Summary
Background
High quantities of tumour-infiltrating lymphocytes (TILs) in primary HER2-positive breast cancer are associated with improved prognosis and response to therapy. We aimed to investigate the prognostic role of host antitumour immunity as represented by baseline quantities of TILs in patients with advanced HER2-positive breast cancer treated with either pertuzumab or placebo in addition to trastuzumab and docetaxel.
Methods
CLEOPATRA was a randomised phase 3 study comparing the addition of either pertuzumab or placebo to first-line therapy with trastuzumab and docetaxel for patients with locally recurrent, unresectable, or metastatic HER2-positive breast cancer. We assessed the quantity of stromal TILs in prospectively collected tumour samples and investigated their association with progression-free survival, overall survival, clinicopathological characteristics, and pertuzumab treatment. We estimated hazard ratios (HR) and 95% CIs with multivariate Cox regression models fitting stromal TILs as a continuous variable (per 10% increment). The CLEOPATRA trial is registered with ClinicalTrials.gov, number NCT00567190.
Findings
Tumour samples from 678 (84%) of 808 participants were evaluable for TILs, including 519 (77%) archival samples, 155 (23%) freshly obtained samples (collected 45 days or fewer before randomisation), and four samples of unknown archival status. Median follow-up was 50 months (IQR 41–54) for progression-free survival and 51 months (IQR 46–57) for overall survival. 519 progression-free survival events occurred and 358 patients died. The median TIL value was 10% (IQR 5–30). Freshly obtained tumour samples had significantly lower TIL values than did archival samples (10·00% [95% CI 5·00–20·00] vs 15·00% [5·00–35·00]; p=0·00036). We detected no significant association between TIL values and progression-free survival (adjusted HR 0·95, 95% CI 0·90–1·00, p=0·063). However, for overall survival, each 10% increase in stromal TILs was significantly associated with longer overall survival (adjusted HR 0·89, 95% CI 0·83–0·96, p=0·0014). The treatment effect of pertuzumab did not differ significantly by stromal TIL value for either progression-free survival (pinteraction=0·23) or overall survival (pinteraction=0·21).
Interpretation
In patients with advanced HER2-positive breast cancer treated with docetaxel, trastuzumab, and pertuzumab or placebo, higher TIL values are significantly associated with improved overall survival, suggesting that the effect of antitumour immunity extends to the advanced setting. Future clinical studies in this cancer subtype should consider TILs as a stratification factor and investigate whether therapies that can augment immunity could potentially further improve survival.
Introduction
Therapeutic advances in HER2-targeted agents have substantially improved outcomes for patients with HER2-positive breast cancer, a breast cancer subtype previously associated with poor prognoses.1 After a median follow-up of 51 months (IQR 46–57), results of the CLEOPATRA (Clinical Evaluation of Pertuzumab and Trastuzumab) study showed that the addition of pertuzumab to docetaxel and trastuzumab significantly improved overall survival compared with that for patients treated with docetaxel, trastuzumab, and placebo, establishing the current standard of care for first-line treatment of metastatic HER2-positive breast cancer.2 Subsequent correlative analyses showed that the effect of the addition of pertuzumab on overall survival was independent of the expression of measured biomarkers, irrespective of PIK3CA genotype.3 However, tumours with PIK3CA mutations had significantly poorer prognosis than did their wild-type counterparts. Evidence from preclinical models of pertuzumab in combination with trastuzumab suggests that the main mechanism of action is through disruption of HER2 dimerisation with EGFR and HER3 and therefore enhanced inhibition of HER2 signalling, although antibody-dependent cell-mediated cytotoxicity also contributes.4–7 The effect of pertuzumab on T-cell-mediated antitumour immunity remains unknown.
Highlights.
Evidence before this study
We searched PubMed for articles published up to Sept 16, 2016, with the terms “tumour-infiltrating lymphocytes”, “HER2-positive breast cancer”, and “prognosis”, with no language restrictions. Studies in early breast cancer have generally shown a positive prognostic association between increasing quantities of tumour-infiltrating lymphocytes (TILs) and survival outcomes, although this association has not always been consistently shown, possibly because of heterogeneity in trial designs (eg, not all assessed patients received trastuzumab). Results from several studies have also shown increased pathological complete responses among patients with higher quantities of TILs who underwent neoadjuvant therapy with chemotherapy and HER2-targeted agents. No studies have examined the prognostic associations of TILs in advanced HER2-positive breast cancer.
Added value of this study
We found a prognostic association between stromal TILs and overall survival, independent of known prognostic clinicopathological variables. To our knowledge, our study is the first to assess the prognostic value of TILs in advanced HER2-positive breast cancer in patients undergoing modern dual HER2-targeted therapy.
Implications of all the available evidence
The association of TILs with clinical outcome suggests an important role for antitumour immunity in the advanced setting of HER2-positive breast cancer. This finding provides rationale for clinical assessment of immunotherapeutic approaches.
Data from many studies in HER2-positive early breast cancer have shown associations between higher quantities of tumour-infiltrating lymphocytes (TILs) and greater proportions of patients achieving pathological complete responses with neoadjuvant chemotherapy and trastuzumab,8,9 as well as improved event-free survival in primary disease treated with trastuzumab and lapatinib.9 The association between TILs and prognosis in the advanced setting is unknown.
Improved understanding of the prognostic role of pre-existing immunity in the advanced setting would have potential implications for future immunotherapeutic approaches. In this study, we retrospectively analysed prospectively collected pretreatment tumour samples from the CLEOPATRA study to investigate two key points. First, we aimed to investigate the relationship between pretreatment TIL quantity and clinical outcomes in newly diagnosed HER2-positive metastatic breast cancer, to analyse the prognostic effect of baseline host antitumour immunity in this setting, irrespective of treatment. Second, we aimed to explore whether the treatment effect of pertuzumab differed with respect to TIL values.
Methods
Study design and participants
The CLEOPATRA trial was a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial done at 204 sites in 25 countries.2,3 Eligible patients were aged 18 years or older and had locally recurrent, unresectable, or metastatic HER2-positive breast cancer. Patients were allowed to have received only one line of previous endocrine therapy before enrolment. Study participants were randomly assigned (1:1) to treatment with trastuzumab and docetaxel plus either pertuzumab or placebo as first-line therapy for metastatic disease. Study drugs were given every 3 weeks intravenously. Pertuzumab was given on day 1 of every cycle, starting at a dose of 840 mg and decreasing to 420 mg from cycle 2 onwards. Placebo was given on the same schedule. Trastuzumab was given at a loading dose of 8 mg/kg and then 6 mg/kg from cycle 2 onwards. Docetaxel was given at 75 mg/m2 on day 2 of cycle 1 and day 1 from cycle 2 onwards. Treatment continued until disease progression or unmanageable toxicity. Six or more cycles of docetaxel were recommended. The trial recruited patients from Feb 12, 2008, to July 7, 2010. The median age of enrolled patients was 54 years (IQR 46–61) and 344 (48%) of 808 patients were positive for oestrogen receptor, progesterone receptor, or both. Approval for the protocol was obtained from an independent ethics committee for each site and written informed consent was obtained from every participant. Details of the study have been reported previously.2,3 All tumour samples were collected before study treatment. Patients analysed in this study had consented for their tumour tissue to be used for future research purposes.
Pathological assessment
Histopathological analysis of the proportion of TILs was done with full-face sections of tumour tissue stained with haematoxylin and eosin. TILs in the tumour stroma were quantified as a percentage of occupied stromal areas by use of a previously reported method.10 In this method, all mononuclear cells, including lymphocytes and plasma cells are scored (granulocytes and polymorphonuclear leucocytes are excluded). TILs in areas occupied by tumour cells are not included in this score. The reproducibility of this method has been described previously.11 To investigate concordance between pathologists in TIL assessment in the advanced setting, samples were independently analysed by two pathologists (RS and SF). Although tumour specimens were prospectively collected, information on whether a tumour biopsy had been freshly obtained for the study was not specifically collected. As such, we defined tumour tissue as fresh if it had been obtained 45 days or fewer before the date of randomisation and the patient had not received any previous endocrine therapy for advanced disease. We defined tumour tissue as archival if the tumour sample was collected more than 45 days from the date of randomisation, or if the patient had received previous endocrine therapy for advanced disease, or both. Details about the sites of metastatic tissue samples were not collected as part of the clinical trial. Therefore only metastatic samples with definite anatomical location as assessed by pathological examination were used in the TIL analysis by organ metastatic site. We assessed HER2 status (by immunohistochemistry and fluorescence in situ hybridisation), oestrogen receptor status, and PIK3CA genotype using previously described methods.2,3
Outcomes
The primary endpoint of the CLEOPATRA study was progression-free survival, defined as the time from randomisation to the first documented radiographic evidence of progressive disease, as assessed with Response Evaluation Criteria in Solid Tumours (RECIST) version 1.0, or death from any cause within 18 weeks after the last investigator assessment of tumours. The secondary endpoint of the CLEOPATRA trial, overall survival, was defined as time from randomisation to death from any cause. The main objective of this secondary analysis was to assess the prognostic association of TILs with progression-free survival and overall survival. We also investigated associations of TILs with clinico pathological factors and pertuzumab treatment effect (ie, the association between TILs and clinical outcome in patients treated with pertuzumab plus trastuzumab and docetaxel vs placebo plus trastuzumab and docetaxel).
Statistical analyses
The statistical analyses for this study were prespecified with stromal TILs (as opposed to intratumoral TILs) as the predefined TIL biomarker (appendix). All analyses were done in the intention-to-treat population of patients with evaluable TILs.
To assess the prognostic effect of TILs on clinical outcome, TILs were included as a continuous variable (per 10% increment) in a Cox regression model based on the TIL-evaluable cohort for the endpoints of progression-free survival and overall survival. We used Cox proportional hazards regression models to test the prognostic value of TILs with a Wald test for significance. On the basis of previous data in early breast cancer,9,12,13 we assumed a SD of 1·75 on the scale of 10% increments in the stromal TIL variable. We calculated that a minimum of 111 progression events would provide 85% power at a two-sided 5% significance level to detect a hazard ratio (HR) of 0·85 for progression-free survival for every 10% increment in stromal TILs in this Cox regression model. We only used the stromal TIL variable, because this variable is more reproducible than intratumoral TILs.10
The Cox model makes the assumption that the TIL covariate has a linear effect on the log hazard function. We have previously found that the prognostic TIL effect is linear.9,12,13 For the present study, we visually verified the linearity assumption after fitting a cubic smoothing spline by 10% increments in stromal TILs (appendix p 2). We verified proportional hazards assumptions with Schoenfeld residuals. We assessed treatment interactions by adding treatment effect and a product interaction term into a Cox model, with the likelihood ratio test for significance. Similarly, we made assessments for interactions between prognostic variables by adding a product interaction term into a Cox model, with the likelihood ratio test for significance. We created a multivariate Cox proportional hazards model using a continuous TIL variable (per 10% increment) and the prespecified clinicopathological characteristics age (<65 years vs ≥65 years), ethnicity (white vs Asian because of the small number of black or African American patients), oestrogen receptor status, PIK3CA genotype, previous treatment status (no previous chemotherapy or trastuzumab vs previous [neo]adjuvant chemotherapy or trastuzumab), and visceral disease status (present or absent at screening). For visualisation with Kaplan-Meier survival curves, we defined subgroups with a cutoff value based on the percentage of stromal TILs (≤20% stromal TILs vs >20% stromal TILs), and made comparisons with the log-rank test. We used a cutoff value of 20% for stromal TILs because this value was the closest decile to the mean stromal TIL value in the study population. Because this study was the first analysis of the prognostic value of TILs in advanced disease, a specific TIL cutoff was not prespecified. The association of TILs with progression-free survival and overall survival by Kaplan-Meier curves was also assessed by median and quartile TIL cutoffs in a post-hoc analysis.
We tested concordance between individual pathologists using the Pearson product-moment correlation coefficient, and associations between TILs and clinico pathological characteristics with Pearson correlation for continuous variables, Mann-Whitney U tests for binary variables, and the Kruskal-Wallis H test for variables with more than two groups. We compared the baseline characteristics of patients with evaluable TILs versus patients with non-evaluable TILs using Mann-Whitney U tests for continuous variables, Fisher’s exact test and χ2 test for categorical variables, and log-rank tests for survival endpoints. We used R software version 3.3.0 for the statistical analyses, and we deemed a two-sided p value less than 0·05 to be significant. The CLEOPATRA trial is registered with ClinicalTrials.gov, number NCT00567190.
Role of the funding source
The funder had a role in the study design and data collection, partly contributed to the cost of the study, and was allowed to edit and review the draft manuscript, but did not influence the data analysis or interpretation. SJL, SM, and SL had full access to the raw data and made the final decision to submit the report for publication. The corresponding author had full access to the data and had final responsibility to submit for publication.
Results
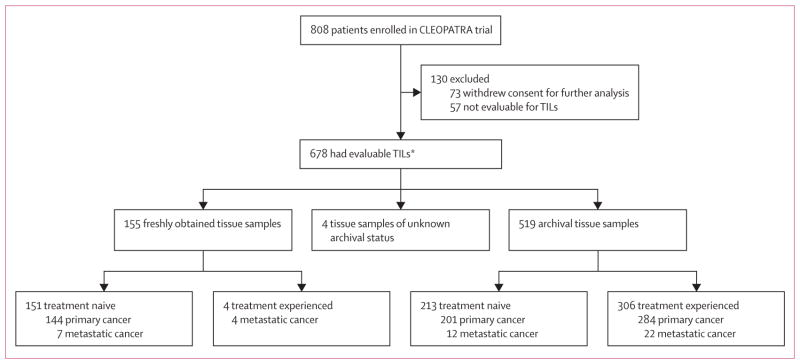
Of the 808 available tumour samples from the CLEOPATRA trial population, slides from 678 (84%) patients had sufficient tumour tissue and were evaluable for TILs (figure 1), whereas 130 (16%) samples were not evaluable. Information on concomitant steroid use at the time of biopsy was unavailable. However, 143 (18%) of 808 patients in the intention-to-treat population were recorded as having received a steroid as a concomitant medication at the time of enrolment and 627 (78%) had received steroids as part of their premedication for docetaxel chemotherapy, which would have been taken after any tumour biopsy.
Figure 1. Trial profile.
Treatment naive was defined as no previous treatment with chemotherapy or trastuzumab (one previous endocrine therapy was allowed) and treatment experienced was defined as previous (neo)adjuvant chemotherapy, trastuzumab, or both. TILs=tumour-infiltrating lymphocytes. *20 paired cases of primary and metastatic cancer were counted only once.
The baseline characteristics of the cohort without evaluable TILs (n=130) were similar to those of the cohort with evaluable TILs, although a smaller proportion of patients in the non-evaluable cohort had visceral disease at screening (table 1). 2-year progression-free survival did not differ significantly between the cohort with evaluable TILs and the non-evaluable TIL cohort (table 1). Median follow-up duration among patients with evaluable TILs was 50 months (IQR 41–54) for progression-free survival and 519 progression-free survival events occurred; median progression-free survival was 12·3 months (95% CI 10·4–13·5) in the placebo group versus 19·2 months (16·6–22·8) in the pertuzumab group. 3-year overall survival was significantly different between the cohort with evaluable TILs and the cohort with non-evaluable TILs, although it was similar between the full study cohort and the TIL-evaluable cohort (table 1). In the TIL-evaluable cohort, median follow-up duration was 51 months (IQR 46–57) for overall survival and 358 patients died; median overall survival was 37·9 months (95% CI 34·2–46·5) in the placebo group versus 55·6 months (49·1–not reached [NR] in the pertuzumab group.
Table 1.
Baseline characteristics
| Study population (n=808) | Patients with evaluable TILs (n=678) | Patients with non-evaluable TILs (n=130) | p value | |
|---|---|---|---|---|
| Age at randomisation (years) | 54 (46–61) | 54 (46–61) | 54 (46–61) | 0·98* |
|
| ||||
| Ethnicity | ·· | ·· | ·· | 0·13† |
| White | 480 (59%) | 391 (58%) | 89 (68%) | ·· |
| Asian | 261 (32%) | 230 (34%) | 31 (24%) | ·· |
| Black or African American | 30 (4%) | 24 (4%) | 6 (5%) | ·· |
| Other | 37 (5%) | 33 (5%) | 4 (3%) | ·· |
|
| ||||
| HER2 FISH status | ·· | ·· | ·· | 1·00† |
| FISH positive | 767 (95%) | 650 (96%) | 117 (90%) | ·· |
| FISH negative | 5 (<1%) | 5 (1%) | 0 | ·· |
| FISH unknown | 36 (4%) | 23 (3%) | 13 (10%) | ·· |
|
| ||||
| HER2 IHC status | ·· | ·· | ·· | 0·15† |
| IHC 0 | 2 (<1%) | 1 (<1%) | 1 (<1%) | ·· |
| IHC 1+ | 4 (<1%) | 2 (<1%) | 2 (2%) | ·· |
| IHC 2+ | 79 (10%) | 69 (10%) | 10 (8%) | ·· |
| IHC 3+ | 721 (89%) | 604 (89%) | 117 (90%) | ·· |
| IHC unknown | 2 (<1%) | 2 (<1%) | 0 | ·· |
|
| ||||
| Histological grade | ·· | ·· | ·· | 0·53‡ |
| Poorly differentiated | 255 (32%) | 213 (31%) | 42 (32%) | ·· |
| Moderately differentiated | 264 (33%) | 228 (34%) | 36 (28%) | ·· |
| Well differentiated | 30 (4%) | 23 (3%) | 7 (5%) | ·· |
| Unknown/other | 259 (32%) | 214 (32%) | 45 (35%) | ·· |
|
| ||||
| Oestrogen receptor status | ·· | ·· | ·· | 0·68‡ |
| Positive | 388 (48%) | 325 (48%) | 63 (48%) | ·· |
| Negative | 408 (50%) | 344 (51%) | 64 (49%) | ·· |
| Unknown | 12 (1%) | 9 (1%) | 3 (2%) | ·· |
|
| ||||
| PIK3CA genotype | ·· | ·· | ·· | 1·00‡ |
| Mutant | 176 (22%) | 147 (22%) | 29 (22%) | ·· |
| Wild type | 381 (47%) | 318 (47%) | 63 (48%) | ·· |
| Unknown | 251 (31%) | 213 (31%) | 38 (29%) | ·· |
|
| ||||
| Previous therapy | ·· | ·· | ·· | 0·56‡ |
| De novo metastatic disease | 432 (53%) | 366 (54%) | 66 (51%) | ·· |
| Previous (neo)adjuvant therapy | 376 (47%) | 312 (46%) | 64 (49%) | ·· |
|
| ||||
| Previous trastuzumab | 88 (11%) | 75 (11%) | 13 (10%) | 0·84‡ |
|
| ||||
| Previous anthracycline | 314 (39%) | 268 (40%) | 46 (35%) | 0·43‡ |
|
| ||||
| Previous taxane | 185 (23%) | 153 (23%) | 32 (25%) | 0·69‡ |
|
| ||||
| Visceral or non-visceral metastatic disease at screening | ·· | ·· | ·· | 0·041‡ |
| Visceral disease | 630 (78%) | 538 (79%) | 92 (71%) | ·· |
| Non-visceral disease | 178 (22%) | 140 (21%) | 38 (29%) | ·· |
|
| ||||
| Treatment group | ·· | ·· | ·· | 0·88‡ |
| Pertuzumab plus trastuzumab plus docetaxel | 402 (50%) | 336 (50%) | 66 (51%) | ·· |
| Placebo plus trastuzumab plus docetaxel | 406 (50%) | 342 (50%) | 64 (49%) | ·· |
|
| ||||
| Clinical endpoints (95% CI) | ||||
| 2-year progression-free survival | 34% (31–38) | 34% (30–38) | 35% (27–46) | 0·89§ |
| 3-year overall survival | 61% (58–65) | 60% (56–64) | 72% (63–83) | 0·0033§ |
Data are n (%) or median (IQR), unless otherwise stated. p values are for comparisons of patients with evaluable TILs vs patients with non-evaluable TILs. Treatment naive was defined as no previous treatment with chemotherapy or trastuzumab (endocrine therapy was allowed). Clinical endpoints are Kaplan-Meier estimates. TILs=tumour-infiltrating lymphocytes. FISH=fluorescence in-situ hybridisation. IHC=immunohistochemistry.
Wilcoxon-Mann-Whitney test p value.
Fisher’s exact test p value.
χ2 test p value.
Log-rank test p value.
In the 678 patients with evaluable TILs, the mean stromal TIL value was 21·07% (SD 22·4), and the median stromal TIL value was 10% (IQR 5–30). Stromal TIL evaluation in a subset of 40 tumour samples was highly concordant between pathologists (r=0·93). 155 tumour samples were freshly obtained, 519 were archival, and four were of unknown archival status. Freshly obtained tumour samples were significantly associated with reduced stromal TIL values compared with archival tumour samples (table 2). Because the 45 day cutoff for archival status was arbitrary, we did a post-hoc analysis with a cutoff of 60 days or less (206 samples obtained ≤60 days before randomisation; 468 samples still regarded as archival), which produced similar results (median 10·00% [IQR 5·00–20·00] in fresh samples vs 15·00% [5·00–35·00] in archival samples, p<0·0001). There were no significant differences in the proportions of primary and metastatic tissue between fresh samples and archival samples (appendix p 1). Analysis of unpaired primary and metastatic samples showed no significant differences in stromal TIL values (table 2). 20 patients had paired primary and metastatic samples available for analysis. In these samples, metastatic tissues had lower stromal TIL values than did primary tissues, although this difference was not significant (table 2).
Table 2.
TIL values and clinicopathological characteristics
| Number of samples with evaluable TILs (n=678) | Stromal TIL value | p value | |
|---|---|---|---|
| Age at randomisation (years) | |||
| <65 | 573 (85%) | 10·00% (5·00–30·00) | 0·47* |
| ≥65 | 105 (15%) | 10·00% (5·00–25·00) | ·· |
|
| |||
| Ethnicity | ·· | ·· | 0·00074† |
| White | 391 (58%) | 10·00% (5·00–25·00) | ·· |
| Asian | 230 (34%) | 15·00% (5·00–35·00) | ·· |
| Black or African American | 24 (4%) | 5·00% (4·00–16·25) | ·· |
| Other | 33 (5%) | ·· | ·· |
|
| |||
| HER2 FISH status | ·· | ·· | 0·65† |
| FISH positive | 650 (96%) | 10·00% (5·00–30·00) | ·· |
| FISH negative | 5 (1%) | 15·00% (10·00–20·00) | ·· |
| FISH unknown | 23 (3%) | ·· | ·· |
|
| |||
| HER2 IHC status | ·· | ·· | 0·38† |
| IHC 0 | 1 (<1%) | 15·00% (15·00–15·00) | ·· |
| IHC 1+ | 2 (<1%) | 12·50% (8·75–16·25) | ·· |
| IHC 2+ | 69 (10%) | 10·00% (5·00–20·00) | ·· |
| IHC 3+ | 604 (89%) | 10·00% (5·00–30·00) | ·· |
| IHC unknown | 2 (<1%) | ·· | ·· |
|
| |||
| Histological grade | ·· | ·· | 0·14† |
| Poorly differentiated | 213 (31%) | 15·00% (5·00–30·00) | ·· |
| Moderately differentiated | 228 (34%) | 10·00% (5·00–30·00) | ·· |
| Well differentiated | 23 (3%) | 10·00% (5·00–22·50) | ·· |
| Unknown or other | 214 (32%) | ·· | ·· |
|
| |||
| Oestrogen receptor status | ·· | ·· | <0·0001* |
| Positive | 325 (48%) | 10·00% (5·00–20·00) | ·· |
| Negative | 344 (51%) | 15·00% (5·00–35·00) | ·· |
| Unknown | 9 (1%) | ·· | ·· |
|
| |||
| PIK3CA genotype | ·· | ·· | 0·035* |
| Mutant | 147 (22%) | 15·00% (5·00–35·00) | ·· |
| Wild type | 318 (47%) | 10·00% (5·00–30·00) | ·· |
| Unknown | 213 (31%) | ·· | ·· |
|
| |||
| Metastatic disease at screening | |||
| Visceral disease | 538 (79%) | 10·00% (5·00–30·00) | 0·16* |
| Non-visceral disease | 140 (21%) | 10·00% (5·00–31·25) | ·· |
|
| |||
| Tissue biopsy type | |||
| Freshly obtained | 155 (23%) | 10·00% (5·00–20·00) | 0·00036* |
| Archival | 519 (77%) | 15·00% (5·00–35·00) | ·· |
| Unknown | 4 (<1%) | ·· | ·· |
|
|
|||
| Unpaired tissue biopsy | |||
| Primary tissue | 631 (93%) | 10·00% (5·00–30·00) | 0·10* |
| Metastatic tissue | 47 (7%) | 10·00% (5·00–20·00) | ·· |
|
| |||
| Paired tissue biopsy (n=20) | |||
| Primary tissue | 20 | 10·00% (5·00–40·00) | 0·068* |
| Metastatic tissue | 20 | 7·50% (5·00–15·00) | ·· |
|
| |||
| Site of metastatic tissue biopsy (n=58) | |||
| Bone | 9 (15%) | 5·00% (1·00–5·00) | 0·0070*‡ |
| Liver | 20 (34%) | 5·00% (1·00–10·00) | ·· |
| Skin | 23 (40%) | 5·00% (4·00–10·00) | ·· |
| Lung | 6 (10%) | 30·00% (11·25–78·75) | ·· |
Data are n (%) or median (IQR) unless stated otherwise. Freshly obtained tissue samples were samples obtained 45 days or fewer before randomisation from patients who had not received any previous endocrine therapy. TIL=tumour-infiltrating lymphocyte. FISH=fluorescence in-situ hybridisation. IHC=immunohistochemistry.
Wilcoxon-Mann-Whitney test p value.
Kruskal-Wallis test p value.
Lung metastases vs non-lung metastases.
A higher percentage of stromal TILs were significantly associated with oestrogen receptor negativity (table 2). Stromal TIL values did not differ significantly by age, histological grade, HER2 status assessed with immunohistochemistry or FISH, or the presence of visceral disease at staging (table 2). Notably, stromal TIL values differed significantly by ethnicity (table 2). In an exploratory analysis comparing Asian participants (n=230) and white participants (n=391), stromal TIL values remained significantly different (p=0·0015). We found no significant differences between ethnicities in the proportions of primary or metastatic tissues and fresh or archival samples (appendix p 1). Tumours with PIK3CA mutations were associated with significantly higher stromal TIL values than were tumours without a PIK3CA mutation. We detected no significant differences by PIK3CA genotype in oestrogen receptor status (p=0·67) or freshly obtained versus archival samples (p=0·46).
Sites of metastatic lesions were recorded for 58 samples with evaluable stromal TILs (table 2). TIL values were low in these sites, except in lung metastases (n=6), which had significantly higher TIL values than did non-lung metastases (n=52; p=0·0070; table 2).
In the cohort with evaluable TILs, we detected no significant association in multivariate analysis between continuous stromal TIL values and progression-free survival (table 3). In the multivariate analysis, ethnicity, PIK3CA genotype, and treatment group were significantly associated with progression-free survival, whereas age, oestrogen receptor status, previous treatment, and visceral disease at screening were not (table 3). Median progression-free survival was similar between patients with TIL values of 20% or lower and patients with TIL values greater than 20% (HR 0·85, 95% CI 0·70–1·03, log-rank p=0·097; figure 2A). Progression-free survival by treatment group is shown in figure 2B.
Table 3.
Univariate and multivariate analyses of progression-free survival and overall survival
| Number of patients | PFS events | OS events | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| Progression-free survival | Overall survival | Progression-free survival | Overall survival | ||||||||
|
|
|
|
|
||||||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR | p value | ||||
| Stromal TILs (per 10% increment) | 678 | 519 | 358 | 0·97 (0·93–1·11) | 0·15 | 0·93 (0·89–0·98) | 0·011 | 0·95 (0·90–1·00) | 0·063 | 0·89 (0·83–0·96) | 0·0014 |
|
| |||||||||||
| Age (<65 vs ≥65 years) | ·· | ·· | ·· | 1·06 (0·83–1·36) | 0·63 | 1·02 (0·76–1·37) | 0·89 | 1·01 (0·74–1·38) | 0·95 | 1·03 (0·70–1·53) | 0·86 |
| <65 years | 573 | 445 | 306 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| ≥65 years | 105 | 74 | 52 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
|
| |||||||||||
| Ethnicity (white vs Asian) | ·· | ·· | ·· | 1·22 (1·01–1·47) | 0·042 | 1·12 (0·90–1·41) | 0·32 | 1·29 (1·03–1·62) | 0·028 | 1·08 (0·82–1·42) | 0·60 |
| White | 391 | 311 | 211 | ·· | ·· | ·· | ·· | ·· | ·· | ·· ·· | ·· |
| Asian | 230 | 169 | 118 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
|
| |||||||||||
| Oestrogen receptor status (positive vs negative) | ·· | ·· | ·· | 1·01 (0·85–1·21) | 0·89 | 0·79 (0·64–0·97) | 0·026 | 1·07 (0·86–1·33) | 0·57 | 0·79 (0·60–1·04) | 0·091 |
| Positive | 325 | 255 | 160 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| Negative | 344 | 257 | 193 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
|
| |||||||||||
| PIK3CA genotype (mutant vs wild type) | ·· | ·· | ·· | 1·64 (1·31–2·04) | <0·0001 | 1·54 (1·18–2·01) | 0·0016 | 1·81 (1·43–2·29) | <0·0001 | 1·65 (1·24–2·19) | 0·00054 |
| Mutant | 147 | 122 | 84 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| Wild type | 318 | 229 | 148 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
|
| |||||||||||
| Previous treatment (treatment naive vs previous [neo] adjuvant therapy) | ·· | ·· | ·· | 0·99 (0·84–1·18) | 0·94 | 0·90 (0·73–1·11) | 0·34 | 1·04 (0·83–1·30) | 0·73 | 0·87 (0·66–1·15) | 0·33 |
| No previous (neo) adjuvant therapy | 366 | 278 | 184 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| Previous (neo)adjuvant therapy | 312 | 241 | 174 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
|
| |||||||||||
| Visceral disease at screening (yes vs no) | 1·26 (1·02–1·57) | 0·034 | 1·84 (1·37–2·46) | <0·0001 | 1·30 (1·00–1·70) | 0·059 | 1·86 (1·27–2·71) | 0·0013 | |||
| Yes | 538 | 417 | 305 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| No | 140 | 102 | 53 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
|
| |||||||||||
| Treatment group (pertuzumab vs placebo) | ·· | ·· | ·· | 0·65 (0·55–0·77) | <0·0001 | 0·64 (0·52–0·79) | <0·0001 | 0·69 (0·55–0·86) | 0·00083 | 0·66 (0·51–0·87) | 0·0029 |
| Pertuzumab | 336 | 241 | 151 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
| Placebo | 342 | 278 | 207 | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· |
All variables included in the univariate and multivariate Cox proportional analyses are shown. HR=hazard ratio. TILs=tumour-infiltrating lymphocytes. PFS=progression-free survival. OS=overall survival.
Figure 2. Stromal TILs and survival.
Kaplan-Meier curves are stratified by mean stromal TIL value (≤20% vs >20%). All analyses were done in the intention-to-treat population of patients with evaluable TILs. Plots show progression-free survival in the whole cohort (A) and in patients grouped by stromal TIL value and treatment group (all patients also received trastuzumab and docetaxel; B), and overall survival in the whole cohort (C) and in patients grouped by stromal TIL value and treatment group (D). HR=hazard ratio for patients with >20% TILs vs those with ≤20% TILs. NR=not reached. TIL= tumour-infiltrating lymphocyte.
In all evaluable patients, high stromal TIL values were significantly associated with longer overall survival by multivariate analysis (table 3). In addition to TILs, in the multivariate analysis, PIK3CA genotype, visceral disease at screening, and treatment group were significantly associated with overall survival, whereas age, ethnicity, oestrogen receptor status, and previous treatment were not (table 3). Median overall survival was shorter in patients with TIL values of 20% or less than in patients with TIL values greater than 20% (HR 0·76, 95% CI 0·60–0·96, p=0·021; figure 2C). Overall survival by treatment group is shown in figure 2D. The 3-year Kaplan-Meier estimates of overall survival by stromal TIL values in the placebo group were 50% (95% CI 44–57) in patients with TIL values of 20% or less versus 55% (46–65) in patients with TIL values greater than 20%. In the pertuzumab group, 3-year overall survival estimates were 64% (58–70) in patients with TIL values of 20% or less versus 78% (69–87) in patients with TIL values greater than 20%. Survival curves showing progression-free survival and overall survival based on median and quartile TIL cutoffs are shown in the appendix (pp 3–4). We detected no significant interactions between TILs and oestrogen receptor status in the TIL-evaluable cohort for progression-free survival (pinteraction=0·072) or overall survival (pinteraction=0·39).
In an exploratory analysis, the association of TILs with overall survival was stronger in patients with freshly obtained tissue (adjusted HR 0·82, 0·67–0·99, p=0·036) than in those with archival tissue (adjusted HR 0·91, 0·84–0·98, p=0·014).
In our analysis of the effect of the addition of pertuzumab and the association between TIL values and clinical outcome, we did not detect significant interactions between stromal TILs as a continuous variable and treatment group in the cohort with evaluable TILs for both progression-free survival and overall survival (figure 3). In an exploratory analysis, the prognostic association between TIL values and overall survival in all subgroups was consistent with the overall result (appendix p 5).
Figure 3. Stromal TILs and effect of pertuzumab.
Forest plots show the HR for each 10% increment in TILs according to treatment group. These HRs were derived with a Cox regression model. pinteraction values represent the test for interaction between TILs and pertuzumab effect—ie, to test if there is a significant difference between the HR point estimates of the effect of the number of TILs on progression-free survival (A) and overall survival (B) by treatment group. All analyses were done in the intention-to-treat population of the cohort with evaluable TILs. Overall HRs were adjusted for clinicopathological factors. TIL=tumour-infiltrating lymphocyte. HR=hazard ratio.
Discussion
In this retrospective analysis of TILs in pretreatment samples from the CLEOPATRA trial, we identified novel associations of TILs with fresh versus archival tissue samples, anatomic location of metastases, and host ethnicity. Additionally, increased quantity of stromal TILs was significantly associated with improved overall survival in patients with HER2-positive metastatic breast cancer treated with either pertuzumab or placebo combined with docetaxel and trastuzumab, and, extending the prognostic role of TILs outside the primary disease setting. To our knowledge, this study is the first to investigate prognostic associations of TILs in advanced HER2-positive disease.
TILs in early breast cancer have been reported to be associated with a T-effector phenotype and an interferon-γ gene signature,8,14 suggesting the presence of an effective antitumour host response. However, immunogenicity is hypothesised to decrease in the metastatic setting because of immunoediting15 and persistent activation of immune-evading mechanisms. Additionally, activation of HER2 signalling might be immunosuppressive.16 Consistent with these findings, proportions of TILs from metastatic lesions have been reported to be lower than primary lesions.17 In our study, we detected significantly lower TIL values in freshly obtained samples than in archival samples, emphasising the potential importance of acquiring new tissue biopsies to examine the immune microenvironment. Notably, we found lower TIL values in metastasis samples than in their paired primary samples, but there were too few paired samples to provide adequate power for this comparison (n=20). Further studies will be needed to confirm whether the quantities of TILs are significantly reduced during the evolution to advanced disease in HER2-positive cancer.
TIL values also differed by metastatic site—all metastatic lesions had generally low TIL values, except for lung metastases, which had significantly more TILs than did the other sites. Anecdotal and retrospective reports have suggested that patients with metastatic burden restricted to the lymph nodes and lungs have better responses to immune checkpoint blockade than do patients with metastases elsewhere.18 Although this finding needs to be validated given the small number of samples, it does raise the possibility that site-specific immunological privileges might exist, which could have implications for the understanding of responses to various immunotherapy approaches.
In this study, oestrogen receptor-negative tumours had more TILs than did oestrogen receptor-positive tumours. Although the exact reasons for this result are unclear, oestrogen has long been thought to have a role in immunosuppression,19 which could extend to the tumour microenvironment. PIK3CA-mutant tumours also had significantly higher proportions of TILs than did PIK3CA wild-type tumours, despite being associated with poorer prognoses in the multivariate analysis. If this observation could be confirmed, it would be of value to characterise the TIL infiltrate to establish the immunosuppressive molecules that might be present. We also noted that TIL values differed by ethnicity. Differences in the incidence of autoimmune diseases have been reported between different ethnicities;20 however, to our knowledge, our findings are the first to show differences in TILs. In our study, black or African American participants, although only representing a small subgroup, had the lowest median TIL value. Other studies have shown worse survival in black or African American women with breast cancer than in women of other ethnicities.21 Host factors governing tumour immune responses remain largely unexplored. The concept of ethnic differences in antitumour immunity warrants further investigation, and could partly account for differences in cancer outcomes.
In the advanced setting, higher stromal TIL values were significantly associated with increased overall survival compared with that in patients with lower TIL values in HER2-positive metastatic disease, irrespective of treatment group. The TIL phenotype, in particular the types of immune checkpoint molecules present, in metastatic disease remains largely unexplored in breast cancer, although our present data suggest that the higher quantities of TILs and better survival are related to a more functional antitumour T-effector cell response. Of note, the prognostic effect of TILs seems to be stronger for overall survival than for progression-free survival, although progression-free survival has been reported to be only a modest surrogate for overall survival in HER2-positive metastatic disease.22 This effect has been previously reported in the context of pertuzumab treatment, with similar differences in progression-free survival and overall survival effects seen in the PHEREXA study.23 Although the reasons remain unclear, we have also noted the difference between disease-free survival and overall survival previously in the early-stage setting.24 Furthermore, analogous improvements in overall survival without significant increases in progression-free survival have also been described in PD-1 immune checkpoint blockade of non-squamous non-small-cell lung cancer and renal cell carcinoma.25,26
It is unclear how pertuzumab adds to the ability of trastuzumab and docetaxel to generate effective anti-tumour immunity. Given that we assessed pretreatment TIL values in this study, this effect of pertuzumab could be through enhanced relief of tumour-mediated immunosuppression due to inhibition of the key oncogene HER2. Enhanced induction of antibody-dependent cell-mediated cytotoxicity or increased enhancement of T-cell-mediated antitumour immunity are other plausible hypotheses.27–29 However, our data provide a new prognostic marker in the setting of advanced disease and suggest that the ability to augment antitumour immune responses will be of therapeutic importance in attempts to further improve survival in advanced disease. Identification of a clinically relevant TIL cutoff value might also help to define a subgroup that could be enriched for patients who respond to checkpoint blockade30 and could contribute to current immunohistochemical immune markers in this setting.
Our study is strengthened by the prospective collection of tumour tissue from nearly all participants who enrolled in the CLEOPATRA clinical trial. Moreover, all participants received chemotherapy and trastuzumab. Our study has the added advantage of a substantial median follow-up of 51 months for overall survival. Although our study was retrospective, there are substantial advantages to biomarker evaluation in datasets from clinical trials because of the accuracy of prospectively collected data. The value of the use of such specimens has been noted previously.31 Limitations of this work include the fact that we did not have material available to make a thorough assessment of the components of the immune infiltrate. However, we believe that stromal TILs in the advanced setting will be similar to those in primary disease, in that they represent an activated T-cell response,8 with the magnitude of the TIL infiltration as the most important factor for prognostic evaluation, and are more pragmatic than individual immune markers.12,13,32
Another limitation of our study was the unavailability of information on the site of all biopsied metastases. As such, our analyses of TIL associations by metastatic site are largely descriptive and therefore need further validation. It should also be noted that only 11% of our cohort had received previous adjuvant or neoadjuvant trastuzumab, so the applicability of our findings to patients with recurrent metastatic disease remains unclear, although we note that the prognostic value of TILs on clinical outcome was greater for freshly obtained samples than for archival samples. However, as more patients with primary disease are cured,33 de-novo metastatic disease is likely to become the main presentation of this breast cancer subtype.
Higher quantities of TILs are significantly associated with improved survival in the context of treatment with taxane-based chemotherapy, trastuzumab, and pertuzumab in advanced HER2-positive breast cancer. Our data suggest that the positive prognostic effect of antitumour immunity persists in the advanced setting and that freshly obtained biopsy samples will be informative for the assessment of pre-existing antitumour immunity. Our data also suggest that the TIL biomarker should be considered as a stratification parameter in future clinical trials to assess both new targeted agents and immunotherapies, and that augmentation of the immune response could further improve survival.
Supplementary Material
Acknowledgments
Funding F Hoffmann-La Roche–Genentech and the Breast Cancer Research Foundation.
The CLEOPATRA study was funded and administered by F Hoffman-La Roche-Genentech. SJL is supported by the University of Melbourne. SL is supported by the Breast Cancer Research Foundation and the John Colebatch Cancer Council Victoria Clinical fellowship.
Footnotes
Contributors
SJL participated in the study design, data collection, data analysis, data interpretation, and writing and editing of the report. RS participated in the study design, data collection, data interpretation, and editing of the report. SF participated in the data collection and editing of the report. PS, JE-W, EC, AK, SS, and JB participated in the editing of the report. SM and SL participated in the study design, data collection, data analysis, data interpretation, and editing of the report. SJL and SL prepared the first draft of the report, and all authors contributed to subsequent drafts.
Declaration of interests
JE-W, EC, and AK are employees of F Hoffmann-La Roche. AK is an employee and reports personal fees from F Hoffmann-La Roche. JE-W is an employee and reports personal fees from Roche-Genentech. EC is an employee of Roche Products, and has an issued patent “Uses for and article of manufacture including HER2 dimerisation inhibitor pertuzumab 13/649591”. SS reports grants, personal fees and non-financial support from Roche-Genentech; personal fees from Clinigen Group, AstraZeneca, OncoPlex Diagnostics, and Pieris Pharmaceuticals; grants and personal fees from Pfizerand EliLilly; and grants from Puma Biotechnology and Merrimack Pharmaceuticals; and has received honoraria and her institution has received research funding from Roche-Genentech. SL reports grants from Roche-Genentech. SL’s institution receives research support from Roche-Genentech. All other authors declare no competing interests.
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–34. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baselga J, Cortes J, Im SA, et al. Biomarker analyses in CLEOPATRA: a phase III, placebo-controlled study of pertuzumab in human epidermal growth factor receptor 2-positive, first-line metastatic breast cancer. J Clin Oncol. 2014;32:3753–61. doi: 10.1200/JCO.2013.54.5384. [DOI] [PubMed] [Google Scholar]
- 4.Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell. 2002;2:127–37. doi: 10.1016/s1535-6108(02)00097-1. [DOI] [PubMed] [Google Scholar]
- 5.Franklin MC, Carey KD, Vajdos FF, Leahy DJ, de Vos AM, Sliwkowski MX. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell. 2004;5:317–28. doi: 10.1016/s1535-6108(04)00083-2. [DOI] [PubMed] [Google Scholar]
- 6.Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–36. doi: 10.1158/0008-5472.CAN-08-4597. [DOI] [PubMed] [Google Scholar]
- 7.Capelan M, Pugliano L, De Azambuja E, et al. Pertuzumab: new hope for patients with HER2-positive breast cancer. Ann Oncol. 2013;24:273–82. doi: 10.1093/annonc/mds328. [DOI] [PubMed] [Google Scholar]
- 8.Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33:983–91. doi: 10.1200/JCO.2014.58.1967. [DOI] [PubMed] [Google Scholar]
- 9.Salgado R, Denkert C, Campbell C, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol. 2015;1:448–54. doi: 10.1001/jamaoncol.2015.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salgado R, Denkert C, Demaria S, et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann Oncol. 2015;26:259–71. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denkert C, Wienert S, Poterie A, et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. 2016;29:1155–64. doi: 10.1038/modpathol.2016.109. [DOI] [PubMed] [Google Scholar]
- 12.Loi S, Michiels S, Salgado R, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol. 2014;25:1544–50. doi: 10.1093/annonc/mdu112. [DOI] [PubMed] [Google Scholar]
- 13.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02–98. J Clin Oncol. 2013;31:860–67. doi: 10.1200/JCO.2011.41.0902. [DOI] [PubMed] [Google Scholar]
- 14.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28:105–13. doi: 10.1200/JCO.2009.23.7370. [DOI] [PubMed] [Google Scholar]
- 15.Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases—elimination, equilibrium and escape. Curr Opin Immunol. 2014;27:16–25. doi: 10.1016/j.coi.2014.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Savas P, Caramia F, Teo ZL, Loi S. Oncogene addiction and immunity: clinical implications of tumour infiltrating lymphocytes in breast cancers overexpressing the HER2/neu oncogene. Curr Opin Oncol. 2014;26:562–67. doi: 10.1097/CCO.0000000000000131. [DOI] [PubMed] [Google Scholar]
- 17.Arnedos M, Filleron T, Dieci MV, et al. Genomic and immune characterization of metastatic breast cancer (MBC): and ancillary studies of the SAFIR01 & M overall survival CATO trials. Ann Oncol. 2014;25(suppl 4):iv116. (abstr 3510) [Google Scholar]
- 18.Tumeh PC, Rosenblum M, Handley N, et al. Abstract 2857: Metastatic site and response to pembrolizumab (anti-PD1 antibody) in melanoma. Cancer Res. 2015;75(15 suppl) abstr 2857. [Google Scholar]
- 19.Svoronos N, Perales-Puchalt A, Allegrezza MJ, et al. Tumor cell-independent estrogen signaling drives disease progression through mobilization of myeloid-derived supressor cells. Cancer Discov. 2016 doi: 10.1158/2159-8290.CD-16-0502. published online Sept 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mori M, Yamada R, Kobayashi K, Kawaida R, Yamamoto K. Ethnic differences in allele frequency of autoimmune-disease-associated SNPs. J Hum Genet. 2005;50:264–66. doi: 10.1007/s10038-005-0246-8. [DOI] [PubMed] [Google Scholar]
- 21.Akinyemiju T, Moore JX, Ojesina AI, Waterbor JW, Altekruse SF. Racial disparities in individual breast cancer outcomes by hormone-receptor subtype, area-level socio-economic status and healthcare resources. Breast Cancer Res Treat. 2016;157:575–86. doi: 10.1007/s10549-016-3840-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michiels S, Pugliano L, Marguet S, et al. Progression-free survival as surrogate end point for overall survival in clinical trials of HER2-targeted agents in HER2-positive metastatic breast cancer. Ann Oncol. 2016;27:1029–34. doi: 10.1093/annonc/mdw132. [DOI] [PubMed] [Google Scholar]
- 23.Urruticoechea A, Rizwanullah M, Im S-A, et al. PHEREXA: a phase III study of trastuzumab (H) + capecitabine (X) ± pertuzumab (P) for patients (pts) who progressed during/after one line of H-based therapy in the HER2-positive metastatic breast cancer (MBC) setting. Proc Am Soc Clin Oncol. 34(suppl) abstr 504. [Google Scholar]
- 24.Loi S. Host antitumor immunity plays a role in the survival of patients with newly diagnosed triple-negative breast cancer. J Clin Oncol. 2014;32:2935–37. doi: 10.1200/JCO.2014.56.7677. [DOI] [PubMed] [Google Scholar]
- 25.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kroemer G, Senovilla L, Galluzzi L, Andre F, Zitvogel L. Natural and therapy-induced immunosurveillance in breast cancer. Nat Med. 2015;21:1128–38. doi: 10.1038/nm.3944. [DOI] [PubMed] [Google Scholar]
- 28.Stagg J, Loi S, Divisekera U, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci USA. 2011;108:7142–47. doi: 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park S, Jiang Z, Mortenson ED, et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell. 2010;18:160–70. doi: 10.1016/j.ccr.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–28. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 31.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst. 2009;101:1446–52. doi: 10.1093/jnci/djp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams S, Gray RJ, Demaria S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32:2959–66. doi: 10.1200/JCO.2013.55.0491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tolaney SM, Barry WT, Dang CT, et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med. 2015;372:134–41. doi: 10.1056/NEJMoa1406281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.