ABSTRACT
Predictors of weight loss responses are not well-defined. We hypothesized that adipose tissue phenotypic features related to remodeling would be associated with bariatric surgery weight loss responses. Visceral and subcutaneous adipose tissues collected from patients during bariatric surgery were studied with flow cytometry, immunohistochemistry, and QRTPCR, and results correlated with weight loss outcomes. Age, male sex, and a diagnosis of type 2 diabetes were associated with less weight loss. Adipocyte size was increased and preadipocyte frequency was decreased in visceral adipose tissue from diabetic subjects. Decreased adipose tissue preadipocyte frequency was associated with less weight loss in women but not men. These data suggest that phenotypic features of adipose tissue remodeling may predict responses to weight loss interventions.
KEYWORDS: adipose tissue, bariatric surgery, diabetes, hypertrophy, preadipocyte, weight loss
Introduction
Bariatric surgery is the most effective treatment of obesity but results are variable, with up to 35% of patients achieving suboptimal weight loss.1,2 Identification of mechanisms underlying this variability and accurate predictors of outcome are critical unmet needs. A diagnosis of type 2 diabetes (DM) is associated with suboptimal surgery-induced weight loss,3-5 but the mechanistic basis of this relationship is not understood. Weight loss involves extensive adipose tissue remodeling, implicating mechanisms underlying adipose tissue plasticity. Adipose tissue homeostasis is regulated by the balance between adipocyte hypertrophy and the size of the preadipocyte pool, which may be disrupted in DM.6 The goal of this study was to determine if adipose tissue phenotypic features associated with remodeling identified before bariatric surgery correlate with post-surgical weight loss. Identification of such predictors would aid in patient selection and identify molecular and cellular targets for research directed toward development of novel weight loss therapies. We performed a longitudinal cohort study in bariatric surgery patients to assess the hypothesis that pre-surgical features of impaired adipose tissue remodeling capacity (DM status, increased adipocyte hypertrophy, decreased preadipocyte content) correlate with decreased surgery-induced weight loss.
Results
DM status, age, and male sex correlate with less surgery-induced weight loss
Ninety-five subjects were recruited. To define clinical characteristics associated with weight loss, we stratified patients into diabetic (DM), pre-diabetic (PRE), and non-diabetic (NDM) subgroups. These group differed significantly with respect to age, HbA1c, and comorbidity prevalence, but were similar with respect to sex and pre-surgical BMI (Table 1). Adjusting for age, sex, and operation, %TWL at 6 and 12 months was less in DM subjects (Fig. 1A). %TWL 6 and 12 months after surgery in the entire cohort ranged from 8–39% and 8–55% respectively (Fig. 1B). Linear regression analysis revealed inverse correlations between %TWL and HbA1c, and between %TWL and age (Fig. 1C, D); no correlation was observed between %TWL and pre-surgical BMI (data not shown). Adjusting for age, HbA1c, and operation, 12-month %TWL was greater in women (Fig. 1E). No difference was observed in age-adjusted HbA1c between men and women (data not shown). These data identify DM status, age, and male sex as predictors of less surgery-induced weight loss.
Table 1.
Subject demographics.
| n = 95 | DM (n = 37) | PRE (n = 26) | NDM (n = 32) | p-value |
|---|---|---|---|---|
| DEMOGRAPHICS, LAB VALUES | ||||
| Gender (F/M, n) | 22/15 | 18/8 | 26/6 | 0.146 |
| Age (mean, years) | 49 | 48 | 38 | <0.001 |
| BMI (mean, kg/m2) | 46 | 48 | 46 | 0.473 |
| HbA1c (mean, %) | 7.2 | 6.0 | 5.4 | <0.001 |
| Fasting plasma glucose (mean, mg/dl) | 146 | 104 | 92 | <0.001 |
| CO-MORBID DISEASES (%) | ||||
| Sleep apnea | 78% | 58% | 41% | 0.003 |
| Hypertension | 81% | 69% | 31% | <0.001 |
| Dyslipidemia | 57% | 50% | 25% | 0.018 |
| MEDICATIONS (%) | ||||
| β-blocker | 27% | 27% | 9% | 0.138 |
| Statin | 51% | 30% | 6% | <0.001 |
| ACE inhibitor | 30% | 23% | 9% | 0.113 |
| Thiazolidinedione | 5% | 0% | 0% | 0.157 |
| Metformin | 84% | 0% | 0% | <0.001 |
| Insulin | 46% | 0% | 0% | <0.001 |
| Sulfonylurea | 27% | 0% | 0% | <0.001 |
Figure 1.
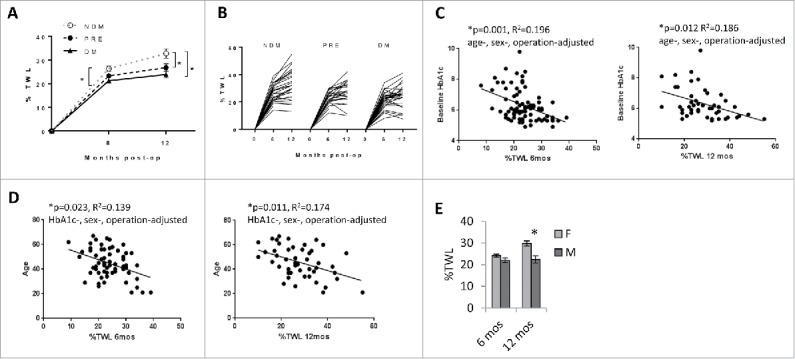
Clinical correlates of surgery-induced weight loss: A. Weight loss is less in DM subjects: %TWL stratified by DM status; *p<0.05 comparing indicated data points at 12 months (PRE or DM) to NDM arm, age-, sex-, operation-adjusted. n = 87 and 83 subjects for 6-month and 12-month %TWL respectively. Error bars represent standard error of mean. B. Range of surgery-induced weight loss in the entire cohort: %TWL 6 and 12 months after bariatric surgery in NDM, PRE, and DM subjects. C. Weight loss correlates inversely with HbA1c: Correlations in all subjects of %TWL with serum HbA1c levels; p-values shown are age-, sex-, operation-adjusted. D. Weight loss correlates inversely with age: Correlations in all subjects of %TWL with age; p-values shown are HbA1c-, sex-, operation-adjusted. E. Weight loss is less in men: %TWL in all subjects stratified by sex; *p = 0.013, age-, HbA1c-, operation-adjusted. n = 25, 24 men and 62, 59 women for 6-month and 12-month %TWL respectively. Error bars represent standard error of mean.
VAT adipocyte size and preadipocyte frequency correlate with DM
To define the relationship of adipocyte size and preadipocyte content with weight loss, we analyzed VAT and SAT with histology and flow cytometry (Fig. 2A, B). Adipocyte size and preadipocyte frequency correlated inversely in VAT and SAT, suggesting a reciprocal relationship between adipocyte hypertrophy and the capacity to generate new adipocytes (Fig. 2C). Adjusting for age and sex, preadipocyte content was decreased and adipocyte size was increased in VAT but not SAT in DM relative to NDM subjects, and in PRE relative to NDM subjects (Fig. 2D, E). No sex differences were observed in preadipocyte frequency in VAT or SAT (Fig. 2F), but greater VAT adipocyte size in men compared with women approached significance (Fig. 2G). Linear regression revealed that HbA1c correlated directly with adipocyte size and inversely with preadipocyte frequency in VAT but not SAT. A direct correlation of adipocyte size with age approached significance in VAT but not SAT; preadipocyte frequency did not correlate with age in either depot (Fig. 2H).
Figure 2.
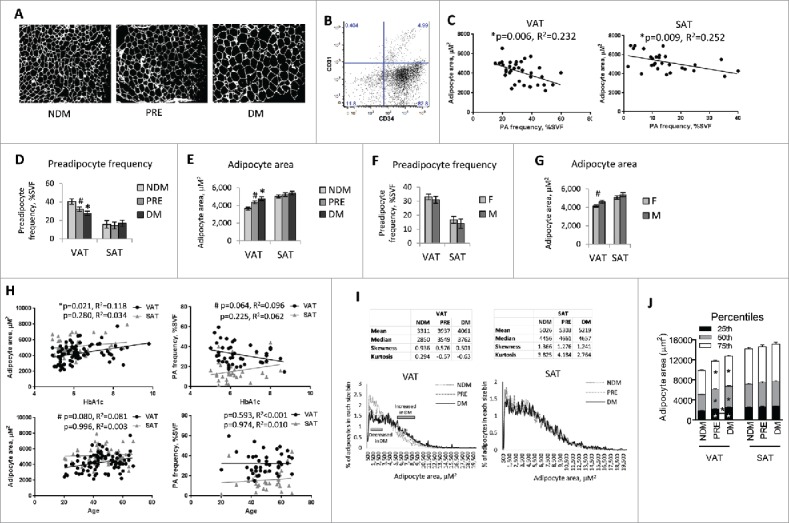
Adipose tissue-based correlates of DM: A. Histologic assessment of adipocyte hypertrophy: Representative fluorescence images in Texas Red channel (595–605 nM) of fixed H&E-stained sectioned adipose tissue used for Image-J reconstruction from NDM, PRE, and DM subjects; adipocyte area measured in 200–500 cells from multiple slides per subject. B. Preadipocyte flow cytometry scatterplot: CD34/CD31 staining within the parent CD45 gate. Preadipocytes are defined as CD45−CD34+CD31− using fluorescence-minus-one controls, quantified as % of all SVF cells after exclusion of doublets and non-viable cells. Antibodies: CD45-FITC, CD31-APC-Cy7, CD34-PerCP-Cy5.5, anti-rabbit-IgG secondary antibody-PE (Biolegend Inc., San Diego, CA, USA). C. Adipocyte hypertrophy and preadipocyte frequency are reciprocally related: Correlations in all subjects of adipocyte area (μM2) with preadipocyte frequency (% all SVF cells); p-values shown are age-, sex-adjusted. D. Preadipocyte frequency is decreased in VAT from DM subjects: Ordinate: preadipocyte frequency as % SVF cells; *: p = 0.004, #:p = 0.051, comparing indicated data point (PRE or DM) to NDM arm, age-, sex-adjusted; n = 11 NDM, 14 PRE, 17 DM subjects for VAT, n = 9 NDM, 9 PRE, 12 DM subjects for SAT. Error bars represent standard error of mean. E. Adipocyte hypertrophy is increased in VAT from DM subjects: Ordinate: adipocyte area (μM2); *: p = 0.002, #: p = 0.102, comparing indicated data point (PRE or DM) to NDM arm, age-, sex-adjusted; n = 31 NDM, 24 PRE, 34 DM subjects for VAT, n = 22 NDM, 17 PRE, 17 DM subjects for SAT. Error bars represent standard error of mean. F. Preadipocyte frequency is similar in men and women: Ordinate: preadipocyte frequency as % SVF cells; no differences observed between men (M) and women (F) with age-, HbA1c-adjustment; n = 17 male, 25 female subjects for VAT, 11 men, 19 women for SAT. Error bars represent standard error of mean. G. Adipocyte hypertrophy is increased in VAT from male subjects: Ordinate; adipocyte area (μM2); #: p = 0.066, comparing men (M) and women (F), age-, HbA1c-adjusted; n = 26 men, 60 women for VAT, 22 men, 33 women for SAT. Error bars represent standard error of mean. H. Correlations of adipocyte size and preadipocyte frequency with HbA1c and age: Correlations in all subjects of adipocyte size (μM2) and preadipocyte frequency (% all SVF cells) with HbA1c and age; p-values shown are adjusted for age and sex when analyzing HbA1c as the dependent variable, and for HbA1c and sex when analyzing age as the dependent variable. I. Adipocyte size distribution: Top: Tables show average mean, median, skewness, and kurtosis for each patient group for adipocyte size data. Bottom: Each line/curve represents counts of adipocyte areas of 200–500 cells for an individual subject; abscissas: adipocyte area (μM2), ordinates: mean % adipocytes in each 100 μM2 increment of all patients in each group (NDM, PRE, DM); gray bars on VAT graph show approximate ranges of adipocytes sizes for which differences in % adipocytes at each 100μM2 increment between DM and NDM subjects. J. Percentile analysis of adipocyte sizes: Stacked graph showing adipocyte size for each patient group at 25th, 50th, and 75th percentiles. Asterisks on bars indicate significant differences for that percentile and group compared with NDM; the single asterisk between bars (VAT, PRE vs. DM) indicates a significant difference between the 2 patient groups at the indicated percentile. Error bars represent standard error of mean.
To further explore the nature of adipocyte hypertrophy associated with DM, we sought to determine if subpopulations of large or small adipocytes correlate disproportionately with DM. We compared adipocyte size distribution between subject groups by mapping the frequency of adipocytes in each 100 μM2 size increment averaged over all patients in each group (NDM, PRE, DM). Compared to NDM subjects, DM and PRE subjects manifested decreased smaller adipocytes (approximately 700–3,300 μM2), along with an increase in larger adipocytes (approximately 5,600–8,700 μM2); these differences were observed in VAT but not SAT (Fig. 2I). For VAT, PRE mean, median, skewness, and kurtosis values for frequency distributions of adipocyte size were consistently between NDM and DM. We also compared adipocyte sizes at 25th, median, and 75th percentiles across groups. DM and PRE subjects had significantly increased size at all percentiles compared with NDM, and at the 25th percentile, DM adipocyte size was also significantly higher than PRE (Fig. 2J). No differences were found for SAT. This analysis of frequency distributions supports fewer small adipocytes and increased larger adipocytes in DM VAT.
Together these observations suggest that an imbalance between adipocyte hypertrophy and preadipocyte hyperplasia contributes to DM pathogenesis in obesity.
Preadipocyte frequency correlates with weight loss
To evaluate the hypothesis that adipocyte hypertrophy-hyperplasia balance contributes to weight loss, we studied correlations of adipocyte size and preadipocyte frequency with surgery-induced weight loss (Fig. 3). Linear regression analysis of all subjects revealed an inverse correlation between 12 month-%TWL and adipocyte size in VAT but not SAT, which remained significant when adjusting for age, and approached significance adjusting for age, HbA1c, and operation. A direct correlation between 12 month-%TWL and VAT preadipocyte frequency approached significance when adjusting for age. Analysis of female subjects revealed a trend toward an inverse correlation between 12 month-%TWL and adipocyte size in VAT but not SAT on univariate analysis that lost significance when adjusting for age, HbA1c, and operation. We also observed direct correlations in female subjects between 12 month-%TWL and preadipocyte frequency that were more robust in SAT than VAT. No correlations were observed between 12 month-%TWL and adipocyte size or preadipocyte frequency in men, or between 6 month-%TWL and adipocyte size or preadipocyte frequency in any patient group (data not shown). These data demonstrate that preadipocyte frequency is associated with weight loss independent of age and diabetes status in women but not men.
Figure 3.
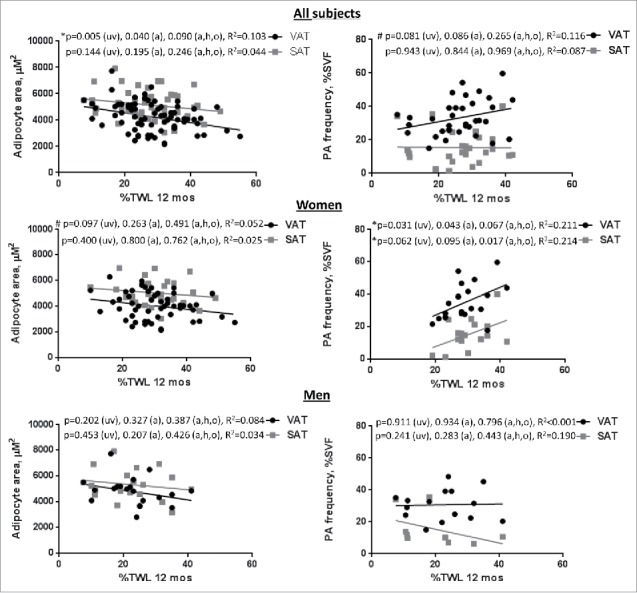
Adipose tissue-based correlates of surgery-induced weight loss: Correlations in all subjects of adipocyte area (μM2) and preadipocyte (PA) frequency (% all SVF cells) with %TWL at 12 months in all subjects and in female and male subgroups; p-values shown are univariate (uv), age-adjusted (a), and age-, HbA1c-, operation-adjusted (a,h,o).
Discussion
We observed that DM status, age, and male sex were negative predictors of surgery-induced weight loss, consistent with prior data.3-5 DM subjects are older in our cohort, and age and male sex correlated inversely with weight loss independent of HbA1c, suggesting that age and sex contribute to weight loss via DM-independent mechanisms.
Adipose tissue-based correlates of metabolic disease and therapeutic responses are poorly defined. Our data add to sparse literature suggesting that adipose tissue phenotypic features regulate weight loss. We observed a correlation between pre-surgical preadipocyte frequency and surgery-induced weight loss, suggesting that defects in this important component of adipose tissue remodeling contribute to suboptimal weight loss. This relationship was observed only in women, suggesting different sex-specific mechanisms of tissue remodeling. We observed modest correlations between adipocyte size and weight loss only in VAT. Adipocyte hypertrophy has been linked to greater reductions in insulin resistance with diet-induced weight loss.7 In contrast, a separate study correlated increased adipocyte hypertrophy with lesser reductions in insulin resistance after gastric bypass,8 suggesting that qualitatively different relationships may exist between hypertrophy and non-surgical and surgical weight loss. Few published data correlate changes in weight with preadipocyte frequency. Induction of obesity with high fat diet in mice is associated with reduced preadipocyte frequency,9 while the replicative capacity in human SAT preadipocytes has been shown to be increased after surgery- and diet-induced weight loss compared with weight-stable patients.10 These observations combined with our results suggest that the preadipocyte pool may regulate adipose tissue responses to weight loss interventions, with increased preadipocytes being associated with greater weight loss. Further research will be necessary to elucidate mechanisms underlying these observations.
We also demonstrate associations between adipose tissue hypertrophy and DM status independent of weight loss. Mouse studies demonstrate that very large adipocytes have altered gene expression and protein content, and are more insulin resistant than smaller adipocytes.11,12 Moreover, consistent with our findings here and in previously published work6 others have shown that larger adipocytes are associated with greater insulin resistance in humans.7,13,14 Of interest, we found that adipocyte hypertrophy in DM affects specific subpopulations of adipocytes based on size, with decreased adipocytes ∼700–3,300 μM2 and increased adipocytes ∼5,600–8,700 μM.2 Others have shown that greater improvements in insulin sensitivity are associated with a reduction of large and very large adipocyte subfractions in DM patients after caloric restriction and exercise-induced weight loss.15 Together these observations raise the intriguing possibility that cells in these size ranges may be metabolically protective and pathogenic respectively. Further research focused on these specific adipocyte subpopulations will be necessary to confirm this hypothesis.
Our finding of an association between DM and decreased preadipocyte frequency are consistent with previous studies,14,16,17 and confirm prior data published by our group in a smaller cohort that constitute a subset of the data in the present manuscript.6 Decreased preadipocyte frequency and proliferative capacity is associated with obesity and metabolic disease in some,6,14,16-18 but not all19 studies. These conflicting literature may result from patient heterogeneity, wide intrinsic variability in human preadipocyte frequency,19,20 and different techniques used to quantify preadipocytes. One study demonstrated a relationship between decreased preadipocyte number and DM restricted to VAT,16 similar to our findings and consistent with the well-established stronger association of metabolic disease with VAT.21
Our cohort is underpowered to control for all potential confounders or study DM remission, issues that will require much larger studies to address. Limitations in available tissue precluded analysis of large numbers of SAT samples. Other adipose tissue functions regulate remodeling, including fibrosis,22 inflammation, and metabolism, targets for future research. Nonetheless, our data support the concept that an imbalance between adipocyte hypertrophy and preadipocyte hyperplasia contributes to DM pathogenesis and surgery-induced weight loss. Furthermore, our findings underscore the importance of sex differences in these effects, and suggest preadipocytes as a target for study of mechanisms underlying response to weight loss interventions.
Patients, methods, materials
Visceral (VAT, greater omentum) and subcutaneous adipose tissue (SAT, abdominal wall) were collected from bariatric surgery patients enrolled with Institutional Review Board approval at University of Michigan and Ann Arbor Veteran's Administration Hospital. Sleeve gastrectomy and gastric bypass comprised 95% and 5% of operations respectively. Per ADA criteria,23 DM subjects were defined by clinical diagnosis requiring medication and HbA1c> = 6.5; PRE subjects had no clinical history of DM, no DM-related medication use, and HbA1c 5.7–6.4; NDM subjects had no clinical history of DM, no DM-related medication use, and HbA1c<5.7. Weight loss data was collected 6 and 12 months after surgery and defined as percent total weight loss from pre-surgical weight (%TWL), with 93% and 88% capture respectively.
Adipocyte sizing was performed as described.6 Briefly, fixed hematoxylin/eosin-stained slides from formalin-fixed, paraffin-embedded, sectioned tissue were imaged on an Olympus IX-81 fluorescent microscope, captured as multiple TIFF-gray-scale images and analyzed with ImageJ software. Pixel areas of all individual cells were averaged for each patient.
Preadipocyte content was quantified by flow cytometry in adipose tissue and expressed as % live cells in the stromovascular cell fraction (SVF) as described.6 Briefly, adipose tissue was digested with Type II collagenase (175 units/ml PBS/2% BSA, Life Technologies Inc., Carlsbad, CA, USA) 60 minutes, 37°C, centrifuged, and the SVF cell pellet isolated and used for flow cytometry analysis. SVF cells were stained with viable dye and antibodies and analyzed on a FACSCanto II flow cytometer (Becton-Dickinson Inc., Franklin Lakes, NJ, USA). Data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR, USA) after exclusion of doublets and non-viable cells using fluorescence-minus-one controls with forward scatter/side scatter gates encompassing all cells with subsequent analysis of CD45- cells. Preadipocytes were defined as CD45-CD34+CD31-. Antibodies: CD45-FITC, CD31-APC-Cy7, CD34-PERCP-Cy5.5 (Biolegend Inc., San Diego, CA, USA), anti-rabbit secondary antibody-PE (Life Technologies, Inc., Carlsbad, CA, USA); CD140a-AlexaFluor 647 (BD Biosciences, Inc., San Jose, CA, USA).
One-way ANOVA was used to compare demographic continuous variables (age, BMI, HbA1c, fasting plasma glucose), and Chi-square test was used to compare dichotomous demographic variables (sex, metabolic disease prevalence, medication use) between DM, PRE, and NDM groups (Table 1). Multiple linear regression was used to determine correlations between dependent variables (%TWL, adipocyte area, preadipocyte frequency) and independent variables (DM status, sex, HbA1c) while adjusting for covariates (age, HbA1c, sex, operation) (Figs. 1, 2C-H). One-way ANOVA with Holm-Sidak correction for multiple comparisons was used to compare adipocyte size percentile data among NDM, PRE, and DM groups (Fig. 2J).
Abbreviations
- BMI
body mass index
- DM
diabetic
- HbA1c
hemoglobin A1c
- NDM
non-diabetic
- PRE
pre-diabetic
- QRTPCR
quantitative real-time polymerase chain reaction
- SAT
subcutaneous adipose tissue
- SVF
stromal-vascular cell fraction
- %TWL
percent total weight loss
- VAT
visceral adipose tissue
Disclosure of potential conflicts of interest
The authors report no conflict of interest.
Acknowledgments
We thank the University of Michigan Center for Statistical Consultation and Research for assistance with statistical analysis, and Colleen Buda, Justin Fahey, Danielle Guerin, Kendra Rogers, and Marilyn Woodruff for assistance with study coordination.
Funding
This work was supported by NIH grants R01DK097449 (RWO), R01DK090262 (CNL), T32DK101357 (LAM), F32DK105676 (LAM), K08DK101755 (KS), and Michigan Institute for Clinical & Health Research T1 Bench to Bedside Translation Pilot Grant 2UL1TR000433 (RWO and CNL).
References
- [1].Brethauer SA, Aminian A, Romero-Talamas H, Batayyah E, Mackey J, Kennedy L, Kashyap SR, Kirwan JP, Rogula T, Kroh M, et al.. Can diabetes be surgically cured? Long-term metabolic effects of bariatric surgery in obese patients with type 2 diabetes mellitus. Ann Surg. 2013; 258(4):628-36; PMID: 24018646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, Horlick M, Kalarchian MA, King WC, Mitchell JE, et al.. Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA 2013; 310(22):2416-2425; PMID: 24189773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Agüera Z, García-Ruiz-de-Gordejuela A, Vilarrasa N, Sanchez I, Baño M, Camacho L, Granero R, Jiménez-Murcia S, Virgili N, Lopez-Urdiales R, et al.. Psychological and personality predictors of weight loss and comorbid metabolic changes after bariatric surgery. Eur Eat Disord Rev 2015; 23(6):509-16; PMID: 26377595; https://doi.org/ 10.1002/erv.2404 [DOI] [PubMed] [Google Scholar]
- [4].Jurowich C, Thalheimer A, Hartmann D, Bender G, Seyfried F, Germer CT, Wichelmann C. Improvement of type 2 diabetes mellitus (T2DM) after bariatric surgery–who fails in the early postoperative course? Obes Surg 2012; 22(10):1521-6; PMID: 22588846; https://doi.org/ 10.1007/s11695-012-0676-2 [DOI] [PubMed] [Google Scholar]
- [5].Ma Y, Pagoto SL, Olendzki BC, Hafner AR, Perugini RA, Mason R, Kelly JJ. Predictors of weight status following laparoscopic gastric bypass. Obes Surg 2006; 16(9):1227-31; PMID: 16989709; https://doi.org/ 10.1381/096089206778392284 [DOI] [PubMed] [Google Scholar]
- [6].Muir LA, Neeley CK, Meyer KA, Baker NA, Brosius AM, Washabaugh AR, Varban OA, Finks JF, Zamarron BF, Flesher CG, et al.. Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity (Silver Spring) 2016; 24(3):597-605; PMID: 26916240; https://doi.org/ 10.1002/oby.21377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Eriksson-Hogling D, Andersson DP, Bäckdahl J, Hoffstedt J, Rössner S, Thorell A, Arner E, Arner P, Rydén M. Adipose tissue morphology predicts improved insulin sensitivity following moderate or pronounced weight loss. Int J Obes (Lond) 2015; 39(6):893-8; PMID: 25666530; https://doi.org/ 10.1038/ijo.2015.18 [DOI] [PubMed] [Google Scholar]
- [8].Cotillard A, Poitou C, Torcivia A, Bouillot JL, Dietrich A, Klöting N, Grégoire C, Lolmede K, Blüher M, Clément K. Adipocyte size threshold matters: link with risk of type 2 diabetes and improved insulin resistance after gastric bypass. J Clin Endocrinol Metab 2014; 99(8):E1466-70; PMID: 24780048; https://doi.org/ 10.1210/jc.2014-1074 [DOI] [PubMed] [Google Scholar]
- [9].Pincu Y, Huntsman HD, Zou K, De Lisio M, Mahmassani ZS, Munroe MR, Garg K, Jensenb T, Boppart MD. Diet-induced obesity regulates adipose-resident stromal cell quantity and extracellular matrix gene expression. Stem Cell Research 2016; 17: 181-190; PMID: 27399175; https://doi.org/ 10.1016/j.scr.2016.07.002 [DOI] [PubMed] [Google Scholar]
- [10].Mitterberger MC, Mattesich M, Zwerschke W. Bariatric surgery and diet-induced long-term caloric restriction protect subcutaneous adipose-derived stromal/progenitor cells and prolong their life span in formerly obese humans. Exp Gerontol 2014; 56:106-13; PMID: 24747059; https://doi.org/ 10.1016/j.exger.2014.03.030 [DOI] [PubMed] [Google Scholar]
- [11].Blüher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell 2002; 3(1):25-38; PMID: 12110165; https://doi.org/ 10.1016/S1534-5807(02)00199-5 [DOI] [PubMed] [Google Scholar]
- [12].Blüher M, Patti ME, Gesta S, Kahn BB, Kahn CR. Intrinsic heterogeneity in adipose tissue of fat-specific insulin receptor knock-out mice is associated with differences in patterns of gene expression. J Biol Chem 2004; 279(30):31891-901; PMID: 15131119; https://doi.org/ 10.1074/jbc.M404569200 [DOI] [PubMed] [Google Scholar]
- [13].Landgraf K, Rockstroh D, Wagner IV, Weise S, Tauscher R, Schwartze JT, Löffler D, Bühligen U, Wojan M, Till H, et al.. Evidence of early alterations in adipose tissue biology and function and its association with obesity-related inflammation and insulin resistance in children. Diabetes 2014; 64(4):1249-61; PMID: 25392242; https://doi.org/ 10.2337/db14-0744 [DOI] [PubMed] [Google Scholar]
- [14].Arner E, Westermark PO, Spalding KL, Britton T, Ryden M, Frisen J, Bernard S, Arner P. Adipocyte turnover: relevance to human adipose tissue morphology. Diabetes 2010; 59(1):105-109; PMID: 19846802; https://doi.org/ 10.2337/db09-0942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pasarica M, Tchoukalova YD, Heilbronn LK, Fang X, Albu JB, Kelley DE, Smith SR, Group Ravussin E; Look AHEAD Adipose Research. Differential effect of weight loss on adipocyte size subfractions in patients with type 2 diabetes. Obesity (Silver Spring) 2009; 17(10):1976-8; PMID: 19629054; https://doi.org/ 10.1038/oby.2009.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bakker AH, Nijhuis J, Buurman WA, van Dielen FM, Greve JW. Low number of omental preadipocytes with high leptin and low adiponectin secretion is associated with high fasting plasma glucose levels in obese subjects. Diabetes Obes Metab 2006; 8(5):585-8; PMID: 16918595; https://doi.org/ 10.1111/j.1463-1326.2006.00558.x [DOI] [PubMed] [Google Scholar]
- [17].Kim SM, Lun M, Wang M, Senyo SE, Guillermier C, Patwari P, Steinhauser ML. Loss of white adipose hyperplastic potential is associated with enhanced susceptibility to insulin resistance. Cell Metab 2014; 20(6):1049-58; PMID: 25456741; https://doi.org/ 10.1016/j.cmet.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Maumus M, Sengenès C, Decaunes P, Zakaroff-Girard A, Bourlier V, Lafontan M, Galitzky J, Bouloumié A. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: influence of fat mass microenvironment and growth. J Clin Endocrinol Metab 2008; 93(10):4098-106; PMID: 18682517; https://doi.org/ 10.1210/jc.2008-0044 [DOI] [PubMed] [Google Scholar]
- [19].Bakker AH, Van Dielen FM, Greve JW, Adam JA, Buurman WA. Preadipocyte number in omental and subcutaneous adipose tissue of obese individuals. Obes Res 2004; 12(3):488-98; PMID: 15044666; https://doi.org/ 10.1038/oby.2004.55 [DOI] [PubMed] [Google Scholar]
- [20].Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci USA 2010; 107(42):18226-31; PMID: 20921416; https://doi.org/ 10.1073/pnas.1005259107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kissebah AH, Vydelingum N, Murray R, Evans DJ, Hartz AJ, Kalkhoff RK, Grégoire C, Lolmede K, Blüher M, Clément K. Relation of body fat distribution to metabolic complications of obesity. J Clin Endocrinol Metab 1982; 54(2):254-60; PMID: 7033275; https://doi.org/ 10.1210/jcem-54-2-254 [DOI] [PubMed] [Google Scholar]
- [22].Divoux A, Tordjman J, Lacasa D, Veyrie N, Hugol D, Aissat A, Basdevant A, Guerre-Millo M, Poitou C, Zucker JD, et al.. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 2010; 59(11):2817-2825; PMID: 20713683; https://doi.org/ 10.2337/db10-0585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chamberlain JJ, Rhinehart AS, Jr Shaefer CF, Neuman A. Diagnosis and Management of Diabetes: Synopsis of the 2016 American Diabetes Association Standards of Medical Care in Diabetes. Ann Intern Med 2016; 164(8):542-52; PMID: 26928912; https://doi.org/ 10.7326/M15-3016 [DOI] [PubMed] [Google Scholar]


