Abstract
Dysregulation of the cyclin D–cyclin-dependent kinase (CDK)4/6–INK4–retinoblastoma (Rb) pathway is an important contributor to endocrine therapy resistance. Recent clinical development of selective inhibitors of CDK4 and CDK6 kinases has led to renewed interest in cell cycle regulators, following experience with relatively nonselective pan-CDK inhibitors that often resulted in limited activity and poor safety profiles in the clinic. The highly selective oral CDK 4/6 inhibitors palbociclib (PD0332991), ribociclib (LEE011), and abemaciclib (LY2835219) are able to inhibit the proliferation of Rb-positive tumor cells and have demonstrated dose-dependent growth inhibition in ER+ breast cancer models. In metastatic breast cancer, all three agents are being explored in combination with endocrine therapy in Phase III studies. Results so far indicate promising efficacy and manageable safety profiles, and led to the FDA approval of palbociclib. Phase II–III studies of these agents, in combination with endocrine therapy, are also underway in early breast cancer in the neoadjuvant and adjuvant settings. Selective CDK 4/6 inhibitors are also being investigated with other targeted agents or chemotherapy in the advanced setting. This article reviews the rationale for targeting cyclin D–CDK 4/6 in hormone receptor-positive (HR+) breast cancer, provides an overview of the available preclinical and clinical data with CDK 4/6 inhibitors in breast cancer to date, and summarizes the main features of ongoing clinical trials of these new agents in breast cancer. Future trials evaluating further combinations strategies with CDK 4/6 backbone and translational studies refining predictive biomarkers are needed to help personalize the optimal treatment regimen for individual patients with ER+ breast cancer.
Keywords: CDK 4/6 inhibitor, ribociclib (LEE011), palbociclib (PD-0332991), abemaciclib (LY2835219), hormone receptor-positive breast cancer
Introduction
Breast cancer is a diverse disease, comprising several molecular subgroups with distinct tumor biology. Approximately 75% of breast cancers express the estrogen receptor (ER) and rely upon ER signaling for growth and survival (Nadji et al., 2005). Endocrine therapy, which antagonizes the growth-promoting activity of estrogen, remains the predominant front-line treatment for these patients (Cardoso et al., 2014). However, not all patients respond (de novo resistance), and a proportion of patients who do respond eventually progress (acquired resistance) (Osborne et al., 2011). Multiple mechanisms for endocrine resistance have been proposed, including the disruption of various components of the ER pathway itself and alterations in the cell cycle and cell survival signaling molecules (Osborne et al., 2011). Among these, dysregulation of the cyclin D–cyclin-dependent kinase (CDK)4/6–INK4–retinoblastoma (Rb) pathway has been associated with a poor response to endocrine therapy (Thangavel et al., 2011) and alterations in this pathway are frequently observed in hormone receptor-positive (HR+) breast cancer (Cancer Genome Atlas Network, 2012). Thus, CDK4 and CDK6 are considered valid targets for therapeutic intervention. This review will focus on the rationale for targeting cyclin D–CDK 4/6 in HR+ breast cancer, and will provide an overview of the preclinical and clinical data with CDK 4/6 inhibitors to date.
Overview of the cyclin D–CDK 4/6–INK4–Rb pathway
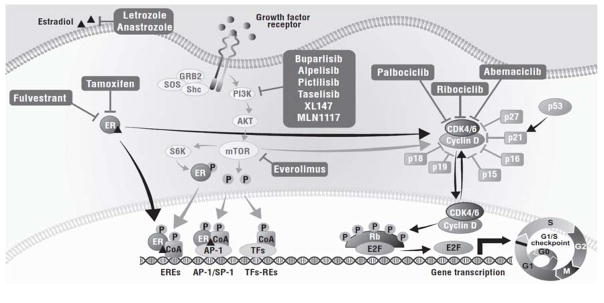
The cell cycle constitutes a series of tightly controlled events that drive DNA replication and cell division (Caldon et al., 2006). The cell cycle is divided into phases: G0 (quiescence) followed by G1 (pre-DNA synthesis), S (DNA synthesis), G2 (pre-division), and M (cell division) (Caldon et al., 2006). The progression from G1 to S is a critical checkpoint in protecting the cell from abnormal replication, and a key regulator of this process is the cyclin D–CDK 4/6–INK4–Rb pathway (Fig. 1) (Lange et al., 2011). A variety of mitogenic signaling pathways, including steroid hormones (such as the ER pathway), PI3K/AKT/mTOR, MAPKs, wnt/β-catenin, STATs, and NF-κB/IKK, upregulate the expression of cyclin D, which associates with CDK 4/6 (Lange et al., 2011). Activation of the cyclin D-CDK 4/6 complex contributes to the hyperphosphorylation of the Rb protein, which causes inactivation of its growth-inhibitory function by decoupling it from E2F transcription factors. The newly released E2F transcription factors allow the transcription of genes promoting entry into the S phase and thus cell-cycle progression (Lange et al., 2011). Association of cyclin D with CDK 4/6 is tightly regulated by inhibitors, such as INK4 (p16INK4A, p15INK4B, p18INK4C, p19INK4D), Cip (21Cip1), and Kip (p27kip1, p57kip2) proteins (Fig. 1) (Ortega et al., 2002).
Fig. 1. Targeting the Cyclin D–CDK 4/6–INK4–Rb pathway.
CDK cyclin-dependent kinase, E2F E2 transcription factor, ER estrogen receptor, GRB2 growth factor receptor-bound protein 2, HR+ hormone receptor-positive, mTOR mammalian target of rapamycin, PI3K phosphatidylinositol 3-kinase, Rb retinoblastoma, RTK receptor tyrosine kinase, S6K S6 kinase
Dysregulation of the cyclin D–CDK 4/6–INK4–Rb pathway in breast cancer
The cyclin D–CDK 4/6–INK4–Rb pathway is critical to cell cycle entry and the majority of cancers disrupt this pathway to promote cell proliferation through mechanisms including overexpression, amplification, and chromosomal translocations of D cyclins; mutations and amplification of CDK 4/6; and loss of inhibitors such as INK4 proteins (Ortega et al., 2002; Asghar et al., 2015; Shapiro, 2006). Loss of the tumor suppressor p16 is one of the most common abnormalities in breast cancer and has been observed in 49% of archival breast tumor specimens (Geradts and Wilson, 1996). Cyclin D1 amplification and overexpression also play important roles (Lundgren et al., 2012), and amplification of the CCND1 gene, which encodes cyclin D1, has been identified in 29–58% of breast cancers (Cancer Genome Atlas Network, 2012). In addition, cyclin D1 protein overexpression, whether due to gene amplification or transcriptional or post-transcriptional dysregulation, is found in up to 50% of breast cancers, where it is believed to drive aberrant phosphorylation and inactivation of Rb protein (Lundgren et al., 2012; Abraham et al., 2014). Notably, a deficiency in Rb is associated with the evolution of a CDK 4/6-independent state, poor prognosis in response to hormonal therapy, and resistance to CDK 4/6 inhibition (Ertel et al., 2009; Dean et al., 2010). However, the majority (>90%) of ER+ breast cancers express functional Rb (Abraham et al., 2014).
Inhibiting CDK 4/6 in HR+ breast cancer
In the past, therapeutic targeting of CDK activity has been limited to pan-CDK inhibitors, which generally resulted in limited activity and challenging safety profiles (Dickson, 2014). However, the development of selective ATP inhibitors of CDK4 and CDK6 kinase activity has the potential to cause dose-dependent G1 arrest in human breast cancers with an improved safety profile. Three CDK 4/6 inhibitors are currently in clinical development: palbociclib (PD0332991; Pfizer Inc., FDA-approved), ribociclib (LEE011; Novartis), and abemaciclib (LY2835219; Lilly, FDA Breakthrough Therapy status).
Preclinical data for single-agent CDK 4/6 inhibitors
Determining the selectivity of CDK 4/6 inhibitors has been an important step in establishing the effectiveness of these compounds versus pan-CDK inhibitors (Fry et al., 2004). Palbociclib, ribociclib, and abemaciclib are highly selective CDK 4/6 inhibitors with IC50 values against CDK4 and CDK6 of <40 nM (Table 1). All three agents inhibit cell proliferation in Rb-positive cells and have demonstrated dose-dependent growth inhibition in tumor xenograft models (Fry et al., 2004; Gelbert et al., 2014; Kim et al., 2013).
Table 1.
IC50 values of CDK 4/6 inhibitors
| IC50 (nM) | |||
|---|---|---|---|
|
| |||
| Palbociclib (Fry et al., 2004) | Ribociclib (Kim et al., 2013) | Abemaciclib (Gelbert et al., 2014) | |
|
| |||
| CDK4-cyclin D1 | 11 | 10 | 2 |
| CDK6-cyclin D1/2/3 | 16 | 39 | 10 |
| CDK1-cyclin B | >10,000 | 113,000 | 1627 |
| CDK2-cyclin A/E | >10,000 | 76,000 | 504 |
| CDK9-cyclin T | NR | NR | 57 |
NR not reported
Preclinical studies have provided rationale for the clinical development of CDK 4/6 inhibitors in specific molecular subgroups of breast cancer. Among a panel of 47 and 50 breast cancer cell lines exposed to palbociclib and ribociclib, respectively, those that were ER+ were the most sensitive to growth inhibition, while basal subtypes were shown to be the most resistant to palbociclib (Finn et al., 2009; O’Brien et al., 2014). Notably, responses were also seen in HER2-amplified cell lines, though primarily those with luminal features (O’Brien et al., 2014). Palbociclib has been shown to slow tumor progression, not only by exerting cell-cycle control, but also via the suppression of epithelial–mesenchymal transition and stem-like properties of cancer cells (Lamb et al., 2013; Arima et al., 2013). In addition, elevated expression of cyclin-D1, Rb and reduced p16 expression have been associated with sensitivity towards palbociclib (Finn et al., 2009).
Clinical data for single-agent CDK 4/6 inhibitors
Safety and preliminary clinical activity
The safety and preliminary antitumor activity of CDK 4/6 inhibitors as single agents have been investigated in several clinical trials. These are summarized in Table 2 and select trials discussed below.
Table 2.
Clinical trials of single-agent CDK 4/6 inhibitors
| Study drug | NCT number/reference | Phase | Patient population | Safety | Clinical activity |
|---|---|---|---|---|---|
| Palbociclib | NCT00141297/ (Flaherty et al., 2012) | I | Advanced solid tumors n = 41 |
|
|
| NCT01037790/ (DeMichele et al., 2015) | II | Refractory solid tumors n = 37 (MBC cohort) |
|
Overall (n = 37)
|
|
| Ribociclib | NCT01237236/ (Infante et al., 2014) | I | Advanced solid tumors or lymphomas n = 128 |
|
Three PRs:
|
| Abemaciclib | NCT01394016/ (Shapiro et al., 2013; Patnaik et al., 2014) | I | Advanced cancer (NSCLC, glioblastoma, breast cancer, melanoma, and colorectal cancer) n = 55 |
|
|
AE adverse event, DCR disease control rate, DLT dose-limiting toxicity, G Grade, HR+ hormone receptor-positive, MBC metastatic breast cancer, NSCLC non-small cell lung cancer, ORR overall response rate, PD progressive disease, PFS progression-free survival, PR partial response, RECIST Response Evaluation Criteria In Solid Tumors, SD stable disease
Palbociclib
Early clinical trials have investigated the safety and clinical activity of palbociclib as a single agent (Table 2). The most common adverse event associated with palbociclib is neutropenia, however, it is distinct from that observed with cytotoxic agents in that it is rapidly reversible, reflecting a cytostatic effect on neutrophil precursors in the bone marrow (Asghar et al., 2015). CDK6 appears to be important in promoting the proliferation of hematologic precursors, and CDK 4/6 inhibition in mice has been associated with a transient growth arrest in hematopoietic precursor cells (Asghar et al., 2015; Johnson et al., 2010). Non-hematologic adverse events related to treatment with palbociclib include nausea, fatigue, diarrhea, stomatitis, and asthenia (Flaherty et al., 2012; Pfizer Inc., 2015; DeMichele et al., 2015).
In the Phase I first-in-human dose-escalation study, patients with advanced solid tumors received oral palbociclib once daily for 21 of 28 days (NCT00141297). Neutropenia was the major toxicity observed and was also dose-limiting. The maximum-tolerated dose (MTD) and recommended Phase II dose (RP2D) were declared as 125 mg given once daily on a 3-weeks-on/1-week-off schedule every 28 days. Ten (27%) of 37 evaluable patients achieved stable disease (SD) for ≥4 cycles, six of whom derived prolonged benefit (>10 cycles) (Flaherty et al., 2012).
Ribociclib
As with palbociclib, hematologic adverse events, including neutropenia, are also common with ribociclib and therefore a 1-week resting period is incorporated into dosing regimens in most trials (Asghar et al., 2015). In the Phase I, first-in-human study, patients with advanced solid tumors or lymphomas received escalating doses of single-agent ribociclib either as part of a 3-weeks-on/1-week-off schedule, or as part of a continuous 28-day schedule (NCT01237236). The MTD and recommended dose for expansion were declared as 900 mg/day and 600 mg/day on a 3-weeks-on/1-week-off dosing schedule, respectively. Out of 110 evaluable patients, three partial responses (PRs) were observed and stable disease (SD) for ≥4 and ≥6 cycles was observed in 24% and 15% of patients, respectively (Table 2) (Infante et al., 2014).
Abemaciclib
Hematologic adverse events, including neutropenia, are somewhat less common in patients receiving abemaciclib. Gastrointestinal-related toxicity appears to be more predominant with abemaciclib, with typical adverse events being nausea, fatigue, diarrhea, and vomiting (Asghar et al., 2015; Shapiro et al., 2013; Patnaik et al., 2014). Notably, abemaciclib is administered on a continuous dosing schedule.
A Phase I first-in-human study is investigating abemaciclib, taken orally every 12 or 24 hours, in patients with advanced cancer in five tumor types, including breast cancer (NCT01394016) (Shapiro et al., 2013). In the expansion cohort, patients with HR+ breast cancer were administered abemaciclib continuously at 150–200 mg orally every 12 hours. Nine of 36 patients with HR+ disease had confirmed PRs with an overall response rate of 25%, and 20 (56%) patients had SD (Patnaik et al., 2014).
Combination approaches with CDK 4/6 inhibitors
Combination with endocrine therapy
Despite the clinical efficacy observed with endocrine therapy, resistance remains a major obstacle in ER+ breast cancer. Treatment of endocrine therapy-resistant cells with palbociclib has been shown to suppress proliferation effectively (Thangavel et al., 2011). Results of key and ongoing combination studies are described in Table 3 with select studies discussed below.
Table 3.
HR+ breast cancer clinical trials of CDK 4/6 inhibitors in combination with other agents
| Combination therapy/setting |
Study drug | Trial reference/ NCT number |
Phase | Patient population | Combination agent(s) |
Primary endpoint |
Results (primary endpoint) |
|---|---|---|---|---|---|---|---|
| Combination with endocrine therapy | |||||||
| Metastatic BC setting | |||||||
| Letrozole | Palbociclib | PALOMA-1/TRIO-18; NCT00721409 | II | ER+, HER2− advanced BC |
|
|
|
| PALOMA-2; NCT01740427 | III | Postmenopausal women with ER+, HER2− advanced BC |
|
|
|
||
| PALOMA-4; NCT02297438 | III | Postmenopausal women with ER+/HER2− advanced BC who have not received prior systemic anticancer therapies for advanced/metastatic disease |
|
|
|
||
| Ribociclib | MONALEESA-2; NCT01958021 | III | Postmenopausal women with HR+, HER2− advanced BC |
|
|
|
|
| Abemaciclib | MONARCH-3; NCT02246621 | III | Postmenopausal women with BC |
|
|
|
|
| Fulvestrant | Palbociclib | PALOMA-3; NCT01942135 | III | HR+, HER2− metastatic BC whose disease has progressed after prior endocrine therapy |
|
|
|
| Ribociclib | MONALEESA-3; NCT02422615 | III | Postmenopausal women with HR+, HER2− advanced BC, who have had no or only one line of prior endocrine treatment |
|
|
|
|
| Abemaciclib | MONARCH-2; NCT02107703 | III | HR+, HER2− locally advanced or metastatic breast cancer, who have had no or only one line of prior endocrine treatment |
|
|
|
|
| NCT01394016 | I | HR+ metastatic BC (among 5 different tumor types) |
|
|
Grade 3 AEs (no Grade 4 AE)
|
||
| Tamoxifen | Ribociclib | MONALEESA-7; NCT02278120 | III | Pre- or peri-menopausal women with HR+, HER2− advanced BC |
|
|
|
| Other/various therapies | Palbociclib | PEARL; NCT02028507 | III | HR+, HER2− metastatic BC with resistance to NSAIs |
|
|
|
| Abemaciclib | NCT02057133 | Ib | HR+, HER2−/HER2+ metastatic BC |
|
|
|
|
| Early BC setting | |||||||
| Neoadjuvant | Palbociclib | NCT01723774 | II | Women with Stage II/III ER+, HER2− BC |
|
|
|
| Palbociclib | PALLET; NCT02296801 | II | Postmenopausal women with ER+, primary BC |
|
|
|
|
| Abemaciclib | neoMONARCH; NCT02441946 | II | Postmenopausal women with HR+, HER2−, early-stage BC |
|
|
|
|
| Adjuvant | Palbociclib | PENELOPE-B; NCT01864746 | III | HR+, HER2− negative BC with residual disease after neoadjuvant chemotherapy |
|
|
|
| Palbociclib | NCT02040857 | II | HR+/HER2− invasive BC |
|
|
|
|
| Palbociclib | ABCSG-42; BIG 03/14 | III | HR+/HER2− Stage II/III BC |
|
|
|
|
| Combination with targeted signaling pathway inhibitors | |||||||
| mTOR inhibitors | Ribociclib | NCT01857193 | Ib | Advanced/metastatic ER+ BC |
|
|
DLTs with triplet therapy:
|
| II | Advanced/metastatic ER+ BC |
|
|
|
|||
| PI3K inhibitors | Ribociclib | NCT01872260 | Ib (dose escalation/expansion) | Advanced ER+ BC (dose expansion phase only includes patients in the first-line setting) |
|
|
|
| II | Advanced ER+ BC (first-line only) |
|
|
|
|||
| Palbociclib | NCT02389842 | Ib | PIK3CA-mutant BC |
|
|
|
|
| Combination with chemotherapy | |||||||
| Palbociclib | NCT01320592 | I | Rb-positive metastatic BC | Paclitaxel | Safety |
|
|
ALT alanine transaminase, AST aspartate transaminase, BC breast cancer, DLT dose-limiting toxicity, ER+ estrogen receptor-positive, HER2− human epidermal growth factor receptor 2-negative, HR+ hormone receptor-positive, iDFS invasive disease-free survival, PFS progression-free survival, NSAI non-steroidal aromatase inhibitors, Rb retinoblastoma, RP2D recommended Phase II dose
In the metastatic setting
Combination with letrozole
Letrozole is an orally administered non-steroidal aromatase inhibitor (NSAI) (Novartis, 2015). Preclinical studies of CDK 4/6 inhibitors in combination with letrozole have shown enhanced clinical activity (O’Brien et al., 2014). The combination of ribociclib and letrozole has also demonstrated sustained tumor control in a PIK3CA-wildtype, ER+ breast cancer model (Parasuraman et al., 2014).
Clinical results
Palbociclib
Perhaps the biggest clinical validation of the CDK 4/6 pathway as an important therapeutic target was provided by the results of the PALOMA-1 trial, a Phase II open-label, randomized trial in advanced ER+, HER2− breast cancer. Patients treated with palbociclib plus letrozole in the first-line setting had a median PFS of 20.2 months (versus 10.2 months with letrozole only; one-sided p = 0.0004; NCT00721409; Table 3) (Finn et al., 2015). Based on these results, palbociclib (Ibrance®; Pfizer), in combination with letrozole, was approved by the United States Food and Drug Administration in February 2015 as a front-line endocrine-based therapy for the treatment of postmenopausal women with ER+, HER2− advanced breast cancer (Pfizer Inc., 2015). Additional subanalyses from PALOMA-1 indicated that the PFS benefit for palbociclib plus letrozole also occurred in patients ≥65 years of age and in those who had not received systemic therapy (Crown et al., 2015; Finn et al., 2015). Clinically meaningful delays in progression in the bone were observed and long-term safety analyses (≥24 months) suggest that the palbociclib–letrozole combination is not associated with cumulative or late-onset toxicities (Slamon et al., 2015). A Phase III confirmatory study is ongoing (NCT01740427; PALOMA-2; Table 3).
Ribociclib
In a Phase Ib/II study of ribociclib in combination with letrozole, an acceptable safety profile and preliminary clinical activity was observed in postmenopausal women with ER+, HER2− advanced breast cancer (NCT01872260; Table 3). The RP2D of ribociclib was declared as 600 mg/day (3-weeks-on/1-week-off) in combination with continuous letrozole 2.5 mg/day (Munster et al., 2014). A Phase III study is currently ongoing (MONALEESA-2; NCT01958021; Table 3).
Abemaciclib
The combination of abemaciclib and either letrozole or anastrozole is being evaluated in the ongoing Phase III, randomized, double-blind, placebo-controlled MONARCH-3 study in postmenopausal women with recurrent or metastatic HR+ breast cancer, who have had no prior systemic therapy for this setting (NCT02246621; Table 3).
Combination with fulvestrant
Fulvestrant is a selective estrogen receptor degrader approved for the treatment of HR+ metastatic breast cancer in postmenopausal women whose disease has progressed following antiestrogen therapy (Howell, 2006; AstraZeneca, 2015).
Clinical results
Palbociclib
The combination of palbociclib and fulvestrant in patients whose disease has progressed after prior endocrine therapy was explored in a Phase III trial (PALOMA-3; NCT01942135; Table 3). After a pre-planned interim analysis, PALOMA-3 was stopped based on an efficacy assessment by an independent data monitoring committee. Addition of palbociclib to fulvestrant significantly prolonged median investigator-assessed PFS compared with fulvestrant alone (9.2 vs 3.8 months; p < 0.001). Interestingly, the palbociclib and fulvestrant combination demonstrated a PFS benefit across all pre-specified patient subgroups, including menopausal status, site of metastatic disease (visceral or non-visceral) and sensitivity to prior hormonal therapy (Turner et al., 2015).
Ribociclib
The combination of ribociclib and fulvestrant is being evaluated in the ongoing Phase III, randomized, double-blind, placebo-controlled MONALEESA-3 study in postmenopausal women with HR+, HER2− advanced breast cancer, who have had one or less prior lines of endocrine treatment (NCT02422615; Table 3).
Abemaciclib
Abemaciclib in combination with fulvestrant was evaluated in a Phase I study, demonstrating an acceptable safety profile and evidence of efficacy (NCT01394016; Table 3) (Patnaik et al., 2014). This combination is now being explored in a Phase III trial (MONARCH-2; NCT02107703; Table 3).
Combination with tamoxifen
Tamoxifen is an estrogen receptor antagonist that has been used for the treatment of HR+ breast cancer for over 30 years (Howell, 2006).
Clinical results
Ribociclib
The combination of ribociclib and tamoxifen is being investigated in peri- or premenopausal women with HR+, HER2− advanced breast cancer. This population typically receives tamoxifen or NSAIs with ovarian function suppression as standard first-line therapy; however, resistance to endocrine therapy and disease progression can occur. The Phase III MONALEESA-7 study is a randomized, double-blind, placebo-controlled study of this population investigating ribociclib combined with either tamoxifen and goserelin or a NSAI and goserelin (NCT02278120) (Tripathy et al., 2015).
Combination with other/various therapies in metastatic breast cancer
In addition to the above studies, a number of trials are investigating CDK 4/6 inhibitors in combination with a variety of other agents (Table 3). One Phase Ib study is evaluating abemaciclib in six patient cohorts where it is combined with letrozole, anastrozole, tamoxifen, exemestane, exemestane plus everolimus, or trastuzumab (NCT02057133; Table 3). Combinations of abemaciclib and endocrine therapy demonstrated manageable safety and early clinical evidence of antitumor activity (Tolaney et al., 2015). A separate Phase III study is underway to compare the efficacy and safety of palbociclib in combination with exemestane with that of capecitabine in postmenopausal women with HR+ metastatic breast cancer whose disease was refractory to prior NSAI (PEARL; NCT02028507; Table 3).
In early breast cancer
In addition to studies in advanced HR+ breast cancer, trials are exploring combinations of CDK 4/6 inhibitors with hormonal agents in early HR+ breast cancer (Table 3).
Neoadjuvant therapy
The potential utility of palbociclib plus anastrozole in the neoadjuvant setting is being investigated in a Phase II trial of women with clinical Stage II/III ER+, HER2− breast cancer (NCT01723774). The ongoing PALLET trial is investigating the combination of palbociclib and letrozole as neoadjuvant therapy in postmenopausal women with ER+ breast cancer (NCT02296801). Another neoadjuvant trial on the horizon is the neoMONARCH Phase II study of neoadjuvant abemaciclib in combination with anastrozole (NCT02441946).
Adjuvant therapy
The ongoing PENELOPE-B trial (NCT01864746) is a Phase III study of palbociclib and standard anti-hormonal therapy in women with HR+, HER2− early breast cancer who did not obtain a pathological complete response after taxane-containing neoadjuvant chemotherapy, and who are at high risk of relapse. A Phase II single-arm trial evaluating the feasibility of 2 years of treatment with palbociclib in combination with adjuvant endocrine therapy (letrozole, anastrozole, or exemestane) in patients with HR+ breast cancer is also ongoing (NCT02040857). The Phase III, double-blind, randomized PALLAS study is evaluating adjuvant palbociclib for 2 years plus endocrine treatment for 5 years versus 5 years of endocrine therapy alone in 4,600 patients with HR+, HER2− Stage II/III breast cancer.
Combination with targeted signaling pathway inhibitors
PI3K-targeted agents are currently being investigated in several Phase III trials in patients with HR+ breast cancer. The rationale for combining these agents with CDK 4/6 inhibitors in patients with HR+ breast cancer is attributed to the increase in cyclin D expression as a result of PI3K/AKT/mTOR pathway activation (Takuwa et al., 1999). In addition, preclinical and clinical data suggest that inhibiting CDK 4/6 activity or the PI3K/AKT/mTOR signaling axis may delay the development of endocrine resistance (Shapiro, 2006). Addition of a pan-PI3K inhibitor (buparlisib [BKM120] or a PI3K-alpha inhibitor (alpelisib [BYL719]) in a triplet combination with ribociclib and an antiestrogen resulted in enhanced, robust tumor regressions, without inducing significant toxicity in preclinical model (O’Brien et al., 2014). In another study, when PIK3CA-mutant breast cancer mouse models, both sensitive and resistant to alpelisib, were exposed to ribociclib, or alpelisib, or a combination of both, the combination regimen led to more enhanced regression, relative to single-agent therapy. The pan-PI3K inhibitor, pictilisib (GDC-0941), in combination with ribociclib, elicited tumor regression in MCF7 and CAL51 xenografts, whereas single-agent treatment did not (Vora et al., 2014). Due to the non-overlapping side effects of PI3K and CDK 4/6 inhibition, combination of these agents was predicted to be well tolerated in patients. Ongoing clinical trials of such combination therapies are summarized in Table 3 and select studies are discussed below.
A Phase Ib/II study of the triplet combination of ribociclib, alpelisib, and letrozole in postmenopausal women with ER+, HER2− advanced breast cancer is currently ongoing (NCT01872260; Table 3). Preliminary pharmacodynamic analyses suggest that, in some patients, alpelisib may help prevent compensatory PI3K/AKT/mTOR pathway activation following treatment with ribociclib and letrozole (Juric et al., 2014). A Phase Ib study is evaluating palbociclib in combination with the PI3K pathway inhibitors taselisib (GDC-0032) or pictilisib (GDC-0941), with the subsequent addition of fulvestrant in patients with PIK3CA-mutant breast cancers (NCT02389842; Table 3). An ongoing Phase Ib/II study is evaluating ribociclib, exemestane, and the mTOR inhibitor everolimus in patients with advanced/metastatic ER+ breast cancer refractory to the NSAIs letrozole or anastrozole (NCT01857193; Table 3). Preliminary results have demonstrated that the triplet combination is feasible, with encouraging signs of clinical activity (Bardia et al., 2014).
Combination with chemotherapy
Co-administration of CDK 4/6 inhibitors with chemotherapy has yielded contrasting results. In Rb-positive triple-negative breast cancer cell lines and xenografts, palbociclib and doxorubicin had an additive cytostatic effect by eliciting a G1 arrest or G2/M arrest, respectively. However, doxorubicin-mediated cell death signaling was also inhibited. Therefore, combination regimens of CDK 4/6 inhibitors with genotoxic compounds that rely heavily on cell proliferation for their cytotoxic effects may need to be approached with caution (McClendon et al., 2012). A Phase I trial investigating the combination of paclitaxel and palbociclib in patients with Rb-positive metastatic breast cancer demonstrated that the combination was well tolerated, and elicited prolonged tumor responses, though dose reductions and interruptions were common due to neutropenia (Clark et al., 2014); NCT01320592; Table 3).
Future challenges
Preclinical and clinical data suggest that CDK 4/6 inhibitors have significant potential in the treatment of breast cancer. In addition, combining CDK 4/6 inhibitors with other well-established therapies has demonstrated enhanced efficacy. Therefore, determining the ideal combinations of CDK 4/6 inhibitors with other targeted agents is an important challenge.
The use of biomarkers may have a role in helping to identify those patients who are most likely to respond to treatment with CDK 4/6 inhibitors. Various clinical trials are investigating the use of biomarkers, such as changes in copy number or expression/activation levels of genes including CCND1, CDKN2A, RB, and changes in the proliferation marker Ki67; however, as yet no potential biomarkers predictive of response have been identified. Results from the PALOMA-1 study demonstrated that both biomarker-positive (CCND1/CEP11 ratio ≥1.5 and/or CDKN2A/CEP9 ratio <0.8) and biomarker-negative patients benefited similarly from treatment with palbociclib and letrozole. In addition, Ki67 staining did not identify a subgroup that benefited more from this treatment combination (Jiang et al., 2014).
Conclusions
Although endocrine therapy is currently the mainstay of treatment for ER+ breast cancer, there is a requirement for novel treatment approaches due to the de novo and acquired resistance that occurs in many patients with advanced disease. CDK 4/6 inhibitors have demonstrated promising antitumor activity, and ongoing studies are exploring the combination of these agents with existing endocrine treatments and with inhibitors of upstream and downstream signaling molecules. Identifying optimal treatment combinations, and refining biomarkers that will predict patients’ responses to treatment, remains a key challenge.
Acknowledgments
The authors thank Eisha Comar PhD for medical editorial assistance with this manuscript.
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. Novartis did not have input on content or approval of the manuscript.
List of abbreviations
- ATP
Adenosine triphosphate
- CDK
Cyclin-dependent kinase
- DLT
Dose-limiting toxicity
- ER
Estrogen receptor
- ER+
Estrogen receptor-positive
- ER−
Estrogen receptor-negative
- HER2−
Human epidermal growth factor receptor 2-negative
- HR+
Hormone receptor-positive
- MTD
Maximum tolerated dose
- mTOR
Mammalian target of rapamycin
- NSAI
Non-steroidal aromatase inhibitor
- NSCLC
Non-small cell lung carcinoma
- PFS
Progression-free survival
- PI3K
Phosphatidylinositol 3-kinase
- Rb
RP2D, RetinoblastomaRecommended Phase II dose
Footnotes
Competing interests
The authors declare that they have no competing interests.
Author’s contributions
Authors LS, AB, and SM participated in all stages of manuscript preparation, and read and approved the final version prior to submission.
References
- Abraham R, VanArsdale T, Shields D, Lee N, Koehler M, Arndt K. Braking the cycle: Inhibition of the cyclin D-CDK 4/6 pathway in breast cancer. Proceedings from the 105th Annual Meeting of the American Association for Cancer Research; April 5–9, 2014; San Diego, CA. Abstract SY34–03. [Google Scholar]
- Arima Y, Hosonaga M, Saya H. Significance of RB-ZEB axis in EMT phenotype of breast cancer and inhibition of ZEB by CDK 4/6 inhibitor. Proceedings from the 104th Annual Meeting of the American Association for Cancer Research; April 6–10, 2013; Washington, DC. Abstract P1-07-02. [Google Scholar]
- Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–46. doi: 10.1038/nrd4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AstraZeneca Pharmaceuticals. [Accessed August 2015];Faslodex® Prescribing Information. http://www1.astrazeneca-us.com/pi/faslodex.pdf.
- Bardia A, Modi S, Chavez-MacGregor M, Kittaneh M, Marino AJ, Matano A, Bhansali S, Hewes B, Cortes J. Phase Ib/II study of LEE011, everolimus, and exemestane in postmenopausal women with ER+/HER2-metastatic breast cancer. J Clin Oncol. 2014;32:5s(Suppl) Abstract 535. [Google Scholar]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldon CE, Daly RJ, Sutherland RL, Musgrove EA. Cell cycle control in breast cancer cells. J Cell Biochem. 2006;97:261–74. doi: 10.1002/jcb.20690. [DOI] [PubMed] [Google Scholar]
- Cardoso F, Costa A, Norton L, Senkus E, Aapro M, André F, Barrios CH, Bergh J, Biganzoli L, Blackwell KL, Cardoso MJ, Cufer T, El Saghir N, Fallowfield L, Fenech D, Francis P, Gelmon K, Giordano SH, Gligorov J, Goldhirsch A, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2) Breast. 2014;23:489–502. doi: 10.1016/j.breast.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Clark AS, O’Dwyer PJ, Heitjan D, Lal P, Feldman MD, Gallagher M, Redlinger C, Colameco C, Lewis D, Zafman K, Langer M, Rosen MA, Gogineni K, Bradbury AR, Domchek SM, Fox KR, DeMichele A. A phase I trial of palbociclib and paclitaxel in metastatic breast cancer. J Clin Oncol. 2014;32:5s(Suppl) Abstract 527. [Google Scholar]
- Crown J, Finn RS, Ettl J, Boer K, Patel R, Thummala A, Randolph S, Kim S, Huang X, Nadanaciva S, Schnell P, Bartlett CH, Slamon DJ. Efficacy and safety of first-line palbociclib plus letrozole compared with letrozole alone in patients aged ≥ 65 years with estrogen receptor-positive, HER2-negative advanced breast cancer: A subgroup analysis by age of the PALOMA-1/TRIO-18 trial. J Clin Oncol. 2015;33(Suppl) Abstract 571. [Google Scholar]
- Dean JL, Thangavel C, McClendon AK, Reed CA, Knudsen ES. Therapeutic CDK 4/6 inhibition in breast cancer: key mechanisms of response and failure. Oncogene. 2010;29:4018–32. doi: 10.1038/onc.2010.154. [DOI] [PubMed] [Google Scholar]
- DeMichele A, Clark AS, Tan KS, Heitjan DF, Gramlich K, Gallagher M, Lal P, Feldman M, Zhang P, Colameco C, Lewis D, Langer M, Goodman N, Domchek S, Gogineni K, Rosen M, Fox K, O’Dwyer P. CDK 4/6 inhibitor palbociclib (PD0332991) in Rb+ advanced breast cancer: phase II activity, safety, and predictive biomarker assessment. Clin Cancer Res. 2015;21:995–1001. doi: 10.1158/1078-0432.CCR-14-2258. [DOI] [PubMed] [Google Scholar]
- Dickson MA. Molecular pathways: CDK4 inhibitors for cancer therapy. Clin Cancer Res. 2014;20:3379–83. doi: 10.1158/1078-0432.CCR-13-1551. [DOI] [PubMed] [Google Scholar]
- Ertel A, Dean JL, Rui H, Liu C, Witkiewicz A, Knudsen KE. RB-pathway disruption in breast cancer: differential association with disease subtypes, disease-specific prognosis and therapeutic response. Cell Cycle. 2010;9:4153–63. doi: 10.4161/cc.9.20.13454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, Ginther C, Atefi M, Chen I, Fowst C, Los G, Slamon DJ. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, Ettl J, Patel R, Pinter T, Schmidt M, Shparyk Y, Thummala AR, Voytko NL, Fowst C, Huang X, Kim ST, Randolph S, Slamon DJ. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- Finn RS, Crown J, Ettl J, Pinter T, Thummala A, Shparyk YV, Patel R, Randolph S, Kim S, Huang X, Jiang Y, Bartlett CH, Slamon DJ. Clinical efficacy and safety profile of palbociclib (P) in combination with letrozole (L) as first-line treatment in patients (pts) with ER+ and HER2- advanced breast cancer (ABC) who have not received any systemic treatment (ST): A subgroup analysis of PALOMA-1/TRIO-18. J Clin Oncol. 2015;33(Suppl) Abstract 575. [Google Scholar]
- Flaherty KT, Lorusso PM, Demichele A, Abramson VG, Courtney R, Randolph SS, Shaik MN, Wilner KD, O’Dwyer PJ, Schwartz GK. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res. 2012;18:568–76. doi: 10.1158/1078-0432.CCR-11-0509. [DOI] [PubMed] [Google Scholar]
- Fry DW, Harvey PJ, Keller PR, Elliott WL, Meade M, Trachet E, Albassam M, Zheng X, Leopold WR, Pryer NK, Toogood PL. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–38. [PubMed] [Google Scholar]
- Gelbert LM, Cai S, Lin X, Sanchez-Martinez C, Del Prado M, Lallena MJ, Torres R, Ajamie RT, Wishart GN, Flack RS, Neubauer BL, Young J, Chan EM, Iversen P, Cronier D, Kreklau E, de Dios A. Preclinical characterization of the CDK 4/6 inhibitor LY2835219: in-vivo cell cycle-dependent/independent anti-tumor activities alone/in combination with gemcitabine. Invest New Drugs. 2014;32:825–37. doi: 10.1007/s10637-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geradts J, Wilson PA. High frequency of aberrant p16(INK4A) expression in human breast cancer. Am J Pathol. 1996;149:15–20. [PMC free article] [PubMed] [Google Scholar]
- Howell A. Pure oestrogen antagonists for the treatment of advanced breast cancer. Endocr Relat Cancer. 2006;13:689–706. doi: 10.1677/erc.1.00846. [DOI] [PubMed] [Google Scholar]
- Infante JR, Shapiro G, Witteveen P, Gerecitano JF, Ribrag V, Chugh R, Issa I, Chakraborty A, Matano A, Zhao X, Parasuraman S, Cassier P. A phase I study of the single-agent CDK 4/6 inhibitor LEE011 in pts with advanced solid tumors and lymphomas. J Clin Oncol. 2014;32;5s(Suppl) Abstract 2528. [Google Scholar]
- Jiang Y, Randolph S, English P, Kim S, Huang X, Press M, et al. Cell cycle biomarker analysis from the PALOMA-1/TRIO 18 palbociclib plus letrozole phase II study in ER-positive/HER2-negative advanced breast cancer. Ann Oncol. 2014;25(Suppl_4) Abstract 4420. [Google Scholar]
- Johnson SM, Torrice CD, Bell JF, Monahan KB, Jiang Q, Wang Y, Ramsey MR, Jin J, Wong KK, Su L, Zhou D, Sharpless NE. Mitigation of hematologic radiation toxicity in mice through pharmacological quiescence induced by CDK 4/6 inhibition. J Clin Invest. 2010;120:2528–36. doi: 10.1172/JCI41402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juric D, Hamilton E, Garcia Estévez L, De Boer RH, Mayer I, Campone M, et al. Phase Ib/II study of LEE011, alpelisib (BYL719), and letrozole in ER+, HER2– breast cancer: safety, preliminary efficacy, and molecular analysis. Proceedings from the San Antonio Breast Cancer Symposium; December 9–13. 2014; San Antonio, TX. Abstract P5-19-24. [Google Scholar]
- Kim S, Loo A, Chopra R, Caponigro G, Huang A, Vora S, et al. LEE011: An orally bioavailable, selective small molecule inhibitor of CDK 4/6--Reactivating Rb in cancer. Proceedings from the AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics; October 19–23, 2013; Boston, MA. Abstract PR02. [Google Scholar]
- Lamb R, Lehn S, Rogerson L, Clarke RB, Landberg G. Cell cycle regulators cyclin D1 and CDK 4/6 have estrogen receptor-dependent divergent functions in breast cancer migration and stem cell-like activity. Cell Cycle. 2013;12:2384–94. doi: 10.4161/cc.25403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange CA, Yee D. Killing the second messenger: targeting loss of cell cycle control in endocrine-resistant breast cancer. Endocr Relat Cancer. 2011;18:C19–24. doi: 10.1530/ERC-11-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren K, Brown M, Pineda S, Cuzick J, Salter J, Zabaglo L, Howell A, Dowsett M, Landberg G TransATAC investigators. Effects of cyclin D1 gene amplification and protein expression on time to recurrence in postmenopausal breast cancer patients treated with anastrozole or tamoxifen: a TransATAC study. Breast Cancer Res. 2012;14:R57. doi: 10.1186/bcr3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClendon AK, Dean JL, Rivadeneira DB, Yu JE, Reed CA, Gao E, Farber JL, Force T, Koch WJ, Knudsen ES. CDK 4/6 inhibition antagonizes the cytotoxic response to anthracycline therapy. Cell Cycle. 2012;11:2747–55. doi: 10.4161/cc.21127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster PN, Hamilton EP, Estevez LG, De Boer RH, Mayer IA, Campone M, Asano S, Bhansali S, Zhang V, Hewes B, Juric D. Ph IB study of LEE011 and BYL719 in combination with letrozole in ER+, HER2- breast cancer. J Clin Oncol. 2014;32(Suppl 26) Abstract 143. [Google Scholar]
- Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5,993 breast cancers. Am J Clin Pathol. 2005;123:21–7. doi: 10.1309/4wv79n2ghj3x1841. [DOI] [PubMed] [Google Scholar]
- Novartis Pharmaceuticals Corporation. [Accessed August 2015];Femara® Prescribing Information. https://www.pharma.us.novartis.com/product/pi/pdf/Femara.pdf.
- O’Brien N, Di Tomaso E, Ayala R, Tong L, Issakhanian S, Linnartz R, et al. In vivo efficacy of combined targeting of CDK 4/6, ER and PI3K signaling in ER+ breast cancer. In: Proceedings from the 105th Annual Meeting of the American Association for Cancer Research; April 5–9, 2014; San Diego, CA. Abstract 4756. [Google Scholar]
- Ortega S, Malumbres M, Barbacid M. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim. Biophys Acta. 2002;1602:73–87. doi: 10.1016/s0304-419x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–47. doi: 10.1146/annurev-med-070909-182917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parasuraman S, Caponigro G, Loo A, Cao Z, Kim S, Issa I, et al. LEE011, a potent and selective CDK 4/6 inhibitor, under preclinical and clinical investigation. Proceedings from the 12th International Congress on Targeted Anticancer Therapies; March 5–7, 2014; Washington, DC. Abstract O4.4. [Google Scholar]
- Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, et al. Clinical activity of LY2835219, a novel cell cycle inhibitor selective for CDK4 and CDK6, in patients with metastatic breast cancer. Proceedings from the 105th Annual Meeting of the American Association for Cancer Research; April 5–9, 2014; San Diego, CA. Abstract CT232. [Google Scholar]
- Patnaik A, Rosen LS, Tolaney SM, Tolcher AW, Goldman JW, Gandhi L, Papadopoulos KP, Beeram M, Rasco DW, Myrand SP, Kulanthaivel P, Andrews JM, Frenzel M, Cronier D, Chan EM, Flaherty K, Wen PY, Geoffrey Shapiro G. LY2835219, a novel cell cycle inhibitor selective for CDK 4/6, in combination with fulvestrant for patients with hormone receptor positive (HR+) metastatic breast cancer. J Clin Oncol. 2014;32:5s(Suppl) Abstract 534. [Google Scholar]
- Pfizer Inc. [Accessed August 2015];Ibrance® Prescribing Information. http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207103s000lblpdf.
- Shapiro GI. Cyclin-dependent kinase pathways as targets for cancer treatment. J Clin Oncol. 2006;24:1770–83. doi: 10.1200/JCO.2005.03.7689. [DOI] [PubMed] [Google Scholar]
- Shapiro G, Rosen LS, Tolcher AW, Goldman JW, Gandhi L, Papadopoulos KP, Tolaney S, Beeram M, Rasco D, Kulanthaivel P, Li Q, Hu T, Cronier D, Chan E, Flaherty K, Wen P, Patnaik A. A first-in-human phase I study of the CDK 4/6 inhibitor, LY2835219, for patients with advanced cancer. J Clin Oncol. 2013;31(Suppl) Abstract 2500. [Google Scholar]
- Slamon DJ, Hurvitz SA, Chen D, André F, Tseng LM, Jerusalem GHM, Wilks S, O’Regan R, Isaacs C, Toi M, Burris HA, He W, Riester M, Robinson D, Taran T, Gianni L. Predictive biomarkers of everolimus efficacy in HER2+ advanced breast cancer: Combined exploratory analysis from BOLERO-1 and BOLERO-3. J Clin Oncol. 2015;33(Suppl) doi: 10.1200/JCO.2015.63.9161. Abstract 512. [DOI] [PubMed] [Google Scholar]
- Takuwa N, Fukui Y, Takuwa Y. Cyclin D1 expression mediated by phosphatidylinositol 3-kinase through mTOR-p70(S6K)-independent signaling in growth factor-stimulated NIH 3T3 fibroblasts. Mol Cell Biol. 1999;19:1346–58. doi: 10.1128/mcb.19.2.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangavel C, Dean JL, Ertel A, Knudsen KE, Aldaz CM, Witkiewicz AK, Clarke R, Knudsen ES. Therapeutically activating RB: reestablishing cell cycle control in endocrine therapy-resistant breast cancer. Endocr Relat Cancer. 2011;18:333–45. doi: 10.1530/ERC-10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolaney SM, Beeram M, Beck JT, Conlin AK, Dees EC, Dickler MN, Helsten TL, Conkling PR, Edenfield WJ, Richards DA, Turner PK, Cai N, Chan EM, Pant S, Becerra C, Kalinsky K, Puhalla S, Rexer BN, Burris HA, Goetz MP. A phase Ib study of abemaciclib with therapies for metastatic breast cancer. J Clin Oncol. 2015;33(Suppl) Abstract 522. [Google Scholar]
- Tripathy D, Bardia A, Hurvitz SA, Harbeck N, Colleoni M, Franke FA, Chow LWC, Im SA, Shao Z, Keyserlingk JR, Soler LM, Horan M, Dhuria SV, Hughes G, Lu YS. Phase III, randomized, double-blind, placebo-controlled study of ribociclib (LEE011) in combination with either tamoxifen and goserelin or a non- steroidal aromatase inhibitor (NSAI) and goserelin for the treatment of premenopausal women with HR+, HER2– advanced breast cancer (aBC): MONALEESA-7. J Clin Oncol. 2015;33(Suppl) Abstract TPS625. [Google Scholar]
- Turner NC, Ro J, André F, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, Huang Bartlett C, Zhang K, Giorgetti C, Randolph S, Koehler M, Cristofanilli M. Palbociclib in hormone-receptor-positive advanced breast cancer. N Engl J Med. 2015;373:209–19. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- Vora SR, Juric D, Kim N, Mino-Kenudson M, Huynh T, Costa C, Lockerman EL, Pollack SF, Liu M, Li X, Lehar J, Wiesmann M, Wartmann M, Chen Y, Cao ZA, Pinzon-Ortiz M, Kim S, Schlegel R, Huang A, Engelman JA. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell. 2014;26:136–49. doi: 10.1016/j.ccr.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]