ABSTRACT
Alzheimer disease is the most prevalent neurodegenerative disease and is associated with aggregation of Amyloid-β42 peptides. In mammals, Amyloid-β42 causes impaired neural stem/progenitor cell (NSPC) proliferation and neurogenesis, which exacerbate with aging. The molecular programs necessary to enhance NSPC proliferation and neurogenesis in our brains to mount successful regeneration are largely unknown. Therefore, to identify the molecular basis of effective brain regeneration, we previously established an Amyloid-β42 model in adult zebrafish that displayed Alzheimer-like phenotypes reminiscent of humans. Interestingly, zebrafish exhibited enhanced NSPC proliferation and neurogenesis after microinjection of Amyloid-β42 peptide. Here, we compare old and young fish to address the effects of aging on regenerative ability after Amyloid-β42 deposition. We found that aging does not affect the rate of NSPC proliferation but reduces the neurogenic response and microglia/macrophage activation after microinjection of Amyloid-β42 in zebrafish, suggesting an important link between aging, neuroinflammation, regenerative neurogenesis and neural stem cell plasticity.
KEYWORDS: Alzheimer disease, amyloid-β 42, Aβ42, aging, inflammation, neurodegeneration, neural stem progenitor cell, neurogenesis, regeneration, microglia, zebrafish
Neurodegenerative diseases entail gradual accumulation of toxic protein aggregates, which impair the physiological functions of neurons including the synaptic transmission, and eventually lead to neuronal loss.1-3 These diseases are age-related, as an extended time period must elapse for the toxicity to manifest. Alzheimer disease (AD) is the most prevalent neurodegenerative disease, and the main pathological culprit is accumulation of short Amyloid-β42 (Aβ42) peptides, the insoluble cleavage product of Amyloid Precursor Protein (APP).4,5 Aβ42 is a self-aggregating peptide that forms toxic β-sheet structures as fibrils and plaques, which are the major pathophysiological hallmarks of the disease.4,6 Although it is debated whether the extracellular Aβ42 plaque load correlates well with the disease progression or whether intracellular Aβ42 aggregates causes the main toxicity in a pre-onset stage of AD,7-9 the role of Aβ42 aggregation in imposing a toxicity to the neurons is well established.10
The main cellular symptoms of AD are loss of synapses, neuronal loss, inflammation through microglial activation and learning deficits.10,11 Additionally, in humans and mammalian animal models of AD, in late stage of disease, where Aβ42 burden is pronounced, neural stem/progenitor cells (NSPCs) decline in their proliferative ability, reduce their neurogenic capacity, and therefore cannot contribute to production of new neurons, which are required to restore the function of the lost neurons.12-16 Furthermore, the existing neurons lose their synaptic connections and die; or a meager amount of newborn neurons in response to disease state cannot survive and integrate into the circuitry.12,13,17 Taken together with reduced stem cell proliferation and hampered neurogenesis, inability of neurons to survive exacerbates the AD pathology and speeds up the disease progression. Therefore, a plausible regenerative therapeutic approach for AD is to increase the proliferation of NSPCs and enhance the survival and integration capacity of newborn neurons in response to Aβ42 toxicity.18
Aging is known to alter the proliferative ability and neurogenic capacity of NSPCs, and the success of a “regenerative response” may vary with age.19-21 Therefore, animal models with regenerative ability would be extremely helpful in addressing whether a successful regenerative response could be mounted after Aβ42 toxicity, how this ability would change with aging, and what should we learn from these animals to design meaningful regenerative therapies in human brains. For instance zebrafish is an excellent model organism for nervous system regeneration owing to their extensive regenerative capacity as adults.22-27 Therefore, by taking the advantage of the regenerative ability of zebrafish, we previously generated an Aβ42 toxicity model in adult zebrafish brain.28 In this model, Aβ42 causes cellular pathological phenotypes that are quite similar to human brains, namely the loss of synaptic connections, neuronal death, elevated immune response, and learning deficits. Interestingly, and in contrast to human brains and rodent models of AD, such a burden of Aβ42 led to increased NSPC proliferation and neurogenesis in adult zebrafish brain using an immune-related signaling through Interleukin-4 between the dying neurons and the stem cells.28 Our previous results therefore suggested a regenerative reaction of the zebrafish brain to Aβ42 toxicity in part by activating specific signaling pathways, and also provided a defined assay system where the effects of age on AD pathology and regenerative response could be assessed.
In mammalian brains, aging reduces the overall plasticity of the brain, including the NSPC proliferation and the neurogenic capacity.29,30 Thus, we aimed to investigate whether in old zebrafish brains, the regenerative response we see in young animals still prevails, and whether we could learn how zebrafish maintains its regenerative ability also in old ages. Such an understanding would be instrumental in designing regenerative therapies for human neurodegenerative diseases, which manifest the onset in elder individuals.
To address whether the Aβ42 pathology, NSPC proliferation and neurogenesis would manifest similarly in young and old zebrafish brains after Aβ42 toxicity, we injected control and TR-Aβ42 peptides28,31 into 1.5 year-old zebrafish, and analyzed the levels of cell death, microglial activation, synaptic degeneration, NSPC proliferation and neurogenesis (Figs. 1–3). Compared to controls (Fig. 1A, C, E, G, G’), TR-Aβ42 injection led to aggregation (Fig. 1B), elevated levels of TUNEL-positive nuclei indicative of cell death (Fig. 1D), microglial activation (Fig. 1F), and reduced levels of Synaptophysin staining indicating synaptic degeneration (Fig. 1H, H’). Quantification of these phenotypes (Fig. 1M–O) showed that the changes in cell death, activation of microglia and synaptic degeneration are statistically significant.
Figure 1.
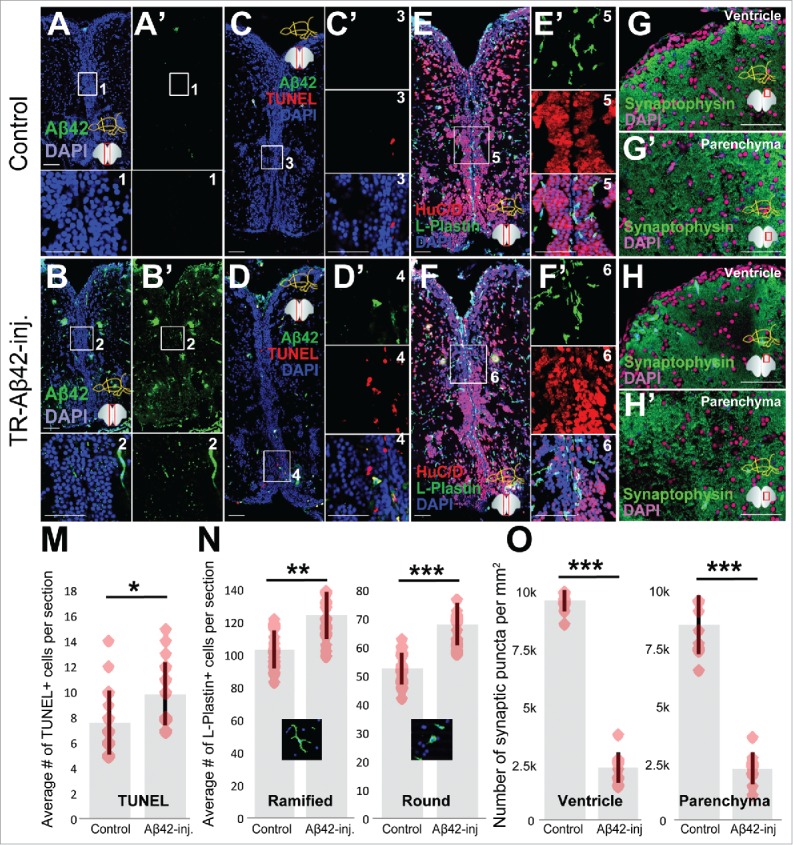
Aβ42 accumulation leads to neurodegeneration phenotypes in old adult zebrafish brain. Aβ42 immunostaining in control (A) and TR-Aβ42-injected brains (B). Insets are magnified images of the boxes. TUNEL detection of apoptotic cells in control (C) and TR-Aβ42-injected brains (D). C’ and D’ are single fluorescent channels of the boxes. Immunostaining for HuC/D (neurons, red) and L-Plastin (macrophages/microglia, green) in control (E) and TR-Aβ42-injected brains (F). E’ and F’ are single fluorescent channels of the boxes in corresponding panels. Synaptophysin immunostaining in ventricular region in control (G), parenchyma of control (G’), ventricular region of TR-Aβ42-injected (H) and parenchyma of TR-Aβ42-injected brain (H’). Quantification graphs for TUNEL (M), L-Plastin (N) and Synaptophysin (O). Data shown as mean ± s.e.m.Red dots are individual data points as an overlaid scatter plot. Representative morphologies of microglia are shown in N at each category. All stainings were performed at 3 d after injection. Scale bars equal 50 μm. n = 3 fish, and 18 sections in total for every staining.
Figure 2.
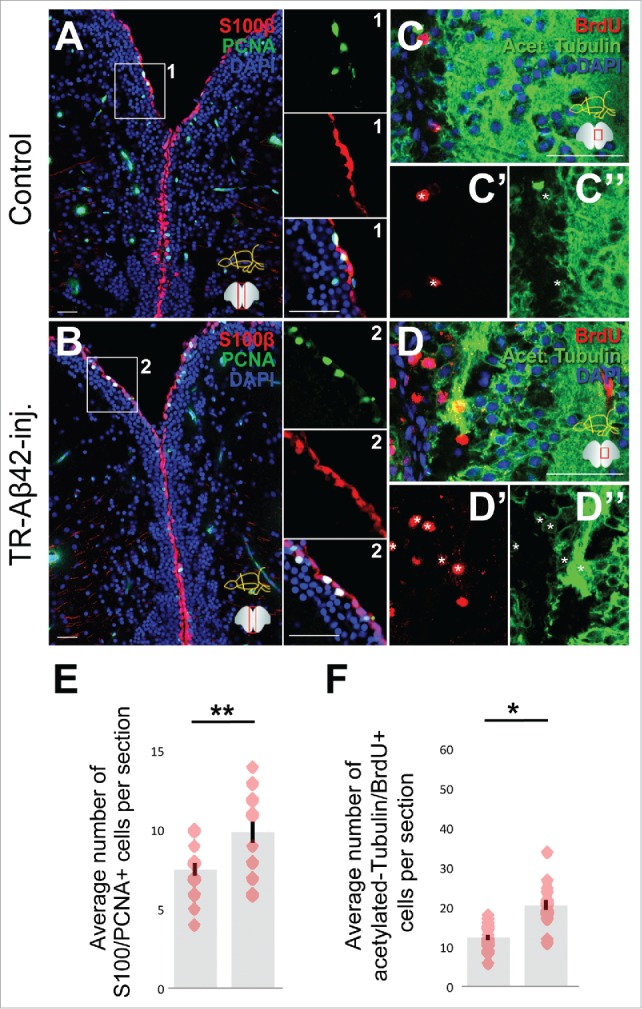
Aβ42 accumulation induces stem cell proliferaton and neurogenesis in old adult zebrafish brain. S100β (neural stem/progenitor cells, red) and PCNA (proliferation marker, green) immunostaining in control (A) and TR-Aβ42-injected brains (B) at 1 day after injection. Insets on the right of the panels are individual channels from the boxes in corresponding panels. Immunostaining for Acetylated tubulin (green, neurons) in control (C) and TR-Aβ42-injected brains (D) at 2 weeks after injection. Insets below the panels (C’, C,“ D’, D”) are individual fluorescent channels. Quantification graphs for S100-PCNA (E) and Acetylated-tubulin-BrdU (F). Data shown as mean ± s.e.m. Red dots are individual data points as an overlaid scatter plot. Scale bars equal 50 μm. n = 5 fish, and 18 sections in total for every staining.
Figure 3.
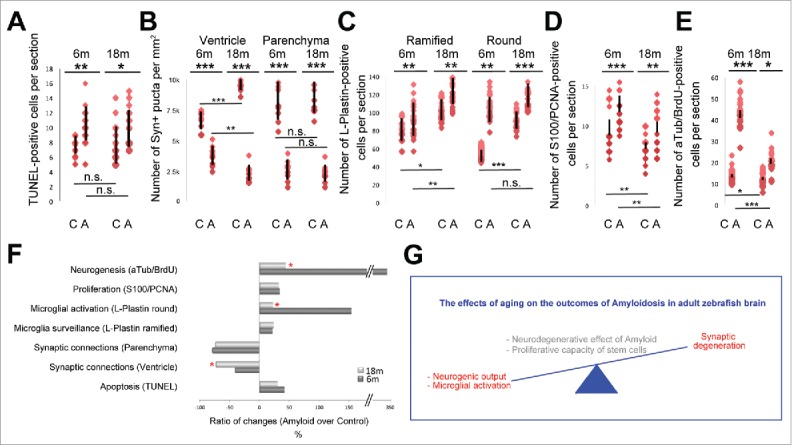
Statistical comparison of young and old zebrafish brains. Quantification of young and old adult zebrafish brains for TUNEL (A), Number of synaptophysin-positive synapses (B), number of L-Plastin positive microglia (C), number of S100/PCNA double positive proliferating glial cells (D), and number of BrdU-positive acetylated tubulin-positive newborn neurons. C: control, A: Amyloid injection. Red dots indicate data points, and black bars are standard deviations. (F) Quantification of the difference between control and Amyloid-injected brains as percentages. (G) Schematic representation of the effects of aging on Amyloidosis in adult zebrafish brain.
In young adult zebrafish brains (6 months of age), Aβ42 deposition led to increased proliferation of NSPCs and subsequent neurogenesis.28 To address whether or not the old fish brain can also respond to Aβ42 toxicity in a similar fashion, we performed immunohistochemical stainings to detect proliferation of glial cells (S100β-PCNA) at 1 day after injection of TR-Aβ42 (Fig. 2A, B), and neurogenesis assay to detect BrdU-incorporating newborn neurons at 2 weeks after TR-Aβ42 injection (Fig. 2C-D”). We found that the old fish brain can also respond to Aβ42 aggregation by enhancing the proliferation of NSPCs (Fig. 2E), and neurogenesis (Fig. 2F) in a statistically significant manner, indicating that zebrafish brain can manifest its regenerative ability regardless of the age.
Aging has been shown to exacerbate the neurodegeneration phenotypes and hamper the proliferative response of stem cells in various models.29,32 Therefore, although the Aβ42-induced phenotypes and the neurogenic response are qualitatively comparable in young and old fish, we hypothesized that the amplitude of the phenotypic effects or the rate of proliferation and neurogenesis could be at varying levels. To investigate whether Aβ42-induced phenotypes would substantiate in older zebrafish, we compared between the young28 and the old fish (this study) the relative changes in every parameter we measured (Fig. 3). For this, we plotted the data in young and old fish for cell death (Fig. 3A), synaptic density (Fig. 3B), number and activation state of immune cells (Fig. 3C), number of proliferating glial cells (Fig. 3D), and the number of newborn neurons (Fig. 3E) in control and Aβ42-injected animals, and quantified the percent changes in the above-mentioned parameters in young and old fish (Fig. 3F). We found that Aβ42 toxicity increases the cell death similarly in young and old fish brains (42.4 ± 18.8% vs 30.7 ± 13.3%, respectively) (Fig. 3F). While the synaptic density in the parenchyma reduces similarly (79.2 ± 11.1% vs. 74.6 ± 9.8%, young and old, respectively), the decline in the density of synapses in the ventricular regions is more pronounced in the old animals (43.2 ± 7.5% vs. 73.6 ± 8.7%) (Fig. 3F). While, Aβ42 increases the amount of ramified L-Plastin cells by 17.8 ± 11.6% in young and 20.4 ± 5.6% in old zebrafish brains), the increase in round L-Plastin cells, which marks the activated stage of microglia/macrophages, is significantly higher in young fish brains (152.4 ± 22.4%) compared with old fish brains (19.1 ± 3.2%) (Fig. 3F). We found that the increase in the proliferation of NSPCs is similar in young (41.6 ± 15.7%) and old zebrafish brains (38.9 ± 6.8%); however, the increase in the number of newborn neurons is significantly higher in young animals (346.1 ± 43.9%) compared with old fish brains (47.4 ± 14.2%) (Fig. 3F). These results show that compared with young animals, in old zebrafish brains Aβ42 toxicity causes synapses to degenerate at a higher level in the ventricular region, and young animals seem to activate macrophages and produce newborn neurons significantly more than the old animals, while the levels of increase in NSPC proliferation, therefore the proliferative capacity of the stem cells, remains independent of the age of the fish (Fig. 3G).
Our results point to a possible link between the microglial activation and synaptic degeneration and neurogenesis. Based on our observations, we hypothesize that activated microglia helps in zebrafish brain to limit the synaptic degeneration, and to promote neurogenesis after Aβ42-induced neurodegeneration. Microglial activity was shown to be required for synaptic pruning33-35 and the role of microglia in disease states is largely thought to be detrimental for synaptic integrity.36-39 In zebrafish, however, the role of microglia might be opposite and work toward promoting the integrity of the circuitry. This role may be facilitated either by the presence of an active survival mechanism of the synapses in microglia, or through the role of microglia in promoting neurogenesis and formation and more synapses. The second option is also favorable because the microglial activity and innate immunity in zebrafish positively affects the regenerative response through immune–related signaling pathways.25,40-42 In mammalian systems, inflammation was also implicated to have positive effects on neurogenesis or neuronal survival,25,41,43-45 yet opposite observations also exist.46,47 Therefore, our results suggest that zebrafish brain would serve as an excellent tool to address the neuro-immune relationship and the molecular mechanisms underlying a successful regeneration response after neurodegeneration, as well as the effects of aging on NSPC biology and neurogenesis. The lessons we might get from zebrafish has the vast potential to be exploited for designing new therapeutic approaches for humans.
Materials and methods
Ethics statement: All animal experiments were performed in accordance with permits of the Landesdirektion Sachsen, Germany (permit number TVV-52/2015 and all relevant amendments to C.K.).
Peptide synthesis: TR-Aβ42 peptide with the amino acid sequence of GWTLNSAGYLLGKINLKALAALAKKILDAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA was synthesized using the standard 9-fluorenylmethoxycarbonyl (Fmoc) chemistry with 2-(1H-benzotriazol-1-yl)-1,1,3,3-tetrame thyluronoiumhexafluorphosphate (HBTU) as coupling reagent on an automated solid-phase peptide synthesizer (ResPep SL, Intavis), and the purity was determined as described before.28 The stock solution was of 1mM concentration in equal volume mixture of acetonitrile:dimethyl-formamide:water solution.
Microinjections: Cerebroventricular microinjections (CVMI) were performed as described.48,49
Tissue preparation and immunohistochemistry: Tissue preparations and stainings were performed as described previously using the following antibodies: anti-ß-Amyloid (Cell Signaling, 8243, 1:500), anti-HuC/D (Life Technologies, A21271, 1:500), anti-PCNA (Dako, M0879, 1:500), anti-S100β (Dako, Z0311, 1:500), anti-L-Plastin (gift from Michael Redd, 1:5000), anti-acetylated-tubulin (Sigma, T6793, 1:500), anti-BrdU (Abd Serotec, MCA2060, 1:500), Synaptophysin (Abcam, Ab32594, 1:500). All secondary antibodies were from Molecular probes and were used at 1:500 dilution.
BrdU experiment: Zebrafish were immersed in freshly prepared 10 mM BrdU (Sigma) solution in E3 for 8 hours per day on 48 and 72 hours post injection. At 2 weeks after injection, zebrafish were killed and zebrafish head were subjected for histological preparations as described.24,42
Imaging and statistical analyses: Images were acquired using an inverted Zeiss AxioImager Z1. Cell counting was performed manually. The statistical evaluation was performed using GraphPad Prism (Version 6.02) for one-way ANOVA followed by a Tukey's post-hoc test and for Student's T-Test. Error bars shown are the s.e.m. and asterisks indicate significance according to: *: p < 0.05, **: p < 0.01, ***: p < 0.001. p > 0.05 is considered not significant (n.s.). Student's T-test was performed for paired samples, and a T-Test for independent measurements. All other analyses were performed as described.28
Abbreviations
- AD
Alzheimer disease
- BrdU
5-bromo-2′-deoxyuridine
- CVMI
Cerebroventricular microinjection
- HuC/D
ELAV Like RNA Binding Protein 3/4
- NSPC
Neural stem/progenitor cell
- PCNA
Proliferating cell nuclear antigen
- S100β
S100 calcium-binding protein B
- TR-Aβ42
Transportan-coupled Amyloid-β42
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
Disclosure of potential conflict of interest
No potential conflict of interest was disclosed.
Acknowledgments
This work was supported by German Center for Neurodegenerative Diseases (DZNE) and Helmholtz Association (VH-NG-1021), Center for Regenerative Therapies Dresden (CRTD) at the TU Dresden (FZ-111, 043_261518) (C.K.). We thank to all members of Kizil laboratory for discussions and help. We apologize to all the colleagues, whose important work could not be cited due to length constraints.
References
- [1].Bertram L, Lill CM & Tanzi RE. The genetics of Alzheimer disease: back to the future. Neuron 2010; 68:270-81; PMID:20955934; https://doi.org/ 10.1016/j.neuron.2010.10.013 [DOI] [PubMed] [Google Scholar]
- [2].Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 2002; 297:353-6; PMID:12130773; https://doi.org/ 10.1126/science.1072994 [DOI] [PubMed] [Google Scholar]
- [3].Selkoe DJ. Alzheimer's disease is a synaptic failure. Science 2002; 298:789-91; PMID:12399581; https://doi.org/ 10.1126/science.1074069 [DOI] [PubMed] [Google Scholar]
- [4].Beyreuther K & Masters CL. Alzheimer's disease. The ins and outs of amyloid-beta. Nature 1997; 389:677-8; PMID:9338775; https://doi.org/ 10.1038/39479 [DOI] [PubMed] [Google Scholar]
- [5].Haass C & Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer's amyloid beta-peptide. Nat Rev Mol Cell Biol 2007; 8:101-12; PMID:17245412; https://doi.org/ 10.1038/nrm2101 [DOI] [PubMed] [Google Scholar]
- [6].Wild-Bode C, Yamazaki T, Capell A, Leimer U, Steiner H, Ihara Y, Haass C. Intracellular generation and accumulation of amyloid beta-peptide terminating at amino acid 42. J Biol Chem 1997; 272:16085-88; PMID:9195901; https://doi.org/ 10.1074/jbc.272.26.16085 [DOI] [PubMed] [Google Scholar]
- [7].LaFerla FM, Green KN, Oddo S. Intracellular amyloid-beta in Alzheimer's disease. Nat Rev Neurosci 2007; 8:499-509; PMID:17551515; https://doi.org/ 10.1038/nrn2168 [DOI] [PubMed] [Google Scholar]
- [8].Selkoe DJ. Folding proteins in fatal ways. Nature 2003; 426:900-904; PMID:14685251; https://doi.org/ 10.1038/nature02264 [DOI] [PubMed] [Google Scholar]
- [9].Kienlen-Campard P, Miolet S, Tasiaux B, Octave JN. Intracellular amyloid-beta 1-42, but not extracellular soluble amyloid-beta peptides, induces neuronal apoptosis. J Biol Chem 2002; 277:15666-70; PMID:11861655; https://doi.org/ 10.1074/jbc.M200887200 [DOI] [PubMed] [Google Scholar]
- [10].LaFerla FM, Green KN. Animal models of Alzheimer disease. Cold Spring Harbor perspectives in medicine 2012; 2:a006320; PMID:23002015; https://doi.org/ 10.1101/cshperspect.a006320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gotz J, Ittner LM. Animal models of Alzheimer's disease and frontotemporal dementia. Nat Rev Neurosci 2008; 9:532-44; PMID:18568014; https://doi.org/ 10.1038/nrn2420 [DOI] [PubMed] [Google Scholar]
- [12].Tincer G, Mashkaryan V, Bhattarai P, Kizil C. Neural stem/progenitor cells in Alzheimer's disease. Yale J Biol Med 2016; 89:23-35; PMID:27505014 [PMC free article] [PubMed] [Google Scholar]
- [13].Taupin P. Adult neurogenesis, neural stem cells and Alzheimer's disease: developments, limitations, problems and promises. Curr Alzheimer Res 2009; 6:461-70; PMID:19747153; https://doi.org/ 10.2174/156720509790147151 [DOI] [PubMed] [Google Scholar]
- [14].Ermini FV, Grathwohl S, Radde R, Yamaguchi M, Staufenbiel M, Palmer TD, Jucker M. Neurogenesis and alterations of neural stem cells in mouse models of cerebral amyloidosis. Am. J. Pathol 2008; 172:1520-28; PMID:18467698; https://doi.org/ 10.2353/ajpath.2008.060520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Marutle A, Ohmitsu M, Nilbratt M, Greig NH, Nordberg A, Sugaya K. Modulation of human neural stem cell differentiation in Alzheimer (APP23) transgenic mice by phenserine. Proceedings of the National Academy of Sciences of the United States of America 2007; 104:12506-11; PMID:17640880; https://doi.org/ 10.1073/pnas.0705346104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Haughey NJ, Nath A, Chan SL, Borchard AC, Rao MS, Mattson MP. Disruption of neurogenesis by amyloid b -peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer ’ s disease. Journal of Neurochemistry 2002; 83:1509-24; PMID:12472904; https://doi.org/ 10.1046/j.1471-4159.2002.01267.x [DOI] [PubMed] [Google Scholar]
- [17].Gomez-Nicola D, Suzzi S, Vargas-Caballero M, Fransen NL, Al-Malki H, Cebrian-Silla A, Garcia-Verdugo JM, Riecken K, Fehse B, Perry VH. Temporal dynamics of hippocampal neurogenesis in chronic neurodegeneration. Brain 2014; 137(Pt 8):2312-28; https://doi.org/ 10.1093/brain/awu155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cosacak MI, Papadimitriou C, Kizil C. Regeneration, Plasticity, and Induced Molecular Programs in Adult Zebrafish Brain. Biomed Res Int 2015; 2015:769763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Conover JC, Todd KL. Development and aging of a brain neural stem cell niche. Exp Gerontol 2016. [Epub ahead of print]; pii: S0531-5565(16)30398-9; PMID:27867091; https://doi.org/10.1016/j.exger.2016.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].DeCarolis NA, Kirby ED, Wyss-Coray T, Palmer TD. The Role of the Microenvironmental Niche in Declining Stem-Cell Functions Associated with Biological Aging. Cold Spring Harbor perspectives in medicine 2015; 5:a025874; PMID:26627453; https://doi.org/ 10.1101/cshperspect.a025874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Silva-Vargas V, Crouch EE, Doetsch F. Adult neural stem cells and their niche: a dynamic duo during homeostasis, regeneration, and aging. Curr Opin Neurobiol 2013; 23:935-42; PMID:24090877; https://doi.org/ 10.1016/j.conb.2013.09.004 [DOI] [PubMed] [Google Scholar]
- [22].Kizil C, Kaslin J, Kroehne V, Brand M. Adult neurogenesis and brain regeneration in zebrafish. Dev Neurobiol 2012; 72:429-61; PMID:21595047; https://doi.org/ 10.1002/dneu.20918 [DOI] [PubMed] [Google Scholar]
- [23].Zupanc GK. Adult neurogenesis and neuronal regeneration in the brain of teleost fish. J Physiol Paris 2008; 102:357-73; PMID:18984045; https://doi.org/ 10.1016/j.jphysparis.2008.10.007 [DOI] [PubMed] [Google Scholar]
- [24].Kizil C, Kyritsis N, Dudczig S, Kroehne V, Freudenreich D, Kaslin J, Brand M. Regenerative neurogenesis from neural progenitor cells requires injury-induced expression of Gata3. Dev Cell 2012; 23:1230-7; PMID:23168169; https://doi.org/ 10.1016/j.devcel.2012.10.014 [DOI] [PubMed] [Google Scholar]
- [25].Kyritsis N, Kizil C, Zocher S, Kroehne V, Kaslin J, Freudenreich D, Iltzsche A, Brand M. Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 2012; 338:1353-6; PMID:23138980; https://doi.org/ 10.1126/science.1228773 [DOI] [PubMed] [Google Scholar]
- [26].Antos CL, Tanaka EM. Vertebrates that regenerate as models for guiding stem cels. Adv Exp Med Biol 2010; 695:184-214; PMID:21222207; https://doi.org/ 10.1007/978-1-4419-7037-4_13 [DOI] [PubMed] [Google Scholar]
- [27].Tanaka EM, Ferretti P. Considering the evolution of regeneration in the central nervous system. Nat Rev Neurosci 2009; 10:713-23; PMID:19763104; https://doi.org/ 10.1038/nrn2707 [DOI] [PubMed] [Google Scholar]
- [28].Bhattarai P, Thomas AK, Cosacak MI, Papadimitriou C, Mashkaryan V, Froc C, Reinhardt S, Kurth T, Dahl A, Zhang Y. IL4/STAT6 signaling activates neural stem cell proliferation and neurogenesis upon Amyloid-β42 aggregation in adult zebrafish brain. Cell Reports 2016; 17:941-8; PMID:27760324; https://doi.org/ 10.1016/j.celrep.2016.09.075 [DOI] [PubMed] [Google Scholar]
- [29].Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature 2016; 539:180-6; PMID:27830812; https://doi.org/ 10.1038/nature20411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kempermann G, Gast D & Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol 2002; 52:135-43; PMID:12210782; https://doi.org/ 10.1002/ana.10262 [DOI] [PubMed] [Google Scholar]
- [31].Kizil C, Iltzsche A, Thomas AK, Bhattarai P, Zhang Y, Brand M. Efficient cargo delivery using a short cell-penetrating peptide in vertebrate brains. PLoS One 2015; 10:e0124073; PMID:25894337; https://doi.org/ 10.1371/journal.pone.0124073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jiang T, Tan L, Zhu XC, Zhou JS, Cao L, Tan MS, Wang HF, Chen Q, Zhang YD, Yu JT. Silencing of TREM2 exacerbates tau pathology, neurodegenerative changes, and spatial learning deficits in P301S tau transgenic mice. Neurobiol Aging 2015; 36:3176-86; PMID:26364736; https://doi.org/ 10.1016/j.neurobiolaging.2015.08.019 [DOI] [PubMed] [Google Scholar]
- [33].Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, et al.. Synaptic pruning by microglia is necessary for normal brain development. Science 2011; 333:1456-58; PMID:21778362; https://doi.org/ 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]
- [34].Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, Ransohoff RM, Greenberg ME, Barres BA, Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 2012; 74:691-705; PMID:22632727; https://doi.org/ 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 2005; 308:1314-8; PMID:15831717; https://doi.org/ 10.1126/science.1110647 [DOI] [PubMed] [Google Scholar]
- [36].Jebelli J, Su W, Hopkins S, Pocock J, Garden GA. Glia: guardians, gluttons, or guides for the maintenance of neuronal connectivity? Ann N Y Acad Sci 2015; 1351:1-10; PMID:25752338; https://doi.org/ 10.1111/nyas.12711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Perry VH, O'Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN Neuro 2010; 2:e00047; PMID:20967131; https://doi.org/ 10.1042/AN20100024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Centonze D, Muzio L, Rossi S, Cavasinni F, De Chiara V, Bergami A, Musella A, D'Amelio M, Cavallucci V, Martorana A, et al.. Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci 2009; 29:3442-52; PMID:19295150; https://doi.org/ 10.1523/JNEUROSCI.5804-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jebelli J, Hooper C, Pocock JM. Microglial p53 activation is detrimental to neuronal synapses during activation-induced inflammation: Implications for neurodegeneration. Neurosci Lett 2014; 583:92-7; PMID:25204787; https://doi.org/ 10.1016/j.neulet.2014.08.049 [DOI] [PubMed] [Google Scholar]
- [40].Kizil C, Kyritsis N, Brand M. Effects of inflammation on stem cells: together they strive? EMBO reports 2015; 16:416-26; PMID:25739812; https://doi.org/ 10.15252/embr.201439702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kyritsis N, Kizil C, Brand M. Neuroinflammation and central nervous system regeneration in vertebrates. Trends Cell Biol 2014; 24:128-35; PMID:24029244; https://doi.org/ 10.1016/j.tcb.2013.08.004 [DOI] [PubMed] [Google Scholar]
- [42].Kizil C, Dudczig S, Kyritsis N, Machate A, Blaesche J, Kroehne V, Brand M. The chemokine receptor cxcr5 regulates the regenerative neurogenesis response in the adult zebrafish brain. Neural Dev 2012; 7:27; PMID:22824261; https://doi.org/ 10.1186/1749-8104-7-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Schwartz M, Kipnis J, Rivest S, Prat A. How do immune cells support and shape the brain in health, disease, and aging? J Neurosci 2013; 33:17587-96; PMID:24198349; https://doi.org/ 10.1523/JNEUROSCI.3241-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kokaia Z, Martino G, Schwartz M, Lindvall O. Cross-talk between neural stem cells and immune cells: the key to better brain repair? Nat Neurosci 2012; 15:1078-87; PMID:22837038; https://doi.org/ 10.1038/nn.3163 [DOI] [PubMed] [Google Scholar]
- [45].Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci 2009; 10:235-41; PMID:19229242; https://doi.org/ 10.1038/nrn2591 [DOI] [PubMed] [Google Scholar]
- [46].Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A 2003; 100:13632-7; PMID:14581618; https://doi.org/ 10.1073/pnas.2234031100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003; 302:1760-65; PMID:14615545; https://doi.org/ 10.1126/science.1088417 [DOI] [PubMed] [Google Scholar]
- [48].Kizil C, Iltzsche A, Kaslin J, Brand M. Micromanipulation of gene expression in the adult zebrafish brain using cerebroventricular microinjection of morpholino oligonucleotides. J Vis Exp 2013; e50415; PMID:23728426; https://doi.org/ 10.3791/50415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Kizil C, Iltzsche A, Kaslin J, Brand M. Micromanipulation of gene expression in the adult zebrafish brain using cerebroventricular microinjection of morpholino oligonucleotides. J Vis Exp 2013; (75):e50415; PMID:23728426; https://doi.org/10.3791/50415 [DOI] [PMC free article] [PubMed] [Google Scholar]


