Abstract
Background & Objectives
Multidisciplinary tumor boards (MDTBs) are frequently employed in cancer centers but their value has been debated. We reviewed the decision-making process and resource utilization of our MDTB to assess its utility in the management of pancreatic and upper gastrointestinal tract conditions.
Methods
A prospectively-collected database was reviewed over a 12-month period. The primary outcome was change in management plan as a result of case discussion. Secondary outcomes included resources required to hold MDTB, survival, and adherence to treatment guidelines.
Results
470 cases were reviewed. MDTB resulted in a change in the proposed plan of management in 101 of 402 evaluable cases (25.1%). New plans favored obtaining additional diagnostic workup. No recorded variables were associated with a change in plan. For newly-diagnosed cases of pancreatic ductal adenocarcinoma (n=33), survival time was not impacted by MDTB (p=.154) and adherence to National Comprehensive Cancer Network guidelines was 100%. The estimated cost of physician time per case reviewed was $190.
Conclusions
Our MDTB influences treatment decisions in a sizeable number of cases with excellent adherence to national guidelines. However, this requires significant time expenditure and may not impact outcomes. Regular assessments of the effectiveness of MDTBs should be undertaken.
Keywords: Multidisciplinary care, pancreas, tumor board, care conference, oncology
INTRODUCTION
Multidisciplinary coordination of care is a requirement for Commission on Cancer accreditation1 and is recommended in guidelines from national organizations including the National Comprehensive Cancer Network (NCCN) 2. Often this coordination of care takes the form of multidisciplinary tumor boards (MDTBs) with the intent to promote decreased variation in practice patterns, assure appropriate use of health care resources, offer educational opportunities for medical professionals, and improve outcomes of cancer care for select patient populations 3. The use and benefits of MDTBs have been reported across various cancer pathologies, including breast 4, 5, gynecologic 6–9, urologic 10, 11, upper gastrointestinal 12, and thoracic 13–15 malignancies.
However, the utility of these conferences has been the subject of debate. In some studies, multidisciplinary review of radiology or pathology altered diagnoses and treatment plans in as many as 50% of reviewed cases 5, 9, 16 and improved survival for solid organ tumors 17–19. Other groups have reported no difference in outcomes, including a review of 138 VA medical centers that failed to demonstrate an association with MDTBs with overall survival and oncologic quality measures 20, 21. Clinician non-adherence to MDTBs recommendations, which has been reported to be as high as 10–15%, can further limit the effectiveness of these meetings, and is often the result of inadequate consideration of patient preference or comorbidities during multidisciplinary discussion 22, 23. Finally, external measures of quality, such as adherence to national guidelines, are infrequently reported but have been at or above 80% in certain series 14, 15.
The time required to organize and implement a MDTB must be considered when assessing utility. These conferences are held regularly and the attendance of a number of clinicians and support staff represents a substantial institutional investment of nonclinical hours. In the UK, multidisciplinary conferences are estimated to require one million person-hours annually at a cost of US$75 million, approximately $120 per case reviewed 24, 25. With such a significant institutional investment and varying reports of utility and quality, institutions might benefit from identifying appropriate clinical inclusion criteria and MDTB formats can maximize resources 3.
Despite prior studies evaluating the multidisciplinary approach in solid organ cancers, the impact of MDTBs in reviewing benign and malignant pancreato-biliary diseases is largely unknown. The purpose of our study was to evaluate the prospectively collected database from our weekly pancreas and upper-gastrointestinal MDTB to 1) determine the impact of MDTB on treatment decisions and identify clinical scenarios or diagnoses that might benefit most from MDTB discussion, 2) assess the impact of MDTB on institutional resource utilization, and 3) determine quality of the MDTB process based on adherence to national guidelines. The results of our study may help to enhance MDTBs both at our own institution and other centers and offer a generalizable method of reporting MDTB data.
METHODS
Setting
The Pancreas and Upper Gastrointestinal Multidisciplinary Conference at the Washington University School of Medicine and Siteman Cancer Center, held weekly, is attended by hepato-pancreato-biliary (HPB) surgeons, medical and radiation oncologists, interventional gastroenterologists, diagnostic radiologists, pathologists, advanced practitioners, and clinical support staff. Cases eligible for presentation include new or existing outpatient or inpatient cases of benign and malignant pancreatic and upper gastrointestinal conditions. A weekly case list is distributed to a diagnostic radiologist and pathologist, who utilize non-protected time to review the cases prior to conference and then lead the MDTB case discussions.
Data Collection and Analysis
Data included in this study were prospectively collected for patients presented during the 12-month period from September 2014 to August 2015. The investigational approach included prospective collection of data from the decision-making process, including recording the pre-conference plan, defined as the submitting physician’s expected next step in the diagnostic or treatment plan, and the post-conference plan, defined as the majority decision for the next step after case discussion at MDTB. If the pre-conference plan was not explicitly stated during MDTB discussion, chart review of pre-MDTB notes was performed; any missing data was coded as such and excluded from subsequent analyses.
The primary outcome was change in management after MDTB discussion. Descriptive and analytic statistical tests including univariate linear regression, multivariate logistic regression, and Fisher’s exact tests were performed using SAS software version 9.4 (SAS Institute, Cary, NC), with all tests two-sided and significance p<.05. Mean survival time, either death date or censored at last known follow-up, were compared using student’s t-test. Adherence to NCCN guidelines for Pancreatic Ductal Adenocarcinoma (PDAC) was determined by retrospective chart review restricted to data available at the time of MDTB presentation and using time-appropriate NCCN guidelines2. Estimates of weekly effort were gathered retrospectively via electronic surveys of six diagnostic radiologists, two support staff, and one pathologist. Estimates of potential physician reimbursement were derived using the following facility-price Current Procedural Terminology codes from the Centers for Medicare and Medicaid Services 2015A26 for metropolitan St. Louis (locality 0530201), one per hour: 99205 (new office visit, Level 5; $168.70), 74177–26 (CT abdomen and pelvis with contrast; $92.32), and 88309 (gross and microscopic pancreatic tissue exam by pathologist; $149.41). Meeting room costs were excluded.
This study was approved by the Institutional Review Board and Human Research Protection Office at Washington University. Reporting of this project follows criteria for the STROBE guidelines for observational studies27, version 4, and applicable SQUIRE guidelines for quality improvement reporting28, version 2.0.
RESULTS
470 cases were presented at MDTB during the 12-month period. The median age at the time of presentation was 61.5 years (range 17 – 89) with 51.2% male. The mean number of cases presented weekly was 10.7 ± 2.7. New diagnoses accounted for 174 cases (37.0%), with the remainder categorized as either currently on chemotherapy or radiation therapy (n=114, 24.3%) or on observation (benign conditions or surveillance after completion of oncologic therapy; n=182, 38.7%). 290 cases were presented by HPB surgeons (61.7%), 122 by medical oncologists (26%), 39 by gastroenterologists (8.3%), 18 by radiation oncologists (3.8%), and one by an interventional radiologist. The most common organ system presented was pancreas (75.5%) (Table 1). Pancreatic adenocarcinoma was the most common pathologic or imaging diagnosis (37%), followed by pancreatic mass (16%) and pancreatic cyst (7%) (Table 2). The most frequent pre-conference plan was to perform surgery (n=125, 26.6%) and the most frequent post-conference plan was to observe (n=109, 23.2%) (Figure 1).
Table 1.
Distribution of case numbers presented at MDTB by organ site.
| Organ Site | n | % |
|---|---|---|
| Pancreas | 355 | 75.5% |
| Bile Duct | 34 | 7.2% |
| Small Bowel | 20 | 4.3% |
| Ampulla | 17 | 3.6% |
| Stomach | 16 | 3.4% |
| Uncharacterized | 16 | 3.4% |
| Other | 12 | 2.6% |
| Total | 470 |
Uncharacterized = undiagnosed primary site. Other = adrenal (n=1), mesenteric (n=2), retroperitoneal (n=3), splenic (n=1), other abdominal mass (n=3), and vascular (n=2).
Table 2.
Pathologic or radiologic diagnoses of all cases reviewed at Multidisciplinary Tumor Board.
| Pathologic or Radiologic Diagnosis | n | % |
|---|---|---|
| Pancreatic adenocarcinoma | 174 | 37.0% |
| Pancreatic mass | 76 | 16.2% |
| Pancreatic cyst | 34 | 7.2% |
| Pancreatitis | 33 | 7.0% |
| Neuroendocrine tumor | 28 | 6.0% |
| Cholangiocarcinoma | 19 | 4.0% |
| Ampullary cancer or mass | 17 | 3.6% |
| Intraductal papillary mucinous neoplasm | 15 | 3.2% |
| Bile duct mass, stricture, obstruction, jaundice | 15 | 3.2% |
| Gastric cancer or mass | 11 | 2.3% |
| Other mass or malignancy | 25 | 5.3% |
| Other benign condition | 23 | 4.9% |
| Total | 470 |
Figure 1.
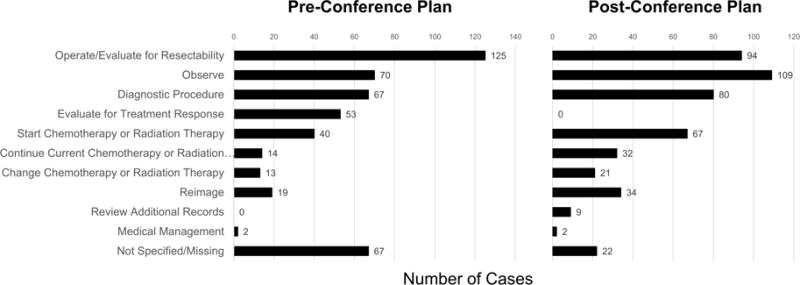
Distribution of pre-conference plans (left) and post-conference plans (right) for all cases reviewed at MDTB
80 patients were presented more than once during the study period (mean 2.3 times, maximum five). Patients were more likely to be presented multiple times if they were listed and presented by medical oncologists (p<.001, OR 2.86, 95% CI 1.65–4.93) or radiation oncologists (p=.016, OR 4.59, 95% CI 1.36–15.45). Surgeons were inversely associated with multiple presentations (p<.001, OR 0.42, 95% CI 0.25–0.69) and gastroenterologists demonstrated no association (p=.190). Malignancy (p<.001, OR 2.92, 95% CI 1.71–4.96) and status as an established patient (p<.001, OR 3.99, 95% CI 2.14–7.43) were also independently associated with additional presentations.
Cases in which MDTB resulted in a change in the treatment plan were further analyzed. 68 cases did not have documented pre-conference plans at the time of MDTB and were removed from the analysis. Of the remaining 402 cases (85.5%), presentation at MDTB resulted in a change in 101 cases (25.1%). The pathologic and radiologic diagnosis of these changed cases are presented in Table 3. MDTB changes tended to favor further workup and less-invasive plans, as the most common pre-conference plan was possible resection (n=47, 46.5%) and the most common post-conference plan was to obtain further diagnostic testing (n=24, 23.8%) (Figure 2). For patients with a pre-conference plan of “operate” (n=47), new post-conference plans were to start chemotherapy or radiation therapy (n=12, 25.5%), undergo a diagnostic procedure (n=11, 23.4%), observe (n=10, 21.3%), reimage (n=7, 14.9%), continue current chemotherapy or radiation therapy (n=5, 10.6%), change chemotherapy or radiation therapy (n=1, 2.1%), and review additional records such as referring facility documentation and pathology (n=1, 2.1%). No variables were associated with a change in the pre-conference plan, including specialty of presenting physician, gender, age, established patient, or malignant diagnosis.
Table 3.
Pathologic or radiologic diagnoses for cases in which the treatment plan was changed after MDTB discussion.
| Pathologic or Radiologic Diagnosis | n | % |
|---|---|---|
| Pancreatic adenocarcinoma | 42 | 41.6% |
| Pancreatic mass | 18 | 17.8% |
| Neuroendocrine tumor | 9 | 8.9% |
| Pancreatitis | 7 | 6.9% |
| Bile duct mass, stricture, obstruction, jaundice | 5 | 5.0% |
| Pancreatic cyst | 4 | 4.0% |
| Cholangiocarcinoma | 4 | 4.0% |
| Ampullary cancer or mass | 2 | 2.0% |
| Intraductal papillary mucinous neoplasm | 3 | 3.0% |
| Gastric cancer or mass | 2 | 2.0% |
| Other mass or malignancy | 3 | 3.0% |
| Other benign condition | 2 | 2.0% |
| Total | 101 |
Figure 2.
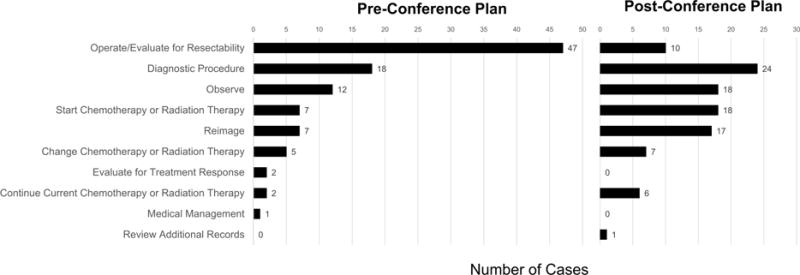
Distribution of pre-conference plans and post-conference plans for cases experiencing a change in plan as a result of presentation at MDTB (n = 101). Post-conference plans favored obtaining additional information including imaging and diagnostic procedures
MDTB recommendations for patients with new diagnoses of PDAC (n=33) were evaluated for differences in survival time and adherence to NCCN guidelines. Mean survival time was no different between cases with a change in plan as a result of MDTB (12.1 ± 5.6 months) and cases without a change in plan (9.0 ± 5.4 months; p=0.154). Adherence to NCCN guidelines was 100% for these 33 cases. Two patients were presented at MDTB with incomplete NCCN diagnostic workup. MDTB recommended completion of NCCN-recommended workup in both cases prior to making a treatment decision. NCCN guideline adherence was 100% for other pathology, including cases of extrahepatic cholangiocarcinoma (n=4) and neuroendocrine tumor (n=9).
To assess institutional resource utilization for our MDTB, weekly time-effort and time to receipt of the MDTB-recommended plan were assessed. Average weekly attendance at MDTB was eleven and weekly time expenditure for preparation was, on average, four (3.92 ± 0.97) hours for a rotating group of six diagnostic radiologists, 0.5 hours for pathology, and one hour for support staff. MDTB therefore represents a total weekly time expenditure of 16.5 hours and a cost of $2035 weekly, $190 per case, if physicians could be performing clinical billable duties instead of preparing for and attending MDTB.
To assess efficiency and adherence to MDTB-recommendations, we evaluated time to receipt of the recommended plan for all cases in which the plan was changed to require new testing or procedures. 17 cases were recommended to receive immediate repeat imaging. 14 of the 17 received recommended reimaging with a mean time after MDTB of 19.5 days (range 1–63; bimodal distribution, less than 30 days n=10, >30 days n=4; reasons for delay >30 days include intentional delay to evaluate for disease progression (n=1), awaiting further workup for other medical conditions (n=1), and delays from outside imaging facilities (n=2)). Reasons for non-adherence to MDTB recommendation included undergoing an additional diagnostic procedure (n=1), pursuit of further chemotherapy at the request of the patient’s local oncologist (n=1), and transfer of care (n=1; patient moved >1,000 miles). 24 cases were recommended to undergo additional diagnostic procedures. This recommendation was carried out in 18 cases (75%), with a mean time after MDTB of 13.8 ±12 days (range 3–50) for 16 cases. The two remaining cases had a documented intentional observation period of over 60 days before the diagnostic procedure. Reasons for non-adherence to MDTB recommendation were decisions to pursue further observation (n=1) or proceed to surgery (n=3, all with a pre-conference plan to evaluate for resection); two patients were lost to follow-up. Finally, 10 cases were recommended to undergo surgery. 4 of 10 patients received an operation, with a mean time after MDTB of 29 ±18.8 days (range 2–44). Reasons for non-adherence to MDTB decision included additional pre-operative workup demonstrating progression of disease (n=3), patients declining surgery (n=2), and plan to extend the period of observation (n=1).
DISCUSSION
Multidisciplinary tumor boards are requirements for cancer center accreditation and have the potential to benefit both patients and participating clinicians in a number of ways. The aim of this study was to determine how we might optimize the benefit or the utility of our MDTB by assessing quality through analyzing decision-making, resource utilization, and adherence to national guidelines. We noted management plans changed in 25% of cases after MDTB presentation and noted no difference in survival and excellent adherence to national guidelines in a subset of patients. When the plans were changed, multidisciplinary discussion led to expeditious coordination of these alterations to the expected plan of care.
The impetus for this study was a general lack of knowledge of the overall resources required to hold these conferences, and the benefits and results of MDTB discussion were largely unknown by the clinicians frequently using them. Previous reports of the utility of multidisciplinary care conferences have addressed the concept of “value-for-time balance” and how multiple factors, including the structure and function of conferences and organizational factors, can greatly impact the often-subjective conclusion of utility or ‘usefulness’ 29. Assessment of value in healthcare relies on identifying some measure of beneficial outcome in comparison to a measure of expenditure 30: in the setting of multidisciplinary care conferences, the beneficial outcome could be short-term – the impact on decision-making – or a more long-term outcome such as patient survival. The measure of expenditure in this report is time invested.
Previous surveys have demonstrated that clinicians are skeptical of the short-term outcome, believing that MDTBs “rarely” change pre-existing treatment plans31, but long-term outcomes including survival have been improved by MDTBs 17, 18. Further complicating assessment of value, one must consider benefits that extend beyond the variables described here and likely beyond what one can quantify: not recorded in our data but noted during MDTB discussion include benefits such as the efficiency of face-to-face communication, opportunity for discussion and possible resolution of conflictual opinions, and enhanced working relationships between physicians, as well as other benefits that have previously been reported including the opportunity to clarify criteria for clinical trials and enroll patients 32, affirmation of individual physician decision-making after collaborative discussion, and enhanced patient perception of patient-centered care when clinicians discuss the results of MDTB in clinical visits 33.
The strengths of our report include descriptive data with which we can regularly assess value-for-time, as well as a data-reporting approach we propose for future reports of MDTBs. We suggest that future reports should review outcomes but allow for internal and external assessments of value through reporting of resources required to maintain a MDTB and quality reporting as assessed by adherence to regional, national, or international treatment guidelines; care compliant with guidelines allows for generalizability and has been associated with increased survival in pancreas and other solid organ gastrointestinal cancers 34, 35.
Suggestions to enhance efficiency of these conferences include limiting pathology to malignant diagnoses or only presenting cases where no standard treatment exists or the patient may be unfit to tolerate the standard treatment 36. In an attempt to identify clinical scenarios that might benefit most from MDTB discussion, we analyzed all cases with a change in the pre-conference plan but were unable to link any recorded variables to this outcome. Other strategies for improving MDTBs include standardization of conference preparation and proceedings 37–39, electronic recording of pre-conference plans 12, 13, and review of the efficacy of teamwork in decision-making 10, 11. Our group has begun revisions to our MDTB through standardization of the electronic listing of patients.
Limitations of our study are associated with our data collection process. 25% of our initial treatment decisions were changed as a result of MDTB yet we are claiming a 100% adherence rate to NCCN guidelines. This might be interpreted that up to 25% of our initial treatment decisions were incorrect. We anticipate that is an overestimate due to data collection bias favoring surgery when in fact the surgeon may have recommended either surgery or chemotherapy for borderline-resectable PDAC pending case discussion at MDTB. Additionally, we were unable to capture instances in which the presenting physician intended to use MDTB to review new or revised imaging reports obtained after a clinical decision was documented. Therefore, pre-conference plans presented here may not represent decision-making taking into account all data available at the time of MDTB. Additional limitations of our study include intrinsic factors related to the make-up of the MDTB at our institution, including our patient population, referral base, and institutional bias, which may make these findings difficult to generalize to other institutions. However, as a tertiary academic medical center with Commission on Cancer accreditation and an NCCN Member, these factors and therefore the data presented here may be generalizable to similar institutions. Finally, the assumptions of cost using retrospective assessment of time-effort and conservative estimates of billing potential have inherent limitations, including assuming physicians would be doing particular billable clinical tasks in the hours spent preparing for or in attendance at MDTB. We addressed this by performing conservative estimates of the potential of one hour of billing activity. Only one other estimate of cost per case, $120, has been published25 but is difficult to compare to our $190 estimate, as it was derived from costs and reimbursement within a different healthcare system.
CONCLUSION
MDTB for pancreas and upper gastrointestinal conditions reviews a wide array of pathologies and influences management decisions in a substantial number of patients. In describing the utility of our MDTB, we advocate for continued emphasis on multidisciplinary collaboration and suggest that regular assessments of multidisciplinary conferences can maximize the significant investment required for efficient and effective coordination of care for our patients, and propose that standardized reporting of multidisciplinary conferences should include outcomes, effort required, and adherence to quality measures.
Acknowledgments
Funding/Support: DGB, MSS, and DES were supported in part by a National Research Service Award from the National Cancer Institute (T32 CA009621).
Abbreviations
- HPB
hepato-pancreato-biliary
- MDTB
multidisciplinary tumor board
- NCCN
National Comprehensive Cancer Network
- PDAC
pancreatic ductal adenocarcinoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: All authors report no conflicts of interest or financial disclosures relevant to this work.
Previous Presentations: This work was presented at the Gastrointestinal Cancers Symposium 2016, January 21–23, San Francisco, CA.
Author Contributions: (CRediT taxonomy)
David G. Brauer: Conceptualization, methodology, software, validation, formal analysis, investigation, data curation, writing – original draft preparation, writing – review and editing, visualization, project administration
Matthew S. Strand: Conceptualization, methodology, validation, formal analysis, investigation, data curation, writing – review and editing
Dominic E. Sanford: Conceptualization, methodology, formal analysis, investigation, data curation, writing – review and editing
Vladimir M. Kushnir: Investigation, writing – review and editing
Kian-Huat Lim: Investigation, writing – review and editing
Daniel K. Mullady: Investigation, writing – review and editing
Benjamin R. Tan Jr: Investigation, writing – review and editing
Andrea Wang-Gillam: Investigation, writing – review and editing
Ashley E. Morton: Investigation, writing – review and editing
Marianna B. Ruzinova: Investigation, writing – review and editing
Parag J. Parikh: Investigation, writing – review and editing
Vamsi R. Narra: Investigation, writing – review and editing
Kathryn J. Fowler: Investigation, writing – review and editing
Majella B. Doyle: Investigation, writing – review and editing
William C. Chapman: Investigation, writing – review and editing
Steven S. Strasberg: Investigation, writing – review and editing
William G. Hawkins: Investigation, writing – review and editing
Ryan C. Fields: Conceptualization, methodology, validation, resources, writing – original draft preparation, writing – review and editing, visualization, supervision, project administration
References
- 1.Cancer Program Standards: Ensuring Patient-Centered Care, 2016 Edition. Chicago: Commission on Cancer, American College of Surgeons; 2016. [Google Scholar]
- 2.Pancreatic Adenocarcinoma (Version 2.2015): National Comprehensive Cancer Network. [updated Mar 6, 2015; cited Jan 7, 2016]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf.
- 3.El Saghir NS, Keating NL, Carlson RW, Khoury KE, Fallowfield L. Tumor boards: optimizing the structure and improving efficiency of multidisciplinary management of patients with cancer worldwide. Am Soc Clin Oncol Educ Book. 2014:e461–6. doi: 10.14694/EdBook_AM.2014.34.e461. [DOI] [PubMed] [Google Scholar]
- 4.Chang JH, Vines E, Bertsch H, Fraker DL, Czerniecki BJ, Rosato EF, et al. The impact of a multidisciplinary breast cancer center on recommendations for patient management: the University of Pennsylvania experience. Cancer. 2001;91(7):1231–7. doi: 10.1002/1097-0142(20010401)91:7<1231::aid-cncr1123>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 5.Newman EA, Guest AB, Helvie MA, Roubidoux MA, Chang AE, Kleer CG, et al. Changes in surgical management resulting from case review at a breast cancer multidisciplinary tumor board. Cancer. 2006;107(10):2346–51. doi: 10.1002/cncr.22266. [DOI] [PubMed] [Google Scholar]
- 6.Santoso JT, Schwertner B, Coleman RL, Hannigan EV. Tumor board in gynecologic oncology. Int J Gynecol Cancer. 2004;14(2):206–9. doi: 10.1111/j.1048-891X.2004.014200.x. [DOI] [PubMed] [Google Scholar]
- 7.Gatcliffe TA, Coleman RL. Tumor board: more than treatment planning–a 1-year prospective survey. J Cancer Educ. 2008;23(4):235–7. doi: 10.1080/08858190802189014. [DOI] [PubMed] [Google Scholar]
- 8.Cohen P, Tan AL, Penman A. The multidisciplinary tumor conference in gynecologic oncology–does it alter management? Int J Gynecol Cancer. 2009;19(9):1470–2. doi: 10.1111/IGC.0b013e3181bf82df. [DOI] [PubMed] [Google Scholar]
- 9.Greer HO, Frederick PJ, Falls NM, Tapley EB, Samples KL, Kimball KJ, et al. Impact of a weekly multidisciplinary tumor board conference on the management of women with gynecologic malignancies. Int J Gynecol Cancer. 2010;20(8):1321–5. doi: 10.1111/IGC.0b013e3181f5871e. [DOI] [PubMed] [Google Scholar]
- 10.Jalil R, Akhter W, Lamb BW, Taylor C, Harris J, Green JS, et al. Validation of team performance assessment of multidisciplinary tumor boards. J Urol. 2014;192(3):891–8. doi: 10.1016/j.juro.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Lamb BW, Green JS, Benn J, Brown KF, Vincent CA, Sevdalis N. Improving decision making in multidisciplinary tumor boards: prospective longitudinal evaluation of a multicomponent intervention for 1,421 patients. J Am Coll Surg. 2013;217(3):412–20. doi: 10.1016/j.jamcollsurg.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 12.van Hagen P, Spaander MC, van der Gaast A, van Rij CM, Tilanus HW, van Lanschot JJ, et al. Impact of a multidisciplinary tumour board meeting for upper-GI malignancies on clinical decision making: a prospective cohort study. Int J Clin Oncol. 2013;18(2):214–9. doi: 10.1007/s10147-011-0362-8. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt HM, Roberts JM, Bodnar AM, Kunz S, Kirtland SH, Koehler RP, et al. Thoracic multidisciplinary tumor board routinely impacts therapeutic plans in patients with lung and esophageal cancer: a prospective cohort study. Ann Thorac Surg. 2015;99(5):1719–24. doi: 10.1016/j.athoracsur.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Freeman RK, Van Woerkom JM, Vyverberg A, Ascioti AJ. The effect of a multidisciplinary thoracic malignancy conference on the treatment of patients with esophageal cancer. Ann Thorac Surg. 2011;92(4):1239–42. doi: 10.1016/j.athoracsur.2011.05.057. discussion 43. [DOI] [PubMed] [Google Scholar]
- 15.Freeman RK, Van Woerkom JM, Vyverberg A, Ascioti AJ. The effect of a multidisciplinary thoracic malignancy conference on the treatment of patients with lung cancer. Eur J Cardiothorac Surg. 2010;38(1):1–5. doi: 10.1016/j.ejcts.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 16.Pillay B, Wootten AC, Crowe H, Corcoran N, Tran B, Bowden P, et al. The impact of multidisciplinary team meetings on patient assessment, management and outcomes in oncology settings: A systematic review of the literature. Cancer Treat Rev. 2016;42:56–72. doi: 10.1016/j.ctrv.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 17.MacDermid E, Hooton G, MacDonald M, McKay G, Grose D, Mohammed N, et al. Improving patient survival with the colorectal cancer multi-disciplinary team. Colorectal Dis. 2009;11(3):291–5. doi: 10.1111/j.1463-1318.2008.01580.x. [DOI] [PubMed] [Google Scholar]
- 18.Bydder S, Nowak A, Marion K, Phillips M, Atun R. The impact of case discussion at a multidisciplinary team meeting on the treatment and survival of patients with inoperable non-small cell lung cancer. Intern Med J. 2009;39(12):838–41. doi: 10.1111/j.1445-5994.2009.02019.x. [DOI] [PubMed] [Google Scholar]
- 19.Abraham NS, Gossey JT, Davila JA, Al-Oudat S, Kramer JK. Receipt of recommended therapy by patients with advanced colorectal cancer. Am J Gastroenterol. 2006;101(6):1320–8. doi: 10.1111/j.1572-0241.2006.00545.x. [DOI] [PubMed] [Google Scholar]
- 20.Keating NL, Landrum MB, Lamont EB, Bozeman SR, Shulman LN, McNeil BJ. Tumor boards and the quality of cancer care. J Natl Cancer Inst. 2013;105(2):113–21. doi: 10.1093/jnci/djs502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boxer MM, Vinod SK, Shafiq J, Duggan KJ. Do multidisciplinary team meetings make a difference in the management of lung cancer? Cancer. 2011;117(22):5112–20. doi: 10.1002/cncr.26149. [DOI] [PubMed] [Google Scholar]
- 22.Blazeby JM, Wilson L, Metcalfe C, Nicklin J, English R, Donovan JL. Analysis of clinical decision-making in multi-disciplinary cancer teams. Ann Oncol. 2006;17(3):457–60. doi: 10.1093/annonc/mdj102. [DOI] [PubMed] [Google Scholar]
- 23.Wood JJ, Metcalfe C, Paes A, Sylvester P, Durdey P, Thomas MG, et al. An evaluation of treatment decisions at a colorectal cancer multi-disciplinary team. Colorectal Dis. 2008;10(8):769–72. doi: 10.1111/j.1463-1318.2007.01464.x. [DOI] [PubMed] [Google Scholar]
- 24.Taylor C, Munro AJ, Glynne-Jones R, Griffith C, Trevatt P, Richards M, et al. Multidisciplinary team working in cancer: what is the evidence? BMJ. 2010;340:c951. doi: 10.1136/bmj.c951. [DOI] [PubMed] [Google Scholar]
- 25.Simcock R, Heaford A. Costs of multidisciplinary teams in cancer are small in relation to benefits. BMJ. 2012;344:e3700. doi: 10.1136/bmj.e3700. [DOI] [PubMed] [Google Scholar]
- 26.Physician Fee Schedule Search. Baltimore, MD: Centers for Medicare and Medicaid Services; Jul 31, 2016. Available from: https://www.cms.gov/apps/physician-fee-schedule/search/search-criteria.aspx. [Google Scholar]
- 27.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573–7. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 28.Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf. 2015 doi: 10.1136/bmjqs-2015-004411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Look Hong NJ, Gagliardi AR, Bronskill SE, Paszat LF, Wright FC. Multidisciplinary cancer conferences: exploring obstacles and facilitators to their implementation. J Oncol Pract. 2010;6(2):61–8. doi: 10.1200/JOP.091085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–81. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 31.Kostaras X, Shea-Budgell MA, Malcolm E, Easaw JC, Roa W, Hagen NA. Is there a role for clinical practice guidelines in multidisciplinary tumor board meetings? A descriptive study of knowledge transfer between research and practice. J Cancer Educ. 2012;27(1):42–5. doi: 10.1007/s13187-011-0263-6. [DOI] [PubMed] [Google Scholar]
- 32.Kuroki L, Stuckey A, Hirway P, Raker CA, Bandera CA, DiSilvestro PA, et al. Addressing clinical trials: can the multidisciplinary Tumor Board improve participation? A study from an academic women’s cancer program. Gynecol Oncol. 2010;116(3):295–300. doi: 10.1016/j.ygyno.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komatsu H, Nakayama K, Togari T, Suzuki K, Hayashi N, Murakami Y, et al. Information sharing and case conference among the multidisciplinary team improve patients’ perceptions of care. Open Nurs J. 2011;5:79–85. doi: 10.2174/1874434601105010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Visser BC, Ma Y, Zak Y, Poultsides GA, Norton JA, Rhoads KF. Failure to comply with NCCN guidelines for the management of pancreatic cancer compromises outcomes. HPB (Oxford) 2012;14(8):539–47. doi: 10.1111/j.1477-2574.2012.00496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boland GM, Chang GJ, Haynes AB, Chiang YJ, Chagpar R, Xing Y, et al. Association between adherence to National Comprehensive Cancer Network treatment guidelines and improved survival in patients with colon cancer. Cancer. 2013;119(8):1593–601. doi: 10.1002/cncr.27935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riquet M, Mordant P, Henni M, Wermert D, Fabre-Guillevin E, Cazes A, et al. Should all cases of lung cancer be presented at Tumor Board Conferences? Thorac Surg Clin. 2013;23(2):123–8. doi: 10.1016/j.thorsurg.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Manual for Cancer Services 2004. London: Department of Health; 2004. [Google Scholar]
- 38.Wright FC, De Vito C, Langer B, Hunter A. Multidisciplinary cancer conferences: a systematic review and development of practice standards. Eur J Cancer. 2007;43(6):1002–10. doi: 10.1016/j.ejca.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 39.Multidisciplinary Treatment Planning Questionnaire: Healthcare Delivery Research Program, Division of Cancer Control and Population Sciences, National Cancer Institute. 2015 [updated 3/13/2015; cited 1/11/2016]. Available from: http://healthcaredelivery.cancer.gov/mtp/


