | 9 | (0.3) | 0 | (0.0) | ||
| Major atrio-ventricular conduction abnormalities | 3 | (0.1) | 0 | (0.0) | ||
|
|
||||||
| Advanced block (complete or second degree) | 0 | 0 | ||||
| Wolff Parkinson White Syndrome | 3 | 0 | ||||
|
|
||||||
| Atrial flutter/fibrillation | 0 | (0.0) | 0 | (0.0) | ||
|
| ||||||
| Total Major ECG Abnormalities | 290 | (10.7) | 13 | (4.9) | P < 0.001 | |
| Minor ECG Abnormalities* | ||||||
| Minimal voltage criteria indicating ventricular hypertrophy | 565 | (23.3) | 56 | (22.0) | ||
|
|
||||||
| Left ventricular hypertrophy without strain pattern | 559 | 56 | ||||
| Right ventricular hypertrophy | 6 | 0 | ||||
|
|
||||||
| Atrial enlargement | 531 | (21.9) | 34 | (13.3) | ||
|
|
||||||
| Right atrial enlargement | 224 | 14 | ||||
| Left atrial enlargement | 392 | 24 | ||||
|
|
||||||
| Minor isolated ST/T wave abnormalities | 478 | (19.7) | 54 | (21.2) | ||
| Incomplete bundle branch block | 319 | (13.2) | 45 | (17.6) | ||
|
|
||||||
| Incomplete right bundle branch block | 129 | 10 | ||||
| Incomplete left bundle branch block | 190 | 35 | ||||
|
|
||||||
| Minor isolated Q/QS waves | 179 | (7.4) | 13 | (5.1) | ||
| Minor atrio-ventricular conduction abnormalities | 125 | (5.2) | 11 | (4.3) | ||
|
|
||||||
| Short PR interval | 119 | 11 | ||||
| Prolonged PR interval (first degree block) | 6 | 0 | ||||
|
|
||||||
| Other rhythm disorders | 121 | (5.0) | 18 | (7.1) | ||
|
|
||||||
| Sinus bradycardia (<50 bpm) | 38 | 15 | ||||
| Sinus tachycardia | 75 | 1 | ||||
| Ectopic atrial rhythm | 8 | 2 | ||||
|
|
||||||
| Non-ischemic ST elevation | 98 | (4.0) | 14 | (5.5) | ||
| Premature ectopic beats | 58 | (2.4) | 2 | (0.8) | ||
|
|
||||||
| Ventricular premature beats | 17 | 0 | ||||
| Atrial premature beats | 41 | 1 | ||||
| Atrial and Ventricular premature beats | 0 | 1 | ||||
|
|
||||||
| Minor QT prolongation | 47 | (1.9) | 7 | (2.7) | ||
| QRS axis deviation | 23 | (1.0) | 0 | (0.0) | ||
|
|
||||||
| Left axis deviation | 21 | 0 | ||||
| Right axis deviation | 2 | 0 | ||||
|
|
||||||
| Low QRS voltage | 17 | (0.7) | 3 | (1.2) | ||
|
| ||||||
| Total Minor ECG Abnormalities | 1581 | (65.2%) | 172 | (67.5%) | P=0.6 | |
Abnormalities are not mutually exclusive, participants may have had more than one abnormality
among 2,425 survivors and 255 controls without major abnormalities
Reasons for pacemaker placement: complete atrioventricular block (n=5), WPW Syndrome (n=1), Sick sinus syndrome (n=1), dilated cardiomyopathy (n=1), ventricular asystole s/p aortic valve replacement (n=1).
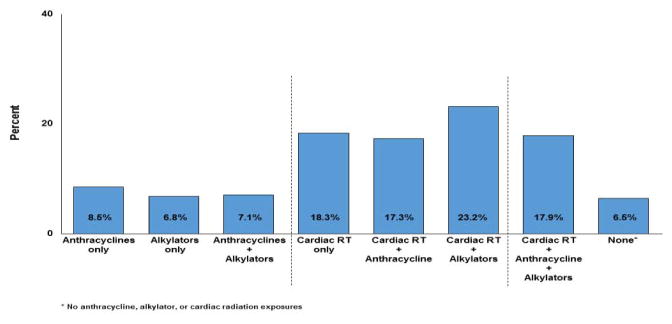
Abnormalities were identified across all treatment exposures (Figure 1). The prevalence of major abnormalities was highest among those exposed to cardiac-directed radiation but also substantial among those treated with chemotherapy only. Among those treated with anthracyclines and/or alkylating agents alone, 8.5% and 6.8%, respectively, had a major abnormality. This increased to 17.3% and 23.2% for those treated with these agents and cardiac-directed radiation. Even among survivors not exposed to cardiotoxic therapies (i.e. radiation, anthracyclines, alkylating agents) 6.5% had a major abnormality.
Figure 1.
Prevalence of major ECG abnormalities by cancer treatment exposure
Table III shows the odds for major or minor ECG abnormalities among cancer survivors as a function of demographic characteristics, lifestyle factors, and treatment exposures. Anthracycline exposure ≥300 mg/m2, increasing doses of cardiac radiation, and hypertension were associated with having a major ECG abnormality. Cardiac radiation, hypertension, and male sex were associated with having a minor ECG abnormality. Cardiac radiation doses ≥3000 cGy conferred the greatest odds for either a major or minor ECG abnormality.
Table III.
Odd Ratios and 95% Confidence Intervals from Multivariable Models of Major and Minor ECG Abnormalities*
| Major ECG Abnormality | Minor ECG Abnormality† | |||
|---|---|---|---|---|
|
| ||||
| OR | (95% CI) | OR | 95% CI | |
|
| ||||
| Gender | ||||
| Female | 1.0 | 1.0 | ||
| Male | 1.2 | (0.9–1.5) | 1.6 | (1.4–1.9) |
| Race | ||||
| White | 1.0 | 1.0 | ||
| Black | 1.4 | (0.9–2.0) | 1.3 | (0.9–1.6) |
| Other | 0.9 | (0.3–2.9) | 0.9 | (0.5–1.7) |
| Body Mass Index (mg/kg2)‡ | 0.9 | (0.9–1.0) | 0.9 | (0.9–1.0) |
| Age at diagnosis‡ | 0.9 | (0.9–1.0) | 0.9 | (0.9–1.0) |
| Age at Evaluation | ||||
| 18–29 years | 1.0 | 1.0 | ||
| 30–39 years | 1.1 | (0.8–1.5) | 0.9 | (0.7–1.1) |
| 40–49 years | 1.3 | (0.9–2.0) | 1.2 | (0.9–1.6) |
| ≥ 50 years | 1.9 | (0.9–3.7) | 1.7 | (0.8–3.4) |
| Anthracycline (mg/m2) | ||||
| None | 1.0 | 1.0 | ||
| 1 – 300 | 1.3 | (0.9–1.8) | 1.0 | (0.8–1.3) |
| ≥ 300 | 1.7 | (1.1–2.5) | 1.1 | (0.8–1.5) |
| Alkylating agents (mg/m2) | ||||
| None | NE | 1.0 | ||
| 1 – 9000 | 1.1 | (0.9–1.3) | ||
| ≥ 9000 | 1.3 | (1.0–1.6) | ||
| Cardiac Radiation (cGy) | ||||
| None | 1.0 | 1.0 | ||
| 1–1999 | 2.1 | (1.5–2.9) | 1.4 | (1.1–1.8) |
| 2000–2999 | 2.6 | (1.6–3.9) | 1.7 | (1.2–2.4) |
| ≥ 3000 | 10.5 | (6.5–16.9) | 4.9 | (2.1–11.7) |
| Hypertension | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.6 | (1.2–2.1) | 1.3 | (1.0–1.6) |
| Smoking | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.1 | (0.8–1.4) | 1.2 | (0.9–1.4) |
| Dyslipidemia | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.0 | (0.8–1.4) | 1.0 | (0.8–1.2) |
| Diabetes | ||||
| No | 1.0 | 1.0 | ||
| Yes | 1.4 | (0.9–2.2) | 1.0 | (0.7–1.5) |
Estimates adjusted for all variables in the table
among 2,425 survivors without major abnormalities
Continuous variables
NE=not estimated, p-value >0.2 on univariate analysis
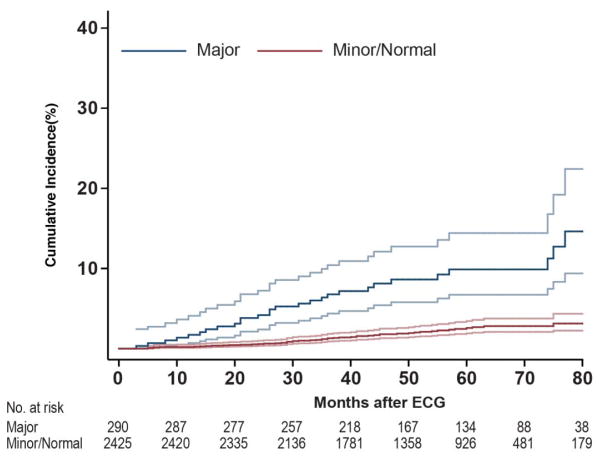
Eighty of the 2,715 (2.9%) survivors died a median of 20 months (3–87) after the initial ECG (a median of 34 years, range: 13–48 years from initial cancer diagnosis). Thirteen of the 80 deaths were cardiac-related (Supplemental Table I). Adjusting for age at diagnosis, current age, gender, race, and smoking, the risk of death was elevated for survivors with a major abnormality (HR 4.0 95% CI 2.1–7.8) (Table IV). The cumulative incidence of death is shown in Figure 2. At 60 months, 10% (95% CI 6.1–13.7) of survivors with a major abnormality had died.
Table IV.
Risk and 95% Confidence Intervals for All-cause Death among Survivors with a Major or Minor ECG Abnormality*
| All-Cause Death | |||
|---|---|---|---|
| HR | (95% CI) | p-value | |
|
|
|||
| Major ECG abnormality | 4.0 | (2.1–7.8) | <0.001 |
| Minor ECG abnormality | 1.3 | (0.7–2.5) | 0.4 |
adjusted for age at diagnosis, current age, gender, race, and smoking status
Figure 2.
Cumulative incidence of all-cause death
Discussion
In this large well-characterized cohort of adults previously treated for childhood cancer, we identified a major ECG abnormality in over 10% of survivors. Cancer survivors had twice the prevalence of major abnormalities compared to controls, and abnormalities were strongly associated with cardiac radiation and higher anthracycline and alkylating chemotherapy exposures. Importantly, major abnormalities appear to predict cardiovascular events and/or all-cause death.
These findings add to a sparse literature concerning rhythm abnormalities following cancer therapy. Larsen and colleagues studied a young adult population of survivors (n=134) a mean of 5±4 years after cardiotoxic exposure and reported the majority of ECGs (66%) to have no abnormalities.13 However, significant findings included 14% with a prolonged QTc (>0.44 sec), 6% with ectopic atrial rhythms, and abnormally flattened or inverted T waves in 2%. Similarly, Schwartz et al. reviewed ECGs among cardiotoxic-exposed survivors returning for follow-up surveillance (n=52).23 Among those exposed to <300 mg/m2 of anthracyclines, 16% (3 of 19) had a QTc interval ≥0.43 sec with 58% (19 of 33) exposed to higher doses having a prolonged QTc. These investigators also performed exercise ECG testing in a subpopulation of eight survivors. Only two responded with normal shortening of the QTc; the remaining six had further prolongation with exercise, raising concern for a possible abnormal neural response. Our study included a broader population further from exposure and found a low prevalence of QTc prolongation (2.2%). Furthermore, we used the QT index that accounts for the distribution of QTc in the general population19 whereas previous investigations have utilized Bazett’s formula to define QTc prolongation, known to overestimate this interval.24
A number of large cohort studies have identified associations between ECG abnormalities and future cardiovascular events. Framingham investigators reported ST/T wave abnormalities among 8.5% of men and 7.7% of women age 44–74 years old25, similar to the prevalence of major ST/T wave abnormalities (7.2%) in our population. With over 30 years of follow-up, Framingham researchers identified an association between ST/T wave abnormalities and incident coronary disease, independent of known cardiovascular risk factors. Among 1,673 men, ages 40–55 years, participating in the Chicago Western Electric Study26, the risk for all-cause death was 1.6 (95% CI: 1.0–2.4) among those with persistent ST/T wave abnormalities compared to those without. While not restricted solely to ST/T wave abnormalities, cancer survivors with a major abnormality were significantly more likely to die compared to those with a minor or no abnormality. Further follow-up will help characterize this group of cancer survivors, with the goal of better informing screening frequencies and defining appropriate screening modalities.
In addition to ST/T wave abnormalities, we identified Q waves (3.7% pathologic and 7.4% minor isolated Q/QS waves) suggestive of myocardial injury/ischemia. In the general population, the prevalence of silent myocardial infarction identified by ECG ranges from 0.3–6.4% and is largely influenced by age.27 In the Reykjavik Study (n=9,141 men), the prevalence at age 50 years was 0.5% and increased to 5% by age 75 years.28 In an older population (mean age 62 years), investigators in Olmsted County, Minnesota reported unrecognized myocardial infarction in 3.9%,29 similar to the prevalence of major Q wave findings in our much younger cohort. Since the prognosis following silent myocardial infarction does not differ from that of acute myocardial infarction, these findings have important implications for the future health of survivors of childhood cancer.
Electrocardiographic estimates of left ventricular hypertrophy (LVH) with the strain pattern (ST segment depression and flat or negative T waves)30 are estimated at 0.8% among men and 0.5% among women.30–32 The prevalence was higher (2.8%) in our population of cancer survivors. Importantly, these ECG findings have been associated with an increased risk of myocardial infarction, heart failure, and cardiovascular death.33 While LVH is associated with hypertension, Framingham investigators reported that a strain pattern is also predictive of hypertension and identifies hypertensive patients with decreased coronary circulation at risk of myocardial damage.33 In childhood cancer survivors, risks for adverse cardiac outcomes are substantially higher in the presence of traditional cardiovascular risk factors, perhaps suggesting an increased susceptibility to conditions of aging. Hypertension may particularly potentiate the therapy-associated risks for coronary disease (RR 6.1 95% CI 3.4–11.2), heart failure (RR 19.4 95% CI 11.4–33.1), and arrhythmia (RR 6.0 95% CI 1.7–21.8) in this population.34 Electrocardiographic LVH with strain pattern may augment echocardiographic findings in a cancer population at high risk for cardiomyopathy.
Cardiovascular screening following cancer therapy has typically focused on ultrasound imaging and assessment of those exposed to known cardiotoxic therapies, such as anthracyclines and/or cardiac-directed radiation. By systematically obtaining ECGs on all survivors in the SJLIFE cohort, we identified abnormalities among those with and without typical cardiotoxic exposures. Even individuals not treated with cardiotoxic therapies had ECG abnormalities (6.5% major), perhaps reflective of the general population but potentially related to prior cancer therapy. Furthermore, many of the findings were suggestive of ischemic heart disease; perhaps expected among irradiated survivors, but less so among those exposed to chemotherapy only. Ischemic disease may not be fully evaluated by echocardiography, thus, missing an opportunity to clinically intervene or provide health counseling. It is possible that current screening parameters underestimate the risk among cancer survivors and a broader cardiac assessment is needed.
In the general population, cardiovascular screening of asymptomatic individuals typically relies on stratification to low, intermediate, or high risk categories (e.g. Framingham Risk Score) to guide the most appropriate screening modalities. In a systematic review, the U.S. Preventive Services Task Force found resting ECG abnormalities to be associated with increased risk for cardiovascular events and suggested considering screening for those at intermediate or high risk.5 Because adult survivors of childhood cancer have added risks related to treatment exposures, determining who to screen and when based on traditional risk calculators is challenging.3 Screening modalities may need to be more carefully tailored, and ECG, a non-invasive, inexpensive modality, may provide an initial broad cardiovascular assessment, augment echocardiographic data, and guide the use of other screening modalities among these at-risk individuals.
A number of limitations should be considered when interpreting our results. Participation required an on-campus evaluation to obtain an ECG recording. Excluding survivors who may not have been able to travel due to commitments, illness, or death, potentially may have affected our findings. Among those who did participate, ECGs were unavailable for 47 survivors due to technical reasons such as suboptimal recording, poor electrical transmission, etc. We assumed the missing to be non-informative and treated these data as missing at random. False positive ECG findings may have led to misclassification bias.
Optimal strategies for cardiovascular risk assessment remain a focus of research and debate within the cardiovascular community, 35 as well as among health care providers caring for cancer survivors. Guidelines developed for the long-term follow-up of childhood cancer survivors suggest a baseline ECG, largely for the purpose of evaluating the QTc interval, but surveillance remains largely focused on echocardiographic imaging.36 These data suggest that ECG evaluation may be more informative than previously recognized. Major ECG abnormalities were predictive of adverse outcomes, identifying a population that may warrant earlier and more comprehensive cardiac assessment and intervention. Additionally, long-term follow-up of minor ECG abnormalities is needed to elucidate the natural progression within this high-risk population.
Supplementary Material
Supplemental Figure 1. Consort diagram
Highlights.
Major ECG abnormalities are common among childhood cancer survivors
ECG abnormalities are predictive of cardiac and all-cause mortality
Abnormalities are associated with anthracycline and cardiac radiation exposure
Acknowledgments
Supported by Cancer Center Support (CORE) Grant (CA21765) to St. Jude Children’s Research Hospital (PI: Dr. Charles W. Roberts), U01 CA195547 (MPI: Drs. Melissa M. Hudson and Leslie L. Robison), and the American Lebanese Syrian Associated Charities (ALSAC), Memphis, TN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Floyd JD, Nguyen DT, Lobins RL, Bashir Q, Doll DC, Perry MC. Cardiotoxicity of cancer therapy. J Clin Oncol. 2005;23:7685–7696. doi: 10.1200/JCO.2005.08.789. [DOI] [PubMed] [Google Scholar]
- 2.Lenihan DJ, Oliva S, Chow EJ, Cardinale D. Cardiac toxicity in cancer survivors. Cancer. 2013;119(Suppl 11):2131–2142. doi: 10.1002/cncr.28061. [DOI] [PubMed] [Google Scholar]
- 3.Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606. doi: 10.1136/bmj.b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulrooney DA, Armstrong GT, Huang S, et al. Cardiac Outcomes in Adult Survivors of Childhood Cancer Exposed to Cardiotoxic Therapy: A Cross-sectional Study. Ann Intern Med. 2016;164:93–101. doi: 10.7326/M15-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chou R, Arora B, Dana T, Fu R, Walker M, Humphrey L. Screening asymptomatic adults with resting or exercise electrocardiography: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2011;155:375–385. doi: 10.7326/0003-4819-155-6-201109200-00006. [DOI] [PubMed] [Google Scholar]
- 6.Ashley EA, Raxwal VK, Froelicher VF. The prevalence and prognostic significance of electrocardiographic abnormalities. Curr Probl Cardiol. 2000;25:1–72. doi: 10.1016/s0146-2806(00)70020-x. [DOI] [PubMed] [Google Scholar]
- 7.Tamene AM, Masri C, Konety SH. Cardiovascular MR imaging in cardio-oncology. Magn Reson Imaging Clin N Am. 2015;23:105–116. doi: 10.1016/j.mric.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong GT, Joshi VM, Ness KK, et al. Comprehensive Echocardiographic Detection of Treatment-Related Cardiac Dysfunction in Adult Survivors of Childhood Cancer: Results From the St. Jude Lifetime Cohort Study. J Am Coll Cardiol. 2015;65:2511–2522. doi: 10.1016/j.jacc.2015.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armstrong GT, Plana JC, Zhang N, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–2884. doi: 10.1200/JCO.2011.40.3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100:1368–1379. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shankar SM, Marina N, Hudson MM, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children’s Oncology Group. Pediatrics. 2008;121:e387–396. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
- 12.Bender KS, Shematek JP, Leventhal BG, Kan JS. QT interval prolongation associated with anthracycline cardiotoxicity. J Pediatr. 1984;105:442–444. doi: 10.1016/s0022-3476(84)80026-8. [DOI] [PubMed] [Google Scholar]
- 13.Larsen RL, Jakacki RI, Vetter VL, Meadows AT, Silber JH, Barber G. Electrocardiographic changes and arrhythmias after cancer therapy in children and young adults. Am J Cardiol. 1992;70:73–77. doi: 10.1016/0002-9149(92)91393-i. [DOI] [PubMed] [Google Scholar]
- 14.Adams MJ, Lipsitz SR, Colan SD, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22:3139–3148. doi: 10.1200/JCO.2004.09.109. [DOI] [PubMed] [Google Scholar]
- 15.Landier W, Armenian SH, Lee J, et al. Yield of screening for long-term complications using the children’s oncology group long-term follow-up guidelines. J Clin Oncol. 2012;30:4401–4408. doi: 10.1200/JCO.2012.43.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56:825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudson MM, Ehrhardt MJ, Bhakta N, et al. Approach for Classification and Severity-grading of Long-term and Late-onset Health Events among Childhood Cancer Survivors in the St. Jude Lifetime Cohort. Cancer Epidemiol Biomarkers Prev. 2016 doi: 10.1158/1055-9965.EPI-16-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prineas RJ, Crow RS, Blackburn H. The Minnesota Code manual of electrocardiographic findings. Littleton, MA: John Wright-PSG; 1982. [Google Scholar]
- 19.Rautaharju PM, Surawicz B, Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53:982–991. doi: 10.1016/j.jacc.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 20.Stovall M, Weathers R, Kasper C, et al. Dose reconstruction for therapeutic and diagnostic radiation exposures: use in epidemiological studies. Radiat Res. 2006;166:141–157. doi: 10.1667/RR3525.1. [DOI] [PubMed] [Google Scholar]
- 21.Le Deley MC, Leblanc T, Shamsaldin A, et al. Risk of secondary leukemia after a solid tumor in childhood according to the dose of epipodophyllotoxins and anthracyclines: a case-control study by the Societe Francaise d’Oncologie Pediatrique. J Clin Oncol. 2003;21:1074–1081. doi: 10.1200/JCO.2003.04.100. [DOI] [PubMed] [Google Scholar]
- 22.Green DM, Nolan VG, Goodman PJ, et al. The cyclophosphamide equivalent dose as an approach for quantifying alkylating agent exposure: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2014;61:53–67. doi: 10.1002/pbc.24679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz CL, Hobbie WL, Truesdell S, Constine LC, Clark EB. Corrected QT interval prolongation in anthracycline-treated survivors of childhood cancer. J Clin Oncol. 1993;11:1906–1910. doi: 10.1200/JCO.1993.11.10.1906. [DOI] [PubMed] [Google Scholar]
- 24.Luo S, Michler K, Johnston P, Macfarlane PW. A comparison of commonly used QT correction formulae: the effect of heart rate on the QTc of normal ECGs. J Electrocardiol. 2004;37(Suppl):81–90. doi: 10.1016/j.jelectrocard.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 25.Kannel WB, Anderson K, McGee DL, Degatano LS, Stampfer MJ. Nonspecific electrocardiographic abnormality as a predictor of coronary heart disease: the Framingham Study. Am Heart J. 1987;113:370–376. doi: 10.1016/0002-8703(87)90280-8. [DOI] [PubMed] [Google Scholar]
- 26.Daviglus ML, Liao Y, Greenland P, et al. Association of nonspecific minor ST-T abnormalities with cardiovascular mortality: the Chicago Western Electric Study. Jama. 1999;281:530–536. doi: 10.1001/jama.281.6.530. [DOI] [PubMed] [Google Scholar]
- 27.Valensi P, Lorgis L, Cottin Y. Prevalence, incidence, predictive factors and prognosis of silent myocardial infarction: a review of the literature. Arch Cardiovasc Dis. 2011;104:178–188. doi: 10.1016/j.acvd.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Sigurdsson E, Thorgeirsson G, Sigvaldason H, Sigfusson N. Unrecognized myocardial infarction: epidemiology, clinical characteristics, and the prognostic role of angina pectoris. The Reykjavik Study. Ann Intern Med. 1995;122:96–102. doi: 10.7326/0003-4819-122-2-199501150-00003. [DOI] [PubMed] [Google Scholar]
- 29.Ammar KA, Samee S, Makwana R, et al. Echocardiographic characteristics of electrocardiographically unrecognized myocardial infarctions in a community population. Am J Cardiol. 2005;96:1069–1075. doi: 10.1016/j.amjcard.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 30.Larsen CT, Dahlin J, Blackburn H, et al. Prevalence and prognosis of electrocardiographic left ventricular hypertrophy, ST segment depression and negative T-wave; the Copenhagen City Heart Study. Eur Heart J. 2002;23:315–324. doi: 10.1053/euhj.2001.2774. [DOI] [PubMed] [Google Scholar]
- 31.De Bacquer D, De Backer G, Kornitzer M, Blackburn H. Prognostic value of ECG findings for total, cardiovascular disease, and coronary heart disease death in men and women. Heart. 1998;80:570–577. doi: 10.1136/hrt.80.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knutsen R, Knutsen SF, Curb JD, Reed DM, Kautz JA, Yano K. The predictive value of resting electrocardiograms for 12-year incidence of coronary heart disease in the Honolulu Heart Program. J Clin Epidemiol. 1988;41:293–302. doi: 10.1016/0895-4356(88)90134-5. [DOI] [PubMed] [Google Scholar]
- 33.Kannel WB. Prevalence and natural history of electrocardiographic left ventricular hypertrophy. Am J Med. 1983;75:4–11. doi: 10.1016/0002-9343(83)90111-0. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khanji MY, Bicalho VV, van Waardhuizen CN, Ferket BS, Petersen SE, Hunink MG. Cardiovascular Risk Assessment: A Systematic Review of Guidelines. Ann Intern Med. 2016;165:713–722. doi: 10.7326/M16-1110. [DOI] [PubMed] [Google Scholar]
- 36.Landier W, Bhatia S, Eshelman DA, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22:4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Consort diagram