Abstract
Objectives
Behavioral weight loss (BWL) programs are the recommended treatment for obesity, yet it is unknown whether these programs change one’s ability to use self-control in food choices and what specific mechanisms support such change. Using experimental economics methods, we investigated whether changes in dietary behavior in individuals with obesity following BWL are driven by one or more of the following potential mechanisms: changes in the perception of the 1) health or 2) taste of food items, and/or 3) shifting decision weights for health versus taste attributes. Therefore, we compared these mechanisms between obese participants and lifetime normal weight controls (NW) both before and after BWL.
Methods
Females with obesity (N=37, mean BMI=33.2) completed a food choice task involving health ratings, taste ratings, and decision-making pre- and post-standard BWL intervention. NW controls (N=30, BMI=22.4) completed the same task.
Results
Individuals with obesity exhibited increased self-control (selecting healthier, less tasty food choices) post-treatment. However, their rates of self-control remained significantly lower than NW. We found no differences in initial health perceptions across groups, and no changes with treatment. In contrast, taste ratings and the relative value of taste versus health decreased following treatment. Although, post-treatment participants continued to perceive unhealthy foods as tastier and used less self-control than NW controls, they showed significant improvements in these domains following a BWL intervention.
Conclusions
To help individuals improve dietary decisions, additional research is needed to determine how to make greater changes in taste preferences and/or the assignment of value to taste versus health attributes in food choices.
Keywords: obesity, decision-making, behavioral weight loss intervention, treatment, behavioral economics
Introduction
Behavioral approaches are recommended as the treatment of choice for mild and moderate obesity(1). These approaches, which help individuals make healthier choices for eating and activity a part of their regular lifestyles(2), typically produce weight losses averaging 7–10% of initial body weight at 6–12 months, although the long-term success of these interventions is lower(3, 4, 5, 6).
Recent studies have shown benefits of behavioral weight loss intervention (BWL) on dietary composition(7), however, the specific mechanisms through which BWL leads to healthier dietary choices remain unclear. Specifically, do BWL programs increase “self-control” over food choices when faced with a concrete challenge, i.e. selection of healthier options over tastier but less healthy food choices, and, if so, what are the mechanisms supporting this change?
Basic decision-making theories suggest three mechanisms could be altered by participation in BWL and subsequently lead to a healthier diet. First, the interventions might change participants’ perceptions about the healthiness of foods. For example, participants might become more knowledgeable about nutrition or more realistic about the dangers of eating specific unhealthy foods like cookies, and the benefits of eating specific healthy foods like broccoli. Indeed, some previous studies have demonstrated improvements in nutrition knowledge through weight loss interventions(8), especially in lower income populations(9, 10). However, although greater knowledge has been linked to better diet quality(11), a large body of previous literature suggests nutrition knowledge does not differ between individuals with overweight/obesity and those with normal weight(12, 13, 14, 15, 16, 17). Treatment seeking individuals with obesity typically possess adequate nutrition and diet knowledge(18) and nutrition knowledge is not sufficient to induce changes in dietary choices(19). Taken together, these previous findings diminish the probability that improvements in health knowledge alone will improve food choices; therefore we do not expect that the behavioral weight loss intervention will alter health perceptions and individuals with obesity will perceive food health similarly to those with normal weight. Nonetheless, we tested for this possible effect in our sample.
Second, these programs might change participants’ taste perceptions of different foods. In particular, participants might learn to like healthy foods and/or dislike the taste of unhealthy items. Taste preferences have been shown to change with weight loss. For instance, preference for fat diminishes after following a lower fat diet(20, 21). Evidence also suggests that food liking may differ as a function of BMI, as greater BMI has been shown to correlate with preference for fatty foods(22, 23, 24, 25, 26, 27). Moreover, individuals with obesity exhibit greater desire for the foods they find tasty, and report thinking about food more often than lean individuals, even in the absence of metabolic hunger(28). Thus we hypothesized that individuals with obesity and normal weight controls will differ in taste preferences and that taste ratings will decrease following treatment, but it is unknown whether these changes will make post-treatment taste ratings equal to those of normal weight individuals.
Third, beyond potential changes in perception, the interventions might also increase the relative weighting given to health versus taste at the time of choice (29). Hare and colleagues demonstrated that healthy eaters differ from unhealthy eaters in the weight placed on health versus taste attributes. Cognitive-behavioral strategies targeted in BWL, such as goal-setting, may help individuals with overweight/obesity modulate their valuation of health over taste, however, such explicit differences in the relative weighting of taste and health at the time of food decisions have not been systematically assessed in those with obesity versus lean individuals, nor examined before and after a BWL intervention, nor has it been determined whether BWL improves self-control in food decision-making.
Here we combine a paradigm from experimental economics(29) with an evidence-based BWL intervention to examine whether individuals with obesity (OB) exhibit greater self-control in food choice decision-making following treatment, and the mechanisms related to this change. We also examine whether food-related decision-making in the OB group post-treatment is more similar to that of normal weight individuals (NW). It is hypothesized that individuals with obesity will increase their use of self-control in food choices following treatment, and based on the above information it is hypothesized that health perceptions will not contribute these changes while taste perceptions may serve as a mechanism for healthier dietary choice. It is further hypothesized that individuals with obesity will place greater value on health rather than taste following the BWL treatment.
Methods
Participants
Women were recruited for this study based upon weight status for either the obese group (OB, N=37) or lifetime normal weight (NW, N=30). A total of 54 women with obesity were recruited via self-referral in response to Internet advertisements within the Lifespan Corporation and community postings to participate in a BWL intervention research study at the Weight Control and Diabetes Research Center in Providence, RI. From this sample 37 women with obesity (OB; mean BMI = 33.2; mean age = 47.1) completed the food choice decision-making task both pre- and post-treatment and are included in the current study. Thirty normal weight women (NW; mean BMI = 22.4; mean age = 44.1) who reported being within the normal weight range (BMI 18.5 – 24.9) throughout their lifetime (excluding pregnancies) were recruited in the same way (i.e., self-referral in response to Internet and community postings seeking women of healthy weight for a research study) for comparison and completed the same task. The sample was restricted to females for multiple reasons. In order to keep sex constant between participant groups, given the disproportionate number of females versus males typically presenting for behavioral weight loss treatment, only females were included. Moreover, previous research employing the food choice decision-making task has focused on females and it is possible there are differences in mechanisms supporting food choice between sexes. All participants were weight stable upon enrollment in the study (defined as within +/−5 lbs. for the past two months) and reported no history of eating disorders. Additionally, a sample of 5 professional licensed nutritionists completed health ratings (described below) to provide expert assessment. Each participant provided informed consent in accordance with the guidelines set by the Institutional Review Boards of The Miriam Hospital and Brown University and received monetary compensation for completing assessments.
Clinical intervention
Individuals in the OB group participated in a BWL intervention incorporating diet, exercise, and behavioral therapy and instructed by Master’s and Doctorate-level interventionists. Interventions were conducted via face-to-face group meetings (n = 31) or via the Internet (n = 6) and varied in duration from 12 weeks (n=18) to 16 weeks (n=19). Core content of the intervention was the same for both the face-to-face and Internet-delivered programs and both durations (12 or 16 weeks). All participants were given the goal of losing 1–2 pounds per week. To help achieve this goal participants were placed on a caloric and fat restricted diet (e.g., 1200–1500 kcals/day depending on initial weight, <30% of calories from fat) and were encouraged to increase their physical activity gradually each week to reach at least 200 minutes per week (using activities similar in intensity to brisk walking in bouts ≥10 minutes). No foods or medications were provided via the intervention. All participants received a fat/calorie guidebook and a diary in which to self-monitor weight, food consumption, and physical activity. All participants were instructed on how to carefully weigh and measure food and caloric beverages consumed to achieve more accurate calorie and fat estimates. Participants in the Internet-delivered programs then entered these data to the study website. Clinicians reviewed these diaries weekly and provided written feedback on participants’ progress to date. Weekly lessons focused on teaching participants standard behavioral strategies for changing eating and activity behavior. These lessons were based off of those used in the Diabetes Prevention Program and the Look AHEAD trials and included topics such as understanding calorie balance, goal setting, problem solving, changing the home environment (i.e., stimulus control), and restaurant eating (30, 31). Individuals in the face-to-face delivered program were able to schedule make-up sessions for any missed classes. Internet-delivered lessons were in video format that participants could view on their own. For the12-week intervention, lessons on similar topics were combined in order to cover the same material in a slightly shortened format.
Assessment procedures
All subjects were weighed using Tanita digital scales (TANITA Corporation of America, Inc, Arlington Heights, IL) and measured via wall-mounted stadiometers. Participants then completed a food choice decision-making task from previous work on the neuroeconomics of self-control(29). The task consists of four stages: 1) health ratings, 2) taste ratings, 3) food choices, and 4) implementation of the choice made in a randomly selected trial. Health and taste ratings were conducted in laboratory testing rooms and the food choices portion of the task was completed during an fMRI scan for all but 1 participant from the OB group who was found to have MRI contraindications at the time of the scan. Body measurements were taken on the same day as ratings for all participants. For 6 OB participants pre-treatment, 7 OB participants post-treatment, and 2 NW participants, food ratings and the decision-making portion of the trial were conducted on the same day. For all others the decision-making portion of the task was completed approximately 1 week following completion of the ratings (mean time between ratings & decision-making = 8.9 days). All Participants consumed the food they had chosen on a randomly selected trial of the decision-making task immediately after the scan. NW participants were measured and completed the full task once, and professional licensed nutritionists completed only health ratings at one time point. Subjects in the OB group were measured and completed the full task twice: just before the beginning of the clinical intervention (maximum lag of 30 days) and after the completion of the program (maximum lag of 30 days). When relevant, visits were scheduled within the estimated follicular phase of the participant’s menstrual cycle. All assessment visits took place in the afternoon and/or evening following 3 hours of fasting since the foods being considered (sweet and salty snacks) in the task are most likely to be eaten during those time periods.
All of the tasks involved high-resolution pictures of 150 different snack food items (e.g., health bars, candy, fruit) that are widely available in supermarkets and convenience stores. The stimulus set is similar to the one used by Hare et al (2009) but includes additional items to allow for more choice trials (see Supplemental Material). In order to ensure adequate self-control challenges, many of the foods items included in the set were selected based on their high calorie, high fat content.
In the health-rating task, subjects were shown pictures of food one item at a time in random order. They were asked to rate how healthy they perceived each food to be, independent of its taste (scale:−2=very unhealthy, −1=unhealthy, 0=neutral, 1=healthy, 2=very healthy). Taste ratings were similar except subjects rated tastiness of each food, independent of its health properties (scale: −2=very bad, −1=bad, 0=neutral, 1=good, 2=very good). The order of the two rating tasks was counterbalanced across participants.
In the food choice task, subjects were then shown one food on every trial and had to make a choice between it and a fixed reference food item. The reference item was customized for each participant by selecting a food rated by that participant as ‘neutral’ in both tastiness and health. Subjects indicated their choices using a 4-point scale (−2=strong no, −1=no, 1=yes, 2=strong yes, where yes/strong yes indicate a selection of the non-reference item). Importantly, at the start of the choice portion of the task each participant was informed that, upon completion of the task, one trial would be selected at random and whatever the participant had chosen on that trial (i.e., the trial food or the reference food) would be served to the participant and she was expected to consume it. In this way, participants were made aware that every single choice ‘counts’, as any trial could be the one selected. For participants in the OB group at post-treatment, if neutral health and taste ratings were provided for the original reference item again at post-treatment, this same reference food item was used (n = 18), however in cases when the former reference item was no longer rated as neutral on both dimensions, a new reference food item was used that fit these criteria.
Statistical Analysis
Descriptive statistics were generated for all variables, including means and standard errors (SEs) for continuous variables and percentages for categorical variables. Analyses were conducted using IBM SPSS Statistics for Windows, Version 20.0 (Armonk, NY: IBM Corp).
At each assessment point, the task used in this study generated 150 observations of health ratings, 150 observations of taste ratings, and 150 choices per participant, corresponding to 150 different food stimuli. Linear mixed effects models (LMMs) were used to estimate the mean and standard error (SE) of health ratings, liking ratings, and choices at each assessment point. Ratings of each individual food for every subject and time point were used to estimate these models. Subject and time were treated as random effects (i.e., subject intercepts and the slope for time were treated as random effects). The LMMs accounted for the inherent lack of independence among observations made by the same participant. Put simply, two ratings of health or liking from the same participant are likely to be more similar than two ratings of health or liking from two different participants. Mixed models account for these within-subject correlations.
LMMs were also used to perform a series of inferential analyses:
Analyses of self-control
This first series of inferential analyses were conducted using a subset of two types of trials from the food choice task that were characterized as requiring self-control. These two types of self-control were examined separately because previous studies using similar choice paradigms have shown that they occur with different frequencies (29, 32, 33). One type of trial required self-control when participants were forced to choose between a food rated high on health but low in taste (e.g., celery) and their neutral reference food (SC Type 1: mean number of trials OB pre-treatment = 3.9, post-treatment = 4.1, NW = 7.6). Exerting self-control in this case involved choosing to eat the healthy but less tasty target food (e.g., celery). The other type of trial required self-control when participants were forced to choose between an unhealthy food they consider highly palatable (e.g., cookie) and their neutral reference food (SC Type 2: mean number of trials OB pre-treatment = 44.9, OB post-treatment = 41.2, NW = 46.7). Successful use of self-control in this type of trial involved selecting the neutral reference food and thus refusing the tasty but unhealthy food. The outcome measure in these analyses (i.e., the likelihood of exerting versus not exerting self-control on trials that required self-control) was modeled using the binomial distribution with a logit link function. The probability of exerting self-control or rate of self-control (reported as the proportion of trials on which self-control was exerted to facilitate interpretation) was compared for: (a) OB at pre- vs. post-treatment, and (b) NWC vs. OB at pre- and post-treatment. These comparisons were made for all self-control trials combined, and each type of self-control trial separately, in separate analyses. Lastly, the pre- to post-treatment change in proportion of self-control trials in which self-control was exerted was correlated with weight loss to determine whether more frequent exertion of self-control was associated with larger weight losses.
Analyses examining food choice decision-making mechanisms
The second set of inferential analyses combined the health ratings, taste ratings, and choice for each food stimulus. Using the full range of choice ratings (+2 to −2) as the outcome, we evaluated health ratings and taste ratings as predictors. These analyses were conducted to evaluate the degree to which health and taste ratings of the food stimuli were associated with choosing versus not choosing a target food over the reference food. The magnitude of the association of health ratings and taste ratings with choice was compared for: (a) OB at pre- vs. post-treatment using health/taste by time interaction terms, and (b) NWC vs. OB at pre- and post-treatment using health/taste by group interaction terms. As an example, the LMM comparing Pre-treatment OB and NW can be represented as:
wherein β0 represents the intercept while β1 and β2 represent slopes. Note that the time or group interaction terms are not shown here for brevity. Subscripts i and j represent food rating and subject respectively with rij accounting for error. The model included random intercepts for subject and groups, as well as random slopes for taste and health by subject.
Finally, we evaluated the degree to which participants’ ratings of health agreed with “objective” ratings of health made by licensed nutritionists for each food stimulus. This was done by calculating an intraclass correlation coefficient (ICC) using the mean of the nutritionists’ ratings of health and the mean of participants’ ratings of health for the 150 food stimuli.
Results
Sample Characteristics
Details regarding the baseline sample characteristics are presented in Table 1. As per definition, there was a significant difference between the OB and NW groups in BMI; however, the two groups did not differ on any other demographic collected (i.e., age, race/ethnicity, or education level; Table 1).
Table 1.
Participant Demographics Baseline
| OB | NW | p-value | |
|---|---|---|---|
| Age (years) | 46.95 (7.9) | 43.97 (8.9) | 0.15 |
| BMI (kg/m2) | 33.5 (3.9) | 22.7 (1.8) | 2.34 E-21* |
| Ethnicity, (% per category) | 0.14 | ||
| African American | 11.4% | 0% | |
| American Indian/Alaskan Native | 0% | 0% | |
| Asian/Pacific Islander | 0% | 3.8% | |
| Non-Hispanic White | 80% | 96.2% | |
| Hispanic/Latino | 11.4% | 7.4% | |
| Education, (% per category) | 0.42 | ||
| High school degree or less | 5.7% | 3.7% | |
| Vocational Training | 1.6% | 1.6% | |
| College Degree | 28.6% | 40.7% | |
| Graduate/Professional Degree | 37.1% | 44.4% |
Values presented are M (SD) unless otherwise noted.
Indicates α < 0.05.
Weight Loss
Overall, participants in the OB group lost an average of 5.82 kg (SE=0.70) through the weight loss intervention, representing a clinically significant loss of 6.62% (SE=0.82%) of initial body weight. There was no significant difference in weight loss between those participating in the face-to-face program (mean percent loss=6.0%, SE=0.78) and those in the Internet-delivered program (mean percent loss=8.7%, SE=2.03; p=0.38) nor were there differences between those in the 12-week intervention (mean percent weight loss = 6.7%, SE =1.1) and those in the 16-week intervention (mean percent weight loss = 6.5%, SE =1.2; p = 0.91).
Exertion of Self-Control
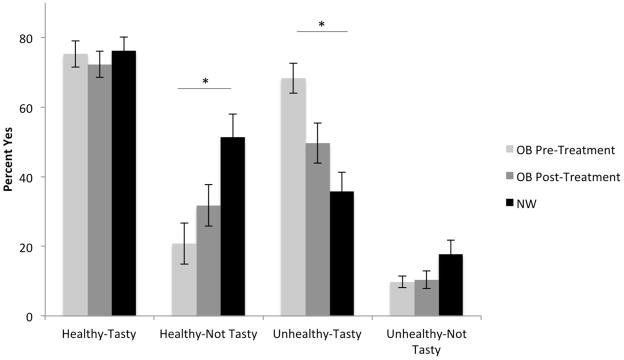
The proportion of trials in which OB participants exercised self-control increased from pre- to post-treatment, and this difference in self-control use trended toward significance (Table 2; mean difference = 10%, p=0.07). NWC participants exerted self-control on significantly more trials than OB both before (mean difference = 32%, SE =0.08; p<0.001) and after treatment (Table 2; mean difference = 21%, SE=0.08, p=0.007). Further analyses separating the two types of trials in which self-control could be used revealed OB participants were able to increase the rate at which they selected healthy, but relatively unpalatable foods (Table 2; self-control type1: mean difference = 14%, SE = 0.05; p=0.008), however, when faced with tasty-unhealthy foods (self-control type2), a smaller, trend-level increase in the use of self-control was observed (mean difference = 10%, SE=0.06 p=0.09). Again, NW participants exerted self-control on both of these trial types significantly more often than OB participants both before (Table 2; mean difference for selecting less tasty-healthy foods NW-OB Pre-Treatment =46%, SE=0.09, p=0.001; mean difference for refusing tastier-unhealthy foods NW-OB Pre-Treatment = 30%, SE=0.08, p=0.001) and after treatment (mean difference =32%, SE=0.09, p=0.001 and mean difference = 20%, SE=0.09, p = 0.02, respectively). Frequency of selecting foods within each of the health-taste categories and these group differences are depicted in Figure 1. Although we observed both increased self-control and substantial weight loss at the group level following the treatment program, individual differences in self-control improvement were not correlated with percentage weight loss (r=0.02, p=0.92).
Table 2.
Self-Control Exertion
| OB Pre-Treatment | OB Post-Treatment | NW | |
|---|---|---|---|
| Overall | 28% (0.05) c | 38% (0.06) c | 59% (0.06)a, b |
| Type I | 15% (0.05) b, c | 29% (0.05) a, c | 62% (0.07) a, b |
| Type II | 29% (0.05) c | 39% (0.06) c | 59% (0.06) a, b |
Values presented are M (Standard Error).
Indicates significant difference (α < 0.05) from OB Pre-Treatment,
indicates significant difference from OB Post-Treatment, and
indicates significant difference from NW.
Figure 1.
Frequency of selecting foods across each health-taste combination. The percentage of trials in which participants responded “yes” for each food type are displayed for individuals with obesity pre- and post-treatment as well as normal weight control participants. Statistical analyses were conducted using non-linear mixed models with logit functions. Significant differences are marked with asterisks. Error bars represent standard error.
Food Choice Decision-Making Mechanisms
Health Ratings
No significant changes in health ratings were observed within the OB group (pre-treatment mean=−0.49, SE=0.06, post-treatment mean=−0.51, SE=0.06; regression coefficient =0.02, t=0.61, p=0.54). Neither pre- nor post-intervention health ratings for OB differed significantly from those of NW subjects (NW mean=−0.51, SE=0.06; comparison to OB pre-treatment regression coefficient = 0.02, p=0.80 and post-treatment coefficient = 0.03, p=0.72,). Moreover, health ratings of the OB and NW subjects were similar to those of the experts (expert mean=−0.52, SE=0.10; interclass correlation coefficient [ICC] with OB pre-treatment=0.96, post-treatment=0.74, NW=0.97). Overall, health ratings were below zero, reflecting a general ‘unhealthy’ perception of the foods by all groups. This coincides with the inclusion of many high calorie, high fat foods in the sample set.
Taste ratings
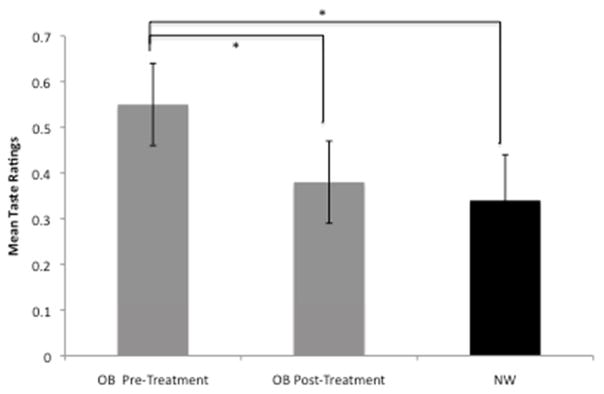
Mean taste ratings of OB participants significantly decreased from 0.55 (SE=0.09) pre-treatment to 0.38 (SE=0.09) post-treatment (regression coefficient = 0.17, t=6.81, p<0.001), indicating that on average the foods were perceived as less tasty following treatment (Figure 2). These changes were not significantly correlated with percent body weight lost during BWL (r= 0.18, p=0.32). To determine if taste preferences changed differentially for unhealthy versus healthy foods, taste ratings of OB pre- and post-treatment were assessed as a function of health using mean ratings from nutritionists to categorize foods as ‘healthy’, ‘unhealthy’, or ‘neutral’. Taste ratings changed within each health category [time × category F(2,147)=39.35, p <0.001; healthy: mean rating pre-treatment=1.11, SE = 0.08, post-treatment = 1.16, SE = 0.07; neutral: pre-treatment = 0.49, SE = 0.10, post-treatment = 0.38, SE = 0.09; unhealthy: pre-treatment = 0.38, SE = .05, post-treatment = 0.11, SE = 0.04), however post-hoc tests revealed absolute change across categories differed [F(2,147) = 15.44, p <0.001) with changes for unhealthy foods significantly greater than that of both neutral (p=0.003) and healthy foods (p<0.001).
Figure 2. Mean Taste Ratings.
Mean taste ratings are plotted for OB pre-treatment, OB post-treatment, and NWC participants. Significant differences are marked with asterisks. Error bars represent standard error.
The mean taste rating for NW subjects was 0.34 (SE=0.10), which was not significantly different from the OB group before the intervention (regression coefficient = 0.21, t = 1.65, p=0.10), or after (regression coefficient = 0.03, t=0.20, p=0.84).
Health and Taste Value in Decision-making
Next, we examined the effect of the intervention on the relative association between health and taste attributes and the choice made via LMM. This analysis took into account changes in taste preferences by using the health and taste ratings at the time of the decision (i.e., pre- or post-treatment) to predict choices at that time. We found that the intervention significantly increased the degree to which health factored into choices (Table 3; p=0.001), and significantly decreased the degree to which taste factored into choices (3; p=0.001) in OB. However, health rather than taste was more strongly associated with choices in NW compared to OB pre- and post-treatment (Table 3; all p’s<0.001).
Table 3.
Health and Taste value in decision
| OB Post-Treatment vs. Pre-Treatment | OB Pre-Treatment vs. NW | OB Post Treatment vs. NW | |
|---|---|---|---|
| Health | 0.07 (4.7) ** | −0.38 (−23.2)** | −0.31 (−18.5)** |
| Taste | −0.10 (−6.2) ** | 0.44 (−24.3)** | 0.34 (18.0)** |
Values presented are regression coefficients with t values in parentheses. Health factored into decisions more for OB following treatment, and NW compared to OB both pre- and post-treatment, whereas taste factored into decisions more for OB Pre-Treatment.
Indicates significant difference (α ≤ 0.001) between groups.
Discussion
Understanding the mechanisms through which successful BWL interventions improve dietary choices is an important open question at the intersection of psychology, economics, and medicine. This study begins to address this by combining a BWL intervention with an experimental economics task, which allowed us to measure self-control in food choice decision-making and the potential change in three basic mechanisms that are often explicitly targeted by BWL treatments: 1) changes in the perception of the healthiness of foods, 2) changes in taste perceptions, and 3) changes in the relative importance of health versus taste when making decisions.
We found that participants with obesity exerted greater self-control after participation in a BWL intervention; particularly in their willingness to consume healthier, but less tasty foods. This result suggests self-control in dietary choice may be a malleable construct that can change with treatment. However, the degree of self-control employed did not reach that of normal weight individuals, suggesting there is room for further improvement. The weight loss protocol employed here encompasses well-established behavioral techniques, including self-monitoring, stimulus control, problem solving, goal setting and assertiveness training, each of which likely contribute to healthy food decision-making, but it remains plausible that more explicitly targeting self-control in food choices may yield added benefit. Moreover, our analysis of the specific situations in which self-control was exerted revealed individuals with obesity significantly increased their use of self-control only on trials in which they were choosing between the neutral reference food and a relatively unpalatable, but healthier food. Three studies employing a similar choice paradigm have also observed differences between the two types of self-control trials(29, 32, 33), supporting the idea that these subtypes may be at least partially distinct or differ in difficulty. Self-control on trials pairing the neutral reference food with tasty, but unhealthy foods showed only marginal improvement. Thus, whereas obese individuals may learn to choose healthy foods that are less appealing in terms of taste, when tempted with highly palatable, unhealthy foods they continue to find it difficult to say ‘no’, making self-control lapses more likely. Although use of self-control was not correlated with amount of weight loss in the current study, increases in self-control may help with longer-term weight loss maintenance. Further research is needed to examine this.
In terms of changes in the two perceptual mechanisms driving dietary choice before and after the weight loss treatment, we found effects on taste, but not health perception. Specifically, we found health ratings of the foods (i.e., perception of food nutrition) did not change as a function of the weight loss program, nor did they differ between individuals with obesity and those with lifetime normal weight. This finding supports previous studies showing no differences in nutrition knowledge between individuals with obesity and lean individuals(12). Moreover, the health ratings of both NW and OB participants were similar to those made by professional nutritionists, suggesting both NW and OB participants had solid perceptions of food item health.
In contrast, taste perceptions did change in individuals with obesity following treatment. On average foods were rated as less tasty post-treatment. This finding is in line with previous studies(20, 21, 22, 23, 24, 25, 26, 27). Furthermore, the decrease in taste ratings was particularly strong for unhealthy foods.
As noted above participants in the BWL intervention showed increases in dietary self-control post treatment. These changes are related to the final mechanism supporting healthier food choices, the degree to which health and taste factor into choices. Valuing longer-term objectives (e.g., healthiness) over short-term desirability (e.g., taste) is critical for employing greater self-control, and tendencies to over-value short-term features can be a source of self-control failure(34). One recent study has shown that self-reported high valuation of taste in food choices within a normal weight population is associated with poorer diet quality, highlighting the need to reduce the association between taste and food choices to improve diet (35). After accounting for the observed changes in taste preferences by using each OB participants’ own new taste and health ratings post-treatment, we found the intervention altered how individuals made food choices. Health attributes became more important and taste less important in their dietary choices. Although these changes made the decision process of individuals with obesity more similar to that of normal weight individuals, participants in the OB group continued to value health less and taste more than normal weight individuals. Again, efforts to render the mechanisms related to food choices of individuals with obesity more similar to those of lifetime normal weight controls via increasing the value of health and decreasing the value of taste may help to produce greater initial and/or longer-term weight loss.
The evidence that BWL enhanced the valuation of health and diminished the valuation of taste raises important questions about the neurobiological changes that are associated with these decisions and how to produce even greater shifts in the relative valuation of these attributes. Previous work has documented neural underpinnings of food choice decision-making in ‘naturally’ healthy and unhealthy eaters (29). This study identified a region of the brain (ventral medial prefrontal cortex) that coded both health and taste value in healthy eaters, but only taste in unhealthy eaters, and a control-based region (dorsal lateral prefrontal cortex, dlPFC) that was more active for healthy eaters on trials requiring self-control. These regions may also differ during food choices of individuals with obesity and normal weight individuals and warrant examination in future work.
Of even greater relevance is a subsequent study showing this process is malleable and can be changed with attentional primes(32). Thus the changes in health/taste valuations that OB participants experienced as a result of BWL may reflect differences in activation of these brain regions. Recent work by Enax and colleagues (2015) has also shown that directing attention to nutritional information using various labeling strategies impacts valuation of foods and activity in vmPFC and dlPFC (36) and others have found cognitive reappraisal strategies increases control-related brain activity in response to viewing appetizing food images(37, 38). Thus lengthening the weight loss program or finding ways to increase the attention paid to health at the time of food choices may lead to more efficient functioning of dlPFC modulatory mechanisms and help make food choices of individuals with obesity more similar to those of normal weight individuals.
A limitation of the present study is the potential for demand characteristics. The possible pressure felt by participants to respond in accordance with lessons of the intervention may have contributed to positive changes observed. The method of randomly selecting a decision trial and having participants actually consume the food they chose on that trial immediately after the task was designed to help overcome this, because participants were aware that their choices had real consequences. Furthermore, we made an effort to separate the decision-making task from the weight loss program by having separate staff collect these data at a different location. Although demand characteristics may still have been operating, we note that following treatment obese participants did not make choices that were fully in accordance with expectations of the BWL program. Specifically, self-control was exhibited on fewer than half of the trials, suggesting these participants were not responding according to expectations of either the weight loss program or notions of social desirability. Another potential limitation is that the normal weight participants only performed the task once, and it is possible that a second exposure to the task and stimuli may have contributed to changes observed in the OB group. Although it is not expected that simply repeating performance of the task would elicit any substantial changes, future studies should balance experience with the task across groups. Another potential limitation is that the food choice task measured taste ratings but did not differentiate ‘liking’ versus ‘wanting’ of foods. More research is needed to determine if liking and wanting have separable impact on food choice behavior. Additionally, only middle-aged females were included in the current sample thus limiting the generalizability of these findings. Future research will aim to include both men and women and explore any potential age and sex-related differences in the mechanisms supporting food choice decision-making.
Despite these limitations, the current study provides novel insight into food decision-making, and the impact of behavioral weight loss treatment on food choices. Individuals with obesity clearly differ from normal weight individuals in food decision-making. Dietary choices become healthier following behavioral weight loss treatment, with changes in taste-ratings and the degree to which health and taste factor into choices both contributing. Nonetheless, post-treatment food decisions continue to differ from those of normal weight individuals and an inability to successfully exert self-control, especially in the face of liked-unhealthy foods, may potentially contribute to the eventual weight regain commonly observed. We believe the current findings provide important insights into key mechanisms of dietary choice in obesity and highlight the importance of conducting future studies to examine whether methods that enhance the valuation of health over taste in food decision-making can further improve treatment outcomes.
Supplementary Material
Acknowledgments
Funding: This work was funded by the National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases K01 (5K01DK090445-04) awarded to KED as well as an NIH NHLBI T32 Fellowship (Fellow: KED, PI: RRW; 5 T32 HL076134-04).
We would like to thank all of the participants who took part in this research, as well as Linda Gay, Interventionist. We thank Sara Cournoyer, Katelyn Gettens, Lucas First, Anne Goldring, and Claudine Yee for their assistance in recruiting participants and collecting assessment data.
Footnotes
Disclosure: None of the authors declare any conflicts of interest.
Author contributions: The authors’ responsibilities were as follows: KED, JMM, TAH, and RRW contributed to the design of the research. TAH designed and provided the food choice decision-making task. KED conducted the research with assistance from KAM. JGT contributed to data analyses. KED was responsible for writing the manuscript, with major contributions from TAH and RRW.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jensen MD, Ryan DH, Donato KA, Apovian CM, Ard JD, Comuzzie AG, et al. Guidelines (2013) for managing overweight and obesity in adults. Obesity. 2014;22:S1–S410. doi: 10.1002/oby.20819. [DOI] [PubMed] [Google Scholar]
- 2.Wing RR. Behavioral Weight Control. In: Wadden TA, Stunkard AJ, editors. Handbook of Obesity Treatment. Guilford Press; New York, NY: 2002. [Google Scholar]
- 3.Jeffery RW, Levy RL, Langer SL, Welsh EM, Flood AP, Jaeb MA, et al. A comparison of maintenance-tailored therapy (MTT) and standard behavior therapy (SBT) for the treatment of obesity. Prev Med. 2009;49:384–389. doi: 10.1016/j.ypmed.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sarwer DB, von Sydow Green A, Vetter ML, Wadden TA. Behavior therapy for obesity: where are we now? Curr Opin Endocrinol Diabetes Obes. 2009;16:347–352. doi: 10.1097/MED.0b013e32832f5a79. [DOI] [PubMed] [Google Scholar]
- 5.Turk MW, Yang K, Hravnak M, Sereika SM, Ewing LJ, Burke LE. Randomized clinical trials of weight loss maintenance: a review. J Cardiovasc Nurs. 2009;24:58–80. doi: 10.1097/01.JCN.0000317471.58048.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wing RR, Crane MM, Thomas JG, Kumar R, Weinberg B. Improving weight loss outcomes of community interventions by incorporating behavioral strategies. Am J Public Health. 2010;100:2513–2519. doi: 10.2105/AJPH.2009.183616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raynor HA, Anderson AM, Miller GD, Reeves R, Delahanty LM, Vitolins MZ, et al. Partial Meal Replacement Plan and Quality of the Diet at 1 Year: Action for Health in Diabetes (Look AHEAD) Trial. J Acad Nutr Diet. 2015;115:731–742. doi: 10.1016/j.jand.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinberg D, Axelson ML, Price S. Changing food knowledge, food choice, and dietary fiber consumption by using tailored messages. Appetite. 2000;35:35–43. doi: 10.1006/appe.2000.0335. [DOI] [PubMed] [Google Scholar]
- 9.Klohe-Lehman DM, Freeland-Graves J, Anderson ER, McDowell T, Clarke KK, Hanss-Nuss H, et al. Nutrition knowledge is associated with greater weight loss in obese and overweight low-income mothers. J Am Diet Assoc. 2006;106:65–75. doi: 10.1016/j.jada.2005.09.047. quiz 76–69. [DOI] [PubMed] [Google Scholar]
- 10.Rustad C, Smith C. Nutrition knowledge and associated behavior changes in a holistic, short-term nutrition education intervention with low-income women. J Nutr Educ Behav. 2013;45:490–498. doi: 10.1016/j.jneb.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Wardle J, Parmenter K, Waller J. Nutrition knowledge and food intake. Appetite. 2000;34:269–275. doi: 10.1006/appe.1999.0311. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien G, Davies M. Nutrition knowledge and body mass index. Health education research. 2007;22:571–575. doi: 10.1093/her/cyl119. [DOI] [PubMed] [Google Scholar]
- 13.Axelson ML, Federline TL, Brinberg D. A meta-analysis of food- and nutrition-related research. Journal of Nutrition Education. 1985;17:51–54. [Google Scholar]
- 14.Shepherd R, Stockley L. Nutrition knowledge, attitudes, and fat consumption. J Am Diet Assoc. 1987;87:615–619. [PubMed] [Google Scholar]
- 15.Shepherd R, Towler G. Nutrition knowledge, attitudes and fat intake: application of the theory of reasoned action. Journal of human nutrition and dietetics: the official journal of the British Dietetic Association. 2007;20:159–169. doi: 10.1111/j.1365-277X.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- 16.Towler G, Shepherd R. Application of Fishbein and Ajzen’s expectancy-value model to understanding fat intake. Appetite. 1992;18:15–27. doi: 10.1016/0195-6663(92)90207-m. [DOI] [PubMed] [Google Scholar]
- 17.Stafleu A, Van Staveren WA, De Graaf C, Burema J, Hautvast JG. Nutrition knowledge and attitudes towards high-fat foods and low-fat alternatives in three generations of women. Eur J Clin Nutr. 1996;50:33–41. [PubMed] [Google Scholar]
- 18.Kaufer-Horwitz M, Villa M, Pedraza J, Dominguez-Garcia J, Vazquez-Velazquez V, Mendez JP, et al. Knowledge of appropriate foods and beverages needed for weight loss and diet of patients in an Obesity Clinic. Eur J Clin Nutr. 2015;69:68–72. doi: 10.1038/ejcn.2014.102. [DOI] [PubMed] [Google Scholar]
- 19.Crites SL, Jr, Aikman SN. Impact of nutrition knowledge on food evaluations. Eur J Clin Nutr. 2005;59:1191–1200. doi: 10.1038/sj.ejcn.1602231. [DOI] [PubMed] [Google Scholar]
- 20.Ledikwe JH, Ello-Martin J, Pelkman CL, Birch LL, Mannino ML, Rolls BJ. A reliable, valid questionnaire indicates that preference for dietary fat declines when following a reduced-fat diet. Appetite. 2007;49:74–83. doi: 10.1016/j.appet.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Mattes RD. Fat preference and adherence to a reduced-fat diet. Am J Clin Nutr. 1993;57:373–381. doi: 10.1093/ajcn/57.3.373. [DOI] [PubMed] [Google Scholar]
- 22.Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: problems, solutions and new perspectives. Philosophical transactions of the Royal Society of London Series B, Biological sciences. 2006;361:1137–1148. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deglaire A, Mejean C, Castetbon K, Kesse-Guyot E, Hercberg S, Schlich P. Associations between weight status and liking scores for sweet, salt and fat according to the gender in adults (The Nutrinet-Sante study) Eur J Clin Nutr. 2015;69:40–46. doi: 10.1038/ejcn.2014.139. [DOI] [PubMed] [Google Scholar]
- 24.Duffy VB, Hayes JE, Sullivan BS, Faghri P. Surveying food and beverage liking: a tool for epidemiological studies to connect chemosensation with health outcomes. Annals of the New York Academy of Sciences. 2009;1170:558–568. doi: 10.1111/j.1749-6632.2009.04593.x. [DOI] [PubMed] [Google Scholar]
- 25.Duffy VB, Lanier SA, Hutchins HL, Pescatello LS, Johnson MK, Bartoshuk LM. Food preference questionnaire as a screening tool for assessing dietary risk of cardiovascular disease within health risk appraisals. J Am Diet Assoc. 2007;107:237–245. doi: 10.1016/j.jada.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura K, Shimai S, Kikuchi S, Tanaka M. Correlation between a liking for fat-rich foods and body fatness in adult Japanese: a gender difference. Appetite. 2001;36:1–7. doi: 10.1006/appe.2000.0377. [DOI] [PubMed] [Google Scholar]
- 27.Mela DJ, Sacchetti DA. Sensory preferences for fats: relationships with diet and body composition. Am J Clin Nutr. 1991;53:908–915. doi: 10.1093/ajcn/53.4.908. [DOI] [PubMed] [Google Scholar]
- 28.Schultes B, Ernst B, Wilms B, Thurnheer M, Hallschmid M. Hedonic hunger is increased in severely obese patients and is reduced after gastric bypass surgery. Am J Clin Nutr. 2010;92:277–283. doi: 10.3945/ajcn.2009.29007. [DOI] [PubMed] [Google Scholar]
- 29.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324:646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- 30.Diabetes Prevention Program Research G. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25:2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Look ARG, Wadden TA, West DS, Delahanty L, Jakicic J, Rejeski J, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring) 2006;14:737–752. doi: 10.1038/oby.2006.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. J Neurosci. 2011;31:11077–11087. doi: 10.1523/JNEUROSCI.6383-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris A, Hare T, Rangel A. Temporally dissociable mechanisms of self-control: early attentional filtering versus late value modulation. J Neurosci. 2013;33:18917–18931. doi: 10.1523/JNEUROSCI.5816-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liberman N, Trope Y. The psychology of transcending the here and now. Science. 2008;322:1201–1205. doi: 10.1126/science.1161958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kourouniotis S, Keast RS, Riddell LJ, Lacy K, Thorpe MG, Cicerale S. The importance of taste on dietary choice, behaviour and intake in a group of young adults. Appetite. 2016;103:1–7. doi: 10.1016/j.appet.2016.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Enax L, Hu Y, Trautner P, Weber B. Nutrition labels influence value computation of food products in the ventromedial prefrontal cortex. Obesity (Silver Spring) 2015;23:786–792. doi: 10.1002/oby.21027. [DOI] [PubMed] [Google Scholar]
- 37.Stice E, Yokum S, Burger K, Rohde P, Shaw H, Gau JM. A pilot randomized trial of a cognitive reappraisal obesity prevention program. Physiol Behav. 2015;138:124–132. doi: 10.1016/j.physbeh.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokum S, Stice E. Cognitive regulation of food craving: effects of three cognitive reappraisal strategies on neural response to palatable foods. Int J Obes (Lond) 2013;37:1565–1570. doi: 10.1038/ijo.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.