Abstract
Glycosaminoglycans (GAGs) are long blocks of negatively charged polysaccharides. They are one of the major components of the extracellular matrix and play multiple roles in different tissues and organs. The accumulation of undegraded GAGs causes mucopolysaccharidoses (MPS). GAGs are associated with other pathological conditions such as osteoarthritis, inflammation, diabetes mellitus, spinal cord injury, and cancer. The need for further understanding of GAG functions and mechanisms of action boosted the development of qualitative and quantitative (alcian blue, toluidine blue, paper and thin layer chromatography, gas chromatography, high pressure liquid chromatography, capillary electrophoresis, 1,9-dimethylmethylene blue, enzyme linked-immunosorbent assay, mass spectrometry) techniques.
The availability of quantitative techniques has facilitated translational research on GAGs into the medical field for: 1) diagnosis, monitoring, and screening for MPS; 2) analysis of GAG synthetic and degradation pathways; and 3) determination of physiological and pathological roles of GAGs.
This review provides a history of development of GAG assays and insights about the use of tandem mass spectrometry and its applications for GAG analysis.
Keywords: Glycosaminoglycans, Mass spectrometry, Alcian blue, ELISA, Chromatography, mucopolysaccharidoses
1. Introduction
Glycosaminoglycans (GAGs) are negatively charged linear polysaccharides composed of repeating disaccharides with variable sulfation levels [1–5]. They are classified in five major groups according to the repeating subunit as: chondroitin sulfate (CS) (glucuronic acid and N-acetylgalactosamine), dermatan sulfate (DS) (iduronic acid or glucuronic acid and N-acetylgalactosamine), heparan sulfate (HS) (iduronic acid or glucuronic acid and N-acetylglucosamine), keratan sulfate (KS) (galactose and N-acetylglucosamine), and hyaluronan (HA) (glucuronic acid and N-acetylglucosamine) (Fig.1) [1–6] (Table 1).
Fig. 1.
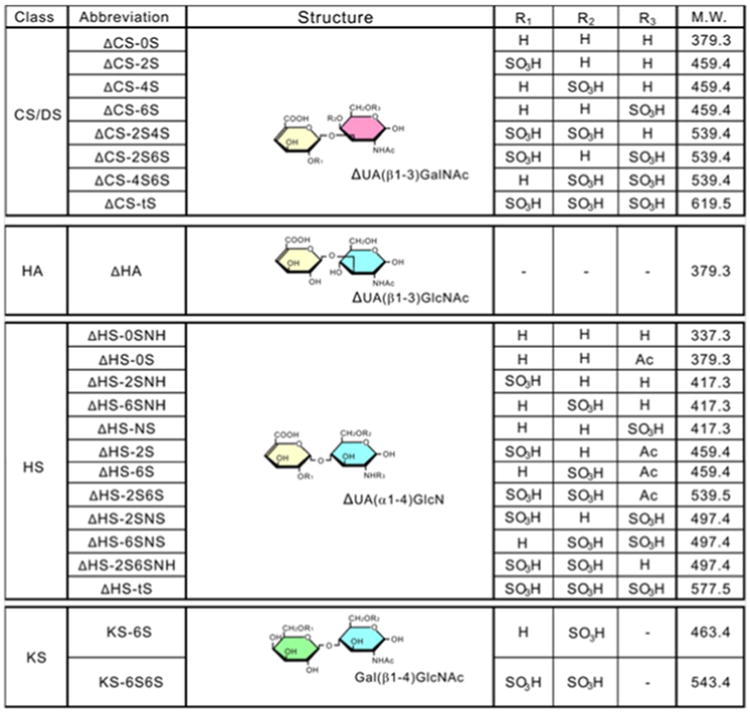
Structure of glycosaminoglycans. CS: chondroitin sulfate, DS: dermatan sulfate, HA: hyaluronic acid, HS: heparan sulfate, KS: keratan sulfate, UA: uronic acid, GlcA: glucuronic acid, Δ: unsaturated. Reproduced with permission from [ref. 152].
Table 1.
History of glycosaminoglycan assay.
| Year | Description | First.author(s) | Ref. |
|---|---|---|---|
| 1856 | Discovery of toluidine blue | Perkin W.H. | [48] |
| 1897 | Development of mass spectrometry | Thomson J.J. | [129] |
| 1956 | Use of alcian blue for staining of acidic carbohydrates | Mowry et al. | [184] |
| 1956 | Use of alcian blue for mucopolysaccharidoses | Runoe et al. | [185] |
| 1969 | Development of GAG thin layer chromatography (TLC) | Teller et al. | [55] |
| 1971 | Spot test azure A dye staining of GAGs | Berman ER | [187] |
| 1974 | Analysis of GAGs with DMB | Humbel et al. | [74] |
| 1974 | Separation of urinary GAGs through gas chromatography | Murphy et al. | [90] |
| 1978 | GAG purification with paper chromatography | Sato et al. | [54] |
| 1983 | Separation of GAGs using paper thin chromatography | Rajendra V. | [53] |
| 1985 | HPLC for heparin | Rice et al. | [92] |
| 1993 | ELISA for C4S/C6S/DS | Shibutani et al. | [123] |
| 1994 | ELISA for KS | Møller et al. | [118] |
| 1996 | GAGs through capillary electrophoresis | Linhardt et al. | [97] |
| 1997 | ELISA for HS | Najjam et al. | [121] |
| 2001 | Single GAG measurement by LC-MS/MS | Oguma et al. | [45–47] |
| 2007 | Multiple GAG assay in plasma/serum or urine | Oguma et al. | [146–147] |
| 2010 | KS levels in plasma of MPS patients | Tomatsu et al. | [188] |
| 2011 | Methanolysis reaction for GAGs | Auray-Blais et al.; Zhang et al. | [155,158] |
| 2012 | ELISA for hyaluronic acid | Yang et al. | [124] |
| 2012 | HPLC-MS/MS for HS | Ruijter et al. | [186] |
| 2012 | Use of non-reducing ends of GAGs as biomarkers | Lawrence et al. | [154] |
| 2013 | Newborn screening for MPS measuring GAGs with LC-MS/MS | Tomatsu et al. | [150] |
| 2014 | Spectrometry for GAGs in cell lines | Kiselova et al. | [4] |
| 2014 | Methanolysis for animal tissue GAGs | Trim et al. | [159] |
| 2014 | Measurement of GAGs in articular cartilage and yellow ligament | Osago et al. | [152] |
| 2014 | Reversed-phase HPLC/MS for GAGs extracts | Zhu et al. | [145] |
| 2014 | Automated high-throughput mass spectrometry for HS | Shimada et al. | [153] |
TLC: thin layer chromatography; GAGs: glycosaminoglycans; DMM: dimethylmethylene blue; HPLC: high performance liquid chromatography; C4S: chondroitin-4sulfate; C6S: chondroitin-6-sulfate; DS: dermatan sulfate; ELISA: enzyme-linked immunosorbent assay; KS: keratan sulfate; HS: heparan sulfate; LC/MS/MS: liquid chromatography tandem mass spectrometry; CSF: cerebrospinal fluid.
GAGs are one of the most important components of extracellular matrix (ECM) and are found in multiple tissues [7]. Polymeric GAGs are covalently bound through a linkage region to core proteins to produce proteoglycans (PGs) or remain as free polysaccharides [4,8,9,10]. PGs are associated with various physiological functions such as hydration and swelling pressure to the tissue to absorb compressional forces, regulation of collagen fibril formation, modification of the activity of transforming growth factor-β, and the major anionic site responsible for the charge selectivity in glomerular filtration. Sulfation patterns in the GAG chains play significant roles by allowing interactions, normally of an ionic nature, with growth factors. The core proteins are not just scaffolds for GAGs, they also contain domains that have particular biological activities [11]. Many PGs are multifunctional molecules that engage in different specific interactions simultaneously. Studies on GAGs and PGs have shown their importance in biological processes such as: cancer progression, angiogenesis, development, growth, microbial pathogenesis, cellular signaling (growth factors, cell surface receptors, cytokines, chemokines, enzymes, complement proteins), and anticoagulation [12–32]. One of the major clinical applications for GAG analysis is with the study of inherited metabolic disorders, particularly mucopolysaccharidoses (MPS). In MPSs, GAG degradation pathways are disrupted due to enzyme deficiency. Enzyme deficiency causes undegraded GAGs to accumulate in multiple tissues leading to organ dysfunction represented by a variety of clinical signs and symptoms such as skeletal dysplasia, short stature, mental retardation, heart valve disease, hearing loss, corneal clouding, hepatosplenomegaly, and umbilical and inguinal hernias. Untreated patients with the severe form die of respiratory failure, heart disease, and brain damage within the first two decades of life [33]. Establishment of GAG measurement facilitates diagnosis, prediction of clinical severity, prognosis, therapy monitoring (biomarker), and disease screening [34].
Several qualitative and quantitative methods (toluidine blue; alcian blue; paper and thin-layer chromatography, 1,9-dimethylmethylene blue; chromatography: gas and high-performance liquid chromatography - HPLC; capillary electrophoresis; enzyme-linked immunosorbent assay - ELISA; mass spectrometry MS) have been developed to determine the significance of many roles of GAGs in biological processes.
Dye-spectrometric methods including alcian blue [35] and dimethylmethylene blue (DMMB) [36–41] have been used to assay total urinary GAG. Thin-layer chromatography (TLC) has been used to separate specific GAGs, but this method has not been adapted to measure GAGs in blood or tissue extracts. Sensitivity and specificity of the dye-spectrometric and TLC methods are not sufficient to detect all types of MPS, especially MPS IV.
HPLC is a sensitive, reproducible, and accurate method to assay each specific GAG but cannot be applied to mass screening because the method is complex and time-consuming [42–44]. ELISA assays for CS, DS, KS, and HS in blood and urine have been established that are rapid and reproducible but expensive. Thus, establishment of a simple, accurate, reproducible, and cost-effective GAG assay method is urgently needed to apply to not only for clinical indications but also for basic research.
Tandem mass spectrometry (MS/MS) has more recently been developed to assay disaccharides derived from CS, DS, HS, and KS in blood, urine, tissues, and/or dried blood spots (DBS) [45–47]. The liquid chromatography (LC) MS/MS method not only shows sensitivity and specificity for detecting all subtypes of GAGs, but is also helpful in elucidating biological roles of GAGs and aiding diagnosis and therapeutic monitoring of MPS. The main limitation of LC processing is the long run time that limits its use in high-throughput screening. An automated high-throughput mass spectrometry (HT-MS/MS) system eliminates the chromatographic process allowing running time in 10 s, while maintaining the quality and accuracy of standard LC/MS/MS platforms.
This review manuscript focuses on the history of GAG assay development with qualitative and quantitative methods with a more detailed discussion on current uses of mass spectrometry (MS/MS) for GAG analysis applications.
2. GAG assay methods
2.1. Toluidine blue staining
Toluidine blue (TB), or tolonium chloride, is a thiazine that has acidophilic metachromatic properties. It was discovered by William H. Perkin in 1856 and is used to detect GAGs due to its high affinity for acidic tissues [48–49].
TB staining is based on metachromasia, a principle in which the dye reacts with electronegative groups in tissues to produce colors of different wavelengths according to the GAG concentration but does not alter the chemical structure of the GAGs [49–50]. The negatively charged sulfates in the GAGs neutralize the positive charge of toluidine blue, leading to dye aggregation by hydrophobic bonding and van der Waals interactions [51]. Detection methods using gel electrophoresis followed by staining with TB have been developed to detect low levels of GAGs in tissue extracts [52]. TB staining is widely used for pathohistology to detect GAG accumulation in tissues sectioned with 0.5 μm thickness providing the best resolution of storage materials (Fig. 2); however, TB method cannot be applied to quantitative analyses of GAGs since TB reacts with other unrelated negatively charged molecules.
Fig. 2.
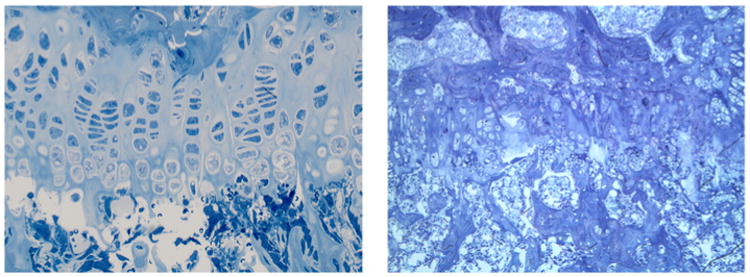
TB staining in growth plate of wild-type (left) and MPS VII (right) mice (12 weeks old). Chondrocytes in a wild-type mouse are stained while chondrocytes in MPS VII is ballooned and vacuolated.
2.2. Paper and thin layer chromatography
GAGs can be separated by paper and thin-layer chromatography [53]. Tissue extracted GAGs can be purified by paper chromatography in the presence of zinc (0.25 M zinc acetate solvent) at pH 4.0 and then precipitated with ethanol to remove impurities [54].
In 1969, Teller et al. developed a TLC method using formate buffer/isopropanol, 60:40, to separate GAGs in 5–6 h [55]. Lipiello et al. used ethanolic solutions (60, 50, 40, 30, 20%) of calcium salts (2.5 and 5% respectively) acidified with acetic acid to separate CS, DS, and KS [56]. Humbel et al. applied the same method to analyze these GAGs in urine samples. They used 5% calcium acetate in 10% ethanol to improve separation of DS [57]. TLC methods separate GAGs based on their size and sulfation levels, and are generally faster and have better sensitivity for small oligosaccharides with low net charge than gel separation methods (polyacrylamide gel electrophoresis-PAGE and fluorophore assisted carbohydrate electrophoresis-FACE) [58].
GAGs can be separated by chromatography on silicated glass paper [59] or filter papers [60]; however both require several elution steps. In 1966, Wusteman et al. reported separation of GAGs using thin layers of silica gel and detection with selective spray reagents (metachromatic spray-toluidine blue or azure A; orcinol-sulphuric acid; nitrous acid-indole; naphthoresorcinol spray; Morgan-Elson spray) [61]. In 1984, Säämänen et al. reported the use of scanning spectrophotometry at 232 nm to detect unsaturated GAGs digested with chondroitinase AC II separated by TLC on cellulose. Samples are scanned by a chromatogram spectrophotometer (Zeiss KM 3, Carl Zeiss) with 232 nm reflectance to measure the light intensity reflected from the plate [62].
Thus, TLC was developed to identify each specific GAG; however, overlapping retention factors (RF) of different GAGs can lead to miss-identification of MPSs. For example, keratan sulfate (KS) that accumulates in MPS IV does not completely separate from C6S, making differential diagnosis of these forms of MPS difficult [63]. Sensitivity and specificity of the TLC methods are not sufficient to detect all types of MPS, especially MPS IV. Another disadvantage of the TLC method is that it is not applicable to blood or tissue extracts without prior protease, nuclease or hyaluronidase digestion. Thus, TLC methods are no longer widely used for GAG analyses.
2.3. Alcian blue
Alcian blue (AB) is a tetravalent cationic dye with a hydrophobic core that contains a copper ion. The dye interacts with high specificity to sulfated GAGs by ionic interactions and has been used to quantify GAGs [35]. It is also used in combination with silver to stain GAGs after separation by PAGE [58,64–66]. However, the use of AB is limited due to problems with precipitation and interference due to interaction with other negatively charged molecules [67–68].
In 1964, Scott et al. demonstrated that AB forms insoluble complexes with acidic GAGs [69]. In 1973, Whiteman added MgCl2 to prevent AB from binding to proteins in acidic solutions [70]. In 1973, Hata et al. reported the use of 0.1% AB in two-dimensional electrophoresis on cellulose acetate strips with 0.1 M pyridine/0.47 M formic acid buffer (pH 3) followed by 0.1 M barium acetate solution (pH 8) [71]. The sensitivity of the assay was improved by adding dimethyl sulphoxide to the assay solution [72].
The AB method can be limited by the presence of negatively charged molecules such as amino acids, which can interfere with the binding of AB to GAGs [73]. The AB staining is used to detect GAGs in combination with electrophoresis as well as tissue staining (Fig. 3). This method is not sensitive and specific enough to measure GAGs in blood or tissue extracts without prior protease, nuclease or hyaluronidase digestion and cannot distinguish specific GAGs by itself.
Fig. 3.
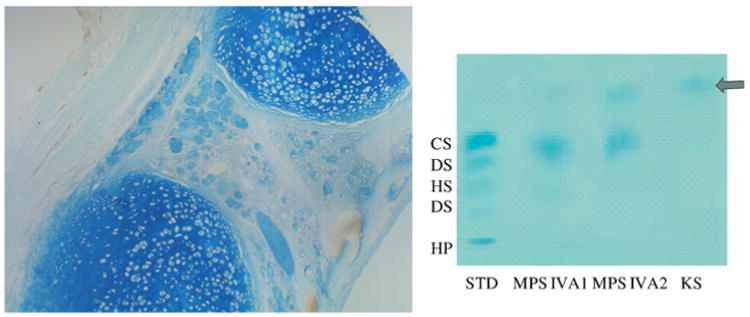
AB staining for trachea with a 23-year-old MPS IVA patient and AB staining for electrophoretic urinary GAG from MPS IVA patients. Chondrocytes and their extracellular matrix as well as tracheal glands were stained with deep blue (left). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.4. Dimethylmethylene blue
Dimethylmethylene blue (DMMB) is another cationic dye that binds to sulfated GAGs and results in an absorbance shift when it binds to GAGs [73]. Reference values for DMMB staining are dependent on age [36]. The DMMB test is one of the most used methodologies to quantify GAGs [74–79]. It takes advantage of the metachromatic properties of the dye, and it can be used in solution [80–81] or in solid-phase [82].
DMMB was first used as a histochemical stain in 1969 by Taylor et al. and as a colorimetric assay in tissues by Humbel et al. in 1974 [83,74]. The assay described by Humbel et al. was limited due to instability of the dye that led to precipitation of the GAG complexes [74]. In 1982, Farndale et al. overcame these limitations by substituting formate for the citrate/phosphate buffer used in the original assay [81].
Sabiston et al. developed an automated method [84], and Panin et al. adapted the method to use DMMB to measure GAGs in cetylpyridinium chloride precipitated urine [85]. Chandrasekhar et al. used DMMB to measure GAGs after chromatographic elution with guanidium chloride (GuHCl) [86] and Whitley et al. refined the method to measure GAGs in small amounts of sample [37].
Heparin should not be used as an anticoagulant because it interferes with the DMMB test, as do drugs that contain artificial coloring agents [37,87]. The test is also limited by purity of the dye because contaminating sulphur can cause false negatives [63]. The amount of protein present in the sample can also lead to false negative results, in which, high protein concentrations were seen with significantly decrease in GAG results [73]. In 1990, Goldberg et al. modified the DMMB protocol by addition of bovine serum albumin (BSA) and phosphate-buffered saline (PBS) to measure GAGs in chondrocyte culture media that contained high levels of protein [88]. de Jong et al. improved the method by increasing the pH (pH 8.8) so that proteins are mostly negatively charged and consequently do not bind to GAGs [78].
Orii's group had first demonstrated that preliminary DMMB test of 10,000 urine samples from 6-month-old infants and under 1-year-old MPS patients provided normal distribution of GAG levels and cut-off values. The same group performed successive MPS screening of around 130,000 urine from the infants from 1993 to 2000. These studies led to the first identification of two patients with MPS II [41]. Thus, DMMB method is still used for the screening of MPS as a feasible, reproducible and economical tool while the disadvantage is that this method cannot be applicable to blood and tissue specimens directly and cannot separate specific GAGs.
2.5. Capillary electrophoresis
The use of capillary electrophoresis (CE) for GAG analysis is beneficial due to high power of separation resolution and simplicity of analysis [97]. Separation is dependent on the amount of GAGs, size, purity, charges, and degree of sulfation [98–103]. In 1979, Cappelletti' group demonstrated that high-resolution electrophoresis of GAGs in tissue and AB staining can differentiate each type of MPS and normal control samples by 560 nm (Gelman Automatic Computing Densitometer ACD-18) followed for GAG analysis in urine by Orii's and Hopwood's groups (Fig. 4) [104–105]. The detection of GAGs separated by CE is based upon either direct UV detection [99], indirect UV detection [106–107], generation of metal complex-copper complexes [108–109], or mass spectrometry [110–112].
Fig. 4.
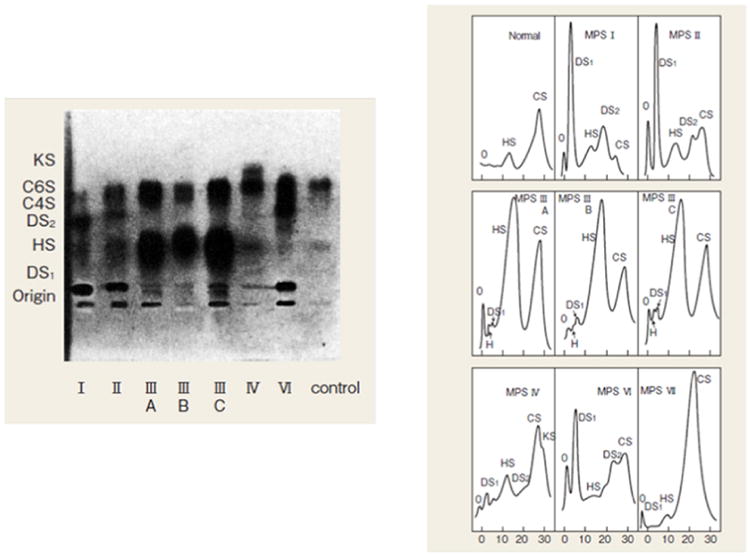
Electrophoresis on a mono-dimensional run (left) and densitography (right) of urinary GAGs from MPS patients. Urinary GAGs was extracted by cetylpyridinium chloride (VPC) method and were separated by the electrophoresis. A mono-dimensional electrophoresis of urinary specimens from MPS I, II, III, IVA, VI, and VII patients and healthy control shows clear separation of specific GAGs (DS1, HS, DS2, C4S, C6S, and KS) (left). A healthy control sample yields C4S, C6S, and HS. Samples from MPS I and II patients provide more DS and HS. Samples from MPS III patients show a strong band of HS and there is no difference of the HS band between subclasses of MPS III. An MPS IVA sample provides a characteristic KS band while an MPS VI sample yields a thick DS band. Each separate GAG band is semi-quantified by Densitometer (right), and each type of MPS provides a unique pattern of densitography apart from the normal control pattern.
The use of electrophoresis method provides the advantage of simultaneous assays for several GAGs with high sensitivity and reproducibility. However, it only allows semi-quantitation and is only applicable to urine. Due to improvements in mass spectrometry and HPLC techniques, it is expected that CE will not be used for GAG analysis in the future.
2.6. Gas chromatography
Gas chromatography is based on vaporization of the compounds that are injected into a heated column with elution in an inert gas. In 1969, Kaplan D. suggested that MPS types could be classified according to the hexosamine content of urinary mucopolysaccharides (GAGs); however, it was extremely hard to identify the relative amount of DS and HS in samples by column chromatography [89]. This led to the use of gas-liquid chromatography (GLC) for GAG analysis in 1974 [90]. In this method, Murphy et al. analyzed GAGs in urine from MPS patients. The GAGs were hydrolyzed with hydrochloric acid to yield glucosamine and galactosamine. The sugars were then acetylated and separated by GLC. CS and DS yield galactosamine while HS yields glucosamine, allowing the discrimination of MPS III from MPS I and II [90].
In 1998, Toida et al. developed a quantitative and qualitative analysis of GAGs by gas chromatography-mass spectrometry (GC/MS). GAG samples were hydrolyzed by methanolysis, and the iduronic and glucuronic acids were derivatized to trimethylsilyl ethers prior to GC–MS [91]. The use of mass spectrometry (MS) coupled with GC allows separation and quantitation of sub-microgram quantities of the sugars.
2.7. High-performance liquid chromatography
High performance liquid chromatography (HPLC) is a separation method based on differential interaction of compounds with adsorbent materials in a column, leading to different elution times for specific compounds. It has been used to quantify GAGs after depolymerization of polysaccharides, followed by separation of resulting disaccharides, detected by UV absorbance or fluorescence [92–93]. In 1984, Kodama et al. quantified disaccharides of CS (ΔDi-0S, ΔDi-4S, ΔDi-6S, ΔDi-diS) by digesting GAGs with chondroitinase and measuring fluorescence of the disaccharides after modification with 2-aminopyridine and separation by HPLC [94]. In 1986, they developed an HPLC method for differential diagnosis of MPS [95].
HPLC is a sensitive, reproducible, and accurate method to assay each specific GAG; however, it cannot be applied to mass screening because the method is complex and time-consuming [43–44,96]. Thus, HPLC protocols are combined with mass spectrometry (MS) to identify and quantify eluted disaccharides with or without modification (see Section 2.9).
2.8. Enzyme-linked immunosorbent assay
Enzyme-linked immunosorbent assay (ELISA) is a technique based on the binding of an antigen to an antibody that is linked to an enzyme and detection by hydrolysis of a substrate to the linked enzymes [113]. There are different types of ELISA: direct [114], indirect [115], sandwich [116], competitive [117].
ELISA assays developed for GAGs can measure levels of KS [118–120], HS [121–122], C4S [123], C6S [123], DS [123], and hyaluronic acid [124]. ELISA protocols have also been developed to detect GAGs in cells and on cell-surfaces [125–126]. Measurement of GAGs by sandwich ELISA is still commonly used, particularly in clinical settings. The advantages of its use are: feasibility, sensitivity, reproducibility and quantitation requiring only a simple ELISA plate reader. Disadvantages are cost since no current assay can detect several GAGs simultaneously requiring multiple assays.
2.9. Mass spectrometry
Mass spectrometry (MS) is a technique that measures compounds based on their mass-to-charge ratio (m/Q, m/q, m/Z, or m/z) [127–129]. Different ionization sources can be used, e.g. electrospray ionization (ESI), atmospheric-pressure chemical ionization (APCI), fast atom bombardment (FAB), chemical ionization (CI), matrix-assisted laser desorption/ionization (MALDI) [130–135].
The principles that led to mass spectrometry were discovered over 100 years ago by the Nobel laureate Sir John Thomson who discovered the electron and was the first to demonstrate separation of isotopes of a stable element [129]. Mass spectrometry has now become one of the most useful analytical techniques due to its specificity, accuracy and sensitivity [127–128,136] and is considered one of the most successful and useful techniques applied for newborn screening (NBS) [137].
GAG analyses have been performed in different types of mass analyzers: time-of-flight (TOF) [138], ion trap (IT) [139–140], Fourier transform ion cyclotron resonance (FTICR) [141], and triple quadrupole (QQQ) [45–47].
MS analysis can be full-spectrum to detect all intact ions in mixtures of unknown compounds, ion monitoring to measure levels of known intact ions, or multiple reaction monitoring (MRM) to measure different intact/product ion pairs that can distinguish compounds that have ions of identical mass/charge ratio but have different fragments [145–147].
Mass spectrometry methods are superior in accuracy, speed, sensitivity, and specificity to other detection methods. There are many protocols described using MS/MS for GAG quantification: e.g. sulfated GAGs in multiple cell lines [4], urinary GAGs [46–47], mono and disaccharides in tissue extracts [45], plasma/serum or urinary GAGs [144–151], GAGs in articular cartilage and yellow ligament [152], and GAGs from dried blood spots [153].
In 2001, Oguma et al. developed an ESI mass spectrometry protocol for quantification of HS and KS from serum and plasma, and in 2007 improved this method by including analysis of DS and adapting the method to measure GAGs in dried blood spots (DBS) [45–47,146–147] (Fig. 5). Polysaccharides were digested with heparitinase, keratanase, and chondroitinase B to release HS, KS and DS, respectively, and the unmodified disaccharides were then detected by LC/ESI/MS/MS [45–47,146–147]. In 2014, Osago et al. described a more complete method for one-shot analysis of disaccharides derived from all four classes of GAGs using LC/ESI/MS/MS (2014) [152] (Fig. 6). This protocol enabled identification and quantitation of 23 different disaccharides (8 CS/DS, 1 hyaluronic acid, 12 HS, and 2 KS) including di- and tri-sulfated species. Applying the method for analysis of disaccharides obtained by enzymatic digestion (chondroitinase ABC, hyaluronidase, heparitinase, and keratanase) of articular cartilage GAGs, they showed the characteristic composition of GAGs in the cartilage. In this method, disaccharides that have the same molecular mass but different structures (isomers) are separated stereospecifically on a porous graphitized carbon column and then identified with MRM transitions having the same Q1 but different Q3 specific to each disaccharide. Thus, the method distinguishes isomers in the different classes as well as in the same class with sulfate(s) at different positions, such as ΔCS-2S, ΔCS-4S, ΔCS-6S, ΔHS-2S, and ΔHS-6S. This method increases the number of disaccharides in different classes that can be measured in a single analysis [152].
Fig. 5.
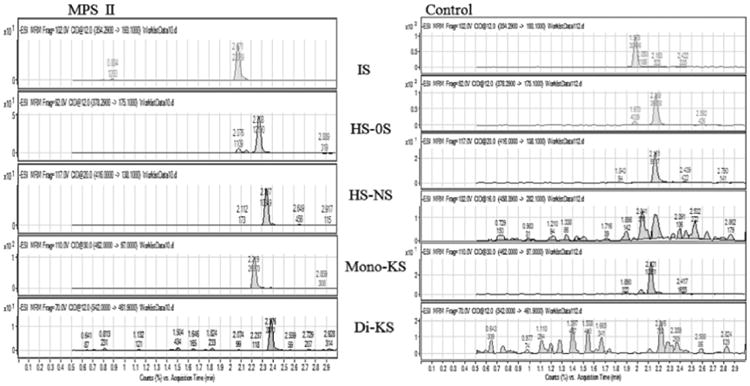
Multiple reaction monitoring (MRM) of DBS samples (control × MPS II patient). Chromatograms for disaccharides of chondrosine (IS), heparan sulfate (HS), mono-sulfated KS, di-sulfated KS. Equipment: 6460 Triple Quad MS/MS with 1260 infinity LC (Agilent Technologies). DBS: dried blood spot; IS: internal standard.
Fig. 6.
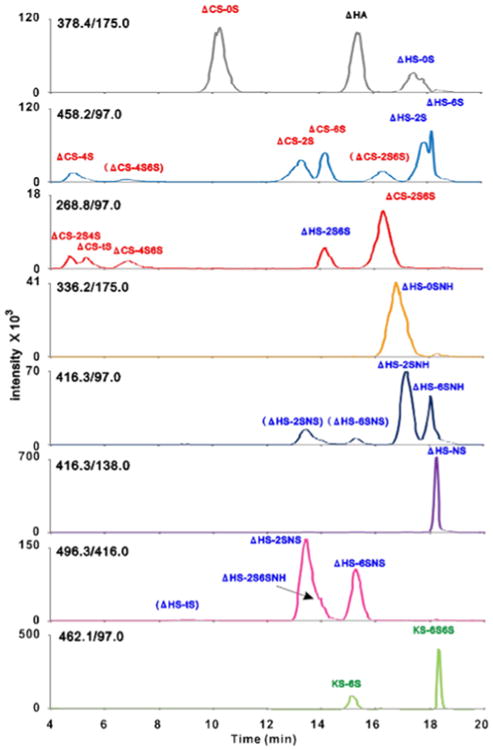
The extracted ion chromatogram of 23 disaccharides derived from four classes of GAGs by the LC/MS/MS analysis. The selected reaction monitoring transitions are shown in each chromatogram. The disaccharides shown in parentheses indicate the signals of their de-sulfated products by in-source fragmentation. Reproduced with permission from [ref. 152].
Lawrence et al. [154] published a protocol for detection of the non-reducing ends of GAGs [154]. In MPS diseases, lack of a specific enzyme leads to accumulation of polysaccharides with a specific sugar with a non-reducing end. After digestion of extracted polysaccharides with bacterial enzymes, non-reducing sugars are labeled by reductive amination with isotopic aniline, and the modified sugars quantified by LC/MS/MS [154]. This method can clearly distinguish 8 different forms of MPS from unaffected controls. This sophisticated derivatization method has not yet been adapted for higher throughput methods needed for routine laboratory use or NBS, but shows promise as a method to identify very selective biomarkers.
An acid-catalyzed chemical process (methanolysis) has been also developed by using a single reagent (methanolic hydrochloric acid) aiming at the analysis of individual GAGs by LC/MS/MS. This procedure was described in 2011 [155] and yields, among other oligosaccharides, desulfated and derivatized disaccharides. Specific disaccharides related to DS and HS were selected, optimized and quantified by MS/MS after chromatographic separation. This versatile procedure has been adapted for the analysis of GAGs from various samples, including urine [155–157], cerebrospinal fluid (CSF) [158], and animal tissues [159]. The same procedure has been used to analyze CS and KS [160]. This method has been used for high-risk screening, diagnosis, and longitudinal evaluation of patients under therapy.
One of the limitations of the LC separation techniques needed to quantify individual GAGs is that the length of time needed for separation of each sample is not compatible with high volume newborn screening programs. MS/MS can be associated with high throughput (HT) platforms to overcome this limitation. Shimada et al. [153] published a study comparing analysis of HS using an automated high-throughput mass spectrometry (HT/MS/MS) with analysis using a conventional LC/MS/MS system [153]. The HT platform used was RapidFire (Agilent Technologies) in which samples are adsorbed onto a matrix for concentration and desalting prior to injection into MS/MS with no chromatographic separation, allowing samples to be analyzed every 10 s.
As an alternative to measuring GAGs, MS has also been used to measure levels of specific enzymes that have reduced activity in MPS. In 2001, Chamoles et al. developed strategies for enzyme assay from re-hydrated dried blood spots (DBS) [161–167], allowing the use of DBS for enzyme assay by LC/MSMS for NBS in many disorders including Gaucher, Niemann-Pick A/B, Pompe, Fabry, Krabbe, Hurler syndrome (MPS I), Maroteaux-Lamy syndrome (MPS VI), and Morquio syndrome type A (MPS IVA) [168–174].
3. Applications of GAG assays
GAGs are widely distributed and associated with physiological and pathological roles depending upon specific GAG as described above. Therefore, establishment of accurate, rapid, sensitive, and specific measurements of specific GAGs has been urgently required. MS/MS based GAG assays are applied to not only diagnosis and therapeutic efficacy for MPS but also other disorders such as: mucolipidoses [120–122], cancer [175–176], osteoarthritis [177], rheumatoid arthritis [178], diabetes [179–180], infectious diseases [181], and spinal cord injury [182] where GAG(s) are down or up regulated.
4. Conclusions
Several methods have been developed for GAG quantification, but most of the earlier developed methods require large amounts of samples and provide limited information about specific GAGs [4,182]. The development of mass spectrometry detection methods allows a fast, sensitive, accurate measurement for GAGs analysis (Figs. 5, 6).
The fastest method, HT/MS/MS, cannot distinguish all isomers of individual disaccharides, but it has a similar sensitivity and reproducibility as conventional LC/MS/MS and thus may be more appropriate for NBS programs to measure elevation of GAGs in MPS. Individual enzyme deficiencies that lead to elevation of GAGs could then be determined in a second screen for individual enzyme activities.
Overall, MS/MS assay contributes greatly to broad fields associated with primary or secondary metabolic pathway of GAGs.
Acknowledgments
This review article was supported by grants from the Austrian MPS Society, The Bennett Foundation, and International Morquio Organization (Carol Ann Foundation). This work was also supported by Japanese MPS Family Society. R.W.M. and S.T. were supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of NIH under grant number P30GM114736. S.T. was supported by National Institutes of Health grant R01HD065767. F.K. was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico from Brazil (CNPq). H. O. was supported by JSPS KAKENHI Grant Numbers 23930010, 25930009 and 26930007. M. T. was supported by JSPS KAKENHI Grant Number 25462371.
Footnotes
Conflict of interest: All the authors contributed to the Review Article and had no conflict of interest with any other party. Francyne Kubaski, Harumi Osago, Robert W. Mason, Seiji Yamaguchi, Hironori Kobayashi, Mikako Tsuchiya, Tadao Orii, and Shunji Tomatsu declare that they have no conflict of interests.
Contributions to the project: Francyne Kubaski is the primary author for this review article and an expert in molecular biology. She has contributed to the concept and planning of the article, collection of previous articles and data, and reporting of the work described.
Harumi Osago is the primary author for this review article and an expert in molecular biology. She has contributed to the planning, data analysis, and reporting of the work described.
Robert W. Mason PhD is a molecular biologist and chemist and has over 30 years of experience in chemistry. He has contributed to the concept, planning of the project, informed consent, analysis of data, and reporting of the work described.
Seiji Yamaguchi MD and PhD is a medical doctor with 40 years of clinical and research experiences in a newborn screening. He has contributed to the planning, data analysis, and reporting of the work described.
Hironori Kobayashi MD and PhD is a medical doctor with 20 years of clinical and research experiences in a newborn screening. He has contributed to the planning, data analysis, and reporting of the work described.
Mikako Tsuchiya MD and PhD is a principal investigator and has 30 years of clinical and research experience in molecular biology. She has contributed to the concept of the project, planning, analysis of data, and reporting of the work described in the review.
Tadao Orii is a medical doctor with 50 years of clinical and research experiences in mucopolysaccharidoses. He published over 300 articles and chapter books in this field. He has contributed to the planning, data analysis, and reporting of the work described.
Shunji Tomatsu MD and PhD is a principal investigator and has 30 years of clinical and research experience in mucopolysaccharidoses, publishing over 160 articles in this field. He has contributed to the concept of the project, planning, analysis of data, and reporting of the work described in the review.
References
- 1.Liu Z, Zhang F, Li L, Li G, He W, Lindhardt RJ. Compositional analysis and structural elucidation of glycosaminoglycans in chicken eggs. Glycoconj J. 2014;31:593–602. doi: 10.1007/s10719-014-9557-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Habuchi O. Diversity and functions of glycosaminoglycan sulfotransferases. Biochim Biophys Acta. 2000;1474:115–117. doi: 10.1016/s0304-4165(00)00016-7. [DOI] [PubMed] [Google Scholar]
- 3.Li G, Li L, Tian F, Zhang L, Xue C, Lindhardt RJ. Glycosaminoglycans of cultured cells using a rapid and sensitive LC–MS/MS approach. ACS Chem Biol. 2015;10:1303–1310. doi: 10.1021/acschembio.5b00011. [DOI] [PubMed] [Google Scholar]
- 4.Kiselova N, Dieker T, Spillmann D, Ramstrom M. An automated mass spectrometry-based screening method for analysis of sulfated glycosaminoglycans. Biochem Biophys Res Commun. 2014;450:598–603. doi: 10.1016/j.bbrc.2014.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Kusche-Gullberg M, Khellén L. Sulfotransferases in glycosaminoglycanbiosynthesis. Curr Opin Struct Biol. 2003;13:605–611. doi: 10.1016/j.sbi.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Sasisekharan R, Raman R, Prabhakar V. Glycomics approach to structure-function relationships of glycosaminoglicans. Annu Rev Biomed Eng. 2006;8:181–231. doi: 10.1146/annurev.bioeng.8.061505.095745. [DOI] [PubMed] [Google Scholar]
- 7.Tomatsu S, Kubaski F, Sawamoto K, Mason RW, Yasuda E, Shimada T, Montano AM, Yamaguchi S, Suzuki Y, Orii T. Newborn screening and diagnosis of mucopolysaccharidoses: application of tandem mass spectrometry. Nihon Masu Sukuriningu Gakkai Shi. 2014;24:19–37. [PMC free article] [PubMed] [Google Scholar]
- 8.Couchman JR, Pataki CA. An introduction to proteoglycans and their localization. J Histochem Cytochem. 2012;60:885–897. doi: 10.1369/0022155412464638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang T. Glycosaminoglycan (GAGS) biosynthesis and GAG-binding proteins. Prog Mol Biol Transl Sci. 93:1–17. doi: 10.1016/S1877-1173(10)93001-9. [DOI] [PubMed] [Google Scholar]
- 10.Esko JD, Kimata K, Lindahl U. Proteoglycans and sulfated glycosaminoglycans. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glyobiology. Cold Spring Harbor; NY: 2009. [PubMed] [Google Scholar]
- 11.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 12.Ucakturk E, Chao C, Li L, Zhang F, Lindhardt RJ. Capillary electrophoresis for total glycosaminoglycan analysis. Anal Bional Chem. 2014;406:4617–4626. doi: 10.1007/s00216-014-7859-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sugahara K, Mikami T, Uyama T, Mizuguchi S, Nomura K, Kitagawa H. Recent advances in the structural biology of chondroitin sulfate and dermatan sulfate. Curr Opin Struct Biol. 2003;13:612–620. doi: 10.1016/j.sbi.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Dai Y, Yang Y, MacLeod V, Yue X, Rapraeger AC, Shriver Z, Venkataram G, Sasisekharan R, Sanderson RD. Sulf-1 and HSulf-2 are potent inhibitors of myeloma tumor growth in vivo. J Biol Chem. 2005;280:40066–40073. doi: 10.1074/jbc.M508136200. [DOI] [PubMed] [Google Scholar]
- 16.Sanderson RD, Yang Y, Kelly T, MacLeod V, Dai Y, Theus A. Enzymatic remodeling of heparan sulfate proteoglycans within the tumor microenvironment: growth regulation and the prospect of new cancer therapies. J Cell Biochem. 2005;96:897–905. doi: 10.1002/jcb.20602. [DOI] [PubMed] [Google Scholar]
- 17.Vlodavsky I, Goldshmidt O. Properties and function of heparanase in cancer metastasis and angiogenesis. Haemostasis. 2001;31:60–63. [PubMed] [Google Scholar]
- 18.Raman R, Sasisekharan V, Sasisekharan R. Structural insights into biological roles of protein–glycosaminoglycan interactions. Chem Biol. 2004;12:267–277. doi: 10.1016/j.chembiol.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 19.Casu B, Guerrini M, Naggi A, Perez M, Torri G, Ribatti D, Carminati P, Gianini G, Penco S, Pisano C, Belleri M, Rusnati M, Presta M. Short heparin sequences spaced by glycolsplit urinate residues are antagonists of fibroblast growth factor 2 and angiogenesis inhibitors. Biochemist. 2002;41:10519–10528. doi: 10.1021/bi020118n. [DOI] [PubMed] [Google Scholar]
- 20.Iozzo RV, San Antonio JD. Heparan sulfate proteoglycans heavy hitters in the angiogenesis arena. J Clin Invest. 2001;108:349–355. doi: 10.1172/JCI13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iozzo RV. Basement membrane proteoglycans: from cellar to ceiling. Nat Rev Mol Cell Bio. 2001;6:646–656. doi: 10.1038/nrm1702. [DOI] [PubMed] [Google Scholar]
- 22.Vlodavsky I, Friedmann Y, Elkin M, Aingorn R, Atzmon R, Ishai-Michaeli R, Bitan M, Pappo O, Peretz T, Michael I, Spector L, Pecker I. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nat Med. 1999:793–802. doi: 10.1038/10518. [DOI] [PubMed] [Google Scholar]
- 23.Vlodavsky I, Goldshmidt O, Zcharia E, Atzmon R, Rangini-Guatta Z, Elkin M, Peretz T, Friedman Y. Mammalian heparanase: involvement in cancer metastasis, angiogenesis and normal development. Semin Cancer Biol. 2002;12:121–129. doi: 10.1006/scbi.2001.0420. [DOI] [PubMed] [Google Scholar]
- 24.Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 25.Carulli D, Laabs T, Geller HM, Fawcett JW. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol. 2005;15:116–120. doi: 10.1016/j.conb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 26.Cavalcante LA, Garcia-Abreu J, Moura Neto V, Silva LC, Weissmuller G. Modulators of axonal growth and guidance at the brain midline with special reference to glial heparan sulfate proteoglycans. An Acad Bras Cienc. 2002;74:691–716. doi: 10.1590/s0001-37652002000400010. [DOI] [PubMed] [Google Scholar]
- 27.Fry EE, Lea SM, Jackson T, Newman J, Ellard FM, Blakemore WE, Abu-Ghazaleh R, Samuel A, King AM, Sutart DI. The structure and function of a foot-and-mouth disease virus-oligosaccharide receptor complex. EMBO J. 1999;18:543–554. doi: 10.1093/emboj/18.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Shriver Z, Pope RM, Thorp SC, Duncan MB, Copeland RJ, Raska CS, Yoshida K, Eisenberg RJ, Cohen G, Lindhardt RJ, Sasisekharan R. Characterization of a heparan sulfate octasaccharide that binds to herpes simplex virus type 1 glycoprotein d. J Biol Chem. 2002;277:33456–33467. doi: 10.1074/jbc.M202034200. [DOI] [PubMed] [Google Scholar]
- 29.Mardberg K, Trybala E, Tufaro F, Bergstrom T. Herpes simplex virus type 1 glycoprotein C is necessary for efficient infection of chondroitin sulfate expressing gro2C cells. J Gen Virol. 2002;83:291–300. doi: 10.1099/0022-1317-83-2-291. [DOI] [PubMed] [Google Scholar]
- 30.Perrimon N, Bernfield M. Cellular functions of proteoglycans—an overview. Cell Dev Biol. 2001;12:65–67. doi: 10.1006/scdb.2000.0237. [DOI] [PubMed] [Google Scholar]
- 31.Casu B, Guerrini M, Torri G. Structural and conformational aspects of the anticoagulant and anti-thrombotic activity of heparin and dermatan sulfate. Curr Pharm Des. 2004;10:939–949. doi: 10.2174/1381612043452794. [DOI] [PubMed] [Google Scholar]
- 32.Fareed J, Hoppensteadt DA, Bick RL. An update on heparins at the beginning of the new millennium. Semin Thromb Hemost. 2000;26:5–21. doi: 10.1055/s-2000-9498. [DOI] [PubMed] [Google Scholar]
- 33.Neufeld E, Muenzer J, Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases of Inherited Disease. 8th. McGraw-Hill; New York: 2001. The mucopolysaccharidoses; pp. 3421–3452. [Google Scholar]
- 34.Tomatsu S, Shimada T, Mason RW, Montano AM, Kelly J, LaMarr WA, Kubaski F, Giugliani R, Guha A, Yasuda E, Mackenzie W, Yamaguchi S, Suzuki Y, Orii T. Establishment of glycosaminoglycan assays for mucopolysaccharidoses. Metabolites. 2014;4:655–679. doi: 10.3390/metabo4030655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Björnsson S. Quantification of proteoglycans as glycosaminoglycans in biological fluids using an alcian blue dot blot analysis. Anal Biochem. 1998;256:229–237. doi: 10.1006/abio.1997.2494. [DOI] [PubMed] [Google Scholar]
- 36.de Jong JG, Wevers RA, Laarakkers C, Poorthuis BJ. Dimethylmethylene blue-based spectrophotometry of glycosaminoglycans in untreated urine: a rapid screening procedure for mucopolysaccharidoses. Clin Chem. 1989;35:1472–1477. [PubMed] [Google Scholar]
- 37.Whitley CB, Ridnour MD, Draper KA, Dutton CM, Neglia JP. Diagnostic test for mucopolysaccharidosis: I. Direct method for quantifying excessive urinary glycosaminoglycan excretion. Clin Chem. 1989;35:374–379. [PubMed] [Google Scholar]
- 38.Whitley CB, Draper KA, Dutton CM, Brown PA, Severson SL, France LA. Diagnostic test for mucopolysaccharidosis. II. Rapid quantification of glycosaminoglycan in urine samples collected on a paper matrix. Clin Chem. 1989;35:2074–2081. [PubMed] [Google Scholar]
- 39.Jong JG, Hasselman JJ, van Landerhem AA, Vader HL, Wevers RA. The spot test is not a reliable screening procedure for mucopolysaccharidoses. Clin Chem. 1991;37:572–575. [PubMed] [Google Scholar]
- 40.Iwata S, Sukegawa K, Sasaki T, Kokuryu M, Yamasita S, Noma A, Iwasa S, Kondo N, Orii T. Mass screening test for mucopolysaccharidoses using the 1,9-dimethylmethylene blue method: positive interference from paper diapers. Clin Chim Acta. 1997;264:245–250. doi: 10.1016/s0009-8981(97)00084-3. [DOI] [PubMed] [Google Scholar]
- 41.Iwata S, Sukegawa K, Kokuryu M, Tomatsu S, Kondo N, Iwasa S, Orii T. Glycosaminoglycans in neonatal urine. Arch Dis Child Fetal Ed Neonatal. 2000 doi: 10.1136/fn.82.1.F78. http://dx.doi.org/10.1136/fn.82.1.F77b. [DOI] [PMC free article] [PubMed]
- 42.Karlsson M, Edfors-Lilja I, Bjornsson S. Binding and detection of glycosaminoglycans immobilized on membranes treated with cationic detergents. Anal Biochem. 2000;286:51–58. doi: 10.1006/abio.2000.4767. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida K, Miyauchi S, Kikuchi H, Tawada A, Tokuyasu K. Analysis of unsaturated disaccharides from glycosaminoglycuronan by high-performance liquid chromatography. Anal Biochem. 1989;177:327–332. doi: 10.1016/0003-2697(89)90061-4. [DOI] [PubMed] [Google Scholar]
- 44.Kinoshita A, Sugahara K. Microanalysis of glycosaminoglycan-derived oligosaccharides labeled with a fluorophore 2-aminobenzamide by high-performance liquid chromatography: application to disaccharide composition analysis and exosequencing of oligosaccharides. Anal Biochem. 1999;269:367–368. doi: 10.1006/abio.1999.4027. [DOI] [PubMed] [Google Scholar]
- 45.Oguma T, Toyoda H, Toida T, Imanari T. Analytical method of chondroitin/dermatan sulfates using high performance liquid chromatography/turbo ion-spray ionization mass spectrometry: application to analyses of the tumor tissue sections on glass slides. Biomed Chromatogr. 2001;5:356–362. doi: 10.1002/bmc.74. [DOI] [PubMed] [Google Scholar]
- 46.Oguma T, Toyoda H, Toida T, Imanari T. Analytical method for heparan sulfates using high-performance liquid chromatography turbo-ionspray ionization tandem mass spectrometry. J Chromat B. 2001;754:153–159. doi: 10.1016/s0378-4347(00)00601-0. [DOI] [PubMed] [Google Scholar]
- 47.Oguma T, Toyoda H, Toida T, Imanari T. Analytical method for keratan sulfate by high-performance liquid chromatography/turbo-ionspray tandem mass spectrometry. Anal Biochem. 2001;290:68–73. doi: 10.1006/abio.2000.4940. [DOI] [PubMed] [Google Scholar]
- 48.Perkin WH. On mauveine and allied colouring matters. J Chem Soc Trans. 1879;35:717–732. [Google Scholar]
- 49.Sridharan G, Shankar AA. Toluidine blue: a review of its chemistry and clinical utility. J Oral Maxillofac Pathol. 2012;16:251–255. doi: 10.4103/0973-029X.99081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Drupy RA, Wallington EA. Carleton's Histological Technique. 5th. Oxford University Press; 1980. [Google Scholar]
- 51.Dietrich CP, Dietrich SM. Electrophoretic behavior of acidic mucopolysaccharides in diamine buffers. Anal Biochem. 1976;70:645–647. doi: 10.1016/0003-2697(76)90496-6. [DOI] [PubMed] [Google Scholar]
- 52.Volpi N, Maccari F. Detection of submicrogram quantities of glycosaminoglycans on agarose gels by sequential staining with toluidine blue and stains-all. Electrophoresis. 2002;23:4060–4066. doi: 10.1002/elps.200290021. [DOI] [PubMed] [Google Scholar]
- 53.Rajendra V. In: Mucopolysaccharides-Glycosaminoglycans-of Body Fluids in Health and Disease. Varma R, Varma RS, editors. de Gruyter; Berlin: New York: 1983. [Google Scholar]
- 54.Sato CF, Gyorkey F. Purification of protease-extracted glycosaminoglycans by short-distance paper chromatography with a zinc acetate solvent. Anal Biochem. 1978;87:540–544. doi: 10.1016/0003-2697(78)90703-0. [DOI] [PubMed] [Google Scholar]
- 55.Teller WM, Ziemann A. Thin layer chromatography of urinary acid glycosaminoglycans as screening procedure for mucopolysaccharidoses. Horm Metab Res. 1969;1:32–35. doi: 10.1055/s-0028-1095171. [DOI] [PubMed] [Google Scholar]
- 56.Lipiello L, Mankin HJ. Thin-layer chromatographic separation of the isomeric chondroitin sulfates, dermatan sulfate, and keratan sulfate. Anal Biochem. 1971;39:54–58. doi: 10.1016/0003-2697(71)90460-x. [DOI] [PubMed] [Google Scholar]
- 57.Humbel R, Chamoles NA. Sequential thin layer chromatography of urinary acidic glycosaminoglycans. Clin Chim Acta. 1972;40:290–293. doi: 10.1016/0009-8981(72)90287-2. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Z, Xie J, Zhang F, Lindhardt RJ. Thin layer chromatography for the analysis of glycosaminoglycan oligosaccharides. Annal Biochem. 2007;371:118–120. doi: 10.1016/j.ab.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berenson GS, Dalferes ER., Jr Identification of acid mucopolysaccharides by glass-paper chromatography. Biochim Biophys Acta. 1962;26:34–40. doi: 10.1016/0006-3002(62)90814-4. [DOI] [PubMed] [Google Scholar]
- 60.Castor CW, Dorstewitz EL. Identification of acid mucopolysaccharides by paper chromatography. J Chromatogr. 1964;13:157–165. doi: 10.1016/s0021-9673(01)95087-3. [DOI] [PubMed] [Google Scholar]
- 61.Wusteman FS, Lloyd AG, Dodgson KS. Thin-layer chromatography and the rapid identification of common acidic glycosaminoglycans. J Chromatog. 1966;21:32–39. doi: 10.1016/s0021-9673(01)91257-9. [DOI] [PubMed] [Google Scholar]
- 62.Säämänen AM, Tammi M. Determination of unsaturated glycosaminoglycans disaccharides by spectrophotometry on thin-layer chromatography plates. Anal Biochem. 1984;140:354–359. doi: 10.1016/0003-2697(84)90177-5. [DOI] [PubMed] [Google Scholar]
- 63.Stone JE, Akhtar N, Botchway S, Pennock CA. Interaction of 1,9-di-methylmethylene blue with glycosaminoglycans. Ann Clin Biochem. 1994;31:147–152. doi: 10.1177/000456329403100206. [DOI] [PubMed] [Google Scholar]
- 64.Edens RE, Al-Hakim A, Weiler JM, Rethwisch DG, Fareed J, Lindhardt RJ. Gradient polyacrylamide gel electrophoresis for determination of molecular weights of heparin preparations and low-molecular-weight heparin derivatives. J Pharm Sci. 1992;81:823–827. doi: 10.1002/jps.2600810821. [DOI] [PubMed] [Google Scholar]
- 65.Min H, Cowman MK. Combined alcian blue and silver staining of glycosaminoglycans in polyacrylamide gels: application to electrophoretic analysis of molecular weight distribution. Anal Biochem. 1986;155:275–285. doi: 10.1016/0003-2697(86)90437-9. [DOI] [PubMed] [Google Scholar]
- 66.Rice KG, Rottink MK, Linhardt RJ. Fractionation of heparin-derived oligosaccharides by gradient polyacrylamide-gel electrophoresis. Biochem J. 1987;244:515–522. doi: 10.1042/bj2440515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pennock CA. A review and selection of simple laboratory methods used for the study of glycosaminoglycan. J Clin Pathol. 1976;29:111–123. doi: 10.1136/jcp.29.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Panin G, Naia S, Dall'Amico R, Chiandetti L, Zachello F, Catassi C, Felici L, Coppa GV. Simple spectrophotometric quantification of urinary excretion of glycosaminoglycan sulfates. Clin Chem. 1986;32:2073–2076. [PubMed] [Google Scholar]
- 69.Scott JE, Quintarelli G, Dellovo MC. The chemical properties of alcian blue. I. The mechanism of alcian blue staining. Histochemie. 1964;4:73–85. doi: 10.1007/BF00306149. [DOI] [PubMed] [Google Scholar]
- 70.Whiteman P. The quantitative measurement of alcian blue-glycosaminoglycan complexes. Biochem J. 1973;131:343–350. doi: 10.1042/bj1310343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hata R, Nagai Y. A rapid and micro method for the separation of acidic glycosaminoglycans by two-dimensional electrophoresis on a cellulose acetate strip. Anal Biochem. 1973;52:652–656. doi: 10.1016/0003-2697(73)90075-4. [DOI] [PubMed] [Google Scholar]
- 72.Newton DJ, Scott JE, Whiteman P. The estimation of acid glycosaminoglycan-alcian blue complexes eluted from electrophoretic strips. Anal Biochem. 1974;62:268–273. doi: 10.1016/0003-2697(74)90386-8. [DOI] [PubMed] [Google Scholar]
- 73.Stone JE. Urine analysis in the diagnosis of mucopolysaccharide disorders. Ann Clin Biochem. 1998;35:207–225. doi: 10.1177/000456329803500204. [DOI] [PubMed] [Google Scholar]
- 74.Humbel R, Etringer S. A colorimetric method for the determination of sulfated glycosaminoglycans. Rev Roum Biochim. 1974;11:21–24. [Google Scholar]
- 75.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883:173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 76.Mabe P, Valiente A, Soto V, Cornejo V, Raiman E. Evaluation of reliability for urine mucopolysaccharidosis screening by dimethylmethylene blue and berry spot tests. Clin Chim Acta. 2004;345:135–140. doi: 10.1016/j.cccn.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 77.de Jong JG, Hasselman JJ, van Landeghem AA, Vader HL, Wevers RA. The spot test is not a reliable screening procedure for mucopolysaccharidoses. Clin Chem. 1991;37:572–575. [PubMed] [Google Scholar]
- 78.Panin G, Naia S, Dall'Amico R, Chiandetti L, Zachello F, Catassi C, Fellici L, Coppa GV. Simple spectrophotometric quantification of urinary excretion of glycosaminoglycan sulfates. Clin Chem. 1986;32:2073–2076. [PubMed] [Google Scholar]
- 79.Carroll GJ. Spectrophotometric measurement of proteoglycans in osteoarthritic synovial flui. Ann Rheum Dis. 1987;46:375–379. doi: 10.1136/ard.46.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gold E. A simple spectrophotometric method for estimating glycosaminoglycan concentrations. Anal Biochem. 1979;99:183–188. doi: 10.1016/0003-2697(79)90061-7. [DOI] [PubMed] [Google Scholar]
- 81.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 82.Bartold PM, Page RC. A microdetermination method for assaying glycosaminoglycans and proteoglycans. Anal Biochem. 1985;150:320–324. doi: 10.1016/0003-2697(85)90517-2. [DOI] [PubMed] [Google Scholar]
- 83.Taylor KB, Jeffree GM. A new basic metachromatic dye 1,9 dimethylmethylene blue. J Hist Chem. 1969;1:199–204. doi: 10.1007/BF01081408. [DOI] [PubMed] [Google Scholar]
- 84.Sabiston P, Adams ME, Ho Y. Automation of 1,9-dimethylmethylene blue dye-binding assay for sulfated glycosaminoglycans with application to cartilage microcultures. Anal Biochem. 1985;149:543–548. doi: 10.1016/0003-2697(85)90611-6. [DOI] [PubMed] [Google Scholar]
- 85.Panin G, Naria S, Dall I, Amico R. Simple spectrophotometric quantification of urinary excretion of glycosaminoglycan sulphates. Clin Chem Acta. 1986;32:2073–2076. [PubMed] [Google Scholar]
- 86.Chandrasekhar S, Esterman MA, Hoffman HA. Microdetermination of proteoglycans and glycosaminoglycans in the presence of guanidine hydrochloride. Anal Biochem. 1987;161:103–108. doi: 10.1016/0003-2697(87)90658-0. [DOI] [PubMed] [Google Scholar]
- 87.Piraud M, Maire I, Mathieu M. Pitfalls of screening for mucopolysaccharidoses by the dimethylmethylene blue test. Clin Chem. 1993;39:163–164. [PubMed] [Google Scholar]
- 88.Goldberg RL, Kolibas LM. An improved method for determining proteoglycans synthesized by chondrocytes in culture. Connect Tissue Res. 1990;24:265–275. doi: 10.3109/03008209009152154. [DOI] [PubMed] [Google Scholar]
- 89.Kaplan D. Classification of the mucopolysaccharidoses based on the pattern of mucopolysacchariduria. Am J Med. 1969;47:721–729. doi: 10.1016/0002-9343(69)90166-1. [DOI] [PubMed] [Google Scholar]
- 90.Murphy D, Pennock CA, London KJ. Gas-liquid chromatographic measurement of glucosamine and galactosamine content of urinary glycosaminoglycans. Clin Chim Acta. 1974;53:145–152. doi: 10.1016/0009-8981(74)90092-8. [DOI] [PubMed] [Google Scholar]
- 91.Toida T, Qiu G, Matsunaga T, Sagehashi Y, Imanari T. Gas chromatography mass spectrometric determinations of iduronic and glucuronic acids in glycosaminoglycans after reduction of carboxylic group using sodium borodeuteride. Anal Sci. 1992;8:799–804. [Google Scholar]
- 92.Rice KG, Kim YS, Grant AC, Merchant ZM, Lindhardt RJ. High-performance liquid chromatographic separation of heparin-derived oligosaccharides. Anal Biochem. 1985;150:325–331. doi: 10.1016/0003-2697(85)90518-4. [DOI] [PubMed] [Google Scholar]
- 93.Volpi N, Maccari F, Galeotti F, Zampini L, Santoro L, Padella L, Galeazzi T, Gabrielli O, Coppa GV. Plasmatic dermatan sulfate and chondroitin sulfate determination in mucopolysaccharidoses. J Pharm Biom Anal. 2013;85:40–45. doi: 10.1016/j.jpba.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 94.Kodama C, Ototani N, Isemura M, Yosizawa Z. High performance liquid chromatography of pyridylamino derivatives of unsaturated disaccharides produced from chondroitin sulfate isomers by chondroitinases. J Biochem. 1984;96:1283–1287. doi: 10.1093/oxfordjournals.jbchem.a134947. [DOI] [PubMed] [Google Scholar]
- 95.Kodama C, Ototani N, Isemura M, Aikawa J, Yosizawa Z. Liquid-chromatographic determination of urinary glycosaminoglycans for differential diagnosis of genetic mucopolysaccharidoses. Clin Chem. 1986;32:30–34. [PubMed] [Google Scholar]
- 96.Yamada H, Miyauchi S, Morita M, Yoshida Y, Yoshihara Y, Kikuchi T, Washimi O, Washimi Y, Terada N, Seki T, Fujikawa K. Content and sulfation pattern of keratan sulfate in hip osteoarthritis using high performance liquid chromatography. J Rheumatol. 2000;27:1721–1724. [PubMed] [Google Scholar]
- 97.Linhardt RJ, Pervin A. Separation of acidic carbohydrates by capillary electrophoresis. J Chromat A. 1996;720:323–335. doi: 10.1016/0021-9673(95)00265-0. [DOI] [PubMed] [Google Scholar]
- 98.Mao W, Thanawiroon C, Linhardt RJ. Capillary electrophoresis for the analysis of glycosaminoglycans and glycosaminoglycan-derived oligosaccharides. Biomed Chromat. 2002;16:77–94. doi: 10.1002/bmc.153. [DOI] [PubMed] [Google Scholar]
- 99.Grimshaw J, Kane A, Trocha-Grimshaw J, Douglas A, Chakravarthy U, Archer D. Quantitative analysis of hyaluronan in vitreous humor using capillary electrophoresis. Electrophoresis. 1994;15:936–940. doi: 10.1002/elps.11501501137. [DOI] [PubMed] [Google Scholar]
- 100.Stefansson M, Novtony M. Separation of complex oligosaccharide mixtures by capillary electrophoresis in the open-tubular format. Anal Chem. 1994;66:1134–1140. doi: 10.1021/ac00079a031. [DOI] [PubMed] [Google Scholar]
- 101.Linhardt RJ, Desai UR, Liu J, Pervin A, Hoppensteadt D, Farred J. Low molecular weight dermatan sulfate as an antithrombotic agent: structure–activity relationship studies. Biochem Pharmacol. 1994;47:1241–1252. doi: 10.1016/0006-2952(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 102.Maslch R, Harenberg J. Purity of glycosaminoglycan-related compounds using capillary electrophoresis. Electrophoresis. 1996;17:401–405. doi: 10.1002/elps.1150170219. [DOI] [PubMed] [Google Scholar]
- 103.Hayse S, Oda Y, Honda S, Kakehi K. High-performance capillary electrophoresis of hyaluronic acid: determination of its amount and molecular mass. J Chromatogr A. 1997;768:295–305. doi: 10.1016/s0021-9673(96)01095-3. [DOI] [PubMed] [Google Scholar]
- 104.Hopwood JJ, Harrison JR. High-resolution electrophoresis of urinary glycosaminoglycans: an improved screening test for the mucopolysaccharidoses. Anal Biochem. 1982;119:120–127. doi: 10.1016/0003-2697(82)90674-1. [DOI] [PubMed] [Google Scholar]
- 105.Cappelletti R, Del Rosso M, Chiarugi VP. A new electrophoretic method for the complete separation of all known animal glycosaminoglycans in a monodimensional run. Anal Biochem. 1979;99:311–315. doi: 10.1016/s0003-2697(79)80012-3. [DOI] [PubMed] [Google Scholar]
- 106.Paules A, Klockow A. Detection of carbohydrates in capillary electrophoresis. J Chromatogr A. 1996;720:353–376. doi: 10.1016/0021-9673(95)00323-1. [DOI] [PubMed] [Google Scholar]
- 107.Grimshaw J. Analysis of glycosaminoglycans and their oligosaccharide fragments by capillary electrophoresis. Electrophoresis. 1997;18:2408–2414. doi: 10.1002/elps.1150181231. [DOI] [PubMed] [Google Scholar]
- 108.Wiley JP. Determination of polycarboxylic acids by capillary electrophoresis with copper complexion. J Chromatogr. 1995;692:267–274. [Google Scholar]
- 109.Toida T, Lindhardt RJ. Detection of glycosaminoglycans as a copper (II) complex in capillary electrophoresis. Electrophoresis. 1996;15:341–346. doi: 10.1002/elps.1150170209. [DOI] [PubMed] [Google Scholar]
- 110.Niessen WMA, Tjaden UR, Greef J. Capillary electrophoresis mass spectrometry. J Chromatogr. 1993;636:3–19. doi: 10.1016/s0021-9673(01)90212-2. [DOI] [PubMed] [Google Scholar]
- 111.Lazar IM, Xin B, Lee ML. Design of a time-of-flight mass spectrometer as a detector for capillary electrophoresis. Anal Chem. 1997;69:3205–3211. [Google Scholar]
- 112.Duteil S, Gareil P, Girault S, Mallet A, Feve C, Siret L. Identification of heparin oligosaccharides by direct coupling of capillary electrophoresis/ion-spray-mass spectrometry. Rapid Commun Mass Spectrom. 1999;13:1889–1898. doi: 10.1002/(SICI)1097-0231(19991015)13:19<1889::AID-RCM719>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 113.Aydin S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides. 2015;72:4–15. doi: 10.1016/j.peptides.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 114.Engvall E. The ELISA enzyme-linked immunosorbent assay. Clin Chem. 2010;56:319–320. doi: 10.1373/clinchem.2009.127803. [DOI] [PubMed] [Google Scholar]
- 115.Lindstöm P, Wager O. IgG autoantibody to human serum albumin studied by the ELISA-technique. Scand J Immunol. 1948;7:419–425. doi: 10.1111/j.1365-3083.1978.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 116.Kato H, Hamaguchi Y, Okawa S, Ishikawa E, Kobayashi K. Use of rabbit antibody IgG bound onto plain and aminoalkylsilyl glass surface for the enzyme-linked sandwich immunoassay. J Biochem. 1977;82:261–266. doi: 10.1093/oxfordjournals.jbchem.a131678. [DOI] [PubMed] [Google Scholar]
- 117.Yorde DE, Sasse EA, Wang TY, Hussa RO, Garancis JC. Competitive enzyme-liked immunoassay with use of soluble enzyme/antibody immune complexes for labeling. I. Measurement of human choriogonadotropin. Clin Chem. 1976;22:1372–1377. [PubMed] [Google Scholar]
- 118.Møller HJ, Larsen FS, Ingemann-Hansen T, Poulsen JH. ELISA for the core protein of the cartilage large aggregating proteoglycan, aggrecan: comparsion with the concentrations of immunogenic keratan sulphate in synovial fluid, serum and urine. Clin Chim Acta. 1994;225:43–55. doi: 10.1016/0009-8981(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 119.Tomatsu S, Okamura K, Taketani T, Orii KO, Nishiota T, Gutierrez MA, Velez-Castrillon S, Fachel AA, Grub JH, Cooper A, Thornley M, Wraith E, Barrera LA, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kircher SG, Paschke E, Yamaguchi S, Ulrich K, Isogai K, Suzuki Y, Orii T, Kondo N, Creer M, Noguchi A. Development and testing of new screening method for keratan sulfate in mucopolysaccharidosis IVA. Pediatr Res. 2004;55:592–597. doi: 10.1203/01.PDR.0000113767.60140.E9. [DOI] [PubMed] [Google Scholar]
- 120.Tomatsu S, Okamura K, Maeda H, Taketani T, Castrillon SV, Gutierrez MA, Nishioka T, Fachel AA, Orii KO, Grubb JH, Cooper A, Thornley M, Wraith E, Barrera LA, Laybauer LS, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kirchner SG, Paschke E, Yamaguchi S, Ulrich K, Haskins M, Isogai K, Suzuki Y, Orii T, Kondo N, Creer M, Okuyama T, Tanaka A, Noguchi A. Keratan sulphate levels in mucopolysaccharidoses and mucolipidoses. JIMD. 2005;28:187–202. doi: 10.1007/s10545-005-5673-3. [DOI] [PubMed] [Google Scholar]
- 121.Najjam S, Gibbs RV, Gordon MY, Rider CC. Characterization of human recombinant interleukin 2 binding to heparin and heparan sulfate using an ELISA approach. Cytokine. 1997;9:1013–1022. doi: 10.1006/cyto.1997.0246. [DOI] [PubMed] [Google Scholar]
- 122.Tomatsu S, Gutierrez MA, Ishimaru T, Pena OM, Montano AM, Maeda H, Velez-Castrillon S, Nishioka T, Fachel AA, Cooper A, Thornley M, Wraith W, Barrera LA, Laybauer LS, Giugliani R, Schwartz IV, Frenking GS, Beck M, Kirchner SG, Paschke E, Yamaguchi S, Ulrich K, Isogai K, Suzuki Y, Orii T, Noguchi A. Heparan sulfate levels in mucopolysaccharidoses and mucolipidoses. JIMD. 2005;28:187–202. doi: 10.1007/s10545-005-0069-y. [DOI] [PubMed] [Google Scholar]
- 123.Shibutani T, Nishino W, Shiraki M, Iwayama Y. ELISA detection of glycosaminoglycan (GAG)-linked proteoglycans in gingival crevicular fluid. J Periodontol Res. 1993;28:17–20. doi: 10.1111/j.1600-0765.1993.tb01045.x. [DOI] [PubMed] [Google Scholar]
- 124.Yang JA, Kim ES, Kwon JH, Kim H, Shin JH, Yun SH, Choi KY, Hahn SK. Transdermal delivery of hyaluronic acid-human growth hormone conjugate. Biomaterials. 2012;33:5947–5954. doi: 10.1016/j.biomaterials.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 125.Bouças RI, Trindade ES, Tersariol IL, Dietrich CP, Nade HB. Development of an enzyme-linked immunosorbent assay (ELISA)-like fluorescence assay to investigate the interactions of glycosaminoglycans to cells. Anal Chim Acta. 2008;618:218–226. doi: 10.1016/j.aca.2008.04.059. [DOI] [PubMed] [Google Scholar]
- 126.Pan T, Wong BS, Liu T, Li R, Petersen RB, Sly MS. Cell-surface prion protein interacts with glycosaminoglycans. Biochem J. 2002;368:81–90. doi: 10.1042/BJ20020773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McLafferty FW. Tandem mass spectrometry. Science. 1981;214:280–287. doi: 10.1126/science.7280693. [DOI] [PubMed] [Google Scholar]
- 128.McLafferty FW. Mass spectrometry across the sciences. PNAS. 2008;105:18088–18089. doi: 10.1073/pnas.0800784105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Thomson JJ. On the Chatode Raus. Proc Camb Philos Soc. 1897;9:243–244. [Google Scholar]
- 130.Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- 131.Carroll DI, Dzidic I, Stillwell RN, Horning MG, Horning EC. Subpicogram detection system for gas phase analysis based upon atmospheric pressure ionization (API) mass spectrometry. Anal Chem. 1974;46:706–710. [Google Scholar]
- 132.Morris HR, Panico M, Barber M, Bordoli RS, Sedgwick RD, Tyler A. Fast atom bombardment: a new spectrometric method for peptide sequence analysis. Biochem Biophys Res Commun. 1981;101:623–631. doi: 10.1016/0006-291x(81)91304-8. [DOI] [PubMed] [Google Scholar]
- 133.Munson MSB, Field FHJ. Chemical ionization mass spectrometry. I. General introduction. J Am Chem Soc. 1966;88:2621–2630. [Google Scholar]
- 134.Karas M, Bachmann D, Hillenkamp F. Influence of the wavelength in high-irradiance ultraviolet laser desorption mass spectrometry of organic molecules. Anal Chem. 1985;57:2935–2939. [Google Scholar]
- 135.Karas M, Kriiger R. Ion formation in MALDI: the cluster ionization mechanism. Chem Rev. 2003;103:427–439. doi: 10.1021/cr010376a. [DOI] [PubMed] [Google Scholar]
- 136.Strathmann FG, Hoofnagle AH. Current and future applications of mass spectrometry to the clinical laboratory. Clinical chem. 2011;136:609–616. doi: 10.1309/AJCPW0TA8OBBNGCK. [DOI] [PubMed] [Google Scholar]
- 137.Ombrone D, Giocaliere E, Forni G, Malvagia S, la Marca G. Expanded newborn screening by mass spectrometry: new tests, future perspectives. Mass Spec Rev. 1999;2015:1–14. doi: 10.1002/mas.21463. [DOI] [PubMed] [Google Scholar]
- 138.Zamfir A, Seidler DG, Schonherr E, Kresse H, Peter-Kataluinic J. On-line sheathless capillary electrophoresis/nanoelectrospray ionization-tandem mass spectrometry for the analysis of glycosaminoglycans oligosaccharides. Electrophoresis. 2004;25:2010–2016. doi: 10.1002/elps.200405925. [DOI] [PubMed] [Google Scholar]
- 139.Desaire H, Sirich TL, Leary JA. Evidence of block and randomly sequenced chondroitin polysaccharides: sequential enzymatic digestion and quantification using ion trap tandem mass spectrometry. Anal Chem. 2001;73:3513–3520. doi: 10.1021/ac010385j. [DOI] [PubMed] [Google Scholar]
- 140.Flangea C, Schiopu C, Sisu E, Serb A, Przybylski M, Seidler DG, Zamfir AD. Determination of sulfation pattern in brain glycosaminoglycans by chip-based electrospray ionization ion trap mass spectrometry. Anal Bioanal Chem. 2009;395:2489–2498. doi: 10.1007/s00216-009-3167-0. [DOI] [PubMed] [Google Scholar]
- 141.Yu Y, Sweeney MD, Saad OM, Crown SE, Handel TM, Leary JA. Chemokine-glycosaminoglycan binding: specificity for CCR2 ligand binding to highly sulfated oligosaccharides using FTICR mass spectrometry. J Biol Chem. 2005;280:32200–32208. doi: 10.1074/jbc.M505738200. [DOI] [PubMed] [Google Scholar]
- 142.Weng N. Mini-review: important roles of chromatography in the quantitation of biomarkers using liquid chromatography and mass spectrometry (LC-MS) Austin Chromatogr. 2014;1:1–4. [Google Scholar]
- 143.Chuang CK, Lin HY, Wang TJ, Tsai CC, Liu HL, Lin SP. A modified liquid chromatography/tandem mass spectrometry method for predominant disaccharide units of urinary glycosaminoglycans in patients with mucopolysaccharidoses. Orphanet J Rare Dis. 2014;9:1–10. doi: 10.1186/s13023-014-0135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kailemia MJ, Park M, Kaplan DA, Venot A, Boons GJ, Li L, Lindhardt RJ, Amster IJ. High-field asymmetric-waveform ion mobility spectrometry and electron detachment dissociation of isobaric mixtures of glycosaminoglycans. J Am Soc Mass Spectrom. 2014;25:258–268. doi: 10.1007/s13361-013-0771-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhu H, Chen X, Zhang X, Liu L, Cong D, Zhao X, Yu G. Acidolysis-based component mapping of glycosaminoglycans by reversed-phase high-performance liquid chromatography with off-line electrospray ionization-tandem mass spectrometry: evidence and tags to distinguish different glycosaminoglycans. Anal Biochem. 2014;466:63–69. doi: 10.1016/j.ab.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 146.Oguma T, Tomatsu S, Montano AM, Okazaki O. Analytical method for the determination of disaccharides derived from keratan, heparan, and dermatan sulfates in human serum and plasma by high-performance liquid chromatography/turbo ionspray ionization tandem mass spectrometry. Anal Biochem. 2007;368:79–86. doi: 10.1016/j.ab.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 147.Oguma T, Tomatsu S, Okazaki O. Analytical method for determination of disaccharides derived from keratan sulfate in human serum and plasma by high-performance liquid chromatography/turbo-ionspray ionization tandem mass spectrometry. Biomed Chromatogr. 2007;31:356–362. doi: 10.1002/bmc.760. [DOI] [PubMed] [Google Scholar]
- 148.Tomatsu S, Montano AM, Oguma T, Dung VC, Oikawa H, Gutierrez ML, Yamaguchi S, Suzuki Y, Fukushi M, Barrera LA, Kida K, Kubota M, Orii T. Validation of disaccharide compositions derived from dermatan sulfate and heparan sulfate in mucopolysaccharidoses and mucopolipidosis II and III by tandem mass spectrometry. MGM. 2010;99:124–131. doi: 10.1016/j.ymgme.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 149.Tomatsu S, Montano AM, Oguma T, Dung VC, Oikawa H, de Carvalho TG, Gutierrez ML, Yamaguchi S, Suzuki Y, Fukushi M, Sakura N, Barrera LA, Kida K, Kubota M, Orii T. Dermatan sulfate and heparan sulfate as a biomarker for mucopolysaccharidosis. JIMD. 2010;33:141–150. doi: 10.1007/s10545-009-9036-3. [DOI] [PubMed] [Google Scholar]
- 150.Tomatsu S, Fujii T, Fukushi M, Oguma T, Shimada T, Maeda M, Kida K, ShibT Y, Futatsumori H, Montano AM, Mason RW, Yamaguchi S, Suzuki Y, Orii T. Newborn screening and diagnosis of mucopolysaccharidoses. MGM. 2013;110:42–53. doi: 10.1016/j.ymgme.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tomatsu S, Shimada T, Mason RW, Kelly J, LaMarr WA, Yasuda E, Shibata Y, Futatsumori H, Montano AM, Yamaguchi S, Suzuki Y, Orii T. Assay for glycosaminoglycans by tandem mass spectrometry and its applications. J Anal Bional Tech. 2014;1:1–25. doi: 10.4172/2155-9872.S2-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Osago H, Shibata T, Hara N, Kuwata S, Kono M, Uchio Y, Tsuchiya M. Quantitative analysis of glycosaminoglycans, chondroitin/dermatan sulfate, hyaluronic acid, heparan sulfate, and keratan sulfate by LC-ESI-MS/MS. Anal Biochem. 2014;467:1–38. doi: 10.1016/j.ab.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 153.Shimada T, Kelly J, LaMarr WA, van Vlies N, Yasuda E, Mason RW, Mackenzie W, Kubaski F, Giugliani R, Chinen Y, Yamaguchi S, Suzuki Y, Orii KE, Fukao T, Orii T, Tomastu S. Novel heparan sulfate assay by using automated high-throughput mass spectrometry. MGM. 2014;113:92–99. doi: 10.1016/j.ymgme.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lawrence R, Brown JR, Al-Mafraji K, Lamanna WC, Beitel JR, Boons GJ, Esko JD, Crawfor BE. Disease-specific non-reducing end carbohydrate biomarkers for mucopolysaccharidoses. Nat Chem Biol. 2012;8:197–204. doi: 10.1038/nchembio.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Auray-Blais C, Bhérer P, Gagnon R, Young SP, Zhang HH, An Y, Clarke JT, Millington DS. Efficient analysis of urinary glycosaminoglycans by LC–MS/MS in mucopolysaccharidoses type I, II and VI. Mol Genet Metab. 2011;102:49–56. doi: 10.1016/j.ymgme.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 156.Auray-Blais C, Lavoie P, Zhang H, Gagnon R, Clarke JT, Maranda B, Young SP, An Y, Millington DS. An improved method for glycosaminoglycan analysis by LC-MS/MS of urine samples collected on filter paper. Clin Chem. 2012;413:771–778. doi: 10.1016/j.cca.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 157.Zhang H, Wood T, Young SP, Mllington DS. A straightforward, quantitative ultra-performance liquid chromatography-tandem mass spectrometric method for heparan sulfate, dermatan sulfate and chondroitin sulfate in urine: an improved clinical screening test for the mucopolysaccharidoses. Mol Genet Metab. 2015;114:123–128. doi: 10.1016/j.ymgme.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 158.Zhang H, Young SP, Auray-Blais C, Orchard PJ, Tolar J, Mllington DS. Analysis of glycosaminoglycans in cerebrospinal fluid from patients with mucopolysaccharidoses by isotope-dilution ultra-performance liquid chromatography-tandem mass spectrometry. Clin Chem. 2011;57:1005–1012. doi: 10.1373/clinchem.2010.161141. [DOI] [PubMed] [Google Scholar]
- 159.Trim PJ, Lau AA, Hopwood JJ, Snel MF. A simple method for early age phenotype confirmation using toe tissue from a mouse model of MPS IIIA. Rapid Commun Mass Spectrom. 2014;28:933–938. doi: 10.1002/rcm.6861. [DOI] [PubMed] [Google Scholar]
- 160.Auray-Blais C, Lavoie P, Maranda B, Boutin M. Evaluation of urinary keratan sulfate disaccharides in MPS IVA patients using UPLC-MS/MS. Bioanalysis. 2016;8:179–191. doi: 10.4155/bio.15.239. [DOI] [PubMed] [Google Scholar]
- 161.Chamoles NA, Blanco M, Gaggioli D. Fabry disease: enzymatic diagnosis in dried blood spots on filter paper. Clin Chim Acta. 2001;308:195–196. doi: 10.1016/s0009-8981(01)00478-8. [DOI] [PubMed] [Google Scholar]
- 162.Chamoles NA, Blanco M, Gaggioli D. Diagnosis of alpha-l-iduronidase deficiency in dried blood spots on filter paper: the possibility of newborn diagnosis. Clin Chem. 2001;47:780–781. [PubMed] [Google Scholar]
- 163.Chamoles NA, Blanco M, Gaggioli D, Casentini C. Tay-Sachs and Sandhoff diseases: enzymatic diagnosis in dried blood spots on filter paper: retrospective diagnoses in newborn-screening cards. Clin Chim Acta. 2002;318:133–137. doi: 10.1016/s0009-8981(02)00002-5. [DOI] [PubMed] [Google Scholar]
- 164.Chamoles NA, Blanco M, Gaggioli D, Casentini C. Gaucher and Niemann-Pick diseases — enzymatic diagnosis in dried blood spots on filter paper: retrospective diagnoses in newborn-screening cards. Clin Chim Acta. 2002;317:191–197. doi: 10.1016/s0009-8981(01)00798-7. [DOI] [PubMed] [Google Scholar]
- 165.Chamoles NA, Blanco MB, Gaggioli D, Casentini C. Hurler-like phenotype: enzymatic diagnosis in dried blood spots on filter paper. Clin Chem. 2001;47:2098–2102. [PubMed] [Google Scholar]
- 166.Chamoles NA, Niizawa G, Blanco M, Casentini C. Glycogen storage disease type II: enzymatic screening in dried blood spots on filter paper. Clin Chim Acta. 2004;347:97–102. doi: 10.1016/j.cccn.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 167.Li, Scott CR, Chamoles NA, Ghavami A, Pinto BM, Turecek F, Gelb MH. Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem. 2004;50:1785–1796. doi: 10.1373/clinchem.2004.035907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Li Y, Brockman K, Turecek F, Scott CR, Gelb MH. Tandem mass spectrometry for the direct assay of enzymes in dried blood spots: application to newborn screening for Krabbe disease. Clin Chem. 2004;50:638–640. doi: 10.1373/clinchem.2003.028381. [DOI] [PubMed] [Google Scholar]
- 169.Blanchard S, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis I. Clin Chem. 2008;54:2067–2070. doi: 10.1373/clinchem.2008.115410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Duffner PK, Caggana M, Orsini JJ, Wenger DA, Patterson MC, Crosley CJ, Kutzberg J, Arnold GL, Escolar ML, Adams DJ, Andriola MR, Aron AM, Ciafaloni E, Djukic A, Erbe RW, Galvin-Parton P, Helton LE, Kolodny EH, Kosofsky BE, Kronn DF, Kwon JM, Levy PA, Miller-Horn J, Naidich TP, Pellegrino JE, Provenzale JM, Rothman SJ, Wasserstein MP. Newborn screening for Krabbe disease: the New York state model. Pediatr Neurol. 2009;40:245–252. doi: 10.1016/j.pediatrneurol.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 171.Gelb MH, Scott CR, Turecek F. Newborn screening for lysosomal storage diseases. Clin Chem. 2015;61:335–346. doi: 10.1373/clinchem.2014.225771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Duffey TA, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis VI (Maroteaux-Lamy syndrome) Anal Chem. 2010;82:9587–9591. doi: 10.1021/ac102090v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Duffey TA, Bellamy G, Elliott S, Fox AC, Glass M, Turecek F, Gelb MH, Scott CR. A tandem mass spectrometry assay for the detection of Fabry, Pompe, and mucopolysaccharidosis-I (Hurler) Clin Chem. 2010;56:1854–1861. doi: 10.1373/clinchem.2010.152009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Khaliq T, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis IVA. Clin Chem. 2011;57:128–131. doi: 10.1373/clinchem.2010.149880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Yip GW, Smollich M, Götte M. Therapeutic value of glycosaminoglycans in cancer. Mol Cancer Ther. 2006;5:2139–2148. doi: 10.1158/1535-7163.MCT-06-0082. [DOI] [PubMed] [Google Scholar]
- 176.Afratis N, Gialeli C, Nikitovic D, Tsegenidis T, Karouscou E, Theocharis AD, Pavão MS, Tzanakakis GN, Karamanos NK. Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J. 2012;279:1177–1197. doi: 10.1111/j.1742-4658.2012.08529.x. [DOI] [PubMed] [Google Scholar]
- 177.Plaas AH, West LA, Wong-Palms S, Nelson FR. Glycosaminoglycan sulfation in human osteoarthritis. Disease-related alterations at the non-reducing termini of chondroitin and dermatan sulfate. J Biol Chem. 1998;15:12642–12649. doi: 10.1074/jbc.273.20.12642. [DOI] [PubMed] [Google Scholar]
- 178.Wang JY, Roehrl MH. Glycosaminoglycans are a potential cause of rheumatoid arthritis. Proc Natl Acad Sci U S A. 2002;99:14362–14367. doi: 10.1073/pnas.222536599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Parthasarathy N, Spiro RG. Effect of diabetes on the glycosaminoglycan component of the human glomerular basement membrane. Diabetes. 1981;31:738–741. doi: 10.2337/diab.31.8.738. [DOI] [PubMed] [Google Scholar]
- 180.Olczyk K, Glowacki A, Kózma EM. Non-insulin-dependent diabetes mellitus associated changes in serum glycosaminoglycans. Pathophysiology. 1997;4:121–129. [Google Scholar]
- 181.Jinno A, Park PW. Role of glycosaminoglycans in infectious disease. Methods Mol Biol. 2015;1229:567–585. doi: 10.1007/978-1-4939-1714-3_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Iaci JF, Vecchione AM, Zimber MP, Caggiano AO. Chondroitin sulfate proteoglycans in spinal cord contusion injury and the effect of chondroitinase treatment. J Neurotrauma. 2007;24:1743–1759. doi: 10.1089/neu.2007.0366. [DOI] [PubMed] [Google Scholar]
- 183.Frazier SB, Roodhouse KA, Hourcade DE. The quantification of glycosaminoglycans: a comparison of HPLC, carbazole, and alcian blue methods. Open Glycosci. 2008;1:31–39. doi: 10.2174/1875398100801010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mowry HW, Winkler CH. The coloration of acidic carbohydrates of bacteria and fungi in tissue sections, with special reference to capsules of cryptococeus neoformans, pneumococci, and staphylococci. Am J Path. 1956;32:628–629. [Google Scholar]
- 185.Runoe H, Ebner H, Lindenschmiot W. Vorzuge der kombinierten Alcian b)au PerjodsFure-Sehiff-Reaktion fur die gynh-kologisehe Histopathologie. Deutsche Med Wchnsehr. 1956;81:1525–1529. [PubMed] [Google Scholar]
- 186.Ruijter J, Ru MH, Wagemans T, Ijlst L, Lund AM, Orchand PJ, Schaefer GB, Wijburg FA, van Vlies N. Heparan sulfate and dermatan sulfate derived disaccharides are sensitive markers for newborn screening for mucopolysaccharidoses type I, II and III. MGM. 2012;107:705–710. doi: 10.1016/j.ymgme.2012.09.024. [DOI] [PubMed] [Google Scholar]
- 187.Berman ER, Vered J, Bach G. A reliable spot test for mucopolysaccharidoses. Clin Chem. 1971;17:886–890. [PubMed] [Google Scholar]
- 188.Tomatsu S, Montano AM, Oguma T, Dung VC, Oikawa H, de Carvalho TG, Gutierrez ML, Yamaguchi S, Suzuki Y, Fukushi M, Kida K, Kubota M, Barrera L, Orii T. Validation of keratan sulfate level in mucopolysaccharidosis type IVA by liquid chromatography-tandem mass spectrometry. JIMD. 2010;33:35–42. doi: 10.1007/s10545-009-9013-x. [DOI] [PubMed] [Google Scholar]


