Abstract
Oscillometric devices are widely used for automatic cuff blood pressure (BP) measurement. These devices estimate BP from the oscillometric cuff pressure waveform using population average methods. Hence, the devices may only be accurate over a limited BP range. The objective was to evaluate a new patient-specific method, which estimates BP by fitting a physiologic model to the same waveform. One-hundred and forty-five cardiac catheterization patients and normal adults were included for study. The oscillometric cuff pressure waveform was obtained with an office device, while reference BP was measured via brachial artery catheterization or auscultation, during baseline and/or nitroglycerin administration. Fifty-seven of the subject records were utilized for refining the patient-specific method, while the remaining 88 subject records were employed for evaluation. The precision errors for all BP levels of the patient-specific method ranged from 6.3 to 7.6 mmHg. These errors were significantly lower than those of the office device (by 29% on average) in subjects with high pulse pressure (>50 mmHg) while being comparable to those of the device in subjects with normal pulse pressure (<50 mmHg). The bias and precision of the differences in repeated estimates for all BP levels of the patient-specific method ranged from 0.1 to 1.1 and 2.1 to 5.9 mmHg, respectively. These precision differences were significantly lower than those of the office device (by 64% on average). The patient-specific method may afford more accurate automatic cuff BP measurement in patients with large artery stiffening while limiting the number of required cuff inflations/deflations per measurement.
Keywords: Arterial stiffness, blood pressure measurement, cuff, oscillometry, parameter estimation, physiologic model
A new patient-specific method for oscillometric blood pressure (BP) estimation was shown to yield significantly lower errors (relative to accurate reference measurements) than widely used population average methods (Omron/Microlife) in subjects with high pulse pressure (PP) without compromising accuracy in subjects with normal PP. Hence, the patient-specific method may afford more accurate automatic cuff BP measurements in patients with large artery stiffening. SP is systolic BP; MP, mean BP; DP, diastolic BP; and RMS, root-mean-square.
I. Introduction
Automatic cuff blood pressure (BP) measurement devices are routinely employed for hypertension detection and control. Most of these devices are based on oscillometry [1]–[3]. Oscillometric devices act as both an actuator to alter the transmural pressure of the brachial artery via cuff inflation/deflation and a sensor to measure the pressure inside the cuff. The measured cuff pressure indicates the applied pressure and is superimposed with small oscillations representing the pulsatile blood volume in the artery. Since the volume-pressure relationship of the brachial artery is nonlinear, the amplitude of the cuff pressure oscillations varies with the applied cuff pressure. BP is estimated from the oscillation amplitude versus cuff pressure function (henceforth referred to as “oscillogram”).
The BP estimation method is proprietary but believed to be based on population averages [1], [2], [4]. For example, the standard method is to first estimate mean BP (MP) as the cuff pressure at which the oscillogram is maximal and then estimate each of systolic and diastolic BP (SP and DP) as the cuff pressure at which the oscillogram is some fixed ratio of its maximal value [1], [2], [5]. The fixed ratio values are determined by obtaining the oscillogram and reference BP from a group of subjects and then finding the values that maximize the agreement between the estimated and reference BP in the group. As a result, the devices may only be accurate in new subjects with typical BP levels. Indeed, it is well known that oscillometric device accuracy degrades in patients with high pulse pressure (PP = SP - DP) due to large artery stiffening [2], [4], which is a common condition that occurs with aging and disease.
We recently proposed a patient-specific method for oscillometric BP measurement [6]. The method represents the oscillogram with a physiologic model and then estimates the patient-specific model parameters, which include BP levels, by optimally fitting the model to the oscillogram. Hence, unlike conventional population average methods, which use the same parameter values in their empirical model for transforming the oscillogram to BP levels in all patients, the new method determines the parameter values of its physiologic model for each patient at the time of measurement. In this way, the accuracy could be maintained over a wider BP range. Furthermore, by employing a physiologic model, the method could be more robust to deviations in the oscillogram caused by respiration and heart rate variability and thus more repeatable.
We also demonstrated that the patient-specific method could indeed provide more accurate BP estimates than population average methods [6]. However, the testing data in this earlier study was limited in that it included only single rather than repeated measurements from 20 patients. Hence, we neither conclusively proved superior accuracy in subjects with atypical BP levels nor showed enhanced repeatability relative to population average methods.
In this study, our aim was to thoroughly assess the patient-specific method. We analyzed data from 145 patients and normal adults whose PP varied from normal levels to high levels due to large artery stiffening. The results of this validation study clearly showed that the method was able to significantly improve upon widely used methods and office devices in terms of precision accuracy, especially in subjects with high PP levels, and repeatability.
II. Material and Methods
A. Patient-Specific Oscillometric Blood Pressure Measurement Method
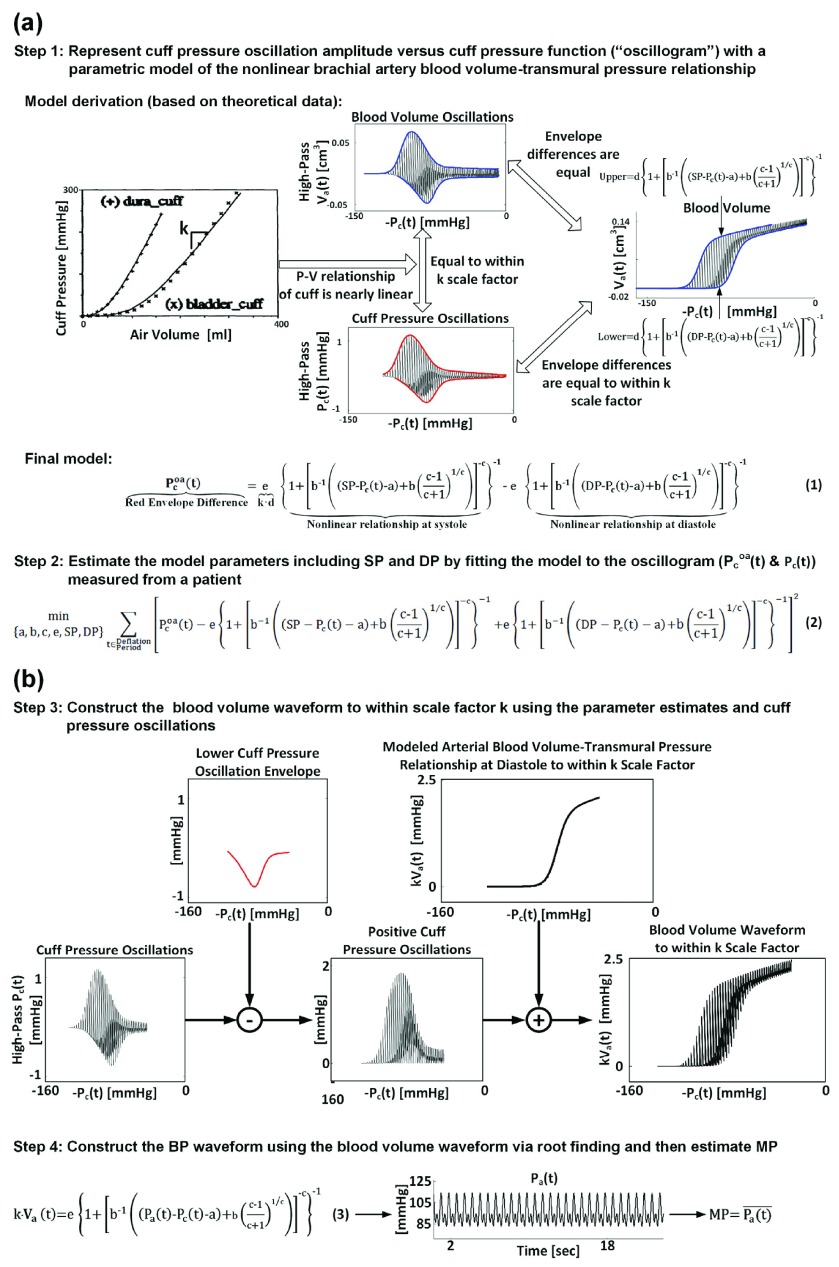
The method is illustrated in Fig. 1 and described in detail elsewhere [6]. Briefly, BP is estimated from a standard oscillogram in four steps. The first two steps produce estimates of SP and DP (see Fig. 1a), while the last two steps yield an estimate of MP (see Fig. 1b). In the first step, the oscillogram (difference between the upper and lower envelopes in red) is represented with a parametric model accounting for the nonlinear brachial artery blood volume-transmural pressure relationship (Eq. (1)). The unknown parameters represent SP, DP, and brachial artery mechanics [a, b, c, e]. In terms of the brachial artery compliance curve (i.e., the derivative of the nonlinear relationship with respect to transmural pressure), a reflects the transmural pressure at which the curve is maximum; b and c denote the width of the curve and extent of asymmetry about its maximum; and e indicates the amplitude of the curve. The parameter e is actually determined by the reciprocal of the cuff compliance [k], which is assumed to be constant as justified by experimental data (see approximately linear cuff pressure-air volume relationships), in addition to brachial artery mechanics. In the second step, the patient-specific parameters are estimated by optimally fitting the model to the oscillogram in the least squares sense (Eq. (2)). In the third step, the blood volume waveform is constructed to within a k scale factor using the parameter estimates and cuff pressure oscillations through a sequence of arithmetic operations. Finally, in the fourth step, the entire BP waveform is derived from the scaled blood volume waveform and the estimated and likewise scaled brachial artery blood volume-transmural pressure relationship (Eq. (3)) via root finding, and MP is computed as the time average of the derived waveform.
FIGURE 1.
The patient-specific method for estimating blood pressure (BP) from the oscillogram [6]: (a) The first two steps produce estimates for systolic and diastolic pressure (SP and DP) and the parameters, a, b, c, and e, which characterize the underlying model of the brachial artery blood volume-transmural pressure relationship. (b) The last two steps use the resulting parameter estimates and measured cuff pressure oscillations to produce an estimate for mean pressure (MP).
B. Human Data
To assess the patient-specific method, a total of 158 human subjects were studied at Taipei Veterans General Hospital (Taiwan). All procedures were approved by the Institutional Review Board of the hospital and adhered to the principles of the Declaration of Helsinki. Written, informed consent was obtained from all subjects prior to study.
Amongst the subjects, 138 were adult patients admitted for diagnostic cardiac catheterization. The study procedures for these subjects are described in detail elsewhere [7], [8]. Briefly, all patients had normal sinus rhythm and inter-arm cuff BP differences of no more than 3 mmHg. A micromanometer-tipped catheter (SPC-320, Millar Instruments, USA) was inserted into a brachial artery to measure the gold standard reference BP waveform. An appropriately sized, inflatable cuff of an office oscillometric device (WatchBP Office, Microlife AG, Switzerland or VP-1000, Omron Colin, Japan) was properly placed over the other brachial artery to measure the raw cuff pressure waveform for analysis and to obtain the BP estimates of the device. The waveforms were simultaneously recorded during baseline and/or sublingual nitroglycerin administration. When the Microlife device was used, two cuff pressure waveforms were recorded per condition via repeated cuff inflation/deflation cycles.
The remaining 20 subjects were normal adults. The inflatable cuff of the Microlife device was placed over a brachial artery to again measure the cuff pressure waveform for analysis and obtain the BP estimates of the device. Using a three-way stopcock, the same cuff was interfaced to a mercury sphygmomanometer to simultaneously obtain reference SP and DP from the same arm via auscultation. The auscultation measurements were performed strictly according to AHA guidelines. Two pairs of cuff pressure waveforms and auscultation measurements were recorded via repeated cuff inflation/deflation cycles.
The cuff pressure waveforms for analysis and invasive reference BP waveforms were visually screened for substantial artifact due to motion or otherwise. All waveforms with such artifact were excluded from subsequent analysis to benchmark method performance. A total of 315 pairs of cuff pressure waveforms and reference BP measurements from 145 patients and normal subjects remained for analysis. The measurement pairs from 57 of the patients were identical to those previously analyzed to demonstrate proof-of-concept of the patient-specific method [6]. Hence, these data were utilized as training data to refine the method, while the remaining new data from 88 patients and normal subjects were utilized as testing data to thoroughly evaluate the method. Note that while patient-specific methods do not require training data in theory, all methods need such data in practice to define any user-selected variables. Table I summarizes the measurement and subject characteristics of the training and testing datasets for analysis. Table II shows the average, standard deviation, and range of reference SP, MP, DP, and PP during baseline and nitroglycerin administration for the patients and normal subjects in the testing dataset. Hence, the BP levels varied widely, with PP and SP ranging from normal levels to high levels due to large artery stiffening. The corresponding statistics for the training dataset, which are reported elsewhere [6], indicated a fairly similar BP range.
TABLE 1. Measurement and Subject Characteristics.
| Training Data | Testing Data | ||||
|---|---|---|---|---|---|
| Cohort 1 | Cohort 2 | Cohort 1 | Cohort 2 | Cohort 3 | |
| Measurement Characteristics | |||||
| Device | Omron | Microlife | Omron | Microlife | Microlife |
| Reference | Invasive | Invasive | Invasive | Invasive | Auscultation |
| # of Subjects | 20 | 37 | 58 | 11 | 19 |
| # of Baseline Measurements | 20 | 37 | 36 | 11 | 19 |
| # of Nitroglycerin Measurements | 8 | 36 | 32 | 11 | 0 |
| # of Repeated Measurements | 0 | 73 | 0 | 16 | 16 |
| Total # of Measurements | 28 | 146 | 68 | 38 | 35 |
| Subject Characteristics | |||||
| Type | Cardiac Catheterization | Normal | |||
| Age [years] | 65.1±15.4 | 65.2±12.3 | 59.8±15.0 | 69.0±12.4 | 34.0±9.4 |
| Weight [kg] | 64.5±12.4 | 74.6±13.4 | 70.12±11.5 | 68.9±14.2 | 60.4±15.8 |
| Height [cm] | 164.0±6.4 | 163.6±8.0 | 161.6±7.8 | 162.2±10.4 | 164.2±9.3 |
| Waist circumference [cm] | 84.6±11.4 | 90.0±12.3 | 91.8±9.5 | 97.2±11.9 | 75.8±11.2 |
| Men [%] | 85 | 75.7 | 74.1 | 72.7 | 36.8 |
| Smoking [%] | 35 | 18.9 | 20.7 | 27.3 | N/A |
| Clinical diagnosis [%] | |||||
| Hypertension | 65 | 59.5 | 56.9 | 90.9 | N/A |
| Type 2 Diabetes Mellitus | 20 | 29.7 | 31 | 54.5 | N/A |
| Dyslipidemia | 45 | 37.8 | 41.4 | 36.4 | N/A |
| Coronary Artery Disease | 40 | 59.5 | 56.9 | 63.6 | N/A |
| Chronic Renal Failure | 5 | 2.7 | 3.4 | 18.2 | N/A |
| Medications [%] | |||||
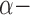 Blockers Blockers |
20 | 13.5 | 12.1 | 27.3 | N/A |
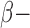 Blockers Blockers |
30 | 43.2 | 37.9 | 63.6 | N/A |
| Calcium Channel Blockers | 25 | 48.6 | 41.4 | 27.3 | N/A |
| Diuretics | 20 | 18.9 | 20.7 | 36.4 | N/A |
| Antiplatelet Agents | 65 | 86.5 | 70.7 | 81.8 | N/A |
TABLE 2. Reference Blood Pressure (BP) Levels in the Testing Data.
| Reference BP | Condition | SP [mmHg] | MP [mmHg] | DP [mmHg] | PP [mmHg] |
|---|---|---|---|---|---|
| Invasive | Baseline | 136±20 (109–192) | 97±13 (76–127) | 72±11 (46–95) | 64±16 (45–107) |
| Nitroglycerin | 130±18 (99–169) | 92±12 (72–115) | 70±11 (46–70) | 60±18 (31–102) | |
| Auscultation | Baseline | 105±11 (88–130) | N/A | 71±10 (54–88) | 34±9 (21–54) |
Values are mean ±SD (minimum-maximum). SP, MP, and DP are systolic, mean, and diastolic BP, respectively, and PP is pulse pressure.
C. Data Analysis
First, the training dataset was analyzed. The requisite oscillogram for BP estimation was constructed from each cuff pressure waveform as described previously [6].
The user-selected variables of the patient-specific method were determined by maximizing the agreement between its BP estimates and the reference BP values while minimizing the number of parameters for estimation in order to enhance robustness. The resulting user-selected variables were similar to those established previously [6]. The only differences were fixing the a parameter, which indicates the peak position of the brachial artery compliance curve, to 1.5 instead of 2.5 mmHg and the b parameter for each value of the c parameter such that the compliance curve was right-skewed by 40 rather than 35% about its maximum. Note that these parameter settings are buttressed by directly measured compliance curves [9]. Hence, the optimized patient-specific method estimated four parameters [SP, DP, c, e] from the oscillogram and yielded BP estimates with similar accuracy to the originally established method on the training dataset (see results elsewhere [6]).
A fixed-ratio method was likewise developed using the training dataset by maximizing the agreement between its BP estimates from the same oscillograms and the reference BP values. The resulting fixed ratio values were 0.57 for SP and 0.75 for DP.
Then, the testing dataset was analyzed. The patient-specific and fixed-ratio methods were applied to oscillograms likewise constructed from the cuff pressure waveforms. The BP estimates of these methods and the office device were compared for accuracy and repeatability.
For accuracy, note that the testing dataset included reference BP via brachial artery catheterization or auscultation (see Table I). Further note that the patient-specific and fixed-ratio methods were trained based on the former reference method (see Table I), whereas the office device was likely developed based on the latter reference method. Since there are systematic differences between the two reference methods (i.e., invasive SP and DP are a few mmHg higher and lower than auscultation SP and DP, respectively) [10], bias accuracy could not be fairly quantified and compared. To quantify precision accuracy, the errors between the SP, MP, DP, and PP estimates and the reference BP values were computed. The bias component of each of these errors for each method in each of the three cohorts in the testing dataset (see Table I) was then removed. In this way, BP error variability introduced by systematic differences in the reference method as well as in the office device and data in each cohort was eliminated. The resulting precision errors were then combined over the three cohorts. These errors were thereafter divided into two groups: normal PP (reference PP < 50 mmHg) and high PP (reference PP > 50 mmHg). Note that a 50 mmHg threshold was chosen so as to arrive at groups of approximately equal size. In the case of repeated measurement pairs, only the first measurement pair was included in the groups. The root-mean-square (RMS) of the errors and percentage of large errors (i.e., percent of absolute errors > 10 and 15 mmHg) in each PP group were then computed. Finally, to compare precision accuracy, the Pittman-Morgan test was applied to the RMS of the errors (which were nearly void of a bias component) of pairs of methods in each PP group [11]. A p < 0.0167 (=0.05/3) was considered significant based on Bonferroni correction for pairwise comparison of three methods.
For repeatability, the mean and standard deviation of the differences between each of the repeated estimates of SP, MP, DP, and PP of each method were computed. The paired t-test and Pittman-Morgan test were then applied to compare the resulting bias and precision of pairs of methods, respectively. A p < 0.0167 was likewise considered significant.
III. Results
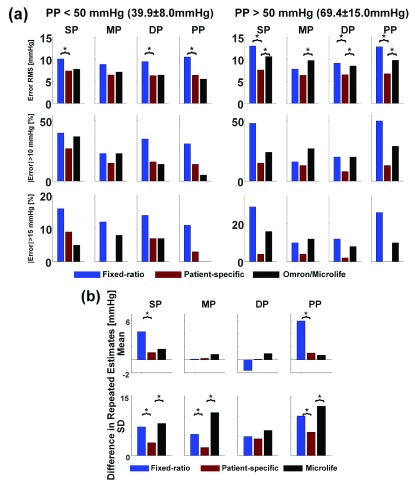
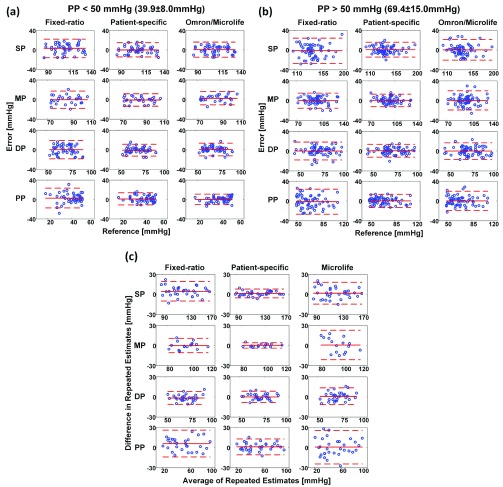
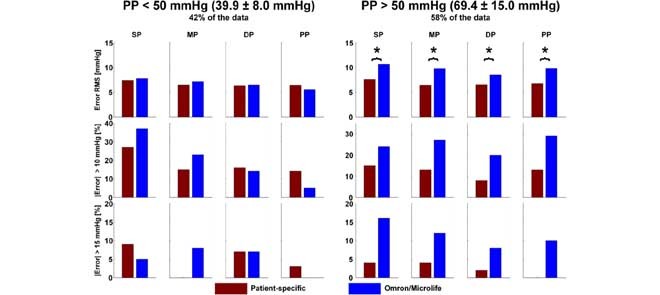
Fig. 2a summarizes the SP, MP, DP, and PP precision accuracy results for the patient-specific method, fixed-ratio method, and Omron/Microlife device in the normal PP and high PP groups of the testing dataset. These results were obtained from 88 subjects wherein the normal PP and high PP groups constituted 42 and 58% of the data, respectively. The mean±SD of PP was 39.9±8.0 mmHg in the normal PP group and 69.4±15.0 mmHg in the high PP group. The reference was either auscultation BP (41% of the data) or invasive BP (59% of the data) in the normal PP group but almost exclusively invasive BP in the high PP group. The RMS errors of the patient-specific method ranged from 6.3 to 7.6 mmHg over both PP groups, and its percentages of large errors were fairly similar between the groups. Hence, the patient-specific method was able to maintain the precision accuracy over a wide PP range. Furthermore, the precision errors of this method were significantly lower (or not different) relative to the fixed-ratio method in both PP groups. In particular, the RMS errors for SP, DP, and PP of the patient-specific method were, on average, 36% smaller than those of the fixed-ratio method, while the absolute precision errors exceeding 10/15 mmHg of the new method were, on average, 50/75% less than the standard method. More notably, the precision errors of the patient-specific method were significantly lower relative to the widely employed Omron/Microlife device in the high PP group while being similar in the normal PP group. Specifically, in the high PP group, the RMS errors for all BP levels of the patient-specific method were, on average, 29% smaller than those of the Omron/Microlife device, while the absolute precision errors exceeding 10/15 mmHg of the new method were, on average, 51/79% less than the office device. Hence, the patient-specific method was able to reduce the number of large precision errors and improve the precision accuracy, especially over the high PP range. Fig. 3ab shows Bland-Altman plots for visual assessment of the precision errors.
FIGURE 2.
Bar graphs of (a) BP precision error metrics in the normal and high reference pulse pressure (PP) groups and (b) BP repeatability metrics for the patient-specific method and two available methods in the testing data. 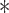 denotes p < 0.0167 compared to the patient-specific method. For precision error, only the root-mean-square (RMS) metrics were statistically compared. Bias errors could not be fairly quantified and compared, as described in the text.
denotes p < 0.0167 compared to the patient-specific method. For precision error, only the root-mean-square (RMS) metrics were statistically compared. Bias errors could not be fairly quantified and compared, as described in the text.
FIGURE 3.
Bland-Altman plots (mean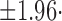 SD) of the (a) precision errors in the normal PP group; (b) precision errors in the high PP group; and (c) differences in repeated estimates for the patient-specific method and two available methods in the testing data.
SD) of the (a) precision errors in the normal PP group; (b) precision errors in the high PP group; and (c) differences in repeated estimates for the patient-specific method and two available methods in the testing data.
Fig. 2b summarizes the SP, MP, DP, and PP repeatability results for the three methods in the testing dataset. These results were obtained from 32 subjects for SP, DP, and PP and 16 subjects for MP. The bias and precision of the differences in repeated estimates for all BP levels of the patient-specific method ranged from 0.1 to 1.1 mmHg and 2.1 to 5.9 mmHg, respectively. These values were significantly lower (or not different) relative to the other methods. In particular, the bias of the differences in repeated estimates for SP and PP of the patient-specific method were, on average, 79% smaller than those of the fixed-ratio method, while the precision of the differences in repeated estimates for SP, MP, and PP of the new method were, on average, 53% smaller than those of the standard method and 64% smaller than those of the Microlife device. Hence, the patient-specific method was able to improve BP measurement repeatability. Fig. 3c shows Bland-Altman plots for visual assessment of the differences.
Secondary results (which are not shown) were as follows. Firstly, and as alluded to earlier, the bias accuracy for the SP and DP estimates of the patient-specific method tended to be superior relative to the Omron/Microlife device when invasive BP was the reference (bias error of −2.4 vs. −5.4 mmHg for SP and −0.1 vs. 1.5 mmHg for DP; p = NS) but tended to be worse compared to the office device when auscultation BP was the reference (4.0 vs. 2.4 mmHg for SP and −6.6 vs. −3.9 mmHg for DP; p = NS). However, the precision accuracy of the patient-specific method and office device were similar in the normal PP group regardless of the reference method employed (precision errors of 6.5-6.9 vs. 6.3-7.8 mmHg for invasive BP and 5.9-8.0 vs. 6.6-7.7 mmHg for auscultation BP). This result, along with the observation that the precision errors of the office device were random (i.e., without obvious trends) in the Bland-Altman plots for the high PP group (see Fig. 3b) wherein invasive BP was essentially the reference, indicate that the bias removal process employed herein may have indeed eliminated the systematic BP differences between the two reference methods employed. Further, the c and e parameter estimates of the patient-specific method were 5.2±0.7 (mean±SD) unitless and 8.2±1.4 mmHg during baseline and 5.9±1.0 unitless and 8.9±1.4 mmHg during nitroglycerin administration, respectively (p ≤ 0.013 via t-tests). Increases in the c and e parameters both correspond to enhanced brachial artery compliance, so the patient-specific method was able to correctly track the drug-induced compliance changes. Finally, and perhaps as a result, the precision accuracy of the patient-specific method tended to be less impacted by nitroglycerin administration than the Omron/Microlife device (average difference in RMS error from baseline to nitroglycerin administration of −0.98 mmHg vs. −1.95 mmHg).
IV. Discussion
Most automatic cuff BP measurement devices employ population average methods to estimate BP from an oscillogram and may thus be accurate only over a limited BP range. We recently proposed a patient-specific method to estimate BP from the oscillogram by leveraging a physiologic model in conjunction with model fitting (see Fig. 1) [6]. In this way, the routinely used devices may not only maintain accuracy over a wider BP range but also be less sensitive to common physiologic deviations in the oscillogram and thus more repeatable. We also demonstrated that the method can offer improved accuracy compared to population average methods [6]. However, this demonstration was based on a limited set of testing data comprising 20 patients without repeated measurements and thus provided only proof-of-concept. In this validation study, we refined the method and thoroughly compared it to existing methods for both accuracy and repeatability in 145 human subjects with normal PP levels and high PP levels induced by large artery stiffening (see Tables I and II).
The patient-specific method achieved BP errors reflecting precision accuracy that ranged from 6.3 to 7.6 mmHg (see Fig. 2a). Hence, the method maintained the precision accuracy over both the normal and high PP ranges. Further, this level of precision accuracy was within the Association for the Advancement of Medical Instrumentation (AAMI) precision limits of 8 mmHg. However, the method did not meet the AAMI standard, because an AAMI data collection protocol was not employed.
The patient-specific method was compared to both the standard fixed-ratio method, which was developed using the same training dataset as the new method, and a currently used office device (Omron or Microlife). Overall, the office device attained greater precision accuracy than the fixed-ratio method (see Fig. 2a), thereby suggesting that the device estimates BP based on other useful features in the oscillogram in addition to, or instead of, amplitude ratios. However, the level of precision accuracy of the office device was not within 8 mmHg for the high PP range. Compared to this device, the patient-specific method revealed significantly lower precision errors for all BP levels in the high PP range (by 29 to 79% on average) while showing similar precision errors with respect to either invasive BP or auscultation BP in the normal PP range (see Figs. 2a and 3ab).
The reference method was almost exclusively invasive BP in the high PP range. The well-known auscultatory gap is strongly related to carotid artery stiffening and aging [12] and thus high PP. Perhaps as a result, the ability of auscultation to stratify risk for stroke and heart disease diminishes with aging [13]. Since auscultation BP was not utilized as the reference in the high PP range, the improvement in precision accuracy attained by the patient-specific method here may be particularly significant. The improved precision accuracy with respect to invasive BP could also be significant in terms of monitoring central BP, which may offer superior cardiovascular risk stratification to brachial BP [14]. That is, a major source of error of non-invasive measurements of central BP is the discrepancy between the BP estimates of current oscillometric devices, which are used to calibrate the tonometry waveforms, and invasive brachial BP [15], [16] Hence, the patient-specific method may be able to enhance the accuracy of non-invasive central BP monitoring.
The bias accuracy of the methods could not be fairly assessed and compared due to the systematic differences in the two reference methods [10] employed for training as well as testing them. While the inability to address bias accuracy represents the main study limitation, precision accuracy may be much more important anyhow. For example, the bias accuracy of the patient-specific method, which was developed using the invasive BP reference, could easily be corrected for an auscultation BP reference by subtracting and adding a constant (e.g., 3-4 mmHg [12]) to its SP and DP estimates, respectively.
The patient-specific method also achieved a bias and precision of the differences in repeated BP estimates that ranged from 0.1 to 1.1 mmHg and 2.1 to 5.9 mmHg, respectively (see Fig. 2b). This level of repeatability was within the AHA recommended limits of 5 mmHg for SP, MP, and DP and near these limits for PP [17].
While the office device was more accurate than the fixed-ratio method, the standard method appeared more repeatable (see Fig. 3c). However, the level of repeatability of the fixed-ratio method was not close to the AHA limits for SP and PP (see Fig. 2b). Compared to this method, the patient-specific method revealed significantly lower bias of the differences in repeated SP and DP estimates (by 79% on average) and precision of the differences in repeated SP, MP, and PP estimates (by 53% on average) (see Figs. 2b and 3c).
In sum, the new information gained from this validation study compared to our previous methods/proof-of-concept study [6] is that the patient-specific method can afford superior precision accuracy, especially in the high PP range, and repeatability compared to widely used, population-based methods. Hence, the new method could improve cardiovascular risk stratification in the elderly and other patients with large artery stiffening while limiting the number of required cuff inflations/deflations per BP measurement [17].
The accuracy of current oscillometric BP measurement devices is also known to degrade in other conditions such as arrhythmias, especially atrial fibrillation, and pre-eclampsia [17], [18], as well as with improper cuff usage such as cuff misplacement and over-cuffing [19]. Future studies would have to be conducted to determine the relative capabilities and limitations of the patient-specific method in such cases. Subsequent studies to confirm the results of this study and assess the cardiovascular risk stratification ability of the method may also be worthwhile.
V. Conclusion
Hypertension detection and control currently represent a major healthcare problem around the world, especially in low resource settings. Effective BP measurement technology is essential to alleviate this problem. Amongst the available technologies, oscillometry offers a number of advantages. In particular, it is non-invasive (unlike catheterization), easy-to-use (unlike manual auscultation or tonometry), inexpensive (unlike volume clamping), unaffected by the auscultatory gap and terminal digit bias (unlike manual auscultation), less sensitive to cuff position and ambient sound (compared to automatic auscultation), environmentally safe (unlike mercury manometers), and more convenient in terms of maintenance (compared to aneroid manometers). However, the disadvantage of oscillometry is that it is not as accurate as other technologies (catheterization and manual auscultation). The reason is that BP is estimated from the oscillogram using population average methods. We evaluated a patient-specific method for estimating BP from a standard oscillogram. The new method showed significantly improved accuracy over a wide PP range as well as repeatability compared to the standard BP estimation method and widely used office devices. With further testing, the patient-specific method could possibly facilitate the management of hypertension by affording more accurate automatic cuff blood pressure measurement in patients with large artery stiffening while limiting the number of required cuff inflations/deflations per measurement.
Biographies

Jiankun Liu received the B.S. degree in physics from Nanjing University, Nanjing, China, in 2006, and the Ph.D. degree from the Department of Electrical and Computer Engineering, Michigan State University, East Lansing, MI, USA, in 2016. His research interests include biomedical computation and modeling and their application on patient monitoring and therapeutics. His current research focuses on ubiquitous vital sign monitoring and ventricular arterial coupling with utilization of multiscale modeling approach.

Hao-Min Cheng received the M.D. degree from the Faculty of Medicine, National Yang-Ming University, Taipei, Taiwan, in 2000, and the Ph.D. degree from the Faculty of Medicine, The University of Adelaide, Australia, in 2013. He is currently a Cardiologist with Taipei Veterans General Hospital, where he is involved in the sub-specialty of interventional cardiology and echocardiography. He is also with National Yang-Ming University, Taipei, where he is an Assistant Professor with the Faculty of Medicine, and a jointly appointed Assistant Professor with the Institute of Public Health. He was a recipient of the International Postgraduate Research Scholarship from the Australian Government and the Young Investigator Award from the Taiwan Society of Cardiology in 2013. He is an Associate Editor of the BMC Cardiovascular Disorders. His research interests include cardiovascular hemodynamics, blood pressure measurements, hypertension management, and evidence-based health care.

Chen-Huan Chen received the M.D. degree from the Faculty of Medicine, National Yang-Ming University, Taiwan, in 1982. He was a Clinical Research Fellow with the Division of Cardiology at the Johns Hopkins Hospital from 1995 to 1997. He has been with the Faculty of the Department of Medicine since 1989, where he is currently a Professor. He completed internal medicine residency training and cardiology fellowship training at Taipei Veterans General Hospital in 1990, where he is also now an Attending Physician. His research interests focus on cardiovascular epidemiology, hemodynamics, vascular aging, arterial stiffness, wave reflections, heart failure, and development of new methods for noninvasive assessment of cardiac and vascular functions. His research works have contributed significantly to the understanding and utilization of the generalized transfer function concept and the optimal clinical assessment of the wave reflections.

Shih-Hsien Sung received the M.D. and Ph.D. degrees from National Yang-Ming University, Taiwan, in 2001 and 2014, respectively. He has been with the Faculty of the Department of Medicine since 2007, where he is currently an Assistant Professor. He completed internal medicine residency training and cardiology fellowship training at Taipei Veterans General Hospital in 2009, where he is also an Attending Physician. His research interests focus on cardiovascular epidemiology, hemodynamics, vascular aging, heart failure, and pulmonary artery hypertension.

Jin-Oh Hahn (M’08) received the B.S. and M.S. degrees in mechanical engineering from Seoul National University, Seoul, South Korea, in 1997 and 1999, respectively, and the Ph.D. degree in mechanical engineering from the Massachusetts Institute of Technology, Cambridge, MA, USA, in 2008. He is currently an Assistant Professor with the Department of Mechanical Engineering, University of Maryland, College Park, MA, USA. His current research interests include systems and controls approach to health monitoring, diagnostics and maintenance of dynamic systems. He was a recipient of the Young Investigator Program Award from the Office of Naval Research in 2014 and the Young Investigator Grant Award from the Korean–American Scientists and Engineers Association in 2013.

Ramakrishna Mukkamala (M’02) received the B.S.E. degree in biomedical/electrical engineering from Duke University, Durham, NC, USA, in 1993, and the S.M. and Ph.D. degrees in electrical engineering and computer science from the Massachusetts Institute of Technology, Cambridge, MA, USA, in 1995 and 2000, respectively. He was a Post-Doctoral Fellow/Research Engineer with the Harvard-MIT Division of Health Sciences and Technology, Cambridge, from 2000 to 2002. He has been with the Faculty of the Department of Electrical and Computer Engineering, Michigan State University, East Lansing, MI, USA, where he is currently a Professor. His research interests include biomedical signal processing and identification, modeling of physiologic systems, cardiovascular physiology, and patient monitoring. He is a member of the IEEE EMBS Technical Committee on Cardiopulmonary Systems. He was a recipient of the AHA Scientist Development Grant, the NSF CAREER Award, and the MSU Teacher–Scholar Award. He is an Associate Editor of the IEEE Transactions on Biomedical Engineering and an Editor of the Cardiovascular and Respiratory Systems Engineering Theme of the IEEE EMBS Conference Proceedings.
Funding Statement
This work was supported in part by the National Institutes of Health under Grant EB-018818 and the National Science Foundation under Grant IIS-1404436 and Grant IIS-1403004.
References
- [1].Alpert B. S., Quinn D., and Gallick D., “Oscillometric blood pressure: A review for clinicians,” J. Amer. Soc. Hypertension, vol. 8, no. 12, pp. 930–938, Dec. 2014. [DOI] [PubMed] [Google Scholar]
- [2].van Montfrans G. A., “Oscillometric blood pressure measurement: Progress and problems,” Blood Pressure Monitor., vol. 6, no. 6, pp. 287–290, Dec. 2001. [DOI] [PubMed] [Google Scholar]
- [3].Smulyan H. and Safar M. E., “Blood pressure measurement: Retrospective and prospective views,” Amer. J. Hypertension, vol. 24, no. 6, pp. 628–634, Jun. 2011. [DOI] [PubMed] [Google Scholar]
- [4].National High Blood Pressure Education Program (NHBPEP)/National Heart, Lung, And Blood Institute (NHLBI) AND American Heart Association (AHA) Working Meeting on Blood Pressure Measurement. Accessed on Jul. 18, 2015. [Online]. Available: https://www.nhlbi.nih.gov/files/docs/resources/heart/bpmeasu.pdf
- [5].Geddes L. A., Voelz M., Combs C., Reiner D., and Babbs C. F., “Characterization of the oscillometric method for measuring indirect blood pressure,” Ann. Biomed. Eng., vol. 10, no. 6, pp. 271–280, Jan. 1982. [DOI] [PubMed] [Google Scholar]
- [6].Liu J., et al. , “Patient-specific oscillometric blood pressure measurement,” IEEE Trans. Biomed. Eng., vol. 63, no. 6, pp. 1220–1228, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cheng H.-M., Sung S.-H., Shih Y.-T., Chuang S.-Y., Yu W.-C., and Chen C.-H., “Measurement of central aortic pulse pressure: Noninvasive brachial cuff-based estimation by a transfer function vs. a novel pulse wave analysis method,” Amer. J. Hypertension, vol. 25, no. 11, pp. 1162–1169, Nov. 2012. [DOI] [PubMed] [Google Scholar]
- [8].Cheng H.-M., Sung S.-H., Shih Y.-T., Chuang S.-Y., Yu W.-C., and Chen C.-H., “Measurement accuracy of a stand-alone oscillometric central blood pressure monitor: A validation report for microlife WatchBP Office Central,” Amer. J. Hypertension, vol. 26, no. 1, pp. 42–50, Jan. 2013. [DOI] [PubMed] [Google Scholar]
- [9].Drzewiecki G., Hood R., and Apple H., “Theory of the oscillometric maximum and the systolic and diastolic detection ratios,” Ann. Biomed. Eng., vol. 22, no. 1, pp. 88–96, 1994. [DOI] [PubMed] [Google Scholar]
- [10].Association for the Advancement of Medical Instrumentation. (2003). American National Standard for Manual, Electronic or Automated Sphygmomanometers. accessed on Jul. 19, 2015. [Online]. Available: https://fmc4me.qa-intranet.fmcna.com/idc/idcplg?IdcService=GET_FILE&Rendition=Primary&RevisionSelectionMethod=Latest&dDocName=PDF_100032384
- [11].Snedecor G. W. and Cochran W. G.. Statistical Methods, accessed on Jul. 19, 2015. [Online]. Available: http://catalog.lib.msu.edu/record=b2300696~S39a [Google Scholar]
- [12].Askey J. M., “The auscultatory gap in sphygmomanometry,” Ann. Internal Med., vol. 80, no. 1, p. 94, Jan. 1974. [DOI] [PubMed] [Google Scholar]
- [13].Lewington S., Clarke R., Qizilbash N., Peto R., and Collins R., “Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies,” Lancet, vol. 360, no. 9349, pp. 1903–1913, Dec. 2002. [DOI] [PubMed] [Google Scholar]
- [14].Vlachopoulos C., Aznaouridis K., O’Rourke M. F., Safar M. E., Baou K., and Stefanadis C., “Prediction of cardiovascular events and all-cause mortality with central haemodynamics: A systematic review and meta-analysis,” Eur. Heart J., vol. 31, no. 15, pp. 1865–1871, Aug. 2010. [DOI] [PubMed] [Google Scholar]
- [15].Shih Y.-T., Cheng H.-M., Sung S.-H., Hu W.-C., and Chen C.-H., “Quantification of the calibration error in the transfer function-derived central aortic blood pressures,” Amer. J. Hypertension, vol. 24, no. 12, pp. 1312–1317, Dec. 2011. [DOI] [PubMed] [Google Scholar]
- [16].Cheng H.-M., Lang D., Tufanaru C., and Pearson A., “Measurement accuracy of non-invasively obtained central blood pressure by applanation tonometry: A systematic review and meta-analysis,” Int. J. Cardiol., vol. 167, no. 5, pp. 1867–1876, Sep. 2013. [DOI] [PubMed] [Google Scholar]
- [17].Pickering T. G., et al. , “Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: A statement for professionals from the subcommittee of professional and public education of the American Heart Association Cou,” Hypertension, vol. 45, no. 1, pp. 142–161, Jan. 2005. [DOI] [PubMed] [Google Scholar]
- [18].O’Brien E., et al. , “European Society of Hypertension recommendations for conventional, ambulatory and home blood pressure measurement,” J. Hypertension, vol. 21, no. 5, pp. 821–848, May 2003. [DOI] [PubMed] [Google Scholar]
- [19].Ringrose J., Millay J., Babwick S. A., Neil M., Langkaas L. A., and Padwal R., “Effect of overcuffing on the accuracy of oscillometric blood pressure measurements,” J. Hypertension, vol. 9, no. 7, pp. 563–568, 2015. [DOI] [PubMed] [Google Scholar]