ABSTRACT
Aging is associated with progressive visceral white adipose tissue (WAT) expansion both in human and mouse. Importantly, WAT enlargement is initiated early in life, suggesting that molecular mechanisms underlying age-dependent obesity are activated at early stages of lifetime. Our recent study found that age-dependent obesity was associated with a specific decline in mitochondrial complex IV activity, which leads to reduced fatty acid oxidation and subsequent adipocyte hypertrophy. At the molecular level, global mitochondrial complex IV inhibition was driven by hypoxia-inducible factor-1α (HIF1α)-mediated repression of some of its key subunits, including cytochrome c oxidase 5b (Cox5b). In this commentary, we compare age-dependent WAT responses with those observed in the high fat diet model of extreme obesity. Furthermore, we discuss the potential scenarios that could initiate age-dependent WAT expansion as well as the mechanisms by which HIF1α could be activated in WAT.
KEYWORDS: aging, HIF-1, hypoxia, mitochondrial complex IV, mitochondrial dysfunction, obesity, white adipocytes
General considerations about age-dependent obesity
Obesity is associated with the development of several metabolic diseases as well as with an increased risk for adverse long-term outcomes, even in the absence of metabolic abnormalities.1,2 Indeed, the American Medical Association (AMA) decided in June 2013 to officially classify obesity as a “disease” (“AMA Adopts New Policies on Second Day of Voting at Annual Meeting.” June 18, 2013; Pollack A. AMA recognizes obesity as a disease. New York Times. June 18, 2013). Moreover, the clinical relevance of obesity has increased considerably because first, obesity is already manifested in middle and early-old aged population and second, WAT mass can decline in advanced old population accompanied by fat redistribution outside WAT depots leading to lipotoxicity.3-5 Obesity is therefore considered a public health priority and a serious and chronic health issue requiring both prevention and treatment.
Several studies in humans have shown that aging is not only associated with adipose tissue expansion but also with a redistribution in the pattern of adiposity.3 Indeed these studies have evidenced a redistribution of fat from subcutaneous to visceral depots that occurs from middle age until old ages, which leads to a preferential visceral WAT expansion during lifetime.3,5,6 As a consequence, a relative increase in visceral fat with aging has been associated to metabolic dysfunction and related maladies.7 However it is important to note again that WAT expansion manifests already in middle age both in human and mouse, occurring much earlier than the onset of metabolic disease.3-5,8,9 Indeed, body weight increase as well as WAT expansion in mice already manifests at 8–12 months of age.8 Therefore, exploring the initiating events of age-dependent obesity in early middle-aged mice - rather than in older mice - could be critical to design targeted interventions to prevent obesity and the metabolic syndrome in the future.
In our recent study, we found that repression of adipocyte mitochondrial complex IV (CIV) activity occurs in ‘aging’ white adipocytes of middle-aged mice, providing a potential molecular basis for age-dependent obesity.10 Here, we use the term ‘aging’, rather than ‘aged’ or ‘old’, since aging refers to mice that are in the process of progressively ‘getting older’. This is in clear contrast to other age-associated molecular and physiologic alterations in aged mice, which usually manifest at later periods of lifetime, for example 22 to 30 months in mice (see also below).11,12
Finally, it is important to note that the majority of studies addressing WAT expansion in animal models commonly use high fat diet (HFD) to provoke obesity. We believe that WAT responses to HFD feeding are likely different to age-dependent WAT alterations for the following reasons: (i) obese/over-weight patients gain weight progressively over time in contrast to the rapid WAT expansion induced in HFD murine models,13,14 (ii) a scenario in which humans are under extreme nutritional overload mimicking that of HFD-fed mice seems unlikely, and (iii) some WAT responses to HFD in mice are not necessarily observed in obese humans. Therefore, we consider that future studies in mice focused on age-dependent WAT expansion are necessary to comprehend metabolic responses (such as mitochondrial CIV repression) that naturally occur in WAT during human aging.
Mitochondrial complex IV vulnerability in aging white adipocytes
Mitochondrial dysfunction is a hallmark of aging.15 We have recently found that mitochondrial oxygen consumption is already repressed in white adipocytes of aging mice as a consequence of an early mitochondrial dysfunction in WAT.10 Remarkably, this is associated with a specific decrease in mitochondrial CIV activity, while the activity of the other complexes as well as mitochondrial content remains unaltered (Fig. 1). Furthermore, this reduction in activity is sufficient to reduce fatty acid oxidation and lead to adipocyte hypertrophy and obesity during aging.10 Restoration of CIV components such as cytochrome c oxidase 5b (Cox5b; see below) using local WAT injection of lentiviruses expressing COX5B counteracts age-dependent WAT expansion10 (Fig. 1). These findings seem to contrast with the general mitochondrial dysfunction described previously in the WAT of HFD-fed mice, or in mice with altered leptin signaling, such as ob/ob and db/db obese mice.16-18 However, it could be considered that a global decline in mitochondrial content would require not only WAT expansion, but also the development of an obesity-driven inflammatory milieu provoked by HFD/ nutrient overload. Indeed, inflammatory mediators are associated with a decline in mitochondrial content17,19-21 and presumably this inflammatory process does not occur—or occurs to a lesser extent—in aging WAT. Significantly, our data are in line with previous data in humans suggesting that decline of white adipocyte mitochondrial content as well as a global mitochondrial dysfunction is not necessarily taking place—or to a mild extent—in obese patients,22-25 but more associated with concomitant diabetes.22 Moreover, our data in humans also show that the expression of mitochondrial CIV components such as COX5B, is specifically reduced during aging.10 However, this cannot be attributed to a general decline in the expression of mitochondrial genes because the mitochondrial marker VDAC1 was not decreased with age.10 Thus, global mitochondrial dysfunction might be a consequence of extreme scenarios such as HFD models or obese patients with concomitant metabolic dysfunction (e.g., diabetes), whereas age-dependent obesity could be considered a milder scenario whereby mitochondria are more gradually affected, with CIV being particularly vulnerable.
Figure 1.
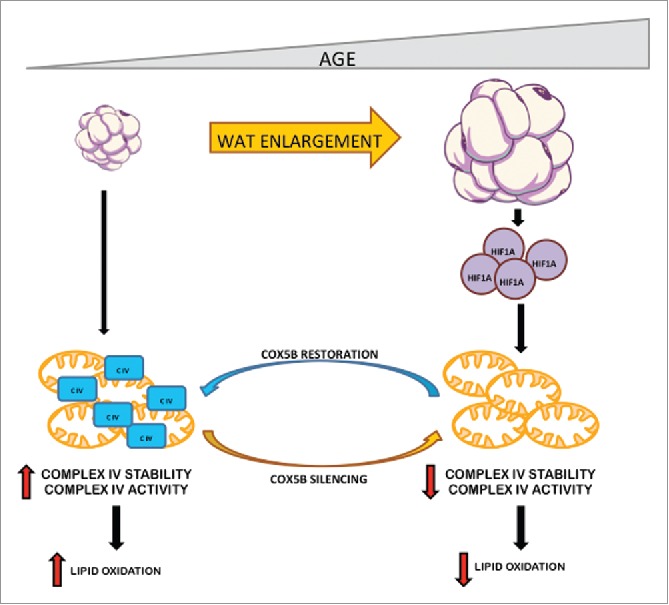
Role of HIF1α-CIV pathway in age-dependent WAT expansion. White adipocyte enlargement is initiated in early phases during aging. During age-dependent WAT expansion HIF1α is stabilized and promotes CIV dysfunction (CIV) (decreased activity and stability). Adipocytes with a dysfunctional CIV are less oxidative and, therefore, accumulate more lipids allowing further WAT expansion. Age-dependent CIV dysfunction can be alleviated by the ectopic overexpression of the nuclear encoded CIV subunit COX5B in aging mice. Conversely, silencing this CIV subunit in young adipocytes promotes adipocyte enlargement.
Age-dependent white adipocyte HIF1α expression
Several studies have shown that WAT expansion in HFD-fed mice is associated with poor oxygenation and consequent white adipocyte activation of HIF1α.8,26-28 Moreover, adipocyte-restricted Hif1α gene inactivation counteracts pathological WAT expansion in HFD-fed mice.8,26-28 In our recent study, we demonstrated that HIF1α expression also increases during age-dependent WAT expansion.10 WAT hypoxia has been detected under normal dietary conditions using the exogenous marker pimonidazole28-30; therefore, HIF1α activation in aging WAT can be a ‘consequence’ of an initial WAT expansion during aging. Nonetheless, HIF1α is also a ‘cause’ of age-dependent WAT hypertrophy because age-dependent WAT expansion requires adipocyte HIF1α activity.10 Indeed, HIF1α promotes white adipocyte fat accumulation by repressing CIV activity, leading to a reduction in adipocyte fatty acid oxidation.10 It therefore seems likely that HIF1α is both a ‘cause’ and a ‘consequence’ of age-dependent WAT expansion, in which initial WAT expansion is accompanied by a degree of hypoxia (perhaps milder than in HFD models) leading to subsequent HIF1α activation, which further exacerbates age-dependent WAT expansion. Regarding the initiating factors of age-dependent WAT enlargement that could provoke HIF1α activation, some studies have shown that aging leads to hypothalamic molecular alterations that, by possibly increasing food intake, result in increased body weight. Indeed, the expression of the NAD+-dependent deacetylase Sirtuin 1 (SIRT1) is progressively reduced in agouti-related peptide (AgRP) neurons in the hypothalamus during aging. This repression has been linked to food intake alterations during aging since restoration of SIRT1 expression in AgRP neurons suppresses food intake.31,32 Moreover, age-dependent over-activation of mammalian target of rapamycin (mTOR) signaling in pro-opiomelanocortin (POMC) neurons in the arcuate nucleus of the hypothalamus contributes to obesity during aging.14 Notably, it has been also described that the dysregulated/altered activities of SIRT1 and mTOR appear in early middle-aged mice and therefore at similar lifetime stages when age-dependent obesity is manifested. Collectively, these studies support the possible involvement of hypothalamic SIRT1 and mTOR signaling in age-dependent obesity. Based on this evidence, age-dependent WAT expansion might be considered a secondary consequence of age-dependent increased food intake, which promotes initial WAT expansion leading to HIF1α activation, which ultimately compromises CIV activity and accelerates WAT expansion in aging mice.
Nevertheless, increased food consumption during aging is a controversial issue,33,34 and this may not be involved in age-dependent body weight gain. Therefore, WAT expansion and obesity during aging could be an autonomous response without the participation of alterations in the extent of food intake, or of other peripheral tissues. Along this line, we failed to detect HIF1α accumulation in liver, skeletal muscle or brown adipose tissue.10 It is conceivable that HIF1α stabilization is inherent to the local WAT depot during aging because lipid accumulation could be inherently associated with a certain degree of adipocyte hypoxia (Fig. 2). However, it is also possible that age-dependent HIF1α activation is triggered by progressive oxidative stress or simply by lipid accumulation, both of which have been reported to activate HIF1α independently of oxygen availability35-37 (Fig. 2). Indeed, reactive oxygen species (ROS) are detected in WAT even at baseline conditions, and their levels progressively increase during aging.38,39 Furthermore, a recent study has shown that cholesterol can lead to HIF1α activation via ROS generation.37 Finally, fat accumulation in HFD-fed mice can also promote HIF1α accumulation through free fatty acid-induced mitochondrial uncoupling and increased oxygen consumption.8 It is however unlikely that this latter mechanism is predominant in age-dependent WAT expansion since mitochondrial oxygen consumption is reduced10 and it is anticipated that the supply of free fatty acids to white adipocytes is much lower than that in HFD models. Independently of the mechanism of basal HIF1α activity in aging WAT, as discussed above, it is probable that initial HIF1α activation during aging triggers a feed-forward mechanism, which further promotes WAT expansion and a more robust HIF1α activity during lifetime.
Figure 2.
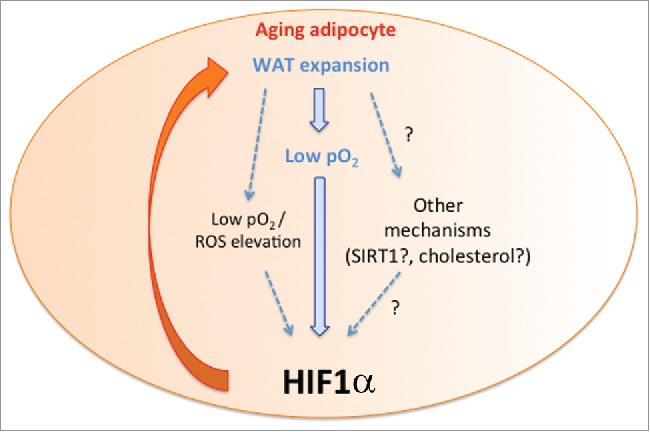
Activation of HIF1α in aging white adipocytes. The figure shows that WAT expansion leads to poor white adipocyte oxygenation (low pO2), which subsequently promotes HIF1α accumulation. In turn HIF1α accumulation also exacerbates WAT expansion involving mitochondrial complex IV repression (see also Fig. 1). This feed-forward mechanism is indicated with the orange arrow. Moreover, white adipocyte HIF1α accumulation could be promoted - not only by hypoxia in itself - but potentially also by intracellular ROS as well as lipid accumulation (e.g., cholesterol) or other metabolic pathways such as SIRT1 involved in HIF1α activation in other tissues during aging.
Interestingly, HIF1α activation has been found in aged tissues, such as skeletal muscle, in old mice (22–30 months)11,40 but not in middle-aged mice.10 Sebastian et al. demonstrated that gain of HIF1α activity in old or very old skeletal muscle leads to global mitochondrial dysfunction or mitochondrial autophagy, providing a molecular basis of skeletal muscle mitochondrial decline during aging.40 In skeletal muscle, Gomes et al. also showed that gain of HIF1α expression has been associated with a decline of nuclear NAD+ and SIRT1 activity and subsequent decline in the levels of the von Hippel-Lindau ubiquitin ligase, which is the principal repressor of HIF1α.11,41 The described SIRT1-dependent mechanism could also be added to the list of potential mechanisms discussed above that trigger HIF1α activity in aging adipocytes (Fig. 2). Independently of the mechanisms involved, it would be interesting to explore whether gain of HIF1α activity is a common signature in aged tissues, although this activation would take place at different lifetime stages in each cell type or tissue.
Age-dependent mitochondrial dysfunction through HIF1α
Numerous studies both in tumor and non-malignant cells have shown that a central response executed by HIF1α is an anaerobic metabolic switch that favors glycolysis and impedes glucose-driven mitochondrial activity.42 Indeed, HIF1α directly induces gene expression of glucose transporter-1 and also glycolytic enzymes including lactate dehydrogenase.43 Moreover, HIF1α induces the expression of pyruvate dehydrogenase kinase-1, −3 and −4 that phosphorylate and inhibit the pyruvate dehydrogenase complex, thereby attenuating the conversion of pyruvate to acetyl-CoA and glucose/pyruvate oxidation.44,45 At the level of mitochondria HIF1α can induce a suite of changes, including the reduction of CI activity through upregulation of Ndufa4l2,46 the reduction of CII activity via a decrease in Sdha expression,47 the rewiring of CIV activity by inducing a subunit switch from Cox4–1 to Cox4–248 and, in some cellular scenarios, can also compromise mitochondrial biogenesis by repressing c-myc activity.49 All the genes mentioned above are simultaneously regulated by HIF1α in hypoxic cells to reduce oxygen consumption and promote the generation of ATP in an oxygen-independent manner. Our gene expression analysis in aging adipocytes found that HIF1α repressed specifically Cox5b and Cox8a, 2 essential components of CIV. However, although Cox5b and Cox8a expression is primarily repressed by HIF1α, it does not necessarily mean that they are the only CIV subunits affected in aging adipocytes. It has been shown that COX5B is essential for assembly of CIV as well as for protein stability of the other CIV subunits.50 In line with this study, we found that silencing Cox5b or Cox8a led to a profound decline in CIV assembly and reduced the protein content (but not gene expression) of other representative CIV subunits, such as NDUFA4 (nuclear-encoded representative) or mt-CO1 (mitochondria-encoded representative), which are also reduced in aging adipocytes.10 This specific repression of Cox5b and Cox8a by HIF1α without affecting other HIF1α-dependent genes might indicate that HIF1α expression/activation in aging adipocytes is not maximal, and the expression of Cox5b and Cox8a subunits is more sensitive to the presumed mild HIF1α activation in aging adipocytes, than other HIF1α-target genes. Therefore, it is conceivable that a more profound activation of HIF1α is required to trigger the full gene expression program mentioned above in white adipocytes. Alternatively, it is possible that some of the HIF1α-dependent metabolic genes identified in other cellular scenarios could be not regulated in white adipocytes. Irrespective of these considerations, it seems clear that future studies should identify the HIF1α-dependent gene expression program that is sufficiently sensitive to the levels of HIF1α present in aging adipocytes as this will undoubtedly help to generate novel insights in age-dependent obesity.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
We acknowledge funding support from the Ministerio de Economia y Competitividad (SAF2013–46058-R and SAF2016–76815-R), RECAVA (RD06/0014/0031), CAM (P2010 / BMD-2542) and CIBERCV (CB16.11.00272).
References
- [1].Collaboration, NCDRF Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet 2016; 387(10026):1377-96; PMID:27115820; https://doi.org/ 10.1016/S0140-6736(16)30054-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Global, BMIMC, Di Angelantonio E, Bhupathiraju Sh N, Wormser D, Gao P, Kaptoge S, Berrington de Gonzalez A, Cairns BJ, Huxley R, Jackson ChL, et al.. Body-mass index and all-cause mortality: individual-participant-data meta-analysis of 239 prospective studies in four continents. Lancet 2016; 388(10046):776-86; PMID:27423262; https://doi.org/ 10.1016/S0140-6736(16)30175-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Res Rev 2009; 8(4):339-48; PMID:19576300; https://doi.org/ 10.1016/j.arr.2009.06.001 [DOI] [PubMed] [Google Scholar]
- [4].Kyle UG, Genton L, Hans D, Karsegard L, Slosman DO, Pichard C. Age-related differences in fat-free mass, skeletal muscle, body cell mass and fat mass between 18 and 94 years. Eur J Clin Nutr 2001; 55(8):663-72; PMID:11477465; https://doi.org/ 10.1038/sj.ejcn.1601198 [DOI] [PubMed] [Google Scholar]
- [5].Cartwright MJ, Tchkonia T, Kirkland JL. Aging in adipocytes: potential impact of inherent, depot-specific mechanisms. Exp Gerontol 2007; 42(6):463-71; PMID:17507194; https://doi.org/ 10.1016/j.exger.2007.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Hughes VA, Roubenoff R, Wood M, Frontera WR, Evans WJ, Fiatarone Singh MA. Anthropometric assessment of 10-y changes in body composition in the elderly. Am J Clin Nutr 2004; 80(2):475-82; PMID:15277173 [DOI] [PubMed] [Google Scholar]
- [7].Slawik M, Vidal-Puig AJ. Lipotoxicity, overnutrition and energy metabolism in aging. Ageing Res Rev 2006; 5(2):144-64; PMID:16630750; https://doi.org/ 10.1016/j.arr.2006.03.004 [DOI] [PubMed] [Google Scholar]
- [8].Lee YS, Kim JW, Osborne O, Oh DY, Sasik R, Schenk S, Chen A, Chung H, Murphy A, Watkins SM, et al.. Increased adipocyte O2 consumption triggers HIF-1alpha, causing inflammation and insulin resistance in obesity. Cell 2014; 157(6):1339-52; PMID:24906151; https://doi.org/ 10.1016/j.cell.2014.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lv Y, Xue L, Cai C, Liu QH, Shen J. Deficiency of myotubularin-related protein 14 influences body weight, metabolism, and inflammation in an age-dependent manner. Cell Biosci 2015; 5:69; PMID:26697164; https://doi.org/ 10.1186/s13578-015-0062-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Soro-Arnaiz I, Li QO, Torres-Capelli M, Melendez-Rodriguez F, Veiga S, Veys K, Sebastian D, Elorza A, Tello D, Hernansanz-Agustin P, et al.. Role of Mitochondrial Complex IV in Age-Dependent Obesity. Cell Rep 2016; 16(11):2991-3002; PMID:27626667; https://doi.org/ 10.1016/j.celrep.2016.08.041 [DOI] [PubMed] [Google Scholar]
- [11].Gomes AP, Price NL, Ling AJ, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, et al., Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013; 155(7):1624-38; PMID:24360282; https://doi.org/ 10.1016/j.cell.2013.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Houtkooper RH, Argmann C, Houten SM, Canto C, Jeninga EH, Andreux PA, Thomas C, Doenlen R, Schoonjans K, Auwerx J. The metabolic footprint of aging in mice. Sci Rep 2011; 1:134; PMID:22355651; https://doi.org/ 10.1038/srep00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sasaki T, Age-Associated Weight Gain, Leptin, and SIRT1: A possible role for hypothalamic SIRT1 in the prevention of weight gain and aging through modulation of leptin sensitivity. Front Endocrinol (Lausanne) 2015; 6:109; PMID:26236282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yang SB, Tien AC, Boddupalli G, Xu AW, Jan YN, Jan LY. Rapamycin ameliorates age-dependent obesity associated with increased mTOR signaling in hypothalamic POMC neurons. Neuron 2012; 75(3):425-36; PMID:22884327; https://doi.org/ 10.1016/j.neuron.2012.03.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013; 153(6):1194-217; PMID:23746838; https://doi.org/ 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Choo HJ, Kim JH, Kwon OB, Lee CS, Mun JY, Han SS, Yoon YS, Yoon G, Choi KM, Ko YG. Mitochondria are impaired in the adipocytes of type 2 diabetic mice. Diabetologia 2006; 49(4):784-91; PMID:16501941; https://doi.org/ 10.1007/s00125-006-0170-2 [DOI] [PubMed] [Google Scholar]
- [17].Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, et al., TNF-alpha downregulates eNOS expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest 2006; 116(10):2791-8; PMID:16981010; https://doi.org/ 10.1172/JCI28570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rong JX, Qiu Y, Hansen MK, Zhu L, Zhang V, Xie M, Okamoto Y, Mattie MD, Higashiyama H, Asano S, et al.. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes 2007; 56(7):1751-60; PMID:17456854; https://doi.org/ 10.2337/db06-1135 [DOI] [PubMed] [Google Scholar]
- [19].Gao CL, Zhu C, Zhao YP, Chen XH, Ji CB, Zhang CM, Zhu JG, Xia ZK, Tong ML, Guo XR. Mitochondrial dysfunction is induced by high levels of glucose and free fatty acids in 3T3-L1 adipocytes. Mol Cell Endocrinol 2010; 320(1–2):25-33; PMID:20144685; https://doi.org/ 10.1016/j.mce.2010.01.039 [DOI] [PubMed] [Google Scholar]
- [20].Kusminski CM, Scherer PE. Mitochondrial dysfunction in white adipose tissue. Trends Endocrinol Metab 2012; 23(9):435-43; PMID:22784416; https://doi.org/ 10.1016/j.tem.2012.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Villarroya J, Giralt M, Villarroya F, Mitochondrial DNA: an up-and-coming actor in white adipose tissue pathophysiology. Obesity (Silver Spring) 2009; 17(10):1814-20; PMID:19461585; https://doi.org/ 10.1038/oby.2009.152 [DOI] [PubMed] [Google Scholar]
- [22].Lindinger A, Peterli R, Peters T, Kern B, von Flue M, Calame M, Hoch M, Eberle AN, Lindinger PW. Mitochondrial DNA content in human omental adipose tissue. Obes Surg 2010; 20(1):84-92; PMID:19826890; https://doi.org/ 10.1007/s11695-009-9987-3 [DOI] [PubMed] [Google Scholar]
- [23].Yin X, Lanza IR, Swain JM, Sarr MG, Nair KS, Jensen MD. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. J Clin Endocrinol Metab 2014; 99(2):E209-16; PMID:24276464; https://doi.org/ 10.1210/jc.2013-3042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kaaman M, Sparks LM, van Harmelen V, Smith SR, Sjolin E, Dahlman I, Arner P. Strong association between mitochondrial DNA copy number and lipogenesis in human white adipose tissue. Diabetologia 2007; 50(12):2526-33; PMID:17879081; https://doi.org/ 10.1007/s00125-007-0818-6 [DOI] [PubMed] [Google Scholar]
- [25].Fischer B, Schottl T, Schempp C, Fromme T, Hauner H, Klingenspor M, Skurk T. Inverse relationship between body mass index and mitochondrial oxidative phosphorylation capacity in human subcutaneous adipocytes. Am J Physiol Endocrinol Metab 2015; 309(4):E380-7; PMID:26081284; https://doi.org/ 10.1152/ajpendo.00524.2014 [DOI] [PubMed] [Google Scholar]
- [26].Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, Gonzalez FJ. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes 2011; 60(10):2484-95; PMID:21873554; https://doi.org/ 10.2337/db11-0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Krishnan J, Danzer C, Simka T, Ukropec J, Walter KM, Kumpf S, Mirtschink P, Ukropcova B, Gasperikova D, Pedrazzini T, et al.. Dietary obesity-associated Hif1alpha activation in adipocytes restricts fatty acid oxidation and energy expenditure via suppression of the Sirt2-NAD+ system. Genes Dev 2012; 26(3):259-70; PMID:22302938; https://doi.org/ 10.1101/gad.180406.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee KY, Gesta S, Boucher J, Wang XL, Kahn CR. The differential role of Hif1beta/Arnt and the hypoxic response in adipose function, fibrosis, and inflammation. Cell Metab 2011; 14(4):491-503; PMID:21982709; https://doi.org/ 10.1016/j.cmet.2011.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, et al.. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 2007; 56(4):901-11; PMID:17395738; https://doi.org/ 10.2337/db06-0911 [DOI] [PubMed] [Google Scholar]
- [30].Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab 2007; 293(4):E1118-28; PMID:17666485; https://doi.org/ 10.1152/ajpendo.00435.2007 [DOI] [PubMed] [Google Scholar]
- [31].Sasaki T, Kikuchi O, Shimpuku M, Susanti VY, Yokota-Hashimoto H, Taguchi R, Shibusawa N, Sato T, Tang L, Amano K, et al.. Hypothalamic SIRT1 prevents age-associated weight gain by improving leptin sensitivity in mice. Diabetologia 2014; 57(4):819-31; PMID:24374551; https://doi.org/ 10.1007/s00125-013-3140-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lafontaine-Lacasse M, Richard D, Picard F. Effects of age and gender on Sirt 1 mRNA expressions in the hypothalamus of the mouse. Neurosci Lett 2010; 480(1):1-3; PMID:20074616; https://doi.org/ 10.1016/j.neulet.2010.01.008 [DOI] [PubMed] [Google Scholar]
- [33].Morley R, The influence of early diet on later development. J Biosoc Sci 1996; 28(4):481-7; PMID:8973005; https://doi.org/ 10.1017/S0021932000022549 [DOI] [PubMed] [Google Scholar]
- [34].Starr ME, Saito H. Age-related increase in food spilling by laboratory mice may lead to significant overestimation of actual food consumption: implications for studies on dietary restriction, metabolism, and dose calculations. J Gerontol A Biol Sci Med Sci 2012; 67(10):1043-8; PMID:22451471; https://doi.org/ 10.1093/gerona/gls009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sena LA, Chandel NS. Physiological roles of mitochondrial reactive oxygen species. Mol Cell 2012; 48(2):158-67; PMID:23102266; https://doi.org/ 10.1016/j.molcel.2012.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A 1998; 95(20):11715-20; PMID:9751731; https://doi.org/ 10.1073/pnas.95.20.11715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Anavi S, Hahn-Obercyger M, Madar Z, Tirosh O, Mechanism for HIF-1 activation by cholesterol under normoxia: a redox signaling pathway for liver damage. Free Radic Biol Med 2014; 71:61-9; PMID:24632196; https://doi.org/ 10.1016/j.freeradbiomed.2014.03.007 [DOI] [PubMed] [Google Scholar]
- [38].Salmon AB, Oxidative stress in the etiology of age-associated decline in glucose metabolism. Longev Healthspan 2012; 1:7; PMID:24764512; https://doi.org/ 10.1186/2046-2395-1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Zhang L, Ebenezer PJ, Dasuri K, Fernandez-Kim SO, Francis J, Mariappan N, Gao Z, Ye J, Bruce-Keller AJ, Keller JN. Aging is associated with hypoxia and oxidative stress in adipose tissue: implications for adipose function. Am J Physiol Endocrinol Metab 2011; 301(4):E599-607; PMID:21586698; https://doi.org/ 10.1152/ajpendo.00059.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Sebastian D, Sorianello E, Segales J, Irazoki A, Ruiz-Bonilla V, Sala D, Planet E, Berenguer-Llergo A, Munoz JP, Sanchez-Feutrie M, et al.. Mfn2 deficiency links age-related sarcopenia and impaired autophagy to activation of an adaptive mitophagy pathway. EMBO J 2016; 35(15):1677-93; PMID:27334614; https://doi.org/ 10.15252/embj.201593084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kaelin WG., Jr The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer 2008; 8(11):865-73; PMID:18923434; https://doi.org/ 10.1038/nrc2502 [DOI] [PubMed] [Google Scholar]
- [42].Aragones J, Fraisl P, Baes M, Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab 2009; 9(1):11-22; PMID:19117543; https://doi.org/ 10.1016/j.cmet.2008.10.001 [DOI] [PubMed] [Google Scholar]
- [43].Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, et al.. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev 1998; 12(2):149-62; PMID:9436976; https://doi.org/ 10.1101/gad.12.2.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab 2006; 3(3):187-97; PMID:16517406; https://doi.org/ 10.1016/j.cmet.2006.01.012 [DOI] [PubMed] [Google Scholar]
- [45].Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab 2006; 3(3):177-85; PMID:16517405; https://doi.org/ 10.1016/j.cmet.2006.02.002 [DOI] [PubMed] [Google Scholar]
- [46].Tello D, Balsa E, Acosta-Iborra B, Fuertes-Yebra E, Elorza A, Ordonez A, Corral-Escariz M, Soro I, Lopez-Bernardo E, Perales-Clemente E, et al.. Induction of the mitochondrial NDUFA4L2 protein by HIF-1alpha decreases oxygen consumption by inhibiting Complex I activity. Cell Metab 2011; 14(6):768-79; PMID:22100406; https://doi.org/ 10.1016/j.cmet.2011.10.008 [DOI] [PubMed] [Google Scholar]
- [47].Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M, Kung AL, Sanso G, Powers JF, Tischler AS, et al.. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet 2005; 1(1):72-80; PMID:16103922; https://doi.org/ 10.1371/journal.pgen.0010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 2007; 129(1):111-22; PMID:17418790; https://doi.org/ 10.1016/j.cell.2007.01.047 [DOI] [PubMed] [Google Scholar]
- [49].Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 2007; 11(5):407-20; PMID:17482131; https://doi.org/ 10.1016/j.ccr.2007.04.001 [DOI] [PubMed] [Google Scholar]
- [50].Galati D, Srinivasan S, Raza H, Prabu SK, Hardy M, Chandran K, Lopez M, Kalyanaraman B, Avadhani NG. Role of nuclear-encoded subunit Vb in the assembly and stability of cytochrome c oxidase complex: implications in mitochondrial dysfunction and ROS production. Biochem J 2009; 420(3):439-49; PMID:19338496; https://doi.org/ 10.1042/BJ20090214 [DOI] [PMC free article] [PubMed] [Google Scholar]


