Summary
Background
Patients with Parkinson’s Disease (PD) may exhibit premotor neurochemical changes in dopaminergic (DA) and nondopaminergic systems. Using positron emission tomography (PET), we studied participants with leucine-rich repeat kinase 2 (LRRK2) mutations and with sporadic PD to assess whether DA and serotonin transporter (SERT) changes were similar in LRRK2 PD and sporadic PD, and whether asymptomatic LRRK2 mutation carriers exhibited PET changes in the absence of motor symptoms.
Methods
Between July 1999 and May 2016, we did two cross sectional PET studies at the Pacific Parkinson’s Research Centre (Vancouver, Canada) with LRRK2 mutation carriers with or without manifest PD, patients with sporadic PD, and age-matched healthy controls, all aged 18 years or older. Patients with PD were diagnosed by a neurologist with movement disorder training in accordance with the UK Parkinson’s Disease Society Brain Bank criteria. LRRK2 carrier status was confirmed by bi-directional Sanger sequencing. First, affected and unaffected LRRK2 carriers seen from July 1999 to January 2012 were imaged with PET tracers for the membrane dopamine transporter (DAT) and dopamine synthesis and storage (18F-6-fluoro-L-dopa; FDOPA) and compared with sporadic PD and age-matched healthy controls. Second, distinct groups of LRRK2 mutation carriers, sporadic PD patients, and age-matched healthy controls seen from November 2012 to May 2016 were studied with tracers for the SERT and vesicular monoamine transporter 2 (VMAT2). Striatal DAT binding, DTBZ binding, FDOPA uptake and SERT binding in multiple brain regions were compared using analysis of covariance adjusted for age.
Findings
Using data from 40 LRRK2 mutation carriers, 63 patients with sporadic PD, and 35 controls, we identified significant group differences in striatal DAT binding (all age ranges p<0·0001 in caudate and putamen) and FDOPA uptake (age 50 or lower in caudate, p=0·0002; all other age ranges p<0·0001; in putamen, all age ranges p<0·0001). Affected LRRK2 mutation carriers (n=15) had reduced striatal DAT binding and FDOPA uptake, comparable to sporadic PD of similar duration. Unaffected carriers (n=25) had greater FDOPA uptake and DAT binding than sporadic PD (n=63), with FDOPA uptake comparable to and DAT binding lower than healthy controls. Unaffected LRRK2 carriers (n=9) had significantly elevated SERT binding in hypothalamus (greater than healthy controls, 7 LRRK2 PD and 13 sporadic PD subjects; p<0·0001), striatum (greater than sporadic PD; p=0·02) and brainstem (greater than affected LRRK2 carriers; p=0·01) after adjustment for age. SERT binding in cortex was not significantly different between groups after age adjustment. Striatal DTBZ binding was reduced in all affected patients and asymmetrically reduced in one unaffected carrier.
Interpretation
Dopaminergic and serotonergic changes progress in a similar fashion in LRRK2 PD and sporadic PD, but unaffected LRRK2 mutation carriers exhibit increased SERT binding in striatum, brainstem and hypothalamus, possibly reflecting compensatory changes in serotonergic innervation preceding the motor onset of PD.
Funding
Canada Research Chairs, Michael J. Fox Foundation, National Institutes of Health, Pacific Alzheimer Research Foundation, Pacific Parkinson’s Research Institute, National Research Council of Canada
INTRODUCTION
Degeneration of dopaminergic neurons in the substantia nigra is already advanced by the time motor symptoms of Parkinson disease (PD) emerge. Non-motor symptoms (including impaired olfaction, autonomic function, sleep, mood, and cognition) often precede motor onset, sometimes by several years,1 and may relate to non-dopaminergic neurodegeneration.2–3 Unaffected carriers of pathogenic mutations known to increase the risk of PD, most commonly in leucine-rich repeat kinase 2 (LRRK2), exhibit dopaminergic deficits similar in distribution to sporadic PD (sPD).4–6 LRRK2 PD also shares non-motor features with sPD, including hyposmia,7,8 constipation, impaired colour discrimination,9 depression, and sleep disturbance. Asymptomatic carriers may therefore demonstrate early non-dopaminergic neurochemical changes underlying these symptoms.
Diffuse reductions of [11C]-3-Amino-4-(2-dimethylaminomethylphenylsulfaryl)-benzonitrile (DASB; a PET tracer with affinity for the serotonin transporter [SERT]) binding have previously been reported in sporadic PD of varying stages between 3 and 15 years since motor onset.10–12 However, in very early PD (mean 2.1 months) DASB binding was preserved and in striatum was inversely correlated to DAT binding,13 suggesting the possibility of a compensatory change in the SERT occurring early in PD.
We compared patients with LRRK2 mutations (with or without manifest PD) to those from an earlier study on sPD14 and age-matched healthy controls. Having established that the dopaminergic deficit in LRRK2 PD evolves in a fashion similar to sPD, we used the DASB tracer to assess changes in the SERT in asymptomatic LRRK2 carriers, LRRK2 PD, sPD, and healthy controls.
METHODS
Study design and participants
Two cross-sectional PET studies were conducted in Vancouver, Canada at a subspecialty clinic and research centre. Subjects were identified locally or referred by subspecialty clinics elsewhere in Canada, the United States, Norway and Japan. This study was approved by the Clinical Research Ethics Board of the University of British Columbia.
For study 1, we recruited adults (age 18 or older) carrying a known pathogenic LRRK2 mutation confirmed by bi-directional Sanger sequencing who had undergone PET with 11C-d-threo-methylphenidate (MP, a marker of the membrane dopamine transporter, DAT) and 18F-6-fluoro-L-dopa (FDOPA, a measure of dopamine synthesis and storage) from July 1999 to January 2012. These subjects were compared with adult subjects with sPD from a previous study at our centre14, and healthy controls studied at our centre. Healthy controls had no personal or family history of neurological or psychiatric illness.
For study 2 we assessed new groups of adult subjects with sPD or a documented LRRK2 mutation from November 2012 to May 2016 using PET with DTBZ and DASB. Those subjects exposed to selective serotonin reuptake inhibitors or serotonin-norepinephrine reuptake inhibitors were excluded. All subjects provided written informed consent.
Procedures
All subjects underwent a neurological examination by a neurologist with subspecialty movement disorders training for determination of the Unified Parkinson’s Disease Rating Scale part III (UPDRS-III) and the diagnosis of PD according to UK Brain Bank criteria. Those in study 2 were also assessed with the Montreal Cognitive Assessment (MoCA) and Beck Depression Inventory (BDI) instruments. Prior to imaging, all anti-parkinsonian medications were withheld for at least 12 hours (18 hours for controlled release levodopa and dopamine agonists).
In study 1, PET scans were performed consecutively in a single day for most cases (MP prior to FDOPA), at least 2·5 hours apart to allow for the decay of radioactivity associated with the previous [11C] tracer. These scans were performed in three-dimensional mode with either the ECAT953B/31 tomograph (CTI Systems/Siemens, Knoxville, TN), or the General Electric (GE) Advance tomograph (General Electric Medical Systems, Milwaukee, WI).
Tracer was injected intravenously in 10 mL of saline over 60 seconds using a Harvard infusion pump (Harvard Apparatus, Holliston, MA; MP, 185MBq; FDOPA, 185–260 MBq). One hour prior to the FDOPA scan subjects received 200 mg of carbidopa orally. MP emission data were collected over 60 minutes into a series of sequential frames (4×1-min, 3× 2-min, 8×5-min, 1×10-min). FDOPA emission data were collected over 90 minutes into a series of sequential frames (ECAT: 9×10-min, Advance: 4×1-min, 3×2-min, 16×5-min).
Phantom- and human imaging-based scanner cross-calibrations were performed on the two scanners and the image reconstruction filter on the GE Advance tomograph was adjusted to match the spatial resolution of the two scanners, with further adjustment for discrepancy in their radial uniformity profiles. The difference between values obtained on the two scanners in age-matched healthy control subjects is ≤5%.
In study 2, scanning took place over a 1–3 day period, with DASB followed by DTBZ and a brain MRI used to aid region of interest placement. Patients were positioned using external lasers aligning the gantry with the inferior orbital-external meatal line, and custom fitted thermoplastic masks were applied to minimize head movement and positioning changes. Radiotracer was administered by intravenous injection over 60 seconds with [11C](+)DTBZ (185 MBq). Data were acquired over 60 min on the GE Advance scanner, which has an effective in-plane resolution of 6·5 mm2 and axial resolution of 6 mm, except for those patients with sPD studied using the Siemens high resolution research tomograph, (HRRT; Knoxville, TN) which has a spatial resolution of 2·3 mm3. Separate groups of healthy controls were studied with DTBZ on the GE Advance and HRRT scanners. The 80-min long [11C]DASB (555 MBq) scans were performed on the HRRT for all subjects. Transmission scans were performed over 10 minutes with 68Ge (Advance) or 137Cs (HRRT) to correct for photon attenuation.
For study 1, striatal regions of interest (ROIs) were defined on an averaged image derived from 4 consecutive image slices (ECAT – slice thickness 3·38 mm), or 3 consecutive image slices (Advance – slice thickness 4·25 mm). Four elliptical ROIs were drawn on the striatal image; one on the caudate, and three covering the full length of the putamen. The same set of image slices was used to define the occipital cortex reference region for the MP and FDOPA scans. Time activity curves (TACs) were extracted for each ROI. The Logan graphical method15 was used to calculate the tissue-input binding potential (BPND) for the MP data. Patlak analysis16 was used to calculate the tissue-input uptake rate constant (Kocc) from the FDOPA data. Age corrected control values for each subject were calculated from healthy control subjects17.
Scan analysis was conducted by a single individual masked to clinical details and genetic status. Left and right mean putaminal Kocc and BPND values were obtained by averaging the three ROIs placed on each putamen. Mean caudate and putaminal values (Kocc or BPND) were obtained by averaging the corresponding left and right values.
DTBZ and DASB binding potentials (BPND) in study 2 were determined using a Logan analysis with occipital cortex reference region (DTBZ) or simplified reference tissue model with cerebellum as the reference region (DASB). BPND was compared with age-matched control values for each tracer. A region of interest template was developed in Montreal Neurological Space (MNI space) using MRI and DASB PET data from healthy controls. Anatomical brain MRI images for each subject were then transformed to the MNI space using Statistical Parametric Mapping (SPM; Wellcome Trust Centre for Neuroimaging, University College London, UK), and the ROI template was inverse transformed from each subject’s MRI scan and applied to their PET data for analysis. DASB binding potentials were then calculated for ROIs in cortex (including anterior cingulate, amygdala, dorsolateral prefrontal cortex and insula), striatum (caudate, putamen and ventral striatum), hypothalamus and brainstem (three ROIs in midbrain, pons and medulla along the median raphe, based on maximal DASB binding in healthy controls; appendix).
Statistical analysis
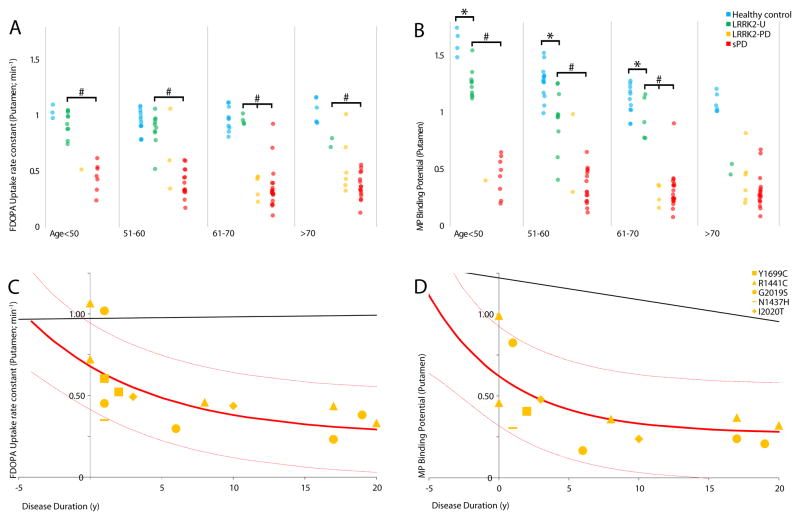
Statistical analysis was performed using SAS (version 8). A normal distribution was assumed for the PET data based on previously published studies14,17, examination of normal probability plots and the Shapiro-Wilk test. Missing data were considered to be missing at random. For study 1, analyses of variance were performed comparing healthy controls, unaffected LRRK2 mutation carriers, symptomatic LRRK2 mutation carriers, and sPD, and Bonferroni correction for multiple comparisons was employed. As both age and disease duration can affect dopaminergic function and we are unable to accurately determine disease duration in premanifesting mutation carriers, separate ANOVAs were performed in four age categories to facilitate comparison of LRRK2 PD: ≤50, 51–60, 61–70 and >70. Due to the effect of age on MP binding and on the likelihood of asymptomatic mutation carriers converting to symptomatic disease, ANOVA was performed at each age group, but only for cell size > 3. The regression of PET data from clinically affected LRRK2 PD patients on disease duration was compared with sPD for each ligand and region as determined in previously published data.18 We examined LRRK2 groups without distinguishing different mutation subgroups due to limited sample size.
For study 2, analyses of covariance compared DASB binding in the striatum, brainstem, hypothalamus and cortex between healthy controls, unaffected LRRK2 mutation carriers, symptomatic LRRK2 carriers and a new set of sPD patients. ANCOVA was performed using age as a covariate; as in study 1, the age of onset was not estimable for unaffected carriers but was similar in symptomatic patients (LRRK2 PD mean 58, sd 12, range 45–76 years; sPD mean 53, sd 10, range 35–68 years. A Holm’s step-down procedure with Duncan’s multiple range test was used to adjust for multiple comparisons. As sPD patients were studied with the HRRT and all LRRK2 mutation carriers with the Advance scanner, the DTBZ data were compared with age matched healthy controls studied with the same scanner, rather than between groups (using t tests). Demographic and clinical information was collected, including age, sex, date of PET, year of symptom onset and diagnosis (for symptomatic patients), and family history of PD.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
40 LRRK2 mutation carriers were studied between July 1999 and January 2012 (Study 1). Nine subjects were studied with the ECAT and 31 with the GE Advance. Only the most recent scans were analysed for nine carriers studied multiple times. One subject was unable to complete FDOPA scanning, and two did not receive MP. 5 subjects (1 affected) were from a Norwegian family with an N1437H mutation,19 4 subjects (2 affected) from a German – Canadian family with a Y1699C mutation, 5 subjects (2 affected) from 2 Japanese families with an I2020T mutation, 10 subjects (3 affected) with a single G2019S mutation and 5 others (2 affected) from a North American kindred with homozygous G2019S mutation, and 11 individuals (5 affected) from a Western Nebraska family with an R1441C mutation. PET findings in many of these subjects have been reported previously.4,5,6,17,19
35 healthy controls studied from February 1997 to July 2008 (19 women; mean age 55, SD 15, range 27–77 years) and 63 individuals with sporadic PD studied between January 1997 and June 2006 (17 women; mean age 63, SD 11, range 35–81 years) were included in study 1.7
Study 2 included 13 people with a diagnosis of sporadic PD studied from November 2012 to May 2016 (6 women; mean age 58, sd 9, range 40–72 years), and 16 with a documented LRRK2 mutation studied from August 2013 to June 2015 (7 with PD: 4 women, mean age 66, SD 15, range 48–86 years; 9 non-manifesting: 5 women, mean age 49, SD 11, range 27–61 years). UPDRS-III data were available for 21 of 25 unaffected carriers in study 1 (mean score 6·2; SD 7·9; table 1). Two of 21 individuals had high UPDRS-III scores deemed not indicative of parkinsonism: one person with a history of poliomyelitis had a score of 32 and another had a score of 23; according to the subjects’ neurologists neither examination was consistent with PD. With these scores excluded only subtle signs were noted (among the remaining 19 unaffected carriers, mean score 3·9, sd 3·3). Scores were measured in 8 of 9 unaffected carriers from study 2. No unaffected carriers had findings consistent with PD and there were only subtle signs on the UPDRS-III exam (mean score 2·3, SD 2·2).
Table 1.
Clinical Characteristics of Subjects
| Group | N | Age (mean, (sd); range) | Age of onset (mean, (sd); range) | Mutations | UPDRS III score (mean, sd) | UPDRS IV score (mean, sd) | BDI (mean, sd) | MoCA (mean, sd) |
|---|---|---|---|---|---|---|---|---|
| FDOPA, MP (Study 1) | ||||||||
| LRRK2-U | 25 (13 female) | 50 (14); 25–77 | - | Y1699C (2), R1441C (6), G2019S (10; 3 homozygous), N1437H (4), I2020T (3) | 6.2 (7·9)* | - | - | - |
| LRRK2- PD | 15 (9 female) | 65 (11); 44–80 | 58 (11); 42–77 | Y1699C (2), R1441C (5), G2019S (5; 2 homozygous), N1437H (1), I2020T (2) | 31·4 (16·5) | - | - | - |
| Sporadic PD | 63 (17 female) | 63 (11); 35–81 | 57 (9); 35–73 | - | 33·3 (0·5) | - | - | - |
| DASB, DTBZ (Study 2) | ||||||||
| LRRK2-U | 9 (5 female) | 49 (11); 27–61 | - | G2019S (9) | 2.3 (2·2) ** | - | 4.0(2·3)# | 28.5 (1·3)## |
| LRRK2- PD | 7 (4 female) | 66 (15); 48–86 | 58 (12); 45–76 | R1441C (1), G2019S (6) | 22·1 (10·5) | 4·0 (3·6)### | 9 (6·8) | 26·7 (2·1) |
| Sporadic PD | 13 (6 female) | 58 (9); 40–72 | 53 (10); 35–68 | - | 16·8 (9·5) | 1·7 (1·7) | 4·9 (3·5) | 28·1 (1·6) |
LRRK2-U= unaffected mutation carrier; LRRK2-PD= affected mutation carrier UPDRS= Unified Parkinson’s Disease Rating Scale; BDI= Beck Depression Inventory; MoCA= Montreal
Cognitive Assessment
UPDRS III data from 21 of 25 unaffected LRRK2 carriers and from **8 of 9 unaffected LRRK2 carriers
BDI data from 5 of 9 unaffected LRRK2 carriers; 2 additional carriers had Geriatric Depression Scores of 0, one had a Hamilton Depression Rating Scale score of 6.
MoCA data from 4 of 9 unaffected LRRK2 carriers.
UPDRS IV data from 3 of 7 LRRK2 PD patients.
In study 1, ANOVA was restricted to cells with at least 3 subjects (see Table 2); most LRRK2-PD patients were age 61 or older, and most unaffected mutation carriers were age 70 or younger. ANOVA demonstrated significant group differences in FDOPA uptake in caudate (age <=50 p = 0·0002; all other age ranges p <0·0001) and in putamen (all age ranges p <0·0001) and in MP binding in caudate and putamen (in both regions, all age ranges p <0·0001). These group differences remained significant after Bonferroni correction for multiple comparisons. LRRK2-PD patients showed lower values for FDOPA uptake and MP binding in the caudate and putamen than healthy controls. The sPD and LRRK2-PD patients had lower FDOPA and MP than healthy controls in caudate and putamen, and in age ranges with sufficient subjects to compare sPD and LRRK2-PD these groups had similar values (putamen shown in Figure 1, caudate and putamen, Table 2). Generally, unaffected LRRK2 mutations carriers had FDOPA values similar to healthy controls, and significantly greater than those with sPD. By contrast, MP values in LRRK2 mutations carriers were significantly lower than healthy controls and significantly higher than sPD, even in younger age groups.
Table 2.
FDOPA uptake, MP binding in Study 1: Group comparisons by age
| Age group | HC (mean, SE, n) | LRRK2-U (mean, SE, n) | LRRK2-PD (mean, SE, n) | sPD (mean, SE, n) | ANOVA |
|---|---|---|---|---|---|
| FDOPA Caudate | |||||
| <=50 | 1·12 (0·22) n=8 |
1·04 (0·22) n=11 |
0·93 (0·22) n=1 |
0·84 (0·22) n=7 |
F(2,23) =12·38; p = 0·0002 HC = LRRK2-U > sPD# |
| 51–60 | 1·15 (0·23) n=6 |
1·08 (0·23) n=10 |
0·82 (0·23) n=2 |
0·77 (0·23) n=15 |
F(2,28) = 34·99; p < 0·0001 HC = LRRK2-U > sPD# |
| 61–70 | 1·08 (0·32) n=8 |
1·18 (0·32) n=3 |
0·7 (0·32) n=5 | 0·68 (0·32) n=21 |
F(3,33) = 18·12; p < 0·0001 HC = LRRK2-U > LRRK2-PD = sPD |
| >70 | 1·24 (0·3) n=9 | 1·06 (0·3) n=2 |
0·86 (0·3) n=5 | 0·76 (0·3) n=16 |
F(2,27) = 28·98; p < 0·0001 HC > LRRK2-PD = sPD* |
| FDOPA Putamen | |||||
| <=50 | 0·95 (0·26) n=8 |
0·9 (0·26) n=11 |
0·52 (0·26) n=1 |
0·45 (0·26) n=7 |
F(2,23) = 30·79; p < 0·0001 HC = LRRK2-U > sPD# |
| 51–60 | 1·01 (0·21) n=6 |
0·92 (0·21) n=10 |
0·48 (0·21) n=2 |
0·4 (0·21) n=15 |
F(2,28) = 109·23; p < 0·0001 HC = LRRK2-U > sPD# |
| 61–70 | 0·94 (0·28) n=8 |
0·98 (0·28) n=3 |
0·37 (0·28) n=5 |
0·35 (0·28) n=21 |
F(3,33) = 43·63; p < 0·0001 HC = LRRK2-U > LRRK2-PD = sPD |
| >70 | 1·02 (0·3) n=9 | 0·76 (0·3) n=2 |
0·53 (0·3) n=5 | 0·37 (0·3) n=16 |
F(2,27) = 52·46; p < 0·0001 HC > LRRK2-PD > sPD* |
| MP Caudate | |||||
| <=50 | 1·75 (0·47) n=7 |
1·38 (0·47) n=11 |
0·74 (0·47) n=1 |
0·86 (0·47) n=8 |
F(2,23) = 26·93; p < 0·0001 HC > LRRK2-U > sPD# |
| 51–60 | 1·38 (0·42) n=6 |
1·19 (0·42) n=11 |
0·55 (0·42) n=1 |
0·63 (0·42) n=16 |
F(2,30) = 36·72; p < 0·0001 HC = LRRK2-U > sPD# |
| 61–70 | 1·25 (0·43) n=8 |
1·06 (0·43) n=3 |
0·45 (0·43) n=4 |
0·52 (0·43) n=21 |
F(3,32) = 25·06; p < 0·0001 HC = LRRK2-U > LRRK2-PD = sPD |
| >70 | 1·16 (0·42) n=9 |
0·82 (0·42) n=2 |
0·66 (0·42) n=5 |
0·52 (0·42) n=18 |
F(2,29) = 26·31; p < 0·0001 HC > LRRK2-PD = sPD* |
| MP Putamen | |||||
| <=50 | 1·51 (0·44) n=7 |
1·18 (0·44) n=11 |
0·41 (0·44) n=1 |
0·44 (0·44) n=8 |
F(2,23) = 44·86; p < 0·0001 HC > LRRK2-U > sPD# |
| 51–60 | 1·23 (0·33) n=6 |
1·03 (0·33) n=11 |
0·3 (0·33) n=1 | 0·35 (0·33) n=16 |
F(2,30) = 82·82; p < 0·0001 HC > LRRK2-U > sPD# |
| 61–70 | 1·08 (0·27) n=8 |
0·83 (0·27) n=3 |
0·28 (0·27) n=4 |
0·31 (0·27) n=21 |
F(3,32) = 68·36; p < 0·0001 HC > LRRK2-U > LRRK2-PD= sPD |
| >70 | 0·96 (0·33) n=9 |
0·5 (0·33) n=2 |
0·41 (0·33) n=5 |
0·31 (0·33) n=18 |
F(2,29) = 44·84; p < 0·0001 HC > LRRK2-PD = sPD* |
HC= healthy control; LRRK2-U= unaffected mutation carrier; LRRK2-PD= affected mutation carrier; sPD = sporadic PD;
LRRK2-U excluded; #LRRK2-PD excluded.
Figure 1.
FDOPA uptake, MP binding grouped by age
18F-FDOPA (F-DOPA) uptake (left panels) and 11C-d-threo-methylphenidate (MP) binding (right panels) in the putamen of healthy controls, LRRK2 mutation carriers with or without manifest PD, and sporadic PD.
A, B: Group comparisons of putamen FDOPA uptake and MP binding.
*LRRK2-unaffected significantly less than healthy controls; p<0·0001. # LRRK2-unaffected significantly greater than LRRK2-PD/sPD; p<0·0001. LRRK2-PD and sPD groups are significantly different from healthy controls for all ages and regions.
C, D: Putamen FDOPA uptake and MP binding as a function of disease duration in symptomatic LRRK2 mutation carriers. Each point represents a single patient; point shape indicates the specific LRRK2 mutation carried by the patient. Solid red line is the equivalent function in patients with sporadic Parkinson’s disease, with 95% confidence limits displayed as light red lines. The solid black line shows the effect of age in healthy controls starting at age 57 (equivalent to duration = 0).
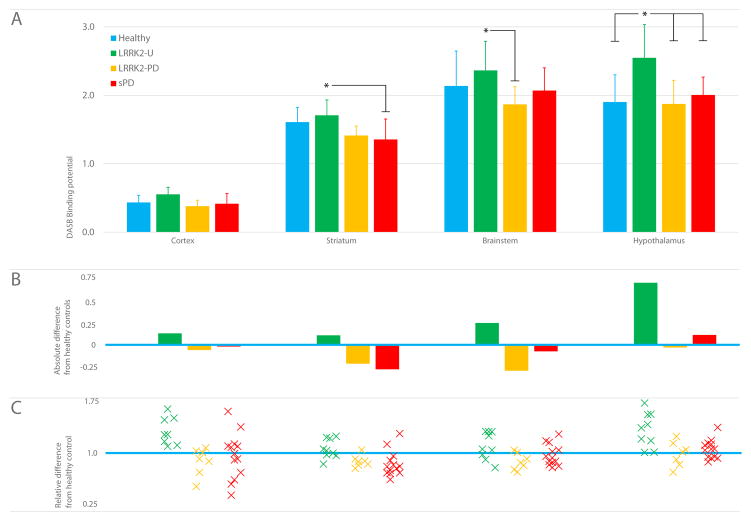
In study 2, ANOVA showed significant group differences in DASB binding in cortex (F3,34=3.49, p=0·026), striatum (F3,34=4·73, p=0·007), and hypothalamus (F3,34=6·34, p=0·002) but not brainstem (F3,34=2·19, p=0·11). ANCOVA demonstrated a significant age effect in brainstem (F1,33=6·96, p=0·013) and hypothalamus (F1,33=11.13, p=0·002). ANCOVA showed significant age-adjusted group differences in hypothalamus, brainstem, and striatum but not in cortex (Table 3, Figure 2). In hypothalamus, unaffected mutation carriers showed significantly higher DASB binding than healthy controls and both groups of affected patients (F3, 33=8·96, p<0·0001). In brainstem, when adjusted for age, unaffected carriers had significantly higher binding than LRRK2-PD patients (F3, 33=3·68, p=0·014). In striatum, DASB binding was significantly higher in unaffected mutation carriers than the sPD patients (F3, 33=3·51, p=0·017). Unaffected mutation carriers had higher DASB binding in cortex than the other groups but when adjusted for age, this was no longer significant (F3, 33=2·56, p=0·057).
Table 3.
DTBZ and DASB binding per group in Study 2
| Group | DASB BP–Cortex (mean, sd) | DASB BP–Striatum (mean, sd) | DASB BP–Brainstem (mean, sd) | DASB BP–Hypothalamus (mean, sd) | DTBZ–putamen (mean, sd)* |
|---|---|---|---|---|---|
| HC | 0·43 (0·10) | 1·61 (0·21) | 2·14 (0·51) | 1·90 (0·40) | - |
| LRRK2-U | 0·55 (0·10) | 1·70 (0·23) | 2·36 (0·43) | 2·55 (0·49) | 1·04 (0·28) |
| LRRK2-PD | 0·38 (0·09) | 1·41 (0·14) | 1·86 (0·26) | 1·87 (0·35) | 0·30 (0·11) |
| sPD | 0·41 (0·15) | 1·35 (0·30) | 2·07 (0·33) | 2·01 (0·33) | 0·25 (0·11) |
HC= healthy control; LRRK2-U= unaffected mutation carrier; LRRK2-PD= affected mutation carrier; sPD = sporadic PD
DTBZ data are expressed as a left/right average normalized to age-matched healthy control values
Figure 2.
Regional DASB Binding
A: Group mean DASB binding potential in different volumes of interest (by reference tissue model); *age-adjusted ANCOVA significantly different; hypothalamus, p<0.0001; striatum, p=0·017; brainstem, p=0·014, corrected for multiple comparisons; whiskers indicate standard deviation.
B: Absolute difference between group mean (bars) and healthy control mean (blue line) in each region
C: Relative difference between healthy control mean (blue line) and binding potential for each participant (as a proportion of the mean healthy control value) in that region
DTBZ binding was significantly reduced in all LRRK2-PD and sPD patients in study 2 compared with healthy controls in both caudate and putamen (all comparisons, p<0·0001); asymmetric reduction was observed in one unaffected LRRK2 mutation carrier (i.e. with this one exception, the changes in DASB binding were not accompanied by abnormal striatal dopaminergic innervation).
DISCUSSION
Based on the results of this study, we suggest that dopaminergic dysfunction in LRRK2 PD progresses in a disease duration-dependent manner and is similar to that observed in sPD. Serotonin transporter binding is elevated rather than reduced in asymptomatic LRRK2 mutation carriers at high genetic risk for PD, in contrast to previous cross sectional studies in established PD showing reduced DASB binding10–12. Consistent with previous cross-sectional studies,4,5 asymptomatic LRRK2 mutation carriers showed reduced MP binding at early ages. This may represent a primary effect on DAT function independent of nerve terminal loss, which does not become apparent until later in life. However, FDOPA uptake was preserved until age 70; we speculate this may persist until impending motor onset.
In our study, DASB binding is elevated in asymptomatic LRRK2 carriers in multiple areas despite normal VMAT2 binding, suggesting that serotonergic changes may occur prior to motor symptoms or dopaminergic dysfunction, though in a direction opposite to expected from previous PET studies in PD10–12 and post-mortem studies of serotonin transporter markers.21
Elevated DASB binding may indicate increased density of serotonin neurons, regulatory changes in synaptic terminal density, or transporter expression. In LRRK2 PD, reactive and/or compensatory sprouting of serotonergic terminals in striatum may occur gradually over a prolonged period, and could theoretically contribute to preserving dopamine synthesis (and thus FDOPA uptake) since these neurons also express aromatic amino acid decarboxylase. Such changes may no longer be apparent when disease is manifest.
Serotonin terminals may also have a functional role in nonmotor aspects of PD. Increased SERT binding in hypothalamus and other areas has been linked to changes in Body Mass Index in PD22. In established PD with fatigue, reduced DASB binding has been reported in striatum, thalamus, cingulate gyrus and amygdala23. A small cross-sectional study of patients with PD and depression showed elevated DASB binding in dorsolateral prefrontal cortex compared with controls; in major depression such changes are thought to represent regulatory changes in transporter expression24. Cortical serotonergic function may affect cognition; increased cortical FDOPA uptake (which can reflect AADC activity in dopaminergic and serotonergic neurons) has been shown in early PD25 and correlates with performance on executive tasks.26,27 Asymptomatic G2019S mutation carriers show engagement of broader networks while performing an executive task,28 suggesting that they are compensating for subtle dysfunction; this may in part be expressed through serotonergic changes.
Our finding of increased DASB binding in asymptomatic LRRK2 mutation carriers requires confirmation in larger cohorts, and with longitudinal observations. The asymptomatic LRRK2 mutation carriers studied were younger than sPD and LRRK2-PD patients, though we corrected for this using ANCOVA. The penetrance of LRRK2 PD is variable and age dependent, and individual mutation groups were too small to examine effects of different mutations on the PET markers; the generalizability of these results to LRRK2 patients may therefore be limited. Data from 9 subjects in the first study were obtained with a different PET scanner, though we demonstrated that the techniques were comparable using a reference object (“phantom”) as well as human subjects. Both FDOPA and MP are potentially affected by regulatory changes and medication effects. We found striatal DASB binding unrelated to UPDRS-III scores but did not attempt to replicate a relationship with medication exposure12.
We have demonstrated a similar relationship between disease duration and dopaminergic dysfunction in sPD and LRRK2 PD, and evidence of serotonergic changes in LRRK2 mutation carriers who do not have PD. Asymptomatic LRRK2 mutation carriers may provide a useful model for apparently sporadic PD, and the early and diverse compensatory responses underway in the brain before disease manifests.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We searched Pubmed for English language articles between January 1, 1970 and April 30, 2016 using the terms “serotonin”, “serotonin transporter” or “DASB” and “Parkinson’s disease” and “positron emission tomography” in human subjects. 15 studies reported original PET data using radiotracers for the presynaptic serotonin transporter in one or more patients. Previous reports are of patients clinically diagnosed with Parkinson’s disease and no asymptomatic patients at risk of Parkinson’s disease (i.e. carrying a known pathogenic mutation) were reported. Serotonin transporter binding in these studies was reduced except in very early clinical disease where a small study reported preserved binding, and two case-control studies showing regional elevations correlating with weight change and depression in Parkinson’s disease. Another study found regional reduction of serotonin transporter binding in patients with Parkinson’s disease and fatigue.
Added value of this study
This is the first report of serotonin transporter binding in patients at high genetic risk for Parkinson disease, without symptoms. We also report age-dependent and disease-duration dependent changes in multiple dopamine markers in patients at genetic risk for Parkinson’s disease.
Implications of all the available evidence
Dopamine changes in genetically determined Parkinson’s disease resemble those in sporadic Parkinson’s disease, but subjects harboring pathogenic mutations exhibit changes in serotonin terminal density before the clinical onset of disease. Those patients with more robust elevations in serotonin terminal density may express certain nonmotor symptoms more readily and this may be the unintended result of adaptive monoamine changes compensating for early dopamine cell loss.
Acknowledgments
We would like to thank all the families involved in this project for their cooperation, Dr. Paul Schaffer and TRIUMF for radiotracer production, Carolyn English and Siobhan McCormick for scanning and analysis. TRIUMF receives federal funding via a contribution agreement with the National Research Council of Canada..
Footnotes
Author contributions:
Conception and design of the study: VS, AJS
Acquisition of the data: KH, JZ, JM, NN, AS, RJU, MG, CPZ, JA, ZKW, MF
Analysis of the data: DJW, PAA, MS, EM, KD, ES, NV, YD, MA, VS, AJS
Drafting a significant portion of the manuscript or figures: DJW, PAA, VS, AJS
Declaration of interests:
Dr. Uitti reports he has a patent for the gene KASPP (United States Patent #8,409,809) with royalties paid to the Mayo Clinic.
Dr. Guttman has participated as a speaker for Novartis in the last 36 months relating to Parkinson's disease diagnosis and owns stock in Neuroderm which is developing products for Parkinson’s disease.
Dr. Zabetian reports grants from NIH/NINDS and salary support from Department of Veterans Affairs.
Dr. Wszolek reports he has patents for the gene KASPP (United States Patent #8,409,809), LRRK2 polynucleotides (#: 8,455,243 B2), and method of screening for LRRK2-related parkinsonism (United States Patent #7,993,841), with royalties paid to the Mayo Clinic.
Dr. Farrer reports grants from Canada Excellence Research Chair, grants from BC Leading Edge Endowment; In addition, Dr. Farrer has a patent on genetic variability in LRRK2 and Parkinson's disease (US8409809, US8455243B2), and on LRRK2 mouse models subsequently developed with royalties paid.
Dr. Stoessl reports grants from Canadian Institutes Health Research, grants from Michael J. Fox Foundation, other from Canada Research Chairs; personal fees from AbbVie, personal fees from Kyowa, personal fees from Pfizer, grants from NTCell; and Grant funding: Cundill Foundation, Pacific Parkinson’s Research Institute, Weston Brain Institute.
All other authors: nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schrag A, Horsfall L, Walters K, Noyce A, Petersen I. Prediagnostic presentations of Parkinson's disease in primary care: a case-control study. Lancet Neurol. 2015;14:57–64. doi: 10.1016/S1474-4422(14)70287-X. [DOI] [PubMed] [Google Scholar]
- 2.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 3.Luk KC, Kehm V, Carroll J, et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–53. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams JR, van Netten H, Schulzer M, et al. PET in LRRK2 mutations: comparison to sporadic Parkinson's disease and evidence for presymptomatic compensation. Brain. 2005;128:2777–85. doi: 10.1093/brain/awh607. [DOI] [PubMed] [Google Scholar]
- 5.Sossi V, de la Fuente-Fernandez R, Nandhagopal R, et al. Dopamine turnover increases in asymptomatic LRRK2 mutations carriers. Mov Disord. 2010;25:2717–23. doi: 10.1002/mds.23356. [DOI] [PubMed] [Google Scholar]
- 6.Hasegawa K, Stoessl AJ, Yokoyama T, Kowa H, Wszolek ZK, Yagishita S. Familial parkinsonism: study of original Sagamihara PARK8 (I2020T) kindred with variable clinicopathologic outcomes. Parkinsonism Relat Disord. 2009;15:300–6. doi: 10.1016/j.parkreldis.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silveira-Moriyama L, Munhoz RP, de Carvalho JM, et al. Olfactory heterogeneity in LRRK2 related Parkinsonism. Mov Disord. 2010;25:2879–83. doi: 10.1002/mds.23325. [DOI] [PubMed] [Google Scholar]
- 8.Saunders-Pullman R, Stanley K, Wang C, et al. Olfactory dysfunction in LRRK2 G2019S mutation carriers. Neurology. 2011;77:319–24. doi: 10.1212/WNL.0b013e318227041c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marras C, Schule B, Munhoz RP, et al. Phenotype in parkinsonian and nonparkinsonian LRRK2 G2019S mutation carriers. Neurology. 2011;77:325–33. doi: 10.1212/WNL.0b013e318227042d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guttman M, Boileau I, Warsh J, et al. Brain serotonin transporter binding in non-depressed patients with Parkinson's disease. Eur J Neurol. 2007;14:523–8. doi: 10.1111/j.1468-1331.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- 11.Albin RL, Koeppe RA, Bohnen NI, Wernette K, Kilbourn MA, Frey KA. Spared caudal brainstem SERT binding in early Parkinson's disease. J Cereb Blood Flow Metab. 2008;28:441–4. doi: 10.1038/sj.jcbfm.9600599. [DOI] [PubMed] [Google Scholar]
- 12.Politis M, Wu K, Loane C, et al. Staging of serotonergic dysfunction in Parkinson's disease: an in vivo 11C-DASB PET study. Neurobiol Dis. 2010;40:216–21. doi: 10.1016/j.nbd.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 13.Strecker K, Wegner F, Hesse S, et al. Preserved serotonin transporter binding in de novo Parkinson's disease: negative correlation with the dopamine transporter. J Neurol. 2011;258:19–26. doi: 10.1007/s00415-010-5666-5. [DOI] [PubMed] [Google Scholar]
- 14.Nandhagopal R, Kuramoto L, Schulzer M, et al. Longitudinal progression of sporadic Parkinson's disease: a multi-tracer positron emission tomography study. Brain. 2009;132:2970–9. doi: 10.1093/brain/awp209. [DOI] [PubMed] [Google Scholar]
- 15.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–40. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Patlak CS, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985;5:584–90. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- 17.Nandhagopal R, Mak E, Schulzer M, et al. Progression of dopaminergic dysfunction in a LRRK2 kindred: a multitracer PET study. Neurology. 2008;71:1790–5. doi: 10.1212/01.wnl.0000335973.66333.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nandhagopal R, Kuramoto L, Schulzer M, et al. Longitudinal evolution of compensatory changes in striatal dopamine processing in Parkinson's disease. Brain. 2011;134:3290–8. doi: 10.1093/brain/awr233. [DOI] [PubMed] [Google Scholar]
- 19.Aasly JO, Vilarino-Guell C, Dachsel JC, et al. Novel pathogenic LRRK2 p.Asn1437His substitution in familial Parkinson's disease. Mov Disord. 2010;25:2156–63. doi: 10.1002/mds.23265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Begareche A, Rodríguez-Oroz MC, Estanga A, et al. DAT Imaging and Clinical Biomarkers in Relatives at Genetic Risk for LRRK2 R1441G Parkinson’s Disease. Mov Disord. 2016;31:335–43. doi: 10.1002/mds.26478. [DOI] [PubMed] [Google Scholar]
- 21.Kish SJ, Tong J, Hornykiewicz O, et al. Preferential loss of serotonin markers in caudate versus putamen in Parkinson's disease. Brain. 2008;131:120–31. doi: 10.1093/brain/awm239. [DOI] [PubMed] [Google Scholar]
- 22.Politis M, Loane C, Wu K, Brooks DJ, Piccini P. Serotonergic mediated body mass index changes in Parkinson’s disease. Neurobiol Dis. 2011;43:609–615. doi: 10.1016/j.nbd.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Pavese N, Metta V, Bose SK, Chaudhuri KR, Brooks DJ. Fatigue in Parkinson’s disease is linked to striatal and limbic serotonergic dysfunction. Brain. 2010;133:3434–43. doi: 10.1093/brain/awq268. [DOI] [PubMed] [Google Scholar]
- 24.Boileau I, Warsh JJ, Guttman M, et al. Elevated Serotonin Transporter Binding in Depressed Patients with Parkinson’s Disease: A Preliminary PET Study with *11C]DASB. Mov Disord. 2008;23:1776–80. doi: 10.1002/mds.22212. [DOI] [PubMed] [Google Scholar]
- 25.Rakshi JS, Uema T, Ito K, et al. Frontal, midbrain and striatal dopaminergic function in early and advanced Parkinson's disease: a 3d [18F]dopa PET study. Brain. 1999;122:1637–50. doi: 10.1093/brain/122.9.1637. [DOI] [PubMed] [Google Scholar]
- 26.Bruck A, Aalto S, Nurmi E, Bergman J, Rinne JO. Cortical 6-[18F]fluoro-l-dopa uptake and frontal cognitive functions in early Parkinson’s disease. Neurobiol Aging. 2005;26:891–8. doi: 10.1016/j.neurobiolaging.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Rinne JO, Portin R, Ruottinen H, et al. Cognitive impairment and the brain dopaminergic system in Parkinson disease. Arch Neurol. 2000;57:470–5. doi: 10.1001/archneur.57.4.470. [DOI] [PubMed] [Google Scholar]
- 28.Thaler A, Mirelman A, Helmich RC, et al. Neural correlates of executive functions in healthy G2019S LRRK2 mutation carriers. Cortex. 2013;49:2501–11. doi: 10.1016/j.cortex.2012.12.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.