Abstract
Background and Purpose
In many neurologic diagnoses significant inter-individual variability exits in the outcomes of rehabilitation. One factor that may impact response to rehabilitation interventions is genetic variation. Genetic variation refers to the presence of differences in the DNA sequence among individuals in a population. Genetic polymorphisms are variations that occur relatively commonly and, while not disease-causing, can impact the function of biological systems. The purpose of this article is to describe genetic polymorphisms that may impact neuroplasticity, motor learning, and recovery after stroke.
Summary of Key Points
Genetic polymorphisms for brain-derived neurotrophic factor (BDNF), dopamine, and apolipoprotein E have been shown to impact neuroplasticity and motor learning. Rehabilitation interventions that rely on the molecular and cellular pathways of these factors may be impacted by the presence of the polymorphism. For example, it has been hypothesized that individuals with the BDNF polymorphism may show a decreased response to neuroplasticity-based interventions, decreased rate of learning, and overall less recovery after stroke. However, research to date has been limited and additional work is needed to fully understand the role of genetic variation in learning and recovery.
Recommendations for Clinical Practice
Genetic polymorphisms should be considered as possible predictors or covariates in studies that investigate neuroplasticity, motor learning, or motor recovery after stroke. Future predictive models of stroke recovery will likely include a combination of genetic factors and other traditional factors (e.g. age, lesion type, corticospinal tract integrity) to determine an individual’s expected response to a specific rehabilitation intervention.
Keywords: polymorphism, motor learning, recovery, predictive models
Introduction
Rehabilitation outcomes in individuals with neurologic diagnoses involve a complex interaction of baseline status, disease severity, individual factors, and treatment content including intensity and dose. Despite a growing body of research in neurologic rehabilitation in the last few decades, the optimal content and dose of rehabilitation interventions in most clinical diagnoses remains unclear. Research on the key ingredients of treatment continues, but an improved understanding of how individual factors interact with these key ingredients is also needed in order to provide clinicians with the information needed to match each client with the most appropriate intervention for optimal outcomes. Increasing evidence suggests that genetic variation may partially explain the frequently reported variability in individual responses to neuroplasticity-based interventions in a manner that could have implications for rehabilitation interventions.1, 2
Genetic variation refers to the presence of differences in the DNA sequence among members of a given population. Most clinicians are familiar with genetic mutation, a relatively uncommon variation in DNA that results in significant functional changes and is often disease-causing (e.g. Huntington’s disease). The focus of this article, however, is genetic polymorphisms. Genetic polymorphisms are relatively frequent variations in DNA among individuals within a population (>1%) that are not directly disease-causing but that can impact underlying systems, especially when interacting with certain other genetic variants or environmental conditions.3, 4 A common type of polymorphism is a single-nucleotide polymorphism (SNP), a variation of the genetic code in a single base pair wherein one nucleotide has been exchanged for another. A single SNP or a set of SNPs can lead to biological variations in cellular and molecular processes that may have functional consequences. Many other types of polymorphisms exist, such as insertion or deletion of a nucleotide or repeats of a set of nucleotides in the DNA sequence, including a variable number of tandem repeats.3, 4 Table 1 provides an overview of several genetic terms and definitions that will be used this article as well as other articles in this research area.
Table 1.
Genetic terms and definitions
| Term | Definition |
|---|---|
| Genetics | Study of genes and genetic variation |
| Epigenetics | Study of how external factors affect gene expression or transcription that are unrelated to the DNA sequence |
| Genetic Mutation | Rare genetic variant (<1% of the population) that results in significant functional change |
| Genetic Polymorphism | Common genetic variant (>1% of the population) that results in relatively small effect on behavior or phenotype |
| Genotype | Set of alleles inherited for a particular gene or polymorphism |
| Allele | One version of a gene or polymorphism; an individual inherits two alleles for each gene or polymorphism, one from each parent |
| Phenotype | Set of behavioral or clinical features that represent the expression of a gene or set of genes and environmental factors |
| Endophenotype | Measurement (behavioral, imaging, biochemical) linked to a genotype that is useful for distinguishing biological subgroups that look the same clinically |
| Epistasis | When the expression of one gene is modified by another gene |
Neuroplasticity serves as the proposed mechanism for numerous interventions in neurologic rehabilitation.5 Specifically, interventions that provide repetitive, challenging and progressive practice of goal-oriented, functional tasks are thought to rely on neuroplastic changes in order to have long lasting effects.6 Additionally, newer techniques aimed at augmenting behavioral practice, such as an acute bout of exercise7 and non-invasive brain stimulation8, are thought to be beneficial through their impact on neuroplasticity. Therefore, SNPs that have an effect on neuroplasticity may be particularly relevant to neurologic rehabilitation. It has been suggested that individuals with genetic variants associated with reductions in some features of neuroplasticity might respond differently to interventions that engage neuroplastic processes, as compared to individuals without those variants.
The purpose of this article is to describe examples of genetic polymorphisms that may impact neuroplasticity, learning, and recovery after a neurologic insult. Examples of how the presence of these polymorphisms can affect individual response to a period of motor practice will be provided. Additionally, using the brain derived neurotrophic factor (BDNF) polymorphism as an example, we will discuss the role of genetic variation in current and future predictive models of motor recovery and response to behavioral interventions after stroke.
Genetic Polymorphisms, Neuroplasticity and Motor Learning
Key examples of genetic polymorphisms that can impact neuroplasticity include polymorphisms for BDNF, dopamine, and apolipoprotein E. While the effect of each polymorphism lies at the cellular/molecular level of the nervous system, these variations may affect neuroplasticity in a manner that impacts learning and recovery through gene-environment interactions. Specifically, in some cases the effect of a polymorphism may not be seen with respect to baseline status but instead emerges when an individual interacts with the environment. Within the context of physical therapy and rehabilitation, ‘environment’ can be viewed as the behavioral interventions or other therapeutic approaches that involve the cellular pathway influenced by the genetic polymorphism. A key point is that response to a therapeutic intervention may be impacted by genetic variation if the mechanism of action of the intervention involves these neuroplastic pathways.
Brain-Derived Neurotrophic Factor Val66Met Polymorphism
BDNF is a neurotrophin found throughout the brain that is important for both neuroprotection and neuroplasticity.9 BDNF plays a crucial role in enhancing synaptic transmission and facilitating long-term potentiation in a manner that supports learning.7, 10, 11 BDNF release is activity-dependent,12 and increased levels of BDNF have been reported in response to a period of skill learning13 and an acute bout of aerobic exercise.14, 15 A relatively common SNP for BDNF (rs6265), where a substitution from guanine to adenine occurs at nucleotide 196, results in an amino acid substitution at codon 66 from valine to methionine (val66met). Individuals with one or two copies of the met allele are defined as having the BDNF val66met polymorphism and show a decrease in activity-dependent release of BDNF.16 The frequency of the BDNF polymorphism varies by ethnic group. Approximately 30% of individuals in the United States of European descent have the BDNF polymorphism. Polymorphism frequencies are higher in Italy and Japan at approximately 50% and 65%, respectively.17
Presence of the BDNF polymorphism can have both neural and behavioral effects. Individuals with the polymorphism show differences in neural plasticity in response to a period of practice of a repetitive finger task.18 In fact, such differences may be seen during simple finger movements in nondisabled individuals19 and in individuals with hemiparetic stroke,20 although effects may be decreased in older adults.21 Additionally, protocols aimed at facilitating neuroplasticity may have differing effects in those with and without the met allele. Response to several non-invasive brain stimulation protocols has been shown to differ based on the presence of one or more copies of the met allele including repetitive transcranial magnetic stimulation,22–24 transcranial direct current stimulation,25 and paired associative stimulation.22, 26 Behaviorally, motor skill learning may also be affected by the BDNF polymorphism. Individuals with the met allele show an overall decrease in learning19, 27 and a reduced rate of learning.28 Studies showing no effect of the BDNF polymorphism on plasticity and motor learning are also present in the literature.29, 30 While these studies had small sample sizes and used stimulation protocols that may have been suboptimal in engaging BDNF processes, they highlight the need for continued work to determine the interaction between BDNF genotype and various intervention approaches and parameters.
Dopamine Polymorphisms
Dopamine is a neurotransmitter that plays an important role in numerous fundamental central nervous system processes including movement, mood, addiction, reward, impulse control, and learning.31–38 Dopamine and dopamine receptors are found both in the basal ganglia and cerebral cortex.39 Interruption of dopamine connections to primary motor cortex interferes with motor skill learning,40, 41 suggesting an essential role for dopamine in this process. Several genetic polymorphisms affect dopamine neurotransmission including within genes that code for catechol-o-methyltransferase (COMT), dopamine transporter protein (DAT), and dopamine receptors D1, D2 and D3. Presence of one or more of these polymorphisms results in either increased or decreased dopamine neurotransmission, depending on the polymorphism.42 Overall, individuals with one of the dopamine polymorphisms that leads to relatively less dopamine activity tend to have conditions associated with a hypodopaminergic state such as poorer working memory,43 attention deficit hyperactivity disorder,44 and reduced dopamine binding to D2 receptors.45
Gene scores that sum the effects of multiple polymorphisms can provide substantially improved insights for some46–48 though not all49, 50 biological systems. Recently, a dopamine gene score combining the effects of five polymorphisms has been studied and found to be a significant predictor of motor learning and its improvement by L-Dopa,42 depression scores in both healthy and depressed populations,51 and impulsivity and its improvement by the dopaminergic drug Ropinirole.52 These findings emphasize the value of considering multiple sources of genetic variation, at least for systems such as brain dopamine, and provide examples of applying genetic research findings to individualized medical care.
Genetic polymorphisms that affect dopamine transmission may be particularly relevant in the management of Parkinson disease. Death of dopaminergic neurons in the substantia nigra lead to dopamine deficiency in individuals with Parkinson disease with dopamine replacement therapy being a common pharmacological intervention.53 While research is still in its early stages, recent work suggests that the presence of polymorphisms in the dopamine system in individuals with Parkinson disease may impact working memory and executive function54–56 and be related to changes in brain activation in cortico-striatal networks.57–59 Additionally, genotype may impact the effectiveness of dopamine drug therapies on motor symptoms60, 61 and motor sequence learning62 in those with Parkinson disease. Continued research is needed to fully understand the interaction between disease severity, drug therapies, motor behavior, and genotype in this clinical population and whether these interactions impact response to rehabilitation interventions.
Apolipoprotein E Polymorphism
Apolipoprotein E (ApoE) is a lipoprotein found in the brain that plays a role in neuronal processes related to repair and recovery.63–65 There are three variants of the ApoE gene at two amino acid positions that can impact the structure and function of ApoE: ε2, ε3, and ε4.2 Several ApoE genotypes occur at varying frequencies in the population and are based on the alleles present at each position (e.g. ε2/ε3, ε3/ε3, ε3/ε4). ApoE ε3 has a neuroprotective effect that protects against neurodegeneration and cognitive decline, while ApoE ε4 has the opposite effect, resulting in an overall negative influence on several neuroplastic processes.66, 67 The ApoE ε4 allele is a risk factor for Alzheimer’s disease68 and is related to poorer outcome after traumatic brain injury69 and stroke.70–72
BDNF Val66Met Polymorphism, Neuroplasticity and Motor Learning after Stroke
While there are several genetic polymorphisms that may impact neuroplasticity and motor learning after stroke, the BDNF polymorphism has received the most attention in the literature and, as such, will be the focus here. As described above, BDNF is thought to play an important role in neuroplasticity. Therefore, the BDNF polymorphism is most likely to have an effect on response to motor practice that engages neuroplastic processes aimed at motor skill learning or re-learning (Figure 1), such as challenging and progressive task-oriented training.6 The BDNF polymorphism may also be relevant in understanding individual response to interventions aimed at augmenting motor practice through the facilitation of neuroplasticity via BDNF dependent processes, such as non-invasive brain stimulation27 or an acute bout of aerobic exercise.73, 74 Overall, research to date suggests that differences in neuroplasticity may be present in individuals post-stroke who have one or more copies of the met allele. Brain activation during hand movement is decreased in individuals post-stroke with the BDNF polymorphism compared to individuals without the polymorphism,20 consistent with previous work in neurologically health individuals.19 In nondisabled individuals, response to several non-invasive brain stimulation protocols has also been shown to differ based on presence of a met allele.22–26 While research is still in its early stages, differences in response to these stimulation techniques after stroke appear to also be present.75, 76 In fact, the BDNF polymorphism has been suggested as one possible factor in explaining the relatively high variability in response to brain stimulation seen in many studies.77
Figure 1.
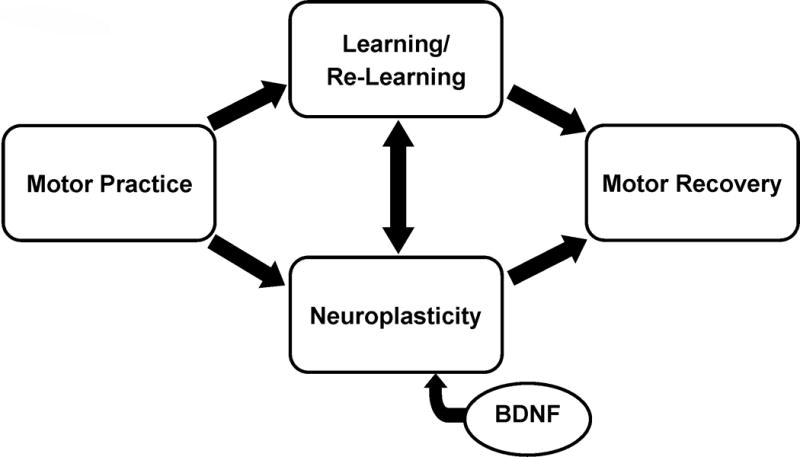
Schematic on the proposed role of BDNF and the val66met BDNF polymorphism in rehabilitation after stroke. Intervention approaches that include motor practice aimed at driving neuroplasticity, and therefore learning or re-learning of skilled actions, are thought to rely on BDNF dependent processes. Individuals with the BDNF polymorphism may have differences in the neuroplastic response to motor practice that may impact motor learning and, ultimately, motor recovery. Rehabilitation approaches aimed at facilitating motor recovery that do not engage these neuroplastic/learning processes may not be affected by the BDNF polymorphism.
The effect of the BDNF polymorphism on motor learning after stroke has been less studied, and to our knowledge only one study has investigated the polymorphism’s effect on motor learning after stroke. In a study of gait adaptation during split-belt treadmill walking in individuals with chronic stroke, while total adaptation did not differ between individuals with and without the polymorphism, the rate of adaption did differ. Individuals without the polymorphism (val/val) showed significant changes in the first 30 trials of adaption, however individuals with the polymorphism (met) did not. Instead, the group with the met allele continued to show change with practice until reaching a similar level of total adaption at the end of practice.78 These findings suggest that individuals post-stroke who have the met allele may need additional practice to achieve the same behavioral outcome compared to those without the met allele, consistent with a similar report in nondisabled individuals.28
BDNF Val66Met Polymorphism and Motor Recovery after Stroke
Several studies have shown that the BDNF polymorphism may impact recovery after stroke. In individuals with one or more copies of the met allele, overall recovery after stroke is less compared to those without the allele.72, 79–82 These studies are an important step in understanding the role of this polymorphism in the variability in recovery seen after stroke. Most studies in this area to date, however, have a few limitations that may impact the application of this work to clinical decision making in physical therapy. First, study endpoints have tended to be a few weeks to a few months post-stroke. In order to fully understand the role of the BDNF polymorphism in motor recovery after stroke, studies that follow individuals for longer durations are needed. Second, description of the content, parameters, and dose of motor behavioral interventions was often not provided. While the optimal content and dose of therapeutic interventions are still under debate, the BDNF polymorphism may not be relevant in predicting motor recovery if interventions do not target neuroplasticity or are of insufficient intensity to drive neuroplasticity. Finally, most studies have measured and defined ‘recovery’ based on scores on the Modified Rankin Scale (mRS) or the National Institutes of Health Stroke Scale (NIHSS). These are valid and reliable scales that are frequently used to measure outcome in research studies in stroke. However, the granularity with which these measures define motor recovery is limited. For example, ‘good’ recovery on the mRS is often defined as a score or 0–1, indicating no significant disability.83 However, individuals with a mRS score of 0–1 often report continued difficulty in using the paretic hand to perform functional tasks and decreased paretic arm use,84 suggesting this measure may not be sufficiently sensitive to change in studies aimed at understanding motor recovery in the upper extremity. Motor system specific outcome measures85 are needed in future studies on genetic variation and motor recovery after stroke.
Two recent studies have included the BDNF polymorphism as a possible predictor of response to a defined period of upper extremity motor training.86, 87 The presence of the met allele did not significantly predict treatment response in either study. Both studies, however, included individuals post-stroke with a wide range of motor impairment and brain structure and function. While variability in clinical presentation can be important to understand some factors that predict treatment response, other factors may be predictors only in certain stroke subtypes or in response to specific interventions. A recent study of BDNF polymorphism found no effect on response to a period of motor training when considering all individuals in a single analysis. However, when individuals were grouped based on level of motor function, presence of the met allele predicted less motor improvement in those with moderate to high motor function but not those with low motor function.88 Subgroups based on lesion type (e.g. cortical, subcortical), level of corticospinal tract integrity,89 or residual motor system functional and structural connectivity90 may also be relevant in determining the role of genetic variation in motor recovery after stroke.
Future Research Directions
While there is evidence that the BDNF polymorphism impacts neuroplasticity and motor learning in humans, additional mechanistic research in this area is needed. For example, while differences in response to non-invasive stimulation in individuals with the met allele have been reported,75, 76 a full understanding of these differences and the development of stimulation protocols optimized for individuals with the met allele have yet to be described. Non-invasive brain stimulation may be an effective adjunct to behavioral practice in individuals with the polymorphism, however, the optimal parameters and dose of stimulation may be different than in individuals without the polymorphism. Additionally, the type and intensity of motor practice may be a factor in the impact of the BDNF polymorphism on motor learning. The type of learning task (e.g. sequence, adaptation, etc.), level of task difficulty, and conditions of practice (e.g. random order practice, external focus of attention, etc.) may impact differences in learning between individuals with and without the met allele. The effect of the BDNF polymorphism on motor learning is likely influenced by the extent to which motor practice engages neuroplasticity and the pathway of action of BDNF.
Future studies on the effect of the BDNF polymorphism on motor recovery after stroke should consider the role of stroke subtype or classification. In other clinical domains, research on stroke risk, prognosis, and treatment often stratify by stroke subtype in order to group clients according to specific criteria. Clinical presentation, treatment approach, and treatment response may be different based on stroke subtype.91 For example, two recent studies in the motor system suggested that neuroplasticity differs as a function of stroke subtype.86, 92 A full understanding of the role of the BDNF polymorphism on motor recovery after stroke will therefore benefit from an investigation of its effect in different stroke subtypes. Stroke classification schemas from other professions may be helpful in understanding the relationship between genetic variation and motor recovery. However, classification schemas specific to recovery and rehabilitation may be needed. For example, the presence of the BDNF polymorphism may not be relevant to motor recovery in individuals with limited motor capacity88 or little to no remaining integrity in important neural pathways such as the corticospinal tract.
This review has focused on a single BDNF polymorphism. However, multiple polymorphisms may combine or interact to have a net effect on neuroplasticity and motor learning. Several polymorphisms that impact the same cellular pathway may combine to impact neuroplasticity, as was described above for the dopamine polygene score. Alternatively, polymorphisms from different cellular pathways may combine or interact. For example, the effect of the BDNF polymorphism on neuroplasticity has been shown to be impacted by the presence of the COMT polymorphism.93 A polygene score that combines the effect of multiple polymorphisms may be useful to best understand the effect of the BDNF polymorphism or other polymorphisms on neuroplasticity, motor learning, and motor recovery after stroke.
Genetic variation alone will not predict motor recovery. While several variables have been reported as possible predictors of upper extremity recovery after stroke such as age, initial level of impairment, degree of corticospinal tract integrity, and measures of brain function,86, 94, 95 consensus in the literature is lacking.96 It will likely be a combination of genetic factors and these traditional variables that best predicts response to rehabilitation interventions.97 Additionally, predictive models may vary based on stroke subtype, intervention content, and the dependent variable being predicted (e.g. measures of motor impairment versus motor function). Large-scale studies that include motor system outcome measures and detailed descriptions of rehabilitation interventions are needed to determine what combination of traditional factors and genetic factors predict motor recovery after stroke. Ultimately, these predictive models must be tested in a prospective manner to understand whether basing clinical decisions about treatment type and dose on a set of baseline variables improves individual rehabilitation outcomes.
Conclusions
Variability in response to rehabilitation interventions is commonly reported in both clinical care and research studies in a variety of neurologic diagnoses. Understanding variables that predict response to specific intervention protocols will provide clinicians with information that can be used in clinical decision-making, improving the ability of physical therapists to match the right intervention content and dose with the right individual. Genetic variation may be one of several factors that impact an individual’s response to an intervention and may be useful in future decision-making models. There are several genetic polymorphisms that can impact neuroplasticity. Individuals with polymorphisms that decrease neuroplasticity may require different types, parameters, or doses of an intervention to achieve similar outcomes to those achieved by individuals without the polymorphisms. Future work will continue to investigate the role of genetic variation, along with other factors, in predicting individual response to rehabilitation interventions.
Acknowledgments
This work was supported by grants NIH R03 HD087481 and American Heart Association 15SDG24970011 to JCS and NIH K24 HD074722 to SCC. Steven C. Cramer has served as a consultant for MicroTransponder, Dart Neuroscience, and Roche.
Funding: NIH R03 HD87481 and AHA 15SDG24970011 to JCS and NIH K24 HD074722 to SCC
Footnotes
Disclosures: Steven C. Cramer has served as a consultant for MicroTransponder, Dart Neuroscience, and Roche.
The content of this manuscript was presented as part of the IV STEP Conference in Columbus, OH in July 2016.
References
- 1.Cheeran BJ, Ritter C, Rothwell JC, Siebner HR. Mapping genetic influences on the corticospinal motor system in humans. Neuroscience. 2009;164(1):156–163. doi: 10.1016/j.neuroscience.2009.01.054. [DOI] [PubMed] [Google Scholar]
- 2.Pearson-Fuhrhop KM, Kleim JA, Cramer SC. Brain plasticity and genetic factors. Top Stroke Rehabil. 2009;16(4):282–299. doi: 10.1310/tsr1604-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Musunuru K, Hickey KT, Al-Khatib SM, et al. Basic concepts and potential applications of genetics and genomics for cardiovascular and stroke clinicians: a scientific statement from the American Heart Association. Circulation Cardiovascular Genetics. 2015;8(1):216–242. doi: 10.1161/HCG.0000000000000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth SM. Genetics Primer for Exercise Science and Health. Champaign, IL: Human Kinetics; 2007. [Google Scholar]
- 5.Cramer SC, Sur M, Dobkin BH, et al. Harnessing neuroplasticity for clinical applications. Brain : a journal of neurology. 2011;134(Pt 6):1591–1609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winstein CJ, Wolf SL. Task-oriented training to promote upper extremity recovery. In: Stein J, RL H, Macko R, Winstein C, Zorowitz R, editors. Stroke recovery & rehabilitation. New York: Demos Medical; 2008. pp. 267–290. [Google Scholar]
- 7.Mang CS, Campbell KL, Ross CJ, Boyd LA. Promoting neuroplasticity for motor rehabilitation after stroke: considering the effects of aerobic exercise and genetic variation on brain-derived neurotrophic factor. Phys Ther. 2013;93(12):1707–1716. doi: 10.2522/ptj.20130053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liew SL, Santarnecchi E, Buch ER, Cohen LG. Non-invasive brain stimulation in neurorehabilitation: local and distant effects for motor recovery. Front Hum Neurosci. 2014;8:378. doi: 10.3389/fnhum.2014.00378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25(6):295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 10.Kleim JA, Jones TA, Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochemical research. 2003;28(11):1757–1769. doi: 10.1023/a:1026025408742. [DOI] [PubMed] [Google Scholar]
- 11.Casey BJ, Glatt CE, Tottenham N, et al. Brain-derived neurotrophic factor as a model system for examining gene by environment interactions across development. Neuroscience. 2009;164(1):108–120. doi: 10.1016/j.neuroscience.2009.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci. 2002;22(23):10399–10407. doi: 10.1523/JNEUROSCI.22-23-10399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ploughman M, Windle V, MacLellan CL, White N, Dore JJ, Corbett D. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke; a journal of cerebral circulation. 2009;40(4):1490–1495. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-Pinilla F, Ying Z, Roy RR, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. Journal of neurophysiology. 2002;88(5):2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- 15.MacLellan CL, Keough MB, Granter-Button S, Chernenko GA, Butt S, Corbett D. A critical threshold of rehabilitation involving brain-derived neurotrophic factor is required for poststroke recovery. Neurorehabilitation and neural repair. 2011;25(8):740–748. doi: 10.1177/1545968311407517. [DOI] [PubMed] [Google Scholar]
- 16.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112(2):257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 17.Shimizu E, Hashimoto K, Iyo M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2004;126b(1):122–123. doi: 10.1002/ajmg.b.20118. [DOI] [PubMed] [Google Scholar]
- 18.Kleim JA, Chan S, Pringle E, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9(6):735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- 19.McHughen SA, Rodriguez PF, Kleim JA, et al. BDNF val66met polymorphism influences motor system function in the human brain. Cereb Cortex. 2010;20(5):1254–1262. doi: 10.1093/cercor/bhp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim DY, Quinlan EB, Gramer R, Cramer SC. BDNF Val66Met Polymorphism Is Related to Motor System Function After Stroke. Phys Ther. 2016;96(4):533–539. doi: 10.2522/ptj.20150135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McHughen SA, Cramer SC. The BDNF val(66)met polymorphism is not related to motor function or short-term cortical plasticity in elderly subjects. Brain Res. 2013;1495:1–10. doi: 10.1016/j.brainres.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheeran B, Talelli P, Mori F, et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J Physiol. 2008;586(23):5717–5725. doi: 10.1113/jphysiol.2008.159905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang JM, Kim YH, Yoon KJ, Uhm KE, Chang WH. Different responses to facilitatory rTMS according to BDNF genotype. Clin Neurophysiol. 2015;126(7):1348–1353. doi: 10.1016/j.clinph.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 24.Lee M, Kim SE, Kim WS, et al. Interaction of motor training and intermittent theta burst stimulation in modulating motor cortical plasticity: influence of BDNF Val66Met polymorphism. PLoS One. 2013;8(2):e57690. doi: 10.1371/journal.pone.0057690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Antal A, Chaieb L, Moliadze V, et al. Brain-derived neurotrophic factor (BDNF) gene polymorphisms shape cortical plasticity in humans. Brain stimulation. 2010;3(4):230–237. doi: 10.1016/j.brs.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Cirillo J, Hughes J, Ridding M, Thomas PQ, Semmler JG. Differential modulation of motor cortex excitability in BDNF Met allele carriers following experimentally induced and use-dependent plasticity. Eur J Neurosci. 2012;36(5):2640–2649. doi: 10.1111/j.1460-9568.2012.08177.x. [DOI] [PubMed] [Google Scholar]
- 27.Fritsch B, Reis J, Martinowich K, et al. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66(2):198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joundi RA, Lopez-Alonso V, Lago A, et al. The effect of BDNF val66met polymorphism on visuomotor adaptation. Exp Brain Res. 2012;223(1):43–50. doi: 10.1007/s00221-012-3239-9. [DOI] [PubMed] [Google Scholar]
- 29.Li Voti P, Conte A, Suppa A, et al. Correlation between cortical plasticity, motor learning and BDNF genotype in healthy subjects. Exp Brain Res. 2011;212(1):91–99. doi: 10.1007/s00221-011-2700-5. [DOI] [PubMed] [Google Scholar]
- 30.Nakamura K, Enomoto H, Hanajima R, et al. Quadri-pulse stimulation (QPS) induced LTP/LTD was not affected by Val66Met polymorphism in the brain-derived neurotrophic factor (BDNF) gene. Neurosci Lett. 2011;487(3):264–267. doi: 10.1016/j.neulet.2010.10.034. [DOI] [PubMed] [Google Scholar]
- 31.Beninger RJ. The role of dopamine in locomotor activity and learning. Brain research. 1983;287(2):173–196. doi: 10.1016/0165-0173(83)90038-3. [DOI] [PubMed] [Google Scholar]
- 32.Nieoullon A. Dopamine and the regulation of cognition and attention. Progress in neurobiology. 2002;67(1):53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- 33.Egerton A, Mehta MA, Montgomery AJ, et al. The dopaminergic basis of human behaviors: A review of molecular imaging studies. Neuroscience and biobehavioral reviews. 2009;33(7):1109–1132. doi: 10.1016/j.neubiorev.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Opmeer EM, Kortekaas R, Aleman A. Depression and the role of genes involved in dopamine metabolism and signalling. Progress in neurobiology. 2010;92(2):112–133. doi: 10.1016/j.pneurobio.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 35.Blum K, Chen AL, Giordano J, et al. The addictive brain: all roads lead to dopamine. J Psychoactive Drugs. 2012;44(2):134–143. doi: 10.1080/02791072.2012.685407. [DOI] [PubMed] [Google Scholar]
- 36.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76(3):470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tritsch NX, Sabatini BL. Dopaminergic modulation of synaptic transmission in cortex and striatum. Neuron. 2012;76(1):33–50. doi: 10.1016/j.neuron.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hosp JA, Luft AR. Dopaminergic Meso-Cortical Projections to M1: Role in Motor Learning and Motor Cortex Plasticity. Front Neurol. 2013;4:145. doi: 10.3389/fneur.2013.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Calabresi P, Picconi B, Tozzi A, Di Filippo M. Dopamine-mediated regulation of corticostriatal synaptic plasticity. Trends in neurosciences. 2007;30(5):211–219. doi: 10.1016/j.tins.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Molina-Luna K, Pekanovic A, Rohrich S, et al. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS One. 2009;4(9):e7082. doi: 10.1371/journal.pone.0007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hosp JA, Pekanovic A, Rioult-Pedotti MS, Luft AR. Dopaminergic projections from midbrain to primary motor cortex mediate motor skill learning. J Neurosci. 2011;31(7):2481–2487. doi: 10.1523/JNEUROSCI.5411-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pearson-Fuhrhop KM, Minton B, Acevedo D, Shahbaba B, Cramer SC. Genetic variation in the human brain dopamine system influences motor learning and its modulation by L-Dopa. PLoS One. 2013;8(4):e61197. doi: 10.1371/journal.pone.0061197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6917–6922. doi: 10.1073/pnas.111134598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gilbert DL, Wang Z, Sallee FR, et al. Dopamine transporter genotype influences the physiological response to medication in ADHD. Brain. 2006;129(Pt 8):2038–2046. doi: 10.1093/brain/awl147. [DOI] [PubMed] [Google Scholar]
- 45.Thompson J, Thomas N, Singleton A, et al. D2 dopamine receptor gene (DRD2) Taq1 A polymorphism: reduced dopamine D2 receptor binding in the human striatum associated with the A1 allele. Pharmacogenetics. 1997;7(6):479–484. doi: 10.1097/00008571-199712000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Zheng SL, Sun J, Wiklund F, et al. Cumulative association of five genetic variants with prostate cancer. The New England journal of medicine. 2008;358(9):910–919. doi: 10.1056/NEJMoa075819. [DOI] [PubMed] [Google Scholar]
- 47.Hamrefors V, Orho-Melander M, Krauss RM, et al. A gene score of nine LDL and HDL regulating genes is associated with fluvastatin-induced cholesterol changes in women. Journal of lipid research. 2010;51(3):625–634. doi: 10.1194/jlr.P001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanchez-Navarro I, Gamez-Pozo A, Pinto A, et al. An 8-gene qRT-PCR-based gene expression score that has prognostic value in early breast cancer. BMC Cancer. 2010;10:336. doi: 10.1186/1471-2407-10-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyssenko V, Jonsson A, Almgren P, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. The New England journal of medicine. 2008;359(21):2220–2232. doi: 10.1056/NEJMoa0801869. [DOI] [PubMed] [Google Scholar]
- 50.Meigs JB, Shrader P, Sullivan LM, et al. Genotype score in addition to common risk factors for prediction of type 2 diabetes. The New England journal of medicine. 2008;359(21):2208–2219. doi: 10.1056/NEJMoa0804742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pearson-Fuhrhop KM, Dunn EC, Mortero S, et al. Dopamine genetic risk score predicts depressive symptoms in healthy adults and adults with depression. PLoS One. 2014;9(5):e93772. doi: 10.1371/journal.pone.0093772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacDonald HJ, Stinear CM, Ren A, et al. Dopamine Gene Profiling to Predict Impulse Control and Effects of Dopamine Agonist Ropinirole. J Cogn Neurosci. 2016;28(7):909–919. doi: 10.1162/jocn_a_00946. [DOI] [PubMed] [Google Scholar]
- 53.Kalia LV, Lang AE. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 54.Fallon SJ, Smulders K, Esselink RA, van de Warrenburg BP, Bloem BR, Cools R. Differential optimal dopamine levels for set-shifting and working memory in Parkinson’s disease. Neuropsychologia. 2015;77:42–51. doi: 10.1016/j.neuropsychologia.2015.07.031. [DOI] [PubMed] [Google Scholar]
- 55.Foltynie T, Goldberg TE, Lewis SG, et al. Planning ability in Parkinson’s disease is influenced by the COMT val158met polymorphism. Movement disorders : official journal of the Movement Disorder Society. 2004;19(8):885–891. doi: 10.1002/mds.20118. [DOI] [PubMed] [Google Scholar]
- 56.Hoogland J, de Bie RM, Williams-Gray CH, Muslimovic D, Schmand B, Post B. Catechol-O-methyltransferase val158met and cognitive function in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2010;25(15):2550–2554. doi: 10.1002/mds.23319. [DOI] [PubMed] [Google Scholar]
- 57.Habak C, Noreau A, Nagano-Saito A, et al. Dopamine transporter SLC6A3 genotype affects cortico-striatal activity of set-shifts in Parkinson’s disease. Brain. 2014;137(Pt 11):3025–3035. doi: 10.1093/brain/awu251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson’s disease. J Neurosci. 2007;27(18):4832–4838. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams-Gray CH, Hampshire A, Barker RA, Owen AM. Attentional control in Parkinson’s disease is dependent on COMT val 158 met genotype. Brain. 2008;131(Pt 2):397–408. doi: 10.1093/brain/awm313. [DOI] [PubMed] [Google Scholar]
- 60.Masellis M, Collinson S, Freeman N, et al. Dopamine D2 receptor gene variants and response to rasagiline in early Parkinson’s disease: a pharmacogenetic study. Brain. 2016;139(Pt 7):2050–2062. doi: 10.1093/brain/aww109. [DOI] [PubMed] [Google Scholar]
- 61.Moreau C, Meguig S, Corvol JC, et al. Polymorphism of the dopamine transporter type 1 gene modifies the treatment response in Parkinson’s disease. Brain. 2015;138(Pt 5):1271–1283. doi: 10.1093/brain/awv063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kwak Y, Bohnen NI, Muller ML, Dayalu P, Burke DT, Seidler RD. Task-dependent interactions between dopamine D2 receptor polymorphisms and L-DOPA in patients with Parkinson’s disease. Behav Brain Res. 2013;245:128–136. doi: 10.1016/j.bbr.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 63.Arendt T, Schindler C, Bruckner MK, et al. Plastic neuronal remodeling is impaired in patients with Alzheimer’s disease carrying apolipoprotein epsilon 4 allele. J Neurosci. 1997;17(2):516–529. doi: 10.1523/JNEUROSCI.17-02-00516.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.White F, Nicoll JA, Roses AD, Horsburgh K. Impaired neuronal plasticity in transgenic mice expressing human apolipoprotein E4 compared to E3 in a model of entorhinal cortex lesion. Neurobiology of disease. 2001;8(4):611–625. doi: 10.1006/nbdi.2001.0401. [DOI] [PubMed] [Google Scholar]
- 65.Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264(5160):850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- 66.Schiefermeier M, Kollegger H, Madl C, et al. Apolipoprotein E polymorphism: survival and neurological outcome after cardiopulmonary resuscitation. Stroke; a journal of cerebral circulation. 2000;31(9):2068–2073. doi: 10.1161/01.str.31.9.2068. [DOI] [PubMed] [Google Scholar]
- 67.Liu Y, Yu JT, Wang HF, et al. APOE genotype and neuroimaging markers of Alzheimer’s disease: systematic review and meta-analysis. Journal of neurology, neurosurgery, and psychiatry. 2015;86(2):127–134. doi: 10.1136/jnnp-2014-307719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poirier J, Minnich A, Davignon J. Apolipoprotein E, synaptic plasticity and Alzheimer’s disease. Annals of medicine. 1995;27(6):663–670. doi: 10.3109/07853899509019253. [DOI] [PubMed] [Google Scholar]
- 69.Lawrence DW, Comper P, Hutchison MG, Sharma B. The role of apolipoprotein E episilon (epsilon)-4 allele on outcome following traumatic brain injury: A systematic review. Brain Inj. 2015;29(9):1018–1031. doi: 10.3109/02699052.2015.1005131. [DOI] [PubMed] [Google Scholar]
- 70.McCarron MO, Weir CJ, Muir KW, et al. Effect of apolipoprotein E genotype on in-hospital mortality following intracerebral haemorrhage. Acta neurologica Scandinavica. 2003;107(2):106–109. doi: 10.1034/j.1600-0404.2003.01365.x. [DOI] [PubMed] [Google Scholar]
- 71.Gallek MJ, Conley YP, Sherwood PR, Horowitz MB, Kassam A, Alexander SA. APOE genotype and functional outcome following aneurysmal subarachnoid hemorrhage. Biol Res Nurs. 2009;10(3):205–212. doi: 10.1177/1099800408323221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cramer SC, Procaccio V. Correlation between genetic polymorphisms and stroke recovery: analysis of the GAIN Americas and GAIN International Studies. Eur J Neurol. 2012;19(5):718–724. doi: 10.1111/j.1468-1331.2011.03615.x. [DOI] [PubMed] [Google Scholar]
- 73.Skriver K, Roig M, Lundbye-Jensen J, et al. Acute exercise improves motor memory: exploring potential biomarkers. Neurobiol Learn Mem. 2014;116:46–58. doi: 10.1016/j.nlm.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 74.Szuhany KL, Bugatti M, Otto MW. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J Psychiatr Res. 2015;60:56–64. doi: 10.1016/j.jpsychires.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang WH, Bang OY, Shin YI, Lee A, Pascual-Leone A, Kim YH. BDNF polymorphism and differential rTMS effects on motor recovery of stroke patients. Brain stimulation. 2014;7(4):553–558. doi: 10.1016/j.brs.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 76.Uhm KE, Kim YH, Yoon KJ, Hwang JM, Chang WH. BDNF genotype influence the efficacy of rTMS in stroke patients. Neurosci Lett. 2015;594:117–121. doi: 10.1016/j.neulet.2015.03.053. [DOI] [PubMed] [Google Scholar]
- 77.Ridding MC, Ziemann U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J Physiol. 2010;588(Pt 13):2291–2304. doi: 10.1113/jphysiol.2010.190314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Helm EE, Tyrell CM, Pohlig RT, Brady LD, Reisman DS. The presence of a single-nucleotide polymorphism in the BDNF gene affects the rate of locomotor adaptation after stroke. Exp Brain Res. 2016;234(2):341–351. doi: 10.1007/s00221-015-4465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim JM, Stewart R, Park MS, et al. Associations of BDNF genotype and promoter methylation with acute and long-term stroke outcomes in an East Asian cohort. PLoS One. 2012;7(12):e51280. doi: 10.1371/journal.pone.0051280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mirowska-Guzel D, Gromadzka G, Mendel T, et al. Impact of BDNF -196 G>A and BDNF -270 C>T polymorphisms on stroke rehabilitation outcome: sex and age differences. Top Stroke Rehabil. 2014;21(Suppl 1):S33–41. doi: 10.1310/tsr21S1-S33. [DOI] [PubMed] [Google Scholar]
- 81.Siironen J, Juvela S, Kanarek K, Vilkki J, Hernesniemi J, Lappalainen J. The Met allele of the BDNF Val66Met polymorphism predicts poor outcome among survivors of aneurysmal subarachnoid hemorrhage. Stroke. 2007;38(10):2858–2860. doi: 10.1161/STROKEAHA.107.485441. [DOI] [PubMed] [Google Scholar]
- 82.Zhao J, Wu H, Zheng L, Weng Y, Mo Y. Brain-derived neurotrophic factor G196A polymorphism predicts 90-day outcome of ischemic stroke in Chinese: a novel finding. Brain Res. 2013;1537:312–318. doi: 10.1016/j.brainres.2013.08.061. [DOI] [PubMed] [Google Scholar]
- 83.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 84.Stewart JC, Cramer SC. Patient-reported measures provide unique insights into motor function after stroke. Stroke. 2013;44(4):1111–1116. doi: 10.1161/STROKEAHA.111.674671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cramer SC, Koroshetz WJ, Finklestein SP. The case for modality-specific outcome measures in clinical trials of stroke recovery-promoting agents. Stroke. 2007;38(4):1393–1395. doi: 10.1161/01.STR.0000260087.67462.80. [DOI] [PubMed] [Google Scholar]
- 86.Burke Quinlan E, Dodakian L, See J, et al. Neural function, injury, and stroke subtype predict treatment gains after stroke. Ann Neurol. 2015;77(1):132–145. doi: 10.1002/ana.24309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Byblow WD, Stinear CM, Barber PA, Petoe MA, Ackerley SJ. Proportional recovery after stroke depends on corticomotor integrity. Ann Neurol. 2015;78(6):848–859. doi: 10.1002/ana.24472. [DOI] [PubMed] [Google Scholar]
- 88.Shiner CT, Pierce KD, Thompson-Butel AG, Trinh T, Schofield PR, McNulty PA. BDNF Genotype Interacts with Motor Function to Influence Rehabilitation Responsiveness Poststroke. Frontiers in neurology. 2016;7:69. doi: 10.3389/fneur.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Riley JD, Le V, Der-Yeghiaian L, et al. Anatomy of stroke injury predicts gains from therapy. Stroke. 2011;42(2):421–426. doi: 10.1161/STROKEAHA.110.599340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bonilha L, Rorden C, Fridriksson J. Assessing the clinical effect of residual cortical disconnection after ischemic strokes. Stroke. 2014;45(4):988–993. doi: 10.1161/STROKEAHA.113.004137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Adams H, Bendixen B, Kappelle L, et al. Classification of subtype of acute ischemic stroke. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke; a journal of cerebral circulation. 1993;24(1):35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 92.Thickbroom GW, Cortes M, Rykman A, et al. Stroke subtype and motor impairment influence contralesional excitability. Neurology. 2015;85(6):517–520. doi: 10.1212/WNL.0000000000001828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Witte AV, Kurten J, Jansen S, et al. Interaction of BDNF and COMT polymorphisms on paired-associative stimulation-induced cortical plasticity. J Neurosci. 2012;32(13):4553–4561. doi: 10.1523/JNEUROSCI.6010-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stinear CM, Barber PA, Petoe M, Anwar S, Byblow WD. The PREP algorithm predicts potential for upper limb recovery after stroke. Brain : a journal of neurology. 2012;135(Pt 8):2527–2535. doi: 10.1093/brain/aws146. [DOI] [PubMed] [Google Scholar]
- 95.Stinear CM, Ward NS. How useful is imaging in predicting outcomes in stroke rehabilitation? International journal of stroke : official journal of the International Stroke Society. 2013;8(1):33–37. doi: 10.1111/j.1747-4949.2012.00970.x. [DOI] [PubMed] [Google Scholar]
- 96.Coupar F, Pollock A, Rowe P, Weir C, Langhorne P. Predictors of upper limb recovery after stroke: a systematic review and meta-analysis. Clin Rehabil. 2012;26(4):291–313. doi: 10.1177/0269215511420305. [DOI] [PubMed] [Google Scholar]
- 97.Falcone GJ, Malik R, Dichgans M, Rosand J. Current concepts and clinical applications of stroke genetics. Lancet Neurol. 2014;13(4):405–418. doi: 10.1016/S1474-4422(14)70029-8. [DOI] [PubMed] [Google Scholar]


