Abstract
The molecular sensor of innocuous (painless) cold sensation is well-established to be Transient Receptor Potential cation channel, subfamily M, member 8 (TRPM8). However, the role of Transient Receptor Potential cation channel, subfamily A, member 1 (TRPA1) in noxious (painful) cold sensation has been controversial. We find that TRPA1 channels contribute to the noxious cold sensitivity of mouse somatosensory neurons, independent of TRPM8 channels, and that TRPA1-expressing neurons are largely non-overlapping with TRPM8-expressing neurons in mouse dorsal-root ganglia (DRG). However, relatively few TRPA1-expressing neurons (e.g., responsive to allyl isothiocyanate or AITC, a selective TRPA1 agonist) respond overtly to cold temperature in vitro, unlike TRPM8-expressing neurons, which almost all respond to cold. Using somatosensory neurons from TRPM8 −/− mice and subtype-selective blockers of TRPM8 and TRPA1 channels, we demonstrate that responses to cold temperatures from TRPA1-expressing neurons are mediated by TRPA1 channels. We also identify two factors that affect the cold-sensitivity of TRPA1-expressing neurons: 1) Cold-sensitive AITC-sensitive neurons express relatively more TRPA1 transcripts than cold-insensitive AITC-sensitive neurons and 2) voltage-gated potassium (KV) channels attenuate the cold-sensitivity of some TRPA1-expressing neurons. The combination of these two factors, combined with the relatively weak agonist-like activity of cold temperature on TRPA1 channels, partially explains why few TRPA1-expressing neurons respond to cold. Blocking KV channels also reveals another subclass of noxious cold-sensitive DRG neurons that do not express TRPM8 or TRPA1 channels. Altogether, the results of this study provide novel insights into the cold-sensitivity of different subclasses of somatosensory neurons.
Keywords: TRPA1, TRPM8, KV1.2, cold pain, ion channel, pharmacology
Introduction
Cold is analgesic when moderate (Proudfoot et al., 2006) and painful when extreme (Foulkes and Wood, 2007). These contradictory effects of cold in warm-blooded animals may be due to differences at the molecular, cellular, or circuit level (Vriens et al., 2014; Palkar et al., 2015; Lolignier et al., 2016). At the molecular level, temperature-sensitive transient receptor potential (thermoTRP) channels such as TRPM8 and TRPA1 have been reported to detect cold temperatures (Talavera et al., 2008). TRPM8, the first molecular sensor of cold-sensation, discovered in 2002, is widely accepted as a cold-sensor (Peier et al., 2002; Madrid et al., 2006; Bautista et al., 2007; Dhaka et al., 2007). TRPA1, on the other hand, was first characterized as a noxious cold-sensor (Story et al., 2003) but some follow-up studies were in conflict with this conclusion, resulting in an ongoing debate about the role of TRPA1 as a cold-sensor (Kwan et al., 2006; Bautista et al., 2007; Fajardo et al., 2008; Caspani and Heppenstall, 2009; Karashima et al., 2009; del Camino et al., 2010; Knowlton et al., 2010; Chen et al., 2013; Ran et al., 2016). Today, TRPA1 is accepted to have a role in pain and inflammation, but its involvement in cold sensation remains controversial.
This controversy encompasses numerous studies that address the issue at the molecular, cellular and behavioral levels (Kwan et al., 2006; Karashima et al., 2009; Cordero-morales et al., 2011; Miyake et al., 2016). One key observation that underlies this controversy is that only a small percentage of TRPA1-expressing somatosensory neurons respond overtly to noxious cold temperatures in neuronal cell culture (Babes et al., 2004; Jordt et al., 2004; Bautista et al., 2006; Fajardo et al., 2008; Karashima et al., 2009). Consistent with these studies, we also find that only a subset of TRPA1-expressing neurons respond to noxious cold stimuli. Even though the cold-sensitivity of TRPA1 channels has been studied at the molecular level (Sawada et al., 2007; Jabba et al., 2014; Miyake et al., 2016), it is difficult to explain why only a subset of the TRPA1-expressing neuronal population responds to cold. Therefore, to investigate why cold-sensitivity is not uniformly observed in TRPA1-expressing neurons, we have characterized this neuronal population to evaluate contributing factors.
Our data strongly suggest that cold responses in TRPA1-expressing neurons are mediated by TRPA1 channels without any contribution from TRPM8 channels. Further, to understand why only a subset of TRPA1-expressing neurons responds to cold, we hypothesized that additional factors, such as excitability brake by KV channels and differences in TRPA1 expression levels among these neurons might influence cold-sensitivity of TRPA1-expressing neurons. Here, we present evidence that these two factors significantly contribute to the cold-sensitivity of TRPA1-expressing neurons.
Experimental Procedures
Dissociated Dorsal-Root Ganglia (DRG) cell cultures from WT and transgenic mice
Cell cultures were prepared from mouse Lumbar DRG at different ages, from postnatal day 3 (P3) to postnatal day 67 (P67). Mouse strains used for cell preparations were wild-type C57BL/6 mice, TRPM8−/− in a C57BL/6 genetic background (The Jackson Laboratory stock # 8198 (Bautista et al., 2007) and TRPA1−/− mice in a mixed genetic background (The Jackson Laboratory stock # 6401 (Kwan et al., 2006). All experimental results reported for a given mouse strain and age were obtained from three or more cell cultures prepared from different mice (in total ~30 mice), consisting of both sexes. All experiments conducted with mouse tissues were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Utah and were conducted in accordance with the National Institutes of Health guide for the care and use of Laboratory animals. Detailed descriptions of DRG cell preparations and calcium-imaging protocols were reported previously (Smith et al. 2013; Teichert et al. 2014; Teichert et al. 2012a; Teichert et al. 2012b).
Briefly, lumbar DRG neurons were dissociated by treating DRGs with trypsin followed by mechanical trituration. Neurons were then plated into the center of a silicone ring that was previously attached to the floor of a 24-well poly-D-lysine coated plate. After allowing the cells to adhere to the floor of the plate for approximately one hour, one mL of media was added to each well. The media (MEM + supplements) consisted of minimal essential media (MEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS), 1X penicillin/streptomycin, 10 mM HEPES, and 0.4% (w/v) Glucose, pH 7.4. The plated cells were placed in a 5% CO2 incubator at 37 °C overnight.
TRPA1 and TRPM8-expressing HEK cell lines
HEK293 cell-lines were kindly provided by Dr. Christopher Reilly (Department of Pharmacology and Toxicology, University of Utah, Salt Lake City, UT) (Deering-Rice et al., 2011). Wild-type HEK293 cells were grown in DMEM:F12 supplemented with 5% FBS and 1X penicillin/streptomycin. HEK293 cell-lines stably overexpressing either human TRPM8 or TRPA1 channels were grown similarly with the addition of 300 μg/mL Geneticin as described previously (Deering-Rice et al., 2011). These cells were treated with trypsin and plated onto the 24-well poly-D-lysine coated plates in the same manner as the DRG cells and incubated overnight for calcium-imaging experiments.
Calcium-imaging experiments and pharmacology
For calcium-imaging experiments, neurons or HEK293 cells were incubated with Fura2-AM dye for 1 hour at 37°C and 0.5 hour at room temperature. All the calcium-imaging experiments were carried out at room-temperature, except when cold bath solutions were used as stimuli. Single distinct cells were visually identified in the field of view and defined as a region of interest (ROI). For each ROI, a calcium-imaging trace was generated with the standard 340nm/380nm ratio as the y-axis and time in minutes as the x-axis. Data points were captured every two or three seconds. The fluorescence of 340nm/380nm excitation ratio (510 nm emission) indicates the relative level of intracellular calcium in the neuron or cell. All the stimuli indicated by arrows in each figure were applied for 15 seconds before washout. Horizontal bars, cold ramp, and triangles in some figures indicate longer incubation times, including 30-second applications of 4 °C (triangles), 2-minute applications of 4 °C (cold ramp) and longer incubations with pharmacological agents (horizontal bars), including the TRPM8 blocker, M8-B (Almeida et al., 2012), the TRPA1 blocker, HC-030031 (Eid et al., 2008), and the KV1.2 blocker, κM-conotoxin RIIIJ (Teichert et al., 2014). Cold bath solutions were placed in an ice bucket and applied either manually for 30-seconds or circulated using a peristaltic pump for 2-minutes, as described in each figure legend. Temperature was monitored by placing a thermocoupler in the experimental well, close to the imaging field of view. To differentiate between neuronal and non-neuronal populations of DRG cells, a depolarizing pulse of 30 mM extracellular potassium, [K+]o, was applied at the beginning of each experiment.
Calcium-imaging experiments were carried out with DRG observation solution consisting of 145 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 1 mM sodium citrate, 10 mM HEPES, and 10 mM glucose, pH 7.4. For [K+]o application, 30mM K+ was made by increasing the concentration of KCl and reducing the concentration of NaCl in DRG observation solution without affecting the osmolarity. All pharmacological agents, including menthol, allyl isothiocyanate (AITC), M8-B, and HC-030031, were purchased from Sigma-Aldrich (except κM-conotoxin RIIIJ). Working concentrations were made in DRG Observation solution from respective stock solutions. Stock solutions were 640 mM menthol in ethanol, 10.2 M AITC oil, 1 mM M8-B and 30 mM HC-030031 in DMSO, and 10 μM κM-conotoxin RIIIJ in DRG Observation solution. For all experiments, the isomer of menthol used was (1R,2S,5R)-(−)-Menthol.
Single-cell RT-qPCR
Immediately following calcium-imaging experiments, individual neurons of interest were harvested with patch pipettes (~3 μm diameter) containing recombinant RNAse Inhibitor (Invitrogen). After collecting each cell in a patch pipette tip, the pipette tip was broken into a PCR tube and the neuronal cell body was transferred to a solution containing 10% Triton X-100 and SuperScript IV VILO Master Mix (Invitrogen) for reverse transcription. The mRNA in each sample was reverse transcribed at 50°C for 10 minutes. 10 μl of this reverse transcription reaction was then divided into two different PCR reactions containing either TRPA1 primers or β-actin primers. TRPA1 primer pairs 5′-AGGTGATTTTTAAAACATTGCTGAG-3′ and 5′-CTCGATAATTGATGTCTCCTAGCAT-3′ yield cDNA fragments of 168 bp, and β-actin primer pairs 5′-GGCCCAGAGCAAGAGAGGTATCC-3′ and 5′-ACGCACGATTTCCCTCTCAGC-3′ yield cDNA fragments of 460 bp (Kwan et al., 2006). Both TRPA1 and β-actin primer pairs span an intron and yield DNA fragments amplified from genomic DNA of 500 bp and 914 bp, respectively. For real-time PCR, the cDNA from RT reaction (4 μl) was denatured at 95°C, annealed at 60°C, and extended at 72°C for 40 cycles with 10 μL KAPA SYBR FAST qPCR MM (Kapa Biosystems) and 400 nM of TRPA1 or 250 nM of β-actin primers in 20 μL PCR reactions. Control samples included cells with no AITC response or extracellular DRG observation solution. Only the cells with β-actin signal were included in the analysis. For data analysis, ΔCq and relative quantities of TRPA1 cDNA molecules in individual cells were determined by normalizing the Cq value of each cell to the Cq cutoff of 34 for TRPA1 reactions (Ståhlberg et al., 2013). Cq cutoff and efficiency of reactions was estimated from a standard curve of RNA isolated from mouse DRG neurons. Finally, 2% agarose gel and melt curves were used to confirm the specificity of the end product.
Data analysis and statistics
For calcium-imaging experiments, cells were defined as regions of interest (ROIs) using the 10X brightfield image. Traces are shown in figures as ratiometric values for each ROI in the series of images taken at two or three-second intervals over the time-frame of the experiment. Ratiometric values are indicators of relative cytosolic calcium levels. The time-course of the ratio was analyzed using a set of functions written in R (www.r-project.org). The maldiquant package (Gibb and Strimmer, 2012) was used to correct baselines, smooth, and detect peaks. All traces were baseline corrected using the estimate Baseline function with the “SNIP” method and smoothed using the smoothIntensity function with the “SavitzkyGolay” method and a half Window Size of 3. Peaks were detected using the detect Peaks function with the “MAD” method, a half Window Size of 30 and a SNR.lim setting of 4. All ROIs were scored as yes/no (binary) response to each input based on the presence of a peak in the response window region. Thresholds were established to define possible peaks in time windows of probable response to the given inputs. We used a signal to noise (SNR) threshold of 4 and a value above baseline threshold of .05. These threshold values give a false positive rate < 0.001 (e.g. peaks during times of no input). Traces with obvious abnormal response patterns were removed from the analysis.
The percentage or frequency of cells responsive to a given stimulus or compound was determined using binary scoring described above. Also, to count the frequency of neuronal responders in DRG cultures, only the cells responding to [K+]o were included in the analysis. The frequency of responses was calculated for each experimental trial and averaged for each culture. Mean frequencies across multiple cultures were determined for each set of experimental conditions. The mantelhaen.test function of R was used to perform a Cochran-Mantel-Haenszel chi-squared test (Agresti, 1990) for equality of response type frequency between mouse lines. The woolf test (Woolf, 1955) was used to test for heterogenity between wells. The cor.test function in R was used to test for significant correlation between dCq and AITC response area. The t.test function in R was used to test for significant differences between dCq values in AITC+ and AITC− neuron populations.
Results
Diverse response profiles observed among cold-sensitive DRG neurons
We used Fura-2 calcium imaging of dissociated adult mouse DRG neurons, coupled with various types of physiological and pharmacological challenges, to identify and characterize different neuronal subclasses. Figure 1 illustrates the diversity of response phenotypes elicited by a depolarizing stimulus (30 mM extracellular K+), cold temperatures (18 °C and 4 °C), menthol and AITC in DRG neurons (n=1465) from wild-type C57BL/6 mice. Notably, a subset of the AITC-sensitive neurons responded to a 30-second application of noxious cold temperature (4 °C) and menthol (Fig. 1A). However, these represented a small minority (7.9% ± 1.2%) of the AITC-sensitive DRG neurons (A+ in Table 1). A larger subset (19.4% ± 1.7%) of AITC-sensitive neurons responded to menthol, but not to cold temperatures (Fig. 1A, Table 1). An additional subset (71.7% ± 1.9%) of DRG neurons responded only to AITC, without robust responses either to cold or menthol (Fig. 1A, Table 1). In contrast to the AITC-sensitive neurons, we observed that a large subset (67.8% ± 4.4%) of menthol-sensitive but AITC-insensitive (M+A− in Table 1) DRG neurons responded to innocuous cool temperatures (e.g. 18 °C, Fig. 1B, Table 1). However, a subset (30.4% ± 4.3%) of these neurons only responded to noxious cold temperatures (e.g. 4 °C, Fig. 1B, Table 1). A large subset of DRG neurons did not respond to cold, menthol or AITC, but did respond to a depolarizing stimulus, which activated voltage-gated calcium channels, eliciting a calcium influx (Fig. 1C). Distributions of these functional response phenotypes as percentages of the total neuronal cell population (K+) have been listed in Table 2.
Figure 1.
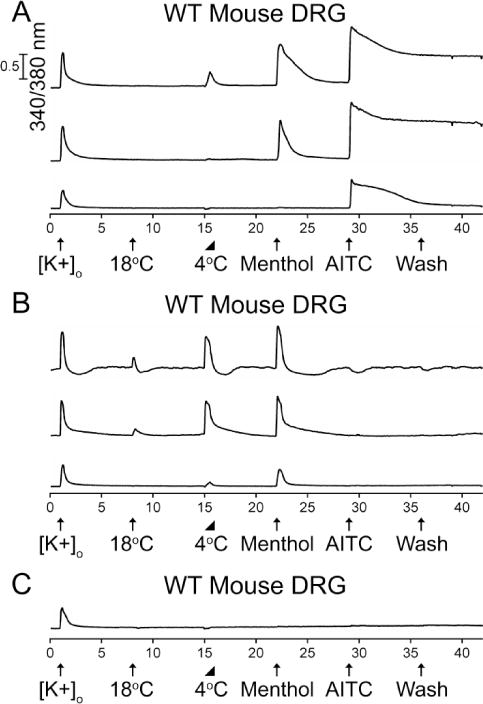
The diversity of neuronal responses from adult (>Postnatal day 45) wild-type mouse DRG to cold, menthol, and AITC. The units of the X-axis are minutes and the units of the Y-axis are the 340/380 nm calcium-imaging ratio described in Materials and Methods. Calibration bar of 0.5 for 340/380 values applies to all the traces. The experimental protocol is depicted below the X-axis. Physiological or pharmacological challenges were administered at 7-minute intervals. Abbreviations are as follows: [K+]o, 30 mM [K+]o; 18°C or 4°C, bath solution at the respective temperature; Menthol, 400 μM menthol; AITC, 100 μM AITC; wash, replacement of static bath solution with identical bath solution. Arrows represent the 15-second application of each challenge. The triangle indicates a 30-second application of 4 °C solution, where the temperature was allowed to gradually rise in the well at room temperature. The aforementioned facts apply to subsequent figures also. (A) Representative traces from AITC-sensitive DRG neurons. A subset of DRG neurons responded to cold, menthol and AITC (2.9% ± 0.4%). Another subset responded to menthol and AITC (7.2% ± 0.7%). An additional subset only responded to AITC (26.5% ± 1.2%). See summary in Table 2. (B) Representative traces from menthol-sensitive and AITC-insensitive DRG neurons. A subset of DRG neurons responded to 18 °C and 4 °C bath applications, and menthol (5.3% ± 0.6%). Another subset only responded to 4 °C and menthol (2.4% ± 0.4%). See summary in Table 2. (C) Representative trace from a large subset of DRG neurons that did not respond to cold, menthol or AITC. Percentage values refer to percentage of [K+]o responsive neurons (n=1465) from 6 experimental trials from 3 mice.
Table 1.
Response phenotypes as percentages of AITC+ (TRPA1-expressing) and Menthol+, AITC− (TRPM8-expressing) mouse DRG neurons or HEK Cells.
| WT Mice | TRPM8 −/− Mice | TRPA1 HEK Cells | ||||
|---|---|---|---|---|---|---|
| % A+ (n=541) | SEM | % A+ (n=478) | SEM | % A+ (n=494) | SEM | |
| 4°+ M+ A+ | 7.9% | 1.2% | 6.7% | 1.1% | 47.2% | 2.2% |
| 4°− M+ A+ | 19.4% | 1.7% | 31.4% | 2.1% | 34.0% | 2.1% |
| 4°− M− A+ | 71.7% | 1.9% | 60.7% | 2.2% | 14.8% | 1.6% |
| 4°+ M− A+ | 0.9% | 0.4% | 1.3% | 0.5% | 4.0% | 0.9% |
| Total A+ Cells | 100.0% | 100.0% | 100.0% | |||
| WT Mice | TRPA1 −/− Mice | TRPM8 HEK Cells | ||||
|---|---|---|---|---|---|---|
| % M+A− (n=115) | SEM | % M+A− (n=74) | SEM | % M+A− (n=880) | SEM | |
| 18°+ 4°+ M+ A− | 67.8% | 4.4% | *47.3% | 5.8% | 89.8% | 1.0% |
| 18°− 4°+ M+ A− | 30.4% | 4.3% | *40.5% | 5.7% | 9.2% | 1.0% |
| 18°− 4°− M+ A− | 1.7% | 1.2% | *12.2% | 3.8% | 1.0% | 0.3% |
| Total M+A− Cells | 100.0% | 100.0% | 100.0% | |||
Abbreviations are the following: 18°, 18° C for 15 seconds; 4°, 4° C for 30-seconds; M, Menthol; A, AITC; +, responsive; −, not responsive; n, number of cells; SEM, standard error of the mean.
The percentages in the table were calculated from the following:
WT Mice: 6 experimental trials from 3 cell cultures (mice).
TRPM8 −/− mice: 7 experimental trials from 4 cell cultures (mice).
TRPA1 −/− mice: 8 experimental trials from 3 cell cultures (mice).
TRPA1 HEK cells: 3 experimental trials from 3 cell cultures.
TRPM8 HEK cells: 4 experimental trials from 3 cell cultures.
We observed significant differences in the frequency of each response phenotype between WT and TRPA1 −/− mice. In general, the M+A− neurons from TRPA1 −/− mice appear to have a higher cold-response threshold than M+A− neurons from WT mice. However, we did not investigate that observation further in this study.
Table 2.
Response phenotypes of AITC+ (TRPA1-expressing) and Menthol+, AITC−(TRPM8-expressing) mouse DRG neurons as percentages of the total neuronal cell population.
| WT Mice | TRPM8 −/− Mice | |||
|---|---|---|---|---|
| % K+ (n=1465) | SEM | % K+ (n=1535) | SEM | |
| 4°+ M+ A+ | 2.9% | 0.4% | 2.1% | 0.4% |
| 4°− M+ A+ | 7.2% | 0.7% | 9.8% | 0.8% |
| 4°− M− A+ | 26.5% | 1.2% | 18.9% | 1.0% |
| 4°+ M− A+ | 0.3% | 0.2% | 0.4% | 0.2% |
| Other Neurons | 63.1% | 1.3% | 68.9% | 1.2% |
| Total Neurons | 100.0% | 100.0% | ||
| WT Mice | TRPA1 −/− Mice | |||
|---|---|---|---|---|
| % K+ (n=1465) | SEM | % K+ (n=1107) | SEM | |
| 18°+ 4°+ M+ A− | 5.3% | 0.6% | 3.2% | 0.5% |
| 18°− 4°+ M+ A− | 2.4% | 0.4% | 2.7% | 0.5% |
| 18°− 4°− M+ A− | 0.1% | 0.1% | 0.8% | 0.3% |
| Other Neurons | 92.2% | 0.7% | 93.3% | 0.8% |
| Total Neurons | 100.0% | 100.0% | ||
Abbreviations are the following: 18°, 18° C for 15 seconds; 4°, 4° C for 30-seconds; M, Menthol; A, AITC; +, responsive; −, not responsive; n, number of cells; SEM, standard error of the mean.
The percentages in the table were calculated from the following:
WT Mice: 6 experimental trials from 3 cell cultures (mice).
TRPM8 −/− mice: 7 experimental trials from 4 cell cultures (mice).
TRPA1 −/− mice: 8 experimental trials from 3 cell cultures (mice).
Cold responses in AITC-sensitive neurons depend on TRPA1 and not TRPM8 channels
The experiment illustrated in figure 1 employed DRG neurons from wild-type mice. We repeated this experimental protocol multiple times with DRG neurons from TRPA1−/− mice and TRPM8−/− mice (Fig. 2). Using DRG neurons from TRPA1−/− mice (n=1107), we observed all of the functional phenotypes shown in Figure 1B (see Fig. 2B, Table 1 and 2), but none of the functional phenotypes exhibited in Figure 1A. These neurons apparently all express TRPM8 channels and not TRPA1 channels because they responded to menthol (a TRPM8 agonist), but not to AITC (a TRPA1 agonist). Using DRG neurons from TRPM8−/− mice (n=1535), we did not observe any of the functional phenotypes shown in Figure 1B and we continued to observe all of the functional phenotypes shown in Figure 1A (see Fig. 2A, Table 1 and 2). We had previously attributed the menthol responses to TRPM8 channels in AITC-sensitive neurons (Teichert et al., 2012a) as menthol was considered a selective agonist of TRPM8 channels (Bautista et al., 2007) and shown to block mouse TRPA1 channels at high concentrations (Karashima et al., 2007; Xiao et al., 2008). However, our results from adult TRPM8−/− mice suggested that the responses to cold and menthol in AITC-sensitive neurons may be solely mediated by TRPA1 channels and not TRPM8 channels. This is consistent with previous studies that used similar concentrations of menthol and reported activation of TRPA1 channels in sensory neurons (Fajardo et al., 2008; Meseguer et al., 2008; Karashima et al., 2009). Furthermore, these results with TRPM8−/− mice suggested that TRPA1 and TRPM8 channels may be expressed in separate, non-overlapping DRG neuronal subclasses.
Figure 2.
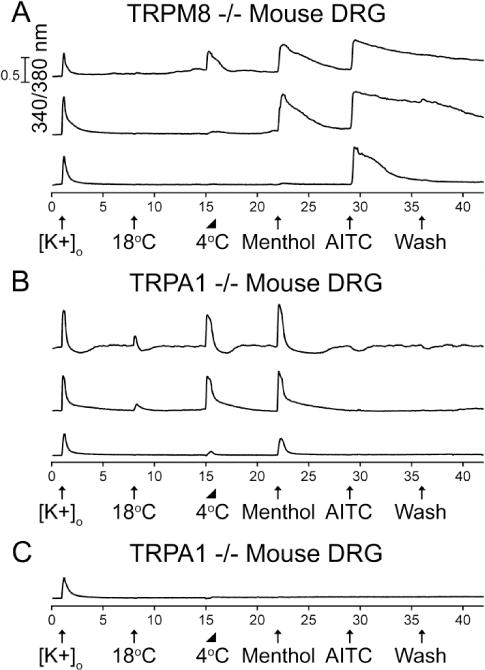
The diversity of neuronal responses to cold, menthol, and AITC from (A) adult TRPM8−/− mouse DRG and (B & C) TRPA1−/− mouse DRG. The experimental protocol is identical to that described in the Figure 1 legend. (A) AITC-sensitive DRG neurons that express TRPA1 channels were observed from TRPM8−/− mice. A subset of DRG neurons responded to cold, menthol and AITC (2.1% ± 0.4%). Another subset responded to menthol and AITC (9.8% ± 0.8%). An additional subset only responded to AITC (18.9% ± 1.0%). See summary in Table 2. (B) Menthol-sensitive DRG neurons that express TRPM8 channels were observed from TRPA1−/− mice. A subset of DRG neurons responded to 18 °C and 4 °C bath applications, and menthol (3.2% ± 0.5%). Another subset only responded to 4 °C and menthol (2.7% ± 0.5%). See summary in Table 2. (C) Representative trace from a large subset of DRG neurons that did not respond to cold, menthol or AITC. In this case, the trace is from a DRG neuron from a TRPA1−/− mouse. Percentage values refer to percentage of [K+]o responsive neurons (n=1535 for TRPM8−/− and n=1107 for TRPA1−/−) from 4 TRPM8−/− and 3 TRPA1−/− mice.
To further test these hypotheses, we utilized subtype-selective antagonists of TRPA1 and TRPM8 channels to block the responses to cold, menthol and AITC in wild-type adult mouse DRG neurons. Figure 3A demonstrates that a selective antagonist of TRPA1 channels, HC-030031 (Eid et al., 2008), completely blocked the responses to cold, menthol and AITC in 96.9% ± 0.7% of the TRPA1-expressing (AITC sensitive) DRG neurons (n=422), but did not block the cold and menthol responses in the TRPM8-expressing DRG neurons (menthol-sensitive but AITC insensitive). In the remaining ~3% of TRPA1-expressing neurons, HC-030031 blocked responses to cold, menthol and AITC by 88.9% ± 6.2%. Figure 3B demonstrates that a selective antagonist of TRPM8 channels, M8-B (Almeida et al., 2012), completely blocked the responses to cold and menthol in 95.0% ± 2.9% of the TRPM8-expressing DRG neurons (n=40), but did not block the cold and menthol responses in the TRPA1-expressing DRG neurons (AITC sensitive). In the remaining ~5% of TRPM8-expressing neurons, M8-B blocked responses to cold and menthol by 92.5% ± 4%. In addition to the results obtained from TRPA1−/− and TRPM8−/− mice, these results confirm that responses to cold and menthol in TRPA1-expressing DRG neurons (AITC sensitive) are solely mediated by TRPA1 channels. Moreover, TRPA1-mediated cold and menthol responses become evident in adult mice compared to neonates as the frequency of AITC-responsive (TRPA1-expressing) neurons increases with age (Fig. 4).
Figure 3.
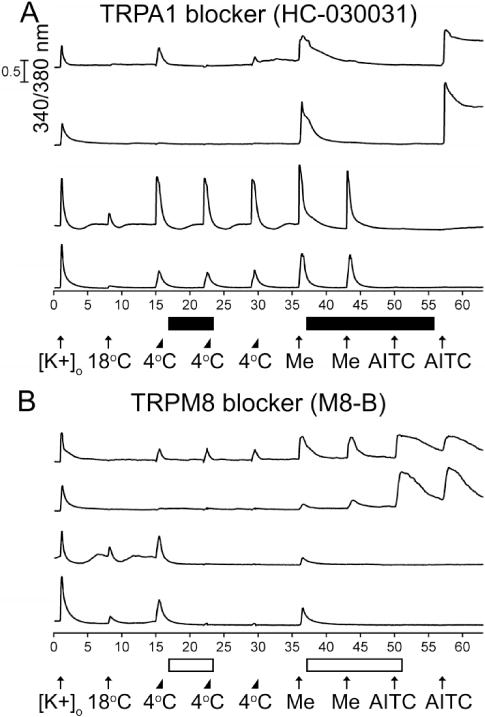
Distribution of TRPA1 and TRPM8 channels in cold-, menthol-and AITC-sensitive adult mouse DRG neurons. The experimental protocol is similar to the protocol outlined in the Figure 1 legend, with a few exceptions described below. Me is an abbreviation for 400 μM Menthol. (A) The TRPA1 antagonist HC-030031 (30 μM) selectively and completely blocked responses to cold, menthol, and AITC in 96.9% ± 0.7% of AITC-sensitive neurons (n=422). The black horizontal bars indicate when HC-030031 was present in the bath solution. (B) The TRPM8 antagonist M8-B (1 μM) selectively and completely blocked responses to cold and menthol in 95.0% ± 2.9% of menthol-sensitive and AITC-insensitive neurons (n=40). The open horizontal bars indicate when M8-B was present in the bath solution.
Figure 4.
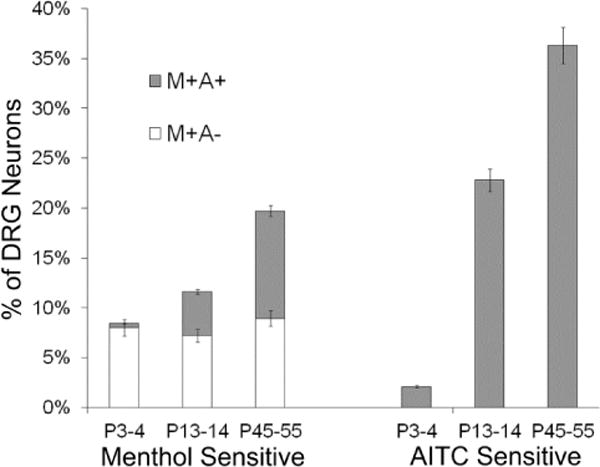
Percentages of lumbar DRG neurons from wild-type mice at different ages that responded to menthol or AITC. At postnatal day 3 or 4 (P3-4), a very low percentage of neurons responded to AITC and nearly all of the menthol-sensitive neurons were insensitive to AITC. The menthol responsive and AITC non-responsive (M+A−) neurons, as a percentage of the neuronal cell population, remained relatively constant across different ages of mice. However, the AITC-sensitive neurons increased dramatically as a percentage of the neuronal cell population during postnatal development. Accordingly, the menthol-sensitive neurons that were also AITC sensitive (M+A+ neurons) increased as a percentage of the neuronal cell population during postnatal development. The legend shown above the stacked bar graphs for menthol-sensitive neurons only applies to the menthol-sensitive neurons. For each age of mice, at least 6 experimental trials were conducted, using at least 3 independently prepared cell cultures from different mice. Total number of neurons scored for each age category was 1412 for P3-4, 1385 for P13-14, and 1465 for P45-55. Error bars are +/− SEM.
KV1.2 channel blocker increases TRPA1-dependent cold responses
It is presently unclear what circumstances or states allow TRPA1 channels to be activated by noxious cold temperature, as only a small fraction of the TRPA1-expressing neurons or HEK cells (Fig. 5, Table 1) responded to a 30-second application of noxious cold temperature in our hands. However, by increasing the duration of the cold bath application (4°C) from 30-seconds to 2-minutes, we observed a significant increase in cold responsiveness from approximately 8% to 24% of TRPA1-expressing mouse DRG neurons and from approximately 47% to 88% of human TRPA1-expressing HEK cells (Table 3). Additionally, in the presence of an antagonist of KV1.2 channels, κM-conotoxin RIIIJ (RIIIJ), the percentage of TRPA1-expressing DRG neurons that responded to a 2-minute application of 4 °C bath solution increased from approximately 24% to 49%, and responses to cold temperature in the absence of RIIIJ were amplified in the presence of RIIIJ (Fig. 6), suggesting that high expression of certain voltage-gated potassium (KV) channels (including but not limited to KV1.2) may blunt the response to cold in TRPA1-expressing neurons to a subthreshold level. These experiments with RIIIJ were conducted using neurons from TRPM8−/− mice (n=2313) to demonstrate that the additional responses obtained in the presence of RIIIJ were mediated by TRPA1 channels and not TRPM8 channels (Fig. 6). Furthermore, the large majority of these cold responses were completely blocked by the TRPA1 channel blocker, HC-030031 (Fig. 6).
Figure 5.
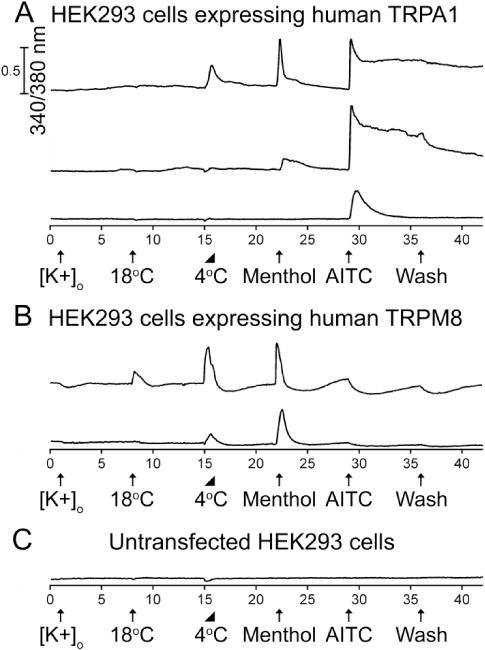
The diversity of cold, menthol, and AITC responses from HEK293 cells stably expressing either human TRPA1 or TRPM8 channels. The experimental protocol is identical to that described in the Figure 1 legend. (A) Representative traces from human TRPA1-expressing HEK cells. A subset responded to cold, menthol and AITC (47.2% ± 2.2%). Another subset responded to menthol and AITC (34.0% ± 2.1%). An additional subset only responded to AITC (14.8% ± 1.6%). See summary in Table 1. (B) Representative traces from human TRPM8-expressing HEK cells. A subset of these neurons responded to 18 °C and 4 °C bath applications, and menthol (89.8% ± 1.0%). Another subset only responded to 4 °C and menthol (9.2% ± 1.0%). See summary in Table 1. (C) Representative trace from untransfected HEK cells.
Table 3.
Frequency of cold-responses from TRPA1-expressing mouse DRG neurons and HEK293 cells stably expressing human TRPA1 for different treatment conditions.
| Treatment | % AITC-responsive Mouse DRG neurons | % HEK293 cells stably expressing human TRPA1 |
|---|---|---|
| 4°C (30 sec) | 7.9 ± 1.2 % (n=541) | 47.2 ± 2.2 % (n= 494) |
| 4°C (2 min) | 24.0 ± 3.6 % (n=1171) | 88.3 ± 2.6 % (n= 894) |
| 4°C + RIIIJ (2min) | 48.5 ± 3.2 % (n=1171) | – |
Figure 6.
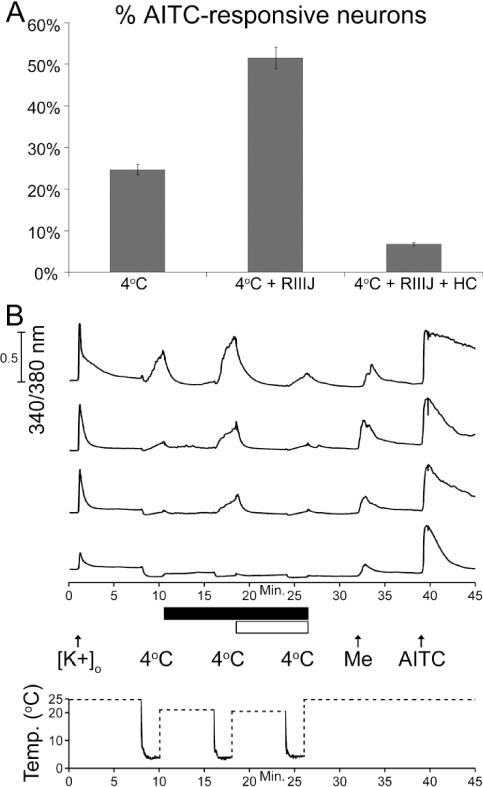
The frequency of responses to cold increased in TRPA1-expressing (AITC responsive) DRG neurons from TRPM8−/− mice when potassium channels that contain KV1.2 subunits were blocked by κM-conotoxin RIIIJ (RIIIJ). Mouse DRG neurons that responded to AITC (n=796) were challenged with a 4 °C bath solution for 2-minutes prior to six-minute incubation with 1 μM RIIIJ and then again after incubation with RIIIJ in the continued presence of RIIIJ (black bar). The 2-minute cold ramp was then repeated again in the presence of RIIIJ and the TRPA1 blocker, HC030031 (white bar). (A) The bar graph shows the percentage of AITC-responsive neurons that also responded to the 4 °C bath solution, 4 °C in the presence of RIIIJ, and 4 °C in the presence of RIIIJ and HC030031. (B) Representative calcium imaging traces and experimental protocol used to obtain the data in A. In these experiments, the bath solution was cooled to a temperature of 4 °C for 2-minutes, according to the temperature ramp shown on the time-scale of the X-axis. The temperature was monitored with a thermocoupler in the bath near the imaging field of view. Dashed lines indicate times when temperature data was not monitored in the bath because the bath solution was replaced by the room-temperature solution.
KV1.2 blocker increases TRPM8-and TRPA1-independent cold responses
In addition to TRPM8-and TRPA1-expressing cold sensitive neurons, there are noxious cold-sensitive neurons that do not express TRPM8 or TRPA1 channels (Babes et al., 2004; Munns et al., 2007; Ran et al., 2016). We have observed that approximately 7% ± 2% of DRG neurons (n=3364) that did not respond to menthol or AITC in fact responded to a 2-minute cold-ramp to 4 °C (CS M-A-neurons). Interestingly, removal of an excitability brake by KV channels that contain KV1.2 subunits increased the frequency of CS M-A-neurons to approximately 12% ± 1% (Fig. 7). The molecular identity of such cold responses remains unknown.
Figure 7.
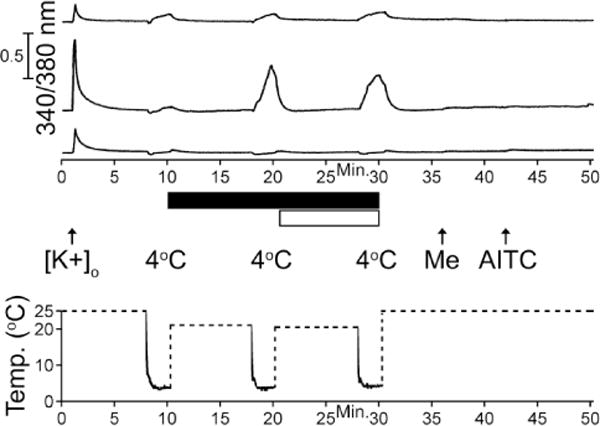
Representative traces of cold-sensitive menthol-and AITC-unresponsive (CS M−A−) neurons. The experimental protocol shown below traces is similar to the protocol in Figure 6, where the cold ramp was applied for 2-minutes with and without κM-RIIIJ, a selective blocker of KV1.2 containing channels (black bar), and HC-030031, a TRPA1 blocker (white bar). About 7% ± 2% of K+ responsive neurons (n=3364) that were unresponsive to menthol or AITC responded to a 2-minute cold ramp to 4 °C as illustrated by the top trace. In the presence of κM-RIIIJ, the frequency of CS M−A− neurons increased to approximately 12% ± 1% of DRG neurons, as shown in the middle trace, while the majority of menthol-and AITC-unresponsive neurons remained unaffected as shown in the bottom trace. The temperature was monitored with a thermocoupler in the bath near the imaging field of view. Dashed lines indicate times when temperature data was not monitored in the bath because the bath solution was rep laced by the room-temperature solution.
TRPA1 expression levels influence cold-sensitivity of TRPA1-expressing neurons
Another factor that could contribute to the cold sensitivity of TRPA1-expressing neurons is functional expression of TRPA1 channels. We have observed that cold-sensitive AITC-sensitive (CS A+) neurons usually exhibit greater menthol and AITC responses than cold-insensitive AITC-sensitive (CI A+) neurons (e.g., AITC responses measured by peak height and time for cytosolic calcium to return to baseline, Fig. 1). Therefore, we hypothesized that CS A+ neurons may express more TRPA1 than CI A+ neurons. To test this hypothesis, we performed single-cell RT-qPCR on cells of interest and determined relative quantities (RQ) of TRPA1 cDNA molecules in CS A+ and CI A+ neurons. Indeed, we find that CS A+ neurons expressed, on an average, ~2.7 fold more TRPA1 transcripts, as compared to CI A+ neurons (Fig. 8A). Furthermore, ACq of TRPA1 in each neuron positively correlated with AITC-elicited Ca2+ responses (Fig. 8B). Most of the neurons that did not respond to AITC also did not have any detectable TRPA1 transcripts but had β-actin transcripts (Fig. 8C), and samples without cells (no-template controls) did not have transcripts of either TRPA1 or β-actin (Fig. 8C). Lastly, the expected size of TRPA1 (~168 bp) and β-Actin (~460 bp) transcripts was confirmed by gel electrophoresis as shown in Fig. 8C. Hence, functional expression levels of TRPA1 channels also play an important role in determining whether a response to cold reaches threshold for detection in TRPA1-expressing neurons.
Figure 8.
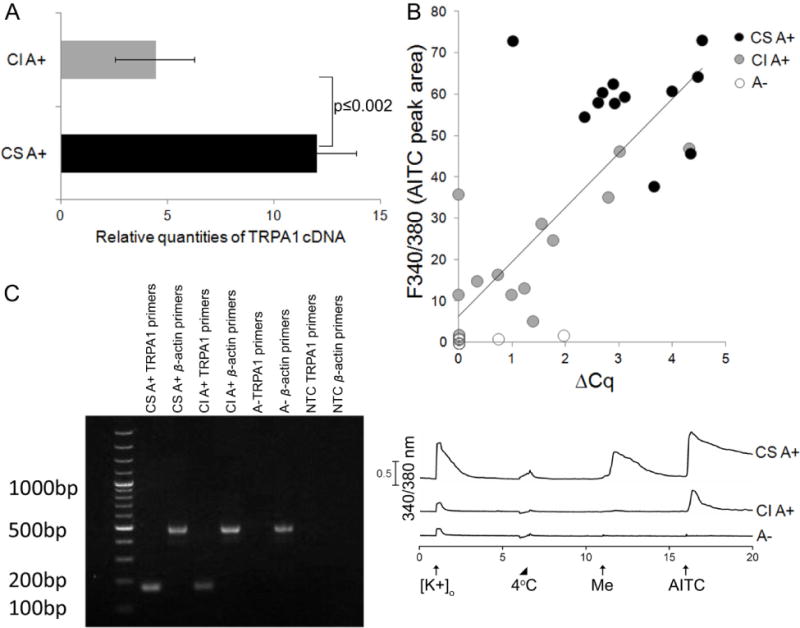
Single-cell RT-qPCR for TRPA1 transcript levels. (A) Relative quantities of TRPA1 cDNA molecules for cold-sensitive AITC-responsive (CS A+) and cold-insensitive AITC-responsive (CI A+) neurons was determined for comparison (CS A+, n=12; CI A+, n=12; p-value=0.002 by Welch Two Sample t test). (B) AITC-elicited Ca2+ responses (peak area over 4-minutes) obtained from calcium imaging experiments positively correlated with ΔCq of TRPA1 for each neuronal cell. This dataset includes CS A+ (black circle, n=12), CI A+ (gray circle, n=12), and AITC-unresponsive neurons abbreviated A− (white circle, n=12). Comparing ΔCq values with AITC response area in the entire data set, there exists a strong correlation (Pearson correlation = 0.81, p-value=2.943e-09). (C) Representative gel image on left showing end product of single-cell RT-qPCR for cells (CS A+, CI A+, A−) and no cell (NTC) samples with calcium imaging response profile shown on right. Expected size of cDNA fragments for TRPA1 was ~168 bp and for β-actin was ~460 bp.
Discussion
The experimental results obtained with DRG neurons from TRPA1−/− mice, TRPM8−/− mice, and wild-type mice, using selective antagonists of TRPA1 and TRPM8 channels, as well as HEK293 cells stably expressing human TRPA1 or TRPM8 channels, cumulatively indicate that responses to cold, menthol and AITC in TRPA1-expressing cells are all mediated by TRPA1 channels. Our finding is consistent with previous reports of TRPA1-dependent cold-and menthol-responses in sensory neurons of trigeminal ganglia (Karashima et al., 2009) and nodose ganglia (Fajardo et al., 2008). Our results also indicate that TRPA1 channels and TRPM8 channels are predominantly expressed in different, largely non-overlapping subsets of mouse DRG neurons, a conclusion that is also supported by recent single-cell RNA sequencing studies of mouse DRG neurons (Usoskin et al., 2014; Li et al., 2015). Therefore, the clear implication of the results is that TRPA1 channels, independent of TRPM8 channels, play a role in the detection of noxious-cold stimuli. In this paper, we have also demonstrated that expression levels of TRPA1 channels and voltage-gated potassium channels (including those that contain KV1.2 subunits) are among the factors that determine whether a TRPA1-expressing neuron responds overtly to noxious cold temperatures in calcium-imaging assays.
In a prior study, we identified putative cold thermosensors, such as those shown in Figures 1B and 2B (which respond to innocuous cold temperatures), and correctly concluded that those neurons express TRPM8 channels, but not TRPA1 channels, because they responded to the application of menthol but not to AITC. However, we also identified putative cold nociceptors (which respond to noxious cold temperatures, but not innocuous cold temperatures), such as those shown in Figures 1A and 2A, but incorrectly concluded that those neurons express both TRPM8 and TRPA1 channels because they responded to both menthol and AITC (Teichert et al. 2012a). However, in this study, we demonstrate conclusively that responses to cold, menthol and AITC are all mediated by TRPA1 channels in the putative cold nociceptors shown in Figures 1A and 2A.
In the same prior study, we demonstrated that the putative cold nociceptors which express TRPA1 channels (see Figs. 1A and 2A) also co-express the voltage-gated Na+ channel, NaV1.8 (Teichert et al. 2012a), which has been shown to be essential for the sensation of cold pain (Zimmermann et al., 2007), but the low-threshold cold-thermosensor neurons (see Figs. 1B and 2B) did not express NaV1.8 (Teichert et al. 2012a). Cumulatively, these data support a role for TRPA1 channels in the sensation of cold-induced pain.
Cold appears to be a weaker agonist of TRPA1 channels than menthol and AITC, which is reflected in the magnitude and frequency of cold responses in AITC-sensitive neurons and TRPA1-expressing HEK cells (Figs. 1A, 2A, 3A, 5A and Tables 1, 2). Cold temperature elicits overt responses (above threshold) in AITC-sensitive (CS A+) neurons that express more TRPA1 transcripts than CI A+ neurons (Fig. 8). Others have hypothesized that TRPA1 expression levels may contribute to observed differences in cold sensitivity among TRPA1-expressing neurons (Karashima et al., 2009). In this study, we have demonstrated it empirically. Moreover, our findings are consistent with previous reports that claim cold-temperature is a weaker and slower activator of TRPA1 channels at the molecular level (Sawada et al., 2007; Karashima et al., 2009).
The weak and slow activation of TRPA1 by cold in some AITC-sensitive neurons can further be blunted by the presence of voltage-gated potassium channels to a sub-threshold level for detection (Fig. 6). KV channels are known to blunt TRPM8-mediated cold responses (Madrid et al., 2009; Teichert et al., 2014) but their contribution to the cold-sensitivity of TRPA1-expressing neurons has not been reported previously. Hence, the combination of relatively low TRPA1 expression levels, relatively high KV-channel expression levels and the relatively weak activation of TRPA1 channels by cold temperatures (compared to AITC) largely explain why most TRPA1-expressing neurons do not respond overtly to cold in calcium-imaging assays.
Even though our results support the hypothesis that TRPA1 channels are activated by cold temperatures, our data do not rule out the possibilities of indirect activation or sensitization of the TRPA1 channels by increases in reactive oxygen species (ROS) and intracellular calcium that may be elicited by cold temperatures (Zurborg et al., 2007; Andersson et al., 2008; del Camino et al., 2010; Miyake et al., 2016). These molecular mechanistic details are beyond the scope of our study but have been addressed elsewhere (Karashima et al., 2009; Moparthi et al., 2014; Miyake et al., 2016). Hence, the cold-sensitivity of TRPA1-expressing neurons is complex and dependent on multiple factors.
The significance of these contributing factors is not just limited to the normal physiology of cold-sensation but extends to its pathophysiology such as cold-allodynia and-hypersensitivity. There is an increase in TRPA1 expression and reduction in KV channel expression during cold-allodynia (Descoeur et al., 2011; Zhao et al., 2012). Also, ROS formation and cytokine release during and following an injury can further sensitize TRPA1 channels, resulting in cold-hypersensitivity (McNamara et al., 2007; del Camino et al., 2010; Bautista et al., 2013; Eberhardt et al., 2014). It is possible that the combination of these mechanisms results in the pain caused by cold via TRPA1. Therefore, therapy for cold-induced neuropathic pain may benefit by targeting multiple mechanisms, including TRPA1 channels and Kv channels.
Highlights.
Three distinct subclasses of sensory neurons mediate cold responses
Cold responses in TRPA1-expressing neurons depend on TRPA1 and not TRPM8 channels
Cold-sensitive TRPA1 neurons express more TRPA1 than cold-insensitive TRP A1 neurons
KV1.2 channels attenuate cold-sensitivity of TRPA1-expressing neurons
Acknowledgments
We are grateful to Dr. Rocio K. Finol-Urdaneta for initial training and continuous advice with single-cell RT-qPCR. We thank Dr. Christopher A. Reilly and Dr. Cassandra Rice, Department of Pharmacology and Toxicology, University of Utah, for providing us the HEK293 cell lines that stably express either TRPM8 or TRPA1 channels and for helpful suggestions regarding our manuscript. We thank Dr. Jean Rivier for peptide synthesis of кM-conotoxin RIIIJ, and My Huynh for assistance in preparing figures.
Funding
This work was supported by the National Institute of General Medical Sciences Grant GM48677.
Abbreviations
- AITC
Allyl isothiocyanate
- TRP
Transient receptor potential channel
- KV
Voltage-gated potassium channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agresti A. Categorical data analysis. Vol. 11. New York: Wiley; 1990. pp. 230–235. [Google Scholar]
- Andersson DA, Gentry C, Moss S, Bevan S. Transient Receptor Potential A1 Is a Sensory Receptor for Multiple Products of Oxidative Stress. J Neurosci. 2008;28:2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babes A, Zorzon D, Reid G. Two populations of cold-sensitive neurons in rat dorsal root ganglia and their modulation by nerve growth factor. Eur J Neurosci. 2004;20:2276–2282. doi: 10.1111/j.1460-9568.2004.03695.x. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt S-E, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Pellegrino M, Tsunozaki M. TRPA1: A gatekeeper for inflammation. Annu Rev Physiol. 2013;75:181–200. doi: 10.1146/annurev-physiol-030212-183811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt S-E, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448:204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- Caspani O, Heppenstall Pa. TRPA1 and cold transduction: an unresolved issue? J Gen Physiol. 2009;133:245–249. doi: 10.1085/jgp.200810136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Kang D, Xu J, Lake M, Hogan JO, Sun C, Walter K, Yao B, Kim D. Species differences and molecular determinant of TRPA1 cold sensitivity. Nat Commun. 2013;4:2501. doi: 10.1038/ncomms3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero-morales JF, Gracheva EO, Julius D. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc Natl Acad Sci U S A. 2011;108:1184–1191. doi: 10.1073/pnas.1114124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deering-Rice CE, Romero EG, Shapiro D, Hughen RW, Light AR, Yost GS, Veranth JM, Reilly Ca. Electrophilic components of diesel exhaust particles (DEP) activate transient receptor potential ankyrin-1 (TRPA1): a probable mechanism of acute pulmonary toxicity for DEP. Chem Res Toxicol. 2011;24:950–959. doi: 10.1021/tx200123z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook Ca, Petrus MJ, Zhao M, D’Amours M, Deering N, Brenner GJ, Costigan M, Hayward NJ, Chong Ja, Fanger CM, Woolf CJ, Patapoutian A, Moran MM. TRPA1 contributes to cold hypersensitivity. J Neurosci. 2010;30:15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Descoeur J, Pereira V, Pizzoccaro A, Francois A, Ling B, Maffre V, Couette B, Busserolles J, Courteix C, Noel J, Lazdunski M, Eschalier A, Authier N, Bourinet E. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol Med. 2011;3:266–278. doi: 10.1002/emmm.201100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Murray AN, Mathur J, Earley TJ, Petrus MJ, Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54:371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- Eberhardt M, et al. H2S and NO cooperatively regulate vascular tone by activating a neuroendocrine HNO-TRPA1-CGRP signalling pathway. Nat Commun. 2014;5:4381. doi: 10.1038/ncomms5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid SR, Crown ED, Moore EL, Liang HYa, Choong KC, Dima S, Henze Da, Kane Sa, Urban MO. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory-and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo O, Meseguer V, Belmonte C, Viana F. TRPA1 Channels Mediate Cold Temperature Sensing in Mammalian Vagal Sensory Neurons: Pharmacological and Genetic Evidence. J Neurosci. 2008;28:7863–7875. doi: 10.1523/JNEUROSCI.1696-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes T, Wood JN. Mechanisms of cold pain. Channels. 2007:154–160. doi: 10.4161/chan.4692. [DOI] [PubMed] [Google Scholar]
- Gibb S, Strimmer K. MALDIquant: a versatile R package for the analysis of mass spectrometry data. Bioinformatics. 2012;28:2270–2271. doi: 10.1093/bioinformatics/bts447. [DOI] [PubMed] [Google Scholar]
- Jabba S, Goyal R, Sosa-Pagán JO, Moldenhauer H, Wu J, Kalmeta B, Bandell M, Latorre R, Patapoutian A, Grandl J. Directionality of temperature activation in mouse TRPA1 ion channel can be inverted by single-point mutations in ankyrin repeat six. Neuron. 2014;82:1017–1031. doi: 10.1016/j.neuron.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt S, Bautista DM, Chuang H, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. J Neurosci. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y, Talavera K, Everaerts W, Janssens A, Kwan KY, Vennekens R, Nilius B, Voets T. TRPA1 acts as a cold sensor in vitro and in vivo. Proc Natl Acad Sci U S A. 2009;106:1273–1278. doi: 10.1073/pnas.0808487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010;150:340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath Ma, Christensen AP, Zhang D-S, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Li C-L, Li K-C, Wu D, Chen Y, Luo H, Zhao J-R, Wang S-S, Sun M-M, Lu Y-J, Zhong Y-Q, Hu X-Y, Hou R, Zhou B-B, Bao L, Xiao H-S, Zhang X. Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. 2015:1–20. doi: 10.1038/cr.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolignier XS, Gkika D, Andersson XD, Leipold E, Vetter XI, Viana F, Noël XJ, Busserolles XJ, Université C, Auvergne U. New Insight in Cold Pain : Role of Ion Channels, Modulation, and Clinical Perspectives. 2016;36:11435–11439. doi: 10.1523/JNEUROSCI.2327-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid R, de la Peña E, Donovan-Rodriguez T, Belmonte C, Viana F. Variable threshold of trigeminal cold-thermosensitive neurons is determined by a balance between TRPM8 and Kv1 potassium channels. J Neurosci. 2009;29:3120–3131. doi: 10.1523/JNEUROSCI.4778-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madrid R, Donovan-Rodríguez T, Meseguer V, Acosta MC, Belmonte C, Viana F. Contribution of TRPM8 channels to cold transduction in primary sensory neurons and peripheral nerve terminals. J Neurosci. 2006;26:12512–12525. doi: 10.1523/JNEUROSCI.3752-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong Ja, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meseguer V, Karashima Y, Talavera K, D’Hoedt D, Donovan-Rodriguez T, Viana F, Nilius B, Voets T. Transient Receptor Potential Channels in Sensory Neurons Are Targets of the Antimycotic Agent Clotrimazole. J Neurosci. 2008;28:576–586. doi: 10.1523/JNEUROSCI.4772-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T, Nakamura S, Zhao M, So K, Inoue K, Numata T, Takahashi N, Shirakawa H, Mori Y, Nakagawa T, Kaneko S. Cold sensitivity of TRPA1 is unveiled by the prolyl hydroxylation blockade-induced sensitization to ROS. Nat Commun. 2016;7:1–10. doi: 10.1038/ncomms12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moparthi L, Survery S, Kreir M, Simonsen C, Kjellbom P, Högestätt ED, Johanson U, Zygmunt PM. Human TRPA1 is intrinsically cold-and chemosensitive with and without its N-terminal ankyrin repeat domain. Proc Natl Acad Sci U S A. 2014;111:16901–16906. doi: 10.1073/pnas.1412689111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns C, AlQatari M, Koltzenburg M. Many cold sensitive peripheral neurons of the mouse do not express TRPM8 or TRPA1. Cell Calcium. 2007;41:331–342. doi: 10.1016/j.ceca.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Palkar R, Lippoldt EK, McKemy DD. The molecular and cellular basis of thermosensation in mammals. Curr Opin Neurobiol. 2015;34:14–19. doi: 10.1016/j.conb.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson Da, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- Proudfoot CJ, Garry EM, Cottrell DF, Rosie R, Anderson H, Robertson DC, Fleetwood-Walker SM, Mitchell R. Analgesia mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr Biol. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- Ran C, Hoon MA, Chen X. The coding of cutaneous temperature in the spinal cord. Nat Neurosci. 2016;19:1201–1209. doi: 10.1038/nn.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y, Hosokawa H, Hori A, Matsumura K, Kobayashi S. Cold sensitivity of recombinant TRPA1 channels. Brain Res. 2007;1160:39–46. doi: 10.1016/j.brainres.2007.05.047. [DOI] [PubMed] [Google Scholar]
- Smith NJ, Hone AJ, Memon T, Bossi S, Smith TE, Mcintosh JM, Olivera BM, Teichert RW. Comparative functional expression of nAChR subtypes in rodent DRG neurons. Front Cell Neurosci. 2013;7:1–11. doi: 10.3389/fncel.2013.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ståhlberg A, Rusnakova V, Forootan A, Anderova M, Kubista M. RT-qPCR work-flow for single-cell data analysis. 2013;59:80–88. doi: 10.1016/j.ymeth.2012.09.007. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson Da, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Talavera K, Nilius B, Voets T. Neuronal TRP channels: thermometers, pathfinders and life-savers. Trends Neurosci. 2008;31:287–295. doi: 10.1016/j.tins.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Teichert RW, Memon T, Aman JW, Olivera BM. Using constellation pharmacology to define comprehensively a somatosensory neuronal subclass. Proc Natl Acad Sci U S A. 2014;111:2319–2324. doi: 10.1073/pnas.1324019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert RW, Raghuraman S, Memon T, Cox JL, Foulkes T, Rivier JE, Olivera BM. Characterization of two neuronal subclasses through constellation pharmacology. Proc Natl Acad Sci U S A. 2012a;109:12758–12763. doi: 10.1073/pnas.1209759109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert RW, Smith NJ, Raghuraman S, Yoshikami D, Light AR, Olivera BM. Functional profiling of neurons through cellular neuropharmacology. Proc Natl Acad Sci U S A. 2012b;109:1388–1395. doi: 10.1073/pnas.1118833109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2014;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- Vriens J, Nilius B, Voets T. Peripheral thermosensation in mammals. Nat Rev Neurosci. 2014;15:573–589. doi: 10.1038/nrn3784. [DOI] [PubMed] [Google Scholar]
- Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19:251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A. Identification of Transmembrane Domain 5 as a Critical Molecular Determinant of Menthol Sensitivity in Mammalian TRPA1 Channels. 2008;28:9640–9651. doi: 10.1523/JNEUROSCI.2772-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Isami K, Nakamura S, Shirakawa H, Nakagawa T, Kaneko S. Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol Pain. 2012;8:55. doi: 10.1186/1744-8069-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN, Reeh PW. Sensory neuron sodium channel Nav1.8 is essential for pain at low temperatures. Nature. 2007;447:855–858. doi: 10.1038/nature05880. [DOI] [PubMed] [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]


