ABSTRACT
The mechanisms by which estradiol modulates adipose lipolysis are poorly understood. We sought to measure basal and β3-stimulated indices of lipoysis (FFAs, glycerol) in vivo in E2 deficient or supplemented rats, and ex vivo with direct acute E2 exposure. For 2 weeks, ovariectomized (OVX) and OVX rats treated with a daily oral dose of E2 (OVX E2) were pairfed to SHAM controls (n = 12 per group). Adipocyte size was modestly (∼40%) increased in OVX rats, but did not reach significance (p = 0.2). After 2 weeks, half of the animals in each group received an in vivo injection of saline or 1 mg/kg of the β3 agonist CL 316, 243. Serum FFA concentrations, but not glycerol, were lower in OVX and OVX E2 rats compared with SHAM controls (p = 0.02). A significant CL response was present in all groups (p<0.001) and HSL activation was unaffected by OVX or OVX E2 in retroperitoneal (r.p.) or inguinal (iWAT) adipose depots in vivo. Ex vivo, CL increased FFA and glycerol accumulation in the media as well as HSL phosphorylation by several fold in r.p. and iWAT explants, but responses from OVX and OVX E2 rats were comparable to SHAMs. To assess whether E2 can directly affect lipolysis, r.p. and iWAT tissue was treated with E2, CL or E2 + CL for 2, 4 or 8 hours using adipose tissue organ culture. CL stimulated FFA release (p<0.001), but was unaffected by E2. Overall, our results indicate that E2 does not directly regulate adipose tissue lipolysis.
KEYWORDS: adipose tissue organ culture, estrogen, in vitro, lipolysis, in vivo, ovariectomy, rat, adipose
Introduction
Estradiol (17-β-estradiol, E2) is a steroid hormone capable of exerting genomic (chronic), and rapid/acute (non-genomic) signaling in target cells.1 The pathways by which E2 regulates adipose tissue metabolism are largely unknown, despite the well-characterized binding of E2 to its nuclear receptor (estrogen receptor, ERα or β 2), and a noted ability to influence food intake, energy expenditure and physical activity.3 Estradiol loss, particularly during menopause in humans, has been shown to cause a shift from subcutaneous to visceral fat storage and derangements in lipolytic responsiveness.4,5 There is some evidence in humans that E2 is antilipolytic through its potentiation of insulin's suppression of lipolysis,6 and inhibition of catecholamine-stimulated lipolysis in subcutaneous adipose tissue.7
Our understanding of the mechanisms by which E2 regulates lipolysis in vivo are largely derived from the ovariectomized (OVX) rodent model. Whole-body circulating FFA concentrations are decreased in OVX mice, but increased after treatment with E2 for 60 d.8 Further evidence points to visceral adipose tissue as a site of impaired lipolyis in the absence of E2. Reduced adenylate cyclase activity and cAMP production have been observed in visceral adipose tissue from OVX animals.9 In OVX mice, there is a reduced translocation of adipose triglyceride lipase (ATGL) to the lipid droplet and blunted Ser660 phosphorylation of hormone sensitive lipase (HSL) in response to acute exercise.10 In visceral adipocytes isolated from OVX animals, basal and catecholamine-stimulated lipolysis are lower compared with controls, which is restored after 4–30 d of E2 replacement.8,11-13 Few studies have explored the relationship between E2 status and subcutaneous adipose tissue lipolysis. In adipocytes isolated from subcutaneous abdominal tissue in humans, Epi-stimulated lipolysis is attenuated in the presence of 100 nM E2 for 24 hours.14 In rats, the evidence is equivocal; in one study, adipocytes isolated from femoral subcutaneous adipose in OVX rats were not different from SHAM controls in their response to isoproterenol.9 In another study, OVX rats showed a 50% reduction in β2-stimulated glycerol release from subcutaneous adipose tissue, as measured by microdialysis, which was restored after 7 d of E2 treatment.15 Although these studies provide some insight into the effects of E2 loss on adipose tissue metabolism, the majority fail to control for hyperphagia by pair-feeding, thereby making it difficult to conclude whether the chronic effects of E2 on lipolysis are directly due to E2 per se, or rather due to secondary effects subsequent to reductions in energy intake16 or changes in fat cell size.8 Altogether, the role of E2 on lipolysis is somewhat discrepant, particularly regarding its ability to modulate subcutaneous adipose tissue.
In terms of rapid, non-genomic signaling, E2 has been shown to directly and acutely activate Akt and AMPK signaling in muscle17,18; however, it is uncertain whether E2 acutely increases functional markers of lipolysis (FFA, glycerol) and relevant signaling pathways in adipose tissue. To address this, one study infused conjugated estrogen (CE) for one hour and found a reduced rate of glycerol appearance from subcutaneous adipose tissue in postmenopausal women.19 However, infusion of rats with the E2 analog moxestrol had no effect on lipolysis.15 Therefore, it remains inconclusive whether E2 can acutely modulate lipolysis in adipose tissue, and whether there might be depot-specific responses to E2.
The overall aim of this study was to determine the role of E2 in regulating adipose tissue lipolysis under basal and stimulated conditions. This was tested in subcutaneous and visceral adipose depots, using both in vivo and tissue culture approaches. OVX rats were pairfed to SHAM controls to control for differences in energy intake imparted by E2 loss, to mitigate potential differences in weight gain due to hyperphagia as a confounding factor.
Results
Pairfeeding does not prevent a greater body mass increase in OVX rats, but daily estradiol treatment slows the rate of gain in OVX rats
Daily caloric intake was similar between groups, indicating successful pair feeding (Fig. 1A). Despite this, body mass was significantly increased in OVX and OVX E2 rats at week one. By week 2 OVX rats were still significantly heavier, but OVX E2 rats were no longer different from SHAM controls (Fig. 1B). Both OVX and OVX E2 groups had significantly more weight gain during weeks 1–2, and this decreased in all groups between weeks 2 and 3 (inset Fig. 1B). Interestingly, total r.p. fat pad mass did not differ between groups, but OVX and OVX E2 rats had significantly more iWAT (3.66 ± 0.25 and 3.14 ± 0.15 versus 2.64 ± 0.15 g, Table 1). Uterine mass was significantly less in OVX and OVX E2 groups compared with SHAM controls, indicating atrophy and successful ovary removal in OVX and OVX E2 rats vs. SHAM controls (0.67 ± 0.07 g SHAM, 0.12 ± 0.01 g OVX and 0.12 ± 0.01 g OVX E2). Circulating estradiol levels at the time of terminal surgery, 24 hours without administration of E2 or vehicle control, were 29.4 ± 3.2, 16.5 ± 2.5 and 26.0 ± 5.0 pg/mL in SHAM, OVX and OVX E2 groups.
Figure 1.
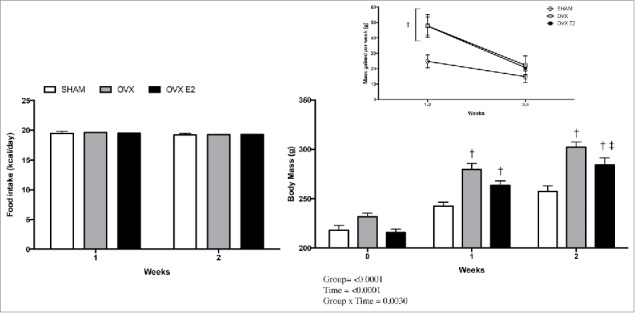
Food intake (A), body mass (B) and (inset) rate of increase in body mass in SHAM, OVX and OVX E2 rats. Data are presented as mean ± SEM. Statistical significance is accepted at p < 0.05; † denotes significant difference compared with SHAM control within weekly time point, ‡ denotes significant difference compared with OVX within weekly time point.
Table 1.
Fat pad mass and serum insulin in SHAM, OVX and OVX E2 rats. Data are presented as mean ± SEM; n = 12 for each group. Statistical significance is accepted at p < 0.05; * denotes significant difference compared with SHAM rats.
| SHAM | OVX | OVX E2 | |
|---|---|---|---|
| r.p fat pad mass (g) | 3.62 ± 0.31 | 3.48 ± 0.25 | 3.41 ± 0.22 |
| iWAT fat pad mass (g) | 2.67 ± 0.15 | 3.67 ± 0.25* | 3.14 ± 0.15 |
| Insulin (pg/mL) | 5.39 ± 0.27 | 4.68 ± 1.45 | 4.43 ± 1.06 |
Adipocyte cross-sectional area of iWAT from OVX rats is slightly elevated
Since iWAT fat pad mass was greater in OVX rats vs the SHAM or OVX E2 groups, we assessed adipocyte size and number in subcutaneous and visceral fat depots in OVX and OVX E2 rats. Although not statistically significant, cross-sectional area was 40% and 26% greater in iWAT from OVX rats compared with SHAM and OVX E2 rats, respectively (Fig. 2A and B). We next measured the frequency distribution of cross-sectional areas. OVX rats tended to have a greater fraction of adipocytes over 6000 um2, but these changes were not significantly different (Fig. 2C and D). Finally, mean adipocyte number was increased 38% in OVX iWAT relative to SHAMs, but this also did not reach statistical significance (data not shown).
Figure 2.
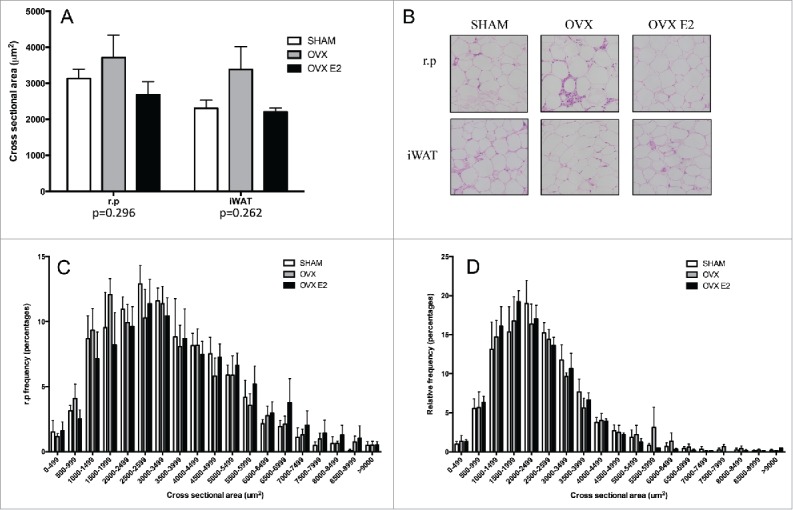
Adipocyte cross sectional area and relative frequency % histograms in r.p. and iWAT tissue. (A) Subcutaneous (iWAT) and r.p cells tend to be larger in OVX rats (p = 0.262 and 0.292, respectively) and (B) shows representative images of H&E stains in both depots. (C) Histogram of retroperitoneal white adipose tissue (r.p.) cross sectional area and (D) inguinal (iWAT). Data are presented as mean ± SEM; n = 5 per adipose depot per group.
In vivo, basal and CL−stimulated serum FFAs are reduced in OVX and OVX E2 rats, but not glycerol or HSL activation
To assess in vivo lipolytic response, rats were injected with a body mass adjusted dose of CL 316, 243, a specific β3 adrenergic agonist. Circulating FFAs under both basal and CL−stimulated conditions were reduced in OVX and OVX E2 rats vs. the SHAM group, as indicated by a significant group effect (Fig. 3A). This was unique to FFAs, as glycerol concentrations were similar among SHAM, OVX and OVX E2 groups in basal and CL stimulated conditions (Fig. 3B).
Figure 3.
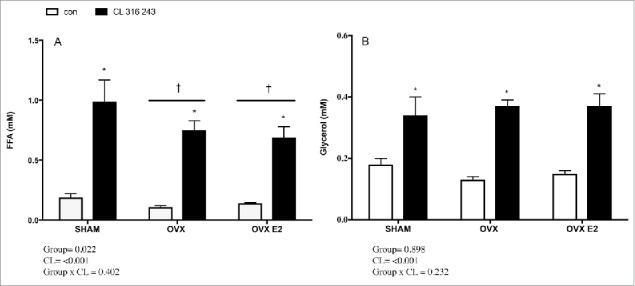
Serum FFA (A) and glycerol (B) concentrations in control or CL 316, 243 treated rats. Data are presented as mean ± SEM; n = 6 for control and CL groups in SHAM, OVX and OVX E2 groups. Statistical significance is accepted at p < 0.05; * denotes significant Treatment (CL) effect compared with own control (ie. SHAM CL vs. SHAM con); † denotes significant group effect compared with SHAM control.
Phosphorylation of HSL activation sites (Ser660 and Ser563) following in vivo injection of CL were measured by western blotting of r.p. (Fig. 4A–B) and iWAT (Fig. 4C–D) samples in an effort to address potential mechanisms underlying reduced FFAs. Adrenergic stimulation increased HSL phosphorylation as expected; however, there were no significant differences between groups in either basal or CL−treated phospho-HSL in either depot. Total HSL content was not different between groups (data not shown).
Figure 4.
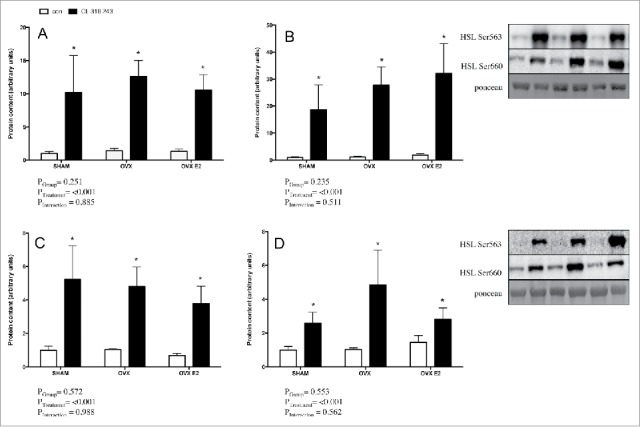
Lipolytic signaling protein content in r.p. and iWAT depots (in vivo) from control or CL 316,243 treated rats. In the r.p. depot A) p-HSL Ser660 and B) p-HSL Ser563; and in the iWAT depot C) p-HSL Ser660 and D) p-HSL Ser563. Data are presented as mean ± SEM; n = 6 for control and CL groups in SHAM, OVX and OVX E2. Statistical significance is accepted at p < 0.05; * denotes significant treatment effect compared with own control.
Circulating insulin is not different in OVX rats vs. E2-treated OVX or SHAM controls
To determine whether the blunted FFA concentrations observed in vivo in fasted OVX rats can be attributed to a systemic factor suppressing lipolysis, we measured circulating insulin concentrations in saline- treated SHAM, OVX and OVX E2 rats. There were no differences in insulin concentrations between groups (Table 1).
Ex vivo lipolytic response is normal in adipose explants from OVX rats
To address whether depot-specific lipolytic alterations exist as a function of E2 status, adipose tissue explant experiments were conducted using subcutaneous (iWAT) and visceral (r.p.) adipose from all 3 groups (SHAM, OVX and OVX E2). CL significantly increased FFA and glycerol accumulation in media from r.p. fragments in all 3 groups, (Fig. 5A and B). There was a trend toward group differences in FFA (p = 0.06) and glycerol (p = 0.08) concentrations, but this was not significant. Robust CL−mediated increases in p-HSL Ser660 and Ser563 were evident (Fig. 5C and D), but were not affected by E2 status. There were no significant changes in total HSL content between groups or with CL treatment (data not shown).
Figure 5.
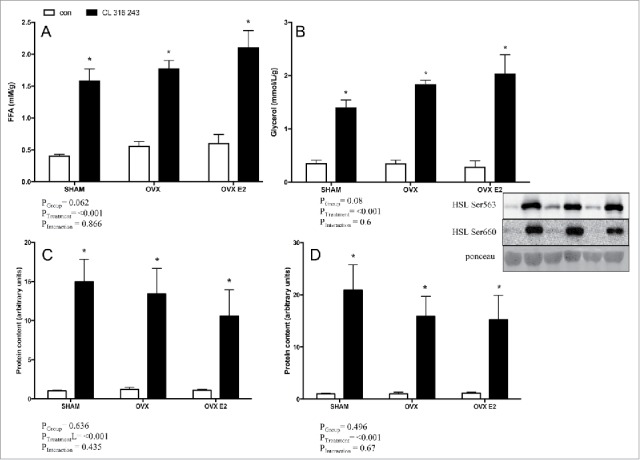
FFA (A) and glycerol (B) concentrations in explant media from r.p. depots in control or CL 316, 243 treated rats. Panels C) and D) show protein content of p-HSL Ser600 p-HSL Ser563 in the r.p. depot. Data are presented as mean ± SEM; n = 6 for control and CL groups in SHAM, OVX and OVX E2 groups. Statistical significance is accepted at p < 0.05; * denotes significant treatment effect compared with own control.
A clear CL effect was also present in functional (FFA, glycerol) and western blotting (p-HSL) measurements in iWAT explants. Similar to the r.p. depot, increases in FFA, glycerol and p-HSL Ser563 and Ser660 were not different between SHAM, OVX, or OVX E2 groups (Fig. 6A–D). Total HSL was not significantly different across SHAM, OVX or OVX E2 groups (data not shown).
Figure 6.
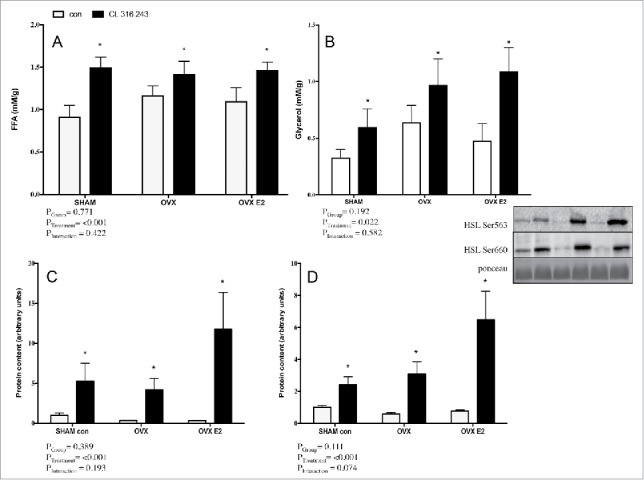
FFA (A) and glycerol (B) concentrations in adipose tissue explant media from iWAT depots in control or CL 316,243 treated rats. Panels C) and D) show protein content of p-HSL Ser600 p-HSL Ser563 in the iWAT depot. Data are presented as mean ± SEM; n = 6 for control and CL groups in SHAM, OVX and OVX E2 groups. Statistical significance is accepted at p < 0.05; * denotes significant treatment effect compared with own control.
Estradiol does not directly alter basal or CL−stimulated lipolysis in adipose tissue organ culture (ATOC)
Since changes in circulating FFA concentrations were observed in OVX rats, we conducted separate adipose tissue organ culture (ATOC) experiments to assess whether E2 has a direct effect on FFA release in isolated adipose tissue. These experiments were performed separately from the ex vivo experiments in which tissue was harvested to assess basal and CL−induced lipolysis in SHAM, OVX and OVX E2 conditions. The ATOC method utilizes ∼5–10 mg adipose fragments, first cultured for 24 hours to wash out systemic factors, followed by treatment to assess the direct effects of CL and E2 on adipose tissue metabolism. In normal Sprague Dawley female rats, treatment with E2 (10 nM) did not have a significant effect on FFA accumulation in the media from either adipose depot at 2, 4 or 8 hours (Fig. 7A and B). As expected, CL markedly increased the accumulation of FFA in the media, but this was not potentiated or suppressed by E2. Thus, differences in lipolytic function observed in Figure 3 do not appear to be directly due to E2, at least at the particular doses used.
Figure 7.
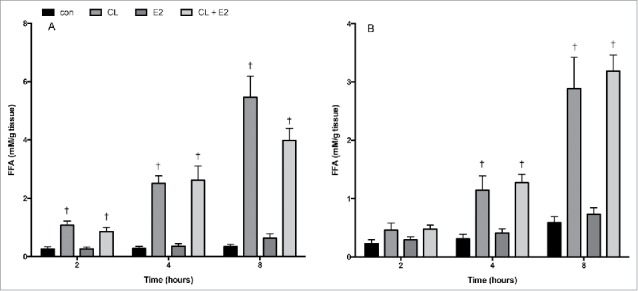
Time course of FFA release in control or CL 316,243 treated adipose fragments. Adipose tissue harvested from r.p. (A) and iWAT (B) were treated ex vivo with CL (1 uM), E2 (10 nM), or CL + E2 (1 uM + 10 nM) for 2, 4 and 8 hrs. Data are presented as mean ± SEM; n = 6 for control and CL groups in SHAM, OVX and OVX E2 groups. Statistical significance is accepted at p < 0.05; † denotes significant difference compared with control at specific time point.
Discussion
Estradiol loss is associated with increased adiposity in women.5,20 E2 or HRT administration can prevent adipose accumulation in rodents,11,21-23 and preserve gynoid fat distribution in women.24 However, the direct effects of E2 on basal and catecholamine-stimulated lipolysis in adipose tissue have been largely unexplored. In the present study, OVX rats demonstrated lower circulating FFA levels, suggestive of impaired lipolysis. However, circulating glycerol concentrations were unaffected by E2 and in vivo lipolytic responsiveness and HSL activation with CL 316, 243 were not reduced in OVX rats. Ex vivo lipolysis and activation of HSL were not impaired in r.p. or iWAT explants from OVX rats, or altered with oral E2 treatment. Lastly, E2 did not directly increase lipolysis in r.p. or iWAT tissue. Our findings suggest that adipose tissue lipolysis is not directly altered by the presence or absence of E2.
The in vivo effects of E2 on lipolysis are sparse and yield conflicting results. In humans, Van Pelt et al. found that acute conjugated estrogen (CE) infusion reduced basal glycerol release from subcutaneous adipose depots in postmenopausal women by 8–16%,19 yet found no differences in systemic, circulating glycerol6 following CE infusion.19 In rats, Darimont et al. reported a 50% decrease in basal lipolysis in parametrial adipose tissue 8 d post-OVX, as measured by in situ microdialysis.15 In contrast to humans, reduced basal lipolysis was increased after 7 d of subcutaneous E2 injection. In our OVX and OVX E2 rats, in vivo serum FFA, but not glycerol concentrations were reduced 2 weeks post-surgery vs. SHAM controls. Daily E2 supplementation did not restore FFA concentrations. Reduced FFAs could be caused by insulin-mediated suppression of lipolysis; however, several studies in accordance with ours, show similar circulating insulin in human subjects with and without CE infusion and between intact and OVX rodents.8,10,19,25
Multiple factors could account for in vivo discrepancies between studies: sampling of subcutaneous vs. parametrial fat pads, CE vs. E2 administration, or species-specificity in β-adrenergic receptor distribution among female humans and rodents.26 Additionally, all studies previous to ours quantified glycerol accumulation or rate of appearance as the sole functional lipolysis marker. It is possible that E2 loss or repletion affect glycerol and FFA efflux or metabolism differently.27 Measuring serum FFAs in circulation imposes limitations to our interpretations, as FFA uptake by peripheral tissues, such as adipose and/or liver, and oxidation by muscle and liver were not measured and would presumably affect systemic concentrations. Furthermore, specific activities of HSL and adipose triglyceride lipase (ATGL) were not measured. While pHSL Ser563 and Ser 660 phosphorylation residues are commonly measured to assess adrenergic activity, it is possible that E2 could be eliciting an effect on enzyme activity at the dose administered in vivo or ex vivo.
Whether E2 status affects catecholamine response in vivo has been sparsely examined and the findings are equivocal. Treatment of female and male rats with E2 has been reported to increase lipolytic responsiveness to catecholamines in isolated adipocytes.13,28 In humans, a single dose of E2 attenuated catecholamine-stimulated lipolysis in femoral subcutaneous adipose.7 In our hands, CL was equally effective in increasing whole-body lipolysis in SHAM, OVX and OVX E2 rats. Unlike Darimont et al.,15 who found increased responsiveness of subcutaneous adipose to isoproterenol infusion after 7 d of E2 injection, we did not observe an augmentation of serum FFA or glycerol in OVX E2 rats after 2 weeks of treatment.
To gain insight into depot-specific lipolytic responses in OVX rats, we sampled visceral (r.p.) and subcutaneous (iWAT) tissues from saline and CL−injected groups to assess HSL signaling. In vivo, CL increased HSL phosphorylation in r.p. adipose tissue at Ser660 and Ser563, but were not different across groups. Observations were similar in iWAT. A low dose of ethinyl E2 for 10 d or high dose for 3 d have each been observed to increase HSL activity in rats with intact ovaries.13 However, our study and others8 suggest that E2 may not directly regulate HSL, since total HSL, p-HSL Ser660 and 563 protein content in adipose and HSL mRNA expression in adipocytes did not change with OVX or OVX E2.8
In vitro, visceral adipocytes from OVX rodents have shown reduced catecholamine responsiveness.9 In vivo E2 treatment of 4–30 d augmented in vitro adipocyte lipolytic response to various β2 agonists in OVX E2 rats vs. OVX.11,29-31 Each of these studies examined adipocytes isolated by collagenase, devoid of additional cell types and typical crosstalk in adipose tissue. In our study, using explants, there were no differences in FFA or glycerol release, or HSL phosphorylation in either adipose depot regardless of E2 status. The changes we32 and others.7,14 have observed in subcutaneous tissue suggest it may be more affected by the chronic absence of E2. Pedersen et al. found that women taking HRT had significantly elevated α2A antilipolytic receptor mRNA which was specific to subcutaneous fat.14 Subcutaneous adipose tissue explants from healthy subjects treated with 100 nM E2 for 24 hours also showed elevated α2A mRNA and receptor binding, coupled with an attenuated response of isolated adipocytes to epinephrine.14 Taken together, this would suggest that E2, at least in humans, could attenuate lipolytic response to catecholamines by upregulating the α2A antilipolytic system, thereby acting as a brake on FFA and/or glycerol release. To gain more insight and further examine possible mechanisms behind direct, acute effects of E2 on lipolysis, we treated isolated iWAT and r.p. adipose fragments (ATOC) from female rats with E2 or E2 + CL for up to 8 hrs ex vivo and measured FFA release. At the E2 dose administered (10 nM), there was no direct effect on stimulating lipolysis either with or without CL.
In conclusion, basal and β3-stimulated FFA, but not glycerol, mobilization in OVX and OVX E2 rats were lower than SHAM controls in vivo, in the absence of any change in circulating insulin concentrations. Ex vivo, basal and CL−stimulated lipolysis and HSL activation were not impaired in subcutaneous or visceral adipose from OVX rats. Neither in vivo or ex vivo indices of lipolysis were altered by physiologic doses of E2 for 2 weeks, but the interaction between SHAM, OVX, OVX E2. Acute E2 had no direct effect on indices of lipolysis, or CL−induced lipolysis in either adipose depot in vitro. Thus, changes in adiposity and circulating FFA in the absence of E2 do not seem to be due to the loss of a direct stimulatory effect of E2 on lipolysis.
Methods
Animal care
All procedures were approved by the University of Guelph Animal Care Committee and followed Canadian Council for Animal Care guidelines. Female Sprague Dawley rats were purchased from Charles River Laboratories at 4 months of age, ∼250 g. Two days before arrival, all rats underwent either bilateral ovariectomy (OVX; 24 animals total) or SHAM surgery (SHAM; 12 animals total) under ketamine–atropine–xylazine anesthesia by Charles River technicians. Proper technical surgery was verified during terminal experiments by recording uterine weight, which was significantly atrophied in OVX and OVX E2 rats vs. SHAM controls (0.67 ± 0.07 g SHAM, 0.12 ± 0.01 g OVX and 0.12 ± 0.01 g OVX E2).
SHAM and OVX animals were housed 4 animals per/cage in a temperature (23°C) controlled room on a 12:12 hour standard light- dark cycle. All rats consumed a phytoestrogen-purified, soy protein free diet (Harlan 2020X) with 16% of calories from fat. Since OVX rats become markedly hyperphagic post-surgery, a pair-feeding regime was used. All rats had body mass recorded weekly. For the 2–8 hour lipolysis time course experiments, female Sprague Dawley rats (with ovaries intact) were received from Charles River at ∼250–300 g and were acclimated in similar housing conditions for one week before final experiments. These rats were allowed ad libitum access to Harlan 2020X diet and were not fasted before tissue harvesting.
Estradiol dosing protocol
Half of the OVX rats (n = 12) were assigned to oral E2 treatment on a daily basis. Rats were administered a dose of 17-β-estradiol (E2) delivered via chocolate hazelnut spread (Nutella), as described by Ingberg et al. (Ingberg et al. 2012). A concentrated stock solution was made weekly by dissolving powdered E2 in sesame oil, which was further diluted and mixed to yield a dose of 28 μg E2/5 μL sesame oil/1 g nutella spread per kg body mass each day. All rats consumed the dose of E2 in less than a minute. SHAM and OVX rats not receiving E2 received a similar amount of Nutella with sesame oil as a control.
Terminal surgeries and in vivo lipolysis assessment
Animals were not fasted overnight before terminal experiments to avoid increases in baseline lipolysis in experiments with adipose explants or adipose tissue organ culture (ATOC). Rats were anaesthetized with an intraperitoneal injection of pentobarbital sodium (6 mg per 100 g body mass). Half of each SHAM, OVX or OVX E2 group (n = 6) received a body mass adjusted saline injection and had ∼200 mg from each depot partitioned toward explant experiments (detailed below). The other half (n = 6) of each group of animals received a 1 mg/kg dose of the β3 agonist CL 316,243 (CL) to assess in vivo lipolytic function. This compound has been used in other studies to stimulate lipolysis in white adipose tissue.33,34 30 minutes post-saline or CL injection, inguinal adipose tissue (iWAT) was removed as a representative subcutaneous depot; retroperitoneal (r.p.) adipose tissue was harvested from behind the kidneys as a representative visceral depot. Total fat pads were carefully removed from each animal and weighed. iWAT and r.p. samples were frozen for subsequent analysis and stored at −80°C. A terminal blood sample was obtained via cardiac puncture, left at room temperature for 30 minutes and centrifuged at 1500 x g for 10 minutes to collect serum, which was then stored at −80°C for subsequent analyses.
Histological analysis
Five micrometer sections were mounted on 1.2- mm Superfrost slides, stained with modified Harris hematoxylin and eosin stock with phloxine (Fisher Scientific), and imaged (Olympus FSX 100 light microscope, Olympus, Tokyo, Japan). Two images per depot from each animal (∼250–300 total cells) were captured and used to determine cross-sectional area (ImageJ software, National Institute of Mental Health, Bethesda, MD). An estimation of cell number was calculated as described previously.17,24 Briefly, the diameter of the cell (determined from cross-sectional area) was used and cells were assumed to be spherical; thus the adipocyte volume could be calculated. The density of the lipid in the cell (0.915 g/mL) was then used to calculate the mass of a single cell.35 Finally, the mass of the fat pad was divided by the mass of a single cell to estimate cell number.
Adipose tissue explants
To assess lipolysis in vitro, adipose tissue explants from SHAM, OVX and OVX E2 rats were prepared immediately after terminal surgeries. Adipose tissue (iWAT and r.p.) was harvested from saline-injected rats and ∼ 150 mg placed into petri dishes with 3 mL of M199 supplemented with 1% antibiotic/antimycotic, 2.5 nM dexamethasone, 0.1 g/L L-glutamine and 2% BSA. Tissue was cut into ∼50 mg fragments and left to equilibrate for 2 hours in a cell incubator at 37 °C and 95% CO2/5% O2. Following 2 hours, cultures were treated with sterile H2O (control) or 1 μM CL 316,243 for another 2 hours. At the end of the experiment, 200 μL of media was collected, adipose tissue was rinsed in ice-cold PBS, strained, frozen in liquid nitrogen and stored at −80°C for further analyses. Tissue mass was recorded to normalize FFA and glycerol accumulation per gram of adipose tissue.
Adipose tissue organ culture
A separate set of experiments was completed using female Sprague Dawley rats to assess the direct and combined effects of E2 and CL on adipose tissue lipolysis. Adipose tissue organ culture (ATOC) is a well-characterized method used to analyze isolated changes in adipose tissue metabolism.36 We used this approach to wash out any effects of circulating E2 on lipolysis before testing for direct effects. Animals in the fed state were anaesthetized with an intraperitoneal injection of pentobarbital sodium (6 mg per 100 g body mass), and iWAT and r.p. depots were excised. Four separate dishes per. depot were prepared with ∼200 mg sections of adipose tissue. Tissue in each dish was minced into 5–10 mg fragments and placed into 4.5 mL M199 supplemented with 1% antibiotic/antimycotic, 50 μU insulin and 2.5 nM dexamethasone. Samples were left to equilibrate for 24 hours at 37°C, at which point the media was removed and changed to M199 supplemented with the same doses of antibiotic and dexamethasone, 2.5% bovine serum albumin (BSA) and no insulin. For the 2–8 hour time course experiment, one dish per depot was treated with each of the following conditions: i) sterile H2O (control), ii) 1 μM CL 316,243, iii) 10 nM 17-β-estradiol, or iv) 1 μM CL 316,243 plus 10 nM 17-β-estradiol. At 2, 4 and 8 hours, 200μL of media was sampled and snap frozen for later analysis. After 8 hours, tissue was rinsed with ice-cold phosphate buffered saline (PBS), strained, and adipose tissue minces collected and frozen in liquid nitrogen and stored at −80°C for later analysis.
Western blotting
Clamp-frozen iWAT and r.p. adipose samples were homogenized in a 2: 1 volume-to-weight ratio of NP40 cell lysis buffer supplemented with Protease Inhibitor Cocktail (PIC) and phenylmethylsulfonyl fluoride (PMSF) using a Fast Prep-24 Homogenizer. Lysed samples were centrifuged for 15 minutes at 2500 g at 4°C to separate the protein supernatant and remaining fat cake. The protein concentration of the supernatant was determined using the BCA method and the CV for this assay is accepted at <5%. Equal amounts of protein (20 μg) were loaded and separated on 10% acrylamide gels and wet transferred onto nitrocellulose membrane for 1 hour at 200 mA. Membranes were blocked in Tris buffered saline–0.01% tween (TBST) supplemented with 5% non-fat dry milk at room temperature for 1 hour with gentle shaking, rinsed with TBST and further incubated in TBST plus 5% BSA-diluted primary antibodies (1:1000) overnight at 4°C with gentle shaking. After 24 hours membranes were briefly washed in TBST and then incubated in TBST–1% non-fat dry milk supplemented with HRP conjugated secondary antibody (donkey anti-rabbit) for 60 minutes at room temperature. Bands were visualized using ECL and captured using the α Innotech Imaging system. Each membrane was stained with ponceau and imaged to confirm equal loading.
Free fatty acid and glycerol concentrations
Free fatty acid (FFA) and glycerol concentrations in serum from saline and CL−injected rats, and from all ATOC/explant media samples, were measured using commercially available kits (Wako Diagnostics, HR Series NEFA(HR), Sigma, MAK117) as described previously.37,38
Insulin and estradiol ELISAs
Serum samples from saline-injected SHAM, OVX and OVX E2 rats were analyzed for insulin concentration in duplicate, using a commercially available kit (Millipore EZMRI-13K), as described previously.39,40 Accuracy and intra-assay comparisons were validated using 2 quality control standards (run in triplicate) provided with each kit. Terminal serum samples were analyzed for estradiol concentration in all groups, using a commercially available kit (Abcam, 108667).
Statistics
All data are expressed as mean ± SEM. For comparing caloric intake and body mass, a 2-way repeated measures ANOVA was used to assess group differences at each week and the interaction between Group x Time was analyzed. For measures involving CL 316,243 treatment in explants from SHAM, OVX and OVX E2 groups, a 2-way ANOVA was used and the interaction between Group x Treatment (CL) was analyzed. A 2-way repeated measures ANOVA was used to assess differences in the 2, 4 and 8 hour ATOC time course experiment. For adipocye size measured by frequency distribution histograms, one way ANOVAs were run at each bin size, per depot. Tukey's post hoc test was used in both ANOVA tests if significant differences were detected. In cases where data failed the Shapiro-Wilk test, a log36 transformation was applied to normalize data. All α values were set to α = 0.05 and significant differences are indicated using symbols visible in the figure legends.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Björnström L, Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 2005; 19:833-842; PMID:15695368; https://doi.org/ 10.1210/me.2004-0486 [DOI] [PubMed] [Google Scholar]
- [2].Foryst-Ludwig A, Kintscher U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J Steroid Biochem Mol Biol 2010; 122:74-81; PMID:20599505; https://doi.org/ 10.1016/j.jsbmb.2010.06.012 [DOI] [PubMed] [Google Scholar]
- [3].Vieira-Potter VJ, Padilla J, Park YM, Welly RJ, Scroggins RJ, Britton SL, Koch LG, Jenkins NT, Crissey JM, Zidon T, Morris EM, Meers GM, Thyfault JP. Female rats selectively bred for high intrinsic aerobic fitness are protected from ovariectomy-associated metabolic dysfunction. Am J Physiol Regul Integr Comp Physiol 2015; 308:R530-42; PMID:25608751; https://doi.org/ 10.1152/ajpregu.00401.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rebuffé-Scrive M, Andersson B, Olbe L, Björntorp P. Metabolism of adipose tissue in intraabdominal depots of nonobese men and women. Metabolism 1989; 38:453-458; PMID:2725284; https://doi.org/ 10.1016/0026-0495(89)90198-4 [DOI] [PubMed] [Google Scholar]
- [5].Toth MJ, Tchernof A, Sites CK, Poehlman ET. Effect of menopausal status on body composition and abdominal fat distribution. Int J Obes 2000; 24:226-231; https://doi.org/ 10.1038/sj.ijo.0801118 [DOI] [PubMed] [Google Scholar]
- [6].Van Pelt RE, Gozansky WS, Schwartz RS, Kohrt WM. Intravenous estrogens increase insulin clearance and action in postmenopausal women. Am J Physiol Endocrinol Metab 2003; 285:E311-7; PMID:12684221; https://doi.org/ 10.1152/ajpendo.00490.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gormsen LC, Høst C, Hjerrild BE, Pedersen SB, Nielsen S, Christiansen JS, Gravholt CH. Estradiol acutely inhibits whole body lipid oxidation and attenuates lipolysis in subcutaneous adipose tissue: a randomized, placebo-controlled study in postmenopausal women. Eur J Endocrinol 2012; 167:543-551; PMID:22872467; https://doi.org/ 10.1530/EJE-12-0422 [DOI] [PubMed] [Google Scholar]
- [8].D'Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, Greenberg AS. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. J Biol Chem 2005; 280:35983-35991; PMID:16109719; https://doi.org/ 10.1074/jbc.M507339200 [DOI] [PubMed] [Google Scholar]
- [9].Lacasa D, Agli B, Pecquery R, Giudicelli Y. Influence of Ovariectomy and Regional Fat Distribution on the Membranous Transducing System Controlling Lipolysis in Rat Fat Cells. Endocrinology 1991; 128:747-753; PMID:1846586; https://doi.org/ 10.1210/endo-128-2-747 [DOI] [PubMed] [Google Scholar]
- [10].Wohlers LM, Jackson KC, Spangenburg EE. Lipolytic signaling in response to acute exercise is altered in female mice following ovariectomy. J Cell Biochem 2011; 112:3675-3684; PMID:21815195; https://doi.org/ 10.1002/jcb.23302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pasquier Y-N, Pecquery R, Giudicelli Y. Increased adenylate cyclase catalytic activity explains how estrogens “in vivo” promote lipolytic activity in rat white fat cells. Biochem Biophys Res Commun 1988; 154:1151-1159; PMID:2457367; https://doi.org/ 10.1016/0006-291X(88)90261-6 [DOI] [PubMed] [Google Scholar]
- [12].Pedersen SB, Børglum JD, Møller-Pedersen T, Richelsen B. Effects of in vivo estrogen treatment on adipose tissue metabolism and nuclear estrogen receptor binding in isolated rat adipocytes. Mol Cell Endocrinol 1992; 85:13-19; PMID:1526313; https://doi.org/ 10.1016/0303-7207(92)90120-U [DOI] [PubMed] [Google Scholar]
- [13].Benoit V, Valette A, Mercier L, Meignen JM, Boyer J. Potentiation of epinephrine-induced lipolysis in fat cells from estrogen-treated rats. Biochem Biophys Res Commun 1982; 109:1186-1191; PMID:7168762; https://doi.org/ 10.1016/0006-291X(82)91902-7 [DOI] [PubMed] [Google Scholar]
- [14].Pedersen SB, Kristensen K, Hermann PA, Katzenellenbogen JA, Richelsen B. Estrogen controls lipolysis by up-regulating α2A-adrenergic receptors directly in human adipose tissue through the estrogen receptor α. Implications for the female fat distribution. J Clin Endocrinol Metab 2004; 89:1869-1878; PMID:15070958; https://doi.org/ 10.1210/jc.2003-031327 [DOI] [PubMed] [Google Scholar]
- [15].Darimont C, Delansorne R, Paris J, Ailhaud G, Negrel R. Influence of estrogenic status on the lipolytic activity of parametrial adipose tissue in vivo: an in situ microdialysis study. Endocrinology 1997; 138:1092-6; PMID:9048614 [DOI] [PubMed] [Google Scholar]
- [16].Ainslie D a, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. Int J Obes Relat Metab Disord 2001; 25:1680-8; PMID:11753591; https://doi.org/ 10.1038/sj.ijo.0801806 [DOI] [PubMed] [Google Scholar]
- [17].Rogers NH, Witczak CA, Hirshman MF, Goodyear LJ, Greenberg AS. Estradiol stimulates Akt, AMP-activated protein kinase (AMPK) and TBC1D1/4, but not glucose uptake in rat soleus. Biochem Biophys Res Commun 2009; 382:646-650; PMID:19265681; https://doi.org/ 10.1016/j.bbrc.2009.02.154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].D'Eon TM, Rogers NH, Stancheva ZS, Greenberg AS. Estradiol and the estradiol metabolite, 2-hydroxyestradiol, activate AMP-activated protein kinase in C2C12 myotubes. Obesity (Silver Spring) 2008; 16:1284-1288; PMID:18421261; https://doi.org/ 10.1038/oby.2008.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Van Pelt RE, Gozansky WS, Hickner RC, Schwartz RS, Kohrt WM. Acute modulation of adipose tissue lipolysis by intravenous estrogens. Obesity 14:2163-72, 2006; PMID:17189542; https://doi.org/ 10.1038/oby.2006.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lovejoy JC, Champagne CM, De Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes 2008; 32:949-58; https://doi.org/ 10.1038/ijo.2008.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gray JM, Wade GN. Food intake, body weight, and adiposity in female rats: actions and interactions of progestins and antiestrogens. Am J Physiol 1981; 240:E474-81; PMID:7195153 [DOI] [PubMed] [Google Scholar]
- [22].Ramirez I. Estradiol-induced changes in lipoprotein lipase, eating, and body weight in rats. Am J Physiol 1981; 240:E533-8; PMID:7235008 [DOI] [PubMed] [Google Scholar]
- [23].Steingrimsdottir L, Brasel J, Greenwood MR. Hormonal modulation of adipose tissue lipoprotein lipase may alter food intake in rats. Am J Physiol Metab 1980; 239:E162-E167 [DOI] [PubMed] [Google Scholar]
- [24].Reubinoff BE, Wurtman J, Rojansky N, Adler D, Stein P, Schenker JG, Brzezinski A. Effects of hormone replacement therapy on weight, body composition, fat distribution, and food intake in early postmenopausal women: a prospective study. FertilSteril 1995; 64:963-968 [DOI] [PubMed] [Google Scholar]
- [25].Godsland IF, Gangar K, Walton C, Cust MP, Whitehead MI, Wynn V, Stevenson JC. Insulin resistance, secretion, and elimination in postmenopausal women receiving oral or transdermal hormone replacement therapy [Online]. Metabolism 1993; 42:846-853; PMID:8345794; https://doi.org/ 10.1016/0026-0495(93)90058-V [DOI] [PubMed] [Google Scholar]
- [26].Langin D, Portillo MP, Saulnier-Blache JS, Lafontan M. Coexistence of three beta-adrenoceptor subtypes in white fat cells of various mammalian species. Eur J Pharmacol 1991; 199:291-301; PMID:1680716; https://doi.org/ 10.1016/0014-2999(91)90492-9 [DOI] [PubMed] [Google Scholar]
- [27].Wohlers LM, Spangenburg EE. 17beta-estradiol supplementation attenuates ovariectomy-induced increases in ATGL signaling and reduced perilipin expression in visceral adipose tissue. J Cell Biochem 2010; 110:420-427; PMID:20336671 [DOI] [PubMed] [Google Scholar]
- [28].Tomita T, Yonekura I, Okada T, Hayashi E. Enhancement in cholesterol-esterase activity and lipolysis due to 17β-estradiol treatment in rat adipose tissue. Horm Metab Res 1984; 16:525-528; PMID:6500487; https://doi.org/ 10.1055/s-2007-1014840 [DOI] [PubMed] [Google Scholar]
- [29].Hansen FM, Fahmy N, Nielsen JH. The influence of sexual hormones on lipogenesis and lipolysis in rat fat cells. [Online]. Acta Endocrinol (Copenh) 1980; 95:566-70; PMID:7456980 [DOI] [PubMed] [Google Scholar]
- [30].Rebuffé-Scrive M. Sex steroid hormones and adipose tissue metabolism in ovariectomized and adrenalectomized rats. Acta Physiol Scand 129:471-7, 1987; PMID:3296658; https://doi.org/ 10.1111/j.1365-201X.1987.tb10620.x [DOI] [PubMed] [Google Scholar]
- [31].Pecquery R, Leneveu M-C, Giudicelli Y. Evidence that the lipolytic defect induced by estradiol-treatment in hamster adipocytes is related to an estrogen receptor-mediated defect in the adenylate cyclase catalytic subunit but not in N S. Biochem Biophys Res Commun 1987; 145:369-375; PMID:3036124; https://doi.org/ 10.1016/0006-291X(87)91331-3 [DOI] [PubMed] [Google Scholar]
- [32].MacDonald TL, Ritchie KL, Davies S, Hamilton MJ, Cervone DT, Dyck DJ. Exercise training is an effective alternative to estrogen supplementation for improving glucose homeostasis in ovariectomized rats. Physiol Rep 2015; 3:e12617; PMID:26603453; https://doi.org/ 10.14814/phy2.12617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Castellani L, Root-Mccaig J, Frendo-Cumbo S, Beaudoin M-S, Wright DC. Exercise training protects against an acute inflammatory insult in mouse epididymal adipose tissue. J Appl Physiol 2014; 116:1272-80; PMID:24674860; https://doi.org/ 10.1152/japplphysiol.00074.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Galitzky J, Langin D, Verwaerde P, Montastruc JL, Lafontan M, Berlan M. Lipolytic effects of conventional beta 3-adrenoceptor agonists and of CGP 12,177 in rat and human fat cells: preliminary pharmacological evidence for a putative beta 4-adrenoceptor. Br J Pharmacol 1997; 122:1244-1250; PMID:9401793; https://doi.org/ 10.1038/sj.bjp.0701523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].MacPherson REK, Gamu D, Frendo‐Cumbo S, Castellani L, Kwon F, Tupling AR, Wright DC. Sarcolipin knockout mice fed a high‐fat diet exhibit altered indices of adipose tissue inflammation and remodeling. Obesity 2016; 24:1499-1505; PMID:27345961; https://doi.org/ 10.1002/oby.21521 [DOI] [PubMed] [Google Scholar]
- [36].Fried SK, Moustaid-Moussa N. Adipose tissue protocols. Vol. 155, Methods in molecular biology. New York: Springer; c2001. Chapter 17, Culture of adipose tissue and isolated adipocytes; pp. 197–212. doi: 10.1385/1-59259-231-7:197. [DOI] [PubMed] [Google Scholar]
- [37].Wan Z, Ritchie I, Beaudoin MS, Castellani L, Chan CB, Wright DC. IL-6 indirectly modulates the induction of glyceroneogenic enzymes in adipose tissue during exercise. PLoS One 2012; 7:e41719; PMID:22844518; https://doi.org/ 10.1371/journal.pone.0041719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wan Z, Root‐Mccaig J, Castellani L, Kemp BE, Steinberg GR, Wright DC. Evidence for the role of AMPK in regulating PGC‐1 alpha expression and mitochondrial proteins in mouse epididymal adipose tissue. Obesity 2014; 22:730-738; PMID:23963743; https://doi.org/ 10.1002/oby.20605 [DOI] [PubMed] [Google Scholar]
- [39].Beaudoin M-S, Snook L a, Arkell AM, Simpson J a, Holloway GP, Wright DC. Resveratrol supplementation improves white adipose tissue function in a depot-specific manner in Zucker diabetic fatty rats. Am J Physiol Regul Integr Comp Physiol 2013; 305:R542-51; PMID:23824959; https://doi.org/ 10.1152/ajpregu.00200.2013 [DOI] [PubMed] [Google Scholar]
- [40].Gulli RA, Tishinsky JM, MacDonald T, Robinson LE, Wright DC, Dyck DJ. Exercise restores insulin, but not adiponectin, response in skeletal muscle of high-fat fed rodents. Am J Physiol Integr Comp Physiol 2012; 303:R1062-R1070; https://doi.org/ 10.1152/ajpregu.00176.2012 [DOI] [PubMed] [Google Scholar]


