Summary
Neutrophils are the first responders to infection and injury and are critical for antimicrobial host defense. Through the generation of reactive oxidants, activation of granular constituents and neutrophil extracellular traps, neutrophils target microbes and prevent their dissemination. While these pathways are beneficial in the context of trauma and infection, their off-target effects in the context of tumor are variable. Tumor-derived factors have been shown to reprogram the marrow, skewing toward the expansion of myelopoiesis. This can result in stimulation of both neutrophilic leukocytosis and the release of immature granulocytic populations that accumulate in circulation and in the tumor microenvironment. While activated neutrophils have been shown to kill tumor cells, there is growing evidence for neutrophil activation driving tumor progression and metastasis through a number of pathways, including stimulation of thrombosis and angiogenesis, stromal remodeling, and impairment of T cell-dependent anti-tumor immunity. There is also growing appreciation of neutrophil heterogeneity in cancer, with distinct neutrophil populations promoting cancer control or progression. In addition to the effects of tumor on neutrophil responses, anti-neoplastic treatment, including surgery, chemotherapy, and growth factors, can influence neutrophil responses. Future directions for research are expected to result in more mechanistic knowledge of neutrophil biology in the tumor microenvironment that may be exploited as prognostic biomarkers and therapeutic targets.
Keywords: cancer, inflammation, injury, neutrophils, wound repair
1 INTRODUCTION
In the late 19th century, Elie Metchnikoff made seminal discoveries pertaining to phagocyte biology. He first discovered the role of phagocytes through his investigation into the inflammatory responses of starfish larvae when impaled with rosebud thorns. He observed that white blood cells from various animals were attracted to bacteria, followed by phagocytosis and killing of pathogens. These studies remain the foundation for our understanding of innate immune responses to infection. There has been an extraordinary expansion in our understanding of neutrophils at the cellular and molecular level. We have learned about bacterial products (e.g. formylated peptides) and host-derived chemoattractant molecules that attract neutrophils to sites of infection and injury. Knowledge has been gained on the molecular mechanisms by which neutrophils sense microbial products and traffic to sites of infection. Key pathways for neutrophil-mediated host defense (e.g. NADPH oxidase and neutrophil granular constituents) have been elucidated. In addition, we have learned about distinct modes of neutrophil death and termination of neutrophilic inflammation.
Neutrophils are the first responders to infection and injury. Pathogen motifs and endogenous products of necrosis (damage-associated molecular patterns; DAMPs) activate similar pathogen recognition receptors and downstream signaling in neutrophils. From a teleological standpoint, recruitment and activation of neutrophils by DAMPs is likely to be of benefit following traumatic injury. Advanced cancer has been likened to a wound that does not heal. Conditions within the tumor microenvironment—hypoxia, nutrient starvation, cellular proliferation, and necrosis—lead to the release of DAMPs that can recruit and activate neutrophils. While beneficial in the context of fighting infection, neutrophilic inflammation in the tumor microenvironment has less predictable and less understood effects. Neutrophils have the capacity to kill tumor cells. However, specific neutrophil responses may promote tumor progression through a number of signaling pathways, including interaction with tumor, inflammatory, and stromal cells in the tumor microenvironment.
A major theme of our review will be to understand neutrophilic inflammation in cancer in the context of a persistent wound. We explore the roles of neutrophils in antimicrobial host defense and how those responses are co-opted in the tumor microenvironment in ways that can promote tumor progression. Tumor-derived factors (e.g. granulocyte-colony stimulating factor; G-CSF) can expand granulopoiesis, leading to an increased accumulation of mature and immature granulocytic populations in circulation, in the primary tumor, and in distant organs where they can create a “premetastatic niche” that facilitates tumor seeding. The interactions between neutrophils and platelets while trafficking to tumor and their potential effects on tumor cell signaling are examined. In addition, we discuss the relationship between activated neutrophils and T cell signaling, including the potential for mature neutrophils to suppress T cell responses in the tumor microenvironment. There is also a growing appreciation for heterogeneity among granulocytic cell populations in the tumor micro-environment. This heterogeneity relates to their maturation status, transcriptome profiles, and functional roles, which can either be pro-tumorigenic or favor tumor control. We consider the effects of cancer therapy on neutrophils and how neutrophils may affect tumor responses. Finally, we propose future directions for research that targets neutrophils as novel strategies to enhance immunotherapy for cancer.
2 IN THE TUMOR MICROENVIRONMENT: THE TWO FACES OF INFLAMMA TION
The presence of white cells within tumors was observed in the 19th century by Rudolf Virchow and raised the notion that inflammation may play a role in cancer. It is now recognized that inflammation plays critical roles at multiple stages of cancer: initiation, growth, metastasis, and response to therapy. While genetic damage is required for tumor initiation, the inflammatory responses may contain or eradicate the tumor or be the “fuel that feeds the flames”.1 Nuclear factor kappa B (NF-κB), a critical driver of inflammation, can promote inflammation-associated cancer,2 but acts in a cell type-specific manner by influencing survival genes in the tumor and pro-inflammatory responses in the tumor microenvironment.3 Importantly, immune subsets that promote tumor progression possess plasticity and can be therapeutically depleted or re-programmed. 4
It is now well-established that cytotoxic T lymphocytes (CTL) are critical effectors of anti-tumor immunity. We observed extraordinary advances in cancer immunotherapy based on strategies that augment anti-tumor CTL function, including the use of checkpoint inhibitors, genetically modified T cells, and vaccine-based approaches. In 2013, Science selected cancer immunotherapy as the “Breakthrough of the Year.” Harnessing natural killer cells and antibody-dependent anti-tumor immunity are additional examples of promising research. With these advances, it becomes even more important to understand immune responses that can enhance or obstruct durable anti-tumor immunity.
There are numerous obstacles to anti-tumor immunity, including low immunogenicity of cancer-specific antigens, poor trafficking of effector T cells to the tumor microenvironment, and induction of immunosuppressive pathways. Tumor-associated macrophages (TAMs) possess a similar phenotype to the alternatively activated (M2) macrophages involved in wound healing, and also suppress T cells.5–8 B7-H4-expressing macrophages constitute a specific TAM population that suppress tumor-specific T cell immunity, and are a target for immunotherapy.5,9 Regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and immune checkpoint molecules (e.g. CTLA-4 and PD-1) are additional examples of immunosuppressive pathways that accumulate in the tumor microenvironment and impede T cell immunity. Seen in this light, both mature neutrophils and immature granulocytic cells are important components of the tumor microenvironment that can influence the balance between tumor control and escape. Neutrophilic recruitment and activation can have broad effects on tumor cells and the microenvironment, which include direct cellular injury from release of oxidants and granular constituents, remodeling of the extracellular matrix, release of pro-angiogenic products, and cross-signaling to other inflammatory cells and tumor stromal constituents. Granulocytic cells can also affect tumor progression independently of T cell immunity. Pekarek et al.10 showed that granulocyte depletion inhibited the growth of tumor cells in nude mice, which are deficient in cellular immunity. However, one of the most translationally important effects of tumor-associated neutrophils (TANs) relates to their effect on CTL responses. To understand the mechanisms by which neutrophils influence the tumor microenvironment, it is of value to understand neutrophil biology in the context of antimicrobial host defense and wound repair.
3 THE GOOD AND B AD OF WOUND REPAIR RESPONSES
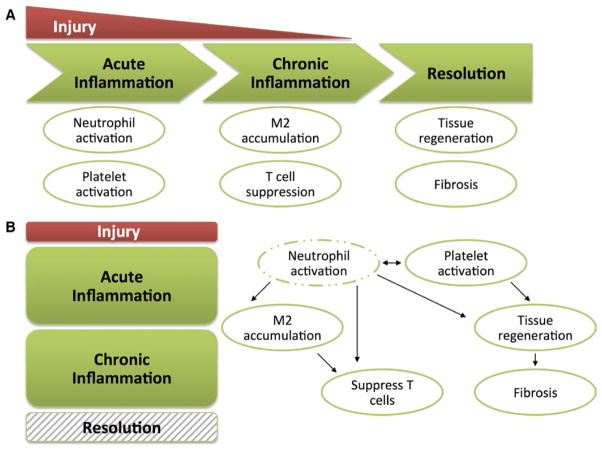
Acute neutrophilic inflammation and wound repair responses evolved to act in a concerted and stepwise manner to target infections and to promote repair of damaged tissue (Fig. 1A). The innate immune system is rapidly activated by injury, such as trauma or bacterial infection. In the first stage, neutrophils and platelets are activated and recruited to the site of injury. Following elimination of the acute insult (e.g. spontaneous drainage of an abscess or removal of a foreign body), the next stage of wound healing involves a transition to a less injurious monocytic inflammatory response. Macrophages help to clear neutrophilic inflammation through efferocytosis (removal of dying cells by phagocytosis). In addition, efferocytosis of apoptotic neutrophils by macrophages can limit neutrophilic inflammation by suppression of IL-23/IL-17-dependent granulopoiesis.11 The next stage involves resolution of the wound, characterized by repair, regeneration, remodeling of tissue, and fibrosis. M2 macrophages are thought to facilitate wound healing by suppressing inflammatory responses and producing pro-angiogenic factors. In contrast, pathological wound healing is characterized by a persistent and disorganized inflammatory response in which neutrophil recruitment and injury are ongoing and regenerative responses are ineffective (Fig. 1B). In this review, we will discuss neutrophil biology in the tumor microenvironment in the context of pathological wound healing.
FIGURE 1.
The good and bad of wound repair mechanisms. (A) When injury (e.g. traumatic injury or acute infection) occurs, the inflammatory response follows an ordered transition during normal wound healing. The first stage of acute inflammation involves neutrophil and platelet recruitment and activation, followed by chronic inflammation involving M2 macrophage accumulation and T cell suppression. Finally, the injurious stimulus is alleviated (e.g. clearance of infection) and resolution of inflammation occurs. Resolution includes tissue regeneration, which requires growth factors, angiogenesis, and fibrosis. (B) Pathologic wound healing is associated with a persistent and disordered inflammatory response. Examples include persistent diabetic wounds and cancer. In these scenarios, neutrophil activation is ongoing, which drives the rest of the inflammatory pathways and prevents resolution from occurring. In the context of cancer, pathologic wound healing can drive tumor progression through a number of pathways that are concurrently activated, including thrombosis and angiogenesis, matrix remodeling, release of growth factors, and T cell suppression
Thirty years ago, Dvorak12 referred to cancer as a wound that does not heal, and noted the similarities between tumor stroma generation and wound healing responses. Indeed, the tumor microenvironment is characterized by persistent injury from a number of causes (e.g. ischemia, nutrient starvation, and inflammation) that co-opts the normal wound healing responses in ways that can promote tumor spread. Pathological wound healing responses result in activation of pro-angiogenic and tissue remodeling pathways that may facilitate metastasis. In addition to these shared features with persistent wounds unrelated to cancer (e.g. from ischemia or infection), cancer has its own unique features, including the release of tumor-derived factors that can influence marrow reprogramming and neutrophil recruitment and activation.
4 GRANULOCYTIC EXP ANSION DURING CANCER
Advanced cancer is associated with an expansion of the granulocytic compartment, including both mature neutrophils and immature granulocytic cells. Elevations in circulating neutrophils, the neutrophil to lymphocyte ratio, and tumor-infiltrating neutrophils have been associated with poor outcomes in several cancers, including hepatocellular, head and neck, lung, and gastrointestinal malignancies.13–20 In patients with advanced ovarian cancer, the pretreatment circulating neutrophil count 21 and the neutrophil to lymphocyte ratio 22 correlated with poor outcomes.
The potential causes for expansion and increased circulation of neutrophils in advanced cancer are multi-factorial. A hallmark of advanced cancer is cellular necrosis, which is associated with the release of DAMPs that may stimulate neutrophilic leukocytosis. In addition, tumor-derived cytokines and growth factors can promote myeloid differentiation. Wu et al.23 showed that circulating hematopoietic stem and progenitor cells (HSPCs) from patients with solid tumors had “myeloid-biased differentiation”. Tumor-derived G-CSF, granulocyte-macrophage-colony stimulating factor (GM-CSF), and interleukin-6 (IL-6) promoted myeloid differentiation of HSPCs, and circulating myeloid precursors (granulocyte-macrophage progenitor; GMP-positive) correlated with advanced disease and were enriched in tumor.23 Casbon et al.24 showed that tumor-derived G-CSF resulted in reprogramming of hematopoiesis in bone marrow that stimulated granulocyte expansion. This reprogramming occurred in early stage disease and resulted in systemic expansion of circulating neutrophils that were T cell suppressive.24 Strauss et al.25 identified the retinoic-acid-related orphan receptor 1 (RORC1) as a key pathway driving tumor-stimulated myelopoiesis in response to colony stimulating factors. This myeloid bias is not unique to cancer. Rather, it is a hallmark of infection, trauma, and other causes of systemic injury. The unique feature of cancer relates to tumor-derived factors that are a persistent stimulus for marrow reprogramming skewed toward myeloid differentiation.
Activated neutrophils can promote metastasis by stimulation of tumor invasion at the primary site. In addition, there is growing recognition of a “premetastatic phase” in which tumor-derived factors stimulate hematopoietic mobilization and tissue-specific responses that enhance metastasis by preparing a distant site for metastatic seeding.26–28 Angiogenesis, which is stimulated by injury and activated myeloid cells, plays a critical role in metastasis. Bv8 proteins (named for the frog Bombina variegata), also known as prokineticin 1 (Prok1) and prokineticin 2 (Prok2), are upregulated by G-CSF and mobilize myeloid cells from marrow and promote angiogenesis in tumors.29 Anti-Bv8 treatment suppressed circulating and tumor-infiltrating myeloid cells and angiogenesis, and reduced tumor growth in mice.29 The benefit of anti-Bv8 on tumor vascularization and growth occurred at early stages of tumor burden.30 In addition, anti-G-CSF or anti-Bv8 antibodies significantly reduced metastasis in tumor-bearing mice.31 These results point to Bv8-expressing granulocytic cells mobilized by G-CSF driving metastasis and as potential therapeutic targets to prevent metastasis at early stages of disease. Wculek et al.32 showed in orthotopic mammary tumor-bearing mice that neutrophils accumulated in the lung before cancer cells and their numbers increased during metastatic progression. Neutrophils in the premetastatic lung augmented the tumorigenic potential of cancer cells in vivo and in vitro, and the pro-metastatic effect of neutrophils was dependent on leukotriene generation.32
Although a growing body of literature points to activated neutrophils driving tumor progression, this is not a universal finding. Granot et al.33 showed that neutrophil accumulation in the lung protected mice from mammary tumor metastasis, an effect that was mediated by reactive oxidant generation and tumor-secreted CCL2. Blaisdell et al.34 identified a protective role for neutrophils in a mouse model of PTEN-deficient uterine cancer. In this model, neutrophils were recruited by the hypoxic tumor microenvironment, and their infiltration led to detachment of tumor cells from the basement membrane, and a reduction in tumor growth and metastasis.34 The potential for TANs to be friends or foes in cancer35 is a recurrent theme of this review, and emphasizes the importance of context-dependent factors in the tumor microenvironment and neutrophil heterogeneity that modulate the interactions between neutrophils and cancer.
MDSCs are a subset of heterogeneous myeloid cells, which have been shown to expand in response to tumor-derived factors, and enhance tumor progression by abrogating T cell immunity and stimulating pro-angiogenic and tissue remodeling responses.36 The most important criterion for defining an MDSC population relates to their ability to suppress stimulated T cell activation. However, this hallmark feature is not unique to MDSCs, since mature neutrophils can acquire a suppressive phenotype. In addition, MDSCs are not unique to cancer. Granulocytic populations mimicking MDSCs based on surface marker expression and capable of suppressing T cell responses have been observed in patients with sepsis.37,38
MDSCs have been classified as monocytic and granulocytic based on surface marker expression. These broad categorizations of MDSCs underestimate their heterogeneity regarding tumor-specific factors that promote their expansion or their prognostic significance in circulation and in the tumor microenvironment.39 Granulocytic MDSCs in mice are typically defined based on a combination of myeloid (CD11b+) and granulocytic (Ly6G+) markers and negative or low expression of the monocytic marker, Ly6C.40,41 In humans, there remains a lack of agreement on the optimal markers for MDSCs. A recent interim study to establish consensus criteria for defining MDSCs identified as many as ten putative MDSC populations from peripheral blood mononuclear cells of normal volunteers.42 Human granulocytic MDSCs were defined as a population of cells resembling granulocytes that express the granulocytic markers, CD15 and CD66b, and do not express CD14.42 This study showed high inter-laboratory variability in putative MDSC populations, especially the granulocytic subsets.42
Another feature commonly associated with MDSCs relates to their immaturity. However, the identification of immunosuppressive mature neutrophils highlights the notion that both the maturational status of granulocytic cells and the inflammatory context can influence their suppressive phenotype. While neutrophils, macrophages, and dendritic cells (DCs) are examples of mature myeloid cells, MDSCs have been proposed to develop from a block in myeloid differentiation. Consistent with this notion, Solito et al.43 identified a human promyelocytic-like population with T cell suppressive activity that was phenotypically similar to MDSCs present in the blood of patients with advanced solid tumors, and correlated with worse prognosis. Very recent evidence points to a key role for HSPCs in promoting tumor expansion through effects on the tumor microenvironment. In tumor-bearing mice, HSPCs are mobilized from the bone marrow to the tumor microenvironment where they differentiate to MDSCs, promoting expansion and metastasis.44 In addition, circulating HSPCs in newly diagnosed cancer patients correlated with increased risk of metastasis.44
Colony stimulating factors and cytokines promoting MDSCs alter the transcriptional regulation of myelopoiesis. Marigo et al.45 noted a key role for activation of C/EBPβ transcription factor by G-CSF, GM-CSF, and IL-6 in the expansion of MDSCs that abrogated tumor-specific T cell immunity. In addition, tumor-derived products can activate STAT3 signaling in myeloid cells that can lead to inhibition of DC maturation and stimulation of MDSC development.46–49 Waight et al.47 identified interferon regulatory factor-8 (IRF-8), a transcriptional regulator of myeloid differentiation and lineage commitment, as a negative regulator of human MDSCs. G-CSF and GM-CSF resulted in IRF-8 downregulation via STAT3-and STAT5-dependent pathways, while IRF-8 overexpression attenuated MDSC accumulation and enhanced immunotherapeutic efficacy in tumor-bearing mice.47 While studies have shown the importance of NOX2 in promoting MDSC accumulation and immunosuppression in tumor-bearing mice,50 we found that the presence of NOX2 did not affect MDSC accumulation or tumor progression.51
Together, studies from patients with cancer and mouse models point to cancers reprogramming the marrow resulting in an expansion of myeloid cells, including granulocytic cells. These granulocytic cells infiltrate tumor and can promote tumor progression by inducing tumor cell proliferation, stimulating angiogenesis and matrix remodeling, and disabling T cell-dependent anti-tumor immunity. In certain contexts, an expansion a specific myeloid lineage occurs, while other settings result in an expansion of multiple heterogeneous lineages. Gaining more mechanistic knowledge about what drives this “myeloid bias” 23 may lead to new therapeutic approaches and opportunities to enhance existing standard treatment.
5 NEUTROPHILS, NEUTR OPHIL EXTRACELLULAR TRAPS, AND PLA TELETS IN TUMOR PR OGRESSION
Neutrophils and platelets are rapidly recruited to sites of injury. This trafficking is mediated by coordinated ligand-receptor interactions involving platelets, neutrophils, and endothelial cells. P-selectin expressed on platelets recognizes and is activated by its ligands, PSGL-1 and CD24, expressed on endothelial cells. Activated platelets induce endothelial expression of ICAM-1, resulting in increased neutrophil adhesion. Consequently, inhibition of neutrophil-platelet interactions protected against acute lung injury in mouse models.52,53 Activation of PSGL-1 on neutrophils results in the redistribution of receptors that mediate neutrophil recruitment and trafficking. PSGL-1 is a docking site for activated platelets during inflammation, and inhibition of PSGL-1 was protective in models of organ injury.54 Platelets release microparticles during inflammation, which are internalized by activated neutrophils and amplify inflammation in arthritis.55,56 In addition, activated platelets release mitochondria into the extracellular environment, which, similar to bacteria, activate and interact with neutrophils in vivo, triggering neutrophil adhesion to the endothelial wall.57 Once neutrophils are recruited to the extravascular space, activation of integrins and leukotriene B4, as well as other chemoattractants, leads to neutrophil clustering in the damaged site and the formation of a wound seal.58 Together, co-activation of neutrophils and platelets serves to contain infection. There is precedent for pathogens evading host defense by abrogating neutrophil-platelet interactions59; however, there is also growing evidence for these same antimicrobial and wound healing pathways accelerating tumor progression.
Activated neutrophils can elicit anti-tumor functions. They are capable of direct lysis of tumor cells60 and killing tumor cells by antibody-dependent cell-mediated cytotoxicity (ADCC).61,62 Mittendorf et al.63 showed that breast cancers cell took up neutrophil elastase (NE) released by TANs, which enhanced their susceptibility to CTL lysis. Despite these anti-tumor properties of neutrophils, the overall effect of granulocytic cell expansion and accumulation in the tumor microenvironment is often weighted toward driving tumor growth and spread. To understand the effect of neutrophils on tumor, it is important to review basic mechanisms of neutrophil-mediated antimicrobial host defense.
Activated neutrophils kill microbes through oxidant-dependent and -independent pathways. The phagocyte NADPH oxidase (NOX2) is a critical enzyme in antimicrobial host defense and in regulating inflammation. Following NOX2 activation, molecular oxygen is converted to superoxide anion and to downstream metabolites, including H2O2 and hydroxyl anion. In neutrophils, myeloperoxidase (MPO) converts H2O2 to hypohalous acid.64–66 In a series of studies by Clark et al.67–70, neutrophil-mediated killing of tumor cells ex vivo was dependent on activation of the MPO-H2O2-halide system. In addition, cationic proteins from human neutrophil granules, which are activated by NOX2,71 were cytotoxic against tumor cells.72 However, activation of the neutrophil NOX2/MPO system can also abrogate T cell and natural killer cell function.73–75 In murine syngeneic ovarian cancer, tumor progression was similar in wildtype and NOX2-deficient mice, demonstrating a lack of effect of NOX2.51 Although ex vivo studies cannot model the complexity of tumor biology in vivo, these studies raise the notion that different tumors and the inflammatory milieu of the tumor microenvironment may influence the sensitivity of tumor cells to neutrophil-generated reactive oxidant attack.
In addition to pathogen killing via oxidant injury from NOX2/MPO activation, NOX2 can augment host defense by intracellular activation of granular proteases71 and generation of neutrophil extracellular traps (NETs).76,77 NETosis is a distinct mode of neutrophil death characterized by the breakdown of membranes and extracellular release of stretches of DNA, histones, and granular constituents.77 NETosis can be stimulated by bacterial and fungal infections,78,79 as well as non-infectious insults that can mimic infection.80–83 It can occur through NOX2-dependent and -independent pathways.84 While apoptosis of neutrophils results in non-inflammatory cell death, NETs release products that augment antimicrobial host defense but also cause tissue injury.82,83,85,86 In the tumor microenvironment, these same pathways that degrade and remodel extracellular matrix may promote tumor cell migration and invasion.
NETs are pro-thrombogenic in vivo, likely due to the released extracellular stretches of chromatin.87–92 In addition, NETs release tissue factor, a key component in the coagulation cascade.93 In mouse models, extracellular chromatin and tissue factor contribute to the pathogenesis of deep venous thrombosis, and depletion of NETs results in limiting thrombosis.91,92 Peptidylarginine deiminase 4 (PAD4), an enzyme that mediates histone citrullination and NETosis, was required for experimental deep venous thrombosis.94 Platelets activated by toll-like receptor 4 signaling can stimulate NETosis during sepsis.95 In addition, P-selectin expressed on platelets, through binding to its ligand PSGL-1 on neutrophils, promotes NETosis.96 Thus, in the context of inflammation and injury, activated neutrophils and platelets can cross-signal in a positive feedback loop. While in the context of infection, NETosis and activation of thrombosis may limit microbial spread, these same pathways may promote tumor progression.
Cancer-associated thrombosis is a major cause of morbidity and mortality. There is growing evidence for NETs playing a role in cancer-associated venous and arterial thrombosis,90,97 and in cancer progression in tumor-bearing mice.98–100 NETs have been identified in a number of cancers in patients, including sarcoma,101 pancreatic cancer, 102 and ovarian cancer (manuscript in preparation). Tumors, as well as their microenvironments, release DAMPs, hypoxia-inducible factors, and pro-inflammatory cytokines, which can attract neutrophils and induce NETosis.100,102,103 Demers et al.90 showed that in tumor-bearing mouse models, chromatin released by NETs stimulated thrombosis. In addition, systemic infection in tumor-bearing mice resulted in large quantities of chromatin release and worsening of the prothrombotic state.90 In small intestinal tumor, lipopolysaccharide induced complement-dependent NETosis resulting in hypercoagulation and metastasis.99 Cools-Lartigue et al.98 showed in a mouse model of concurrent sepsis and metastatic tumor that NETs trapped circulating tumor cells and promoted metastasis. It is unclear from these results whether targeting tumor-stimulated NETs in the absence of concurrent sepsis would limit metastasis. In patients undergoing curative-intent liver resection for metastatic colorectal cancer, Tohme et al.100 observed that increased postoperative NET formation was associated with a significantly shorter disease-free survival. Consistent with these results, ischemia reperfusion injury in tumor-bearing mice worsened metastasis, an effect that was abrogated by inhibition of NETosis.100
NETs may facilitate tumor progression through promotion of thrombosis and angiogenesis. Thrombosis can accelerate tumor progression through several pathways, including recruitment of inflammatory cells to the tumor microenvironment, and stimulation of tumor cell migration and adhesion to endothelial cells and of the production of vascular endothelial growth factor (VEGF) and other pro-angiogenic products that increase metastatic potential.104 In addition, NETs in tumor-bearing mice were associated with vessel damage and organ injury.105
There is also growing evidence that activated platelets can drive tumor progression. Thrombocytosis has been observed in patients with advanced solid tumors as well as other conditions associated with increased inflammation, such as autoimmune disease. Thrombocytosis at diagnosis of advanced ovarian cancer predicted worse survival.106 Paraneoplastic thrombocytosis, in addition to being a correlate of advanced cancer, can drive tumor progression through a number of pathways, including stimulation of tumor cell aggregation and proliferation, and the release of growth factors and pro-angiogenic factors. 107 In addition, activated platelets cross-signal to tumor cells to induce epithelial-mesenchymal transition (EMT), which is associated with greater metastatic potential and chemotherapy resistance.108–110 Platelet-derived transforming growth factor-beta (TGF-β) plays a key role in EMT, and can act in concert with platelet-derived growth factors and platelet-tumor cell contacts to promote EMT.108–110
TGF-β has multiple signaling properties depending on the inflammatory context. In the context of wound healing, TGF-β plays an essential role in fibroblast activation and scar formation.111,112 The effect of TGF-β on T cells varies based on the inflammatory context, and has the potential to stimulate both IL-17-producing lymphocytes and Tregs113,114 and to impair CTL activity.115 Within the tumor microenvironment, TGF-β can also induce a population of neutrophils with an immunosuppressive pro-tumorigenic phenotype.116,117 Labelle et al.118 showed that platelets stimulate recruitment of neutrophils to tumors that promote metastasis at early stages of cancer in tumor-bearing mice. These results suggest that during pathologic wound healing in cancer, TGF-β derived from platelets and other cells may have important roles in shaping neutrophil responses and tumor cell biology that promote metastasis. Together, these results support a model in which the tumor cells or their environment stimulate neutrophil and platelet activation through a number of pathways: tumor-derived products, DAMPs, production of pro-inflammatory cytokines and chemokines, and vascular injury and extravasation. These stimuli lead to an inflammatory and thrombogenic cascade that mimic those induced by infection and trauma, and serve to control infection, stop bleeding, and promote wound healing. In the context of cancer where injury is persistent, these antimicrobial and wound repair pathways may promote tumor progression and metastasis through their direct influence on tumor cell biology, and through their influence on inflammation and thrombosis in the tumor microenvironment that create a metastatic niche.
6 EFFECTS OF NEUTR OPHILS ON CELL-MEDIATED IMMUNITY
The mechanisms by which neutrophils may alter CTL responses are likely to be the most translationally important, as understanding the roles that neutrophils play in the context of tumor progression and response to immunotherapy may lead to novel strategies to enhance treatment. Following accumulation at sites of infection, neutrophils are able to migrate to draining lymph nodes.119,120 This new finding demonstrates the potential for neutrophils to engage with lymphocytes and antigen-presenting cells, at the precise site of inflammation and also at the draining lymph nodes. Neutrophils may interact at these sites either by cell contact or via mediators, such as reactive oxidants, release of granular constituents, and cytokines; and the overall effect may be to prime or dampen antigen-dependent immune responses. During experimental bacterial skin infection, neutrophils migrated from inflamed skin into lymph nodes where they augmented lymphocyte proliferation.119 Lim et al.121 showed that during influenza infection in mice, the early recruitment of neutrophils to lungs was essential for cell-mediated immune protection. Neutrophils released CXCL12 required for CD8+ T cell recruitment and effector functions.121 In other contexts, activated neutrophils can suppress T cell responses. Immunosuppressive neutrophils are not unique to the tumor microenvironment. During acute systemic inflammation induced by lipopolysaccharide, release of hydrogen peroxide from a subset of activated neutrophils led to suppression of T cell proliferation.122 Neutrophils were also shown to limit the spread of T cell responses from draining to distal lymph nodes following protein immunizations.123
TANs exhibit heterogeneity in their capacity to promote or impede T cell immunity. There is growing evidence that neutrophils promote tumor progression through a dual effect of inhibition of CTL responses and promotion of myeloid inflammation and angiogenesis. Langowski et al.124 showed that IL-23 promoted tumor incidence and growth in mice. IL-23 stimulated the expansion of IL-17A-producing lymphocytes that, in turn, stimulated myeloid growth factors and pro-inflammatory cytokines that drive neutrophilic inflammation. While important for antimicrobial host defense, the IL-23/IL-17A axis is also associated with autoimmunity. In tumor-bearing mice, IL-23 increased neutrophil and macrophage accumulation in tumors and stimulated angiogenesis and upregulation of matrix metalloprotease-9 (MMP-9), but reduced CD8+ T cell infiltration into the tumor.124 MMP-9 is principally expressed in neutrophils, macrophages, and mast cells, and contributes to tumor invasiveness by tissue remodeling and angiogenesis.125–127 In tumor-bearing mice, IL-17 stimulated the expression of G-CSF, leading to myeloid cell mobilization and recruitment of Bv8-positive granulocytes to the tumor microenvironment, and promoted resistance to anti-VEGF treatment.128 These results point to the IL-23/IL-17 axis as another mechanism to reprogram tumor-associated inflammation from anti-tumor CTL responses towards pro-inflammatory and pro-angiogenic pathways that promote tumor progression.129
In a mouse model of colorectal tumor, IL-23 produced by myeloid cells augmented intratumoral IL-17 response and promoted tumor growth and progression.130 The authors proposed a model in which barrier erosion by tumor enabled mucosal invasion by bowel flora that triggered IL-23-dependent inflammation and acceleration of tumor growth.130 These results are consistent with tumor-infiltrating CD8+ and Th1 cells predicting prolonged disease-free survival, and Th17 inflammation predicting poor prognosis in patients with colorectal cancer.131 Coffelt et al.132 showed that IL-1β stimulated IL-17 expression from γδ T cells, resulting in G-CSF-dependent expansion of neutrophils in a mouse model of spontaneous mammary tumor development. Tumor-induced neutrophils suppressed CTL responses, and depletion of IL-17 or G-CSF and absence of γδ T cells prevented neutrophil accumulation within tumors and abrogated the T cell suppressive phenotype of neutrophils. In addition, the absence of γδ T cells and neutrophils limited tumor metastasis without affecting the size of the primary tumor. Activated neutrophils can suppress T cell responses through a number of mechanisms. TANs, for instance, can stimulate T cell exhaustion. Koyama et al.133 showed that ablating the tumor suppressor genes STK11/LKB1 in a mouse model of KRAS-driven non-small cell lung cancer resulted in the accumulation of neutrophils with T cell suppressive function, and increased expression of exhaustion markers on T cells. TANs were also shown to contribute to an immunosuppressive environment through recruitment of Tregs.134
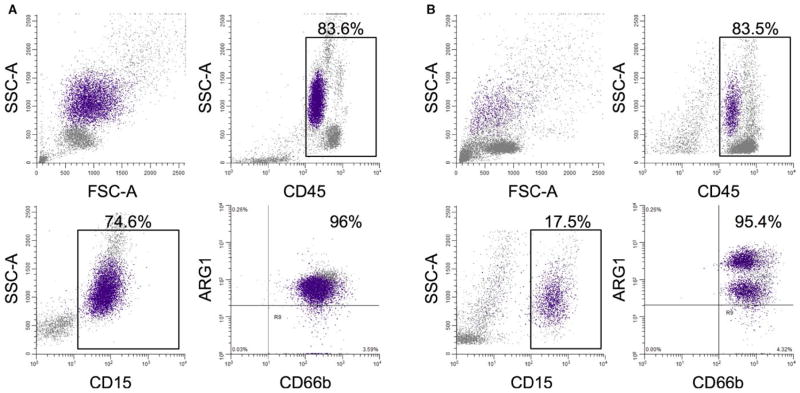
Arginase-1 (Arg-1) is expressed in neutrophil tertiary granules, and released during neutrophil activation.135 Arg-1 converts arginine to ornithine, a precursor of proline required for wound healing. Release of Arg-1 by neutrophils can lead to depletion of arginine, resulting in T cell suppression.136,137 Activation of neutrophils can stimulate Arg-1 release and induce phenotypic and functional changes similar to granulocytic MDSCs.138 As shown in Fig. 2, there can be heterogeneity among neutrophil populations in the tumor microenvironment regarding Arg-1 expression. In mice with spontaneous mammary tumor development, iNOS expression in neutrophils was also associated with a T cell suppressive phenotype.132 These results point to modulation of arginine metabolism as a potential mechanism for activated neutrophils to suppress T cell responses.
FIGURE 2.
Neutrophils within the tumor microenvironment possess heterogeneity in regard to arginase-1 (Arg-1) expression. Flow cytometric analysis of peripheral blood (A) and ascites (B) from a patient with newly diagnosed ovarian cancer prior to therapy. Blood was collected pre-operatively on the day of surgery, and ascites was collected intra-operatively prior to tumor resection. The upper left panel shows the ungated scatter of all cells within the sample, the upper right panel is the proportion of CD45+ cells in the ungated population, the lower left is the proportion of CD15+ cells in the ungated population, and the lower right panel shows the CD66b and Arg-1 expression of the CD15+ cells. In blood there is a single population of CD15+ CD66b+ Arg-1+ cells, while in ascites two distinct populations were observed: CD15+ CD66b+ Arg-1 low and CD15+ CD66b+ Arg-1 intermediate. The difference in Arg-1 expression may relate to differences in transcription or Arg-1 release from activated neutrophils. Neutrophils can be tracked throughout the plots by the purple-colored cells
7 REVISITING THE C ONCEPT OF NEUTROPHIL HETER OGENEITY
Modern tools for cellular and transcriptome analyses have led to an appreciation for the extraordinary heterogeneity among macrophages regarding their cellular origin and biological properties.139,140 The notion of neutrophil heterogeneity—that neutrophils are made up of biologically distinct populations—has been suggested for decades. Earlier studies largely relied on surface marker expression on neutrophils and differences in neutrophil responses to ex vivo stimuli to define unique populations.141–145 In a prescient paper published more than 30 years ago, Gallin146 noted the existence of neutrophil heterogeneity, but asked whether it was meaningful. With regard to tumor immunology, the answer is almost certainly yes. There has been a greater appreciation for the potential of neutrophil heterogeneity, including distinct modes of neutrophil death (apoptosis, necrosis, NETosis), reverse transendothelial migration, the ability to present antigen, and acquisition of phenotypes that promote or inhibit adaptive immunity.
One of the major distinctions between neutrophil populations in cancer relates to the N1 versus N2 classification. N1 TANs can promote anti-tumor effector responses both by directly targeting tumor cells and by stimulation of T cell immunity. In contrast, N2 TANs facilitate tumor progression by suppressing T cell responses and upregulating angiogenic factors (e.g. VEGF and MMP-9).127,147,148 Sagiv et al.117 recently expanded on these results by characterizing three distinct neutrophil populations in cancer: mature high-density neutrophils, mature low-density neutrophils, and immature low-density neutrophils. The mature high-density neutrophils had an N1-like phenotype and the capacity to kill tumor cells. The low-density population included immature neutrophils (granulocytic MDSCs) and mature neutrophils with T cell suppressive capacity. When treated with TGF-β, the mature high-density neutrophils polarized to become low-density neutrophils, and acquired a suppressive phenotype. Importantly, the immunosuppressive low-density neutrophils increased in relation to tumor burden.
Neutrophils expressing major histocompatibility complex class II and costimulatory molecules have been detected at inflammatory sites, and can mimic properties of professional antigen-presenting cells. Similar to professional antigen-presenting cells, neutrophils recruited T cells via CXCL9 and CXCL10116,149 and cross-primed CD8+ T cells in vivo and stimulated CTL effector functions.150 Duffy et al.151 showed that following intradermal injection of a vaccinia virus, neutrophils transported virus from the dermis to the bone marrow and were required for generation of virus-specific CD8+ memory T cells. Interestingly, neutrophils were also shown to differentiate into a hybrid population expressing surface markers for both neutrophils and DCs, which were capable of clearing bacteria and generating NETs (characteristics of neutrophils) and presenting antigens to naïve CD4+ T cells (characteristic of DCs).152,153 It is unknown if these neutrophil-DC hybrids are relevant in the tumor microenvironment or in the context of immunotherapy. However, at a conceptual level, these observations raise the potential for reprogramming neutrophils to a DC phenotype that could be exploitable for tumor antigen presentation and augmentation of cellular immunity.
Neutrophil variability in cancer can result from changes at multiple levels, including epigenetics, metabolic, oxidant generation, post-translational changes, and cross-signaling between cells. We do not know how durable these changes are. As we learn more about the cues that regulate neutrophil diversity, new approaches for their therapeutic modulation are expected to emerge.
8 THE O VARIAN CANCER MICROENVIRONMENT: A CASE STUD Y ON PERSISTENT INFLAMMA TION AND IMMUNOSUPPRESSION
Ovarian cancer is commonly diagnosed at advanced stages. Standard treatment for advanced disease involves cytoreductive surgery and chemotherapy. Although the majority of patients respond to chemotherapy, the median progression-free survival for women with stage III epithelial ovarian cancer was 17 months.154 There is growing recognition that immune responses in the pretreatment tumor microenvironment are both prognostic biomarkers and potential targets for therapeutic modulation in patients with advanced ovarian cancer. The critical role of immune surveillance in ovarian cancer was demonstrated by correlation of survival with tumor-infiltrating lymphocytes.155 Intraepithelial CD8+ cell accumulation and a high CD8+/Treg ratio were associated with favorable prognosis156 while increased Treg accumulation predicted worse outcome157 in patients with advanced ovarian cancer.
There is growing evidence that the tumor microenvironment in ovarian cancer is inflammatory, characterized by mature and immature myeloid cell accumulation and expression of pro-inflammatory cytokines and chemokines, as well as immunosuppressive.158–164 In pretreatment ascites of patients with ovarian cancer, the myeloid cell population consists of mature macrophages, immature myeloid cells, and granulocytic cells with variable immunosuppressive phenotypes. 8,165 Accumulation of B7-H4-expressing macrophages in the tumor microenvironment impeded T cell responses and correlated with more rapid tumor progression.5,166 Tumor cells and TAMs produce the chemokine CCL22, which mediates trafficking of Tregs to the tumor leading to suppression of tumor-specific T cell immunity.157
It has been recognized for several years that the ascites in patients with ovarian cancer is thrombogenic, and that coagulation and fibrinolysis could affect tumor invasion.167–169 Stone et al.106 showed that paraneoplastic thrombocytosis in advanced ovarian cancer was driven by IL-6, and correlated with poor prognosis. As previously described, there are several mechanisms by which activated platelets can affect tumor progression, including release of pro-angiogenic factors, cross-signaling with neutrophils, stimulation of EMT, and promotion of a premetastatic niche110,118 In addition, platelets increase the proliferation of ovarian cancer cells through TGF-β-dependent signaling.170 In murine ovarian cancer, platelets were shown to reduce the efficacy of chemotherapy.171
The tumor microenvironment of ovarian cancer is associated with release of products of necrosis that activate innate immune responses. We found that when treated with cell-free ascites, normal donor neutrophils underwent NETosis and suppressed stimulated T cell proliferation in a cell contact-dependent manner (Singel K.L., A.N.H. Khan, T.R. Emmons, P.C. Mayor, K.B. Moysich, K. Odunsi, and B.H. Segal. 2016. Ovarian cancer ascites-activated neutrophils suppress T cell proliferation in a contact-dependent mechanism. J. Immunol. 196: 211.16 (Abstr.)). Together these results support a model in which DAMPs in the ovarian cancer tumor microenvironment stimulate innate immune responses and platelet activation that normally function to control infection and promote wound repair. We speculate that these responses may augment tumor cell invasion and metastasis through several pathways (e.g. enhanced tumor cell seeding onto serosa, release of growth factors and stimulation of angiogenesis, and cross-signaling to tumor cells to promote proliferation and EMT).
9 EFFECTS OF ANTI-NEOPLASTIC TREATMENT ON NEUTR OPHILS
In the clinic, myeloid cell responses are not a static process. Cytotoxic chemotherapy results in marrow hypoplasia followed by recovery. Cytotoxic chemotherapy and radiation cause death of tumor cells, but also cause bystander injury to non-tumor cells that can affect the skin, mucosa, and visceral organs. Chemotherapy and radiation are administered in cycles, resulting in repeated tissue injury and wound repair responses. Some of the immune responses to cytotoxic regimens may be beneficial. For example, chemotherapy-and radiation-induced tissue necrosis can result in DAMP release that activates DCs. In addition, specific chemotherapy regimens can target MDSCs, and enhance anti-tumor T cell immunity.172,173 However, Bruchard et al.174 showed that chemotherapy also triggered inflammasome activation in MDSCs that activated IL-1β and IL-17 responses and inhibited anti-tumor immunity. Treatment with an IL-1 receptor antagonist enhanced the effect of chemotherapy. In addition, myeloid cell recruitment may be an obstacle to radiation therapy. Inhibition of Mac-1 (CD11b/CD18) enhanced tumor response to radiation by reducing myeloid cell recruitment in tumor-bearing mice.175
Major surgery, including tumor resection, can activate innate immune responses that mimic infection (e.g. fever and leukocytosis). Wound repair entails rapid activation of thrombosis followed by regenerative responses. Particularly in the setting of non-curative intent surgery in which non-resected tumor remains (e.g. primary debulking for metastatic ovarian cancer or nephrectomy for metastatic renal cancer), we do not adequately understand how wound repair responses to surgery affect cancer progression. Resection of tumor is expected to reduce the levels of tumor-derived factors that drive granulocytic responses, including TANs and MDSCs. However, at the same time, wound repair responses activated by surgery may also drive angiogenesis and immunosuppressive responses. Predina et al.176 showed that incomplete resection of tumor in mice led to the accumulation of immunosuppressive M2 macrophages and Tregs in recurrent tumors that were barriers to anti-tumor vaccines. Surgery can also stimulate NETs that stimulate progression of metastatic tumor.100
A common side effect for patients undergoing chemotherapeutic treatment for cancer is febrile neutropenia. The National Comprehensive Cancer Network177 and American Society of Clinical Oncology guidelines178 recommend a myeloid growth factor as prophylaxis in patients receiving regimens associated with a high risk for neutropenic fever and those with a high risk of infectious complications during neutropenia. The use of recombinant G-CSF to accelerate neutrophil recovery from chemotherapy will, by design, stimulate granulocytic differentiation. We do not fully understand the effects of repeated cycles of chemotherapy and G-CSF on myeloid cell populations within the tumor microenvironment and how they influence tumor cell biology. In murine models, tumor-derived G-CSF can expand MDSCs, worsen tumor progression, and promote resistance to chemotherapy.24,47,179–181 These results should not influence clinical practice regarding growth factor support; such decisions should be based on clinical trial data and authoritative guidelines. However, they do underscore the need for additional research regarding the effects of chemotherapy and growth factor support on the tumor microenvironment with the ultimate goal of improving chemotherapy efficacy and limiting toxicity.
10 FUTURE THERAPEUTIC STRA TEGIES TARGETING NEUTR OPHILS IN THE TUMOR MICROENVIRONMENT
Future therapeutic options that target neutrophils, both mature and immature granulocytic cells, should be based on an understanding of the heterogeneity of these cells and their diverse roles as both promoters of tumor control and promoters of immune evasion and tumor progression. Detailed studies of neutrophil biology in the tumor microenvironment, including modern approaches for transcriptome profiling at the population and potentially single cell level will add insight into neutrophil heterogeneity, and may form the basis for targeted approaches against populations that drive tumor progression. As we learn more about the plasticity of neutrophils,182 including the N1/N2 axis, we envision that newer approaches will emerge to skew TANs toward an anti-tumor phenotype. As an example, Andzinski et al.183 found that type I interferons induced polarization of TANs to an anti-tumor N1 phenotype in tumor-bearing mice, and similar changes in neutrophil activation were observed in melanoma patients receiving type I interferon therapy. Therapeutic approaches to reprogram neutrophils would need to consider the dynamic phenotypes of TANs both in relation to early versus advanced disease and location within the tumor (peripheral versus central) that affect neutrophil biology based on several factors, including oxygen levels, DAMPs, and cytokines.
As previously discussed, there is growing evidence for NETs driving tumor progression. There are currently several experimental approaches that can deplete NETs, including DNase, antibodies against NET constituents, and small molecule inhibitors of signaling pathways required for NETosis.82,83,98,184,185 Small molecule inhibitors of peptidylarginine deiminase inhibit NET generation and have been protective in murine models of lupus and atherosclerosis.186–188 Prostaglandin E2 was recently shown to inhibit NET generation.189 Targeting NETs or specific NET constituents merits further investigation as adjunctive therapies for cancer, initially in tumor-bearing mice.
Since neutrophils traffick to sites of injury, including the tumor microenvironment, they are potentially exploitable for delivery of antineoplastic agents. As a proof of principle, Chu et al.190 observed in murine melanoma that neutrophils enhanced the delivery of nanoparticles to the tumor and augmented the effect of antibody-mediated immunotherapy.
While tumor-derived factors and the tumor microenvironment can reprogram marrow to drive granulopoiesis and generation of neutrophils with distinct phenotypes, other factors may also do so. For example, recent studies point to the microbiome influencing neutrophil lifespan and function, including the propensity to generate NETs.191,192 The influence of the microbiome on neutrophil biology may be of particular relevance in patients with cancer where microbiome alterations can result from several factors, including mucosal injury from chemotherapy and radiation and the widespread use of antibiotics. There is tremendous excitement about learning how the bowel microbiome affects cancer risk and response to therapy,193 including to checkpoint inhibitors.194,195 Seen in this light, the microbiome in patients with cancer might also influence neutrophil phenotypes that are relevant to control or progression of cancer, and can potentially be therapeutically modified.
Another emerging concept of potential relevance to tumor immunology is innate immune memory or “trained immunity”. Memory characteristics of innate immunity involves a priming event that modifies innate immune cells such that re-exposure to identical or heterologous stimuli results in a heightened response and augmented host defense.196 The acquisition of memory is associated with epigenetic and metabolic changes.197,198 In a chronic parasitic infection model, activated neutrophils can prime a long-lived effector macrophage phenotype that augments host defense.199 We speculate that as greater knowledge is gained about trained immunity, including in the context of cancer, that newer vaccines that combine adaptive and innate immune memory will be developed.200
Acknowledgments
Supported by the NIH: T32 CA085183 (K. L. S.) and R01 CA188900 (B. H. S.).
Footnotes
CONFLICTS OF INTEREST
Neither of the authors (K. L. S. and B. H. S.) have any potential conflicts of interest to disclose.
References
- 1.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Pikarsky E, Porat RM, Stein I, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 3.DiDonato JA, Mercurio F, Karin M. NF-kappaB and the link between inflammation and cancer. Immunol Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 4.Palucka AK, Coussens LM. The basis of oncoimmunology. Cell. 2016;164:1233–1247. doi: 10.1016/j.cell.2016.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kryczek I, Zou L, Rodriguez P, et al. B7-H4 expression identifies a novel suppressive macrophage population in human ovarian carcinoma. J Exp Med. 2006;203:871–881. doi: 10.1084/jem.20050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez D, Silvera R, Carrio R, et al. Tumor microenvironment profoundly modifies functional status of macrophages: peritoneal and tumor-associated macrophages are two very different subpopulations. Cell Immunol. 2013;283:51–60. doi: 10.1016/j.cellimm.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torroella-Kouri M, Silvera R, Rodriguez D, et al. Identification of a subpopulation of macrophages in mammary tumor-bearing mice that are neither M1 nor M2 and are less differentiated. Cancer Res. 2009;69:4800–4809. doi: 10.1158/0008-5472.CAN-08-3427. [DOI] [PubMed] [Google Scholar]
- 8.Khan AN, Kolomeyevskaya N, Singel KL, et al. Targeting myeloid cells in the tumor microenvironment enhances vaccine efficacy in murine epithelial ovarian cancer. Oncotarget. 2015;6:11310–11326. doi: 10.18632/oncotarget.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dangaj D, Lanitis E, Zhao A, et al. Novel recombinant human b7-h4 antibodies overcome tumoral immune escape to potentiate T-cell antitumor responses. Cancer Res. 2013;73:4820–4829. doi: 10.1158/0008-5472.CAN-12-3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–294. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 13.Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol. 2013;23:200–207. doi: 10.1016/j.semcancer.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 14.Absenger G, Szkandera J, Pichler M, et al. A derived neutrophil to lymphocyte ratio predicts clinical outcome in stage II and III colon cancer patients. Br J Cancer. 2013;109:395–400. doi: 10.1038/bjc.2013.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao Y, Yuan D, Liu H, Gu X, Song Y. Pretreatment neutrophil to lymphocyte ratio is associated with response to therapy and prognosis of advanced non-small cell lung cancer patients treated with first-line platinum-based chemotherapy. Cancer Immunol Immunother. 2013;62:471–479. doi: 10.1007/s00262-012-1347-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non-small cell lung cancer (NSCLC): a retrospective review. BMC Cancer. 2013;13:158. doi: 10.1186/1471-2407-13-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motomura T, Shirabe K, Mano Y, et al. Neutrophil-lymphocyte ratio reflects hepatocellular carcinoma recurrence after liver transplantation via inflammatory microenvironment. J Hepatol. 2013;58:58–64. doi: 10.1016/j.jhep.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 18.Zhou SL, Dai Z, Zhou ZJ, et al. Overexpression of CXCL5 mediates neutrophil infiltration and indicates poor prognosis for hepatocellular carcinoma. Hepatology. 2012;56:2242–2254. doi: 10.1002/hep.25907. [DOI] [PubMed] [Google Scholar]
- 19.Wang GY, Yang Y, Li H, et al. A scoring model based on neutrophil to lymphocyte ratio predicts recurrence of HBV-associated hepatocellular carcinoma after liver transplantation. PLoS ONE. 2011;6:e25295. doi: 10.1371/journal.pone.0025295. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Dumitru CA, Bankfalvi A, Gu X, Zeidler R, Brandau S, Lang S. AHNAK and inflammatory markers predict poor survival in laryngeal carcinoma. PLoS ONE. 2013;8:e56420. doi: 10.1371/journal.pone.0056420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerjee S, Rustin G, Paul J, et al. A multicenter, randomized trial of flat dosing versus intrapatient dose escalation of single-agent carboplatin as first-line chemotherapy for advanced ovarian cancer: an SGCTG (SCOTROC 4) and ANZGOG study on behalf of GCIG. Ann Oncol. 2013;24:679–687. doi: 10.1093/annonc/mds494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho H, Hur HW, Kim SW, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother. 2009;58:15–23. doi: 10.1007/s00262-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu WC, Sun HW, Chen HT, et al. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc Natl Acad Sci USA. 2014;111:4221–4226. doi: 10.1073/pnas.1320753111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Casbon AJ, Reynaud D, Park C, et al. Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci USA. 2015;112:E566–E575. doi: 10.1073/pnas.1424927112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strauss L, Sangaletti S, Consonni FM, et al. RORC1 regulates tumor-promoting “emergency” granulo-monocytopoiesis. Cancer Cell. 2015;28:253–269. doi: 10.1016/j.ccell.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 26.van Kempen LC, Coussens LM. MMP9 potentiates pulmonary metastasis formation. Cancer Cell. 2002;2:251–252. doi: 10.1016/s1535-6108(02)00157-5. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol. 2006;8:1369–1375. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 29.Shojaei F, Wu X, Zhong C, et al. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 30.Shojaei F, Singh M, Thompson JD, Ferrara N. Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci USA. 2008;105:2640–2645. doi: 10.1073/pnas.0712185105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kowanetz M, Wu X, Lee J, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G+Ly6C+ granulocytes. Proc Natl Acad Sci USA. 2010;107:21248–21255. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wculek SK, Malanchi I. Neutrophils support lung colonization of metastasis-initiating breast cancer cells. Nature. 2015;528:413–417. doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell. 2011;20:300–314. doi: 10.1016/j.ccr.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blaisdell A, Crequer A, Columbus D, et al. Neutrophils oppose uterine epithelial carcinogenesis via debridement of hypoxic tumor cells. Cancer Cell. 2015;28:785–799. doi: 10.1016/j.ccell.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–955. doi: 10.1093/carcin/bgs123. [DOI] [PubMed] [Google Scholar]
- 36.Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Janols H, Bergenfelz C, Allaoui R, et al. A high frequency of MDSCs in sepsis patients, with the granulocytic subtype dominating in gram-positive cases. J Leukoc Biol. 2014;96:685–693. doi: 10.1189/jlb.5HI0214-074R. [DOI] [PubMed] [Google Scholar]
- 38.Darcy CJ, Minigo G, Piera KA, et al. Neutrophils with myeloid derived suppressor function deplete arginine and constrain T cell function in septic shock patients. Crit Care. 2014;18:R163. doi: 10.1186/cc14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci. 2014;1319:47–65. doi: 10.1111/nyas.12469. [DOI] [PubMed] [Google Scholar]
- 40.Sinha P, Parker KH, Horn L, Ostrand-Rosenberg S. Tumor-induced myeloid-derived suppressor cell function is independent of IFN-γ and IL-4Rα. Eur J Immunol. 2012;42:2052–2059. doi: 10.1002/eji.201142230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mandruzzato S, Brandau S, Britten CM, et al. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: results from an interim study. Cancer Immunol Immunother. 2016;65:161–169. doi: 10.1007/s00262-015-1782-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Solito S, Falisi E, Diaz-Montero CM, et al. A human promyelocyticlike population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118:2254–2265. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giles AJ, Reid CM, Evans JD, et al. Activation of hematopoietic stem/progenitor cells promotes immunosuppression within the pre-metastatic niche. Cancer Res. 2016;76:1335–1347. doi: 10.1158/0008-5472.CAN-15-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marigo I, Bosio E, Solito S, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 46.Farren MR, Carlson LM, Netherby CS, et al. Tumor-induced STAT3 signaling in myeloid cells impairs dendritic cell generation by decreasing PKCbetaII abundance. Sci Signal. 2014;7:ra16. doi: 10.1126/scisignal.2004656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Waight JD, Netherby CS, Hensen ML, et al. Myeloid-derived suppressor cell development is regulated by a STAT/IRF-8 axis. J Clin Invest. 2013;123:4464–4478. doi: 10.1172/JCI68189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vasquez-Dunddel D, Pan F, Zeng Q, et al. STAT3 regulates arginase-I in myeloid-derived suppressor cells from cancer patients. J Clin Invest. 2013;123:1580–1589. doi: 10.1172/JCI60083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mace TA, Ameen Z, Collins A, et al. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013;73:3007–3018. doi: 10.1158/0008-5472.CAN-12-4601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corzo CA, Cotter MJ, Cheng P, et al. Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol. 2009;182:5693–5701. doi: 10.4049/jimmunol.0900092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Godoy HE, Khan AN, Vethanayagam RR, et al. Myeloid-derived suppressor cells modulate immune responses independently of NADPH oxidase in the ovarian tumor microenvironment in mice. PLoS ONE. 2013;8:e69631. doi: 10.1371/journal.pone.0069631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grommes J, Alard JE, Drechsler M, et al. Disruption of platelet-derived chemokine heteromers prevents neutrophil extravasation in acute lung injury. Am J Respir Crit Care Med. 2012;185:628–636. doi: 10.1164/rccm.201108-1533OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sreeramkumar V, Adrover JM, Ballesteros I, et al. Neutrophils scan for activated platelets to initiate inflammation. Science. 2014;346:1234–1238. doi: 10.1126/science.1256478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boilard E, Nigrovic PA, Larabee K, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duchez AC, Boudreau LH, Naika GS, et al. Platelet microparticles are internalized in neutrophils via the concerted activity of 12-lipoxygenase and secreted phospholipase A2-IIA. Proc Natl Acad Sci USA. 2015;112:E3564–E3573. doi: 10.1073/pnas.1507905112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boudreau LH, Duchez AC, Cloutier N, et al. Platelets release mitochondria serving as substrate for bactericidal group IIA-secreted phospholipase A2 to promote inflammation. Blood. 2014;124:2173–2183. doi: 10.1182/blood-2014-05-573543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lammermann T, Afonso PV, Angermann BR, et al. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Herron MJ, Nelson CM, Larson J, Snapp KR, Kansas GS, Goodman JL. Intracellular parasitism by the human granulocytic ehrlichiosis bacterium through the P-selectin ligand, PSGL-1. Science. 2000;288:1653–1656. doi: 10.1126/science.288.5471.1653. [DOI] [PubMed] [Google Scholar]
- 60.Lichtenstein A, Kahle J. Anti-tumor effect of inflammatory neutrophils: characteristics of in vivo generation and in vitro tumor cell lysis. Int J Cancer. 1985;35:121–127. doi: 10.1002/ijc.2910350119. [DOI] [PubMed] [Google Scholar]
- 61.Otten MA, Rudolph E, Dechant M, et al. Immature neutrophils mediate tumor cell killing via IgA but not IgG Fc receptors. J Immunol. 2005;174:5472–5480. doi: 10.4049/jimmunol.174.9.5472. [DOI] [PubMed] [Google Scholar]
- 62.Stockmeyer B, Beyer T, Neuhuber W, et al. Polymorphonuclear granulocytes induce antibody-dependent apoptosis in human breast cancer cells. J Immunol. 2003;171:5124–5129. doi: 10.4049/jimmunol.171.10.5124. [DOI] [PubMed] [Google Scholar]
- 63.Mittendorf EA, Alatrash G, Qiao N, et al. Breast cancer cell uptake of the inflammatory mediator neutrophil elastase triggers an anticancer adaptive immune response. Cancer Res. 2012;72:3153–3162. doi: 10.1158/0008-5472.CAN-11-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harrison JE, Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251:1371–1374. [PubMed] [Google Scholar]
- 65.Albrich JM, McCarthy CA, Hurst JK. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc Natl Acad Sci USA. 1981;78:210–214. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weiss SJ, Klein R, Slivka A, Wei M. Chlorination of taurine by human neutrophils. Evidence for hypochlorous acid generation. J Clin Invest. 1982;70:598–607. doi: 10.1172/JCI110652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Clark RA, Klebanoff SJ. Neutrophil-mediated tumor cell cytotoxicity: role of the peroxidase system. J Exp Med. 1975;141:1442–1447. doi: 10.1084/jem.141.6.1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clark RA, Klebanoff SJ. Role of the myeloperoxidase-H2O2-halide system in concanavalin A-induced tumor cell killing by human neutrophils. J Immunol. 1979;122:2605–2610. [PubMed] [Google Scholar]
- 69.Clark RA, Klebanoff SJ, Einstein AB, Fefer A. Peroxidase-H2O2-halide system: cytotoxic effect on mammalian tumor cells. Blood. 1975;45:161–170. [PubMed] [Google Scholar]
- 70.Clark RA, Szot S. The myeloperoxidase-hydrogen peroxide-halide system as effector of neutrophil-mediated tumor cell cytotoxicity. J Immunol. 1981;126:1295–1301. [PubMed] [Google Scholar]
- 71.Reeves EP, Lu H, Jacobs HL, et al. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 2002;416:291–297. doi: 10.1038/416291a. [DOI] [PubMed] [Google Scholar]
- 72.Clark RA, Olsson I, Klebanoff SJ. Cytotoxicity for tumor cells of cationic proteins from human neutrophil granules. J Cell Biol. 1976;70:719–723. doi: 10.1083/jcb.70.3.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.El-Hag A, Clark RA. Down-regulation of human natural killer activity against tumors by the neutrophil myeloperoxidase system and hydrogen peroxide. J Immunol. 1984;133:3291–3297. [PubMed] [Google Scholar]
- 74.el-Hag A, Clark RA. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol. 1987;139:2406–2413. [PubMed] [Google Scholar]
- 75.el-Hag A, Lipsky PE, Bennett M, Clark RA. Immunomodulation by neutrophil myeloperoxidase and hydrogen peroxide: differential susceptibility of human lymphocyte functions. J Immunol. 1986;136:3420–3426. [PubMed] [Google Scholar]
- 76.Bianchi M, Hakkim A, Brinkmann V, et al. Restoration of NET formation by gene therapy in CGD controls aspergillosis. Blood. 2009;114:2619–2622. doi: 10.1182/blood-2009-05-221606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fuchs TA, Abed U, Goosmann C, et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 79.Rohm M, Grimm MJ, D’Auria AC, Almyroudis NG, Segal BH, Urban CF. NADPH oxidase promotes neutrophil extracellular trap formation in pulmonary aspergillosis. Infect Immun. 2014;82:1766–1777. doi: 10.1128/IAI.00096-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Itagaki K, Kaczmarek E, Lee YT, et al. Mitochondrial DNA released by trauma induces neutrophil extracellular traps. PLoS ONE. 2015;10:e0120549. doi: 10.1371/journal.pone.0120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arai Y, Nishinaka Y, Arai T, et al. Uric acid induces NADPH oxidase-independent neutrophil extracellular trap formation. Biochem Biophys Res Commun. 2014;443:556–561. doi: 10.1016/j.bbrc.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 82.Caudrillier A, Kessenbrock K, Gilliss BM, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122:2661–2671. doi: 10.1172/JCI61303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomas GM, Carbo C, Curtis BR, et al. Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood. 2012;119:6335–6343. doi: 10.1182/blood-2012-01-405183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Douda DN, Khan MA, Grasemann H, Palaniyar N. SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci USA. 2015;112:2817–2822. doi: 10.1073/pnas.1414055112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Narasaraju T, Yang E, Samy RP, et al. Excessive neutrophils and neutrophil extracellular traps contribute to acute lung injury of influenza pneumonitis. Am J Pathol. 2011;179:199–210. doi: 10.1016/j.ajpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rossaint J, Herter JM, Van Aken H, et al. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap mediated sterile inflammation. Blood. 2014;123:2573–2584. doi: 10.1182/blood-2013-07-516484. [DOI] [PubMed] [Google Scholar]
- 87.Semeraro F, Ammollo CT, Morrissey JH, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118:1952–1961. doi: 10.1182/blood-2011-03-343061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martinod K, Wagner DD. Thrombosis: tangled up in NETs. Blood. 2014;123:2768–2776. doi: 10.1182/blood-2013-10-463646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Demers M, Krause DS, Schatzberg D, et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci USA. 2012;109:13076–13081. doi: 10.1073/pnas.1200419109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Brill A, Fuchs TA, Savchenko AS, et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. 2012;10:136–144. doi: 10.1111/j.1538-7836.2011.04544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.von Bruhl ML, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209:819–835. doi: 10.1084/jem.20112322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stakos DA, Kambas K, Konstantinidis T, et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J. 2015;36:1405–1414. doi: 10.1093/eurheartj/ehv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Martinod K, Demers M, Fuchs TA, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci USA. 2013;110:8674–8679. doi: 10.1073/pnas.1301059110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 96.Etulain J, Martinod K, Wong SL, Cifuni SM, Schattner M, Wagner DD. P-selectin promotes neutrophil extracellular trap formation in mice. Blood. 2015;126:242–246. doi: 10.1182/blood-2015-01-624023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thalin C, Demers M, Blomgren B, et al. NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb Res. 2016;139:56–64. doi: 10.1016/j.thromres.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cools-Lartigue J, Spicer J, McDonald B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446–3458. doi: 10.1172/JCI67484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guglietta S, Chiavelli A, Zagato E, et al. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat Commun. 2016;7:11037. doi: 10.1038/ncomms11037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tohme S, Yazdani HO, Al-Khafaji AB, et al. Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 2016;76:1367–1380. doi: 10.1158/0008-5472.CAN-15-1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berger-Achituv S, Brinkmann V, Abed UA, et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol. 2013;4:48. doi: 10.3389/fimmu.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Boone BA, Orlichenko L, Schapiro NE, et al. The receptor for advanced glycation end products (RAGE) enhances autophagy and neutrophil extracellular traps in pancreatic cancer. Cancer Gene Ther. 2015;22:326–334. doi: 10.1038/cgt.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alfaro C, Teijeira A, Onate C, et al. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs) Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-2463. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 104.Palumbo JS, Kombrinck KW, Drew AF, et al. Fibrinogen is an important determinant of the metastatic potential of circulating tumor cells. Blood. 2000;96:3302–3309. [PubMed] [Google Scholar]
- 105.Cedervall J, Zhang Y, Huang H, et al. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Cancer Res. 2015;75:2653–2662. doi: 10.1158/0008-5472.CAN-14-3299. [DOI] [PubMed] [Google Scholar]
- 106.Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. N Engl J Med. 2012;366:610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sharma D, Brummel-Ziedins KE, Bouchard BA, Holmes CE. Platelets in tumor progression: a host factor that offers multiple potential targets in the treatment of cancer. J Cell Physiol. 2014;229:1005–1015. doi: 10.1002/jcp.24539. [DOI] [PubMed] [Google Scholar]
- 108.Gotzmann J, Fischer AN, Zojer M, et al. A crucial function of PDGF in TGF-beta-mediated cancer progression of hepatocytes. Oncogene. 2006;25:3170–3185. doi: 10.1038/sj.onc.1209083. [DOI] [PubMed] [Google Scholar]
- 109.Jechlinger M, Sommer A, Moriggl R, et al. Autocrine PDGFR signaling promotes mammary cancer metastasis. J Clin Invest. 2006;116:1561–1570. doi: 10.1172/JCI24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20:576–590. doi: 10.1016/j.ccr.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ishida Y, Kondo T, Takayasu T, Iwakura Y, Mukaida N. The essential involvement of cross-talk between IFN-gamma and TGF-beta in the skin wound-healing process. J Immunol. 2004;172:1848–1855. doi: 10.4049/jimmunol.172.3.1848. [DOI] [PubMed] [Google Scholar]
- 112.Reynolds LE, Conti FJ, Lucas M, et al. Accelerated re-epithelialization in beta3-integrin-deficient-mice is associated with enhanced TGF-beta1 signaling. Nat Med. 2005;11:167–174. doi: 10.1038/nm1165. [DOI] [PubMed] [Google Scholar]
- 113.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 114.Mucida D, Park Y, Kim G, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 115.Terabe M, Matsui S, Park JM, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sagiv JY, Michaeli J, Assi S, et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015;10:562–573. doi: 10.1016/j.celrep.2014.12.039. [DOI] [PubMed] [Google Scholar]
- 118.Labelle M, Begum S, Hynes RO. Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci USA. 2014;111:E3053–E3061. doi: 10.1073/pnas.1411082111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hampton HR, Bailey J, Tomura M, Brink R, Chtanova T. Microbe-dependent lymphatic migration of neutrophils modulates lymphocyte proliferation in lymph nodes. Nat Commun. 2015;6:7139. doi: 10.1038/ncomms8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hampton HR, Chtanova T. The lymph node neutrophil. Semin Immunol. 2016;28:129–136. doi: 10.1016/j.smim.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 121.Lim K, Hyun YM, Lambert-Emo K, et al. Neutrophil trails guide influenza-specific CD8(+) T cells in the airways. Science. 2015;349:aaa4352. doi: 10.1126/science.aaa4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pillay J, Kamp VM, van Hoffen E, et al. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327–336. doi: 10.1172/JCI57990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Yang CW, Unanue ER. Neutrophils control the magnitude and spread of the immune response in a thromboxane A2-mediated process. J Exp Med. 2013;210:375–387. doi: 10.1084/jem.20122183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Langowski JL, Zhang X, Wu L, et al. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 125.Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nozawa H, Chiu C, Hanahan D. Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci USA. 2006;103:12493–12498. doi: 10.1073/pnas.0601807103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120:1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chung AS, Wu X, Zhuang G, et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19:1114–1123. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 129.Langowski JL, Kastelein RA, Oft M. Swords into plowshares: IL-23 repurposes tumor immune surveillance. Trends Immunol. 2007;28:207–212. doi: 10.1016/j.it.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 130.Grivennikov SI, Wang K, Mucida D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tosolini M, Kirilovsky A, Mlecnik B, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 132.Coffelt SB, Kersten K, Doornebal CW, et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Koyama S, Akbay EA, Li YY, et al. STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res. 2016;76:999–1008. doi: 10.1158/0008-5472.CAN-15-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zhou SL, Zhou ZJ, Hu ZQ, et al. Tumor-associated neutrophils recruit macrophages and t-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology. 2016;150:1646–1658. doi: 10.1053/j.gastro.2016.02.040. [DOI] [PubMed] [Google Scholar]
- 135.Jacobsen LC, Theilgaard-Monch K, Christensen EI, Borregaard N. Arginase 1 is expressed in myelocytes/metamyelocytes and localized in gelatinase granules of human neutrophils. Blood. 2007;109:3084–3087. doi: 10.1182/blood-2006-06-032599. [DOI] [PubMed] [Google Scholar]
- 136.Rotondo R, Barisione G, Mastracci L, et al. IL-8 induces exocytosis of arginase 1 by neutrophil polymorphonuclears in nonsmall cell lung cancer. Int J Cancer. 2009;125:887–893. doi: 10.1002/ijc.24448. [DOI] [PubMed] [Google Scholar]
- 137.Rotondo R, Bertolotto M, Barisione G, et al. Exocytosis of azurophil and arginase 1-containing granules by activated polymorphonuclear neutrophils is required to inhibit T lymphocyte proliferation. J Leukoc Biol. 2011;89:721–727. doi: 10.1189/jlb.1109737. [DOI] [PubMed] [Google Scholar]
- 138.Rodriguez PC, Ernstoff MS, Hernandez C, et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gomez Perdiguero E, Klapproth K, Schulz C, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 2015;518:547–551. doi: 10.1038/nature13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brown CC, Malech HL, Jacobson RJ, et al. Unique human neutrophil populations are defined by monoclonal antibody ED12F8C10. Cell Immunol. 1991;132:102–114. doi: 10.1016/0008-8749(91)90010-9. [DOI] [PubMed] [Google Scholar]
- 142.Gallin JI, Jacobson RJ, Seligmann B, et al. A marker of neutrophil heterogeneity reveals two forms of chronic myelogenous leukemia. Trans Assoc Am Physicians. 1985;98:166–170. [PubMed] [Google Scholar]
- 143.Seligmann B, Chused TM, Gallin JI. Human neutrophil heterogeneity identified using flow microfluorometry to monitor membrane potential. J Clin Invest. 1981;68:1125–1131. doi: 10.1172/JCI110356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Seligmann B, Malech HL, Melnick DA, Gallin JI. An antibody to a subpopulation of neutrophils demonstrates antigenic heterogeneity which correlates with response heterogeneity. Trans Assoc Am Physicians. 1984;97:319–324. [PubMed] [Google Scholar]
- 145.Seligmann B, Malech HL, Melnick DA, Gallin JI. An antibody binding to human neutrophils demonstrates antigenic heterogeneity detected early in myeloid maturation which correlates with functional heterogeneity of mature neutrophils. J Immunol. 1985;135:2647–2653. [PubMed] [Google Scholar]
- 146.Gallin JI. Human neutrophil heterogeneity exists, but is it meaningful? Blood. 1984;63:977–983. [PubMed] [Google Scholar]
- 147.Mishalian I, Bayuh R, Levy L, Zolotarov L, Michaeli J, Fridlender ZG. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol Immunother. 2013;62:1745–1756. doi: 10.1007/s00262-013-1476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Tazawa H, Okada F, Kobayashi T, et al. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol. 2003;163:2221–2232. doi: 10.1016/S0002-9440(10)63580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Scapini P, Lapinet-Vera JA, Gasperini S, Calzetti F, Bazzoni F, Cassatella MA. The neutrophil as a cellular source of chemokines. Immunol Rev. 2000;177:195–203. doi: 10.1034/j.1600-065x.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 150.Beauvillain C, Delneste Y, Scotet M, et al. Neutrophils efficiently cross-prime naive T cells in vivo. Blood. 2007;110:2965–2973. doi: 10.1182/blood-2006-12-063826. [DOI] [PubMed] [Google Scholar]
- 151.Duffy D, Perrin H, Abadie V, et al. Neutrophils transport antigen from the dermis to the bone marrow, initiating a source of memory CD8+ T cells. Immunity. 2012;37:917–929. doi: 10.1016/j.immuni.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 152.Geng S, Matsushima H, Okamoto T, et al. Emergence, origin, and function of neutrophil-dendritic cell hybrids in experimentally induced inflammatory lesions in mice. Blood. 2013;121:1690–1700. doi: 10.1182/blood-2012-07-445197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Matsushima H, Geng S, Lu R, et al. Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood. 2013;121:1677–1689. doi: 10.1182/blood-2012-07-445189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Winter WE, 3rd, Maxwell GL, Tian C, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 155.Zhang L, Conejo-Garcia JR, Katsaros D, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 156.Sato E, Olson SH, Ahn J, et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 158.Simpson-Abelson MR, Loyall JL, Lehman HK, et al. Human ovarian tumor ascites fluids rapidly and reversibly inhibit T cell receptor-induced NF-kappaB and NFAT signaling in tumor-associated T cells. Cancer Immun. 2013;13:14. [PMC free article] [PubMed] [Google Scholar]
- 159.Obermajer N, Wong JL, Edwards RP, et al. Induction and stability of human Th17 cells require endogenous NOS2 and cGMP-dependent NO signaling. J Exp Med. 2013;210:1433–1445. doi: 10.1084/jem.20121277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mhawech-Fauceglia P, Wang D, Ali L, et al. Intraepithelial T cells and tumor-associated macrophages in ovarian cancer patients. Cancer Immun. 2013;13:1. [PMC free article] [PubMed] [Google Scholar]
- 161.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71:7463–7470. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lo CW, Chen MW, Hsiao M, et al. IL-6 trans-signaling in formation and progression of malignant ascites in ovarian cancer. Cancer Res. 2011;71:424–434. doi: 10.1158/0008-5472.CAN-10-1496. [DOI] [PubMed] [Google Scholar]
- 163.Lane D, Matte I, Rancourt C, Piche A. Prognostic significance of IL-6 and IL-8 ascites levels in ovarian cancer patients. BMC Cancer. 2011;11:210. doi: 10.1186/1471-2407-11-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Giuntoli RL, 2nd, Webb TJ, Zoso A, et al. Ovarian cancer-associated ascites demonstrates altered immune environment: implications for antitumor immunity. Anticancer Res. 2009;29:2875–2884. [PubMed] [Google Scholar]
- 165.Obermajer N, Muthuswamy R, Odunsi KO, Edwards R, Kalinski P. PGE2-dependent CXCL12 production and CXCR4 expression control the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71:7463–7470. doi: 10.1158/0008-5472.CAN-11-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kryczek I, Wei S, Zhu G, et al. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67:8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 167.Naldini A, Morena E, Belotti D, Carraro F, Allavena P, Giavazzi R. Identification of thrombin-like activity in ovarian cancer associated ascites and modulation of multiple cytokine networks. Thromb Haemost. 2011;106:705–711. doi: 10.1160/TH11-05-0311. [DOI] [PubMed] [Google Scholar]
- 168.Wilhelm O, Hafter R, Henschen A, Schmitt M, Graeff H. Role of plasmin in the degradation of the stroma-derived fibrin in human ovarian carcinoma. Blood. 1990;75:1673–1678. [PubMed] [Google Scholar]
- 169.Zhang T, Ma Z, Wang R, et al. Thrombin facilitates invasion of ovarian cancer along peritoneum by inducing monocyte differentiation toward tumor-associated macrophage-like cells. Cancer Immunol Immunother. 2010;59:1097–1108. doi: 10.1007/s00262-010-0836-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cho MS, Bottsford-Miller J, Vasquez HG, et al. Platelets increase the proliferation of ovarian cancer cells. Blood. 2012;120:4869–4872. doi: 10.1182/blood-2012-06-438598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bottsford-Miller J, Choi HJ, Dalton HJ, et al. Differential platelet levels affect response to taxane-based therapy in ovarian cancer. Clin Cancer Res. 2015;21:602–610. doi: 10.1158/1078-0432.CCR-14-0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bunt SK, Mohr AM, Bailey JM, Grandgenett PM, Hollingsworth MA. Rosiglitazone and Gemcitabine in combination reduces immune suppression and modulates T cell populations in pancreatic cancer. Cancer Immunol Immunother. 2012;62:225–236. doi: 10.1007/s00262-012-1324-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vincent J, Mignot G, Chalmin F, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–3061. doi: 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- 174.Bruchard M, Mignot G, Derangere V, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med. 2013;19:57–64. doi: 10.1038/nm.2999. [DOI] [PubMed] [Google Scholar]
- 175.Ahn GO, Tseng D, Liao CH, Dorie MJ, Czechowicz A, Brown JM. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc Natl Acad Sci USA. 2010;107:8363–8368. doi: 10.1073/pnas.0911378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Predina J, Eruslanov E, Judy B, et al. Changes in the local tumor microenvironment in recurrent cancers may explain the failure of vaccines after surgery. Proc Natl Acad Sci USA. 2013;110:E415–E424. doi: 10.1073/pnas.1211850110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Crawford J, Armitage J, Balducci L, et al. Myeloid growth factors. J Natl Compr Canc Netw. 2013;11:1266–1290. doi: 10.6004/jnccn.2013.0148. [DOI] [PubMed] [Google Scholar]
- 178.Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24:3187–3205. doi: 10.1200/JCO.2006.06.4451. [DOI] [PubMed] [Google Scholar]
- 179.Abrams SI, Waight JD. Identification of a G-CSF-Granulocytic MDSC axis that promotes tumor progression. Oncoimmunology. 2012;1:550–551. doi: 10.4161/onci.19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kawano M, Mabuchi S, Matsumoto Y, et al. The significance of G-CSF expression and myeloid-derived suppressor cells in the chemoresistance of uterine cervical cancer. Sci Rep. 2015;5:18217. doi: 10.1038/srep18217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Takeuchi S, Baghdadi M, Tsuchikawa T, et al. Chemotherapy-derived inflammatory responses accelerate the formation of immunosuppressive myeloid cells in the tissue microenvironment of human pancreatic cancer. Cancer Res. 2015;75:2629–2640. doi: 10.1158/0008-5472.CAN-14-2921. [DOI] [PubMed] [Google Scholar]
- 182.Granot Z, Fridlender ZG. Plasticity beyond cancer cells and the “immunosuppressive switch”. Cancer Res. 2015;75:4441–4445. doi: 10.1158/0008-5472.CAN-15-1502. [DOI] [PubMed] [Google Scholar]
- 183.Andzinski L, Kasnitz N, Stahnke S, et al. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int J Cancer. 2016;138:1982–1993. doi: 10.1002/ijc.29945. [DOI] [PubMed] [Google Scholar]
- 184.McInturff AM, Cody MJ, Elliott EA, et al. Mammalian target of rapamycin regulates neutrophil extracellular trap formation via induction of hypoxia-inducible factor 1 alpha. Blood. 2012;120:3118–3125. doi: 10.1182/blood-2012-01-405993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hakkim A, Fuchs TA, Martinez NE, et al. Activation of the Raf-MEK-ERK pathway is required for neutrophil extracellular trap formation. Nat Chem Biol. 2011;7:75–77. doi: 10.1038/nchembio.496. [DOI] [PubMed] [Google Scholar]
- 186.Knight JS, Zhao W, Luo W, et al. Peptidylarginine deiminase inhibition is immunomodulatory and vasculoprotective in murine lupus. J Clin Invest. 2013;123:2981–2993. doi: 10.1172/JCI67390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Knight JS, Luo W, O’Dell AA, et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res. 2014;114:947–956. doi: 10.1161/CIRCRESAHA.114.303312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Knight JS, Subramanian V, O’Dell AA, et al. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann Rheum Dis. 2014;74:2199–2206. doi: 10.1136/annrheumdis-2014-205365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shishikura K, Horiuchi T, Sakata N, et al. Prostaglandin E2 inhibits neutrophil extracellular trap formation through production of cyclic AMP. Br J Pharmacol. 2016;173:319–331. doi: 10.1111/bph.13373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chu D, Zhao Q, Yu J, Zhang F, Zhang H, Wang Z. Nanoparticle targeting of neutrophils for improved cancer immunotherapy. Adv Healthc Mater. 2016;5:1088–1093. doi: 10.1002/adhm.201500998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhang D, Chen G, Manwani D, et al. Neutrophil ageing is regulated by the microbiome. Nature. 2015;525:528–532. doi: 10.1038/nature15367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Hergott CB, Roche AM, Tamashiro E, et al. Peptidoglycan from the gut microbiota governs the lifespan of circulating phagocytes at homeostasis. Blood. 2016;127:2460–2471. doi: 10.1182/blood-2015-10-675173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zitvogel L, Galluzzi L, Viaud S, et al. Cancer and the gut microbiota: an unexpected link. Sci Transl Med. 2015;7:271ps1. doi: 10.1126/scitranslmed.3010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science. 2015;350:1084–1089. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Vetizou M, Pitt JM, Daillere R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350:1079–1084. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Netea MG, Latz E, Mills KH, O’Neill LA. Innate immune memory: a paradigm shift in understanding host defense. Nat Immunol. 2015;16:675–679. doi: 10.1038/ni.3178. [DOI] [PubMed] [Google Scholar]
- 197.Cheng SC, Quintin J, Cramer RA, et al. mTOR-and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Saeed S, Quintin J, Kerstens HH, et al. Epigenetic programming of monocyte-to-macrophage differentiation and trained innate immunity. Science. 2014;345:1251086. doi: 10.1126/science.1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Chen F, Wu W, Millman A, et al. Neutrophils prime a long-lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol. 2014;15:938–946. doi: 10.1038/ni.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Netea MG, Joosten LA, Latz E, et al. Trained immunity: a program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]