Abstract
Over the past 30 years, cumulative evidence has indicated that cerebellar function extends beyond sensorimotor control. This view has emerged from studies of neuroanatomy, neuroimaging, neuropsychology and brain stimulation, with the results implicating the cerebellum in domains as diverse as attention, language, executive function and social cognition. Although the literature provides sophisticated models of how the cerebellum helps refine movements, it remains unclear how the core mechanisms of these models can be applied when considering a broader conceptualization of cerebellar function. In light of recent multidisciplinary findings, we consider two key concepts that have been suggested as general computational principles of cerebellar function, prediction and error-based learning, examining how these might be relevant in the operation of cognitive cerebro-cerebellar loops.
Keywords: cerebellum, cognition, prediction, learning, language, social cognition
Understanding the Cognitive Cerebellum
Although the function of the cerebellum has historically been associated with the sensorimotor system, the cognitive neuroscience literature has, from its emergence in the 1980’s, challenged this perspective. For example, a seminal functional brain imaging study revealed cerebellar activity during language processing, even when the overt motor requirements were equated [1]. In an individual with an extensive right posterior inferior cerebellar artery stroke, marked impairments were found in semantic knowledge, associative learning and verb generation, along with poor awareness of performance errors despite high scores on standard intelligence tests [2]. Similar findings from many neuroimaging studies, as well as neuropsychological research [3], has led to considerable effort to understand cerebellar involvement in high-level cognitive functions, including research on a range of psychiatric and developmental disorders. However, discussion of the cerebellum in broader studies and concepts of cognitive brain networks has remained scarce, and the mechanisms by which the cerebellum may contribute to cognition largely obscure.
The goal of this opinion piece is to offer an integrative picture of recent progress from diverse methodologies and theoretical perspectives. The first part will briefly review how the cerebellum helps refine movement through its predictive capacity and, as a consequence, through error-based learning. A core concept is the forward model, an internal representation of the environment and agent that, in sensorimotor control, serves to predict the sensory outcome of a motor act (see Glossary and [4] for a recent review). We then evaluate how this functional concept may extend to the realms of language comprehension and social cognition. Although prediction and error-based learning may serve as general principles for understanding cerebellar function, we also critically consider the utility and limitations of current attempts to apply them to cognitive domains.
Functional Neuroanatomy of Cerebro-Cerebellar Interplay
Cerebro-cerebellar communication is organized in a series of parallel loops (Box 1): Areas in the cerebral cortex project to the same cerebellar regions from which they receive input [5–7]. Motor-related activation is mainly found in the anterior cerebellum (lobules I–IV and V) and anterior aspects of lobule VI [8–10], regions that exhibit resting-state functional connectivity (rsFC) with contralateral primary motor and somatosensory cortices [11, 12]. Interestingly, the cerebellum contains two sensorimotor somatotopic representations: lobules II-V and VIII/IX for lower limbs, lobules V/VI and VIII for upper limbs and lobules VI and VIII for the face [8–10].
Box 1. Pathways Underlying Cerebro-Cerebellar Interaction.
The primary reciprocal connections between the cerebral cortex and the cerebellar hemispheres are contralaterally organized [181]. Resting state functional connectivity (rsFC) data indicate multiple cortico-cerebellar networks [11–14], consistent with the idea that cerebellar processing has a broad influence on cortical activity. In general, the cortico-ponto-cerebello-dentato-thalamo-cortical pathways form a series of parallel loops, terminating in regions that were the source of input to the cerebellum [6]. Recent task-related causal functional (effective) connectivity and diffusion tensor imaging analyses reveal the cerebellum interacts not only with prefrontal but also temporal cortex during language processing and social cognition, in particular the lobules Crus I/II [30–32, 117].
Input from the cortex to the cerebellum is disynaptic, via the ipsilateral pontine nuclei [26], which, in turn, send mossy fibers that decussate, pass through the middle cerebellar peduncle and form excitatory synapses in the contralateral cerebellum [181]. The second important cerebro-cerebellar pathway is via the ipsilateral inferior olive. Projections from the sensorimotor cortex are particularly prominent in the cortico-olivary pathway [182, 183], but electrophysiological evidence in cats and rats also suggests indirect signaling from the prefrontal [166] and parietal cortices [168, 184]. The indirect projections’ relay may be located in the mesodiencephalic junction [60, 167, 185], itself a target of excitatory cerebellar output. Through the inferior cerebellar peduncle, the inferior olive sends crossing excitatory climbing fibers to the deep cerebellar nuclei and the cerebellar cortex, forming sagittally aligned zones.
The cerebellar cortex projects to the deep cerebellar nuclei that mediate all output from the cerebellum. The largest of these nuclei, the dentate, is a target of powerful convergence from the lateral cerebellar hemispheres. Most of the output from the dentate nucleus ascends back to the cerebral cortex: The axons pass through the superior cerebellar peduncle, decussate at the level of the brainstem, and, through the contralateral cerebral peduncle, reach the thalamic ventral lateral nucleus. These thalamic regions then project to the cerebral cortices [186].
Output from the other deep cerebellar nuclei, the interposed nucleus (composed of the globose and emboliform nuclei) and the fastigial nucleus, influences the descending extrapyramidal tracts, but also reaches the cerebral cortex. Some data also indicate monosynaptic connections from the fastigial nucleus to the amygdala, hippocampus and middle temporal gyri [105], pathways that may mediate involvement of the cerebellum in emotional processing and regulation [103].
Figure I. Interactions between Cerebellum and Cognitive Cortical Networks.
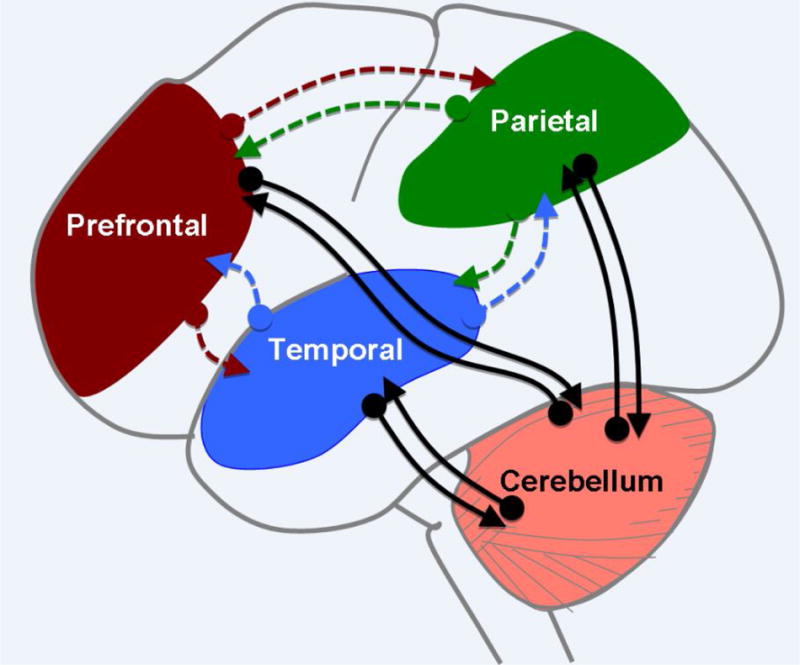
The cerebellum receives input from cortical areas via the pons and projects back to similar areas via the thalamus, forming a closed-loop architecture (black arrows). Complex cognitive processes such as language or social cognition require interactions between distributed regions in the cerebral cortex (colored, dashed arrows). Thus, the closed-loop architecture would suggest that the cerebellar outputs modulate singular network components, instead of affecting a complex cognitive process as a whole. However, cortico-cortical connections provide a means to expand the influence of the cerebellum.
Large swaths of the cerebellar hemispheres communicate with non-motor, cognitive associative cerebral areas [11–14]. To highlight just a few of these regions, rsFC studies show that cerebellar lobule VI interacts with the salience network (processing pertinent stimuli in a competitive and context-dependent manner), lobules Crus I and II with the cerebral executive control circuitry, and lobule IX with the default-mode network [11].
An extensive meta-analysis of imaging studies reveals that right cerebellar lobules VI and Crus I are engaged during language function and left lobule VI during visuo-spatial processing, confirming that the functional lateralization of the cerebellar hemispheres mirrors to some degree, the picture observed in the cerebral cortex [15]. The rsFC data reveal two (possibly three) representations of cortical space (Figure 1), running along the anterior—posterior axis of the cerebellar cortex [12].
Figure 1. Functional Topography of the Cerebellar Cortex.
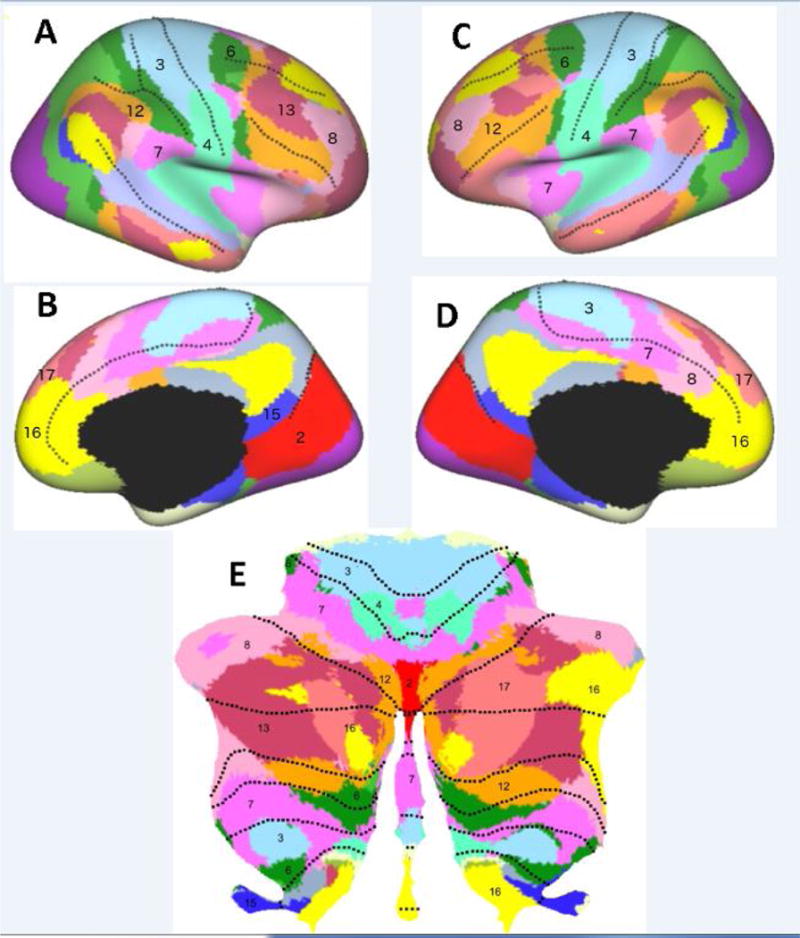
The cerebellar cortical sheet (E), when graphically flattened into a 2-D map has a huge surface area. Resting state functional connectivity (rsFC) studies show that each cerebellar area can be linked to a region in the cerebral cortex (A–D). There appears to be a repeated mapping with, for example, prefrontal cortical areas (label 16 in C, D) represented in anterior and posterior lobes of the cerebellum (E). Adapted from [12], [178] and [19], with permissions.
We note that cerebellar activations in neuroimaging studies are generally labeled in terms of lobules, yet, functionally defined areas often cross the fissures between lobules. The organization of these modules is reminiscent of immunohistological data on zebrin-positive and zebrin-negative stripes across the cerebellar cortex [16–18], a more nuanced picture that is best seen in flat map fMRI representations [19]. The positive and negative stripes for zebrin differ in their electrophysiological properties and innervation [17, 20, 21]. Zebrin expression is also distributed in an anterior-posterior fashion, with more zebrin-positive stripes in the posterior cerebellum [18, 22]. As deficits in motor coordination are primarily observed after stroke in the anterior cerebellum and cognitive dysfunction is mainly related to more posterior cerebellar damage [23], zebrin-positive stripes may be associated with more complex motor and higher cognitive processes (Box 2).
Box 2. Cerebellar Stripes and Zebrin.
Immunohistochemical and recent electrophysiological studies have revealed a surprising picture of variations across the cerebellar cortex. Zebrin II is an antigen expressed on the enzyme aldolase C, involved in glycolysis and gluconeogenesis, and therefore in the formation of adenosine triphosphate (ATP) [187]. When visualized in the cerebellum, prominent stripes can be seen along the sagittal axis, with zebrin II positive regions interleaved with zebrin-negative regions [16]. This pattern is observed across avian and mammalian species [16, 22, 188], including humans [18].
Purkinje cells that expression zebrin II exhibit lower intrinsic simple spike rates while zebrin-negative Purkinje cells fire at higher frequencies. Perhaps related, long-term potentiation (LTP) represents the predominant form of plasticity in zebrin-positive stripes while LTD prevails in zebrin-negative zones [20, 21]. Moreover, zebrin-positive zones mainly receive climbing fiber input from inferior olive subdivisions innervated by the cerebral cortex and mesodiencephalic junction, whereas climbing fiber signals inputs to zebrin-negative zones are associated with peripheral sensory information [17, 189]. Interestingly, climbing fiber inputs in pigeons’ vestibulocerebellum indicate zebrin-positive and negative stripes may complement each other to form functional units [190] and sensory stimulation synchronizes otherwise asynchronous complex spikes in zebrin-positive and negative modules in mice [191].
The identification of other markers of functional diversity represents a promising avenue for future research on functional specialization within the cerebellum. At present, there is an intriguing association of posterior cerebellar involvement in cognition [23, 104], the predominance of zebrin-positive zones in the posterior cerebellum [18, 22] and the innervation of these zones by climbing fibers carrying information from the cerebral cortex [17, 189], potentially linking zebrin positivity and higher cognitive processing.
The main output of the primate cerebellum, the dentate nucleus, also exhibits functional parcellation: dorsal and ventral portions project, via the thalamus, to motor and associative areas, respectively [24]. Prefrontal and parietal cortices appear to be the main cognitive hubs interacting with the cerebellum [14, 25]. However, neuroanatomical and neurophysiological data in non-human primates suggest the temporal cortex also projects to the cerebellum via the pons [26–28], and recent human brain imaging indicates direct reciprocal connections between the cerebellum and temporal cortex [29–33]. The connectivity with association areas stands in contrast with the absence of connectivity with primary visual and auditory areas [12]. The cerebellum thus interacts with cortical areas essential for many aspects of cognition.
Based on neuroimaging and neuropsychological findings (Box 3), hypotheses of how the cerebellum contributes to cognition have focused on diverse processes associated with attention, working memory and temporal representation [34–37]. The uniformity of cerebellar cytoarchitecture suggests similar computations on distinct, incoming signals, with function dictated by the specific cerebro-cerebellar loop [6, 38]. If principles of information processing are indeed similar across the cerebellum, we should consider mechanistic hypotheses that would be consistent across task domains [35, 36]. Hence, understanding cerebellar involvement in cognition may benefit from considering the substantial knowledge we have as to how the cerebellum contributes to sensorimotor control.
Box 3. Neuropsychological Profile of Patients with Cerebellar Dysfunction.
An impressive literature has emerged characterizing the neuropsychological profile of individuals with cerebellar stroke, tumors, or degeneration. Despite considerable heterogeneity, the general picture resembles that in frontal lobe dysfunction, with altered working memory, verbal fluency and cognitive control [37, 192–194]. Explicit memory is generally spared. Visuospatial perceptual deficits and involvement in multisensory integration have also been reported [176, 195]. Schmahmann and colleagues [95] have described a cerebellar cognitive affective syndrome (CCAS).
Both focal insult and degeneration of the cerebellum can affect neuropsychological performance. Nonetheless, it is difficult to draw strong inferences concerning function and mechanism from these observations. First, due to limited patient numbers, inferences have often been drawn across patients exhibiting different etiologies and/or considerable variation in the locus of pathology. We would never consider inferring the contribution of cerebral areas to different task domains by pooling together the data of patients with occipital and frontal lesions. Furthermore, although the most consistent problems are observed in patients with degenerative disorders, the pathology in some of these syndromes extends into extracerebellar regions such as the brainstem, basal ganglia and cerebral cortex. Second, movement disorders may negatively impact performance on neuropsychological tests. For patients with coordination problems, even the simple act of button pressing may divert attentional resources from the test material. Third, the cerebellum may also be an extremely plastic system, allowing for rapid reorganization to compensate for acquired damage (as evident from impressive motor recovery if the deep cerebellar nuclei are spared) [177, 192].
Cognitive consequences of cerebellar damage may also depend on age of onset. Despite a somewhat heterogeneous picture, more overt neuropsychological deficits are generally observed in children [196–199]. Given its protracted course of maturation [200, 201], damage to the cerebellum during vulnerable phases may contribute to more pronounced neuropsychological dysfunction. The time course of cerebellar development and its variation across the lobules [200] may also offer an important window for understanding how cerebellar abnormalities contribute to neuropsychiatric disorders, such as altered interplay within specific cerebro-cerebellar loops relevant for social cognition and interaction during development thought to be involved in ASD pathogenesis [202].
Taken together, the neuropsychological evidence presents a rather mixed picture and emphasizes the need for probes designed to assess subtle functional impairments as opposed to the rather marked neuropsychological consequences of cerebral damage (e.g., aphasia, agnosia, amnesia).
Sensorimotor Coordination, Prediction and Error-Based Learning
The hallmark of cerebellar dysfunction is a loss of sensorimotor coordination. Patients typically have normal muscle tone and strength, and exhibit no difficulty in selecting the appropriate muscles. However, the pattern of muscular activity is disrupted: A delay in the onset of the antagonists can result in dysmetria with the limb shooting past the target [39], or a failure to account for interactional torques can result in systematic directional errors [40]. These motor impairments – termed ataxias – are especially pronounced during rapid movement. Furthermore, the intact cerebellum ensures smoothness of movement, such as in pursuit eye movements [41].
Theoretical models of sensorimotor control have emphasized the predictive role of the cerebellum [42–44], with a focus on forward models. This idea, supported by a substantial literature involving various tasks and species, centers on the idea that the cerebellum receives a copy of the motor command [45–47] and, in combination with exteroceptive and proprioceptive sensory inputs, generates a representation of the expected sensory consequences of that command [45, 48–50]. An alternative and potentially complementary theory argues that the cerebellum contributes to the generation of motor commands, functioning as an inverse model [51–53].
The sensory predictions generated by a forward model can be used to coordinate motor output [54, 55], providing a means to anticipate the consequences of a motor command [56] and to update a state estimate of the motor system, processes especially essential for actions that involve coordination across multiple effectors [57]. These predictions can also be compared with afferent input. If the predicted and actual signals match, the afferent signals can be cancelled [58]. When they mismatch, the difference constitutes a sensory prediction error (Figure 2). These error signals are essential for sensorimotor control, allowing for rapid adjustments in the motor output. They are also essential for learning, to refining future sensory predictions and reduce the prediction error signal on subsequent movements. The inferior olive is assumed to be an essential component in this comparison process (Box 1), as its neurons receive convergent input: excitatory from cortical, subcortical or spinal structures and inhibitory from the cerebellum [59, 60].
Figure 2. Forward Models and Prediction.
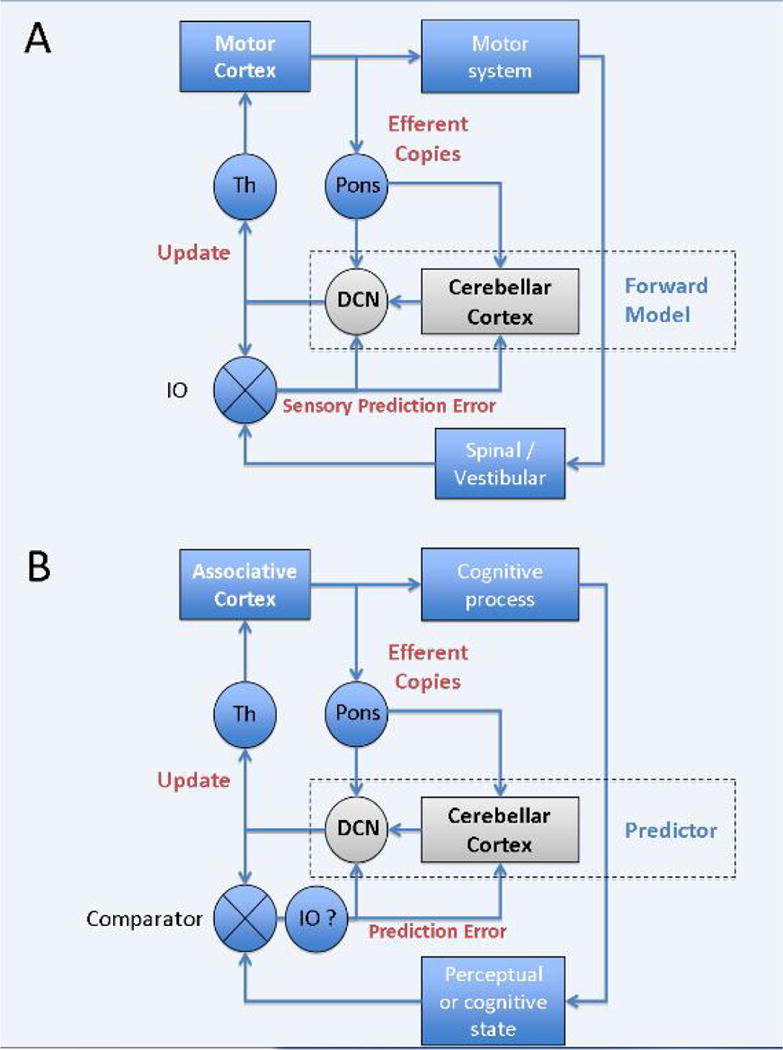
(A) The motor cerebellum has been hypothesized to operate as a forward model or state estimator, using efferent copies of motor commands to predict the sensory consequences of actions. Sensory prediction errors, the difference between the predicted and actual outcome, are conveyed to the cerebellum through the climbing fibres of the inferior olive (IO). Whether cerebellar output is the state estimate or a signal updating a state estimate represented elsewhere, for example in motor or parietal cortex, is unclear. (B) By analogy, the cognitive cerebellum might predict changes in perceptual or mental states, and feed these updates to associative areas. It is uncertain if the calculation of errors (comparator) is within the IO or elsewhere, and fed to the cerebellum via the IO. For example, there is a loop between mesodiencephalic junction, inferior olive, cerebellar cortex, DCN and back to the mesodiencephalic junction that may contribute to this computation. Although these diagrams only depict cortical inputs to the pons, there are many other sources of pontine input. DCN: deep cerebellar nuclei; Th: thalamus.
Most models of cerebellar learning have been inspired by the unique anatomy and physiology of the cerebellum. Dating from influential theories by Marr and Albus [61, 62], experimental evidence has demonstrated that climbing fiber inputs from the inferior olive to the cerebellar cortex can result in long-term depression (LTD) of the parallel fiber synapses onto Purkinje cells [44]. LTD modifies signal processing in the cerebellar cortex, sculpting the powerful, converging inhibition by Purkinje cells on the deep cerebellar nuclear cells. Hence, the Purkinje cell simple spike activity could be viewed as constituting the sensory prediction [45, 46, 63] (although it may also contribute to the motor command), while the climbing fiber activity resulting in Purkinje cell complex spikes provides a representation of sensory prediction errors [64–66], driving cerebellar learning. It should be noted that, although this model has been highly influential in theories of cerebellar-based learning, there remains considerable debate over the functional role of the climbing fiber signals and their interaction with simple spike activity [50, 64, 67]. Furthermore, recent physiological data in mice and rats suggest cerebellar modules vary in the firing rate of simple spike activity and exhibit plasticity tuned to different time intervals between parallel and climbing fiber inputs [20, 21, 68]. These recent findings may represent a first glimpse at how the cerebellum could support diverse functions associated with different constraints, despite its relatively uniform structure.
Damage to the cerebellum may not only affect sensorimotor coordination but also cognitive function, an issue that has been extensively explored in neuropsychological studies (Box 3). We focus here on language and social cognition, reviewing evidence implicating the cerebellum in these domains and examining the relevance of unifying computational concepts derived from studies of sensorimotor control.
Cerebellar Contribution to Language Functions
Many neuroimaging studies, in combination with more limited neuropsychological literature, point to a role for right Crus I and Crus II in language processing and verbal working memory [15, 69]. This has inspired numerous studies of how the cerebellum may support language processing. Early functional suggestions focused on a motor-inspired hypothesis, namely that, similar to its role in overt speech production, the cerebellum may be essential for covert articulation or internal rehearsal, a core process of verbal working memory. However, these studies failed to provide a parsimonious account of the activation in healthy individuals [70] or the behavioural impairments observed in patients [37, 71].
Other aspects of language processing also engage the cerebellum, and here a functional explanation based on putative “motor” operations seems even less plausible. For example, there is evidence for Crus I/II being involved in vocabulary acquisition [72, 73] and verbal retrieval, as assessed in fluency tasks [74]. Perturbation of the right cerebellum induced by transcranial magnetic stimulation (TMS) has been suggested to disrupt the retrieval of lexical associations [75] (but see also [76]). In a complementary vein, the cerebellum may be essential for representing the temporal dimension, facilitating language processes and/or semantic associations at particular time-points [77]. There are also suggestions of a cerebellar link with developmental [78] and acquired dyslexia [79], that may be related to the temporal aspects of language processing (for review, see [80]).
No single cognitive process comfortably fits with all these aspects of language. We consider here how the predictive capability of the cerebellum may offer a useful functional hypothesis. As described previously, in sensorimotor control, forward models generate predictions of the anticipated sensory consequences of outgoing motor commands. Applied to language, a forward model would be a way for a listener or reader to use the current context to anticipate forthcoming linguistic and paralinguistic information.
This predictive aspect of language comprehension was originally suggested without reference to the cerebellum [81]. Motivated by the idea that a common function might underlie its contribution across motor and non-motor domains, we have recently explored the role of the cerebellum in the generation and evaluation of linguistic predictions. In a functional magnetic resonance imaging (MRI) experiment [82], greater cerebellar activation (Figure 3B) was observed in right Crus I and II for sequentially presented sentences when the last word was predictable (e.g., “Two plus two is four.”) compared to trials in which the word order was scrambled, and thus the stimulus sequence lacked predictability. In a related study, a very similar area of the cerebellum showed greater activation in anticipation of a word that was highly predictable given the context, compared to identical sentence stems in context that limited predictability [83].
Figure 3. The Cerebellum and Linguistic Prediction.
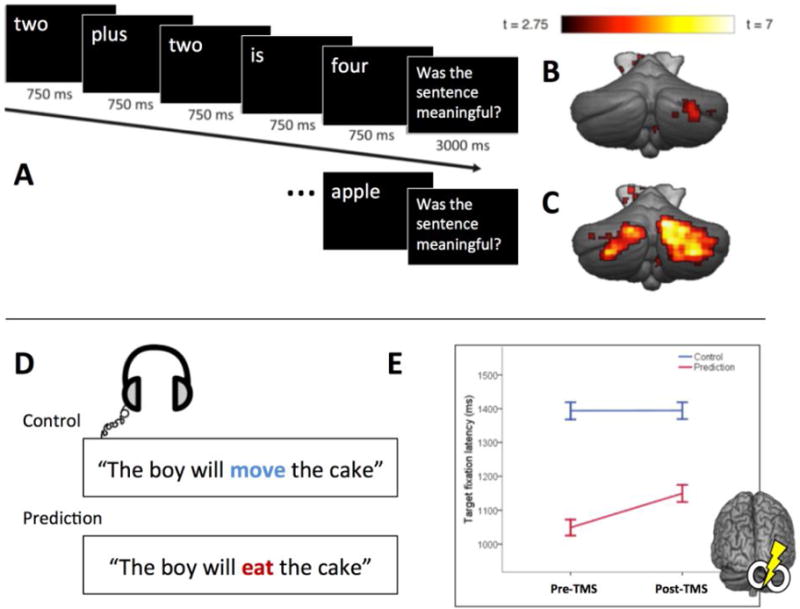
(A) The predictability of visually presented sentences can be high. (B) A small region in right posterior lateral cerebellum was more active in the high predictable condition compared to when predictability was reduced by scrambling the order of the presented words [82]. If the expected ending of the sentence was violated (“Two plus two is apple”), a broad, bilateral region of the cerebellar cortex was activated. (C, contrast between incongruent and scrambled trials). (D,E) Evidence that right posterior lateral cerebellum is causally involved in linguistic prediction [83]. The latency advantage in fixating the object of the predictable spoken sentences compared to unpredictable control sentences was significantly reduced after rTMS to the right lateral cerebellum. Illustrations adapted and reprinted with permissions.
Evidence offering a more direct test of a causal role for this region in language comes from a study [84] in which eye movements were tracked as participants listened to spoken sentences. When the sentence was predictable (e.g., “The boy will eat the … ”), participants looked at a picture of a cake, the only edible object in the display, in anticipation of the final word. Repetitive TMS over the right posterior cerebellum reduced the anticipatory advantage for predictive sentences, without altering latencies of the reactive eye movements to objects named in non-predictable sentences. This result was recently replicated and extended using anodal and cathodal transcranial direct current stimulation (tDCS) over the right lateral cerebellar hemisphere to show a polarity-specific effect [85], enhancing and reducing the predictive advantage, respectively.
Both our imaging studies of semantic prediction [82, 83] also examined error representation in the cerebellum. The key comparison here was between sentences in which the last word was congruent with its context and sentences in which the expectation was violated (“Two plus two is apple”, Figure 3C). A large increase in activation spanning the bilateral posterior cerebellum was observed in response to the incongruent stimuli, reflecting the cerebellar response to the linguistic prediction error. By analogy to studies of sensory prediction errors in motor tasks [86], this error signal may lead to an update of a cerebellar forward model, although the content of these linguistic signals remains to be elucidated (see Constraints on Cerebellar Prediction and Error Processing in Cognition).
It is important to emphasize that these responses were not unique to the cerebellum. The same contrasts in both studies also revealed cortical activations in temporal, parietal and frontal regions, encompassing the cortical language network. Thus, forward modelling and error sensitivity (two closely related processes fundamental to adaptive prediction) may characterize cerebellar function within a broader, linguistic network. A major gap in the literature is the lack of a systems-level description of the interactions between cerebellar and cortical language areas, and specific hypotheses of what aspects of language each area underpins.
The Cerebellum in Social Cognition and Affective Processing
We turn next to our third domain, social cognition and affective processing. We note at the outset that the case for a cerebellar role in this domain is not well developed. Much of the evidence comes from functional imaging studies where causality cannot be assessed and, in many studies of social cognition and affect, the cerebellum was of secondary interest. We first review the imaging and neuropsychology literature suggesting a role of the cerebellum in affective and socio-cognitive processing. We also highlight some neuropsychiatric disorders marked by impairments in social cognition that have been associated with cerebellar abnormalities, examining the relevance of prediction and error processing to these impairments [87].
Social cognition represents the understanding of intentions and emotions of others conveyed by verbal and non-verbal signals such as facial expression, prosody and body language [88, 89]. Affective processing and social cognition are closely intertwined [90]. Disrupted perception or experience of emotions may lead to deficits in social cognition. The representation of emotional states is essential for empathy and social learning, allowing us to appreciate the context of another individual’s behavior. Conversely, misinterpretation due to socio-cognitive dysfunction may contribute to affective disorders [91].
Generally speaking, social cognition has been hypothesized to entail two distinct processes, an immediate affective response and a delayed, more rational response [92, 93]. The affective component refers to emotional contagion, for example the instantaneous visceral feeling of fear when we perceive another fearful person. The cognitive component refers to a more reflective representation based upon perspective taking and mentalizing. In our example, this would be an attempt to understand why this person is scared. At the level of prefrontal cortex, lesion data indicate the inferior frontal gyrus is a core component of the affective response, whereas ventromedial prefrontal cortex is associated with the cognitive component [94]. As in the prefrontal cortex, the two processes appear topographically dissociated in the cerebellum, with the posterior vermis of the cerebellum being involved in affective processing and the posterior lateral cerebellum in more reflective, cognitive components of these tasks.
Flattened affect and reduced emotional expressivity, sometimes with disinhibited, childish behavior, have been observed in patients with lesions of the cerebellar vermis [95], a constellation of symptoms that has come to be referred to as cerebellar cognitive and affective syndrome (CCAS). Although somewhat inconsistent, patients with cerebellar degeneration or stroke also present some deficits in recognizing emotion from faces [96], matching facial emotion with prosody [97], and in the attribution of emotions to characters in short stories [98]. Pathological laughter and crying have been linked to dysfunction of prefronto-ponto-cerebellar loops [99, 100] and inhibitory TMS over the cerebellar vermis results in impaired emotional regulation [101]. There is also evidence of altered EEG signatures of emotional processing in patients with cerebellar degeneration [102]. Coupled with functional imaging data, it has been proposed that the posterior vermis should be viewed as the “limbic cerebellum” [103, 104]. Anatomical data on direct vermis-amygdala connections are relatively controversial [105], but rsFC has been found between the vermis and hippocampus, cingulate cortex, prefrontal cortex, precuneus, and superior temporal cortex [106, 107].
Although few neuroimaging studies on social cognition have specifically focused on the cerebellum, a recent meta-analysis of 350 whole-brain functional MRI studies revealed consistent activation of the posterior cerebellar hemispheres (Figure 4), and in particular Crus I, in tasks requiring abstract social inferences [108]. To date, few studies have focused on the performance of patients with cerebellar dysfunction on tests of social cognition, nor have non-invasive brain stimulation studies in healthy individuals been conducted to test specific causal hypotheses. Impaired performance on Theory of Mind (ToM) tasks has been recently reported in heterogeneous patient groups with cerebellar damage, including stroke, atrophy and spinocerebellar ataxia (SCA) [109–111]. Most ToM tests require attribution of mental states, beliefs and intentions to others on the basis of short stories, animations or eye expressions. While social predictions and models of agents’ mental states play a crucial role here [112], the topography and mechanistic contributions of the cerebellum remains unclear.
Figure 4. Social Cognition and the Cerebellum.
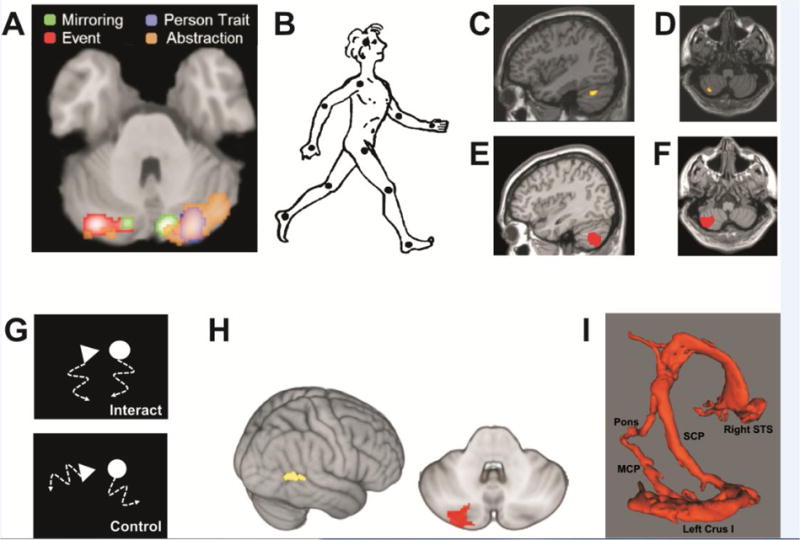
(A) Summary of cerebellar findings from meta-analysis of 350 whole-brain neuroimaging studies of social cognition. Most consistent cerebellar activation is elicited by abstraction in trait inferences, in bilateral Crus I, with a right hemispheric predominance. Bilateral activation is also found during mirroring (observation of human body motion). In contrast, event (e.g., observation of social interaction depicted by moving geometric shapes) and person trait mentalizing appear left- and right-lateralized, respectively. Adapted from [108], with permission. (B–F) Series of studies specifically focused on cerebellar engagement in social cognition. (B) Observation of human body motion represented by point lights on the head and the main joints (reprinted from [179] with automatic permission from SAGE Publications) elicits activation in the left cerebellar lobules (C) Crus I and (D) VIIB. Reprinted from [30] with permission by Oxford University Press. (E+F) Patients with tumors topographically overlapping regions left lateral cerebellum exhibit deficits in perception of body motion. Reprinted from [116] with permission by Oxford University Press. (G) Illustration of geometric shapes that move as if they would socially interact (upper panel) or in a random fashion (control; lower panel). Adapted from [180]. (H) As with visual perception of body motion, observation of the interacting geometric shapes results in activation of left cerebellar Crus I/II, and effective connectivity with the right superior temporal sulcus (STS). Adapted from [117], with permission by Oxford University Press. (I) Diffusion tensor imaging revealing a structural loop between the left lateral cerebellar lobule Crus I and right STS. Reprinted from [31] with permission by Oxford University Press.
Understanding the purpose of agents’ motion and body language is another rich source of social cues [88]. Patients with midline cerebellar pathology exhibit deficits in perception of inanimate object motion [113–115], but only lesions of the left lateral cerebellum are associated with altered perception of body motion [116], potentially related to disrupted communication between Crus I and right posterior superior temporal sulcus (STS) [30] (Figure 4). STS and the adjacent temporo-parietal junction (TPJ) are key components of brain networks engaged in the perception of body language and social cognition [88]. Imitation of finger movements and animations showing geometric shapes that seem to socially interact also activate the left lateral cerebellum, with effective connectivity between Crus I/II and STS [29, 117, 118].
Can the concepts of prediction and error processing be useful in understanding cerebellar contributions to social cognition and affective processing? Prediction is, of course, fundamental to socio-emotional processing [87, 119, 120]. Having a sense of another individual’s state of mind not only requires creating a mental model of that individual, but also being able to simulate how these mental states might influence their behavior. Recognizing deviations in the outcome of a social interaction from expected behavior, and using that information to adapt future social predictions in a fluid and automated manner such as the cerebellum can provide would appear useful for adaptive social behavior.
Observing the intentional actions of others has been shown to engage neural pathways associated with sensorimotor internal models [121–125]. Thus, to some extent, sensorimotor internal models are thought to participate in socio-cognitive processes, an idea captured by the notion of embodied cognition [126]. However, more abstract, predictive modeling and error-based learning may also be essential for social cognition [87, 127]. For instance, we need to predict the social consequences of how we act or what we say across various contexts (analogous to forward modeling), and we have to try and understand what caused our own or an agent’s specific behavior (inverse modeling), and how these behaviors may impact the social situation.
Cerebellar contribution to these more abstract forms of prediction remains unclear. At present, despite providing important preliminary insight, most research has been largely restricted to descriptive reports, with the cerebellum link being one of “guilt by association.” Furthermore, most social cognition paradigms and studies have not been specifically designed to test adaptive prediction and error-based learning. For processing errors in ToM, the most relevant contrast would be between scenarios in which a social prediction is confirmed or violated. In one study providing such a contrast, violations of social norms elicited higher activation in a fronto-temporal network associated with social cognition, along with activation in the cerebellum [128]. Additional studies using a range of complementary methods are needed to directly assess whether and how the cerebellum plays a part in social predictions.
Although controversial, the cerebellum has been linked to a number of neuropsychiatric conditions marked by deficits in social cognition and affective processing, including autistic spectrum disorders (ASD) and schizophrenia. Starting with the seminal structural MRI findings of Courchesne and colleagues [129], there has been considerable interest in the association between cerebellar pathology and ASD. Abnormalities in cerebellar grey matter volume, microstructure, and connectivity are prominent in ASD, with a particular focus on the lobules Crus I and II [130]. Altered rsFC between the cerebellum (in particular, left Crus I) and cortical regions associated with ToM tasks (e.g., right TPJ) is found in patients with ASD [131, 132]. Diffusion tensor imaging (DTI) and psychophysiological interaction (PPI) analyses in individuals with ASD show that social deficits correlate with altered left cerebellar output to the right cerebral cortex, and in particular the right STS [133, 134]. Deficits in body motion perception are well documented in ASD patients [135–138]. These data, linking pathway integrity to function, suggest the Crus I – STS loop involved in processing of dynamic visual social signals may contribute to socio-cognitive dysfunction in ASD. In addition, a contralateral reciprocal connection could be involved in the linguistic deficits in ASD [139].
In schizophrenia, the data currently appear more heterogeneous. Reduced grey matter volume across the cerebellum has been reported in patients with in schizophrenia [140, 141], although there is also a report of increased vermis volume in individuals with positive psychotic symptoms and thought disorder [142]. Reduced rsFC is found between bilateral cerebellar lobules IV and V and the dorsal attention network [143], and between cerebellum and thalamus [144]. In contrast, rsFC is increased between ventral attention, salience and default-mode networks and the cerebellum, and in particular, Crus II [143, 145]. DTI studies have shown globally altered microstructure of cerebellar tracts [146] and disrupted modular architecture, also predominant in Crus II [147]. At the behavioral level, schizophrenia patients exhibit deficits in visual body motion perception [148, 149]. As compared with healthy subjects, higher yet delayed left cerebellar activation has been demonstrated in patients with schizophrenia during observation of abstract social interactions [150], but lower right cerebellar PET activation during verbal mental state attribution [151]. Overall, these data indicate structural and physiological abnormalities of the cerebellum in schizophrenia, but also highlight substantial pathophysiological and clinical heterogeneity. Of note, alteration and dysfunction of cerebro-cerebellar networks are not evident in all neuropsychiatric conditions, as evidenced by normal cerebellar volume and cerebello-thalamic tracts in patients with bipolar disorder [141, 144]. However, further efforts are needed to better understand cerebellar contribution to socio-cognitive deficits in schizophrenia.
Alteration of forward modeling is considered a core feature of the thought disorder in schizophrenia [152, 153]. Indeed, an inability to distinguish between internal states arising from one’s own thoughts and external events would create conditions ripe for hallucinations. Mechanistically, one intriguing line of work on an indirect link between schizophrenia and cerebellar function examined how the N1 response, an event-related potential (ERP) observed 100 ms after a tone, is modulated by prediction. In healthy individuals, this response is attenuated when the participant makes a movement (e.g., button press) to produce the tone, presumably due to the predictable link between the movement and the forthcoming sensory event. Interestingly, this sensory attenuation is largely absent in individuals with focal cerebellar lesions [154] or schizophrenia [155], suggesting both groups have impaired motor-sensory predictions – the output of a forward model.
What remains unclear is whether such prediction deficits can be generalized. That is, would patients with altered cerebellar function, either from a neurological disorder or a psychiatric condition, exhibit similar patterns if tested on predictions that did not require a motor component? For example, would they be impaired if the tone was predicted by another sensory event, or does it require a motor-sensory prediction? And what if the predictions were more abstract, such as those required for social cognition? Would ERP abnormalities be evident to these types of predictions or prediction violations, and would they be similar for patients with focal cerebellar lesions, cerebellar degeneration and neuropsychiatric disorders related to the cerebellum? Experiments such as the ones described here will be essential to illuminate how the cerebellum might contribute to social cognition, and test hypotheses that postulate a causal link between cerebellar abnormalities and these psychiatric conditions.
Constraints on Cerebellar Prediction and Error Processing in Cognition
We have argued that insights garnered from studies of sensorimotor control might help develop general mechanistic accounts of cerebellar function, focusing on two core concepts: prediction and error processing. Prediction has figured prominently in hypotheses concerning cerebellar processing in non-motor domains. The cerebellum, with its high level of convergence and reciprocal connections to much of the cerebral cortex, seems well positioned to integrate multiple inputs in order to generate estimates of future states, an idea formalized by forward models. This predictive capacity may be hierarchical, with predictions at lower levels anticipating how specific actions alter sensory inputs, while predictions at higher levels anticipate longer chains of events. This hypothesis would fit with observations of impaired sequencing after cerebellar damage [156, 157], as well as imaging data suggesting the cerebellum represents higher-order associative rules [158].
Of course prediction is not unique to the cerebellum; indeed, all brain processing may entail some form of prediction [159]. It will be important to identify constraints on cerebellar predictive capabilities (e.g. is embodiment a constraint) to see if these help in understanding the cognitive deficits observed in people with cerebellar pathology, or in neuropsychiatric conditions associated with cerebellar abnormalities.
One idea that has been useful in the sensory and motor domains is that cerebellar predictive capabilities are temporally constrained. This may be in terms of temporal extent—that the predictive capabilities of the cerebellum span relatively short time intervals relevant for skilled movement (e.g., up to a second or so), or that cerebellar predictions are temporally precise, representing when, as well as what is anticipated. This temporal constraint has received little attention in domains such as language and social cognition. Given the importance of temporal patterning in speech, the need for a system that generates predictions with real-time precision seems clear. Temporal constraints appear somewhat less straightforward in the social domain where interactions typically unfold over longer intervals of time. Nonetheless, social competence depends on the timing of our interactions with others [160]: We are very aware of atypical delays in a conversational exchange, as exemplified by transatlantic telephone calls [161].
Constraints are also relevant when considering how the cerebellum contributes to error representation. In the sensorimotor domain, cerebellar-dependent, error-based learning is used to calibrate forward models. Indeed, supervised learning is a hallmark of cerebellar function, driven by the sensory prediction error signal [42]. Notably, this signal is temporally constrained; slight delays in the delivery of an error signal markedly reduce the rate of learning [162, 163], a constraint that is much weaker in basal ganglia-dependent, reinforcement learning [164]. The exquisite temporal precision of cerebellar processing may be a key characteristic to differentiate cerebellar processing from that in the cerebral cortex [165].
Moreover, sensory prediction errors are vectorial, signaling not only the occurrence of an error but also providing metrical information concerning the direction of the error (e.g., a reach action to the right of its target). As such, sensory prediction errors not only tell you that an error has occurred, but they also provide information on how to correct future movements. Again, this contrasts with reinforcement learning where the signal might be binary (“correct or incorrect”) and where a reward prediction error is used to update the value associated with a potential choice. Reward errors can signal magnitude (e.g., a very bad action), but they do not typically provide information about how to improve performance.
More generally, our concepts of error processing in non-motor domains are limited. While we can easily appreciate how mis-reaching to the right of an object requires a leftward adjustment of a subsequent movement, how do we characterize the dimensions that define semantic relationships or social interactions? It is not obvious how a forward model in the linguistic system would be adjusted after a semantic expectancy is violated as in the sentence “The ice was purple”, or how our semantic system would be recalibrated were we to land on a planet where the water was, in fact, purple. At present, our inability to conceptualize the metrics of these abstract representational spaces may be a limiting factor. The reliance on sensory prediction errors in the cerebellum for calibrating coordinated movements may be just one specific instantiation of a more general error-based learning system.
It is also important to distinguish between the representation of errors and the use of error signals. Climbing fiber activity, stemming from the inferior olive, has long been hypothesized to constitute an error signal, one that will sculpt the cerebellar cortical output onto the deep cerebellar nuclei [64–66]. However, the comparison essential for the computation of an error may be extracerebellar: predictions generated in the cerebellum may be compared with actual input in subcortical or cortical areas, with the difference serving as the generator of the error signal. For example, the cerebellum could help generate a temporally constrained expectancy of a word in semantic areas or an agent’s reaction in social brain areas. A mismatch would then define the error, relayed back to the cerebellum to refine future predictions (Figure 2). By this view, the focus shifts back to the cerebellar role in prediction. Its sensitivity to error may be unrelated to the generation of an error signal, but rather to the exploitation of these error signals to improve future predictions and/or produce on-line changes in processing or behavior in response to the error.
Little is known about the cerebellar representation of errors outside the sensorimotor domain. There are indicators that neurophysiological correlates of error may be observed in the cerebellum, but this question has not been systematically explored.
Despite some evidence of projections from associative areas of the cerebral cortex to the inferior olive [60, 166–168], it remains unclear whether these projections contribute to perceptual and cognitive error signals that may supervise cerebellar learning. Given the diversity of parallel cognitive processes, whether and how associative error inputs are filtered and assigned represents another significant open question. Neural modeling may help refine such concepts of cerebellar function and plasticity, offering a way to integrate information that incorporates our knowledge of cerebellar cell types and architecture, cerebro-cerebellar connectivity, and behaviors associated with these networks [169]. More generally, as briefly outlined in this article, interdisciplinary efforts bridging the gap between methods that range from immunohistochemistry and electrophysiology to computational and clinical neuroscience appear indispensable to substantially promote our understanding of the cerebellum. To date, supervised learning and its constraints remain insufficiently targeted in cognitive studies on the cerebellum.
This idea of a cerebellar predictor resonates with various metaphors that have been used, describing the cerebellum as a system that facilitates cortical processing. These metaphors build on the classic observation that, while not essential for movement, the cerebellum allows actions to be performed in an effortlessly coordinated manner. Extended to non-motor domains, this has led to metaphors in which the cerebellum “coordinates” shifts of attention [170], “facilitates” the retrieval of information in memory [171] or helps “automatize” cortical processing [6, 172]. These hypotheses describe how the cerebellum may support specific cortical processes, rather than articulating computations that might be unique to the cerebellum (e.g., precise representation of temporal relationships, or model-based prediction). Fronto-parietal networks are known to provide critical representations for attentional selection and orientation, and fronto-temporal for social interactions. The cerebellum may help ensure smooth coordinated processing within these networks. “Cognitive dysmetria” and “dysmetria of thought” are phrases coined to capture this metaphor, arguing that cortical processing loses its fluidity due to cerebro-cerebellar circuitry dysfunction in patients with schizophrenia [173] and those with acquired damage to the cerebellum [174].
A limitation with these metaphorical descriptions is that they become difficult to test. While one can formulate experiments to evaluate computationally-specified mechanisms (e.g., temporally-limited vs. temporally-extended; forward vs. inverse model), it is harder to define conditions that specify how thought becomes less coordinated. These metaphors are perhaps just restatements of the observed phenomena—people with schizophrenia have frazzled cognition and, thus, the cerebellar link is attributed to a problem in coordinating thought.
Yet one could also see how the vast pattern recognition capacity of the cerebellum, coupled with its broad connectivity to the cortex, could prime or bias cortical processing in particular directions, and so be central to the coordination and automatization of thought. This kind of supportive function need not require a prediction, per se. Simply recognizing current neural states, and using that information to prod the cortex to its associated patterns could be sufficient to promote more fluid cortical processing. However, this operation would be even more efficient if the cerebellum were, as a forward modeling device, using the input to anticipate future states, either of the world or of cortical activity.
Concluding Remarks and Future Perspectives
In this article, we have provided an overview of emerging perspectives on how the functional role of the cerebellum might extend to cognition, using language and social cognition as two representative domains. The motivation for this new conceptualization has been largely inspired by neuroanatomical and neuroimaging studies. The neuropsychological long-term picture is more nuanced: Although there is certainly evidence of impaired language function, altered affect, and rather heterogeneous problems in social cognition, these deficits appear mild in patients with cerebellar pathology acquired in adulthood, with executive dysfunction being the most likely to persist, even if rather modestly [175, 176]. The impressive degree of recovery may be viewed as a challenge to models in which the cerebellum provides essential computations, or it may reflect an extraordinary capacity for reorganization [177]. Deeper insight into how cerebellar insults impact cognition will depend on asking more refined questions in behavioral and imaging studies with patient populations, and on meticulous lesion-symptom mapping on sufficiently powered groups of patients with more homogeneous lesion topography and etiology.
Our arguments have been guided by the belief that there will be commonalities in cerebellar function across different processing domains. We recognize that this assumption – although suggested by cerebellar architecture and shared by most researchers studying non-motor functions of the cerebellum – may be misguided. The belief that computational models of the cerebellum based in sensorimotor control are important for understanding how it contributes to language and social cognition could prove to be an impediment to progress. The growing evidence on functional diversity of structurally similar cerebellar modules could not only signify an enlarged spectrum for a universal computational principle, but also open the window for other functional processes. These issues, as well as those highlighted in the previous section, require critical consideration as we seek to expand our understanding of how the cerebellum supports cognition.
Nonetheless, as we argue here, some form of prediction may represent a universal principle of cerebellar function, applied to both motor and cognitive domains. Moreover, error-based learning is of crucial value for predictive processing, and it is useful to consider how to build on known constraints associated with cerebellar learning in developing a broader perspective on cerebellar function. Multimodal integrative research efforts based on sound theoretical reasoning will surely help to further explicate the principles underlying cerebellar involvement in learning and cognition.
Trends.
Multidisciplinary evidence implicates a role for the cerebellum in various aspects of cognition.
Due to its uniform cytoarchitecture and extensive reciprocal connections with frontal, parietal and temporal associative cortices, theorists have sought to identify cerebellar computations that are universal across sensorimotor and associative processes. Two key concepts are prediction and error-based learning.
Recently uncovered physiological diversity of structurally similar cerebellar modules may explain how similar principles of cerebellar processing subserve diverse functional domains.
Knowledge has substantially evolved on cerebellar involvement in language and social cognition, providing representative domains to evaluate functional hypotheses of the “cognitive” cerebellum, and to consider how disturbances of cerebellar function may contribute to developmental and neuropsychiatric disorders.
Outstanding Questions.
Have efforts to understand of cerebellar function been helped or hindered by a bias to look for computational hypotheses that offer a unified account in domains as diverse as sensorimotor control and social cognition?
Is it best to consider the reciprocal loops between the cerebral cortex and cerebellum as closed? While there is currently little evidence to support interaction between cerebellar modules, could significant information exchange take place at the thalamic or cortical levels?
What makes cerebellar prediction unique, relative to processing in other regions of the brain, and how might this form of prediction be useful across a range of functional domains? Are temporal precision and supervised learning key constraints on cerebellar processing, suggesting that insight into cerebellar function may benefit by specifically manipulating these constraints in tests of higher cognition?
Climbing fiber inputs from the inferior olive are thought to drive cerebellar adaptation of sensorimotor forward models, by signaling sensory prediction errors. Is the inferior olive also involved in cognitive error-based learning and, if so, what is the information content of its signals?
What is the functional significance of the two (or three) cortical representation maps in the cerebellum? Do these maps differentially contribute to adaptive prediction providing different variants of the same model or do they provide different types of prediction (e.g., for on-line processing or prospective model adaptation)?
What are the consequences of functionally diverse modules in the cerebellum challenging classic notions of a uniform physiology across the cerebellar cortex?
What are cerebellar and extracerebellar neurobiological correlates of recovery from focal cerebellar lesions? How does this pattern of recovery bear on hypotheses concerning cerebellar cognitive function?
How might insights gained from the study of the “cognitive” cerebellum be useful in reexamining cerebellar involvement in sensorimotor control and learning?
Acknowledgments
The authors wish to thank Richard Frackowiak and Chris De Zeeuw for valuable discussion. A.A.S. was supported by a research grant for junior academic clinicians by the fund of the Research Committee of the Faculty for Biology and Medicine, University of Lausanne, Switzerland. R.B.I. was supported by grants NS092079 and NS074917 from the National Institute of Health (USA). R.C.M was supported by grants from the Wellcome Trust (WT087554) and MRC (MR/J012610/1).
Glossary
- Ataxia
A neurological condition characterized by abnormal motor coordination, due to alteration in sensory, vestibular or cerebellar systems. Signs include problems with balance, eye movements, volitional movements and speech, especially when the movements are produced quickly and require coordination of multiple joints
- Default-mode network
A physiological network identified by resting state functional connectivity (rsFC), spanning medial prefrontal, medial temporal, posterior cingulate cortex and precuneus, activated in the absence of a specific external task and thought to be involved in mental simulation for planning, self-evaluation and social interaction
- Dysmetria
Refers to movements lacking the appropriate metrics due to ataxia. Spatially, the movements may over- or undershoot the target, with the problems evident with eye, arm or leg movements
- Error-based learning
Process by which changes in behavior are driven by errors occurring as a result of the production of that behavior in a similar context. In ideal circumstances, the amount of error is reduced in a continuous manner from trial to trial, continuing until performance is error-free
- Forward model
Representation of the predictable relationship between the input and output of a system, thus providing an estimation of a new state or outcome given an input. Forward models are typically adaptive, updated by experience. In sensorimotor systems, a forward model can take the motor command (or efference copy of the command) to predict the forthcoming sensory consequences of the movement
- Inverse model
Representation that takes a desired sensory state as the input and generates the motor command required to achieve that state. Inverse models perform the inverse operation of a forward model
- Long-term depression (LTD)
Physiological mechanism of learning manifest as the reduction of synaptic strength between two neurons, typically resulting from a strong excitatory input. In the cerebellar cortex, LTD of parallel fiber synapses onto Purkinje cells is triggered by powerful climbing fiber input
- Sensory prediction error
Signal arising from the mismatch between the expected sensory consequences of a stimulus or movement and the actual sensory input. As such, sensory prediction errors are a violation of a prediction. For example, when walking down the stairs, a missing tread would result in a sensory prediction error based on the absence of expected somatosensory input
- Spinocerebellar ataxia (SCA)
Heritable degenerative disease that produces ataxia, through degeneration of the cerebellum and/or structures sending critical inputs to the cerebellum. At present there are more than 40 known types, each related to specific gene mutations. Pathophysiological correlates and symptomology vary as a function of type
- Theory of mind (ToM)
Psychological construct that refers to the ability to infer the mental states of other individuals, and in particular their beliefs, feelings and intentions
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Petersen SE, et al. Positron emission tomographic studies of the processing of single words. J Cogn Neurosci. 1989;1:153–170. doi: 10.1162/jocn.1989.1.2.153. [DOI] [PubMed] [Google Scholar]
- 2.Fiez JA, et al. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study. Brain. 1992;115(Pt 1):155–178. doi: 10.1093/brain/115.1.155. [DOI] [PubMed] [Google Scholar]
- 3.Ivry RB, Fiez JA. Cerebellar contributions to cognition and imagery. In: Gazzaniga M, editor. The Cognitive Neurosciences. 2nd. MIT Press; 2000. pp. 999–1011. [Google Scholar]
- 4.Ishikawa T, et al. The cerebro-cerebellum: Could it be loci of forward models? Neurosci Res. 2016;104:72–79. doi: 10.1016/j.neures.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Middleton FA, Strick PL. Cerebellar projections to the prefrontal cortex of the primate. J Neurosci. 2001;21:700–712. doi: 10.1523/JNEUROSCI.21-02-00700.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strick PL, et al. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. [DOI] [PubMed] [Google Scholar]
- 7.Salmi J, et al. Cognitive and motor loops of the human cerebro-cerebellar system. J Cogn Neurosci. 2010;22:2663–2676. doi: 10.1162/jocn.2009.21382. [DOI] [PubMed] [Google Scholar]
- 8.Grodd W, et al. Sensorimotor mapping of the human cerebellum: fMRI evidence of somatotopic organization. Hum Brain Mapp. 2001;13:55–73. doi: 10.1002/hbm.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rijntjes M, et al. Multiple somatotopic representations in the human cerebellum. Neuroreport. 1999;10:3653–3658. doi: 10.1097/00001756-199911260-00035. [DOI] [PubMed] [Google Scholar]
- 10.Nitschke MF, et al. Somatotopic motor representation in the human anterior cerebellum. A high-resolution functional MRI study. Brain. 1996;119(Pt 3):1023–1029. doi: 10.1093/brain/119.3.1023. [DOI] [PubMed] [Google Scholar]
- 11.Habas C, et al. Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci. 2009;29:8586–8594. doi: 10.1523/JNEUROSCI.1868-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckner RL, et al. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–2345. doi: 10.1152/jn.00339.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dobromyslin VI, et al. Distinct functional networks within the cerebellum and their relation to cortical systems assessed with independent component analysis. Neuroimage. 2012;60:2073–2085. doi: 10.1016/j.neuroimage.2012.01.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Reilly JX, et al. Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex. 2010;20:953–965. doi: 10.1093/cercor/bhp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoodley CJ, Schmahmann JD. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44:489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 16.Hawkes R, Leclerc N. Antigenic map of the rat cerebellar cortex: the distribution of parasagittal bands as revealed by monoclonal anti-Purkinje cell antibody mabQ113. J Comp Neurol. 1987;256:29–41. doi: 10.1002/cne.902560104. [DOI] [PubMed] [Google Scholar]
- 17.Sugihara I, Shinoda Y. Molecular, topographic, and functional organization of the cerebellar cortex: a study with combined aldolase C and olivocerebellar labeling. J Neurosci. 2004;24:8771–8785. doi: 10.1523/JNEUROSCI.1961-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buono P, et al. Differential distribution of aldolase A and C in the human central nervous system. J Neurocytol. 2001;30:957–965. doi: 10.1023/a:1021828421792. [DOI] [PubMed] [Google Scholar]
- 19.Diedrichsen J, Zotow E. Surface-Based Display of Volume-Averaged Cerebellar Imaging Data. PLoS One. 2015;10:e0133402. doi: 10.1371/journal.pone.0133402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci. 2005;8:1329–1334. doi: 10.1038/nn1539. [DOI] [PubMed] [Google Scholar]
- 21.Zhou H, et al. Cerebellar modules operate at different frequencies. Elife. 2014;3:e02536. doi: 10.7554/eLife.02536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marzban H, Hawkes R. On the architecture of the posterior zone of the cerebellum. Cerebellum. 2011;10:422–434. doi: 10.1007/s12311-010-0208-3. [DOI] [PubMed] [Google Scholar]
- 23.Stoodley CJ, et al. Location of lesion determines motor vs. cognitive consequences in patients with cerebellar stroke. Neuroimage Clin. 2016;12:765–775. doi: 10.1016/j.nicl.2016.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dum RP, Strick PL. An unfolded map of the cerebellar dentate nucleus and its projections to the cerebral cortex. J Neurophysiol. 2003;89:634–639. doi: 10.1152/jn.00626.2002. [DOI] [PubMed] [Google Scholar]
- 25.Ramnani N, et al. The evolution of prefrontal inputs to the cortico-pontine system: diffusion imaging evidence from Macaque monkeys and humans. Cereb Cortex. 2006;16:811–818. doi: 10.1093/cercor/bhj024. [DOI] [PubMed] [Google Scholar]
- 26.Brodal P. The corticopontine projection in the rhesus monkey. Origin and principles of organization. Brain. 1978;101:251–283. doi: 10.1093/brain/101.2.251. [DOI] [PubMed] [Google Scholar]
- 27.Glickstein M, et al. Visual pontocerebellar projections in the macaque. J Comp Neurol. 1994;349:51–72. doi: 10.1002/cne.903490105. [DOI] [PubMed] [Google Scholar]
- 28.Schmahmann JD, Pandya DN. Projections to the basis pontis from the superior temporal sulcus and superior temporal region in the rhesus monkey. J Comp Neurol. 1991;308:224–248. doi: 10.1002/cne.903080209. [DOI] [PubMed] [Google Scholar]
- 29.Jack A, et al. Subcortical contributions to effective connectivity in brain networks supporting imitation. Neuropsychologia. 2011;49:3689–3698. doi: 10.1016/j.neuropsychologia.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 30.Sokolov AA, et al. Biological motion processing: The left cerebellum communicates with the right superior temporal sulcus. Neuroimage. 2012;59:2824–2830. doi: 10.1016/j.neuroimage.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 31.Sokolov AA, et al. Structural loop between the cerebellum and the superior temporal sulcus: evidence from diffusion tensor imaging. Cereb Cortex. 2014;24:626–632. doi: 10.1093/cercor/bhs346. [DOI] [PubMed] [Google Scholar]
- 32.Booth JR, et al. The role of the basal ganglia and cerebellum in language processing. Brain Res. 2007;1133:136–144. doi: 10.1016/j.brainres.2006.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kellermann T, et al. Effective connectivity of the human cerebellum during visual attention. J Neurosci. 2012;32:11453–11460. doi: 10.1523/JNEUROSCI.0678-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Allen G, et al. Attentional activation of the cerebellum independent of motor involvement. Science. 1997;275:1940–1943. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- 35.Ide JS, Li CS. A cerebellar thalamic cortical circuit for error-related cognitive control. Neuroimage. 2011;54:455–464. doi: 10.1016/j.neuroimage.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivry RB, Keele SW. Timing functions of the cerebellum. J Cogn Neurosci. 1989;1:136–152. doi: 10.1162/jocn.1989.1.2.136. [DOI] [PubMed] [Google Scholar]
- 37.Ravizza SM, et al. Cerebellar damage produces selective deficits in verbal working memory. Brain. 2006;129:306–320. doi: 10.1093/brain/awh685. [DOI] [PubMed] [Google Scholar]
- 38.Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 39.Hore J, et al. Cerebellar dysmetria at the elbow, wrist, and fingers. J Neurophysiol. 1991;65:563–571. doi: 10.1152/jn.1991.65.3.563. [DOI] [PubMed] [Google Scholar]
- 40.Bastian AJ, et al. Cerebellar ataxia: abnormal control of interaction torques across multiple joints. J Neurophysiol. 1996;76:492–509. doi: 10.1152/jn.1996.76.1.492. [DOI] [PubMed] [Google Scholar]
- 41.Lisberger SG. Visual guidance of smooth-pursuit eye movements: sensation, action, and what happens in between. Neuron. 2010;66:477–491. doi: 10.1016/j.neuron.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doya K. What are the computations of the cerebellum, the basal ganglia and the cerebral cortex? Neural Netw. 1999;12:961–974. doi: 10.1016/s0893-6080(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 43.Kawato M, Gomi H. The cerebellum and VOR/OKR learning models. Trends Neurosci. 1992;15:445–453. doi: 10.1016/0166-2236(92)90008-v. [DOI] [PubMed] [Google Scholar]
- 44.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 45.Requarth T, Sawtell NB. Plastic corollary discharge predicts sensory consequences of movements in a cerebellum-like circuit. Neuron. 2014;82:896–907. doi: 10.1016/j.neuron.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tomatsu S, et al. Information processing in the hemisphere of the cerebellar cortex for control of wrist movement. J Neurophysiol. 2016;115:255–270. doi: 10.1152/jn.00530.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Green AM, et al. A reevaluation of the inverse dynamic model for eye movements. J Neurosci. 2007;27:1346–1355. doi: 10.1523/JNEUROSCI.3822-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hewitt AL, et al. Representation of limb kinematics in Purkinje cell simple spike discharge is conserved across multiple tasks. J Neurophysiol. 2011;106:2232–2247. doi: 10.1152/jn.00886.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laurens J, et al. Computation of linear acceleration through an internal model in the macaque cerebellum. Nat Neurosci. 2013;16:1701–1708. doi: 10.1038/nn.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herzfeld DJ, et al. Encoding of action by the Purkinje cells of the cerebellum. Nature. 2015;526:439–442. doi: 10.1038/nature15693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawato M, Gomi H. A computational model of four regions of the cerebellum based on feedback-error learning. Biol Cybern. 1992;68:95–103. doi: 10.1007/BF00201431. [DOI] [PubMed] [Google Scholar]
- 52.Shidara M, et al. Inverse-dynamics model eye movement control by Purkinje cells in the cerebellum. Nature. 1993;365:50–52. doi: 10.1038/365050a0. [DOI] [PubMed] [Google Scholar]
- 53.Lisberger SG. Internal models of eye movement in the floccular complex of the monkey cerebellum. Neuroscience. 2009;162:763–776. doi: 10.1016/j.neuroscience.2009.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ebner TJ, et al. What features of limb movements are encoded in the discharge of cerebellar neurons? Cerebellum. 2011;10:683–693. doi: 10.1007/s12311-010-0243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu X, et al. Neuronal activity related to the visual representation of arm movements in the lateral cerebellar cortex. J Neurophysiol. 2003;89:1223–1237. doi: 10.1152/jn.00817.2002. [DOI] [PubMed] [Google Scholar]
- 56.Wolpert DM, Kawato M. Multiple paired forward and inverse models for motor control. Neural Netw. 1998;11:1317–1329. doi: 10.1016/s0893-6080(98)00066-5. [DOI] [PubMed] [Google Scholar]
- 57.Frens MA, Donchin O. Forward models and state estimation in compensatory eye movements. Front Cell Neurosci. 2009;3:13. doi: 10.3389/neuro.03.013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brooks JX, Cullen KE. The primate cerebellum selectively encodes unexpected self-motion. Curr Biol. 2013;23:947–955. doi: 10.1016/j.cub.2013.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Zeeuw CI, et al. Mesodiencephalic and cerebellar terminals terminate upon the same dendritic spines in the glomeruli of the cat and rat inferior olive: an ultrastructural study using a combination of [3H]leucine and wheat germ agglutinin coupled horseradish peroxidase anterograde tracing. Neuroscience. 1990;34:645–655. doi: 10.1016/0306-4522(90)90171-y. [DOI] [PubMed] [Google Scholar]
- 60.De Zeeuw CI, et al. Microcircuitry and function of the inferior olive. Trends Neurosci. 1998;21:391–400. doi: 10.1016/s0166-2236(98)01310-1. [DOI] [PubMed] [Google Scholar]
- 61.Marr D. A theory of cerebellar cortex. J Physiol. 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- 63.Brooks JX, et al. Learning to expect the unexpected: rapid updating in primate cerebellum during voluntary self-motion. Nat Neurosci. 2015;18:1310–1317. doi: 10.1038/nn.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ohmae S, Medina JF. Climbing fibers encode a temporal-difference prediction error during cerebellar learning in mice. Nat Neurosci. 2015;18:1798–1803. doi: 10.1038/nn.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gibson AR, et al. Activation of climbing fibers. Cerebellum. 2004;3:212–221. doi: 10.1080/14734220410018995. [DOI] [PubMed] [Google Scholar]
- 66.Apps R. Movement-related gating of climbing fibre input to cerebellar cortical zones. Prog Neurobiol. 1999;57:537–562. doi: 10.1016/s0301-0082(98)00068-9. [DOI] [PubMed] [Google Scholar]
- 67.Lang EJ, et al. The Roles of the Olivocerebellar Pathway in Motor Learning and Motor Control. A Consensus Paper. Cerebellum. 2016 doi: 10.1007/s12311-016-0787-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suvrathan A, et al. Timing Rules for Synaptic Plasticity Matched to Behavioral Function. Neuron. 2016;92:959–967. doi: 10.1016/j.neuron.2016.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marien P, Beaton A. The enigmatic linguistic cerebellum: clinical relevance and unanswered questions on nonmotor speech and language deficits in cerebellar disorders. Cerebellum Ataxias. 2014;1:12. doi: 10.1186/2053-8871-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chein JM, Fiez JA. Dissociation of verbal working memory system components using a delayed serial recall task. Cereb Cortex. 2001;11:1003–1014. doi: 10.1093/cercor/11.11.1003. [DOI] [PubMed] [Google Scholar]
- 71.Justus T, et al. Reduced phonological similarity effects in patients with damage to the cerebellum. Brain Lang. 2005;95:304–318. doi: 10.1016/j.bandl.2005.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lesage E, et al. Cerebellar BOLD signal during the acquisition of a new lexicon predicts its early consolidation. Brain Lang. 2015 doi: 10.1016/j.bandl.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raboyeau G, et al. Lexical learning of the English language: a PET study in healthy French subjects. Neuroimage. 2004;22:1808–1818. doi: 10.1016/j.neuroimage.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 74.Arasanz CP, et al. The cerebellum and its role in word generation: a cTBS study. Cortex. 2012;48:718–724. doi: 10.1016/j.cortex.2011.02.021. [DOI] [PubMed] [Google Scholar]
- 75.Argyropoulos GP, et al. theta-burst stimulation of the right neocerebellar vermis selectively disrupts the practice-induced acceleration of lexical decisions. Behav Neurosci. 2011;125:724–734. doi: 10.1037/a0025134. [DOI] [PubMed] [Google Scholar]
- 76.Argyropoulos GP, Muggleton NG. Effects of cerebellar stimulation on processing semantic associations. Cerebellum. 2013;12:83–96. doi: 10.1007/s12311-012-0398-y. [DOI] [PubMed] [Google Scholar]
- 77.Oliveri M, et al. Motor and linguistic linking of space and time in the cerebellum. PLoS One. 2009;4:e7933. doi: 10.1371/journal.pone.0007933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nicolson RI, et al. Developmental dyslexia: the cerebellar deficit hypothesis. Trends Neurosci. 2001;24:508–511. doi: 10.1016/s0166-2236(00)01896-8. [DOI] [PubMed] [Google Scholar]
- 79.Marien P, et al. Cognitive, linguistic and affective disturbances following a right superior cerebellar artery infarction: a case study. Cortex. 2009;45:527–536. doi: 10.1016/j.cortex.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 80.Moberget T, Ivry RB. Cerebellar contributions to motor control and language comprehension: searching for common computational principles. Ann N Y Acad Sci. 2016;1369:154–171. doi: 10.1111/nyas.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pickering MJ, Clark A. Getting ahead: forward models and their place in cognitive architecture. Trends Cogn Sci. 2014;18:451–456. doi: 10.1016/j.tics.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 82.Moberget T, et al. Generalized role for the cerebellum in encoding internal models: evidence from semantic processing. J Neurosci. 2014;34:2871–2878. doi: 10.1523/JNEUROSCI.2264-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lesage E, et al. Soc for Neurosci. Washington DC: 2014. Cerebellar BOLD response to linguistic stimuli is modulated by predictability. [Google Scholar]
- 84.Lesage E, et al. Cerebellar rTMS disrupts predictive language processing. Curr Biol. 2012;22:R794–795. doi: 10.1016/j.cub.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miall RC, et al. Modulation of linguistic prediction by TDCS of the right lateral cerebellum. Neuropsychologia. 2016;86:103–109. doi: 10.1016/j.neuropsychologia.2016.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Imamizu H, et al. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- 87.Koster-Hale J, Saxe R. Theory of mind: a neural prediction problem. Neuron. 2013;79:836–848. doi: 10.1016/j.neuron.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pavlova MA. Biological motion processing as a hallmark of social cognition. Cereb Cortex. 2012;22:981–995. doi: 10.1093/cercor/bhr156. [DOI] [PubMed] [Google Scholar]
- 89.Frith CD, Frith U. Mechanisms of social cognition. Annu Rev Psychol. 2012;63:287–313. doi: 10.1146/annurev-psych-120710-100449. [DOI] [PubMed] [Google Scholar]
- 90.Olsson A, Ochsner KN. The role of social cognition in emotion. Trends Cogn Sci. 2008;12:65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 91.Weightman MJ, et al. A review of the role of social cognition in major depressive disorder. Front Psychiatry. 2014;5:179. doi: 10.3389/fpsyt.2014.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Waal FB. Putting the altruism back into altruism: the evolution of empathy. Annu Rev Psychol. 2008;59:279–300. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- 93.Davis M. Empathy: A social psychological approach. Westview Press; 1996. [Google Scholar]
- 94.Shamay-Tsoory SG, et al. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–627. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- 95.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 96.D’Agata F, et al. The recognition of facial emotions in spinocerebellar ataxia patients. Cerebellum. 2011;10:600–610. doi: 10.1007/s12311-011-0276-z. [DOI] [PubMed] [Google Scholar]
- 97.Adamaszek M, et al. Impairment of emotional facial expression and prosody discrimination due to ischemic cerebellar lesions. Cerebellum. 2014;13:338–345. doi: 10.1007/s12311-013-0537-0. [DOI] [PubMed] [Google Scholar]
- 98.Sokolovsky N, et al. A preliminary characterisation of cognition and social cognition in spinocerebellar ataxia types 21 and 7. Behav Neurol. 2010;23:17–29. doi: 10.3233/BEN-2010-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Parvizi J, et al. Pathological laughter and crying: a link to the cerebellum. Brain. 2001;124:1708–1719. doi: 10.1093/brain/124.9.1708. [DOI] [PubMed] [Google Scholar]
- 100.Parvizi J, et al. Pathological laughter and crying in patients with multiple system atrophy-cerebellar type. Mov Disord. 2007;22:798–803. doi: 10.1002/mds.21348. [DOI] [PubMed] [Google Scholar]
- 101.Schutter DJ, van Honk J. The cerebellum in emotion regulation: a repetitive transcranial magnetic stimulation study. Cerebellum. 2009;8:28–34. doi: 10.1007/s12311-008-0056-6. [DOI] [PubMed] [Google Scholar]
- 102.Adamaszek M, et al. Neural correlates of impaired emotional face recognition in cerebellar lesions. Brain Res. 2015;1613:1–12. doi: 10.1016/j.brainres.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 103.Schmahmann JD, et al. The neuropsychiatry of the cerebellum - insights from the clinic. Cerebellum. 2007;6:254–267. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- 104.Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Heath RG, Harper JW. Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: evoked potential and histological studies in monkeys and cats. Exp Neurol. 1974;45:268–287. doi: 10.1016/0014-4886(74)90118-6. [DOI] [PubMed] [Google Scholar]
- 106.Bernard JA, et al. Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front Neuroanat. 2012;6:31. doi: 10.3389/fnana.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sang L, et al. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage. 2012;61:1213–1225. doi: 10.1016/j.neuroimage.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 108.Van Overwalle F, et al. Social cognition and the cerebellum: a meta-analysis of over 350 fMRI studies. Neuroimage. 2014;86:554–572. doi: 10.1016/j.neuroimage.2013.09.033. [DOI] [PubMed] [Google Scholar]
- 109.Hoche F, et al. Cerebellar Contribution to Social Cognition. Cerebellum. 2015 doi: 10.1007/s12311-015-0746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parente A, et al. Selective theory of mind impairment and cerebellar atrophy: a case report. J Neurol. 2013;260:2166–2169. doi: 10.1007/s00415-013-6985-0. [DOI] [PubMed] [Google Scholar]
- 111.Garrard P, et al. Cognitive and social cognitive functioning in spinocerebellar ataxia: a preliminary characterization. J Neurol. 2008;255:398–405. doi: 10.1007/s00415-008-0680-6. [DOI] [PubMed] [Google Scholar]
- 112.Frith C, Frith U. Theory of mind. Curr Biol. 2005;15:R644–646. doi: 10.1016/j.cub.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 113.Nawrot M, Rizzo M. Motion perception deficits from midline cerebellar lesions in human. Vision Res. 1995;35:723–731. doi: 10.1016/0042-6989(94)00168-l. [DOI] [PubMed] [Google Scholar]
- 114.Ivry RB, Diener HC. Impaired velocity perception in patients with lesions to the cerebellum. J Cogn Neurosci. 1991;3:355–366. doi: 10.1162/jocn.1991.3.4.355. [DOI] [PubMed] [Google Scholar]
- 115.Jokisch D, et al. Differential involvement of the cerebellum in biological and coherent motion perception. Eur J Neurosci. 2005;21:3439–3446. doi: 10.1111/j.1460-9568.2005.04145.x. [DOI] [PubMed] [Google Scholar]
- 116.Sokolov AA, et al. Cerebellar engagement in an action observation network. Cereb Cortex. 2010;20:486–491. doi: 10.1093/cercor/bhp117. [DOI] [PubMed] [Google Scholar]
- 117.Jack A, Pelphrey KA. Neural Correlates of Animacy Attribution Include Neocerebellum in Healthy Adults. Cereb Cortex. 2015;25:4240–4247. doi: 10.1093/cercor/bhu146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gobbini MI, et al. Two takes on the social brain: a comparison of theory of mind tasks. J Cogn Neurosci. 2007;19:1803–1814. doi: 10.1162/jocn.2007.19.11.1803. [DOI] [PubMed] [Google Scholar]
- 119.Barsalou LW. Simulation, situated conceptualization, and prediction. Philos Trans R Soc Lond B Biol Sci. 2009;364:1281–1289. doi: 10.1098/rstb.2008.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown EC, Brune M. The role of prediction in social neuroscience. Front Hum Neurosci. 2012;6:147. doi: 10.3389/fnhum.2012.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gazzola V, Keysers C. The observation and execution of actions share motor and somatosensory voxels in all tested subjects: single-subject analyses of unsmoothed fMRI data. Cereb Cortex. 2009;19:1239–1255. doi: 10.1093/cercor/bhn181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fuentes CT, Bastian AJ. ‘Motor cognition’ - what is it and is the cerebellum involved? Cerebellum. 2007;6:232–236. doi: 10.1080/14734220701329268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci. 2010;11:264–274. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- 124.Miall RC. Connecting mirror neurons and forward models. Neuroreport. 2003;14:2135–2137. doi: 10.1097/00001756-200312020-00001. [DOI] [PubMed] [Google Scholar]
- 125.Wolpert DM, et al. A unifying computational framework for motor control and social interaction. Philos Trans R Soc Lond B Biol Sci. 2003;358:593–602. doi: 10.1098/rstb.2002.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Barsalou LW. Perceptual symbol systems. Behav Brain Sci. 1999;22:577–609. doi: 10.1017/s0140525x99002149. discussion 610–560. [DOI] [PubMed] [Google Scholar]
- 127.Mahon BZ, Caramazza A. A critical look at the embodied cognition hypothesis and a new proposal for grounding conceptual content. J Physiol Paris. 2008;102:59–70. doi: 10.1016/j.jphysparis.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 128.Berthoz S, et al. An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain. 2002;125:1696–1708. doi: 10.1093/brain/awf190. [DOI] [PubMed] [Google Scholar]
- 129.Courchesne E, et al. Hypoplasia of cerebellar vermal lobules VI and VII in autism. N Engl J Med. 1988;318:1349–1354. doi: 10.1056/NEJM198805263182102. [DOI] [PubMed] [Google Scholar]
- 130.D’Mello AM, Stoodley CJ. Cerebro-cerebellar circuits in autism spectrum disorder. Front Neurosci. 2015;9:408. doi: 10.3389/fnins.2015.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Igelstrom KM, et al. Functional Connectivity Between the Temporoparietal Cortex and Cerebellum in Autism Spectrum Disorder. Cereb Cortex. 2016 doi: 10.1093/cercor/bhw079. [DOI] [PubMed] [Google Scholar]
- 132.Olivito G, et al. Resting-State Functional Connectivity Changes Between Dentate Nucleus and Cortical Social Brain Regions in Autism Spectrum Disorders. Cerebellum. 2016 doi: 10.1007/s12311-016-0795-8. [DOI] [PubMed] [Google Scholar]
- 133.Jack A, Morris JP. Neocerebellar contributions to social perception in adolescents with autism spectrum disorder. Dev Cogn Neurosci. 2014;10:77–92. doi: 10.1016/j.dcn.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Catani M, et al. Altered cerebellar feedback projections in Asperger syndrome. Neuroimage. 2008;41:1184–1191. doi: 10.1016/j.neuroimage.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 135.Blake R, et al. Visual recognition of biological motion is impaired in children with autism. Psychol Sci. 2003;14:151–157. doi: 10.1111/1467-9280.01434. [DOI] [PubMed] [Google Scholar]
- 136.Klin A, et al. Two-year-olds with autism orient to non-social contingencies rather than biological motion. Nature. 2009;459:257–261. doi: 10.1038/nature07868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Koldewyn K, et al. The psychophysics of visual motion and global form processing in autism. Brain. 2010;133:599–610. doi: 10.1093/brain/awp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nackaerts E, et al. Recognizing biological motion and emotions from point-light displays in autism spectrum disorders. PLoS One. 2012;7:e44473. doi: 10.1371/journal.pone.0044473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tager-Flusberg H. A psychological approach to understanding the social and language impairments in autism. Int Rev Psychiatry. 1999;11:325–334. doi: 10.1080/09540269974203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Roman-Urrestarazu A, et al. Brain structure in different psychosis risk groups in the Northern Finland 1986 birth cohort. Schizophr Res. 2014;153:143–149. doi: 10.1016/j.schres.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 141.Laidi C, et al. Cerebellar volume in schizophrenia and bipolar I disorder with and without psychotic features. Acta Psychiatr Scand. 2015;131:223–233. doi: 10.1111/acps.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Levitt JJ, et al. Quantitative volumetric MRI study of the cerebellum and vermis in schizophrenia: clinical and cognitive correlates. Am J Psychiatry. 1999;156:1105–1107. doi: 10.1176/ajp.156.7.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guo W, et al. Resting-state cerebellar-cerebral networks are differently affected in first-episode, drug-naive schizophrenia patients and unaffected siblings. Sci Rep. 2015;5:17275. doi: 10.1038/srep17275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Anticevic A, et al. Mediodorsal and visual thalamic connectivity differ in schizophrenia and bipolar disorder with and without psychosis history. Schizophr Bull. 2014;40:1227–1243. doi: 10.1093/schbul/sbu100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shinn AK, et al. Aberrant cerebellar connectivity in motor and association networks in schizophrenia. Front Hum Neurosci. 2015;9:134. doi: 10.3389/fnhum.2015.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kanaan RA, et al. Microstructural organization of cerebellar tracts in schizophrenia. Biol Psychiatry. 2009;66:1067–1069. doi: 10.1016/j.biopsych.2009.07.028. [DOI] [PubMed] [Google Scholar]
- 147.Kim DJ, et al. Disrupted modular architecture of cerebellum in schizophrenia: a graph theoretic analysis. Schizophr Bull. 2014;40:1216–1226. doi: 10.1093/schbul/sbu059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim J, et al. Impaired visual recognition of biological motion in schizophrenia. Schizophr Res. 2005;77:299–307. doi: 10.1016/j.schres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 149.Kim J, et al. Deficient biological motion perception in schizophrenia: results from a motion noise paradigm. Front Psychol. 2013;4:391. doi: 10.3389/fpsyg.2013.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pedersen A, et al. Theory of mind in patients with schizophrenia: is mentalizing delayed? Schizophr Res. 2012;137:224–229. doi: 10.1016/j.schres.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 151.Andreasen NC, et al. Theory of mind and schizophrenia: a positron emission tomography study of medication-free patients. Schizophr Bull. 2008;34:708–719. doi: 10.1093/schbul/sbn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Frith CD, et al. Explaining the symptoms of schizophrenia: abnormalities in the awareness of action. Brain Res Brain Res Rev. 2000;31:357–363. doi: 10.1016/s0165-0173(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 153.Ford JM, Mathalon DH. Anticipating the future: automatic prediction failures in schizophrenia. Int J Psychophysiol. 2012;83:232–239. doi: 10.1016/j.ijpsycho.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Knolle F, et al. Cerebellar contribution to the prediction of self-initiated sounds. Cortex. 2013;49:2449–2461. doi: 10.1016/j.cortex.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 155.Ford JM, et al. Did I do that? Abnormal predictive processes in schizophrenia when button pressing to deliver a tone. Schizophr Bull. 2014;40:804–812. doi: 10.1093/schbul/sbt072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Leggio MG, et al. Cognitive sequencing impairment in patients with focal or atrophic cerebellar damage. Brain. 2008;131:1332–1343. doi: 10.1093/brain/awn040. [DOI] [PubMed] [Google Scholar]
- 157.Keele SW, et al. The cognitive and neural architecture of sequence representation. Psychol Rev. 2003;110:316–339. doi: 10.1037/0033-295x.110.2.316. [DOI] [PubMed] [Google Scholar]
- 158.Balsters JH, et al. Cerebellum and cognition: evidence for the encoding of higher order rules. Cereb Cortex. 2013;23:1433–1443. doi: 10.1093/cercor/bhs127. [DOI] [PubMed] [Google Scholar]
- 159.Friston K. The free-energy principle: a unified brain theory? Nat Rev Neurosci. 2010;11:127–138. doi: 10.1038/nrn2787. [DOI] [PubMed] [Google Scholar]
- 160.Levinson SC. Turn-taking in Human Communication–Origins and Implications for Language Processing. Trends Cogn Sci. 2016;20:6–14. doi: 10.1016/j.tics.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 161.Schoenenberg K, et al. On interaction behaviour in telephone conversations under transmission delay. Speech Commun. 2014:63–64. [Google Scholar]
- 162.Kitazawa S, et al. Effects of delayed visual information on the rate and amount of prism adaptation in the human. J Neurosci. 1995;15:7644–7652. doi: 10.1523/JNEUROSCI.15-11-07644.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brudner SN, et al. Delayed feedback during sensorimotor learning selectively disrupts adaptation but not strategy use. J Neurophysiol. 2016;115:1499–1511. doi: 10.1152/jn.00066.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fiorillo CD, et al. The temporal precision of reward prediction in dopamine neurons. Nat Neurosci. 2008;11:966–973. doi: 10.1038/nn.2159. [DOI] [PubMed] [Google Scholar]
- 165.De Zeeuw CI, Ten Brinke MM. Motor Learning and the Cerebellum. Cold Spring Harb Perspect Biol. 2015;7:a021683. doi: 10.1101/cshperspect.a021683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Watson TC, et al. Electrophysiological mapping of novel prefrontal - cerebellar pathways. Front Integr Neurosci. 2009;3:18. doi: 10.3389/neuro.07.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kuypers HG, Lawrence DG. Cortical projections to the red nucleus and the brain stem in the Rhesus monkey. Brain Res. 1967;4:151–188. doi: 10.1016/0006-8993(67)90004-2. [DOI] [PubMed] [Google Scholar]
- 168.Sasaki K, et al. Electrophysiological studies of the projections from the parietal association area to the cerebellar cortex. Exp Brain Res. 1975;23:91–102. doi: 10.1007/BF00238732. [DOI] [PubMed] [Google Scholar]
- 169.D’Angelo E, et al. Distributed Circuit Plasticity: New Clues for the Cerebellar Mechanisms of Learning. Cerebellum. 2016;15:139–151. doi: 10.1007/s12311-015-0711-7. [DOI] [PubMed] [Google Scholar]
- 170.Akshoomoff NA, et al. Attention coordination and anticipatory control. Int Rev Neurobiol. 1997;41:575–598. doi: 10.1016/s0074-7742(08)60371-2. [DOI] [PubMed] [Google Scholar]
- 171.Desmond JE, et al. Dissociation of frontal and cerebellar activity in a cognitive task: evidence for a distinction between selection and search. Neuroimage. 1998;7:368–376. doi: 10.1006/nimg.1998.0340. [DOI] [PubMed] [Google Scholar]
- 172.Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Andreasen NC, et al. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. [DOI] [PubMed] [Google Scholar]
- 174.Schmahmann JD. Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci. 1998;2:362–371. doi: 10.1016/s1364-6613(98)01218-2. [DOI] [PubMed] [Google Scholar]
- 175.Alexander MP, et al. Cognitive impairments due to focal cerebellar injuries in adults. Cortex. 2012;48:980–990. doi: 10.1016/j.cortex.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 176.Malm J, et al. Cognitive impairment in young adults with infratentorial infarcts. Neurology. 1998;51:433–440. doi: 10.1212/wnl.51.2.433. [DOI] [PubMed] [Google Scholar]
- 177.Sokolov AA, et al. Recovery of biological motion perception and network plasticity after cerebellar tumor removal. Cortex. 2014;59:146–152. doi: 10.1016/j.cortex.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 178.Yeo BT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pavlova M, et al. Recognition of point-light biological motion displays by young children. Perception. 2001;30:925–933. doi: 10.1068/p3157. [DOI] [PubMed] [Google Scholar]
- 180.Pavlova M, et al. Cortical response to social interaction is affected by gender. Neuroimage. 2010;50:1327–1332. doi: 10.1016/j.neuroimage.2009.12.096. [DOI] [PubMed] [Google Scholar]
- 181.Brodal P. The pontocerebellar projection in the rhesus monkey: an experimental study with retrograde axonal transport of horseradish peroxidase. Neuroscience. 1979;4:193–208. doi: 10.1016/0306-4522(79)90082-4. [DOI] [PubMed] [Google Scholar]
- 182.Brown JT, et al. A study of afferent input to the inferior olivary complex in the rat by retrograde axonal transport of horseradish peroxidase. J Comp Neurol. 1977;176:1–22. doi: 10.1002/cne.901760102. [DOI] [PubMed] [Google Scholar]
- 183.Berkley KJ, Worden IG. Projections to the inferior olive of the cat. I. Comparisons of input from the dorsal column nuclei, the lateral cervical nucleus, the spino-olivary pathways, the cerebral cortex and the cerebellum. J Comp Neurol. 1978;180:237–251. doi: 10.1002/cne.901800204. [DOI] [PubMed] [Google Scholar]
- 184.Oka H, et al. The parieto-rubro-olivary pathway in the cat. Exp Brain Res. 1979;37:115–125. doi: 10.1007/BF01474258. [DOI] [PubMed] [Google Scholar]
- 185.Monakow KH, et al. Projections of precentral and premotor cortex to the red nucleus and other midbrain areas in Macaca fascicularis. Exp Brain Res. 1979;34:91–105. doi: 10.1007/BF00238343. [DOI] [PubMed] [Google Scholar]
- 186.Sakai ST. Cerebellar thalamic and thalamocortical projections. In: Manto M, et al., editors. Handbook of Cerebellum and Cerebellar Disorders. Springer; 2013. pp. 529–547. [Google Scholar]
- 187.Arakaki TL, et al. Structure of human brain fructose 1,6-(bis)phosphate aldolase: linking isozyme structure with function. Protein Sci. 2004;13:3077–3084. doi: 10.1110/ps.04915904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Cogn Sci. 1998;2:307–313. doi: 10.1016/s1364-6613(98)01210-8. [DOI] [PubMed] [Google Scholar]
- 189.Voogd J, et al. The distribution of climbing and mossy fiber collateral branches from the copula pyramidis and the paramedian lobule: congruence of climbing fiber cortical zones and the pattern of zebrin banding within the rat cerebellum. J Neurosci. 2003;23:4645–4656. doi: 10.1523/JNEUROSCI.23-11-04645.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Pakan JM, et al. Climbing fiber projections in relation to zebrin stripes in the ventral uvula in pigeons. J Comp Neurol. 2014;522:3629–3643. doi: 10.1002/cne.23626. [DOI] [PubMed] [Google Scholar]
- 191.Tsutsumi S, et al. Structure-function relationships between aldolase C/zebrin II expression and complex spike synchrony in the cerebellum. J Neurosci. 2015;35:843–852. doi: 10.1523/JNEUROSCI.2170-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Koziol LF, et al. Consensus paper: the cerebellum’s role in movement and cognition. Cerebellum. 2014;13:151–177. doi: 10.1007/s12311-013-0511-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Marien P, et al. Consensus paper: Language and the cerebellum: an ongoing enigma. Cerebellum. 2014;13:386–410. doi: 10.1007/s12311-013-0540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ackermann H, et al. The contribution of the cerebellum to speech production and speech perception: clinical and functional imaging data. Cerebellum. 2007;6:202–213. doi: 10.1080/14734220701266742. [DOI] [PubMed] [Google Scholar]
- 195.Baumann O, et al. Consensus paper: the role of the cerebellum in perceptual processes. Cerebellum. 2015;14:197–220. doi: 10.1007/s12311-014-0627-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Aarsen FK, et al. Long-term sequelae in children after cerebellar astrocytoma surgery. Neurology. 2004;62:1311–1316. doi: 10.1212/01.wnl.0000120549.77188.36. [DOI] [PubMed] [Google Scholar]
- 197.Steinlin M. Cerebellar disorders in childhood: cognitive problems. Cerebellum. 2008;7:607–610. doi: 10.1007/s12311-008-0083-3. [DOI] [PubMed] [Google Scholar]
- 198.Tavano A, et al. Disorders of cognitive and affective development in cerebellar malformations. Brain. 2007;130:2646–2660. doi: 10.1093/brain/awm201. [DOI] [PubMed] [Google Scholar]
- 199.Timmann D, Daum I. How consistent are cognitive impairments in patients with cerebellar disorders? Behav Neurol. 2010;23:81–100. doi: 10.3233/BEN-2010-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zecevic N, Rakic P. Differentiation of Purkinje cells and their relationship to other components of developing cerebellar cortex in man. J Comp Neurol. 1976;167:27–47. doi: 10.1002/cne.901670103. [DOI] [PubMed] [Google Scholar]
- 201.ten Donkelaar HJ, et al. Development and developmental disorders of the human cerebellum. J Neurol. 2003;250:1025–1036. doi: 10.1007/s00415-003-0199-9. [DOI] [PubMed] [Google Scholar]
- 202.Wang SS, et al. The cerebellum, sensitive periods, and autism. Neuron. 2014;83:518–532. doi: 10.1016/j.neuron.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


