Preamble
Since 1980, the American College of Cardiology (ACC) and American Heart Association (AHA) have translated scientific evidence into clinical practice guidelines with recommendations to improve cardiovascular health. These guidelines, based on systematic methods to evaluate and classify evidence, provide a cornerstone of quality cardiovascular care.
In response to reports from the Institute of Medicine1,2 and a mandate to evaluate new knowledge and maintain relevance at the point of care, the ACC/AHA Task Force on Clinical Practice Guidelines (Task Force) modified its methodology.3–5 The relationships among guidelines, data standards, appropriate use criteria, and performance measures are addressed elsewhere.5
Intended Use
Practice guidelines provide recommendations applicable to patients with or at risk of developing cardiovascular disease. The focus is on medical practice in the United States, but guidelines developed in collaboration with other organizations may have a broader target. Although guidelines may be used to inform regulatory or payer decisions, the intent is to improve quality of care and align with patients' interests. Guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances, and should not replace clinical judgment. Guidelines are reviewed annually by the Task Force and are official policy of the ACC and AHA. Each guideline is considered current until it is updated, revised, or superseded by published addenda, statements of clarification, focused updates, or revised full-text guidelines. To ensure that guidelines remain current, new data are reviewed biannually to determine whether recommendations should be modified. In general, full revisions are posted in 5-year cycles.3–6
Modernization
Processes have evolved to support the evolution of guidelines as “living documents” that can be dynamically updated. This process delineates a recommendation to address a specific clinical question, followed by concise text (ideally <250 words) and hyperlinked to supportive evidence. This approach accommodates time constraints on busy clinicians and facilitates easier access to recommendations via electronic search engines and other evolving technology.
Evidence Review
Writing committee members review the literature; weigh the quality of evidence for or against particular tests, treatments, or procedures; and estimate expected health outcomes. In developing recommendations, the writing committee uses evidence-based methodologies that are based on all available data.3–7 Literature searches focus on randomized controlled trials (RCTs) but also include registries, nonrandomized comparative and descriptive studies, case series, cohort studies, systematic reviews, and expert opinion. Only selected references are cited.
The Task Force recognizes the need for objective, independent Evidence Review Committees (ERCs) that include methodologists, epidemiologists, clinicians, and biostatisticians who systematically survey, abstract, and assess the evidence to address systematic review questions posed in the PICOTS format (P=population, I=intervention, C=comparator, O=outcome, T=timing, S=setting).2,4–6 Practical considerations, including time and resource constraints, limit the ERCs to evidence that is relevant to key clinical questions and lends itself to systematic review and analysis that could affect the strength of corresponding recommendations.
Guideline-Directed Management and Treatment
The term “guideline-directed management and therapy” (GDMT) refers to care defined mainly by ACC/AHA Class I recommendations. For these and all recommended drug treatment regimens, the reader should confirm dosage with product insert material and carefully evaluate for contraindications and interactions. Recommendations are limited to treatments, drugs, and devices approved for clinical use in the United States.
Class of Recommendation and Level of Evidence
The Class of Recommendation (COR; ie, the strength of the recommendation) encompasses the anticipated magnitude and certainty of benefit in proportion to risk. The Level of Evidence (LOE) rates evidence supporting the effect of the intervention on the basis of the type, quality, quantity, and consistency of data from clinical trials and other reports (Table 1).3–5 Unless otherwise stated, recommendations are sequenced by COR and then by LOE. Where comparative data exist, preferred strategies take precedence. When >1 drug, strategy, or therapy exists within the same COR and LOE and no comparative data are available, options are listed alphabetically.
Relationships With Industry and Other Entities
The ACC and AHA sponsor the guidelines without commercial support, and members volunteer their time. The Task Force zealously avoids actual, potential, or perceived conflicts of interest that might arise through relationships with industry or other entities (RWI). All writing committee members and reviewers are required to disclose current industry relationships or personal interests, from 12 months before initiation of the writing effort. Management of RWI involves selecting a balanced writing committee and assuring that the chair and a majority of committee members have no relevant RWI (Appendix 1). Members are restricted with regard to writing or voting on sections to which their RWI apply. For transparency, members' comprehensive disclosure information is available online. Comprehensive disclosure information for the Task Force is also available online.
The Task Force strives to avoid bias by selecting experts from a broad array of backgrounds representing different geographic regions, sexes, ethnicities, intellectual perspectives/biases, and scopes of clinical practice, and by inviting organizations and professional societies with related interests and expertise to participate as partners or collaborators.
Individualizing Care in Patients With Associated Conditions and Comorbidities
Managing patients with multiple conditions can be complex, especially when recommendations applicable to coexisting illnesses are discordant or interacting.8 The guidelines are intended to define practices meeting the needs of patients in most, but not all, circumstances. The recommendations should not replace clinical judgment.
Clinical Implementation
Management in accordance with guideline recommendations is effective only when followed. Adherence to recommendations can be enhanced by shared decision making between clinicians and patients, with patient engagement in selecting interventions on the basis of individual values, preferences, and associated conditions and comorbidities. Consequently, circumstances may arise in which deviations from these guidelines are appropriate.
Keywords: AHA Scientific Statements, peripheral artery disease, claudication, critical limb ischemia, acute limb ischemia, antiplatelet agents, supervised exercise, endovascular procedures, bypass surgery, limb salvage, smoking cessation
1. Introduction
1.1. Methodology and Evidence Review
The recommendations listed in this guideline are, whenever possible, evidence based. An initial extensive evidence review, which included literature derived from research involving human subjects, published in English, and indexed in MEDLINE (through PubMed), EMBASE, the Cochrane Library, the Agency for Healthcare Research and Quality, and other selected databases relevant to this guideline, was conducted from January through September 2015. Key search words included but were not limited to the following: acute limb ischemia, angioplasty, ankle-brachial index, anticoagulation, antiplatelet therapy, atypical leg symptoms, blood pressure lowering/hypertension, bypass graft/bypass grafting/surgical bypass, cilostazol, claudication/intermittent claudication, critical limb ischemia/severe limb ischemia, diabetes, diagnostic testing, endovascular therapy, exercise rehabilitation/exercise therapy/exercise training/supervised exercise, lower extremity/foot wound/ulcer, peripheral artery disease/peripheral arterial disease/peripheral vascular disease/lower extremity arterial disease, smoking/smoking cessation, statin, stenting, and vascular surgery. Additional relevant studies published through September 2016, during the guideline writing process, were also considered by the writing committee, and added to the evidence tables when appropriate. The final evidence tables included in the Online Data Supplement summarize the evidence utilized by the writing committee to formulate recommendations. Additionally, the writing committee reviewed documents related to lower extremity peripheral artery disease (PAD) previously published by the ACC and AHA.9,10 References selected and published in this document are representative and not all-inclusive.
As stated in the Preamble, the ACC/AHA guideline methodology provides for commissioning an independent ERC to address systematic review questions (PI-COTS format) to inform recommendations developed by the writing committee. All other guideline recommendations (not based on the systematic review questions) were also subjected to an extensive evidence review process. For this guideline, the writing committee in conjunction with the Task Force and ERC Chair identified the following systematic review questions: 1) Is antiplatelet therapy beneficial for prevention of cardiovascular events in the patient with symptomatic or asymptomatic lower extremity PAD? 2) What is the effect of revascularization, compared with optimal medical therapy and exercise training, on functional outcome and quality of life (QoL) among patients with claudication? Each question has been the subject of recently published, systematic evidence reviews.11–13 The quality of these evidence reviews was appraised by the ACC/AHA methodologist and a vendor contracted to support this process (Doctor Evidence [Santa Monica, CA]). Few substantive randomized or nonrandomized studies had been published after the end date of the literature searches used for the existing evidence reviews, so the ERC concluded that no additional systematic review was necessary to address either of these critical questions.
A third systematic review question was then identified: 3) Is one revascularization strategy (endovascular or surgical) associated with improved cardiovascular and limb-related outcomes in patients with critical limb ischemia (CLI)? This question had also been the subject of a high-quality systematic review that synthesized evidence from observational data and an RCT14; additional RCTs addressing this question are ongoing.15–17 The writing committee and the Task Force decided to expand the survey to include more relevant randomized and observational studies. Based on evaluation of this additional evidence the ERC decided that further systematic review was not needed to inform the writing committee on this question. Hence, the ERC and writing committee concluded that available systematic reviews could be used to inform the development of recommendations addressing each of the 3 systematic review questions specified above. The members of the Task Force and writing committee thank the members of the ERC that began this process and their willingness to participate in this volunteer effort. They include Aruna Pradhan, MD, MPH (ERC Chair); Natalie Evans, MD; Peter Henke, MD; Dharam J. Kumbhani, MD, SM, FACC; and Tamar Polonsky, MD.
1.2. Organization of the Writing Committee
The writing committee consisted of clinicians, including noninvasive and interventional cardiologists, exercise physiologists, internists, interventional radiologists, vascular nurses, vascular medicine specialists, and vascular surgeons, as well as clinical researchers in the field of vascular disease, a nurse (in the role of patient representative), and members with experience in epidemiology and/or health services research. The writing committee included representatives from the ACC and AHA, American Association of Cardiovascular and Pulmonary Rehabilitation, Inter-Society Consensus for the Management of Peripheral Arterial Disease, Society for Cardiovascular Angiography and Interventions, Society for Clinical Vascular Surgery, Society of Interventional Radiology, Society for Vascular Medicine, Society for Vascular Nursing, Society for Vascular Surgery, and Vascular and Endovascular Surgery Society.
1.3. Document Review and Approval
This document was reviewed by 2 official reviewers nominated by the ACC and AHA; 1 to 2 reviewers each from the American Association of Cardiovascular and Pulmonary Rehabilitation, Inter-Society Consensus for the Management of Peripheral Arterial Disease, Society for Cardiovascular Angiography and Interventions, Society for Clinical Vascular Surgery, Society of Interventional Radiology, Society for Vascular Medicine, Society for Vascular Nursing, Society for Vascular Surgery, and Vascular and Endovascular Surgery Society; and 16 additional individual content reviewers. Reviewers' RWI information was distributed to the writing committee and is published in this document (Appendix 2).
Appendix 2. Reviewer Relationships With Industry and Other Entities (Comprehensive)—2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease (March 2016).
| Reviewer | Representation | Employment | Consultant | Speakers Bureau |
Ownership/Partnership /Principal |
Personal Research |
Institutional,Organizational, or Other FinancialBenefit |
Expert Witness |
|---|---|---|---|---|---|---|---|---|
| Deepak L. Bhatt | Official Reviewer—ACC Board of Trustees | Brigham and Women's Hospital— Executive Director of Interventional Cardiovascular Programs; Harvard Medical School—Professor of Medicine |
|
None | None |
|
None | |
| Mark A. Creager | Official Reviewer—AHA | Dartmouth-Hitchcock Medical Center— Director | None | None | None | None |
|
None |
| Philip Goodney | Official Reviewer—AHA | Dartmouth-Hitchcock—Associate Professor of Surgery and The Dartmouth Institute Director | None | None | None |
|
|
None |
| John S. Ikonomidis | Official Reviewer—ACC/ AHA Task Force on Clinical Practice Guidelines | Medical University of South Carolina—Chief | None | None | None | None | None | None |
| Amy W. Pollak | Official Reviewer—AHA | Mayo Clinic—Cardiovascular Medicine Physician | None | None | None | None | None | None |
| Michael D. White | Official Reviewer—ACC Board of Governors | Catholic Health Initiatives—Chief Academic Officer |
|
None | None |
|
None | None |
| Ehrin J. Armstrong | Organizational Reviewer—SVM | University of Colorado—Director, Interventional Cardiology |
|
None | None | None | None | None |
| Bernadette Aulivola | Organizational Reviewer—VESS | Loyola University medical Center, Stritch School of Medicine—Director, Division of Vascular Surgery and Endovascular Therapy; Associate Professor, Department of Surgery; Program Director, Vascular Surgery Fellowship; Medical Director, Vascular Noninvasive lab | None | None | None | None | None | None |
| Alison Bailey | Organizational Reviewer—AACVPR | University of Tennessee Chattanooga—Cardiologist | None | None | None |
|
|
None |
| Todd Brown | Organizational Reviewer—AACVPR | University of Alabama at Birmingham—Associate Professor | None | None | None | None | None | |
| Kristen Columbia | Organizational Reviewer—SVN | University of Maryland Baltimore Washington Medical Center, Maryland Vascular Center—Nurse practitioner | None | None | None | None | None | None |
| Michael S. Conte | Organizational Reviewer—SVS | University of California San Francisco—Professor and Chief |
|
None | None |
|
|
None |
| Alik Farber | Organizational Reviewer—SCVS | Boston Medical Center—Chief, Division of Vascular Surgery |
|
None | None | None | None | None |
| Robert Feezor | Organizational Reviewer—VESS | University of Florida—Associate Professor of Surgery, Division of Vascular Surgery and Endovascular Therapy |
|
None | None |
|
|
|
| Dmitriy N. Feldman | Organizational Reviewer—SCAI | Weill Cornell Medical College, New York Presbyterian Hospital—Associate Professor of Medicine |
|
|
None | None |
|
None |
| Jonathan Golledge | Organizational Reviewer—TASC | James Cook University—Professor, Department of Surgery, Head of Vascular Biology Unit | None | None | None |
|
None | None |
| Bruce H. Gray | Organizational Reviewer—SCAI | Greenville Health System—Director of Clinical Trials, Department of Surgery | None |
|
None | None | ||
| William R. Hiatt | Organizational Reviewer—TASC | Colorado Prevention Center—Professor of Medicine | None | None | None | None | ||
| Joseph Mills | Organizational Reviewer—SVS | Baylor College of Medicine—Professor and Chief, Division of Vascular surgery and Endovascular Therapy | None | None | None | None |
|
None |
| Mohammad Reza Rajebi | Organizational Reviewer—SIR | University of Colorado Denver— Assistant Professor | None | None | None | None | None | None |
| Mitchell J. Silver | Organizational Reviewer—SVM | McConnell Heart Hospital for Critical Limb Care—Director of Vascular Imaging |
|
|
None |
|
None | |
| Lily Thomson | Organizational Reviewer—SVN | Hôpital St-Boniface Hospital—Clinical Research Coordinator, Vascular Surgery Nurse, Section of Vascular Surgery, Health Sciences Centre | None | None | None | None | None | None |
| Sana M. Al-Khatib | Content Reviewer—ACC/ AHA Task Force on Clinical Practice Guidelines | Duke Clinical Research Institute—Associate Professor of Medicine | None | None | None | None | ||
| Herbert Aronow | Content Reviewer—ACC Peripheral Vascular Disease Member Section | Rhode Island Hospital—Director of Cardiac Catheterization Laboratories | None | None | None | |||
| Joshua A. Beckman | Content Reviewer | Vanderbilt University Medical Center— Director | None |
|
|
|||
| James C. Blankenship | Content Reviewer | Geisinger Medical Center—Staff Physician; Director, Cardiac Catheterization Laboratory | None | None | None | None | ||
| Biykem Bozkurt | Content Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | Michael E. DeBakey VA Medical Center—The Mary and Gordon Cain Chair and Professor of Medicine | None | None | None |
|
None | None |
| Joaquin E. Cigarroa | Content Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | Oregon Health and Science University—Clinical Professor of Medicine | None | None | None | None | None | |
| Federico Gentile | Content Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | Centro Medico Diagnostico—Director, Cardiovascular Disease | None | None | None | None | None | None |
| Anuj Gupta | Content Reviewer—ACC Peripheral Vascular Disease Member Section | University of Maryland—Assistant Professor of Medicine | None | None | None | None | ||
| John Jeb Hallett | Content Reviewer | Medical University of South Carolina—Clinical Professor of Surgery | None | None | None | None | None | None |
| Alan Hirsch | Content Reviewer | University of Minnesota Medical School—Professor of Medicine, Epidemiology and Community Health, and Director Vascular Medicine Program | None | None | None | |||
| Mark A. Hlatky | Content Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | Stanford University School of Medicine—Professor of Health Research and Policy, Professor of Medicine |
|
None | None |
|
None | |
| Michael R. Jaff | Content Reviewer | Newton-Wellesley Hospital; Harvard Medical School— Professor of Medicine |
|
None | None | |||
| José A. Joglar | Content Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | UT Southwestern Medical Center—Professor of Internal Medicine; Clinical Cardiac Electrophysiology—Fellowship Program Director | None | None | None | None | None | None |
| Glenn N. Levine | Content Reviewer—ACC/AHA Task Force on Clinical Practice Guidelines | Baylor College of Medicine—Professor of Medicine; Director, Cardiac Care Unit | None | None | None | None | None | None |
| Khusrow Niazi | Content Reviewer—ACC Peripheral Vascular Disease Member Section | Emory University Department of Medicine—Associate Professor of Medicine | None |
|
None |
|
None |
|
| Paul D. Varosy | Content Reviewer—Task Force on Performance Measures | VA Eastern Colorado Health Care System—Associate Professor | None | None | None |
|
|
None |
| Christopher J. White | Content Reviewer | Ochsner Clinical School, University of Queensland—Chairman, Department of Cardiology |
|
None | None |
|
|
None |
This table represents all relationships of reviewers with industry and other entities that were reported by authors, including those not deemed to be relevant to this document, at the time this document was under development. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity, or ownership of ≥$5000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person's gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted. Please refer to http://www.acc.org/guidelines/about-guidelines-and-clinical-documents/ relationships-with-industry-policy for definitions of disclosure categories or additional information about the ACC/AHA Disclosure Policy for Writing Committees.
Significant relationship.
No financial benefit.
AACVPR indicates American Association of Cardiovascular and Pulmonary Rehabilitation; ACC, American College of Cardiology; ACE, Accreditation for Cardiovascular Excellence; AHA, American Heart Association; AMA, American Medical Association; DSMB, data and safety monitoring board; EUCLID, Effects of Ticagrelor and Clopidogrel in Patients with Peripheral Artery Disease; FDA, US Food and Drug Administration; HRS, Heart Rhythm Society; MI, myocardial infarction; NCDR, National Cardiovascular Data Registry; NIH, National Institutes of Health; NHLBI, National Heart, Lung, and Blood Institute; PCORI, Patient-Centered Outcomes Research Institute; PI, primary investigator; PLX-PAD, placental-derived adherent stromal cell; SCAI, Society for Cardiovascular Angiography and Interventions; SCVS, Society for Clinical Vascular Surgery; SIR, Society of Interventional Radiology; SVM, Society for Vascular Medicine; SVN, Society for Vascular Nursing; SVS, Society for Vascular Surgery; TASC, Trans-Atlantic Inter-Society Consensus for the Management of Peripheral Arterial Disease; VA, Veterans Affairs; VESS, Vascular and Endovascular Surgery Society; and VIVA, Vascular Intervention Advances.
This document was approved for publication by the governing bodies of the ACC and the AHA and endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation, Inter-Society Consensus for the Management of Peripheral Arterial Disease, Society for Cardiovascular Angiography and Interventions, Society for Clinical Vascular Surgery, Society of Interventional Radiology, Society for Vascular Medicine, Society for Vascular Nursing, Society for Vascular Surgery, and Vascular and Endovascular Surgery Society.
1.4. Scope of Guideline
Lower extremity PAD is a common cardiovascular disease that is estimated to affect approximately 8.5 million Americans above the age of 40 years and is associated with significant morbidity, mortality, and QoL impairment.18 It has been estimated that 202 million people worldwide have PAD.19 The purpose of this document is to provide a contemporary guideline for diagnosis and management of patients with lower extremity PAD. This document supersedes recommendations related to lower extremity PAD in the “ACC/AHA 2005 Guidelines for the Management of Patients With Peripheral Arterial Disease”9 and the “2011 ACCF/AHA Focused Update of the Guideline for the Management of Patients With Peripheral Artery Disease.”10 The scope of this guideline is limited to atherosclerotic disease of the lower extremity arteries (PAD) and includes disease of the aortoiliac, femoropopliteal, and infrapopliteal arterial segments. It does not address nonatherosclerotic causes of lower extremity arterial disease, such as vasculitis, fibromuscular dysplasia, physiological entrapment syndromes, cystic adventitial disease, and other entities. Future guidelines will address aneurysmal disease of the abdominal aorta and lower extremity arteries and diseases of the renal and mesenteric arteries.
In developing the “2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease,” the writing committee reviewed the evidence to support recommendations in the relevant ACC/AHA guidelines noted in Table 2 and affirms the ongoing validity of the related recommendations, thus obviating the need to repeat existing guideline recommendations in the current guideline. Table 2 also contains a list of other statements that may be of interest to the reader. Table 3 includes definitions for PAD key terms used throughout the guideline.
Table 2. Important Guideline Policy.
| Title | Organization | Publication Year (Reference) |
|---|---|---|
| ACC/AHA Guideline policy relevant to the management of lower extremity PAD | ||
| Duration of dual antiplatelet therapy in patients with coronary artery disease | ACC/AHA | 201620 |
| Perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery | ACC/AHA | 201421 |
| Lifestyle management to reduce cardiovascular risk | AHA/ACC | 201322 |
| Assessment of cardiovascular risk | ACC/AHA | 201323 |
| Blood cholesterol to reduce atherosclerotic cardiovascular risk in adults | ACC/AHA | 201324 |
| PAD (lower extremity, renal, mesenteric, and abdominal aortic) | ACC/AHA | 20059 and 201110 |
| Secondary prevention and risk-reduction therapy for patients with coronary and other atherosclerotic vascular disease | AHA/ACC | 201125 |
| Other related publications | ||
| Atherosclerotic occlusive disease of the lower extremities guideline | SVS | 201526 |
| Measurement and interpretation of the ankle-brachial index | AHA | 201227 |
| Cardiac disease evaluation and management among kidney and liver transplantation candidates | AHA/ACC | 201228 |
| Intensive glycemic control and the prevention of cardiovascular events | ADA/ACC/AHA | 200929 |
| Influenza vaccination as secondary prevention for cardiovascular disease | AHA/ACC | 200630 |
| Indications for renal arteriography at the time of coronary arteriography | AHA/CLCD/CVRI/KCVD | 200631 |
| Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7)* | NHLBI | 200332 |
A revision to the current document is being prepared, with publication expected in 2017. The new title is expected to be “ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Detection, Evaluation, Prevention and Management of High Blood Pressure.”
AAPA indicates American Academy of Physician Assistants; ABC, Association of Black Cardiologists; ACC, American College of Cardiology; ACPM, American College of Preventive Medicine; ADA, American Diabetes Association; AGS, American Geriatrics Society; AHA, American Heart Association; APhA, American Pharmacists Association; ASH, American Society of Hypertension; ASPC, American Society for Preventive Cardiology; CLCD, Council on Clinical Cardiology; CVRI, Council on Cardiovascular Radiology and Intervention; KCVD, Council on the Kidney in Cardiovascular Disease; NHLBI, National Heart, Lung, and Blood Institute; NMA, National Medical Association; PAD, peripheral artery disease; PCNA, Preventive Cardiovascular Nurses Association; and SVS, Society for Vascular Surgery.
Table 3. Definition of PAD Key Terms.
| Term | Definition |
|---|---|
| Claudication | Fatigue, discomfort, cramping, or pain of vascular origin in the muscles of the lower extremities that is consistently induced by exercise and consistently relieved by rest (within 10 min). |
| Acute limb ischemia (ALI) | Acute (<2 wk), severe hypoperfusion of
the limb characterized by these features: pain, pallor, pulselessness,
poikilothermia (cold), paresthesias, and paralysis.
|
| Tissue loss | Type of tissue loss:
|
| Critical limb ischemia (CLI) | A condition characterized by chronic
(≥2 wk) ischemic rest pain, nonhealing wound/ulcers, or gangrene
in 1 or both legs attributable to objectively proven arterial occlusive
disease.
|
| In-line blood flow | Direct arterial flow to the foot, excluding collaterals. |
| Functional status | Patient's ability to perform normal daily activities required to meet basic needs, fulfill usual roles, and maintain health and well-being. Walking ability is a component of functional status. |
| Nonviable limb | Condition of extremity (or portion of extremity) in which loss of motor function, neurological function, and tissue integrity cannot be restored with treatment. |
| Salvageable limb | Condition of extremity with potential to secure viability and preserve motor function to the weight-bearing portion of the foot if treated. |
| Structured exercise program | Planned program that provides individualized
recommendations for type, frequency, intensity, and duration of
exercise.
|
| Supervised exercise program | Structured exercise program that takes place
in a hospital or outpatient facility in which intermittent walking
exercise is used as the treatment modality.
|
| Structured community- or home-based exercise program | Structured exercise program that takes place
in the personal setting of the patient rather than in a clinical
setting.41,47–51
|
| Emergency versus urgent |
|
| Interdisciplinary care team | A team of professionals representing different
disciplines to assist in the evaluation and management of the patient
with PAD.
|
| Cardiovascular ischemic events | Acute coronary syndrome (acute MI, unstable angina), stroke, or cardiovascular death. |
| Limb-related events | Worsening claudication, new CLI, new lower extremity revascularization, or new ischemic amputation. |
ABI indicates ankle-brachial index; ALI, acute limb ischemia; CLI, critical limb ischemia; MI, myocardial infarction; PAD, peripheral artery disease; TBI, toe-brachial index; and TcPO2, transcutaneous oxygen pressure.
2. Clinical Assessment for PAD
Evaluating the patient for PAD begins with the clinical history, review of symptoms, and physical examination.
2.1. History and Physical Examination: Recommendations
|
| ||
| Recommendations for History and Physical Examination | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | B-NR | Patients at increased risk of PAD (Table 4) should undergo a comprehensive medical history and a review of symptoms to assess for exertional leg symptoms, including claudication or other walking impairment, ischemic rest pain, and nonhealing wounds.52–57 |
|
| ||
| See Online Data Supplement 1. | The symptoms and signs of PAD are variable. Patients with PAD may experience the classic symptom of claudication or may present with advanced disease, including CLI. Studies have demonstrated that the majority of patients with confirmed PAD do not have typical claudication but have other non–joint-related limb symptoms or are asymptomatic.53,55 Atypical lower extremity symptoms related to PAD may include pain or discomfort that begins at rest but worsens with exertion, pain or discomfort that does not stop an individual from walking, and pain or discomfort that begins with exertion but is not alleviated within 10 minutes of rest.54 Patients with PAD who do not have typical claudication but have other leg symptoms, or who are asymptomatic, have been shown to have functional impairment comparable to patients with claudication.54 Thus, all patients at increased risk of PAD should be asked not only about claudication but also about other exertional non–joint-related limb symptoms and perceived walking impairment. | |
|
| ||
| I | B-NR | Patients at increased risk of PAD (Table 4) should undergo vascular examination, including palpation of lower extremity pulses (ie, femoral, popliteal, dorsalis pedis, and posterior tibial), auscultation for femoral bruits, and inspection of the legs and feet.56,58,59 |
|
| ||
| See Online Data Supplements. | A thorough lower extremity vascular examination and careful inspection of the legs and feet are important components of the clinical assessment for PAD. To perform a thorough examination, legs and feet are examined with lower garments (pants/skirt, shoes, and socks) removed. Examination findings suggestive of PAD are shown in Table 5. Lower extremity pulses should be assessed and rated as follows: 0, absent; 1, diminished; 2, normal; or 3, bounding. Reproducibility of pulse assessment is better for detection of normal versus absent pulse than for normal versus diminished pulse.56 Absence of the dorsalis pedis pulse is less accurate for diagnosis of PAD than is absence of the posterior tibial pulse because the dorsalis pedis pulse can be absent on examination in a significant percentage of healthy patients.56,58 The presence of multiple abnormal physical findings (ie, multiple pulse abnormalities, bruits) increases the likelihood of confirmed PAD.56,58,59 Abnormal physical findings, such as a pulse abnormality, require confirmation with the ankle-brachial index (ABI) to establish the diagnosis of PAD. Similarly, an entirely normal pulse examination and absence of bruits decreases the likelihood of confirmed PAD.56,58 The presence of nonhealing lower extremity wounds may be a sign of CLI. Findings of cool or discolored skin and delayed capillary refill are not reliable for PAD diagnosis.56 To confirm the diagnosis of PAD, abnormal physical examination findings must be confirmed with diagnostic testing (Section 3), generally with the ABI as the initial test. | |
|
| ||
| I | B-NR | Patients with PAD should undergo noninvasive blood pressure measurement in both arms at least once during the initial assessment.60–62 |
|
| ||
| See Online Data Supplement 1. | An inter-arm blood pressure difference of >15 to 20 mm Hg is abnormal and suggestive of subclavian (or innominate) artery stenosis. Patients with PAD are at increased risk of subclavian artery stenosis.60–62 Measuring blood pressure in both arms identifies the arm with the highest systolic pressure, a requirement for accurate measurement of the ABI.27 Identification of unequal blood pressures in the arms also allows for more accurate measurement of blood pressure in the treatment of hypertension (ie, blood pressure is taken at the arm with higher measurements). Although a difference in arm systolic pressures of >15 to 20 mm Hg suggests subclavian (or innominate) artery stenosis, in the absence of symptoms (eg, arm claudication or symptoms of vertebral artery steal), no further imaging or intervention is warranted. | |
|
| ||
Table 4. Patients at Increased Risk of PAD.
| Age ≥65 y |
| Age 50–64 y, with risk factors for atherosclerosis (eg, diabetes mellitus, history of smoking, hyperlipidemia, hypertension) or family history of PAD63 |
| Age <50 y, with diabetes mellitus and 1 additional risk factor for atherosclerosis |
| Individuals with known atherosclerotic disease in another vascular bed (eg, coronary, carotid, subclavian, renal, mesenteric artery stenosis, or AAA) |
AAA indicates abdominal aortic aneurysm; PAD, peripheral artery disease.
Table 5. History and/or Physical Examination Findings Suggestive of PAD.
| History |
| Claudication |
| Other non–joint-related exertional lower extremity symptoms (not typical of claudication) |
| Impaired walking function |
| Ischemic rest pain |
| Physical Examination |
| Abnormal lower extremity pulse examination |
| Vascular bruit |
| Nonhealing lower extremity wound |
| Lower extremity gangrene |
| Other suggestive lower extremity physical findings (eg, elevation pallor/dependent rubor) |
PAD indicates peripheral artery disease.
3. Diagnostic Testing for the Patient with Suspected Lower Extremity PAD (Claudication or CLI)
3.1. Resting ABI for Diagnosing PAD: Recommendations
|
| ||
| Recommendations for Resting ABI for Diagnosing PAD | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | B-NR | In patients with history or physical examination findings suggestive of PAD (Table 5), the resting ABI, with or without segmental pressures and waveforms, is recommended to establish the diagnosis.64–69 |
|
| ||
| See Online Data Supplement 4. | The resting ABI is obtained by measuring systolic blood pressures at the arms (brachial arteries) and ankles (dorsalis pedis and posterior tibial arteries) in the supine position by using a Doppler device. The ABI of each leg is calculated by dividing the higher of the dorsalis pedis or posterior tibial pressure by the higher of the right or left arm blood pressure.27 In patients with a history or physical examination suggestive of PAD, the ABI has good validity as a first-line test in the diagnosis of PAD, as shown by vascular imaging, with sensitivities ranging from 68% to 84% and specificities from 84% to 99%.64–69 Segmental lower extremity blood pressures and Doppler or plethysmographic waveforms (pulse volume recordings) can be used to localize anatomic segments of disease (eg, aortoiliac, femoropopliteal, infrapopliteal).34,70,71 | |
|
| ||
| I | C-LD | Resting ABI results should be reported as abnormal (ABI ≤0.90), borderline (ABI 0.91–0.99), normal (1.00–1.40), or noncompressible (ABI >1.40).27,67–69,72 |
|
| ||
| See Online Data Supplement 4. | Standardized reporting improves communication among healthcare providers. Calculated ABI values should be recorded to 2 decimal places. Patients with ABI ≤0.90 are diagnosed with PAD.67–69 Those with ABI 0.91 to 0.99 may possibly have PAD and should undergo exercise ABI, if the clinical suspicion of PAD is significant (Tables 4 and 5).73,74 Values >1.40 indicate that the arteries were not able to be compressed, which is more common among individuals with diabetes mellitus and/or advanced chronic kidney disease. In the setting of noncompressible ABI values, additional imaging can be used to diagnose PAD if the clinical suspicion is significant (Figures 1 and 2).72 These cutpoints for ABI interpretation have been previously proposed and represent a reasonable standardized categorization.27 | |
|
| ||
| IIa | B-NR | In patients at increased risk of PAD (Table 4) but without history or physical examination findings suggestive of PAD (Table 5), measurement of the resting ABI is reasonable.54,55,75–97 |
|
| ||
| See Online Data Supplements 3 and 4. | The ABI test is noninvasive, is simple to perform, and has minimal risks, making it suitable for use in asymptomatic individuals. Previous studies have demonstrated a significant prevalence of abnormal resting ABI among asymptomatic patients with risk factors for PAD.55,79,95 A significant body of evidence demonstrates that patients with an abnormal ABI who are asymptomatic have poorer cardiovascular morbidity and mortality outcomes than do patients with normal ABI.79–87 While there is no conclusive evidence that aspirin treatment changes cardiovascular or limb outcomes in this population, in 1 cohort study of 5480 patients with asymptomatic PAD, statin treatment improved cardiovascular outcomes.75–78,96 | |
|
| ||
| There is also evidence that asymptomatic patients with a low resting ABI have a poorer functional status and a more rapid rate of functional decline than do patients with a normal ABI.54,88–92 Although physical activity has been shown to be associated with improvement in functional status in patients with asymptomatic PAD,93,94 the benefit of resting ABI testing to identify asymptomatic patients who are at increased risk of functional decline and may benefit from structured exercise programs remains to be determined. | ||
|
| ||
| III: No Benefit | B-NR | In patients not at increased risk of PAD (Table 4) and without history or physical examination findings suggestive of PAD (Table 5), the ABI is not recommended.95,98 |
|
| ||
| See Online Data Supplement 4. | The prevalence of PAD among individuals without risk factors for atherosclerosis and who are <50 years of age is low. Data from population-based cohort studies have demonstrated a low prevalence (approximately 1%) of abnormal resting ABI among individuals <50 years of age.95,98 In the NHANES (National Health and Nutrition Study), approximately 95% of participants with an abnormal resting ABI had at least 1 risk factor for atherosclerosis.95 The yield of ABI testing among younger, asymptomatic individuals without risk factors for atherosclerosis is low, and these patients should not be routinely tested for PAD.95,98 | |
|
| ||
Figure 1. Diagnostic Testing for Suspected PAD.
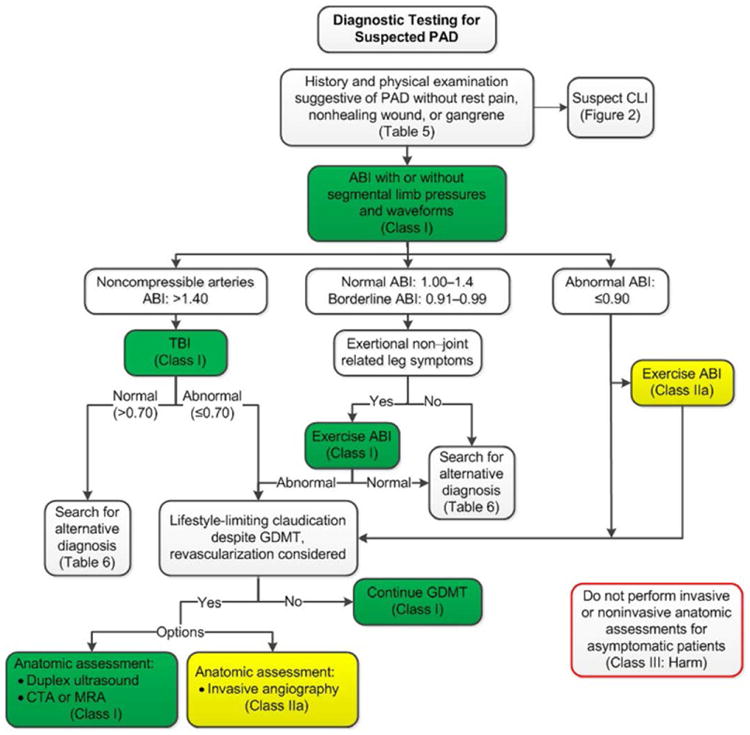
Colors correspond to Class of Recommendation in Table 1. ABI indicates ankle-brachial index; CLI, critical limb ischemia; CTA, computed tomography angiography; GDMT, guideline-directed management and therapy; MRA, magnetic resonance angiography; PAD, peripheral artery disease; and TBI, toe-brachial index.
Figure 2. Diagnostic Testing for Suspected CLI.
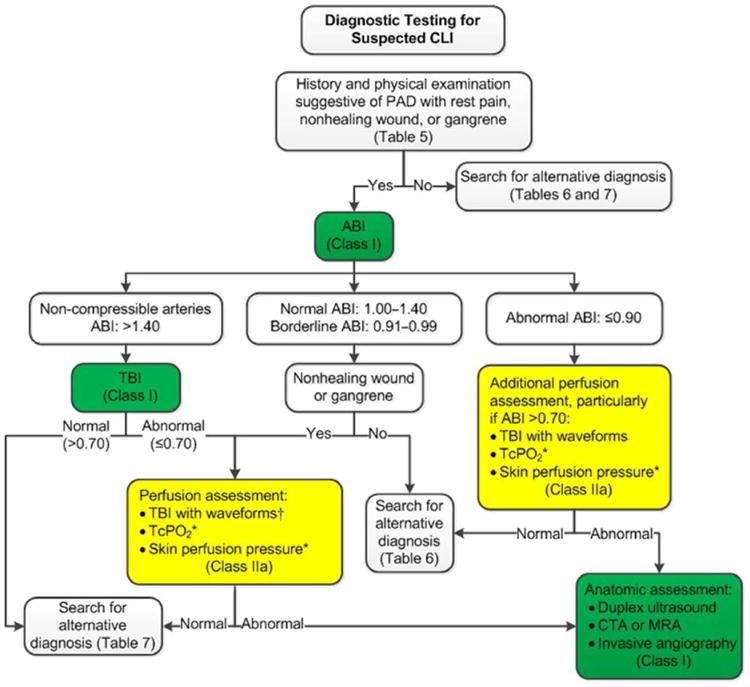
Colors correspond to Class of Recommendation in Table 1. *Order based on expert consensus. †TBI with waveforms, if not already performed. ABI indicates ankle-brachial index; CLI, critical limb ischemia; CTA, computed tomography angiography; MRA, magnetic resonance angiography; TcPO2, transcutaneous oxygen pressure; and TBI, toe-brachial index.
3.2. Physiological Testing: Recommendations
|
| ||
| Recommendations for Physiological Testing | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | B-NR | Toe-brachial index (TBI) should be measured to diagnose patients with suspected PAD when the ABI is greater than 1.40.72,99–102 |
|
| ||
| See Online Data Supplement 5. | TBI is a noninvasive test that is useful to evaluate for PAD in patents with noncompressible arteries, which cause an artificial elevation of the ABI.99,100,102,103 A TBI ≤0.70 is abnormal and diagnostic of PAD because the digital arteries are rarely noncompressible.99–102,104,105 Patients with longstanding diabetes mellitus72,101 or advanced chronic kidney disease106 have a high incidence of noncompressible arteries. Therefore, TBI assessment allows for the diagnosis of PAD in these patients with noncompressible arteries who have history or physical examination findings suggestive of PAD (Figure 1). | |
|
| ||
| I | B-NR | Patients with exertional non–joint-related leg symptoms and normal or borderline resting ABI (>0.90 and ≤1.40) should undergo exercise treadmill ABI testing to evaluate for PAD.71,74,107–110 |
|
| ||
| See Online Data Supplement 5. | Exercise treadmill ABI testing is important to objectively measure symptom limitations and diagnose PAD.71,74,107–110 It is useful in establishing the diagnosis of lower extremity PAD in the symptomatic patient when resting ABIs are normal or borderline and to differentiate claudication from pseudoclaudication in individuals with exertional leg symptoms. If the post-exercise treadmill ABI is normal, alternative causes of leg pain are considered (Table 6). If a treadmill is not available, the pedal plantarflexion ABI test is a reasonable alternative because the results correlate well with treadmill ABIs (Figure 1).111 | |
|
| ||
| IIa | B-NR | In patients with PAD and an abnormal resting ABI (≤0.90), exercise treadmill ABI testing can be useful to objectively assess functional status.71,74,107–110 |
|
| ||
| See Online Data Supplement 5. | In patients with PAD, exercise treadmill ABI testing can objectively assess symptoms, measure change in ABI in response to exercise, and assess functional status71,74,107–110 (Figure 1). It can be useful to correlate exertional lower extremity symptoms to a decline in ABI after treadmill exercise. Exercise treadmill ABI testing can document the magnitude of symptom limitation in patients with PAD and provide objective data that can demonstrate the safety of exercise and help to individualize exercise prescriptions in patients with PAD before initiation of a formal program of structured exercise training. Exercise ABI may also be used to objectively measure the functional improvement obtained in response to claudication treatment (eg, structured exercise program or revascularization). Administration of a 6-minute walk test in a corridor is a reasonable alternative to treadmill ABI testing for assessment of functional status.54 | |
|
| ||
| IIa | B-NR | In patients with normal (1.00–1.40) or borderline (0.91–0.99) ABI in the setting of nonhealing wounds or gangrene, it is reasonable to diagnose CLI by using TBI with waveforms, transcutaneous oxygen pressure (TcPO2), or skin perfusion pressure (SPP).112–116 |
|
| ||
| See Online Data Supplement 5. | The toe pressure and TBI may be discordant with the ABI 0.90 to 1.40 in some patients with diabetes mellitus and a nonhealing wound (Figure 2).115,116 A TBI ≤0.70 is considered diagnostic of PAD.101,104,105 Doppler or plethysmographic waveforms taken at the toe supplement the toe pressure and TBI measurement and may be severely dampened in the setting of CLI. The likelihood of wound healing decreases with toe pressure <30 mm Hg.100 Perfusion assessment measures (ie, TBI with waveforms, TcPO2, SPP) are obtained in a warm room to prevent arterial vasoconstriction in response to the cold. TcPO2 measurements are performed with a standardized protocol and are taken at multiple sites.117 Correlation between TBI, TcPO2, and SPP has been reported.113 TcPO2 >30 mm Hg has been used to predict ulcer healing.118 SPP ≥30 to 50 mm Hg is associated with increased likelihood of wound healing.113 If perfusion measures are normal or only mildly impaired, alternative causes of the nonhealing wounds are considered (Table 7). TcPO2 and SPP can be used in angiosome-targeted assessment for revascularization.119 | |
|
| ||
| IIa | B-NR | In patients with PAD with an abnormal ABI (≤0.90) or with noncompressible arteries (ABI >1.40 and TBI ≤0.70) in the setting of nonhealing wounds or gangrene, TBI with waveforms, TcPO2, or SPP can be useful to evaluate local perfusion.112–116 |
|
| ||
| See Online Data Supplement 5. | Perfusion assessment measures (eg, TBI with waveforms, TcPO2, SPP) can be useful when the ABI is only mildly reduced (eg, ABI 0.70–0.90) to determine whether factors other than PAD may be contributing to impaired wound healing (Figure 2). These perfusion assessment measures are obtained in a warm room to prevent arterial vasoconstriction in response to the cold. TcPO2 measurements are performed with a standardized protocol and are taken at multiple sites.117 The likelihood of wound healing decreases with toe pressure <30 mm Hg.100 There is correlation between TBI, TcPO2, and SPP. TcPO2 >30 mm Hg has been used to predict ulcer healing.118 SPP ≥30 to 50 mm Hg is associated with increased likelihood of wound healing.113 TcPO2 and SPP can be used in angiosome-targeted assessment for revascularization.119 Additional perfusion assessment may also be useful for patients with nonhealing wounds or gangrene who have noncompressible arteries (ABI >1.40) but who have a diagnosis of PAD that is based on an abnormal TBI (ABI ≤0.70). | |
|
| ||
Table 6. Alternative Diagnoses for Leg Pain or Claudication With Normal Physiological Testing (Not PAD-Related).
| Condition | Location | Characteristic | Effect of Exercise | Effect of Rest | Effect of Position | Other Characteristics |
|---|---|---|---|---|---|---|
| Symptomatic Baker's cyst | Behind knee, down calf | Swelling, tenderness | With exercise | Also present at rest | None | Not intermittent |
| Venous claudication | Entire leg, worse in calf | Tight, bursting pain | After walking | Subsides slowly | Relief speeded by elevation | History of iliofemoral deep vein thrombosis; edema; signs of venous stasis |
| Chronic compartment syndrome | Calf muscles | Tight, bursting pain | After much exercise (jogging) | Subsides very slowly | Relief with rest | Typically heavy muscled athletes |
| Spinal stenosis | Often bilateral buttocks, posterior leg | Pain and weakness | May mimic claudication | Variable relief but can take a long time to recover | Relief by lumbar spine flexion | Worse with standing and extending spine |
| Nerve root compression | Radiates down leg | Sharp lancinating pain | Induced by sitting, standing, or walking | Often present at rest | Improved by change in position | History of back problems; worse with sitting; relief when supine or sitting |
| Hip arthritis | Lateral hip, thigh | Aching discomfort | After variable degree of exercise | Not quickly relieved | Improved when not weight bearing | Symptoms variable; history of degenerative arthritis |
| Foot/ankle arthritis | Ankle, foot, arch | Aching pain | After variable degree of exercise | Not quickly relieved | May be relieved by not bearing weight | Symptoms variable; may be related to activity level or present at rest |
Modified from Norgren L et al.35
PAD indicates peripheral artery disease.
Table 7. Alternative Diagnoses for Nonhealing Wounds With Normal Physiological Testing (Not PAD-Related).
| Condition | Location | Characteristics and Causes |
|---|---|---|
| Venous ulcer | Distal leg, especially above medial mellolus | Develops in regions of skin changes due to chronic venous disease and local venous hypertension Typically wet (ie, wound drainage) rather than dry lesion |
| Distal small arterial occlusion (microangiopathy) | Toes, foot, leg | Diabetic microangiopathy End-stage renal disease Thromboangiitis obliterans (Buerger's) Sickle cell anemia Vasculitis (eg, Churg-Strauss, Henoch-Schonlein purpura, leukocytoclastic vasculitis, microscopic polyangiitis, polyarteritis nodosa) Scleroderma Cryoagglutination Embolic (eg, cholesterol emboli, thromboemboli, endocarditis) Thrombotic (eg, antiphospholipid antibody syndrome, Sneddon's syndrome, warfarin skin necrosis, disseminated intravascular coagulation, livedoid vasculitis, protein C or S deficiency, prolonged vasospasm) |
| Local injury | Toes, foot, leg | Trauma Insect or animal bite Burn |
| Medication related | Toes, foot, leg | Drug reactions (eg, erythema
multiforme) Medication direct toxicity (eg, doxorubicin, hydroxyurea, some tyrosine kinase inhibitors) |
| Neuropathic | Pressure zones of foot | Hyperkeratosis surrounds the
ulcer Diabetes mellitus with peripheral neuropathy Peripheral neuropathy without diabetes mellitus Leprosy |
| Autoimmune injury | Toes, foot, leg | With blisters (eg, pemphigoid, pemphigus,
epidermolysis bullosa) Without blisters (eg, dermatomyositis, lupus, scleroderma) |
| Infection | Toes, foot, leg | Bacterial (eg, pseudomonas, necrotizing
streptococcus) Fungal (eg, blastomycosis, Madura foot, chromomycosis) Mycobacterial Parasitic (eg, Chagas, leishmaniasis) Viral (eg, herpes) |
| Malignancy | Toes, foot, leg | Primary skin malignancy Metastatic malignancy Malignant transformation of ulcer |
| Inflammatory | Toes, foot, leg | Necrobiosis lipoidica Pyoderma gangrenosum Granuloma annulare |
PAD indicates peripheral artery disease.
3.3. Imaging for Anatomic Assessment: Recommendations
|
| ||
| Recommendations for Imaging for Anatomic Assessment | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | B-NR | Duplex ultrasound, computed tomography angiography (CTA), or magnetic resonance angiography (MRA) of the lower extremities is useful to diagnose anatomic location and severity of stenosis for patients with symptomatic PAD in whom revascularization is considered.118,120–122 |
|
| ||
| See Online Data Supplement 6. | For symptomatic patients in whom ABI/TBI confirms PAD and in whom revascularization is considered, additional imaging with duplex ultrasonography, CTA, or MRA is useful to develop an individualized treatment plan, including assistance in selection of vascular access sites, identification of significant lesions, and determination of the feasibility of and modality for invasive treatment. All 3 of these noninvasive imaging methods have good sensitivity and specificity as compared with invasive angiography.118,120–122 Renal function does not affect the safety of duplex ultrasonography, although duplex offers lower spatial resolution than CTA and MRA in the setting of arterial calcification. The tomographic data from CTA and MRA afford 3-dimensional reconstruction of the vessels examined. The iodinated contrast used in CTA confers risk of contrast-induced nephropathy and (rarely) severe allergic reaction123,124; CTA uses ionizing radiation. MRA does not use ionizing radiation; however, gadolinium contrast used frequently in MRA studies confers risk of nephrogenic systemic sclerosis for patients with advanced renal dysfunction and is therefore contraindicated in this population.125 The choice of the examination should be determined in an individualized approach to the anatomic assessment for each patient, including risk–benefit assessment of each study type. If these noninvasive tests are nondiagnostic, then invasive angiography may be required to delineate anatomy and plan revascularization. | |
|
| ||
| I | C-EO | Invasive angiography is useful for patients with CLI in whom revascularization is considered. |
|
| ||
| N/A | By definition, CLI results from extensive PAD that limits tissue perfusion. Because timely diagnosis and treatment are essential to preserve tissue viability in CLI, it is often most effective and expeditious to pursue invasive angiography with endovascular revascularization directly, without delay and potential risk of additional noninvasive imaging. | |
|
| ||
| IIa | C-EO | Invasive angiography is reasonable for patients with lifestyle-limiting claudication with an inadequate response to GDMT for whom revascularization is considered. |
|
| ||
| N/A | For patients with lifestyle-limiting claudication despite GDMT (including structured exercise therapy) for whom revascularization is being considered, proceeding directly to invasive angiography for anatomic assessment and to determine revascularization strategy is reasonable. In certain clinical settings, noninvasive imaging studies for anatomic assessment (ie, duplex ultrasound, CTA, or MRA) may not be available because of lack of local resources or expertise. In addition, there are clinical scenarios in which noninvasive studies for anatomic assessment may be perceived to confer greater risk to the patient than invasive angiography (eg, patient with advanced chronic kidney disease for whom contrast dose for invasive angiography would be lower than that required for CTA). | |
|
| ||
| III: Harm | B-R | Invasive and noninvasive angiography (ie, CTA, MRA) should not be performed for the anatomic assessment of patients with asymptomatic PAD.123,124,126 |
|
| ||
| See Online Data Supplements 6 and 7. | Angiography, either noninvasive or invasive, should not be performed for the anatomic assessment of patients with PAD without leg symptoms because delineation of anatomy will not change treatment for this population. This lack of benefit occurs in the setting of risk of contrast-induced nephropathy, patient discomfort, and allergic reactions.123,124,126 This recommendation does not address assessment of lower extremity aneurysmal disease or nonatherosclerotic causes of arterial disease, which is beyond the scope of this document. | |
|
| ||
4. Screening for Atherosclerotic Disease in Other Vascular Beds for the Patient with PAD
4.1. Abdominal Aortic Aneurysm: Recommendation
|
| ||
| Recommendation for Abdominal Aortic Aneurysm | ||
|
| ||
| COR | LOE | Recommendation |
|
| ||
| IIa | B-NR | A screening duplex ultrasound for abdominal aortic aneurysm (AAA) is reasonable in patients with symptomatic PAD.127–129 |
|
| ||
| See Online Data Supplement 8. | PAD has been recognized as a risk factor for AAA. In observational studies, the prevalence of AAA (aortic diameter ≥3 cm) was higher in patients with symptomatic PAD than in the general population127,129 and in a population of patients with atherosclerotic risk factors.128 The prevalence of AAA among patients with PAD increased with age, beginning in patients ≥55 years of age, and was highest in patients ≥75 years of age.129 There are no data on AAA screening in patients with asymptomatic PAD. This recommendation refers to screening patients with symptomatic PAD for AAA regardless of patient age, sex, smoking history, or family history of AAA. Recommendations for screening the general population with risk factors for AAA (based on age, sex, smoking history, and family history) have been previously published.9 | |
|
| ||
4.2. Screening for Asymptomatic Atherosclerosis in Other Arterial Beds (Coronary, Carotid, and Renal Arteries)
The prevalence of atherosclerosis in the coronary, carotid, and renal arteries is higher in patients with PAD than in those without pad.128,130–135 However, intensive atherosclerosis risk factor modification in patients with PAD is justified regardless of the presence of disease in other arterial beds. Thus, the only justification for screening for disease in other arterial beds is if revascularization results in a reduced risk of myocardial infarction (MI), stroke, or death, and this has never been shown. Currently, there is no evidence to demonstrate that screening all patients with PAD for asymptomatic atherosclerosis in other arterial beds improves clinical outcome. Intensive treatment of risk factors through GDMT is the principle method for preventing adverse cardiovascular ischemic events from asymptomatic disease in other arterial beds.
5. Medical Therapy for the Patient with PAD
Patients with PAD should receive a comprehensive program of GDMT, including structured exercise and lifestyle modification, to reduce cardiovascular ischemic events and improve functional status. Smoking cessation is a vital component of care for patients with PAD who continue to smoke. A guideline-based program of pharmacotherapy to reduce cardiovascular ischemic events and limb-related events should be prescribed for each patient with PAD and is customized to individual risk factors, such as whether the patient also has diabetes mellitus. Previous studies have demonstrated that patients with PAD are less likely to receive GDMT than are patients with other forms of cardiovascular disease, including coronary artery disease (CAD).136–138
5.1. Antiplatelet Agents: Recommendations
|
| ||
| Recommendations for Antiplatelet Agents | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | A | Antiplatelet therapy with aspirin alone (range 75–325 mg per day) or clopidogrel alone (75 mg per day) is recommended to reduce MI, stroke, and vascular death in patients with symptomatic PAD. 139–142 |
|
| ||
| See Online Data Supplement 13. | The effect of antiplatelet therapy on cardiovascular events has been systematically reviewed by the Antithrombotic Trialists' Collaboration.139 Of note, this meta-analysis included studies of antiplatelet agents other than aspirin or clopidogrel. Among patients with symptomatic PAD treated with antiplatelet therapy, there was a 22% odds reduction for cardiovascular events, including MI, stroke, or vascular death.139 Symptomatic patients with lower extremity PAD included both those with claudication and those with prior lower extremity revascularization. The Antithrombotic Trialists' Collaboration meta-analysis also compared the efficacy of different doses of aspirin.139 The proportional reduction in vascular events was 32% with 75 to 150 mg daily, 26% with 160 to 325 mg daily, and 19% with 500 to 1500 mg daily, whereas there was a significantly smaller (13%) reduction in cardiovascular events in patients being treated with <75 mg of aspirin per day.139 CLIPS (Critical Leg Ischaemia Prevention Study) demonstrated a benefit of aspirin (100 mg daily) compared with placebo in preventing vascular events, but the study was too small to derive meaningful conclusions.140 A meta-analysis of trials of aspirin (alone or in combination with dipyridamole) for prevention of cardiovascular events in patients with PAD found a non–statistically significant reduction in the primary endpoint of cardiovascular death, MI, and stroke and a statistically significant reduction in the secondary endpoint of nonfatal stroke with aspirin versus placebo.141 The CAPRIE (Clopidogrel Versus Aspirin in Patients at Risk of Ischemic Events) trial demonstrated a benefit of clopidogrel as compared with aspirin in cardiovascular risk reduction and bleeding events in a population of patients with symptomatic atherosclerotic vascular disease, including a subgroup of patients with symptomatic PAD.142 | |
|
| ||
| IIa | C-EO | In asymptomatic patients with PAD (ABI ≤0.90), antiplatelet therapy is reasonable to reduce the risk of MI, stroke, or vascular death. |
|
| ||
| See Online Data Supplement 13. | Patients with PAD (ie, ABI ≤0.90) who do not have claudication may have leg symptoms atypical for claudication or may be too functionally limited to allow for adequate leg symptom assessment. Patients with PAD without claudication are at increased cardiovascular risk.79 Subgroup analysis in a trial evaluating asymptomatic patients did not show an effect of aspirin in patients with an abnormally low ABI (<0.80 or ≤0.90).76 However, the trial was not powered to analyze subgroups, and the uncertainty of the result does not rule out the possibility that aspirin could provide benefit in such patients, especially in those at increased risk of cardiovascular events. Another trial that included asymptomatic patients was too small to derive meaningful conclusions.140 | |
|
| ||
| IIb | B-R | In asymptomatic patients with borderline ABI (0.91–0.99), the usefulness of antiplatelet therapy to reduce the risk of MI, stroke, or vascular death is uncertain.75,76 |
|
| ||
| See Online Data Supplement 13. | In asymptomatic patients with an abnormal or borderline ABI, 2 RCTs found that aspirin had no effect in reducing cardiovascular events75,76 and might increase bleeding.76 However, the trials were not powered to examine patients with borderline ABI separately. Given that cardiovascular risk is lower in patients with borderline ABI than in those with abnormal ABI,80 it would be unlikely that aspirin would have a meaningful effect in this subgroup when there was no evidence of an effect in the total trial populations. | |
|
| ||
| IIb | B-R | The effectiveness of dual antiplatelet therapy (DAPT) (aspirin and clopidogrel) to reduce the risk of cardiovascular ischemic events in patients with symptomatic PAD is not well established.143,144 |
|
| ||
| See Online Data Supplement 13. | Based on findings from a subset of patients with PAD in the CHARISMA (Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance) trial, DAPT with aspirin plus clopidogrel may be considered for patients with PAD at particularly high risk of cardiovascular ischemic events who are not at high risk of bleeding.143,144 Currently, there are sparse data on newer P2Y12 antagonists for PAD. There is uncertainty about the net benefit of long-term DAPT for patients with PAD—specifically the balance of risks of cardiovascular ischemic events versus major bleeding. Additional clinical trials are needed in the population with PAD. Refer to the DAPT guideline focused update for DAPT recommendations specifically for CAD.20 | |
|
| ||
| IIb | C-LD | DAPT (aspirin and clopidogrel) may be reasonable to reduce the risk of limb-related events in patients with symptomatic PAD after lower extremity revascularization.145–148 |
|
| ||
| See Online Data Supplements 13 and 14. | There are sparse data on DAPT after lower extremity revascularization. Still, DAPT is prescribed in up to 55% of patients after endovascular revascularization for CLI.146 One small RCT of aspirin or aspirin plus clopidogrel in patients undergoing endovascular revascularization demonstrated that patients with DAPT had fewer repeat revascularization procedures for clinical symptoms.145 A subsequent small RCT of aspirin plus placebo or aspirin plus clopidogrel in patients after endovascular revascularization also showed a decrease in the need for repeat revascularization at 6 months in patients receiving clopidogrel.147 An RCT of aspirin plus placebo or aspirin plus clopidogrel in patients who underwent below-knee bypass graft showed a decrease in limb-related events only in the prespecified subgroup of patients with prosthetic bypass grafts.148 Refer to the DAPT guideline focused update for DAPT recommendations specifically for CAD.20 | |
|
| ||
| IIb | B-R | The overall clinical benefit of vorapaxar added to existing antiplatelet therapy in patients with symptomatic PAD is uncertain.149–152 |
|
| ||
| See Online Data Supplement 13. | This novel antagonist of protease-activated receptor-1 added to existing antiplatelet therapy reduced the risk of cardiovascular ischemic events in patients with atherosclerosis who were receiving standard therapy in an RCT.150,151 However, it also increased the risk of moderate or severe bleeding. Although the cardiovascular benefit was not demonstrated in the subgroup with symptomatic PAD, there was a reduction in limb-related events with vorapaxar, specifically in acute limb ischemia (ALI) and peripheral revascularization.149,152 More than half of ALI events in the PAD subset were due to thrombosis of lower extremity bypass grafts.149 Unfortunately, the benefit in limb events in patients with PAD was accompanied by an increased risk of bleeding.149,152 Therefore, the overall clinical benefit of vorapaxar in patients with PAD is uncertain. | |
|
| ||
5.2. Statin Agents: Recommendation
|
| ||
| Recommendation for Statin Agents | ||
|
| ||
| COR | LOE | Recommendation |
|
| ||
| I | A | Treatment with a statin medication is indicated for all patients with PAD.96,153–157 |
|
| ||
| See Online Data Supplements 15 and 16. | Statin therapy improves both cardiovascular and limb outcomes in patients with PAD.157 In a subgroup of 6748 patients with PAD in the HPS (Heart Protection Study), simvastatin 40 mg daily reduced the rate of first major vascular event by 22% relative to placebo.155 | |
|
| ||
| In a multinational registry, statin use among patients with PAD reduced 4-year adverse limb-related events (ie, worsening claudication, new CLI, new lower extremity revascularization, new ischemic amputation) compared with no statin.153 Use of simvastatin in the HPS reduced relative risk of peripheral vascular events (including noncoronary revascularization, aneurysm repair, major amputation, or PAD death) compared with placebo.155 In Medicare patients undergoing lower extremity revascularization, 1-year limb salvage rates were improved among those receiving statin medication.154 In a multicenter RCT, use of atorvastatin 80 mg daily improved pain-free walking time and community-based walking at 12 months compared with placebo.156 In 1 cohort study of 5480 patients with asymptomatic PAD, statin treatment improved cardiovascular outcomes.96 Guidelines for dosing of statin medications have been previously published.24 | ||
|
| ||
5.3. Antihypertensive Agents: Recommendations
|
| ||
| Recommendations for Antihypertensive Agents | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | A | Antihypertensive therapy should be administered to patients with hypertension and PAD to reduce the risk of MI, stroke, heart failure, and cardiovascular death.158–162 |
|
| ||
| See Online Data Supplements 17 and 18. | Treatment of elevated blood pressure is indicated to lower the risk of cardiovascular events.162 Target blood pressure and selection of antihypertensive therapy should be consistent with current published guidelines for hypertension management. Concerns have been raised that antihypertensive therapy may reduce limb perfusion. However, multiple studies have demonstrated that blood pressure treatment, including the use of beta blockers, does not worsen claudication symptoms or impair functional status in patients with PAD.163–165 There is no evidence that one class of antihypertensive medication or strategy is superior for blood pressure lowering in PAD.158,166,167 An updated multisocietal guideline on the management of high blood pressure is anticipated in 2017. | |
|
| ||
| IIa | A | The use of angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers can be effective to reduce the risk of cardiovascular ischemic events in patients with PAD.161,168,169 |
|
| ||
| See Online Data Supplement 17. | The effect of ramipril versus placebo on cardiovascular events was studied in high-risk patients free of heart failure in the HOPE (Heart Outcomes Prevention Evaluation) trial.168,169 Patients were normotensive on average at the time of enrollment. In a subgroup of 4051 patients with PAD, ramipril reduced the risk of MI, stroke, or vascular death by 25%, similar to the efficacy in the entire study population.168,169 The efficacy was similar in patients with PAD with symptomatic disease and asymptomatic low ABI.168 ONTARGET (Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial) compared telmisartan, ramipril, and combination therapy in patients with cardiovascular disease, including PAD, and/or diabetes mellitus.161 All 3 treatments had similar cardiovascular event rates with higher rates of adverse events (including hypotension, syncope, and renal failure) in the combination-therapy group. The efficacy of telmisartan was similar in the subgroup of 3468 patients with PAD, which supports the use of angiotensin-receptor blockers as an alternative to angiotensin-converting enzyme inhibitors.161 The effect of angiotensin-receptor blockers in asymptomatic PAD has not been studied. | |
|
| ||
5.4. Smoking Cessation: Recommendations
|
| ||
| Recommendations for Smoking Cessation | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | A | Patients with PAD who smoke cigarettes or use other forms of tobacco should be advised at every visit to quit.170–172 |
|
| ||
| See Online Data Supplements 19 and 20. | Tobacco use is a strong risk factor for the development and progression of PAD.173,174 Sparse evidence exists with regard to the association of novel tobacco product use, including electronic cigarettes, and PAD.175 Observational studies suggest that smoking cessation is associated with lower rates of cardiovascular ischemic events, limb-related events, bypass graft failure, amputation, and death in patients with PAD.172,176–178 Clinician advice increases quit rates, which supports simple provider-based measures as a component of smoking cessation programs.22,171,179 | |
|
| ||
| I | A | Patients with PAD who smoke cigarettes should be assisted in developing a plan for quitting that includes pharmacotherapy (ie, varenicline, bupropion, and/or nicotine replacement therapy) and/or referral to a smoking cessation program.170,180–182 |
|
| ||
| See Online Data Supplements 19 and 20. | Coordinated smoking cessation interventions that include nonpharmacological and pharmacological approaches have the greatest efficacy. An RCT of a follow-up program and smoking cessation medications provided to hospitalized patients, including those with PAD, demonstrated a modest increase in quit rates.181 In an RCT of patients with PAD specifically, a comprehensive smoking cessation program combining counseling and pharmacological agents increased the rates of smoking cessation to 21.3%, compared with 6.8% with standard advice.170 Three pharmacological approaches (ie, varenicline, bupropion, and nicotine replacement therapy) used alone or in combination all increase smoking cessation rates.179,180,182 Two meta-analyses of RCTs of smoking cessation medications showed no evidence of increased cardiovascular event rates with nicotine replacement, bupropion, or varenicline.183,184 Sparse data suggest that electronic cigarettes have no benefit on smoking cessation rates.179 | |
|
| ||
| I | B-NR | Patients with PAD should avoid exposure to environmental tobacco smoke at work, at home, and in public places.185,186 |
|
| ||
| See Online Data Supplement 20. | Passive smoke exposure has been associated with the development of PAD.186 Observational studies have shown lower cardiovascular and cerebrovascular event rates in the general population after enactment of smoke-free legislation.185 The effects of avoidance of passive smoke exposure on limb-related events are not known. | |
|
| ||
5.5. Glycemic Control: Recommendations
|
| ||
| Recommendations for Glycemic Control | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | C-EO | Management of diabetes mellitus in the patient with PAD should be coordinated between members of the healthcare team. |
|
| ||
| N/A | Diabetes mellitus is an important risk factor for the development of PAD.187 Furthermore, the presence of diabetes mellitus increases the risk of adverse outcomes among patients with PAD, including progression to CLI, amputation, and death.188,189 A comprehensive care plan for patients with PAD and diabetes mellitus is important and may include diet and weight management, pharmacotherapy for glycemic control and management of other cardiovascular risk factors, and foot care and ulcer prevention.25,190 Guidelines for glycemic control among patients with diabetes mellitus and atherosclerotic vascular disease have been previously published.25,29 Regular follow-up with and communication among the patient's healthcare providers, including vascular specialists and diabetes care providers (eg, primary care physicians, endocrinologists) constitute an important component of care for patients with PAD and diabetes mellitus. | |
|
| ||
| IIa | B-NR | Glycemic control can be beneficial for patients with CLI to reduce limb-related outcomes.191,192 |
|
| ||
| See Online Data Supplement 22. | In a cohort of 1974 participants with diabetes mellitus from the Strong Heart Study, compared with patients without PAD, patients with PAD and a Hg A1c level <6.5% had lower age-adjusted odds of major amputation compared to patients with PAD and hemoglobin A1c 6.5% to 9.5% and hemoglobin A1c >9.5%.188 Glycemic control is particularly important for patients with PAD and diabetes mellitus who have CLI. Single-center observational studies have demonstrated improved limb-related outcomes, including lower rates of major amputation and improved patency after infrapopliteal intervention, among patients with CLI who have more optimized glycemic control parameters compared with patients with inferior glycemic control.191,192 | |
|
| ||
5.6. Oral Anticoagulation: Recommendations
|
| ||
| Recommendations for Oral Anticoagulation | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| IIb | B-R | The usefulness of anticoagulation to improve patency after lower extremity autogenous vein or prosthetic bypass is uncertain.193–195 |
|
| ||
| See Online Data Supplements 23 and 24. | Two RCTs evaluating the effectiveness of oral anticoagulation (warfarin) in improving lower extremity bypass patency demonstrated improved patency among the subgroup of patients with autogenous vein bypass grafts.193,194 However, a Cochrane systematic review showed no patency benefit with the use of anticoagulation compared with antiplatelet therapy.195 All RCTs and observational studies evaluating the effect of anticoagulants on bypass patency demonstrated increased bleeding complications associated with anticoagulant use. One RCT evaluating the effectiveness of oral anticoagulation (warfarin) in addition to aspirin in improving lower extremity bypass patency demonstrated improved patency in a subgroup of patients with 6-mm polytetrafluoroethylene (known as PTFE) bypass graft.196 Randomization to anticoagulation plus aspirin was associated with increased risk of death and major hemorrhage versus aspirin alone. | |
|
| ||
| III: Harm | A | Anticoagulation should not be used to reduce the risk of cardiovascular ischemic events in patients with PAD.194,196–198 |
|
| ||
| See Online Data Supplements 23 and 24 | RCTs and observational studies have uniformly demonstrated that oral anticoagulation therapy aimed at decreasing major cardiovascular ischemic events provided no benefit and resulted in increased morbidity.194,196–198 In the WAVE (Warfarin Antiplatelet Vascular Evaluation) trial of patients with atherosclerotic vascular disease, including PAD, there was no difference in cardiovascular ischemic events among patients randomized to oral anticoagulation and antiplatelet therapy versus antiplatelet therapy alone.198 In addition, there was an increase in bleeding endpoints including life-threatening and intracranial bleeding.198 One RCT demonstrated increased death rate among patients randomized to warfarin plus aspirin versus aspirin alone after lower extremity bypass grafting.196 | |
|
| ||
5.7. Cilostazol: Recommendation
|
| ||
| Recommendation for Cilostazol | ||
|
| ||
| COR | LOE | Recommendation |
|
| ||
| I | A | Cilostazol is an effective therapy to improve symptoms and increase walking distance in patients with claudication.199,200 |
|
| ||
| See Online Data Supplement 25. | In a Cochrane review including 15 double-blind RCTs with a total of 3718 participants, cilostazol was associated with improvement in claudication symptoms but no changes in cardiovascular deaths or QoL when compared with placebo.199 In 1 RCT, cilostazol was more effective than pentoxifylline or placebo.200 Side effects include headache, abnormal stool (diarrhea), dizziness, and palpitations. Cilostazol is contraindicated in patients with congestive heart failure.201 In 1 trial, 20% of patients discontinued cilostazol within 3 months.202 | |
|
| ||
5.8. Pentoxifylline: Recommendation
|
| ||
| Recommendation for Pentoxifylline | ||
|
| ||
| COR | LOE | Recommendation |
|
| ||
| III: No Benefit | B-R | Pentoxifylline is not effective for treatment of claudication.200,203 |
|
| ||
| See Online Data Supplement 26. | In a Cochrane review of 24 studies with 3377 participants, there was large variability in study design and results between individual studies, and therefore the review's effectiveness was unclear.203 Pentoxifylline was shown to be generally well tolerated.203 In a multicenter RCT of pentoxifylline, cilostazol, or placebo for patients with moderate-to-severe claudication, there was no difference between pentoxifylline and placebo in the primary endpoint of maximal walking distance.200 Therefore, pentoxifylline is not recommended as treatment for claudication. | |
|
| ||
5.9. Chelation Therapy: Recommendation
|
| ||
| Recommendation for Chelation Therapy | ||
|
| ||
| COR | LOE | Recommendation |
|
| ||
| III: No Benefit | B-R | Chelation therapy (eg, ethylenediaminetetraacetic acid) is not beneficial for treatment of claudication.204 |
|
| ||
| See Online Data Supplement 27. | In a Cochrane review of 5 studies with 260 participants, chelation therapy showed no significant difference in symptoms (maximal and pain-free walking distance) compared with placebo.204 | |
|
| ||
5.10. Homocysteine Lowering: Recommendation
|
| ||
| Recommendation for Homocysteine Lowering | ||
|
| ||
| COR | LOE | Recommendation |
|
| ||
| III: No Benefit | B-R | B-complex vitamin supplementation to lower homocysteine levels for prevention of cardiovascular events in patients with PAD is not recommended.205–207 |
|
| ||
| See Online Data Supplements 28 and 29. | Although patients with PAD have been shown to have increased plasma homocysteine levels compared with patients without PAD, there is no evidence that B-complex vitamin supplementation improves clinical outcomes in patients with PAD.207 The HOPE-2 trial randomized 5522 patients with atherosclerotic vascular disease, including symptomatic PAD, or diabetes mellitus with additional risk factors to receive folic acid/vitamin B6/vitamin B12 or placebo.205,206 Despite lowering of homocysteine levels in the vitamin supplementation arm, there was no improvement in the primary endpoint of cardiovascular death, MI, or stroke. | |
|
| ||
5.11. Influenza Vaccination: Recommendation
|
| ||
| Recommendation for Influenza Vaccination | ||
|
| ||
| COR | LOE | Recommendation |
|
| ||
| I | C-EO | Patients with PAD should have an annual influenza vaccination. |
|
| ||
| See Online Data Supplements 30 and 31. | Observational studies have demonstrated reduced cardiovascular event rates among patients with cardiovascular disease who have received an influenza vaccination.30 Two RCTs that enrolled patients with CAD demonstrated a benefit of an influenza vaccination on the prevention of cardiovascular events, particularly coronary ischemic events.208,209 Although these trials did not specifically enroll participants with PAD, a majority of patients with PAD also have CAD.30 On the basis of this evidence, an annual influenza vaccination is recommended as a component of medical therapy for patients with PAD. | |
|
| ||
6. Structured Exercise Therapy: Recommendations
Structured exercise therapy is an important element of care for the patient with PAD. Components of structured exercise programs for PAD are outlined in Table 8.
Table 8. Structured Exercise Programs for PAD: Definitions.
| Supervised exercise program (COR I, LOE A) |
| Program takes place in a hospital or outpatient facility. |
| Program uses intermittent walking exercise as the treatment modality. |
| Program can be standalone or within a cardiac rehabilitation program. |
| Program is directly supervised by qualified healthcare provider(s). |
| Training is performed for a minimum of 30–45 min/session; sessions are performed at least 3 times/wk for a minimum of 12 wk.36–46 |
| Training involves intermittent bouts of walking to moderate-to-maximum claudication, alternating with periods of rest. |
| Warm-up and cool-down periods precede and follow each session of walking. |
| Structured community- or home-based exercise program (COR IIa, LOE A) |
| Program takes place in the personal setting of the patient rather than in a clinical setting.41,47–51 |
| Program is self-directed with guidance of healthcare providers. |
| Healthcare providers prescribe an exercise regimen similar to that of a supervised program. |
| Patient counseling ensures understanding of how to begin and maintain the program and how to progress the difficulty of the walking (by increasing distance or speed). |
| Program may incorporate behavioral change techniques, such as health coaching or use of activity monitors. |
COR indicates Class of Recommendation; LOE, Level of Evidence; and PAD, peripheral artery disease.
|
| ||
| Recommendations for Structured Exercise Therapy | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | A | In patients with claudication, a supervised exercise program is recommended to improve functional status and QoL and to reduce leg symptoms.36–38,40–46,48,210,211 |
|
| ||
| See Online Data Supplement 32. | The data supporting the efficacy of supervised exercise training as an initial treatment for claudication continue to develop and remain convincing, building on many earlier RCTs.40–46,48,210,211 Trials with long-term follow-up from 18 months37,38 to 7 years36 have demonstrated a persistent benefit of supervised exercise in patients with claudication. Data also support a benefit of supervised exercise for patients with symptomatic PAD and diabetes mellitus.212 The risk–benefit ratio for supervised exercise in PAD is favorable, with an excellent safety profile in patients screened for absolute contraindications to exercise such as exercise-limiting cardiovascular disease, amputation or wheelchair confinement, and other major comorbidities that would preclude exercise.36,39,49,213–216 Despite the health benefits associated with supervised exercise in patients with PAD, initiating and maintaining a high level of adherence remain challenging. Frequent contact with patients both when performing exercise in the supervised setting and at home has been somewhat effective in promoting retention.37,38 | |
|
| ||
| I | B-R | A supervised exercise program should be discussed as a treatment option for claudication before possible revascularization.36–38 |
|
| ||
| See Online Data Supplement 32. | The CLEVER (Claudication: Exercise Versus Endoluminal Revascularization) trial randomized patients with symptomatic aortoiliac PAD and showed comparable benefits for supervised exercise and stent revascularization at 6 and 18 months, with each therapy being superior to optimal medical care.37,38 Overall, the safety profile for supervised exercise was excellent. An RCT that compared 7-year effectiveness of supervised exercise or endovascular revascularization in patients with stable claudication with iliac or femoropopliteal disease found no differences in improved walking and QoL outcomes.36 Although more secondary interventions occurred in the exercise group, the total number of interventions was greater in the endovascular revascularization group. Collectively, these studies provide strong support for offering patients a supervised exercise program for reducing claudication symptoms and for improving functional status and QoL. | |
|
| ||
| A 3-month RCT that compared percutaneous transluminal angioplasty (PTA), supervised exercise, and combined treatment for claudication found that both supervised exercise and PTA improved clinical and QoL outcomes, whereas PTA plus supervised exercise produced greater benefits than either therapy alone.217 The ERASE (Endovascular Revascularization and Supervised Exercise) study randomized participants with claudication to endovascular revascularization plus supervised exercise or supervised exercise alone. After 1 year, patients in both groups had significant improvements in walking distances and health-related QoL, with greater improvements in the combined-therapy group.218 Collectively, these studies support the continued provision of supervised exercise to patients with claudication, whether as a monotherapy or combined with revascularization. | ||
|
| ||
| IIa | A | In patients with PAD, a structured community- or home-based exercise program with behavioral change techniques can be beneficial to improve walking ability and functional status.49,88,94,213 |
|
| ||
| See Online Data Supplement 32. | Unstructured community-based or home-based walking programs that consist of providing general recommendations to patients with claudication to simply walk more are not efficacious.50 Studies supporting structured community- or home-based programs for patients with symptomatic PAD (claudication and/or leg symptoms atypical for claudication) are more recent than studies supporting supervised exercise programs, and have provided strong evidence in support of the community- or home-based approach.47,49,51,88,94,213 For example, the GOALS (Group Oriented Arterial Leg Study) trial94 included patients with confirmed PAD with and without claudication (atypical lower extremity symptoms or no symptoms) and showed increases in several parameters of functional status for both of these patient cohort subgroups, versus nonexercising controls, after 6 months,88 with improvement maintained at 12 months.94 | |
|
| ||
| As with supervised exercise programs, despite proven benefit, initiating and maintaining a high level of adherence to community- or home-based exercise programs remains challenging. Studies that have incorporated behavioral change techniques, such as health coaching and activity tracking used in supervised settings, appear to reduce attrition and promote higher levels of adherence, thereby improving functional and QoL outcomes, both short term and long term.49,88,94 | ||
|
| ||
| IIa | A | In patients with claudication, alternative strategies of exercise therapy, including upper-body ergometry, cycling, and pain-free or low-intensity walking that avoids moderate-to-maximum claudication while walking, can be beneficial to improve walking ability and functional status.39,215,219,220 |
|
| ||
| See Online Data Supplements 32 and 33. | Protocols for exercise therapy for PAD traditionally have recommended intermittent walking bouts to moderate or higher pain levels interspersed with short periods of rest. Although these protocols are efficacious, intolerance of pain may lead to poor exercise adherence. An increasing number of studies have shown that modalities of exercise that avoid claudication or walking performed at intensities that are pain free or produce only mild levels of claudication can achieve health benefits comparable to walking at moderate or higher levels of claudication pain.39,41,215,219–221 | |
|
| ||
7. Minimizing Tissue Loss in Patients with PAD: Recommendations
|
| ||
| Recommendations for Minimizing Tissue Loss in Patients With PAD | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | C-LD | Patients with PAD and diabetes mellitus should be counseled about self–foot examination and healthy foot behaviors.222,223 |
|
| ||
| See Online Data Supplement 34. | Some RCTs have suggested that patient education may help reduce the incidence of serious foot ulcers and lower extremity amputations, but the quality of evidence supporting patient education is low.222 Educational efforts generally include teaching patients about healthy foot behaviors (eg, daily inspection of feet, wearing of shoes and socks; avoidance of barefoot walking), the selection of proper footwear, and the importance of seeking medical attention for new foot problems.223 Educational efforts are especially important for patients with PAD who have diabetes mellitus with peripheral neuropathy. | |
|
| ||
| I | C-LD | In patients with PAD, prompt diagnosis and treatment of foot infection are recommended to avoid amputation.224–228 |
|
| ||
| See Online Data Supplement 34. | Foot infections (infection of any of the structures distal to the malleoli) may include cellulitis, abscess, fasciitis, tenosynovitis, septic joint space infection, and osteomyelitis. Studies have investigated the accuracy of physical findings for identification of infection and determining infection severity and risk of amputation.224–226 Because of the consequences associated with untreated foot infection—especially in the presence of PAD—clinicians should maintain a high index of suspicion.228 It is also recognized that the presence of diabetes mellitus with peripheral neuropathy and PAD may make the presentation of foot infection more subtle than in patients without these problems. Foot infection should be suspected if the patient presents with local pain or tenderness; periwound erythema; periwound edema, induration or fluctuance; pretibial edema; any discharge (especially purulent); foul odor; visible bone or a wound that probes-to-bone; or signs of a systemic inflammatory response (including temperature >38°C or <36°C, heart rate >90/min, respiratory rate >20/min or Paco2 <32 mm Hg, white blood cell count >12 000 or <4000/mcL or >10% immature forms).226 Probe-to-bone test is moderately predictive for osteomyelitis but is not pathognomonic.227 | |
|
| ||
| IIa | C-LD | In patients with PAD and signs of foot infection, prompt referral to an interdisciplinary care team (Table 9) can be beneficial.228–230 |
|
| ||
| See Online Data Supplement 34. | The EuroDIALE (European Study Group on Diabetes and the Lower Extremity) study demonstrated that the presence of both PAD and foot infection conferred a nearly 3-fold higher risk of leg amputation than either infection or PAD alone.228 The treatment of deep soft-tissue infection typically requires prompt surgical drainage; vascular imaging and expeditious revascularization generally follow. Experienced clinical teams have reported very good outcomes when this is performed in a coordinated and timely fashion.229,230 Previous groups have described various combinations of functions of interdisciplinary care teams (See Online Data Supplement 34a for a complete list of functions). See Section 9.2 for recommendations related to the role of the interdisciplinary care team in wound healing therapies for CLI. | |
|
| ||
| IIa | C-EO | It is reasonable to counsel patients with PAD without diabetes mellitus about self–foot examination and healthy foot behaviors. |
|
| ||
| N/A | Although there are limited data to support patient education about self–foot examination and foot care for patients with diabetes mellitus, there are no data that have evaluated this practice in a population of patients with PAD but without diabetes mellitus. Nonetheless, this is a very low-risk intervention with potential for benefit. Educational efforts generally include teaching patients about healthy foot behaviors (eg, daily inspection of feet; foot care and hygiene, including appropriate toenail cutting strategies; avoidance of barefoot walking), the selection of appropriately fitting shoes, and the importance of seeking medical attention for new foot problems.223 | |
|
| ||
| IIa | C-EO | Biannual foot examination by a clinician is reasonable for patients with PAD and diabetes mellitus. |
|
| ||
| N/A | A history of foot ulcers, foot infections, or amputation identifies patients with a very high (>10%) yearly incidence of recurrent ulcers.231 Examination includes a visual inspection for foot ulcers (full-thickness epithelial defects) and structural (bony) deformities, monofilament testing for sensory neuropathy, and palpation for pedal pulses. | |
|
| ||
Table 9. Interdisciplinary Care Team for PAD.
| A team of professionals representing different disciplines to assist in the evaluation and management of the patient with PAD. For the care of patients with CLI, the interdisciplinary care team should include individuals who are skilled in endovascular revascularization, surgical revascularization, wound healing therapies and foot surgery, and medical evaluation and care. |
| Interdisciplinary care team members may include: |
| Vascular medical and surgical specialists (ie, vascular medicine, vascular surgery, interventional radiology, interventional cardiology) |
| Nurses |
| Orthopedic surgeons and podiatrists |
| Endocrinologists |
| Internal medicine specialists |
| Infectious disease specialists |
| Radiology and vascular imaging specialists |
| Physical medicine and rehabilitation clinicians |
| Orthotics and prosthetics specialists |
| Social workers |
| Exercise physiologists |
| Physical and occupational therapists |
| Nutritionists/dieticians |
CLI indicates critical limb ischemia; and PAD, peripheral artery disease.
8. Revascularization for Claudication
An individualized approach to revascularization for claudication is recommended for each patient to optimize outcome. Revascularization is but one component of care for the patient with claudication, as each patient should have a customized care plan that also includes medical therapy (Section 5), structured exercise therapy (Section 6), and care to minimize tissue loss (Section 7). If a strategy of revascularization for claudication is undertaken, the revascularization strategy should be evidence based and can include endovascular revascularization, surgery, or both.
Because of the variability of ischemic limb symptoms and impact of these symptoms on functional status and QoL, patients should be selected for revascularization on the basis of severity of their symptoms. Factors to consider include a significant disability as assessed by the patient, adequacy of response to medical and structured exercise therapy, status of comorbid conditions, and a favorable risk–benefit ratio. Patient preferences and goals of care are important considerations in the evaluation for revascularization. The revascularization strategy should have a reasonable likelihood of providing durable relief of symptoms. A general recommendation for revascularization as a treatment option for claudication is provided below followed by specific recommendations for endovascular (Section 8.1.1) and surgical (Section 8.1.2) procedures if a revascularization strategy is undertaken.
8.1. Revascularization for Claudication: Recommendation
|
| ||
| Recommendation for Revascularization for Claudication | ||
|
| ||
| COR | LOE | Recommendation |
|
| ||
| IIa | A | Revascularization is a reasonable treatment option for the patient with lifestyle-limiting claudication with an inadequate response to GDMT.12,37,38,232,233 |
|
| ||
| See Online Data Supplements 35 and 36. | A minority of patients with claudication (estimated at <10% to 15% over 5 years or more) will progress to CLI.234–237 Therefore, the role of revascularization in claudication is improvement in claudication symptoms and functional status, and consequently in QoL, rather than limb salvage. Revascularization is reasonable when the patient who is being treated with GDMT (including structured exercise therapy) presents with persistent lifestyle-limiting claudication.12,37,38,232,233 Lifestyle-limiting claudication is defined by the patient rather than by any test. It includes impairment of activities of daily living and/ or vocational and/or recreational activities due to claudication. There should be clear discussion with the patient about expected risks and benefits of revascularization, as well as discussion of the durability of proposed procedures. | |
|
| ||
8.1.1. Endovascular Revascularization for Claudication: Recommendations
Endovascular techniques to treat claudication include balloon dilation (angioplasty), stents, and atherectomy. These techniques continue to involve and now include covered stents, drug-eluting stents (DES), cutting balloons, and drug-coated balloons. The technique chosen for endovascular treatment is related to lesion characteristics (eg, anatomic location, lesion length, degree of calcification) and operator experience. Assessment of the appropriateness of specific endovascular techniques for specific lesions for the treatment of claudication is beyond the scope of this document.
Revascularization is performed on lesions that are deemed to be hemodynamically significant, and stenoses selected for endovascular treatment should have a reasonable likelihood of limiting perfusion to the distal limb. Stenoses of 50% to 75% diameter by angiography may not be hemodynamically significant, and resting or provoked intravascular pressure measurements may be used to determine whether lesions are significant.238,239 Multiple RCTs have compared endovascular procedures to various combinations of medical treatment with or without supervised or unsupervised exercise programs.12,37,38,217,232,233,240–251 These trials have used different endpoints and enrolled patients with anatomic disease distribution at different levels.
|
| ||
| Recommendations for Endovascular Revascularization for Claudication | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | A | Endovascular procedures are effective as a revascularization option for patients with lifestyle-limiting claudication and hemodynamically significant aortoiliac occlusive disease.12,37,38,232,240,242,246 |
|
| ||
| See Online Data Supplements 35 and 36. | Two separate systematic analyses that included RCTs that enrolled patients with aortoiliac disease reported that endovascular treatment of claudication improved walking parameters and QoL.11,12,233 The CLEVER trial enrolled only patients with aortoiliac disease and compared endovascular therapy to supervised exercise therapy and to medications alone.37,38 At 6-month follow-up, both the endovascular therapy and supervised exercise groups had improved peak walking time compared with medication alone, with a greater improvement in the supervised exercise group.37 By 18 months, there was no significant difference between the endovascular therapy and supervised exercise groups, with a sustained benefit versus medication alone.38 Other RCTs that included patients with aortoiliac disease have shown QoL, as assessed by questionnaires and time to onset of claudication, may be superior with endovascular treatment in combination with a medical and an exercise treatment plan, compared versus medical treatment alone.232,233,246 The ERASE trial randomized patients with claudication and aortoiliac (as well as femoropopliteal) disease to endovascular revascularization plus supervised exercise or supervised exercise alone. After 1 year, patients in both groups had significant improvements in walking distances and health-related QoL, with greater improvements in the combined-therapy group.218 The long-term comparative efficacy of endovascular revascularization versus supervised exercise therapy and medical therapy compared to supervised exercise therapy and medical therapy without revascularization for aortoiliac disease is unknown. | |
|
| ||
| IIa | B-R | Endovascular procedures are reasonable as a revascularization option for patients with lifestyle-limiting claudication and hemodynamically significant femoropopliteal disease.217,232,243–245,250,251 |
|
| ||
| See Online Data Supplement 35. | Multiple RCTs have demonstrated short-term efficacy with endovascular treatment of femoropopliteal disease for claudication versus supervised exercise training or medical therapy, with benefit that diminishes by 1 year.217,232,240–246,250,251 Two separate systematic reviews that included RCTs that enrolled patients with femoropopliteal disease, reported that endovascular treatment of claudication improved walking parameters and QoL.11,12,233 The durability of endovascular treatment for claudication is directly related to vessel patency. Long-term patency is greater in the iliac artery than in the femoropopliteal segment. Furthermore, durability is diminished with greater lesion length, occlusion rather than stenosis, the presence of multiple and diffuse lesions, poor-quality runoff, diabetes mellitus, chronic kidney disease, renal failure, and smoking.252–255 The choice of endovascular therapy as a revascularization approach for claudication due to femoropopliteal disease therefore should include a discussion of outcomes, addressing the risk of restenosis and repeat intervention, particularly for lesions with poor likelihood of long-term durability. | |
|
| ||
| IIb | C-LD | The usefulness of endovascular procedures as a revascularization option for patients with claudication due to isolated infrapopliteal artery disease is unknown.256–258 |
|
| ||
| See Online Data Supplement 35. | Isolated infrapopliteal disease is unlikely to cause claudication. Incidence of in-stent restenosis is high and long-term benefit lacking with bare-metal stenting of the infrapopliteal arteries.256 Studies that have enrolled patients with claudication as well as CLI have demonstrated a benefit of DES versus bare-metal stents or versus drug-coated balloons for revascularization of infrapopliteal lesions.257,258 However, these differences were mainly for patency and restenosis endpoints, and neither of these studies included patient-oriented outcomes, such as walking function or QoL parameters. Additional efficacy data on the use of infrapopliteal drug-coated balloon or DES for the treatment of claudication are likely to be published in the near future. | |
|
| ||
| III: Harm | B-NR | Endovascular procedures should not be performed in patients with PAD solely to prevent progression to CLI.234–237,259–261 |
|
| ||
| See Online Data Supplements 36 and 38. | There are no data to support a practice paradigm of performing endovascular procedures on patients with PAD for the purpose of preventing progression of claudication symptoms to CLI. Reported rates of amputation or progression to CLI from prospective cohort studies of patients with claudication are <10% to 15% over 5 years or more, and increased mortality rate associated with claudication is usually the result of cardiovascular events rather than limb-related events.234–237,262 Similarly, there are no data to support revascularization in patients with asymptomatic PAD. Procedural risks include bleeding, renal failure from contrast-induced nephropathy, and the possibility of adverse limb outcomes.259–261 Therefore, the known risks of endovascular procedures outweigh any hypothetical benefit of preventing progression from asymptomatic PAD or claudication to CLI. | |
|
| ||
8.1.2. Surgical Revascularization for Claudication: Recommendations
|
| ||
| Recommendations for Surgical Revascularization for Claudication | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | A | When surgical revascularization is performed, bypass to the popliteal artery with autogenous vein is recommended in preference to prosthetic graft material.263–271 |
|
| ||
| See Online Data Supplements 37 and 38. | The superficial femoral and proximal popliteal arteries are the most common anatomic sites of stenosis or occlusion among individuals with claudication. Femoral-popliteal bypass is therefore one of the most common surgical procedures for claudication and may be performed under general or regional anesthesia. The type of conduit and site of popliteal artery anastomosis (above versus below knee) are major determinants of outcomes associated with femoral-popliteal bypass. Systematic reviews and meta-analyses have identified a clear and consistent primary patency benefit for autogenous vein versus to prosthetic grafts for popliteal artery bypass.270,271 Prosthetic grafts to the popliteal artery above the knee have reduced patency rates and increased rates of repeat intervention.263,266,269,272 Sparse evidence suggests a long-term patency advantage for Dacron over polytetrafluoroethylene (known as PTFE) graft for above-knee bypass,270 although this finding has not been consistently demonstrated in all RCTs.266,273,274 | |
|
| ||
| IIa | B-NR | Surgical procedures are reasonable as a revascularization option for patients with lifestyle-limiting claudication with inadequate response to GDMT, acceptable perioperative risk, and technical factors suggesting advantages over endovascular procedures.232,265,275–277 |
|
| ||
| See Online Data Supplements 37 and 38. | Systematic reviews have concluded that surgical procedures are an effective treatment for claudication and have a positive impact on QoL and walking parameters but have identified sparse evidence supporting the effectiveness of surgery compared with other treatments.11,233,278,279 Although symptom and patency outcomes for surgical interventions may be superior versus less invasive endovascular treatments for specific patients, surgical interventions are also associated with greater risk of adverse perioperative events.280–286 Treatment selection should therefore be individualized on the basis of the patient's goals, perioperative risk, and anticipated benefit. Surgical procedures for claudication are usually reserved for individuals who a) do not derive adequate benefit from nonsurgical therapy, b) have arterial anatomy favorable to obtaining a durable result with surgery, and c) have acceptable risk of perioperative adverse events. Acceptable risk is defined by the individual patient and provider on the basis of symptom severity, comorbid conditions, and appropriate GDMT risk evaluation. Guidelines for the evaluation and management of patients undergoing noncardiac surgery, including vascular surgical procedures, have been previously published.21 | |
|
| ||
| III: Harm | B-R | Femoral-tibial artery bypasses with prosthetic graft material should not be used for the treatment of claudication.287–289 |
|
| ||
| See Online Data Supplement 37. | Bypasses to the tibial arteries with prosthetic material for treatment of claudication should be avoided because of very high rates of graft failure and amputation.287–289 | |
|
| ||
| III: Harm | B-NR | Surgical procedures should not be performed in patients with PAD solely to prevent progression to CLI.234–237,262 |
|
| ||
| See Online Data Supplements 37 and 38. | Claudication does not commonly progress to CLI. Reported rates of amputation or progression to CLI from prospective cohort studies of patients with claudication are <10% to 15% for 5 years or more, and increased mortality rate associated with claudication is usually the result of cardiovascular events rather than limb-related events.234–237,262 Surgical intervention should not be performed primarily to prevent disease progression, given the risk of adverse perioperative events without potential for significant benefit. Similarly, there are no data to support surgical revascularization in patients with asymptomatic PAD to prevent progression to CLI. | |
|
| ||
9. Management of CLI
Patients with CLI are at increased risk of amputation and major cardiovascular ischemic events. Care of the patient with CLI includes evaluation for revascularization and wound healing therapies, with the objective to minimize tissue loss, completely heal wounds, and preserve a functional foot. Medical therapy to prevent cardiovascular ischemic events is also an important component of care for the patient with CLI (Section 5).
9.1. Revascularization for CLI: Recommendations
|
| ||
| Recommendation for Revascularization for CLI | ||
|
| ||
| COR | LOE | Recommendation |
|
| ||
| I | B-NR | In patients with CLI, revascularization should be performed when possible to minimize tissue loss.290 |
|
| ||
| See Online Data Supplement 39. | Patients with CLI are at high risk of major cardiovascular ischemic events, as well as nonhealing wounds and major amputation. In a systematic review of 13 studies of patients with CLI who did not receive revascularization, which included patients enrolled in medical and angiogenic therapy trials, there was a 22% all-cause mortality rate and a 22% rate of major amputation at a median follow-up of 12 months.290 The goal of surgical or endovascular revascularization is to provide in-line blood flow to the foot through at least 1 patent artery, which will help decrease ischemic pain and allow healing of any wounds, while preserving a functional limb. Multiple RCTs comparing contemporary surgical and endovascular treatment for patients with CLI are ongoing.15–17 Revascularization is not warranted in the setting of a nonviable limb. | |
|
| ||
| I | C-EO | An evaluation for revascularization options should be performed by an interdisciplinary care team (Table 9) before amputation in the patient with CLI. |
|
| ||
| N/A | Patients with CLI should be evaluated by an interdisciplinary care team. Before amputation, evaluation generally includes imaging for assessment of revascularization options (eg, duplex ultrasound, CTA, MRA, or catheter-based angiogram). The objective of this strategy is to minimize tissue loss and preserve a functional limb with revascularization. | |
|
| ||
9.1.1. Endovascular Revascularization for CLI: Recommendations
|
| ||
| Recommendations for Endovascular Revascularization for CLI | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | B-R | Endovascular procedures are recommended to establish in-line blood flow to the foot in patients with nonhealing wounds or gangrene.292,293 |
|
| ||
| See Online Data Supplement 39. | The technique chosen for endovascular treatment of CLI is related to anatomic location of lesions, lesion characteristics, and operator experience. Revascularization is performed on hemodynamically significant stenoses that are likely to be limiting blood flow to the limb. For stenoses of 50% to 75%, where the hemodynamic significance is unclear, intravascular pressure measurements may be used to determine hemodynamic significance.294 The BASIL (Bypass versus Angioplasty in Severe Ischemia of the Leg) RCT demonstrated that endovascular revascularization is an effective option for patients with CLI as compared with open surgery.292,293 The primary endpoint of amputation-free survival was the same in the endovascular and surgical arms. Of note, the endovascular arm used only PTA.292,293 Multiple RCTs comparing contemporary surgical and endovascular treatment for patients with CLI are ongoing.15–17 Table 10 addresses factors that may prompt an endovascular versus surgical approach to the patient with CLI. | |
|
| ||
| IIa | C-LD | A staged approach to endovascular procedures is reasonable in patients with ischemic rest pain.295,296 |
|
| ||
| N/A | For patients with multilevel disease who suffer from ischemic rest pain, in-flow lesions are generally addressed first.295,296 Depending on procedural characteristics, including contrast volume used, radiation exposure, and procedure time, out-flow lesions can be addressed in the same setting or at a later time if symptoms persist. This strategy for ischemic rest pain is distinct from the strategy recommended for CLI in the patient with a nonhealing wound or gangrene. In that scenario, restoration of direct in-line flow to the foot is essential for wound healing. | |
|
| ||
| IIa | B-R | Evaluation of lesion characteristics can be useful in selecting the endovascular approach for CLI.297,298 |
|
| ||
| See Online Data Supplement 39. | The lesion characteristics to consider include length, anatomic location, and extent of occlusive disease. For example, if an adequate angioplasty result can be achieved with PTA alone for short (<10 cm) stenoses in the femoropopliteal segment, then stent placement is not necessary.297,298 Presence of thrombosis or calcification at the lesion site will also affect the endovascular approach. In general, the advantages of DES and drug-coated balloons over PTA alone or bare-metal stents are more consistent in the femoropopliteal segment than for infrapopliteal interventions.257,258,299–309 However, these differences are mainly for patency, restenosis, and repeat-revascularization endpoints. Most studies were underpowered or did not examine other patient-oriented outcomes, such as amputation or wound healing in CLI. Endovascular techniques continue to evolve rapidly, and there has been limited literature comparing techniques with regard to clinically significant outcomes, such as amputation or wound healing. | |
|
| ||
| IIb | B-NR | Use of angiosome-directed endovascular therapy may be reasonable for patients with CLI and nonhealing wounds or gangrene.310–319 |
|
| ||
| See Online Data Supplements 39 and 40. | During the past decade, the goal of care with regard to endovascular therapy for the treatment of nonhealing wounds due to CLI has been establishment of direct in-line blood flow to the affected limb. The angiosome concept has also been described in the literature in relation to the treatment of nonhealing wounds. Angiosome-directed treatment entails establishing direct blood flow to the infrapopliteal artery directly responsible for perfusing the region of the leg or foot with the nonhealing wound. Multiple retrospective studies and 1 small nonrandomized prospective study assessing the efficacy of this concept have been published.119,310–321 Meta-analyses of these studies found improved wound healing and limb salvage with angiosome-guided therapy but cautioned that the quality of the evidence was low.322,323 Although the angiosome concept is theoretically satisfying, randomized data comparing the establishment of in-line flow versus angiosome-guided therapy have yet to be published. Furthermore, there is no evidence yet to demonstrate the potential benefit of treating additional infrapopliteal arteries once in-line flow has been established in one artery, regardless of angiosome. Important considerations with regard to angiosome-guided therapy include the potential for longer procedural times, more contrast exposure, and more technically complex procedures. The impact of all these factors needs to be weighed against the likelihood of a technically successful procedure providing hypothetical added benefit over the establishment of in-line blood flow. | |
|
| ||
Table 10. Therapy for CLI: Findings That Prompt Consideration of Surgical or Endovascular Revascularization.
| Findings That Favor Consideration of Surgical Revascularization | Examples |
|---|---|
| Factors associated with technical failure or poor durability with endovascular treatment | Lesion involving common femoral artery, including origin of deep femoral artery |
| Long segment lesion involving the below-knee popliteal and/or infrapopliteal arteries in a patient with suitable single-segment autogenous vein conduit | |
| Diffuse multilevel disease that would require endovascular revascularization at multiple anatomic levels | |
| Small-diameter target artery proximal to site of stenosis or densely calcified lesion at location of endovascular treatment | |
| Endovascular treatment likely to preclude or complicate subsequent achievement of in-line blood flow through surgical revascularization | Single-vessel runoff distal to ankle |
| Findings That Favor Consideration of Endovascular Revascularization | Examples |
| The presence of patient comorbidities may place patients at increased risk of perioperative complications from surgical revascularization. In these patients, an endovascular-first approach should be used regardless of anatomy | Patient comorbidities, including coronary ischemia, cardiomyopathy, congestive heart failure, severe lung disease, and chronic kidney disease |
| Patients with rest pain and disease at multiple levels may undergo a staged approach as part of endovascular-first approach | In-flow disease can be addressed first, and out-flow disease can be addressed in a staged manner, when required, if clinical factors or patient safety prevent addressing all diseased segments at one setting |
| Patients without suitable autologous vein for bypass grafts | Some patients have had veins harvested for previous coronary artery bypass surgery and do not have adequate remaining veins for use as conduits. Similarly, patients may not have undergone prior saphenous vein harvest, but available vein is of inadequate diameter |
CLI indicates critical limb ischemia.
9.1.2. Surgical Revascularization for CLI: Recommendations
|
| ||
| Recommendations for Surgical Revascularization for CLI | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | A | When surgery is performed for CLI, bypass to the popliteal or infrapopliteal arteries (ie, tibial, pedal) should be constructed with suitable autogenous vein.263,266,269,272 |
|
| ||
| See Online Data Supplement 37. | Many large RCTs have demonstrated that bypasses above the knee should be autogenous vein either reversed or in situ vein.263,266,269,272 There are large single-center trials showing the efficacy of autogenous vein to distal tibial vessels.324,325 In addition, composite sequential femoropopliteal-tibial bypass and bypass to an isolated popliteal arterial segment that has collateral out flow to the foot are both acceptable methods of revascularization and should be considered when no other form of bypass with adequate autogenous conduit is possible.326,327 | |
|
| ||
| I | C-LD | Surgical procedures are recommended to establish in-line blood flow to the foot in patients with nonhealing wounds or gangrene.328–330 |
|
| ||
| See Online Data Supplement 42. | In patients presenting with nonhealing ulcers or gangrene, surgical procedures should be performed to establish in-line blood flow to the foot.328–330 Table 10 addresses factors that may prompt a surgical approach to the patient with CLI. | |
|
| ||
| IIa | B-NR | In patients with CLI for whom endovascular revascularization has failed and a suitable autogenous vein is not available, prosthetic material can be effective for bypass to the below-knee popliteal and tibial arteries.331–333 |
|
| ||
| See Online Data Supplement 42. | There are studies demonstrating that patients for whom endovascular treatment for CLI has failed can be treated successfully with autogenous vein bypass graft332,333 or prosthetic material.331 Although autogenous vein is the preferred conduit for surgical revascularization, prosthetic conduit is a secondary option for patients with CLI without suitable saphenous vein who require surgical revascularization. | |
|
| ||
| IIa | C-LD | A staged approach to surgical procedures is reasonable in patients with ischemic rest pain.334–336 |
|
| ||
| N/A | It is reasonable to perform a staged approach to revascularization in patients with ischemic rest pain with multilevel disease. For example, aortoiliac (inflow) disease may be treated first with endovascular treatment or by surgical reconstruction, depending on lesion characteristics, patient comorbidities, and patient preference.337,338 Combined percutaneous and surgical revascularization may require separate interventions, typically with the most proximal procedure performed first. | |
|
| ||
9.2. Wound Healing Therapies for CLI: Recommendations
|
| ||
| Recommendations for Wound Healing Therapies for CLI | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | B-NR | An interdisciplinary care team should evaluate and provide comprehensive care for patients with CLI and tissue loss to achieve complete wound healing and a functional foot.229,339–341 |
|
| ||
| See Online Data Supplement 44. | The management of patients with CLI and nonhealing wounds should include coordinated efforts for both revascularization and wound healing, because the risk of limb-threatening infections remains until complete wound healing is achieved. The structure and activities of interdisciplinary care teams for CLI may vary according to several factors, including the local availability of resources. Previous groups have described various combinations of activities of this team, which are in addition to revascularization and include functions such as wound care, infection management, orthotics, and prosthetics (see Online Data Supplement 34a for a complete list of functions). Coordination of these activities and some degree of organized team structure are recommended, as opposed to ad hoc or unstructured referrals among various specialty clinicians not involved in interdisciplinary care. | |
|
| ||
| Ambulatory patients with PAD and nonhealing foot ulcers should be considered for efforts to prevent amputation. The components of this effort may include revascularization, offloading, treatment of infection, and wound care. The long-term outcome of the limb is excellent when complete wound healing can be achieved.339Revascularization should be coordinated with the efforts of clinicians who manage foot infections, provide offloading, and achieve complete wound healing, either through medical therapy, surgical options, or a combination thereof. Coordinated and timely interdisciplinary care can achieve excellent limb outcomes for patients with PAD and nonhealing foot wounds.229,339–341 | ||
|
| ||
| I | C-LD | In patients with CLI, wound care after revascularization should be performed with the goal of complete wound healing.339 |
|
| ||
| See Online Data Supplement 44. | A comprehensive plan for treatment of CLI must include a plan for achieving an intact skin surface on a functional foot. One study demonstrated a limb salvage rate of 100% at 3 years in a cohort of patients with CLI who achieved complete wound healing with endovascular revascularization and dedicated wound care.339 Before revascularization, the interdisciplinary care team should devise a plan to achieve the goal of complete wound healing. After successful revascularization, most patients with gangrene of the foot are evaluated for minor amputation with staged/delayed primary closure or surgical reconstruction when feasible.342–344 Negative-pressure wound therapy dressings are helpful to achieve wound healing after revascularization and minor (ie, digit or partial foot) amputation when primary or delayed secondary closure is not feasible.345,346 Spontaneous amputation, or autoamputation, of gangrenous digits should be reserved for palliation in patients without options for revascularization.345,347,348 | |
|
| ||
| Other evidence-based guidelines relevant to those with nonhealing foot wounds following revascularization cover the full spectrum of diabetic foot problems349 or separately consider the management of infection,225,350 offloading,351 and wound care.352 To date, there are no RCTs or high-quality studies that have focused on wound healing adjuncts in limbs with severe PAD (eg, topical cytokine ointments, skin substitutes, cell-based therapies intended to optimize wound healing). | ||
|
| ||
| IIb | B-NR | In patients with CLI, intermittent pneumatic compression (arterial pump) devices may be considered to augment wound healing and/or ameliorate severe ischemic rest pain.353 |
|
| ||
| See Online Data Supplement 44. | A systematic review of studies that used intermittent pneumatic compression devices specifically designed to augment arterial perfusion of the lower extremities suggests that these may provide modest clinical benefit (specifically, decreased amputation rates and improved QoL) in patients with CLI who were ineligible for revascularization.353 The potential benefit appears to outweigh the low risk associated with the use of these devices. | |
|
| ||
| IIb | C-LD | In patients with CLI, the effectiveness of hyperbaric oxygen therapy for wound healing is unknown.354 |
|
| ||
| See Online Data Supplement 44. | The literature evaluating the utility of hyperbaric oxygen therapy has focused on patients without severe PAD and has not demonstrated a long-term benefit on wound healing or improving amputation-free survival when compared with sham treatment.355 There are no published studies evaluating the role of hyperbaric oxygen therapy for patients with nonreconstructible PAD. One small RCT that focused on patients with foot ulcers and PAD (ABI <0.80 or TBI <0.70) for whom no revascularization was planned demonstrated a significant decrease in ulcer area at 6 weeks, but no significant differences in ulcer size at 6 months, complete ulcer healing at 6 weeks or 6 months, and major or minor amputations.354 Further research on the utility of hyperbaric oxygen therapy in this context is needed. | |
|
| ||
| III: No Benefit | B-R | Prostanoids are not indicated in patients with CLI.356 |
|
| ||
| See Online Data Supplement 43. | A systematic review and meta-analysis concluded that RCTs have not demonstrated meaningful long-term clinical benefit from the administration of prostanoids to patients with CLI attributable to nonreconstructible PAD.356 | |
|
| ||
10. Management of ALI
ALI is one of the most treatable and potentially devastating presentations of PAD. Timely recognition of arterial occlusion as the cause of an ischemic, cold, painful leg is crucial to successful treatment. The writing committee has used a standard definition of ALI in which symptom duration is <2 weeks (Table 3).33,34 Category I refers to viable limbs that are not immediately threatened. Category II refers to threatened limbs. Category IIa limbs are marginally threatened and salvageable, if promptly treated. Category IIb are immediately threatened limbs that require immediate revascularization if salvage is to be accomplished. Category III are irreversibly damaged limbs, in which case resultant major tissue loss or permanent nerve damage is inevitable.34
10.1. Clinical Presentation of ALI: Recommendations
|
| ||
| Recommendations for Clinical Presentation of ALI | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | C-EO | Patients with ALI should be emergently evaluated by a clinician with sufficient experience to assess limb viability and implement appropriate therapy. |
|
| ||
| N/A | Patients with ALI should be rapidly evaluated by a vascular specialist if one is available. Depending on local clinical expertise, the vascular specialist may be a vascular surgeon, interventional radiologist, cardiologist, or a general surgeon with specialized training and experience in treating PAD. If such expertise is not locally or rapidly available, there should be strong consideration of transfer of the patient to a facility with such resources. The more advanced the degree of ischemia, the more rapidly the communication (including communication about potential patient transfer) needs to occur. | |
|
| ||
| I | C-LD | In patients with suspected ALI, initial clinical evaluation should rapidly assess limb viability and potential for salvage and does not require imaging.357–361 |
|
| ||
| See Online Data Supplements 45 and 46. | ALI is a medical emergency and must be recognized rapidly. The time constraint is due to the period that skeletal muscle will tolerate ischemia—roughly 4 to 6 hours.362 A rapid assessment of limb viability and ability to restore arterial blood flow should be performed by a clinician able to either complete the revascularization or triage the patient.358 Lower extremity symptoms in ALI can include both pain and loss of function. The longer these symptoms are present, the less likely the possibility of limb salvage.360,361 Clinical assessment must include symptom duration, pain intensity, and motor and sensory deficit severity to distinguish a threatened from a nonviable extremity (Figure 3). The bedside assessment should include arterial and venous examination with a handheld continuous-wave Doppler because of the inaccuracy of pulse palpation.34 The loss of dopplerable arterial signal indicates that the limb is threatened. The absence of both arterial and venous Doppler signal indicates that the limb may be irreversibly damaged (nonsalvageable). Comorbidities should be investigated and managed aggressively, but this must not delay therapy. Even in the setting of rapid and effective revascularization, the 1-year morbidity and mortality rates associated with ALI are high.360,363 | |
|
| ||
Figure 3. Diagnosis and Management of ALI.
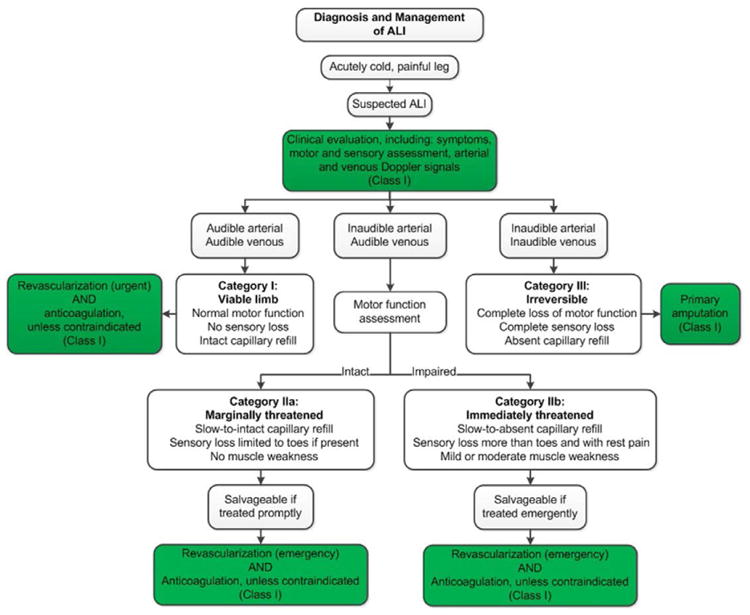
33,34, Colors correspond to Class of Recommendation in Table 1. ALI indicates acute limb ischemia.
10.2. Medical Therapy for ALI: Recommendations
|
| ||
| Recommendation for ALI Medical Therapy | ||
|
| ||
| COR | LOE | Recommendation |
|
| ||
| I | C-EO | In patients with ALI, systemic anticoagulation with heparin should be administered unless contraindicated. |
|
| ||
| N/A | Heparin (generally intravenous unfractionated heparin) is given to all patients acutely.35,364 This can stop thrombus propagation and may provide an anti-inflammatory effect that lessens the ischemia. Patients who have received heparin before the onset of ALI and have a decrease in platelet count may have heparin-induced thrombocytopenia.365,366 In this situation, a direct thrombin inhibitor is given, rather than heparin, if heparin-induced thrombocytopenia with thrombosis is suspected. | |
|
| ||
10.3. Revascularization for ALI: Recommendations
|
| ||
| Recommendations for Revascularization for ALI | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | C-LD | In patients with ALI, the revascularization strategy should be determined by local resources and patient factors (eg, etiology and degree of ischemia).367–369 |
|
| ||
| See Online Data Supplement 47. | For marginally or immediately threatened limbs (Category IIa and IIb ALI [Figure 3]), revascularization should be performed emergently (within 6 hours). For viable limbs (Category I ALI [Figure 3]), revascularization should be performed an on urgent basis (within 6–24 hours). The revascularization strategy can range from catheter-directed thrombolysis to surgical thromboembolectomy. Available facilities and clinical expertise are factors that should be considered when determining the revascularization strategy. The technique that will provide the most rapid restoration of arterial flow with the least risk to the patient should be selected. For example, catheter-directed thrombolysis can provide rapid restoration of arterial flow to a viable or marginally threatened limb, particularly in the setting of recent occlusion, thrombosis of synthetic grafts, and stent thrombosis.367 If this is not available locally, surgical options for timely revascularization should be considered, along with the feasibility of timely transfer to a facility with the necessary expertise. | |
|
| ||
| I | A | Catheter-based thrombolysis is effective for patients with ALI and a salvageable limb.367–371 |
|
| ||
| See Online Data Supplement 47. | Assessment of the comparative effectiveness of catheter-based thrombolysis versus open surgery is complicated by variable definitions of ALI in this literature. Four RCTs comparing catheter-based thrombolysis to surgery,367,369–371 as well as a meta-analysis,368 have demonstrated similar limb salvage rates between the 2 approaches but better survival with catheter-based therapy. The survival advantage of catheter-based therapy may be at least in part attributable to multiple comorbidities found among the population of patients who present with ALI. Increased comorbidities are likely to contribute to increased perioperative risk. Several of the RCTs included patients with relatively chronic ischemia. Acuity and severity are both factors in the decision to consider thrombolysis.367,369–371 | |
|
| ||
| I | C-LD | Amputation should be performed as the first procedure in patients with a nonsalvageable limb.372,373 |
|
| ||
| See Online Data Supplement 48. | For patients with Category III ALI (Figure 3), amputation should be performed as the index procedure. Prolonged duration of ischemia is the most common factor in patients requiring amputation for treatment of ALI. The risks associated with reconstruction outweigh the potential benefit in a limb that is already insensate or immobile because of prolonged ischemia. Patients who have an insensate and immobile limb in the setting of prolonged ischemia (>6 to 8 hours) are unlikely to have potential for limb salvage.34,362 In addition, in this setting the reperfusion and circulation of ischemic metabolites can result in multiorgan failure and cardiovascular collapse. However, if pain can be controlled and there is no evidence of infection, amputation may be deferred if this meets with the patient's goals. | |
|
| ||
| I | C-LD | Patients with ALI should be monitored and treated (eg, fasciotomy) for compartment syndrome after revascularization.372,373 |
|
| ||
| See Online Data Supplement 48. | The lower extremity muscles reside in compartments, surrounded by fascia and bones. Reperfusion to ischemic muscles can cause cellular edema, resulting in increased compartment pressure. When compartment pressure is >30 mm Hg, there is capillary and venule compression that leads to malperfusion of the muscle; this is compartment syndrome. Fasciotomy is indicated when the compartment pressure increases. Measurement of intracompartment pressure is not always easily accessible. In such cases, evaluation for fasciotomy is prompted by development of increased pain, tense muscle, or nerve injury. Fasciotomy should be considered for patients with Category IIb ischemia for whom the time to revascularization is >4 hours. | |
|
| ||
| IIa | B-NR | In patients with ALI with a salvageable limb, percutaneous mechanical thrombectomy can be useful as adjunctive therapy to thrombolysis.374–378 |
|
| ||
| See Online Data Supplements 49 and 50. | Multiple nonrandomized studies have suggested that percutaneous mechanical thrombectomy in combination with pharmacological therapy can be beneficial in the treatment of threatened limbs.374–378 | |
|
| ||
| IIa | C-LD | In patients with ALI due to embolism and with a salvageable limb, surgical thromboembolectomy can be effective.379–381 |
|
| ||
| See Online Data Supplements 49 and 50. | Patients with arterial embolism and an absent pulse ipsilateral to the ischemic limb can be treated by exposure of an artery in the affected limb and balloon-catheter thromboembolectomy. These patients may benefit from adjunctive intraoperative fibrinolytics. In the event that thromboembolectomy does not restore arterial flow, bypass can be performed.381–383 | |
|
| ||
| IIb | C-LD | The usefulness of ultrasound-accelerated catheter-based thrombolysis for patients with ALI with a salvageable limb is unknown.384–386 |
|
| ||
| See Online Data Supplements 47 and 50. | The use of ultrasound-accelerated catheter delivery of thrombolytic agents has been published in case series384 and retrospective analyses.385 However, the single RCT comparing this technique to standard catheter-based thrombolytic therapy failed to demonstrate a difference in outcomes, including bleeding, despite a lower total amount of lytic delivered.386 | |
|
| ||
10.4. Diagnostic Evaluation of the Cause of ALI: Recommendations
|
| ||
| Recommendations for Diagnostic Evaluation of the Cause of ALI | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | C-EO | In the patient with ALI, a comprehensive history should be obtained to determine the cause of thrombosis and/or embolization. |
|
| ||
| N/A | In addition to identifying a known history of PAD, the history should focus on uncovering clinical evidence of other conditions that can result in ALI through either embolic or thrombotic mechanisms. These conditions include atrial fibrillation, left ventricular thrombus, aortic dissection, trauma, hypercoagulable state, and presence of a limb artery bypass graft. The clinical history should identify the presence or absence of a history of MI, symptoms and signs of left ventricular dysfunction resulting in congestive heart failure, or possible endocarditis. The history should evaluate for possibility of deep vein thrombosis with intracardiac shunt (eg, patent foramen ovale or other that may result in paradoxical arterial embolism), hypercoagulable state, and family history of thrombosis. | |
|
| ||
| IIa | C-EO | In the patient with a history of ALI, testing for a cardiovascular cause of thromboembolism can be useful. |
|
| ||
| N/A | Treatment of ALI should not be delayed for testing for the underlying cause of the limb ischemia. Delay from symptom onset to revascularization is a major determinant of outcome.360,361 The evaluation of a cardiovascular cause of ALI is most useful in the patient without underlying PAD. Evaluation for cardiovascular cause includes electrocardiogram or additional heart rhythm monitoring to detect atrial fibrillation, electrocardiogram to detect evidence of MI, and echocardiography to further determine whether there is a cardiac etiology for thromboembolism, such as valvular vegetation, left atrial or left ventricular thrombus, or intracardiac shunt.387 | |
|
| ||
11. Longitudinal Follow-Up: Recommendations
PAD is a lifelong chronic medical condition. Ongoing care focuses on cardiovascular risk reduction with medical therapy, optimizing functional status with structured exercise and, when indicated, revascularization.
|
| ||
| Recommendations for Longitudinal Follow-Up | ||
|
| ||
| COR | LOE | Recommendations |
|
| ||
| I | C-EO | Patients with PAD should be followed up with periodic clinical evaluation, including assessment of cardiovascular risk factors, limb symptoms, and functional status. |
|
| ||
| N/A | A comprehensive care plan for patients with PAD includes periodic clinical evaluation by a healthcare provider with experience in the care of vascular patients. Clinical evaluation should include assessment of cardiovascular risk factors, assessment of adherence to medical therapy, and re-evaluation of smoking cessation efforts. Comprehensive lifestyle modification, including heart-healthy nutrition, is encouraged.22 Patients with PAD should also undergo periodic assessment of limb symptoms, functional status, and their ability to participate in vocational and recreational activities. Ongoing participation in a structured exercise program should be facilitated. Foot examination and patient counseling about healthy foot behaviors in PAD are addressed in Section 7. | |
|
| ||
| I | C-EO | Patients with PAD who have undergone lower extremity revascularization (surgical and/or endovascular) should be followed up with periodic clinical evaluation and ABI measurement. |
|
| ||
| N/A | In addition to the clinical evaluation of cardiovascular risk factors, functional status, and adherence to medical therapy and smoking cessation, patients with PAD who have previously undergone lower extremity revascularization (surgical and/or endovascular) require additional ongoing assessment and care. Follow-up visits after revascularization should include reassessment of the patient's limb symptoms and interval change in functional status, as well as participation in a structured exercise program. Pulse examination and ABI are included in the assessment. A change in ABI of 0.15 is considered clinically significant.388 | |
|
| ||
| IIa | B-R | Duplex ultrasound can be beneficial for routine surveillance of infrainguinal, autogenous vein bypass grafts in patients with PAD.389–395 |
|
| ||
| See Online Data Supplements 51 and 52. | A general surveillance schedule may be at 4 to 6 weeks, 6 months, and 12 months in the first year and yearly thereafter. It is important that testing frequency is individualized to the patient, type of arterial bypass, and any prior duplex scan findings. Duplex graft surveillance focuses on the identification of high-grade stenosis (eg, peak systolic velocity >300 cm/s and peak systolic velocity ratio across the stenosis >3.5) or impending graft failure (eg, PSV <40 cm/s).392,395 Detection of a graft stenosis prompts the consideration of further revascularization to treat the stenosis and maintain graft patency. Duplex may detect significant stenoses that may not be detected by a decline in ABI.394 Although case series have demonstrated high rates of primary assisted patency with a duplex ultrasound-surveillance strategy, RCTs of duplex surveillance versus clinical surveillance with the ABI have demonstrated mixed results in terms of a benefit on patency and limb outcomes.391,393,396 | |
|
| ||
| IIa | C-LD | Duplex ultrasound is reasonable for routine surveillance after endovascular procedures in patients with PAD.397–399 |
|
| ||
| See Online Data Supplement 52. | Studies have developed duplex ultrasound diagnostic criteria for diagnosing restenosis at the site of endovascular revascularization. Diagnostic criteria need to be customized to the location (eg, iliac or superficial femoral artery) and type of intervention (eg, angioplasty, uncovered stent, or covered stent). The optimal timing for surveillance after endovascular procedures is unclear.397–399 There are limited outcome data on routine duplex surveillance versus clinical surveillance plus the ABI after endovascular revascularization.397–399 The value of duplex ultrasound may be greater in cases with higher rates of restenosis, such as after interventions to treat very long lesions or occlusions.400 | |
|
| ||
| IIb | B-R | The effectiveness of duplex ultrasound for routine surveillance of infrainguinal prosthetic bypass grafts in patients with PAD is uncertain.393,401–403 |
|
| ||
| See Online Data Supplements 51 and 52. | Duplex ultrasound of prosthetic bypass grafts may be used to characterize mid-graft velocity, because low velocities can predict impending graft failure.401–403 Outcome studies of duplex surveillance of prosthetic grafts have not shown consistent benefit.393,401–403 One RCT of duplex versus clinical surveillance with the ABI for femoropopliteal grafts did not show a benefit of duplex on outcome in the subset of patients with prosthetic grafts, though there was a benefit of duplex surveillance for vein bypass grafts.393 | |
|
| ||
12. Evidence Gaps and Future Research Directions
In performing the evidence review and in developing the present guidelines, the writing committee identified the following critical evidence gaps and future directions for PAD-related research:
Basic science and translational studies to better understand the vascular biology of endovascular therapies and bypass grafting and to develop new methods for preventing restenosis after revascularization.
Determination of risk factors for progression from asymptomatic PAD to symptomatic disease, including CLI.
RCTs needed to determine the value of using the ABI to identify asymptomatic patients with PAD for therapies to reduce cardiovascular risk (eg, antiplatelet agents, statins, and other therapies).
Advancement in PAD diagnostics, such as technologies for simplified yet highly accurate measurement of the ABI and tools for more reliable noninvasive perfusion assessment in CLI.
Comparative-effectiveness studies to determine the optimal antiplatelet therapy (drug or drugs and dosage) for prevention of cardiovascular and limb-related events in patients with PAD.
Development of additional medical therapies for claudication–an area of unmet medical need with a currently limited research pipeline.404
Studies to investigate the role of dietary intervention, in addition to statin therapy, to improve outcome and modify the natural history of PAD.
Additional research to identify the best community-or home-based exercise programs for patients with PAD to maximize functional status and improve QoL, as well as the role of such exercise programs before or in addition to revascularization.
Development and validation of improved clinical classification systems for PAD that incorporate symptoms, anatomic factors, and patient-specific risk factors and can be used to predict clinical outcome and optimize treatment approach. An example of a recently developed classification system is the Society for Vascular Surgery limb classification system, based on wound, ischemia, and foot infection (WIfI), which has been validated in different populations and may permit more meaningful prognosis in patients with CLI.405–409
Comparative- and cost-effectiveness studies of the different endovascular technologies for treatment of claudication and CLI, including drug-coated balloons and DES. Studies should include patient-centered end-points, such as functional parameters, time to wound healing, and QoL, in addition to standard patency-focused outcomes. These studies could then be incorporated into value-based clinical algorithms for approach to revascularization for claudication and CLI.
Additional studies to demonstrate the impact of multisocietal registries on clinical outcomes and appropriate use. At present, these include the Vascular Quality Initiative (VQI), the National Cardiovascular Data Registry Peripheral Vascular Intervention Registry™ (PVI Registry™), and the National Radiology Data Registry for Interventional Radiology (NRDR). These registries provide an opportunity to obtain “real-world” data on surgical and endovascular procedures for PAD and to improve quality by providing feedback to participating centers. Future efforts should incorporate these registries into interventional RCTs and postmarketing studies of PAD-related devices.
13. Advocacy Priorities
The writing committee identified 3 priorities for multi-societal advocacy initiatives to improve health care for patients with PAD. First, the writing committee supports the availability of the ABI as the initial diagnostic test to establish the diagnosis of PAD in patients with history or physical examination findings suggestive of PAD (Table 5). Although the ABI test is generally reimbursed by third-party payers for patients with classic claudication or lower extremity wounds, payers may not provide reimbursement for the ABI with other findings suggestive of PAD, such as lower extremity pulse abnormalities or femoral bruits. The writing committee affirms the importance of confirming the diagnosis of PAD in such patients to allow for GDMT as delineated in this document. Second, the writing committee supports the vital importance of insuring access to supervised exercise programs for patients with PAD. Although extensive high-quality evidence supports supervised exercise programs to improve functional status and QoL, only a minority of patients with PAD participate in such programs because of lack of reimbursement by third-party payers. Third, the writing committee recognizes the need for incorporation of patient-centered outcomes into the process of regulatory approval of new medical therapies and revascularization technologies. For revascularization technologies, regulatory approval is driven primarily by data on angiographic efficacy (ie, target lesion patency) and safety endpoints. The nature of the functional limitation associated with PAD warrants the incorporation of patient-centered outcomes, such as functional parameters and QoL, into the efficacy outcomes for the approval process.
Supplementary Material
Evidence Table 1. Nonrandomized Trials, Observational Studies, and/or Registries of History for Clinical Assessment for PAD–Section 2.1.
Evidence Table 2. Nonrandomized Trials, Observational Studies, and/or Registries of Physical Examination for Clinical Assessment for PAD–Section 2.1.
Evidence Table 3. RCTs of Resting ABI for Diagnosing PAD–Section 3.1.
Evidence Table 4. Nonrandomized Trials, Observational Studies, and/or Registries of Resting ABI for Diagnosing PAD–Section 3.1.
Evidence Table 5. Nonrandomized Trials, Observational Studies, and/or Registries of Physiological Testing–Section 3.2.
Evidence Table 6. Nonrandomized Trials, Observational Studies, and/or Registries of Imaging for Anatomic Assessment (Ultrasound, CTA, MRA, Angiography)–Section 3.3.
Evidence Table 7. RCTs of Imaging for Anatomic Assessment (Ultrasound, CTA, MRA, Angiography)–Section 3.3.
Evidence Table 8. Nonrandomized Trials, Observational Studies, and/or Registries for Abdominal Aortic Aneurysm–Section 4.1.
Evidence Table 9. Nonrandomized Trials, Observational Studies, and/or Registries of Coronary Artery Disease Screening in PAD–Section 4.2.
Evidence Table 10. RCTs for CAD Screening in PAD–Section 4.2.
Evidence Table 11. Nonrandomized Trials, Observational Studies, and/or Registries of Screening in Carotid Artery Disease–Section 4.3.
Evidence Table 12. Nonrandomized Trials, Observational Studies, and/or Registries for Renal Artery Disease–Section 4.4.
Evidence Table 13. RCTs Evaluating Antiplatelet Agents– Section 5.1.
Evidence Table 14. Nonrandomized Trials, Observational Studies, and/or Registries of Antiplatelet Agents–Section 5.2.
Evidence Table 15. Randomized Trials Comparing Statin Agents–Section 5.2.
Evidence Table 16. Nonrandomized Trials, Observational Studies, and/or Registries of Statin Agents–Section 5.2.
Evidence Table 17. RCTs for Antihypertensive Agents– Section 5.3.
Evidence Table 18. Nonrandomized Trials, Observational Studies, and/or Registries of Antihypertensive Agents–Section 5.3.
Evidence Table 19. RCTs for Smoking Cessation–Section 5.4.
Evidence Table 20. Nonrandomized Trials, Observational Studies, and/or Registries of Smoking Cessation–Section 5.4.
Evidence Table 21. RCTs Evaluating Glycemic Control in Patients with PAD and Diabetes Mellitus–Section 5.5.
Evidence Table 22. Nonrandomized Trials, Observational Studies, and/or Registries of Glycemic Control–Section 5.5.
Evidence Table 23. RCTs Evaluating Oral Anticoagulation–Section 5.6.
Evidence Table 24. Nonrandomized Trials, Observational Studies, and/or Registries of Oral Anticoagulation–Section 5.6.
Evidence Table 25. RCTs and Observational Studies of Cilostazol–Section 5.7.
Evidence Table 26. Nonrandomized Trials, Observational Studies, and/or Registries of Pentoxifylline–Section 5.8.
Evidence Table 27. Systematic Review of Chelation Therapy–Section 5.9.
Evidence Table 28. Nonrandomized Trials, Observational Studies, and/or Registries of Homocysteine Lowering Therapy for Lower Extremity PAD in Patients with Diabetes Mellitus–Section 5.10.1.
Evidence Table 29. RCTs Comparing Additional Medical Therapies of Homocysteine Lowering Therapy for Lower Extremity PAD–Section 5.10.1.
Evidence Table 30. RCTs for Influenza Vaccination–Section 5.10.2.
Evidence Table 31. Nonrandomized Trials for Influenza Vaccination–Section 5.10.2.
Evidence Table 32. RCTs for Exercise Therapy–Section 6.
Evidence Table 33. Nonrandomized Trials, Observational Studies, and/or Registries for Exercise Therapy–Section 6.
Evidence Table 34. Nonrandomized Trials and Observational Studies of Minimizing Tissue Loss in Patients with PAD–Section 7.
Data Supplement 34a. Functions of a Multidisciplinary Foot Care / Amputation Prevention Team–Section 7.
Evidence Table 35. RCTs Comparing Endovascular Treatment and Endovascular Versus Noninvasive Treatment of Claudication–Section 8.1.
Evidence Table 36. Nonrandomized Trials, Observational Studies, and/or Registries of Endovascular and Endovascular Versus Noninvasive Treatment of Claudication–Section 8.1.
Evidence Table 37. RCTs Evaluating Surgical Treatment for Claudication–Section 8.1.2.
Evidence Table 38. Nonrandomized Trials, Observational Studies, and/or Registries of Surgical Treatment for Claudication–Section 8.1.2.
Evidence Table 39. RCTs Comparing Endovascular Revascularization for Chronic CLI–Section 8.2.
Evidence Table 40. Nonrandomized Trials, Observational Studies, and/or Registries of Endovascular Revascularization for Chromic CLI–Section 8.2.1.
Evidence Table 41. RCTs of Surgical Revascularization for Chronic CLI–Section 8.2.
Evidence Table 42. Nonrandomized Trials, Observational Studies, and/or Registries for Surgical Revascularization for Chronic CLI–Section 8.2.
Evidence Table 43. RCT Comparing Prostanoids for End-Stage Peripheral Artery Disease–Section 8.2.3.
Evidence Table 44. Nonrandomized Trials, Observational Studies, and/or Registries for Would Healing Therapies for CLI–Section 8.2.3.
Evidence Table 45. Nonrandomized Trials, Observational Studies, and/or Registries of Acute Limb Ischemia–Section 9.1.
Evidence Table 46. Nonrandomized Trials, Observational studies, and/or Registries Comparing Evaluating Noninvasive Testing and Angiography for ALI–Section 9.1.
Evidence Table 47. RCTs of Revascularization Strategy for ALI–Section 9.2.2.
Evidence Table 48. Nonrandomized Trials, Observational Studies, and/or Registries of Clinical Presentation of ALI–Section 9.2.2.
Evidence Table 49. Nonrandomized Trials, Observational Studies, and/or Registries of Diagnostic Evaluation of the Cause of ALI–Section 9.2.2.
Evidence Table 50. Nonrandomized Trials, Observational Studies, and/or Registries of Revascularization Strategy for ALI–Section 9.2.2.
Evidence Table 51. RCTs for Longitudinal Follow-Up–Section 10.
Evidence Table 52. Nonrandomized Trials, Observational Studies, and/or Registries for Longitudinal Follow-Up–Section 10.
References.
Table 1. ACC/AHA Recommendation System: Applying Class of Recommendation and Level of Evidence to Clinical Strategies, Interventions, Treatments, or Diagnostic Testing in Patient Care* (Updated August 2015).
| CLASS (STRENGTH) OF RECOMMENDATION | |
|---|---|
| CLASS 1 (STRONG) | Benefit >>> Risk |
Suggested phrases for writing recommendations:
| |
| CLASS IIa (MODERATE) | Benefit >> Risk |
Suggested phrases for writing recommendations;
| |
| CLASS IIb (WEAK) | Benefit ≥ Risk |
Suggested phrases for writing recommendations:
| |
| CLASS III: No Benefit (MODERATE) (Generally, LOE A or B use only) | Benefit = Risk |
Suggested phrases for writing recommendations:
| |
| CLASS III: Harm (STRONG) | Risk > Benefit |
Suggested phrases for writing recommendations:
| |
| LEVEL (QUALITY) OF EVIDENCE‡ | |
| LEVEL A | |
| |
| LEVEL B-R | (Randomized) |
| |
| LEVEL B-NR | (Nonrandomized) |
| |
| LEVEL C-LD | (Limited Data) |
| |
| LEVEL C-EO | (Expert Opinion) |
| |
COR and LOE are determined independently (any COR may be paired with any LOE),
A recommendation with LOE C does not imply that the recommendation is weak. Many important clinical questions addressed in guidelines do not lend themselves to clinical trials. Although RCTs are unavailable, there may be a very dear clinical consensus that a particular test or therapy is useful or effective.
The outcome or result of the intervention should be specified (an improved clinical outcome or increased diagnostic accuracy or incremental prognostic information).
For comparative-effectiveness recommendations (COR I and lla; LOE A and B only), studies that support the use of comparator verbs should involve direct comparisons of the treatments or strategies being evaluated.
The method of assessing quality is evolving, including the application of standardized, widely used, and preferably validated evidence grading tools; and for systematic reviews, the incorporation of an Evidence Review Committee.
COR indicates Class of Recommendation; EO, expert opinion; LD, limited data; LOE, Level of Evidence; NR, nonrandomized; R, randomized; and RCT randomized controlled trial.
Appendix 1. Author Relationships With Industry and Other Entities (Relevant)—2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease (March 2014).
| Committee Member | Employment | Consultant | Speakers Bureau |
Ownership/Partnership /Principal |
Personal Research |
Institutional, Organizational, or Other Financial Benefit |
Expert Witness |
Voting Recusals by Section* |
|---|---|---|---|---|---|---|---|---|
| Marie D. Gerhard-Herman, Chair | Harvard Medical School—Associate Professor | None | None | None | None | None | None | None |
| Heather L. Gornik, Vice Chair | Cleveland Clinic Foundation, Cardiovascular Medicine—Medical Director, Noninvasive Vascular Laboratory | None | None |
|
|
None | None | 3.1, 3.2, 5.1–5.3, and 5.6. |
| Coletta Barrett | Our Lady of the Lake Regional Medical Center—Vice President | None | None | None | None | None | None | None |
| Neal R. Barshes | Baylor College of Medicine, Division of Vascular Surgery and Endovascular Therapy Michael E. DeBakey Department of Surgery—Assistant Professor | None | None | None | None | None | None | None |
| Matthew A. Corriere | University of Michigan—Frankel Professor of Cardiovascular Surgery, Associate Professor of Surgery | None | None | None | None | None | None | None |
| Douglas E. Drachman | Massachusetts General Hospital—Training Director |
|
None | None |
|
None | None | 4, 8.1.1–9.1.2, and 10.2.2. |
| Lee A. Fleisher | University of Pennsylvania Health System Department of Anesthesiology and Critical Care—Chair | None | None | None | None | None | None | None |
| Francis Gerry R. Fowkes | University of Edinburgh—Emeritus Professor of Epidemiology |
|
None | None | None | None | None | 5.1–5.3, 5.6, 5.10, 7, and 9.2. |
| Naomi M. Hamburg | Boston University School of Medicine, Cardiovascular Medicine Section—Associate Professor of Medicine | None | None | None | None | None | None | None |
| Scott Kinlay | VA Boston Healthcare System—Associate Chief, Cardiology Director, Cardiac Catheterization Laboratory & Vascular Medicine | None | None | None | None | None | 4, 5.6, 8.1.1, 9.1.1, 10.2.1, and 10.2.2. | |
| Robert Lookstein | Mount Sinai Medical Center—Chief, Interventional Radiology; Professor of Radiology and Surgery; Vice Chair, Department of Radiology |
|
|
None |
|
None | None | 4, 5.6, 8.1.1, 9.1.1, 10.2.1, and 10.2.2. |
| Sanjay Misra | Mayo Clinic, Division of Vascular and Interventional Radiology—Professor; Department of Radiology— Interventional Radiologist | None | None | None |
|
None | None | 4, 7, 8, and 10.2.2. |
| Leila Mureebe | Duke University Medical Center—Associate Professor of Surgery, Division of Vascular Surgery | None | None | None | None | None | None | None |
| Jeffrey W. Olin | Ichan School of Medicine at Mount Sinai, Zena and Michael A. Wiener Cardiovascular Institute and Marie-Josée and Henry R. Kravis Center for Cardiovascular Health—Professor of Medicine, Cardiology; Director, Vascular Medicine |
|
None |
|
|
None | None | 5.1–5.3, 5.6, 5.10, and 12. |
| Rajan A.G. Patel | John Ochsner Heart & Vascular Center, Ochsner Clinical School, University of Queensland School of Medicine— Senior Lecturer | None | None | None | None | None | None | None |
| Judith G. Regensteiner | University of Colorado, Health Sciences Center, Division of Cardiology—Associate Professor of Medicine | None | None | None | None | None | None | None |
| Andres Schanzer | University of Massachusetts Medical School—Professor of Surgery and Quantitative Health Sciences; Program Director, Vascular Surgery Residency |
|
None | None | None | None | None | 4, 8.1.1, 9.1.1, and 10.2.2. |
| Mehdi H. Shishehbor | Cleveland Clinic, Interventional Cardiology and Vascular Medicine— Director, Endovascular Services | None | None | None |
|
None | 4, 8.1.1– 9.1.2, and 10.2.2. | |
| Kerry J. Stewart | Johns Hopkins University, School of Medicine; Johns Hopkins Bayview Medical Center— Professor of Medicine; Director, Clinical and Research Exercise Physiology | None | None | None | None | None | None | None |
| Diane Treat-Jacobson | University of Minnesota, School of Nursing— Professor | None | None | None | None | None | None | None |
| M. Eileen Walsh | University of Toledo, College of Nursing— Professor | None | None | None | None | None | None | None |
This table represents the relationships of committee members with industry and other entities that were determined to be relevant to this document. These relationships were reviewed and updated in conjunction with all meetings and/or conference calls of the writing committee during the document development process. The table does not necessarily reflect relationships with industry at the time of publication. A person is deemed to have a significant interest in a business if the interest represents ownership of ≥5% of the voting stock or share of the business entity, or ownership of ≥$5000 of the fair market value of the business entity; or if funds received by the person from the business entity exceed 5% of the person's gross income for the previous year. Relationships that exist with no financial benefit are also included for the purpose of transparency. Relationships in this table are modest unless otherwise noted. According to the ACC/AHA, a person has a relevant relationship IF: a) the relationship or interest relates to the same or similar subject matter, intellectual property or asset, topic, or issue addressed in the document; or b) the company/entity (with whom the relationship exists) makes a drug, drug class, or device addressed in the document, or makes a competing drug or device addressed in the document; or c) the person or a member of the person's household, has a reasonable potential for financial, professional, or other personal gain or loss as a result of the issues/content addressed in the document.
Writing committee members are required to recuse themselves from voting on sections to which their specific relationships with industry and other entities may apply.
Significant relationship.
No financial benefit.
ACC indicates American College of Cardiology; AHA, American Heart Association; DSMB, data safety monitoring board; and VA, Veterans Affairs.
Appendix 3. Abbreviations.
| AAA = abdominal aortic aneurysm |
| ABI = ankle-brachial index |
| ALI = acute limb ischemia |
| CAD = coronary artery disease |
| CLI = critical limb ischemia |
| CTA = computed tomography angiography |
| DAPT = dual antiplatelet therapy |
| DES = drug-eluting stent(s) |
| GDMT = guideline-directed management and therapy |
| MI = myocardial infarction |
| MRA = magnetic resonance angiography |
| PAD = peripheral artery disease |
| PTA = percutaneous transluminal angioplasty |
| RCT = randomized controlled trial |
| SPP = skin perfusion pressure |
| TBI = toe-brachial index |
| TcPO2 = transcutaneous oxygen pressure |
| QoL = quality of life |
The American Heart Association requests that this document be cited as follows: Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FGR, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RAG, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, Walsh ME. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines.
ACC/AHA Task Force Members
Jonathan L. Halperin, MD, FACC, FAHA, Chair; Glenn N. Levine, MD, FACC, FAHA, Chair-Elect; Sana M. Al-Khatib, MD, MHS,FACC, FAHA; Kim K. Birtcher, PharmD, MS, AACC; Biykem Bozkurt, MD, PhD, FACC, FAHA; Ralph G. Brindis, MD, MPH, MACC; Joaquin E. Cigarroa, MD, FACC; Lesley H. Curtis, PhD, FAHA; Lee A. Fleisher, MD, FACC, FAHA; Federico Gentile, MD, FACC; Samuel Gidding, MD, FAHA; Mark A. Hlatky, MD, FACC; John Ikonomidis, MD, PhD, FAHA; José Joglar, MD, FACC, FAHA; Susan J. Pressler, PhD, RN, FAHA; Duminda N. Wijeysundera, MD, PhD
Presidents and Staff
American College of Cardiology: Richard A. Chazal, MD, FACC, President
Shalom Jacobovitz, Chief Executive Officer
William J. Oetgen, MD, MBA, FACC, Executive Vice President, Science, Education, Quality, and Publishing
Amelia Scholtz, PhD, Publications Manager, Science, Education, Quality, and Publishing
American College of Cardiology/American Heart Association: Katherine Sheehan, PhD, Director, Guideline Strategy and Operations
Lisa Bradfield, CAE, Director, Guideline Methodology and Policy
Abdul R. Abdullah, MD, Associate Science and Medicine Advisor
Allison Rabinowitz, MPH, Project Manager, Clinical Practice Guidelines
American Heart Association: Steven R. Houser, PhD, FAHA, President
Nancy Brown, Chief Executive Officer
Rose Marie Robertson, MD, FAHA, Chief Science and Medicine Officer
Gayle R. Whitman, PhD, RN, FAHA, FAAN, Senior Vice President, Office of Science Operations
Comilla Sasson, MD, PhD, FACEP, Vice President, Science and Medicine
Jody Hundley, Production Manager, Scientific Publications, Office of Science Operations
The Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIR.0000000000000471/-/DC2.
Copies: This document is available on the World Wide Web sites of the American College of Cardiology (www.acc.org) and the American Heart Association (professional.heart.org). A copy of the document is available at http://professional.heart. org/statements by selecting either the “By Topic” link or the “By Publication Date” link. To purchase additional reprints, call 843-216-2533 or kelle.ramsay@wolterskluwer.com.
Expert peer review of AHA Scientific Statements is conducted by the AHA Office of Science Operations. For more on AHA statements and guidelines development, visit http://professional.heart.org/statements and select the “Policies and Development” link.
References
- 1.Committee on Standards for Developing Trustworthy Clinical Practice Guidelines, Institute of Medicine. Clinical Practice Guidelines We Can Trust. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 2.Committee on Standards for Systematic Reviews of Comparative Effectiveness Research, Institute of Medicine. Finding What Works in Health Care: Standards for Systematic Reviews. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 3.ACCF/AHA Task Force on Practice Guidelines. American College of Cardiology and American Heart Association; 2010. [Accessed January 23, 2015]. Methodology Manual and Policies From the ACCF/AHA Task Force on Practice Guidelines. http://assets.cardiosource.com/Methodology_Manual_for_ACC_AHA_Writing_Committees.pdf and http://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/documents/downloadable/ucm_319826.pdf. [Google Scholar]
- 4.Halperin JL, Levine GN, al-Khatib S, et al. Further evolution of the ACC/AHA clinical practice guideline recommendation classification system: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2016;133:1426–28. doi: 10.1161/CIR.0000000000000312. [DOI] [PubMed] [Google Scholar]
- 5.Jacobs AK, Kushner FG, Ettinger SM, et al. ACCF/AHA clinical practice guideline methodology summit report: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:268–310. doi: 10.1161/CIR.0b013e31827e8e5f. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs AK, Anderson JL, Halperin JL. The evolution and future of ACC/AHA clinical practice guidelines: a 30-year journey: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:1208–17. doi: 10.1161/CIR.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 7.Anderson JL, Heidenreich PA, Barnett PG, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. Circulation. 2014;129:2329–45. doi: 10.1161/CIR.0000000000000042. [DOI] [PubMed] [Google Scholar]
- 8.Arnett DK, Goodman RA, Halperin JL, et al. AHA/ACC/HHS strategies to enhance application of clinical practice guidelines in patients with cardiovascular disease and comorbid conditions: from the American Heart Association, American College of Cardiology, and U.S. Department of Health and Human Services. Circulation. 2014;130:1662–7. doi: 10.1161/CIR.0000000000000128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary: a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) Circulation. 2006;113:e463–654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 10.Rooke TW, Hirsch AT, Misra S, et al. 2011 ACCF/AHA focused update of the guideline for the management of patients with peripheral artery disease (updating the 2005 guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2020–45. doi: 10.1161/CIR.0b013e31822e80c3. [DOI] [PubMed] [Google Scholar]
- 11.Jones WS, Schmit KM, Vemulapalli S, et al. Treatment Strategies for Patients With Peripheral Artery Disease. [Accessed September 25, 2016];Comparative Effectiveness Review No 118 The Duke Evidence-based Practice Center under Contract No 290-2007-10066-I. 2013 Available at: http://www.effectivehealthcare.ahrq.gov/ehc/products/368/1415/Peripheral-Artery-Disease-Treatment-130301.pdf.
- 12.Vemulapalli S, Dolor RJ, Hasselblad V, et al. Comparative effectiveness of medical therapy, supervised exercise, and revascularization for patients with intermittent claudication: a network metaanalysis. Clin Cardiol. 2015;38:378–86. doi: 10.1002/clc.22406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmit K, Dolor RJ, Jones WS, et al. Comparative effectiveness review of antiplatelet agents in peripheral artery disease. J Am Heart Assoc. 2014;3:e001330. doi: 10.1161/JAHA.113.001330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones WS, Dolor RJ, Hasselblad V, et al. Comparative effectiveness of endovascular and surgical revascularization for patients with peripheral artery disease and critical limb ischemia: systematic review of revascularization in critical limb ischemia. Am Heart J. 2014;167:489–98.e7. doi: 10.1016/j.ahj.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 15.Menard MT, Farber A. The BEST-CLI trial: a multidisciplinary effort to assess whether surgical or endovascular therapy is better for patients with critical limb ischemia. Semin Vasc Surg. 2014;27:82–4. doi: 10.1053/j.semvascsurg.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 16.Popplewell MA, Davies H, Jarrett H, et al. Bypass versus angio plasty in severe ischaemia of the leg - 2 (BASIL-2) trial: study protocol for a randomised controlled trial. Trials. 2016;17:11. doi: 10.1186/s13063-015-1114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Menard MT, Farber A, Assmann SF, et al. Design and rationale of the Best Endovascular Versus Best Surgical Therapy for Patients With Critical Limb Ischemia (BEST-CLI) Trial. J Am Heart Assoc. 2016;5:e003219. doi: 10.1161/JAHA.116.003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133:e38–360. doi: 10.1161/CIR.0000000000000350. published correction appears in Circulation. 2016;133:e599. [DOI] [PubMed] [Google Scholar]
- 19.Flowkes FG, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382:1329–40. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 20.Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2016;134:e123–55. doi: 10.1161/CIR.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 21.Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:e278–333. doi: 10.1161/CIR.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 22.Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S76–99. doi: 10.1161/01.cir.0000437740.48606.d1. [DOI] [PubMed] [Google Scholar]
- 23.Goff DC, Lloyd-Jones D, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;63(suppl 2):S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 24.Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 25.Smith SC, Benjamin EJ, Bonow RO, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–73. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 26.Conte MS, Pomposelli FB, Clair DG, et al. Society for Vascular Surgery practice guidelines for atherosclerotic occlusive disease of the lower extremities: management of asymptomatic disease and claudication. J Vasc Surg. 2015;61:2S–41S. doi: 10.1016/j.jvs.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Aboyans V, Criqui MH, Abraham P, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126:2890–909. doi: 10.1161/CIR.0b013e318276fbcb. [DOI] [PubMed] [Google Scholar]
- 28.Lentine KL, Costa SP, Weir MR, et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation. 2012;126:617–63. doi: 10.1161/CIR.0b013e31823eb07a. [DOI] [PubMed] [Google Scholar]
- 29.Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA Diabetes Trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119:351–57. doi: 10.1161/CIRCULATIONAHA.108.191305. [DOI] [PubMed] [Google Scholar]
- 30.Davis MM, Taubert K, Benin AL, et al. Influenza vaccination as secondary prevention for cardiovascular disease: a science advisory from the American Heart Association/American College of Cardiology. Circulation. 2006;114:1549–53. doi: 10.1161/CIRCULATIONAHA.106.178242. [DOI] [PubMed] [Google Scholar]
- 31.White CJ, Jaff MR, Haskal ZJ, et al. Indications for renal arteriography at the time of coronary arteriography: a science advisory from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology, and the Councils on Cardiovascular Radiology and Intervention and on Kidney in Cardiovascular Disease. Circulation. 2006;114:1892–5. doi: 10.1161/CIRCULATIONAHA.106.178777. [DOI] [PubMed] [Google Scholar]
- 32.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–52. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 33.Creager MA, Belkin M, Bluth EI, et al. 2012 ACCF/AHA/ACR/SCAI/SIR/STS/SVM/SVN/SVS key data elements and definitions for peripheral atherosclerotic vascular disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Clinical Data Standards for Peripheral Atherosclerotic Vascular Disease) Circulation. 2012;125:395–467. doi: 10.1161/CIR.0b013e31823299a1. [DOI] [PubMed] [Google Scholar]
- 34.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg. 1997;26:517–38. doi: 10.1016/s0741-5214(97)70045-4. [DOI] [PubMed] [Google Scholar]
- 35.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-society consensus for the management of peripheral arterial disease (TASC II) Eur J Vasc Endovasc Surg. 2007;33(1):S1–75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 36.Fakhry F, Rouwet EV, Tden Hoed P, et al. Long-term clinical effectiveness of supervised exercise therapy versus endovascular revascularization for intermittent claudication from a randomized clinical trial. Br J Surg. 2013;100:1164–71. doi: 10.1002/bjs.9207. [DOI] [PubMed] [Google Scholar]
- 37.Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise versus primary stenting for claudication resulting from aortoiliac peripheral artery disease: six-month outcomes from the claudication: exercise versus endoluminal revascularization (CLEVER) study. Circulation. 2012;125:130–9. doi: 10.1161/CIRCULATIONAHA.111.075770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy TP, Cutlip DE, Regensteiner JG, et al. Supervised exercise, stent revascularization, or medical therapy for claudication due to aortoiliac peripheral artery disease: the CLEVER study. J Am Coll Cardiol. 2015;65:999–1009. doi: 10.1016/j.jacc.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Treat-Jacobson D, Bronas UG, Leon AS. Efficacy of arm-ergometry versus treadmill exercise training to improve walking distance in patients with claudication. Vasc Med. 2009;14:203–13. doi: 10.1177/1358863X08101858. [DOI] [PubMed] [Google Scholar]
- 40.Hiatt WR, Regensteiner JG, Hargarten ME, et al. Benefit of exercise conditioning for patients with peripheral arterial disease. Circulation. 1990;81:602–9. doi: 10.1161/01.cir.81.2.602. [DOI] [PubMed] [Google Scholar]
- 41.Parmenter BJ, Dieberg G, Smart NA. Exercise training for management of peripheral arterial disease: a systematic review and meta-analysis. Sports Med. 2015;45:231–44. doi: 10.1007/s40279-014-0261-z. [DOI] [PubMed] [Google Scholar]
- 42.Parmenter BJ, Dieberg G, Phipps G, et al. Exercise training for health-related quality of life in peripheral artery disease: a systematic review and meta-analysis. Vasc Med. 2015;20:30–40. doi: 10.1177/1358863X14559092. [DOI] [PubMed] [Google Scholar]
- 43.Pilz M, Kandioler-Honetz E, Wenkstetten-Holub A, et al. Evaluation of 6- and 12-month supervised exercise training on strength and endurance parameters in patients with peripheral arterial disease. Wien Klin Wochenschr. 2014;126:383–9. doi: 10.1007/s00508-014-0548-y. [DOI] [PubMed] [Google Scholar]
- 44.Regensteiner JG, Steiner JF, Hiatt WR. Exercise training improves functional status in patients with peripheral arterial disease. J Vasc Surg. 1996;23:104–15. doi: 10.1016/s0741-5214(05)80040-0. [DOI] [PubMed] [Google Scholar]
- 45.Regensteiner JG. Exercise in the treatment of claudication: assessment and treatment of functional impairment. Vasc Med. 1997;2:238–42. doi: 10.1177/1358863X9700200313. [DOI] [PubMed] [Google Scholar]
- 46.Stewart KJ, Hiatt WR, Regensteiner JG, et al. Exercise training for claudication. N Engl J Med. 2002;347:1941–51. doi: 10.1056/NEJMra021135. [DOI] [PubMed] [Google Scholar]
- 47.Collins TC, Lunos S, Carlson T, et al. Effects of a home-based walking intervention on mobility and quality of life in people with diabetes and peripheral arterial disease: a randomized controlled trial. Diabetes Care. 2011;34:2174–9. doi: 10.2337/dc10-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fakhry F, Spronk S, de Ridder M, et al. Long-term effects of structured home-based exercise program on functional capacity and quality of life in patients with intermittent claudication. Arch Phys Med Rehabil. 2011;92:1066–73. doi: 10.1016/j.apmr.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Gardner AW, Parker DE, Montgomery PS, et al. Step-monitored home exercise improves ambulation, vascular function, and inflammation in symptomatic patients with peripheral artery disease: a randomized controlled trial. J Am Heart Assoc. 2014;3:e001107. doi: 10.1161/JAHA.114.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mays RJ, Rogers RK, Hiatt WR, et al. Community walking programs for treatment of peripheral artery disease. J Vasc Surg. 2013;58:1678–87. doi: 10.1016/j.jvs.2013.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McDermott MM, Domanchuk K, Liu K, et al. The Group Oriented Arterial Leg Study (GOALS) to improve walking performance in patients with peripheral arterial disease. Contemp Clin Trials. 2012;33:1311–20. doi: 10.1016/j.cct.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ. 1962;27:645–58. [PMC free article] [PubMed] [Google Scholar]
- 53.McDermott MM, Mehta S, Greenland P. Exertional leg symptoms other than intermittent claudication are common in peripheral arterial disease. Arch Intern Med. 1999;159:387–92. doi: 10.1001/archinte.159.4.387. [DOI] [PubMed] [Google Scholar]
- 54.McDermott MM, Greenland P, Liu K, et al. Leg symptoms in peripheral arterial disease: associated clinical characteristics and functional impairment. JAMA. 2001;286:1599–606. doi: 10.1001/jama.286.13.1599. [DOI] [PubMed] [Google Scholar]
- 55.Hirsch AT, Criqui MH, Treat-Jacobson D, et al. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–24. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 56.Khan NA, Rahim SA, Anand SS, et al. Does the clinical examination predict lower extremity peripheral arterial disease? JAMA. 2006;295:536–46. doi: 10.1001/jama.295.5.536. [DOI] [PubMed] [Google Scholar]
- 57.Criqui MH, Denenberg JO, Bird CE, et al. The correlation between symptoms and non-invasive test results in patients referred for peripheral arterial disease testing. Vasc Med. 1996;1:65–71. doi: 10.1177/1358863X9600100112. [DOI] [PubMed] [Google Scholar]
- 58.Armstrong DWJ, Tobin C, Matangi MF. The accuracy of the physical examination for the detection of lower extremity peripheral arterial disease. Can J Cardiol. 2010;26:e346–50. doi: 10.1016/s0828-282x(10)70467-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cournot M, Boccalon H, Cambou JP, et al. Accuracy of the screening physical examination to identify subclinical atherosclerosis and peripheral arterial disease in asymptomatic subjects. J Vasc Surg. 2007;46:1215–21. doi: 10.1016/j.jvs.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 60.Clark CE, Taylor RS, Shore AC, et al. Association of a difference in systolic blood pressure between arms with vascular disease and mortality: a systematic review and meta-analysis. Lancet. 2012;379:905–14. doi: 10.1016/S0140-6736(11)61710-8. [DOI] [PubMed] [Google Scholar]
- 61.Singh S, Sethi A, Singh M, et al. Simultaneously measured inter-arm and inter-leg systolic blood pressure differences and cardiovascular risk stratification: a systemic review and meta-analysis. J Am Soc Hypertens. 2015;9:640–650. doi: 10.1016/j.jash.2015.05.013. e12. [DOI] [PubMed] [Google Scholar]
- 62.Shadman R, Criqui MH, Bundens WP, et al. Subclavian artery stenosis: prevalence, risk factors, and association with cardiovascular diseases. J Am Coll Cardiol. 2004;44:618–23. doi: 10.1016/j.jacc.2004.04.044. [DOI] [PubMed] [Google Scholar]
- 63.Wassel CL, Loomba R, Ix JH, et al. Family history of peripheral artery disease is associated with prevalence and severity of peripheral artery disease: the San Diego Population Study. J Am Coll Cardiol. 2011;58:1386–92. doi: 10.1016/j.jacc.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schröder F, Diehm N, Kareem S, et al. A modified calculation of ankle-brachial pressure index is far more sensitive in the detection of peripheral arterial disease. J Vasc Surg. 2006;44:531–6. doi: 10.1016/j.jvs.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 65.Premalatha G, Ravikumar R, Sanjay R, et al. Comparison of colour duplex ultrasound and ankle-brachial pressure index measurements in peripheral vascular disease in type 2 diabetic patients with foot infections. J Assoc Physicians India. 2002;50:1240–4. [PubMed] [Google Scholar]
- 66.Allen J, Oates CP, Henderson J, et al. Comparison of lower limb arterial assessments using color-duplex ultrasound and ankle/brachial pressure index measurements. Angiology. 1996;47:225–32. doi: 10.1177/000331979604700302. [DOI] [PubMed] [Google Scholar]
- 67.Lijmer JG, Hunink MG, van den Dungen JJ, et al. ROC analysis of noninvasive tests for peripheral arterial disease. Ultrasound Med Biol. 1996;22:391–8. doi: 10.1016/0301-5629(96)00036-1. [DOI] [PubMed] [Google Scholar]
- 68.Guo X, Li J, Pang W, et al. Sensitivity and specificity of ankle-brachial index for detecting angiographic stenosis of peripheral arteries. Circ J. 2008;72:605–10. doi: 10.1253/circj.72.605. [DOI] [PubMed] [Google Scholar]
- 69.Niazi K, Khan TH, Easley KA. Diagnostic utility of the two methods of ankle brachial index in the detection of peripheral arterial disease of lower extremities. Catheter Cardiovasc Interv. 2006;68:788–92. doi: 10.1002/ccd.20906. [DOI] [PubMed] [Google Scholar]
- 70.Eslahpazir BA, Allemang MT, Lakin RO, et al. Pulse volume recording does not enhance segmental pressure readings for peripheral arterial disease stratification. Ann Vasc Surg. 2014;28:18–27. doi: 10.1016/j.avsg.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 71.Raines JK, Darling RC, Buth J, et al. Vascular laboratory criteria for the management of peripheral vascular disease of the lower extremities. Surgery. 1976;79:21–9. [PubMed] [Google Scholar]
- 72.Aboyans V, Ho E, Denenberg JO, et al. The association between elevated ankle systolic pressures and peripheral occlusive arterial disease in diabetic and nondiabetic subjects. J Vasc Surg. 2008;48:1197–203. doi: 10.1016/j.jvs.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 73.Ouriel K, McDonnell AE, Metz CE, et al. Critical evaluation of stress testing in the diagnosis of peripheral vascular disease. Surgery. 1982;91:686–93. [PubMed] [Google Scholar]
- 74.Stein R, Hriljac I, Halperin JL, et al. Limitation of the resting ankle-brachial index in symptomatic patients with peripheral arterial disease. Vasc Med. 2006;11:29–33. doi: 10.1191/1358863x06vm663oa. [DOI] [PubMed] [Google Scholar]
- 75.Belch J, MacCuish A, Campbell I, et al. The Prevention of Progression of Arterial Disease and Diabetes (POPADAD) trial: factorial randomised placebo controlled trial of aspirin and antioxidants in patients with diabetes and asymptomatic peripheral arterial disease. BMJ. 2008;337:a1840. doi: 10.1136/bmj.a1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Flowkes FG, Price JF, Stewart MC, et al. Aspirin for prevention of cardiovascular events in a general population screened for a low ankle brachial index: a randomized controlled trial. JAMA. 2010;303:841–8. doi: 10.1001/jama.2010.221. [DOI] [PubMed] [Google Scholar]
- 77.Alahdab F, Wang AT, Elraiyah TA, et al. A systematic review for the screening for peripheral arterial disease in asymptomatic patients. J Vasc Surg. 2015;61:42S–53S. doi: 10.1016/j.jvs.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 78.Lin JS, Olson CM, Johnson ES, et al. The ankle-brachial index for peripheral artery disease screening and cardiovascular disease prediction among asymptomatic adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med. 2013;159:333–41. doi: 10.7326/0003-4819-159-5-201309030-00007. [DOI] [PubMed] [Google Scholar]
- 79.Diehm C, Allenberg JR, Pittrow D, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation. 2009;120:2053–61. doi: 10.1161/CIRCULATIONAHA.109.865600. [DOI] [PubMed] [Google Scholar]
- 80.Flowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300:197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ratanakorn D, Keandoungchun J, Tegeler CH. Prevalence and association between risk factors, stroke subtypes, and abnormal ankle brachial index in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2012;21:498–503. doi: 10.1016/j.jstrokecerebrovasdis.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 82.Sen S, Lynch DR, Kaltsas E, et al. Association of asymptomatic peripheral arterial disease with vascular events in patients with stroke or transient ischemic attack. Stroke. 2009;40:3472–7. doi: 10.1161/STROKEAHA.109.559278. [DOI] [PubMed] [Google Scholar]
- 83.Bundó M, Muñoz L, Pérez C, et al. Asymptomatic peripheral arterial disease in type 2 diabetes patients: a 10-year follow-up study of the utility of the ankle brachial index as a prognostic marker of cardiovascular disease. Ann Vasc Surg. 2010;24:985–93. doi: 10.1016/j.avsg.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 84.Bouisset F, Bongard V, Ruidavets JB, et al. Prognostic usefulness of clinical and subclinical peripheral arterial disease in men with stable coronary heart disease. Am J Cardiol. 2012;110:197–202. doi: 10.1016/j.amjcard.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 85.Hiramoto JS, Katz R, Ix JH, et al. Sex differences in the prevalence and clinical outcomes of subclinical peripheral artery disease in the Health, Aging, and Body Composition (Health ABC) study. Vascular. 2014;22:142–8. doi: 10.1177/1708538113476023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jiménez M, Dorado L, Hernández-Pérez M, et al. Ankle-brachial index in screening for asymptomatic carotid and intracranial atherosclerosis. Atherosclerosis. 2014;233:72–5. doi: 10.1016/j.atherosclerosis.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 87.Tsivgoulis G, Bogiatzi C, Heliopoulos I, et al. Low ankle-brachial index predicts early risk of recurrent stroke in patients with acute cerebral ischemia. Atherosclerosis. 2012;220:407–12. doi: 10.1016/j.atherosclerosis.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 88.McDermott MM, Liu K, Guralnik JM, et al. Home-based walking exercise intervention in peripheral artery disease: a randomized clinical trial. JAMA. 2013;310:57–65. doi: 10.1001/jama.2013.7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.McDermott MM, Fried L, Simonsick E, et al. Asymptomatic peripheral arterial disease is independently associated with impaired lower extremity functioning: the Women's Health and Aging Study. Circulation. 2000;101:1007–12. doi: 10.1161/01.cir.101.9.1007. [DOI] [PubMed] [Google Scholar]
- 90.McDermott MM, Applegate WB, Bonds DE, et al. Ankle brachial index values, leg symptoms, and functional performance among community-dwelling older men and women in the Lifestyle Interventions and Independence for Elders Study. J Am Heart Assoc. 2013;2:e000257. doi: 10.1161/JAHA.113.000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McDermott MM, Liu K, Greenland P, et al. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–61. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- 92.McDermott MM, Ferrucci L, Liu K, et al. Leg symptom categories and rates of mobility decline in peripheral arterial disease. J Am Geriatr Soc. 2010;58:1256–62. doi: 10.1111/j.1532-5415.2010.02941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McDermott MM, Liu K, Ferrucci L, et al. Physical performance in peripheral arterial disease: a slower rate of decline in patients who walk more. Ann Intern Med. 2006;144:10–20. doi: 10.7326/0003-4819-144-1-200601030-00005. [DOI] [PubMed] [Google Scholar]
- 94.McDermott MM, Guralnik JM, Criqui MH, et al. Home-based walking exercise in peripheral artery disease: 12-month follow-up of the GOALS randomized trial. J Am Heart Assoc. 2014;3:e000711. doi: 10.1161/JAHA.113.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999–2000. Circulation. 2004;110:738–43. doi: 10.1161/01.CIR.0000137913.26087.F0. [DOI] [PubMed] [Google Scholar]
- 96.Ramos R, García-Gil M, Comas-Cufí M, et al. Statins for prevention of cardiovascular events in a low-risk population with low ankle brachial index. J Am Coll Cardiol. 2016;67:630–40. doi: 10.1016/j.jacc.2015.11.052. [DOI] [PubMed] [Google Scholar]
- 97.Flowkes FG, Murray GD, Butcher I, et al. Development and validation of an ankle brachial index risk model for the prediction of cardiovascular events. Eur J Prev Cardiol. 2014;21:310–20. doi: 10.1177/2047487313516564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Criqui MH, Vargas V, Denenberg JO, et al. Ethnicity and peripheral arterial disease: the San Diego Population Study. Circulation. 2005;112:2703–7. doi: 10.1161/CIRCULATIONAHA.105.546507. [DOI] [PubMed] [Google Scholar]
- 99.Carter SA, Tate RB. Value of toe pulse waves in addition to systolic pressures in the assessment of the severity of peripheral arterial disease and critical limb ischemia. J Vasc Surg. 1996;24:258–65. doi: 10.1016/s0741-5214(96)70101-5. [DOI] [PubMed] [Google Scholar]
- 100.Ramsey DE, Manke DA, Sumner DS. Toe blood pressure. A valuable adjunct to ankle pressure measurement for assessing peripheral arterial disease. J Cardiovasc Surg (Torino) 1983;24:43–8. [PubMed] [Google Scholar]
- 101.Vincent DG, Salles-Cunha SX, Bernhard VM, et al. Noninvasive assessment of toe systolic pressures with special reference to diabetes mellitus. J Cardiovasc Surg (Torino) 1983;24:22–8. [PubMed] [Google Scholar]
- 102.Carter SA. Clinical measurement of systolic pressures in limbs with arterial occlusive disease. JAMA. 1969;207:1869–74. [PubMed] [Google Scholar]
- 103.Carter SA. Indirect systolic pressures and pulse waves in arterial occlusive diseases of the lower extremities. Circulation. 1968;37:624–37. doi: 10.1161/01.cir.37.4.624. [DOI] [PubMed] [Google Scholar]
- 104.Weinberg I, Giri J, Calfon MA, et al. Anatomic correlates of supra-normal ankle brachial indices. Catheter Cardiovasc Interv. 2013;81:1025–30. doi: 10.1002/ccd.24604. [DOI] [PubMed] [Google Scholar]
- 105.Park SC, Choi CY, Ha YI, et al. Utility of toe-brachial index for diagnosis of peripheral artery disease. Arch Plast Surg. 2012;39:227–31. doi: 10.5999/aps.2012.39.3.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Covic A, Kanbay M, Voroneanu L, et al. Vascular calcification in chronic kidney disease. Clin Sci. 2010;119:111–21. doi: 10.1042/CS20090631. [DOI] [PubMed] [Google Scholar]
- 107.Mahe G, Pollak AW, Liedl DA, et al. Discordant diagnosis of lower extremity peripheral artery disease using American Heart Association postexercise guidelines. Medicine (Baltimore) 2015;94:e1277. doi: 10.1097/MD.0000000000001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nicolaï SP, Viechtbauer W, Kruidenier LM, et al. Reliability of treadmill testing in peripheral arterial disease: a meta-regression analysis. J Vasc Surg. 2009;50:322–9. doi: 10.1016/j.jvs.2009.01.042. [DOI] [PubMed] [Google Scholar]
- 109.Laing SP, Greenhalgh RM. Standard exercise test to assess peripheral arterial disease. Br Med J. 1980;280:13–6. doi: 10.1136/bmj.280.6206.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sumner DS, Strandness DE. The relationship between calf blood flow and ankle blood pressure in patients with intermittent claudication. Surgery. 1969;65:763–71. [PubMed] [Google Scholar]
- 111.McPhail IR, Spittell PC, Weston SA, et al. Intermittent claudication: an objective office-based assessment. J Am Coll Cardiol. 2001;37:1381–5. doi: 10.1016/s0735-1097(01)01120-2. [DOI] [PubMed] [Google Scholar]
- 112.Biotteau E, Mahe G, Rousseau P, et al. Transcutaneous oxygen pressure measurements in diabetic and non-diabetic patients clinically suspected of severe limb ischemia: a matched paired retrospective analysis. Int Angiol. 2009;28:479–83. [PubMed] [Google Scholar]
- 113.Yamada T, Ohta T, Ishibashi H, et al. Clinical reliability and utility of skin perfusion pressure measurement in ischemic limbs—comparison with other noninvasive diagnostic methods. J Vasc Surg. 2008;47:318–23. doi: 10.1016/j.jvs.2007.10.045. [DOI] [PubMed] [Google Scholar]
- 114.Castronuovo JJ, Adera HM, Smiell JM, et al. Skin perfusion pressure measurement is valuable in the diagnosis of critical limb ischemia. J Vasc Surg. 1997;26:629–37. doi: 10.1016/s0741-5214(97)70062-4. [DOI] [PubMed] [Google Scholar]
- 115.Bunte MC, Jacob J, Nudelman B, et al. Validation of the relationship between ankle-brachial and toe-brachial indices and infra-genicular arterial patency in critical limb ischemia. Vasc Med. 2015;20:23–9. doi: 10.1177/1358863X14565372. [DOI] [PubMed] [Google Scholar]
- 116.Shishehbor MH, Hammad TA, Zeller T, et al. An analysis of IN.PACT DEEP randomized trial on the limitations of the societal guidelines-recommended hemodynamic parameters to diagnose critical limb ischemia. J Vasc Surg. 2016;63:1311–7. doi: 10.1016/j.jvs.2015.11.042. [DOI] [PubMed] [Google Scholar]
- 117.Fife CE, Smart DR, Sheffield PJ, et al. Transcutaneous oximetry in clinical practice: consensus statements from an expert panel based on evidence. Undersea Hyperb Med. 2009;36:43–53. [PubMed] [Google Scholar]
- 118.Burbelko M, Augsten M, Kalinowski MO, et al. Comparison of contrast-enhanced multi-station MR angiography and digital subtraction angiography of the lower extremity arterial disease. J Magn Reson Imaging. 2013;37:1427–35. doi: 10.1002/jmri.23944. [DOI] [PubMed] [Google Scholar]
- 119.Söderstrom M, Albäck A, Biancari F, et al. Angiosome-targeted infrapopliteal endovascular revascularization for treatment of diabetic foot ulcers. J Vasc Surg. 2013;57:427–35. doi: 10.1016/j.jvs.2012.07.057. [DOI] [PubMed] [Google Scholar]
- 120.Shareghi S, Gopal A, Gul K, et al. Diagnostic accuracy of 64 multidetector computed tomographic angiography in peripheral vascular disease. Catheter Cardiovasc Interv. 2010;75:23–31. doi: 10.1002/ccd.22228. [DOI] [PubMed] [Google Scholar]
- 121.Ota H, Takase K, Igarashi K, et al. MDCT compared with digital subtraction angiography for assessment of lower extremity arterial occlusive disease: importance of reviewing cross-sectional images. AJR Am J Roentgenol. 2004;182:201–9. doi: 10.2214/ajr.182.1.1820201. [DOI] [PubMed] [Google Scholar]
- 122.de Vries SO, Hunink MG, Polak JF. Summary receiver operating characteristic curves as a technique for meta-analysis of the diagnostic performance of duplex ultrasonography in peripheral arterial disease. Acad Radiol. 1996;3:361–9. doi: 10.1016/s1076-6332(96)80257-1. [DOI] [PubMed] [Google Scholar]
- 123.Andreucci M, Solomon R, Tasanarong A. Side effects of radiographic contrast media: pathogenesis, risk factors, and prevention. Biomed Res Int. 2014;2014:741018. doi: 10.1155/2014/741018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stacul F, van der Molen AJ, Reimer P, et al. Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011;21:2527–41. doi: 10.1007/s00330-011-2225-0. [DOI] [PubMed] [Google Scholar]
- 125.Zhang B, Liang L, Chen W, et al. An updated study to determine association between gadolinium-based contrast agents and nephrogenic systemic fibrosis. PLoS ONE. 2015;10:e0129720. doi: 10.1371/journal.pone.0129720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McCullough PA, Capasso P. Patient discomfort associated with the use of intra-arterial iodinated contrast media: a meta-analysis of comparative randomized controlled trials. BMC Med Imaging. 2011;11:12. doi: 10.1186/1471-2342-11-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Barba A, Estallo L, Rodríguez L, et al. Detection of abdominal aortic aneurysm in patients with peripheral artery disease. Eur J Vasc Endovasc Surg. 2005;30:504–8. doi: 10.1016/j.ejvs.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 128.Kurvers HA, van der Graaf Y, Blankensteijn JD, et al. Screening for asymptomatic internal carotid artery stenosis and aneurysm of the abdominal aorta: comparing the yield between patients with manifest atherosclerosis and patients with risk factors for atherosclerosis only. J Vasc Surg. 2003;37:1226–33. doi: 10.1016/s0741-5214(02)75140-9. [DOI] [PubMed] [Google Scholar]
- 129.Giugliano G, Laurenzano E, Rengo C, et al. Abdominal aortic aneurysm in patients affected by intermittent claudication: prevalence and clinical predictors. BMC Surg. 2012;12(1):S17. doi: 10.1186/1471-2482-12-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lee JY, Lee SW, Lee WS, et al. Prevalence and clinical implications of newly revealed, asymptomatic abnormal ankle-brachial index in patients with significant coronary artery disease. JACC Cardiovasc Interv. 2013;6:1303–13. doi: 10.1016/j.jcin.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 131.McFalls EO, Ward HB, Moritz TE, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795–804. doi: 10.1056/NEJMoa041905. [DOI] [PubMed] [Google Scholar]
- 132.Sultan S, Chua BY, Hamada N, et al. Preoperative vascular screening in the presence of aortic, carotid and peripheral pathology for patients undergoing their first arterial intervention: 18 month follow-up. Int Angiol. 2013;32:281–90. [PubMed] [Google Scholar]
- 133.Hansen KJ, Edwards MS, Craven TE, et al. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg. 2002;36:443–51. doi: 10.1067/mva.2002.127351. [DOI] [PubMed] [Google Scholar]
- 134.Leertouwer TC, Pattynama PM, van den Berg-Huysmans A. Incidental renal artery stenosis in peripheral vascular disease: a case for treatment? Kidney Int. 2001;59:1480–3. doi: 10.1046/j.1523-1755.2001.0590041480.x. [DOI] [PubMed] [Google Scholar]
- 135.Olin JW, Melia M, Young JR, et al. Prevalence of atherosclerotic renal artery stenosis in patients with atherosclerosis elsewhere. Am J Med. 1990;88:46N–51N. [PubMed] [Google Scholar]
- 136.Krishnamurthy V, Munir K, Rectenwald JE, et al. Contemporary outcomes with percutaneous vascular interventions for peripheral critical limb ischemia in those with and without poly-vascular disease. Vasc Med. 2014;19:491–9. doi: 10.1177/1358863X14552013. [DOI] [PubMed] [Google Scholar]
- 137.Selvin E, Hirsch AT. Contemporary risk factor control and walking dysfunction in individuals with peripheral arterial disease: NHANES 1999–2004. Atherosclerosis. 2008;201:425–33. doi: 10.1016/j.atherosclerosis.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pande RL, Perlstein TS, Beckman JA, et al. Secondary prevention and mortality in peripheral artery disease: National Health and Nutrition Examination Study, 1999 to 2004. Circulation. 2011;124:17–23. doi: 10.1161/CIRCULATIONAHA.110.003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Catalano M, Born G, Peto R. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med. 2007;261:276–84. doi: 10.1111/j.1365-2796.2006.01763.x. [DOI] [PubMed] [Google Scholar]
- 141.Berger JS, Krantz MJ, Kittelson JM, et al. Aspirin for the prevention of cardiovascular events in patients with peripheral artery disease: a meta-analysis of randomized trials. JAMA. 2009;301:1909–19. doi: 10.1001/jama.2009.623. [DOI] [PubMed] [Google Scholar]
- 142.CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348:1329–39. doi: 10.1016/s0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 143.Cacoub PP, Bhatt DL, Steg PG, et al. Patients with peripheral arterial disease in the CHARISMA trial. Eur Heart J. 2009;30:192–201. doi: 10.1093/eurheartj/ehn534. [DOI] [PubMed] [Google Scholar]
- 144.Bhatt DL, Flather MD, Hacke W, et al. Patients with prior myocardial infarction, stroke, or symptomatic peripheral arterial disease in the CHARISMA trial. J Am Coll Cardiol. 2007;49:1982–8. doi: 10.1016/j.jacc.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 145.Tepe G, Bantleon R, Brechtel K, et al. Management of peripheral arterial interventions with mono or dual antiplatelet therapy—the MIRROR study: a randomised and double-blinded clinical trial. Eur Radiol. 2012;22:1998–2006. doi: 10.1007/s00330-012-2441-2. [DOI] [PubMed] [Google Scholar]
- 146.Armstrong EJ, Anderson DR, Yeo KK, et al. Association of dual-antiplatelet therapy with reduced major adverse cardiovascular events in patients with symptomatic peripheral arterial disease. J Vasc Surg. 2015;62:157–65. doi: 10.1016/j.jvs.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 147.Strobl FF, Brechtel K, Schmehl J, et al. Twelve-month results of a randomized trial comparing mono with dual antiplatelet therapy in endovascularly treated patients with peripheral artery disease. J Endovasc Ther. 2013;20:699–706. doi: 10.1583/13-4275MR.1. [DOI] [PubMed] [Google Scholar]
- 148.Belch JJ, Dormandy J, et al. CASPAR Writing Committee. Results of the randomized, placebo-controlled Clopidogrel and Acetylsalicylic Acid in Bypass Surgery for Peripheral Arterial Disease (CASPAR) trial. J Vasc Surg. 2010;52:825–33. doi: 10.1016/j.jvs.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 149.Bonaca MP, Scirica BM, Creager MA, et al. Vorapaxar in patients with peripheral artery disease: results from TRA2oP-TIMI 50. Circulation. 2013;127:1522–9. doi: 10.1161/CIRCULATIONAHA.112.000679. [DOI] [PubMed] [Google Scholar]
- 150.Morrow DA, Braunwald E, Bonaca MP, et al. Vorapaxar in the secondary prevention of atherothrombotic events. N Engl J Med. 2012;366:1404–13. doi: 10.1056/NEJMoa1200933. [DOI] [PubMed] [Google Scholar]
- 151.Bohula EA, Aylward PE, Bonaca MP, et al. Efficacy and safety of vorapaxar with and without a thienopyridine for secondary prevention in patients with previous myocardial infarction and no history of stroke or transient ischemic attack: results from TRA 2oP-TIMI 50. Circulation. 2015;132:1871–9. doi: 10.1161/CIRCULATIONAHA.114.015042. [DOI] [PubMed] [Google Scholar]
- 152.Bonaca MP, Gutierrez JA, Creager MA, et al. Acute limb ischemia and outcomes with vorapaxar in patients with peripheral artery disease: results from the Trial to Assess the Effects of Vorapaxar in Preventing Heart Attack and Stroke in patients With Atherosclerosis-Thrombolysis in Myocardial Infarction 50 (TRA2oP-TIMI 50) Circulation. 2016;133:997–1005. doi: 10.1161/CIRCULATIONAHA.115.019355. [DOI] [PubMed] [Google Scholar]
- 153.Kumbhani DJ, Steg PG, Cannon CP, et al. Statin therapy and long-term adverse limb outcomes in patients with peripheral artery disease: insights from the REACH registry. Eur Heart J. 2014;35:2864–72. doi: 10.1093/eurheartj/ehu080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Vogel TR, Dombrovskiy VY, Galiñanes EL, et al. Preoperative statins and limb salvage after lower extremity revascularization in the Medicare population. Circ Cardiovasc Interv. 2013;6:694–700. doi: 10.1161/CIRCINTERVENTIONS.113.000274. [DOI] [PubMed] [Google Scholar]
- 155.Heart Protection Study Collaborative Group. Randomized trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes in 20,536 people with peripheral arterial disease and other high-risk conditions. J Vasc Surg. 2007;45:645–54. doi: 10.1016/j.jvs.2006.12.054. [DOI] [PubMed] [Google Scholar]
- 156.Mohler ER, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108:1481–6. doi: 10.1161/01.CIR.0000090686.57897.F5. [DOI] [PubMed] [Google Scholar]
- 157.Aung PP, Maxwell HG, Jepson RG, et al. Lipid-lowering for peripheral arterial disease of the lower limb. Cochrane Database Syst Rev. 2007:CD000123. doi: 10.1002/14651858.CD000123.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bavry AA, Anderson RD, Gong Y, et al. Outcomes Among hypertensive patients with concomitant peripheral and coronary artery disease: findings from the INternational VErapamil-SR/Trandolapril STudy. Hypertension. 2010;55:48–53. doi: 10.1161/HYPERTENSIONAHA.109.142240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Feringa HH, van Waning VH, Bax JJ, et al. Cardioprotective medication is associated with improved survival in patients with peripheral arterial disease. J Am Coll Cardiol. 2006;47:1182–7. doi: 10.1016/j.jacc.2005.09.074. [DOI] [PubMed] [Google Scholar]
- 160.Sleight P. The HOPE Study (Heart Outcomes Prevention Evaluation) J Renin Angiotensin Aldosterone Syst. 2000;1:18–20. doi: 10.3317/jraas.2000.002. [DOI] [PubMed] [Google Scholar]
- 161.Yusuf S, Teo KK, Pogue J, et al. Telmisartan, ramipril, or both in patients at high risk for vascular events. N Engl J Med. 2008;358:1547–59. doi: 10.1056/NEJMoa0801317. [DOI] [PubMed] [Google Scholar]
- 162.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research GroupThe Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 163.Diehm C, Pittrow D, Lawall H. Effect of nebivolol vs. hydrochlorothiazide on the walking capacity in hypertensive patients with intermittent claudication. J Hypertens. 2011;29:1448–56. doi: 10.1097/HJH.0b013e3283471151. [DOI] [PubMed] [Google Scholar]
- 164.Paravastu SC, Mendonca DA, Da Silva A. Beta blockers for peripheral arterial disease. Cochrane Database Syst Rev. 2013:CD005508. doi: 10.1002/14651858.CD005508.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Espinola-Klein C, Weisser G, Jagodzinski A, et al. β-Blockers in patients with intermittent claudication and arterial hypertension: results from the nebivolol or metoprolol in arterial occlusive disease trial. Hypertension. 2011;58:148–54. doi: 10.1161/HYPERTENSIONAHA.110.169169. [DOI] [PubMed] [Google Scholar]
- 166.Piller LB, Simpson LM, Baraniuk S, et al. Characteristics and long-term follow-up of participants with peripheral arterial disease during ALLHAT. J Gen Intern Med. 2014;29:1475–83. doi: 10.1007/s11606-014-2947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zanchetti A, Julius S, Kjeldsen S, et al. Outcomes in subgroups of hypertensive patients treated with regimens based on valsartan and amlodipine: an analysis of findings from the VALUE trial. J Hypertens. 2006;24:2163–8. doi: 10.1097/01.hjh.0000249692.96488.46. [DOI] [PubMed] [Google Scholar]
- 168.Ostergren J, Sleight P, Dagenais G, et al. Impact of ramipril in patients with evidence of clinical or subclinical peripheral arterial disease. Eur Heart J. 2004;25:17–24. doi: 10.1016/j.ehj.2003.10.033. [DOI] [PubMed] [Google Scholar]
- 169.Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–53. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 170.Hennrikus D, Joseph AM, Lando HA, et al. Effectiveness of a smoking cessation program for peripheral artery disease patients: a randomized controlled trial. J Am Coll Cardiol. 2010;56:2105–12. doi: 10.1016/j.jacc.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 171.Stead LF, Buitrago D, Preciado N, et al. Physician advice for smoking cessation. Cochrane Database Syst Rev. 2013:CD000165. doi: 10.1002/14651858.CD000165.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hoel AW, Nolan BW, Goodney PP, et al. Variation in smoking cessation after vascular operations. J Vasc Surg. 2013;57:1338–44. doi: 10.1016/j.jvs.2012.10.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Willigendael EM, Teijink JA, Bartelink ML, et al. Influence of smoking on incidence and prevalence of peripheral arterial disease. J Vasc Surg. 2004;40:1158–65. doi: 10.1016/j.jvs.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 174.Duval S, Long KH, Roy SS, et al. The contribution of tobacco use to high health care utilization and medical costs in peripheral artery disease: a state-based cohort analysis. J Am Coll Cardiol. 2015;66:1566–74. doi: 10.1016/j.jacc.2015.06.1349. [DOI] [PubMed] [Google Scholar]
- 175.Bhatnagar A, Whitsel LP, Ribisl KM, et al. Electronic cigarettes: a policy statement from the American Heart Association. Circulation. 2014;130:1418–36. doi: 10.1161/CIR.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Armstrong EJ, Wu J, Singh GD, et al. Smoking cessation is associated with decreased mortality and improved amputation-free survival among patients with symptomatic peripheral artery disease. J Vasc Surg. 2014;60:1565–71. doi: 10.1016/j.jvs.2014.08.064. [DOI] [PubMed] [Google Scholar]
- 177.Clair C, Rigotti NA, Porneala B, et al. Association of smoking cessation and weight change with cardiovascular disease among adults with and without diabetes. JAMA. 2013;309:1014–21. doi: 10.1001/jama.2013.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Selvarajah S, Black JH, 3rd, Malas MB, et al. Preoperative smoking is associated with early graft failure after infrainguinal bypass surgery. J Vasc Surg. 2014;59:1308–14. doi: 10.1016/j.jvs.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 179.Patnode CD, Henderson JT, Thompson JH, et al. Rockville (MD): Agency for Healthcare Research and Quality (US); 2015. [Accessed September 25, 2016]. Behavioral counseling and pharmacotherapy interventions for tobacco cessation in adults, including pregnant women: a review of reviews for the U.S Preventive Services Task Force. Available at: http://www.ncbi.nlm.nih.gov/books/NBK321744/ [PubMed] [Google Scholar]
- 180.Rigotti NA, Pipe AL, Benowitz NL, et al. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121:221–9. doi: 10.1161/CIRCULATIONAHA.109.869008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Rigotti NA, Regan S, Levy DE, et al. Sustained care intervention and postdischarge smoking cessation among hospitalized adults: a randomized clinical trial. JAMA. 2014;312:719–28. doi: 10.1001/jama.2014.9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tonstad S, Farsang C, Klaene G, et al. Bupropion SR for smoking cessation in smokers with cardiovascular disease: a multicentre, randomised study. Eur Heart J. 2003;24:946–55. doi: 10.1016/s0195-668x(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 183.Mills EJ, Thorlund K, Eapen S, et al. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129:28–41. doi: 10.1161/CIRCULATIONAHA.113.003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856. doi: 10.1136/bmj.e2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tan CE, Glantz SA. Association between smoke-free legislation and hospitalizations for cardiac, cerebrovascular, and respiratory diseases: a meta-analysis. Circulation. 2012;126:2177–83. doi: 10.1161/CIRCULATIONAHA.112.121301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lu L, Mackay DF, Pell JP. Association between level of exposure to secondhand smoke and peripheral arterial disease: cross-sectional study of 5,686 never smokers. Atherosclerosis. 2013;229:273–6. doi: 10.1016/j.atherosclerosis.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 187.Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116:1509–26. doi: 10.1161/CIRCRESAHA.116.303849. [DOI] [PubMed] [Google Scholar]
- 188.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the Strong Heart Study. Circulation. 2004;109:733–9. doi: 10.1161/01.CIR.0000112642.63927.54. [DOI] [PubMed] [Google Scholar]
- 189.Jude EB, Oyibo SO, Chalmers N, et al. Peripheral arterial disease in diabetic and nondiabetic patients: a comparison of severity and outcome. Diabetes Care. 2001;24:1433–7. doi: 10.2337/diacare.24.8.1433. [DOI] [PubMed] [Google Scholar]
- 190.American Diabetes Association. Peripheral arterial disease in people with diabetes. Diabetes Care. 2003;26:3333–41. doi: 10.2337/diacare.26.12.3333. [DOI] [PubMed] [Google Scholar]
- 191.Singh S, Armstrong EJ, Sherif W, et al. Association of elevated fasting glucose with lower patency and increased major adverse limb events among patients with diabetes undergoing infrapopli-teal balloon angioplasty. Vasc Med. 2014;19:307–14. doi: 10.1177/1358863X14538330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Takahara M, Kaneto H, Iida O, et al. The influence of glycemic control on the prognosis of Japanese patients undergoing percutaneous transluminal angioplasty for critical limb ischemia. Diabetes Care. 2010;33:2538–42. doi: 10.2337/dc10-0939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sarac TP, Huber TS, Back MR, et al. Warfarin improves the outcome of infrainguinal vein bypass grafting at high risk for failure. J Vasc Surg. 1998;28:446–57. doi: 10.1016/s0741-5214(98)70130-2. [DOI] [PubMed] [Google Scholar]
- 194.Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral Anticoagulants or Aspirin Study): a randomised trial. Lancet. 2000;355:346–51. [PubMed] [Google Scholar]
- 195.Bedenis R, Lethaby A, Maxwell H, et al. Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery. Cochrane Database Syst Rev. 2015:CD000535. doi: 10.1002/14651858.CD000535.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Johnson WC, Williford WO. Department of Veterans Affairs Cooporative Study #362Benefits, morbidity, and mortality associated with long-term administration of oral anticoagulant therapy to patients with peripheral arterial bypass procedures: a prospective randomized study. J Vasc Surg. 2002;35:413–21. doi: 10.1067/mva.2002.121847. [DOI] [PubMed] [Google Scholar]
- 197.Alonso-Coello P, Bellmunt S, McGorrian C, et al. Antithrombotic therapy in peripheral artery disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e669S–90S. doi: 10.1378/chest.11-2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Anand S, Yusuf S, Xie C, et al. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med. 2007;357:217–27. doi: 10.1056/NEJMoa065959. [DOI] [PubMed] [Google Scholar]
- 199.Bedenis R, Stewart M, Cleanthis M, et al. Cilostazol for intermittent claudication. Cochrane Database Syst Rev. 2014:CD003748. doi: 10.1002/14651858.CD003748.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dawson DL, Cutler BS, Hiatt WR, et al. A comparison of cilostazol and pentoxifylline for treating intermittent claudication. Am J Med. 2000;109:523–30. doi: 10.1016/s0002-9343(00)00569-6. [DOI] [PubMed] [Google Scholar]
- 201.Pletal (cilostazol) Rockville, MD: Otsuka America Pharmaceutical, Inc; 1999. Package insert. [Google Scholar]
- 202.Lee C, Nelson PR. Effect of cilostazol prescribed in a pragmatic treatment program for intermittent claudication. Vasc Endovascular Surg. 2014;48:224–9. doi: 10.1177/1538574413518121. [DOI] [PubMed] [Google Scholar]
- 203.Salhiyyah K, Senanayake E, Abdel-Hadi M, et al. Pentoxifylline for intermittent claudication. Cochrane Database Syst Rev. 2012;1:CD005262. doi: 10.1002/14651858.CD005262.pub2. [DOI] [PubMed] [Google Scholar]
- 204.Villarruz MV, Dans A, Tan F. Chelation therapy for atherosclerotic cardiovascular disease. Cochrane Database Syst Rev. 2002:CD002785. doi: 10.1002/14651858.CD002785. [DOI] [PubMed] [Google Scholar]
- 205.Lonn E, Held C, Arnold JM, et al. Rationale, design and baseline characteristics of a large, simple, randomized trial of combined folic acid and vitamins B6 and B12 in high-risk patients: the Heart Outcomes Prevention Evaluation (HOPE)-2 trial. Can J Cardiol. 2006;22:47–53. doi: 10.1016/s0828-282x(06)70238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 207.Khandanpour N, Loke YK, Meyer FJ, et al. Homocysteine and peripheral arterial disease: systematic review and meta-analysis. Eur J Vasc Endovasc Surg. 2009;38:316–22. doi: 10.1016/j.ejvs.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 208.Gurfinkel EP, Leon de la Fuente R, Mendiz O, et al. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) Study. Eur Heart J. 2004;25:25–31. doi: 10.1016/j.ehj.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 209.Ciszewski A, Bilinska ZT, Brydak LB, et al. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. Eur Heart J. 2008;29:1350–8. doi: 10.1093/eurheartj/ehm581. [DOI] [PubMed] [Google Scholar]
- 210.Brenner I, Parry M, Brown CA. Exercise interventions for patients with peripheral arterial disease: a review of the literature. Phys Sportsmed. 2012;40:41–55. doi: 10.3810/psm.2012.05.1964. [DOI] [PubMed] [Google Scholar]
- 211.Lane R, Ellis B, Watson L, et al. Exercise for intermittent claudication. Cochrane Database Syst Rev. 2014:CD000990. doi: 10.1002/14651858.CD000990.pub3. [DOI] [PubMed] [Google Scholar]
- 212.Allen JD, Stabler T, Kenjale AA, et al. Diabetes status differentiates endothelial function and plasma nitrite response to exercise stress in peripheral arterial disease following supervised training. J Diabetes Complicat. 2014;28:219–25. doi: 10.1016/j.jdiacomp.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gardner AW, Parker DE, Montgomery PS, et al. Efficacy of quantified home-based exercise and supervised exercise in patients with intermittent claudication: a randomized controlled trial. Circulation. 2011;123:491–8. doi: 10.1161/CIRCULATIONAHA.110.963066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Guidon M, McGee H. One-year effect of a supervised exercise programme on functional capacity and quality of life in peripheral arterial disease. Disabil Rehabil. 2013;35:397–404. doi: 10.3109/09638288.2012.694963. [DOI] [PubMed] [Google Scholar]
- 215.Saxton JM, Zwierska I, Blagojevic M, et al. Upper- versus lower-limb aerobic exercise training on health-related quality of life in patients with symptomatic peripheral arterial disease. J Vasc Surg. 2011;53:1265–73. doi: 10.1016/j.jvs.2010.10.125. [DOI] [PubMed] [Google Scholar]
- 216.Gommans LN, Fokkenrood HJ, van Dalen HC, et al. Safety of supervised exercise therapy in patients with intermittent claudication. J Vasc Surg. 2015;61:512–8. doi: 10.1016/j.jvs.2014.08.070. [DOI] [PubMed] [Google Scholar]
- 217.Mazari FA, Gulati S, Rahman MN, et al. Early outcomes from a randomized, controlled trial of supervised exercise, angioplasty, and combined therapy in intermittent claudication. Ann Vasc Surg. 2010;24:69–79. doi: 10.1016/j.avsg.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 218.Fakhry F, Spronk S, van der Laan L, et al. Endovascular revascularization and supervised exercise for peripheral artery disease and intermittent claudication: a randomized clinical trial. JAMA. 2015;314:1936–44. doi: 10.1001/jama.2015.14851. [DOI] [PubMed] [Google Scholar]
- 219.Langbein WE, Collins EG, Orebaugh C, et al. Increasing exercise tolerance of persons limited by claudication pain using polestrid-ing. J Vasc Surg. 2002;35:887–93. doi: 10.1067/mva.2002.123756. [DOI] [PubMed] [Google Scholar]
- 220.Walker RD, Nawaz S, Wilkinson CH, et al. Influence of upper- and lower-limb exercise training on cardiovascular function and walking distances in patients with intermittent claudication. J Vasc Surg. 2000;31:662–9. doi: 10.1067/mva.2000.104104. [DOI] [PubMed] [Google Scholar]
- 221.Mika P, Konik A, Januszek R, et al. Comparison of two treadmill training programs on walking ability and endothelial function in intermittent claudication. Int J Cardiol. 2013;168:838–42. doi: 10.1016/j.ijcard.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 222.Dorresteijn JA, Kriegsman DM, Assendelft WJ, et al. Patient education for preventing diabetic foot ulceration. Cochrane Database Syst Rev. 2014:CD001488. doi: 10.1002/14651858.CD001488.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Miller JD, Carter E, Shih J, et al. How to do a 3-minute diabetic foot exam. J Fam Pract. 2014;63:646–56. [PubMed] [Google Scholar]
- 224.Gardner SE, Hillis SL, Frantz RA. Clinical signs of infection in diabetic foot ulcers with high microbial load. Biol Res Nurs. 2009;11:119–28. doi: 10.1177/1099800408326169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lipsky BA, Berendt AR, Cornia PB, et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin Infect Dis. 2012;54:e132–73. doi: 10.1093/cid/cis346. [DOI] [PubMed] [Google Scholar]
- 226.Pickwell K, Siersma V, Kars M, et al. Predictors of lower-extremity amputation in patients with an infected diabetic foot ulcer. Diabetes Care. 2015;38:852–7. doi: 10.2337/dc14-1598. [DOI] [PubMed] [Google Scholar]
- 227.Dinh MT, Abad CL, Safdar N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: meta-analysis. Clin Infect Dis. 2008;47:519–27. doi: 10.1086/590011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Prompers L, Schaper N, Apelqvist J, et al. Prediction of outcome in individuals with diabetic foot ulcers: focus on the differences between individuals with and without peripheral arterial disease. The EURODIALE Study. Diabetologia. 2008;51:747–55. doi: 10.1007/s00125-008-0940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Vartanian SM, Robinson KD, Ofili K, et al. Outcomes of neuroisch-emic wounds treated by a multidisciplinary amputation prevention service. Ann Vasc Surg. 2015;29:534–42. doi: 10.1016/j.avsg.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 230.Clerici G, Faglia E. Saving the limb in diabetic patients with ischemic foot lesions complicated by acute infection. Int J Low Extrem Wounds. 2014;13:273–93. doi: 10.1177/1534734614549416. [DOI] [PubMed] [Google Scholar]
- 231.Lavery LA, Peters EJ, Williams JR, et al. Reevaluating the way we classify the diabetic foot: restructuring the diabetic foot risk classification system of the International Working Group on the Diabetic Foot. Diabetes Care. 2008;31:154–6. doi: 10.2337/dc07-1302. [DOI] [PubMed] [Google Scholar]
- 232.Nordanstig J, Taft C, Hensäter M, et al. Improved quality of life after 1 year with an invasive versus a noninvasive treatment strategy in claudicants: one-year results of the Invasive Revascularization or Not in Intermittent Claudication (IRONIC) Trial. Circulation. 2014;130:939–47. doi: 10.1161/CIRCULATIONAHA.114.009867. [DOI] [PubMed] [Google Scholar]
- 233.Malgor RD, Alahdab F, Elraiyah TA, et al. A systematic review of treatment of intermittent claudication in the lower extremities. J Vasc Surg. 2015;61:54S–73S. doi: 10.1016/j.jvs.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 234.Leng GC, Lee AJ, Flowkes FG, et al. Incidence, natural history and cardiovascular events in symptomatic and asymptomatic peripheral arterial disease in the general population. Int J Epidemiol. 1996;25:1172–81. doi: 10.1093/ije/25.6.1172. [DOI] [PubMed] [Google Scholar]
- 235.Dormandy J, Mahir M, Ascady G, et al. Fate of the patient with chronic leg ischaemia. A review article. J Cardiovasc Surg (Torino) 1989;30:50–7. [PubMed] [Google Scholar]
- 236.Jelnes R, Gaardsting O, Hougaard Jensen K, et al. Fate in intermittent claudication: outcome and risk factors. Br Med J (Clin Res Ed) 1986;293:1137–40. doi: 10.1136/bmj.293.6555.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Bloor K. Natural history of arteriosclerosis of the lower extremities: Hunterian lecture delivered at the Royal College of Surgeons of England on 22nd April 1960. Ann R Coll Surg Engl. 1961;28:36–52. [PMC free article] [PubMed] [Google Scholar]
- 238.Tetteroo E, van Engelen AD, Spithoven JH, et al. Stent placement after iliac angioplasty: comparison of hemodynamic and angiographic criteria. Dutch Iliac Stent Trial Study Group. Radiology. 1996;201:155–9. doi: 10.1148/radiology.201.1.8816537. [DOI] [PubMed] [Google Scholar]
- 239.Udoff EJ, Barth KH, Harrington DP, et al. Hemodynamic significance of iliac artery stenosis: pressure measurements during angiography. Radiology. 1979;132:289–93. doi: 10.1148/132.2.289. [DOI] [PubMed] [Google Scholar]
- 240.Spronk S, Bosch JL, den Hoed PT, et al. Intermittent claudication: clinical effectiveness of endovascular revascularization versus supervised hospital-based exercise training—randomized controlled trial. Radiology. 2009;250:586–95. doi: 10.1148/radiol.2501080607. [DOI] [PubMed] [Google Scholar]
- 241.Gelin J, Jivegård L, Taft C, et al. Treatment efficacy of intermittent claudication by surgical intervention, supervised physical exercise training compared to no treatment in unselected randomised patients, I: one year results of functional and physiological improvements. Eur J Vasc Endovasc Surg. 2001;22:107–13. doi: 10.1053/ejvs.2001.1413. [DOI] [PubMed] [Google Scholar]
- 242.Greenhalgh RM, Belch JJ, Brown LC, et al. The adjuvant benefit of angioplasty in patients with mild to moderate intermittent claudication (MIMIC) managed by supervised exercise, smoking cessation advice and best medical therapy: results from two randomised trials for stenotic femoropopliteal and aortoiliac arterial disease. Eur J Vasc Endovasc Surg. 2008;36:680–8. doi: 10.1016/j.ejvs.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 243.Hobbs SD, Marshall T, Fegan C, et al. The constitutive proco-agulant and hypofibrinolytic state in patients with intermittent claudication due to infrainguinal disease significantly improves with percutaneous transluminal balloon angioplasty. J Vasc Surg. 2006;43:40–6. doi: 10.1016/j.jvs.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 244.Mazari FA, Khan JA, Carradice D, et al. Randomized clinical trial of percutaneous transluminal angioplasty, supervised exercise and combined treatment for intermittent claudication due to femoropopliteal arterial disease. Br J Surg. 2012;99:39–48. doi: 10.1002/bjs.7710. [DOI] [PubMed] [Google Scholar]
- 245.Nordanstig J, Gelin J, Hensäter M, et al. Walking performance and health-related quality of life after surgical or endovascular invasive versus non-invasive treatment for intermittent claudication—a prospective randomised trial. Eur J Vasc Endovasc Surg. 2011;42:220–7. doi: 10.1016/j.ejvs.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 246.Nylaende M, Abdelnoor M, Stranden E, et al. The Oslo Balloon Angioplasty versus Conservative Treatment study (OBACT)—the 2-years results of a single centre, prospective, randomised study in patients with intermittent claudication. Eur J Vasc Endovasc Surg. 2007;33:3–12. doi: 10.1016/j.ejvs.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 247.Perkins JM, Collin J, Creasy TS, et al. Reprinted article “Exercise training versus angioplasty for stable claudication. Long and medium term results of a prospective, randomised trial”. Eur J Vasc Endovasc Surg. 2011;42(1):S41–5. doi: 10.1016/j.ejvs.2011.06.021. [DOI] [PubMed] [Google Scholar]
- 248.Spronk S, Bosch JL, den Hoed PT, et al. Cost-effectiveness of endovascular revascularization compared to supervised hospital-based exercise training in patients with intermittent claudication: a randomized controlled trial. J Vasc Surg. 2008;48:1472–80. doi: 10.1016/j.jvs.2008.06.016. [DOI] [PubMed] [Google Scholar]
- 249.Taft C, Karlsson J, Gelin J, et al. Treatment efficacy of intermittent claudication by invasive therapy, supervised physical exercise training compared to no treatment in unselected randomised patients, II: one-year results of health-related quality of life. Eur J Vasc Endovasc Surg. 2001;22:114–23. doi: 10.1053/ejvs.2001.1406. [DOI] [PubMed] [Google Scholar]
- 250.Whyman MR, Flowkes FG, Kerracher EM, et al. Randomised controlled trial of percutaneous transluminal angioplasty for intermittent claudication. Eur J Vasc Endovasc Surg. 1996;12:167–72. doi: 10.1016/s1078-5884(96)80102-x. [DOI] [PubMed] [Google Scholar]
- 251.Whyman MR, Flowkes FG, Kerracher EM, et al. Is intermittent claudication improved by percutaneous transluminal angioplasty? A randomized controlled trial. J Vasc Surg. 1997;26:551–7. doi: 10.1016/s0741-5214(97)70052-1. [DOI] [PubMed] [Google Scholar]
- 252.Capek P, McLean GK, Berkowitz HD. Femoropopliteal angioplasty. Factors influencing long-term success. Circulation. 1991;83:I70–80. [PubMed] [Google Scholar]
- 253.Löfberg AM, Karacagil S, Ljungman C, et al. Percutaneous transluminal angioplasty of the femoropopliteal arteries in limbs with chronic critical lower limb ischemia. J Vasc Surg. 2001;34:114–21. doi: 10.1067/mva.2001.113486. [DOI] [PubMed] [Google Scholar]
- 254.Clark TW, Groffsky JL, Soulen MC. Predictors of long-term patency after femoropopliteal angioplasty: results from the STAR registry. J Vasc Interv Radiol. 2001;12:923–33. doi: 10.1016/s1051-0443(07)61570-x. [DOI] [PubMed] [Google Scholar]
- 255.Johnston KW, Rae M, Hogg-Johnston SA, et al. 5-year results of a prospective study of percutaneous transluminal angioplasty. Ann Surg. 1987;206:403–13. doi: 10.1097/00000658-198710000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Schulte KL, Pilger E, Schellong S, et al. Primary self-expanding nitinol stenting vs balloon angioplasty with optional bailout stent-ing for the treatment of infrapopliteal artery disease in patients with severe intermittent claudication or critical limb ischemia (EXPAND Study) J Endovasc Ther. 2015;22:690–697. doi: 10.1177/1526602815598955. [DOI] [PubMed] [Google Scholar]
- 257.Rastan A, Tepe G, Krankenberg H, et al. Sirolimus-eluting stents vs. bare-metal stents for treatment of focal lesions in infrapopliteal arteries: a double-blind, multi-centre, randomized clinical trial. Eur Heart J. 2011;32:2274–81. doi: 10.1093/eurheartj/ehr144. [DOI] [PubMed] [Google Scholar]
- 258.Siablis D, Kitrou PM, Spiliopoulos S, et al. Paclitaxel-coated balloon angioplasty versus drug-eluting stenting for the treatment of infrapopliteal long-segment arterial occlusive disease: the IDEAS randomized controlled trial. JACC Cardiovasc Interv. 2014;7:1048–56. doi: 10.1016/j.jcin.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 259.Sachs T, Pomposelli F, Hamdan A, et al. Trends in the national outcomes and costs for claudication and limb threatening ischemia: angioplasty vs bypass graft. J Vasc Surg. 2011;54:1021–31. doi: 10.1016/j.jvs.2011.03.281. [DOI] [PubMed] [Google Scholar]
- 260.Shammas NW, Shammas GA, Dippel EJ, et al. Predictors of distal embolization in peripheral percutaneous interventions: a report from a large peripheral vascular registry. J Invasive Cardiol. 2009;21:628–31. [PubMed] [Google Scholar]
- 261.Matsi PJ, Manninen HI. Complications of lower-limb percutaneous transluminal angioplasty: a prospective analysis of 410 procedures on 295 consecutive patients. Cardiovasc Intervent Radiol. 1998;21:361–6. doi: 10.1007/s002709900281. [DOI] [PubMed] [Google Scholar]
- 262.Kannel WB, Skinner JJ, Schwartz MJ, et al. Intermittent claudication. Incidence in the Framingham Study. Circulation. 1970;41:875–83. doi: 10.1161/01.cir.41.5.875. [DOI] [PubMed] [Google Scholar]
- 263.AbuRahma AF, Robinson PA, Holt SM. Prospective controlled study of polytetrafluoroethylene versus saphenous vein in claudicant patients with bilateral above knee femoropopliteal bypasses. Surgery. 1999;126:594–602. [PubMed] [Google Scholar]
- 264.Archie JP. Femoropopliteal bypass with either adequate ipsilater-al reversed saphenous vein or obligatory polytetrafluoroethylene. Ann Vasc Surg. 1994;8:475–84. doi: 10.1007/BF02133068. [DOI] [PubMed] [Google Scholar]
- 265.Eugster T, Marti R, Gurke L, et al. Ten years after arterial bypass surgery for claudication: venous bypass is the primary procedure for TASC C and D lesions. World J Surg. 2011;35:2328–31. doi: 10.1007/s00268-011-1237-x. [DOI] [PubMed] [Google Scholar]
- 266.Green RM, Abbott WM, Matsumoto T, et al. Prosthetic above-knee femoropopliteal bypass grafting: five-year results of a randomized trial. J Vasc Surg. 2000;31:417–25. [PubMed] [Google Scholar]
- 267.Hunink MG, Wong JB, Donaldson MC, et al. Patency results of percutaneous and surgical revascularization for femoropopliteal arterial disease. Med Decis Making. 1994;14:71–81. doi: 10.1177/0272989X9401400109. [DOI] [PubMed] [Google Scholar]
- 268.Johnson WC, Lee KK. Comparative evaluation of externally supported Dacron and polytetrafluoroethylene prosthetic bypasses for femorofemoral and axillofemoral arterial reconstructions. Veterans Affairs Cooperative Study #141. J Vasc Surg. 1999;30:1077–83. doi: 10.1016/s0741-5214(99)70046-7. [DOI] [PubMed] [Google Scholar]
- 269.Klinkert P, Schepers A, Burger DH, et al. Vein versus polytetrafluoroethylene in above-knee femoropopliteal bypass grafting: five-year results of a randomized controlled trial. J Vasc Surg. 2003;37:149–55. doi: 10.1067/mva.2002.86. [DOI] [PubMed] [Google Scholar]
- 270.Twine CP, McLain AD. Graft type for femoro-popliteal bypass surgery. Cochrane Database Syst Rev. 2010:CD001487. doi: 10.1002/14651858.CD001487.pub2. [DOI] [PubMed] [Google Scholar]
- 271.Pereira CE, Albers M, Romiti M, et al. Meta-analysis of femoropopliteal bypass grafts for lower extremity arterial insufficiency. J Vasc Surg. 2006;44:510–17. doi: 10.1016/j.jvs.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 272.Johnson WC, Lee KK. A comparative evaluation of polytetrafluoroethylene, umbilical vein, and saphenous vein bypass grafts for femoral-popliteal above-knee revascularization: a prospective randomized Department of Veterans Affairs cooperative study. J Vasc Surg. 2000;32:268–77. doi: 10.1067/mva.2000.106944. [DOI] [PubMed] [Google Scholar]
- 273.Jensen LP, Lepäntalo M, Fossdal JE, et al. Dacron or PTFE for above-knee femoropopliteal bypass, a multicenter randomised study. Eur J Vasc Endovasc Surg. 2007;34:44–9. doi: 10.1016/j.ejvs.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 274.van Det RJ, Vriens BH, van der Palen J, et al. Dacron or ePTFE for femoro-popliteal above-knee bypass grafting: short- and long-term results of a multicentre randomised trial. Eur J Vasc Endovasc Surg. 2009;37:457–63. doi: 10.1016/j.ejvs.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 275.Feinglass J, McCarthy WJ, Slavensky R, et al. Functional status and walking ability after lower extremity bypass grafting or angioplasty for intermittent claudication: results from a prospective outcomes study. J Vasc Surg. 2000;31:93–103. doi: 10.1016/s0741-5214(00)70071-1. [DOI] [PubMed] [Google Scholar]
- 276.Koivunen K, Lukkarinen H. One-year prospective health-related quali-tyof-life outcomes in patients treated with conservative method, en-dovascular treatment or open surgery for symptomatic lower limb atherosclerotic disease. Eur J Cardiovasc Nurs. 2008;7:247–56. doi: 10.1016/j.ejcnurse.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 277.Mori E, Komori K, Kume M, et al. Comparison of the long-term results between surgical and conservative treatment in patients with intermittent claudication. Surgery. 2002;131:S269–74. doi: 10.1067/msy.2002.119966. [DOI] [PubMed] [Google Scholar]
- 278.Antoniou GA, Chalmers N, Georgiadis GS, et al. A meta-analysis of endovascular versus surgical reconstruction of femoropopliteal arterial disease. J Vasc Surg. 2013;57:242–53. doi: 10.1016/j.jvs.2012.07.038. [DOI] [PubMed] [Google Scholar]
- 279.Flowkes F, Leng GC. Bypass surgery for chronic lower limb isch-aemia. Cochrane Database Syst Rev. 2008:CD002000. doi: 10.1002/14651858.CD002000.pub2. [DOI] [PubMed] [Google Scholar]
- 280.Aihara H, Soga Y, Mii S, et al. Comparison of long-term outcome after endovascular therapy versus bypass surgery in claudication patients with Trans-Atlantic Inter-Society Consensus-II C and D femoropopliteal disease. Circ J. 2014;78:457–64. doi: 10.1253/circj.cj-13-1147. [DOI] [PubMed] [Google Scholar]
- 281.Chiesa R, Marone EM, Tshomba Y, et al. Aortobifemoral bypass grafting using expanded polytetrafluoroethylene stretch grafts in patients with occlusive atherosclerotic disease. Ann Vasc Surg. 2009;23:764–9. doi: 10.1016/j.avsg.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 282.Goodney PP, Likosky DS, Cronenwett JL, et al. Predicting ambulation status one year after lower extremity bypass. J Vasc Surg. 2009;49:1431–9. doi: 10.1016/j.jvs.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 283.Lo RC, Bensley RP, Dahlberg SE, et al. Presentation, treatment, and outcome differences between men and women undergoing revascularization or amputation for lower extremity peripheral arterial disease. J Vasc Surg. 2014;59:409–18. doi: 10.1016/j.jvs.2013.07.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Nguyen BN, Amdur RL, Abugideiri M, et al. Postoperative complications after common femoral endarterectomy. J Vasc Surg. 2015;61:1489–94. doi: 10.1016/j.jvs.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 285.Sachwani GR, Hans SS, Khoury MD, et al. Results of iliac stenting and aortofemoral grafting for iliac artery occlusions. J Vasc Surg. 2013;57:1030–7. doi: 10.1016/j.jvs.2012.09.038. [DOI] [PubMed] [Google Scholar]
- 286.Siracuse JJ, Gill HL, Schneider DB, et al. Assessing the perioperative safety of common femoral endarterectomy in the endovascular era. Vasc Endovascular Surg. 2014;48:27–33. doi: 10.1177/1538574413508827. [DOI] [PubMed] [Google Scholar]
- 287.Veith FJ, Gupta SK, Ascer E, et al. Six-year prospective multi-center randomized comparison of autologous saphenous vein and expanded polytetrafluoroethylene grafts in infrainguinal arterial reconstructions. J Vasc Surg. 1986;3:104–14. doi: 10.1067/mva.1986.avs0030104. [DOI] [PubMed] [Google Scholar]
- 288.Schweiger H, Klein P, Lang W. Tibial bypass grafting for limb salvage with ringed polytetrafluoroethylene prostheses: results of primary and secondary procedures. J Vasc Surg. 1993;18:867–74. [PubMed] [Google Scholar]
- 289.Baldwin ZK, Pearce BJ, Curi MA, et al. Limb salvage after infrainguinal bypass graft failure. J Vasc Surg. 2004;39:951–7. doi: 10.1016/j.jvs.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 290.Abu Dabrh AM, Steffen MW, Undavalli C, et al. The natural history of untreated severe or critical limb ischemia. J Vasc Surg. 2015;62:1642–51. doi: 10.1016/j.jvs.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 291.Deleted in press.
- 292.Adam DJ, Beard JD, Cleveland T, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet. 2005;366:1925–34. doi: 10.1016/S0140-6736(05)67704-5. [DOI] [PubMed] [Google Scholar]
- 293.Bradbury AW, Adam DJ, Bell J, et al. Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial. Health Technol Assess. 2010;14:1–210. iii–iv. doi: 10.3310/hta14140. [DOI] [PubMed] [Google Scholar]
- 294.Panaich SS, Arora S, Patel N, et al. Intravascular ultrasound in lower extremity peripheral vascular interventions: variation in utilization and impact on in-hospital outcomes from the Nationwide Inpatient Sample (2006–2011) J Endovasc Ther. 2016;23:65–75. doi: 10.1177/1526602815620780. [DOI] [PubMed] [Google Scholar]
- 295.Gray BH, Laird JR, Ansel GM, et al. Complex endovascular treatment for critical limb ischemia in poor surgical candidates: a pilot study. J Endovasc Ther. 2002;9:599–604. doi: 10.1177/152660280200900509. [DOI] [PubMed] [Google Scholar]
- 296.Ryer EJ, Trocciola SM, DeRubertis B, et al. Analysis of outcomes following failed endovascular treatment of chronic limb ischemia. Ann Vasc Surg. 2006;20:440–6. doi: 10.1007/s10016-006-9101-4. [DOI] [PubMed] [Google Scholar]
- 297.Krankenberg H, Schlüter M, Steinkamp HJ, et al. Nitinol stent implantation versus percutaneous transluminal angioplasty in superficial femoral artery lesions up to 10 cm in length: the Femoral Artery Stenting Trial (FAST) Circulation. 2007;116:285–92. doi: 10.1161/CIRCULATIONAHA.107.689141. [DOI] [PubMed] [Google Scholar]
- 298.Schillinger M, Sabeti S, Dick P, et al. Sustained benefit at 2 years of primary femoropopliteal stenting compared with balloon angioplasty with optional stenting. Circulation. 2007;115:2745–9. doi: 10.1161/CIRCULATIONAHA.107.688341. [DOI] [PubMed] [Google Scholar]
- 299.Liistro F, Porto I, Angioli P, et al. Drug-eluting balloon in peripheral intervention for below the knee angioplasty evaluation (DEBATE-BTK): a randomized trial in diabetic patients with critical limb ischemia. Circulation. 2013;128:615–21. doi: 10.1161/CIRCULATIONAHA.113.001811. [DOI] [PubMed] [Google Scholar]
- 300.Tepe G, Schnorr B, Albrecht T, et al. Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the THUNDER trial. JACC Cardiovasc Interv. 2015;8:102–8. doi: 10.1016/j.jcin.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 301.Dake MD, Ansel GM, Jaff MR, et al. Durable clinical effectiveness with paclitaxel-eluting stents in the femoropopliteal artery: 5-year results of the Zilver PTX Randomized Trial. Circulation. 2016;133:1472–83. doi: 10.1161/CIRCULATIONAHA.115.016900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Liistro F, Grotti S, Porto I, et al. Drug-eluting balloon in peripheral intervention for the superficial femoral artery: the DEBATE-SFA randomized trial (drug eluting balloon in peripheral intervention for the superficial femoral artery) JACC Cardiovasc Interv. 2013;6:1295–302. doi: 10.1016/j.jcin.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 303.Bosiers M, Scheinert D, Peeters P, et al. Randomized comparison of everolimus-eluting versus bare-metal stents in patients with critical limb ischemia and infrapopliteal arterial occlusive disease. J Vasc Surg. 2012;55:390–8. doi: 10.1016/j.jvs.2011.07.099. [DOI] [PubMed] [Google Scholar]
- 304.Katsanos K, Spiliopoulos S, Diamantopoulos A, et al. Wound healing outcomes and health-related quality-of-life changes in the ACHILLES trial: 1-year results from a prospective randomized controlled trial of infrapopliteal balloon angioplasty versus sirolimus-eluting stenting in patients with ischemic peripheral arterial disease. JACC Cardiovasc Interv. 2016;9:259–67. doi: 10.1016/j.jcin.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 305.Scheinert D, Katsanos K, Zeller T, et al. A prospective randomized multicenter comparison of balloon angioplasty and infrapopliteal stenting with the sirolimus-eluting stent in patients with ischemic peripheral arterial disease: 1-year results from the ACHILLES trial. J Am Coll Cardiol. 2012;60:2290–5. doi: 10.1016/j.jacc.2012.08.989. [DOI] [PubMed] [Google Scholar]
- 306.Zeller T, Baumgartner I, Scheinert D, et al. Drug-eluting balloon versus standard balloon angioplasty for infrapopliteal arterial revascularization in critical limb ischemia: 12-month results from the IN.PACT DEEP randomized trial. J Am Coll Cardiol. 2014;64:1568–76. doi: 10.1016/j.jacc.2014.06.1198. [DOI] [PubMed] [Google Scholar]
- 307.Rosenfield K, Jaff MR, White CJ, et al. Trial of a paclitaxel-coat-ed balloon for femoropopliteal artery disease. N Engl J Med. 2015;373:145–53. doi: 10.1056/NEJMoa1406235. [DOI] [PubMed] [Google Scholar]
- 308.Scheinert D, Duda S, Zeller T, et al. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv. 2014;7:10–9. doi: 10.1016/j.jcin.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 309.Werk M, Albrecht T, Meyer DR, et al. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: evidence from the randomized PACIFIER trial. Circ Cardiovasc Interv. 2012;5:831–40. doi: 10.1161/CIRCINTERVENTIONS.112.971630. [DOI] [PubMed] [Google Scholar]
- 310.Acín F, Varela C, López de Maturana I, et al. Results of infrapopliteal endovascular procedures performed in diabetic patients with critical limb ischemia and tissue loss from the perspective of an angiosome-oriented revascularization strategy. Int J Vasc Med. 2014;2014:270539. doi: 10.1155/2014/270539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Alexandrescu VA, Hubermont G, Philips Y, et al. Selective primary angioplasty following an angiosome model of reperfusion in the treatment of Wagner 1-4 diabetic foot lesions: practice in a multidisciplinary diabetic limb service. J Endovasc Ther. 2008;15:580–93. doi: 10.1583/08-2460.1. [DOI] [PubMed] [Google Scholar]
- 312.Azuma N, Uchida H, Kokubo T, et al. Factors influencing wound healing of critical ischaemic foot after bypass surgery: is the angiosome important in selecting bypass target artery? Eur J Vasc Endovasc Surg. 2012;43:322–8. doi: 10.1016/j.ejvs.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 313.Fossaceca R, Guzzardi G, Cerini P, et al. Endovascular treatment of diabetic foot in a selected population of patients with below-the-knee disease: is the angiosome model effective? Cardiovasc Intervent Radiol. 2013;36:637–44. doi: 10.1007/s00270-012-0544-4. [DOI] [PubMed] [Google Scholar]
- 314.Iida O, Soga Y, Hirano K, et al. Long-term results of direct and indirect endovascular revascularization based on the angiosome concept in patients with critical limb ischemia presenting with isolated below-the-knee lesions. J Vasc Surg. 2012;55:363–70. doi: 10.1016/j.jvs.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 315.Kabra A, Suresh KR, Vivekanand V, et al. Outcomes of angiosome and non-angiosome targeted revascularization in critical lower limb ischemia. J Vasc Surg. 2013;57:44–9. doi: 10.1016/j.jvs.2012.07.042. [DOI] [PubMed] [Google Scholar]
- 316.Kret MR, Cheng D, Azarbal AF, et al. Utility of direct angiosome revascularization and runoff scores in predicting outcomes in patients undergoing revascularization for critical limb ischemia. J Vasc Surg. 2014;59:121–8. doi: 10.1016/j.jvs.2013.06.075. [DOI] [PubMed] [Google Scholar]
- 317.Lejay A, Georg Y, Tartaglia E, et al. Long-term outcomes of direct and indirect below-the-knee open revascularization based on the angiosome concept in diabetic patients with critical limb ischemia. Ann Vasc Surg. 2014;28:983–9. doi: 10.1016/j.avsg.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 318.Neville RF, Attinger CE, Bulan EJ, et al. Revascularization of a specific angiosome for limb salvage: does the target artery matter? Ann Vasc Surg. 2009;23:367–73. doi: 10.1016/j.avsg.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 319.Osawa S, Terashi H, Tsuji Y, et al. Importance of the six angio-somes concept through arterial-arterial connections in CLI. Int Angiol. 2013;32:375–85. [PubMed] [Google Scholar]
- 320.Rashid H, Slim H, Zayed H, et al. The impact of arterial pedal arch quality and angiosome revascularization on foot tissue loss healing and infrapopliteal bypass outcome. J Vasc Surg. 2013;57:1219–26. doi: 10.1016/j.jvs.2012.10.129. [DOI] [PubMed] [Google Scholar]
- 321.Varela C, Acín F, de Haro J, et al. The role of foot collateral vessels on ulcer healing and limb salvage after successful endovascular and surgical distal procedures according to an angiosome model. Vasc Endovascular Surg. 2010;44:654–60. doi: 10.1177/1538574410376601. [DOI] [PubMed] [Google Scholar]
- 322.Bosanquet DC, Glasbey JC, Williams IM, et al. Systematic review and meta-analysis of direct versus indirect angiosomal revascularisation of infrapopliteal arteries. Eur J Vasc Endovasc Surg. 2014;48:88–97. doi: 10.1016/j.ejvs.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 323.Biancari F, Juvonen T. Angiosome-targeted lower limb revascularization for ischemic foot wounds: systematic review and metaanalysis. Eur J Vasc Endovasc Surg. 2014;47:517–22. doi: 10.1016/j.ejvs.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 324.Eugster T, Stierli P, Guerke L, et al. Present status of infrainguinal arterial bypass procedures following an all autogenous policy—long-term results of a single center. Swiss Surg. 2002;8:171–5. doi: 10.1024/1023-9332.8.4.171. [DOI] [PubMed] [Google Scholar]
- 325.Reifsnyder T, Arhuidese IJ, Hicks CW, et al. Contemporary outcomes for open infrainguinal bypass in the endovascular era. Ann Vasc Surg. 2016;30:52–8. doi: 10.1016/j.avsg.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 326.Chew DK, Conte MS, Donaldson MC, et al. Autogenous composite vein bypass graft for infrainguinal arterial reconstruction. J Vasc Surg. 2001;33:259–64. doi: 10.1067/mva.2001.112699. [DOI] [PubMed] [Google Scholar]
- 327.Belkin M, Conte MS, Donaldson MC, et al. Preferred strategies for secondary infrainguinal bypass: lessons learned from 300 consecutive reoperations. J Vasc Surg. 1995;21:282–93. doi: 10.1016/s0741-5214(95)70269-5. [DOI] [PubMed] [Google Scholar]
- 328.Fogle MA, Whittemore AD, Couch NP, et al. A comparison of in situ and reversed saphenous vein grafts for infrainguinal reconstruction. J Vasc Surg. 1987;5:46–52. [PubMed] [Google Scholar]
- 329.Leather RP, Karmody AM. In-situ saphenous vein arterial bypass for the treatment of limb ischemia. Adv Surg. 1986;19:175–219. [PubMed] [Google Scholar]
- 330.Taylor LM, Edwards JM, Porter JM. Present status of reversed vein bypass grafting: five-year results of a modern series. J Vasc Surg. 1990;11:193–205. doi: 10.1067/mva.1990.17235. [DOI] [PubMed] [Google Scholar]
- 331.Nolan BW, De Martino RR, Stone DH, et al. Prior failed ipsilateral percutaneous endovascular intervention in patients with critical limb ischemia predicts poor outcome after lower extremity bypass. J Vasc Surg. 2011;54:730–5. doi: 10.1016/j.jvs.2011.03.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Santo VJ, Dargon P, Azarbal AF, et al. Lower extremity autologous vein bypass for critical limb ischemia is not adversely affected by prior endovascular procedure. J Vasc Surg. 2014;60:129–35. doi: 10.1016/j.jvs.2014.01.013. [DOI] [PubMed] [Google Scholar]
- 333.Uhl C, Hock C, Betz T, et al. Pedal bypass surgery after crural endovascular intervention. J Vasc Surg. 2014;59:1583–7. doi: 10.1016/j.jvs.2013.11.071. [DOI] [PubMed] [Google Scholar]
- 334.Nishibe T, Maruno K, Iwahori A, et al. The role of common femoral artery endarterectomy in the endovascular era. Ann Vasc Surg. 2015;29:1501–7. doi: 10.1016/j.avsg.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 335.Okadome J, Matsumoto T, Aoyagi Y, et al. Long-term results of a hybrid revascularization procedure for peripheral arterial disease. Fukuoka Igaku Zasshi. 2015;106:254–61. [PubMed] [Google Scholar]
- 336.Starodubtsev V, Karpenko A, Ignatenko P. Hybrid and open surgery of Trans-Atlantic Inter-Society II type C and D iliac occlusive disease and concomitant lesion of common femoral artery. Int Angiol. 2016;35:484–91. [PubMed] [Google Scholar]
- 337.Kasemi H, Marino M, Dionisi CP, et al. Seven-year approach evolution of the aortoiliac occlusive disease endovascular treatment. Ann Vasc Surg. 2016;30:277–85. doi: 10.1016/j.avsg.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 338.Bredahl K, Jensen LP, Schroeder TV, et al. Mortality and complications after aortic bifurcated bypass procedures for chronic aortoiliac occlusive disease. J Vasc Surg. 2015;62:75–82. doi: 10.1016/j.jvs.2015.02.025. [DOI] [PubMed] [Google Scholar]
- 339.Kobayashi N, Hirano K, Nakano M, et al. Prognosis of critical limb ischemia patients with tissue loss after achievement of complete wound healing by endovascular therapy. J Vasc Surg. 2015;61:951–9. doi: 10.1016/j.jvs.2014.11.065. [DOI] [PubMed] [Google Scholar]
- 340.Armstrong DG, Bharara M, White M, et al. The impact and outcomes of establishing an integrated interdisciplinary surgical team to care for the diabetic foot. Diabetes Metab Res Rev. 2012;28:514–8. doi: 10.1002/dmrr.2299. [DOI] [PubMed] [Google Scholar]
- 341.Chung J, Modrall JG, Ahn C, et al. Multidisciplinary care improves amputation-free survival in patients with chronic critical limb ischemia. J Vasc Surg. 2015;61:162–9. doi: 10.1016/j.jvs.2014.05.101. [DOI] [PubMed] [Google Scholar]
- 342.García-Morales E, Lázaro-Martínez JL, Aragón-Sánchez J, et al. Surgical complications associated with primary closure in patients with diabetic foot osteomyelitis. Diabet Foot Ankle. 2012;3:19000. doi: 10.3402/dfa.v3i0.19000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Berceli SA, Brown JE, Irwin PB, et al. Clinical outcomes after closed, staged, and open forefoot amputations. J Vasc Surg. 2006;44:347–51. doi: 10.1016/j.jvs.2006.04.043. [DOI] [PubMed] [Google Scholar]
- 344.Shaikh N, Vaughan P, Varty K, et al. Outcome of limited forefoot amputation with primary closure in patients with diabetes. Bone Joint J. 2013;95-B:1083–7. doi: 10.1302/0301-620X.95B8.31280. [DOI] [PubMed] [Google Scholar]
- 345.Armstrong DG, Lavery LA. Diabetic Foot Study ConsortiumNegative pressure wound therapy after partial diabetic foot amputation: a multicentre, randomised controlled trial. Lancet. 2005;366:1704–10. doi: 10.1016/S0140-6736(05)67695-7. [DOI] [PubMed] [Google Scholar]
- 346.Sepúlveda G, Espíndola M, Maureira M, et al. Negative-pressure wound therapy versus standard wound dressing in the treatment of diabetic foot amputation. A randomised controlled trial. Cir Esp. 2009;86:171–7. doi: 10.1016/j.ciresp.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 347.Barshes NR, Gold B, Garcia A, et al. Minor amputation and palliative wound care as a strategy to avoid major amputation in patients with foot infections and severe peripheral arterial disease. Int J Low Extrem Wounds. 2014;13:211–9. doi: 10.1177/1534734614543663. [DOI] [PubMed] [Google Scholar]
- 348.Fikri R, Bicknell CD, Bloomfield LM, et al. Awaiting autoamputation: a primary management strategy for toe gangrene in diabetic foot disease. Diabetes Care. 2011;34:e134. doi: 10.2337/dc11-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Centre for Clinical practice at NICE (UK) London: National Institute for Health and Clinical Excellence (UK); 2011. [Accessed September 25, 2016]. Diabetic Foot Problems: Inpatient Management of Diabetic Foot Problems. Available at: http://www.ncbi.nlm.nih.gov/books/NBK82350/ [PubMed] [Google Scholar]
- 350.Lipsky BA, Aragón-Sánchez J, Diggle M, et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab Res Rev. 2016;32(1):45–74. doi: 10.1002/dmrr.2699. [DOI] [PubMed] [Google Scholar]
- 351.Bus SA, van Deursen RW, Armstrong DG, et al. Footwear and offloading interventions to prevent and heal foot ulcers and reduce plantar pressure in patients with diabetes: a systematic review. Diabetes Metab Res Rev. 2016;32(suppl 1):99–118. doi: 10.1002/dmrr.2702. [DOI] [PubMed] [Google Scholar]
- 352.Game FL, Apelqvist J, Attinger C, et al. IWGDF guidance on use of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev. 2016;32(1):75–83. doi: 10.1002/dmrr.2700. [DOI] [PubMed] [Google Scholar]
- 353.Moran PS, Teljeur C, Harrington P, et al. A systematic review of intermittent pneumatic compression for critical limb ischaemia. Vasc Med. 2015;20:41–50. doi: 10.1177/1358863X14552096. [DOI] [PubMed] [Google Scholar]
- 354.Abidia A, Laden G, Kuhan G, et al. The role of hyperbaric oxygen therapy in ischaemic diabetic lower extremity ulcers: a double-blind randomised-controlled trial. Eur J Vasc Endovasc Surg. 2003;25:513–8. doi: 10.1053/ejvs.2002.1911. [DOI] [PubMed] [Google Scholar]
- 355.Kranke P, Bennett MH, Martyn-St James M, et al. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst Rev. 2012:CD004123. doi: 10.1002/14651858.CD004123.pub3. [DOI] [PubMed] [Google Scholar]
- 356.Ruffolo AJ, Romano M, Ciapponi A. Prostanoids for critical limb ischaemia. Cochrane Database Syst Rev. 2010:CD006544. doi: 10.1002/14651858.CD006544.pub2. [DOI] [PubMed] [Google Scholar]
- 357.Baril DT, Patel VI, Judelson DR, et al. Outcomes of lower extremity bypass performed for acute limb ischemia. J Vasc Surg. 2013;58:949–56. doi: 10.1016/j.jvs.2013.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Londero LS, Nørgaard B, Houlind K. Patient delay is the main cause of treatment delay in acute limb ischemia: an investigation of pre- and in-hospital time delay. World J Emerg Surg. 2014;9:56. doi: 10.1186/1749-7922-9-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Manojlović V, Popović V, Nikolić D, et al. Analysis of associated diseases in patients with acute critical lower limb ischemia. Med Pregl. 2013;66:41–5. doi: 10.2298/mpns1302041m. [DOI] [PubMed] [Google Scholar]
- 360.Duval S, Keo HH, Oldenburg NC, et al. The impact of prolonged lower limb ischemia on amputation, mortality, and functional status: the FRIENDS registry. Am Heart J. 2014;168:577–87. doi: 10.1016/j.ahj.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 361.Morris-Stiff G, D'Souza J, Raman S, et al. Update experience of surgery for acute limb ischaemia in a district general hospital—are we getting any better? Ann R Coll Surg Engl. 2009;91:637–40. doi: 10.1308/003588409X12486167521271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg. 2002;10:620–30. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 363.Saarinen E, Vuorisalo S, Kauhanen P, et al. The benefit of revascularization in nonagenarians with lower limb ischemia is limited by high mortality. Eur J Vasc Endovasc Surg. 2015;49:420–5. doi: 10.1016/j.ejvs.2014.12.027. [DOI] [PubMed] [Google Scholar]
- 364.Blaisdell FW, Steele M, Allen RE. Management of acute lower extremity arterial ischemia due to embolism and thrombosis. Surgery. 1978;84:822–34. [PubMed] [Google Scholar]
- 365.Altoijry A, MacKenzie KS, Corriveau MM, et al. Heparin-induced thrombocytopenia causing graft thrombosis and bowel ischemia postendovascular aneurysm repair. J Vasc Surg. 2015;61:234–6. doi: 10.1016/j.jvs.2013.08.086. [DOI] [PubMed] [Google Scholar]
- 366.Turba UC, Bozlar U, Simsek S. Catheter-directed thrombolysis of acute lower extremity arterial thrombosis in a patient with heparin-induced thrombocytopenia. Catheter Cardiovasc Interv. 2007;70:1046–50. doi: 10.1002/ccd.21304. [DOI] [PubMed] [Google Scholar]
- 367.Comerota AJ, Weaver FA, Hosking JD, et al. Results of a prospective, randomized trial of surgery versus thrombolysis for occluded lower extremity bypass grafts. Am J Surg. 1996;172:105–12. doi: 10.1016/S0002-9610(96)00129-8. [DOI] [PubMed] [Google Scholar]
- 368.Diffin DC, Kandarpa K. Assessment of peripheral intraarterial thrombolysis versus surgical revascularization in acute lower-limb ischemia: a review of limb-salvage and mortality statistics. J Vasc Interv Radiol. 1996;7:57–63. doi: 10.1016/s1051-0443(96)70734-0. [DOI] [PubMed] [Google Scholar]
- 369.Ouriel K, Veith FJ, Sasahara AA. A comparison of recombinant urokinase with vascular surgery as initial treatment for acute arterial occlusion of the legs. Thrombolysis or Peripheral Arterial Surgery (TOPAS) Investigators. N Engl J Med. 1998;338:1105–11. doi: 10.1056/NEJM199804163381603. [DOI] [PubMed] [Google Scholar]
- 370.Results of a prospective randomized trial evaluating surgery versus thrombolysis for ischemia of the lower extremity. The STILE trial. Ann Surg. 1994;220:266–8. doi: 10.1097/00000658-199409000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Ouriel K, Shortell CK, DeWeese JA, et al. A comparison of thrombolytic therapy with operative revascularization in the initial treatment of acute peripheral arterial ischemia. J Vasc Surg. 1994;19:1021–30. doi: 10.1016/s0741-5214(94)70214-4. [DOI] [PubMed] [Google Scholar]
- 372.Eliason JL, Wakefield TW. Metabolic consequences of acute limb ischemia and their clinical implications. Semin Vasc Surg. 2009;22:29–33. doi: 10.1053/j.semvascsurg.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 373.Henke PK. Contemporary management of acute limb ischemia: factors associated with amputation and in-hospital mortality. Semin Vasc Surg. 2009;22:34–40. doi: 10.1053/j.semvascsurg.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 374.Ansel GM, Botti CF, Silver MJ. Treatment of acute limb ischemia with a percutaneous mechanical thrombectomy-based endovascular approach: 5-year limb salvage and survival results from a single center series. Catheter Cardiovasc Interv. 2008;72:325–30. doi: 10.1002/ccd.21641. [DOI] [PubMed] [Google Scholar]
- 375.Gupta R, Hennebry TA. Percutaneous isolated pharmaco-mechanical thrombolysis-thrombectomy system for the management of acute arterial limb ischemia: 30-day results from a single-center experience. Catheter Cardiovasc Interv. 2012;80:636–43. doi: 10.1002/ccd.24283. [DOI] [PubMed] [Google Scholar]
- 376.Silva JA, Ramee SR, Collins TJ, et al. Rheolytic thrombectomy in the treatment of acute limb-threatening ischemia: immediate results and six-month follow-up of the multicenter AngioJet registry. Possis Peripheral AngioJet Study AngioJet Investigators. Cathet Cardiovasc Diagn. 1998;45:386–93. doi: 10.1002/(sici)1097-0304(199812)45:4<386::aid-ccd7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 377.Taha AG, Byrne RM, Avgerinos ED, et al. Comparative effectiveness of endovascular versus surgical revascularization for acute lower extremity ischemia. J Vasc Surg. 2015;61:147–54. doi: 10.1016/j.jvs.2014.06.109. [DOI] [PubMed] [Google Scholar]
- 378.Leung DA, Blitz LR, Nelson T, et al. Rheolytic pharmacom-echanical thrombectomy for the management of acute limb ischemia: results from the PEARL Registry. J Endovasc Ther. 2015;22:546–57. doi: 10.1177/1526602815592849. [DOI] [PubMed] [Google Scholar]
- 379.Zaraca F, Ponzoni A, Sbraga P, et al. Factors affecting long-term outcomes after thromboembolectomy for acute lower limb ischemia. Minerva Chir. 2012;67:49–57. [PubMed] [Google Scholar]
- 380.Ender Topal A, Nesimi Eren M, Celik Y. Management of non-traumatic acute limb ischemia and predictors of outcome in 270 thrombembolectomy cases. Int Angiol. 2011;30:172–80. [PubMed] [Google Scholar]
- 381.Fogarty TJ, Cranley JJ, Krause RJ, et al. A method for extraction of arterial emboli and thrombi. Surg Gynecol Obstet. 1963;116:241–4. [PubMed] [Google Scholar]
- 382.Creager MA, Kaufman JA, Conte MS. Clinical practice. Acute limb ischemia. N Engl J Med. 2012;366:2198–206. doi: 10.1056/NEJMcp1006054. [DOI] [PubMed] [Google Scholar]
- 383.O'Connell JB, Quiñones-Baldrich WJ. Proper evaluation and management of acute embolic versus thrombotic limb ischemia. Semin Vasc Surg. 2009;22:10–6. doi: 10.1053/j.semvascsurg.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 384.Schrijver AM, Reijnen MM, van Oostayen JA, et al. Initial results of catheter-directed ultrasound-accelerated thrombolysis for thromboembolic obstructions of the aortofemoral arteries: a feasibility study. Cardiovasc Intervent Radiol. 2012;35:279–85. doi: 10.1007/s00270-011-0164-4. [DOI] [PubMed] [Google Scholar]
- 385.Schrijver A, Vos J, Hoksbergen AW, et al. Ultrasound-accelerated thrombolysis for lower extremity ischemia: multicenter experience and literature review. J Cardiovasc Surg (Torino) 2011;52:467–76. [PubMed] [Google Scholar]
- 386.Schrijver AM, Reijnen MM, van Oostayen JA, et al. Dutch randomized trial comparing standard catheter-directed thrombolysis versus ultrasound-accelerated thrombolysis for thromboembolic infrainguinal disease (DUET): design and rationale. Trials. 2011;12:20. doi: 10.1186/1745-6215-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Bekwelem W, Connolly SJ, Halperin JL, et al. Extracranial systemic embolic events in patients with nonvalvular atrial fibrillation: incidence, risk factors, and outcomes. Circulation. 2015;132:796–803. doi: 10.1161/CIRCULATIONAHA.114.013243. [DOI] [PubMed] [Google Scholar]
- 388.Baker JD, Dix DE. Variability of Doppler ankle pressures with arterial occlusive disease: an evaluation of ankle index and brachial-ankle pressure gradient. Surgery. 1981;89:134–7. [PubMed] [Google Scholar]
- 389.Jongsma H, Bekken JA, van Buchem F, et al. Secondary interventions in patients with autologous infrainguinal bypass grafts strongly improve patency rates. J Vasc Surg. 2016;63:385–90. doi: 10.1016/j.jvs.2015.08.100. [DOI] [PubMed] [Google Scholar]
- 390.Carter A, Murphy MO, Halka AT, et al. The natural history of stenoses within lower limb arterial bypass grafts using a graft surveillance program. Ann Vasc Surg. 2007;21:695–703. doi: 10.1016/j.avsg.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 391.Ihlberg L, Luther M, Albäck A, et al. Does a completely accomplished duplex-based surveillance prevent vein-graft failure? Eur J Vasc Endovasc Surg. 1999;18:395–400. doi: 10.1053/ejvs.1999.0935. [DOI] [PubMed] [Google Scholar]
- 392.Westerband A, Mills JL, Kistler S, et al. Prospective validation of threshold criteria for intervention in infrainguinal vein grafts undergoing duplex surveillance. Ann Vasc Surg. 1997;11:44–8. doi: 10.1007/s100169900008. [DOI] [PubMed] [Google Scholar]
- 393.Lundell A, Lindblad B, Bergqvist D, et al. Femoropopliteal-crural graft patency is improved by an intensive surveillance program: a prospective randomized study. J Vasc Surg. 1995;21:26–33. doi: 10.1016/s0741-5214(95)70241-5. [DOI] [PubMed] [Google Scholar]
- 394.Mills JL, Harris EJ, Taylor LM, et al. The importance of routine surveillance of distal bypass grafts with duplex scanning: a study of 379 reversed vein grafts. J Vasc Surg. 1990;12:379–86. [PubMed] [Google Scholar]
- 395.Bandyk DF, Cato RF, Towne JB. A low flow velocity predicts failure of femoropopliteal and femorotibial bypass grafts. Surgery. 1985;98:799–809. [PubMed] [Google Scholar]
- 396.Davies AH, Hawdon AJ, Sydes MR, et al. Is duplex surveillance of value after leg vein bypass grafting? Principal results of the Vein Graft Surveillance Randomised Trial (VGST) Circulation. 2005;112:1985–91. doi: 10.1161/CIRCULATIONAHA.104.518738. [DOI] [PubMed] [Google Scholar]
- 397.Back MR, Novotney M, Roth SM, et al. Utility of duplex surveillance following iliac artery angioplasty and primary stenting. J Endovasc Ther. 2001;8:629–37. doi: 10.1177/152660280100800617. [DOI] [PubMed] [Google Scholar]
- 398.Baril DT, Marone LK. Duplex evaluation following femoropopliteal angioplasty and stenting: criteria and utility of surveillance. Vasc Endovascular Surg. 2012;46:353–7. doi: 10.1177/1538574412448684. [DOI] [PubMed] [Google Scholar]
- 399.Troutman DA, Madden NJ, Dougherty MJ, et al. Duplex ultrasound diagnosis of failing stent grafts placed for occlusive disease. J Vasc Surg. 2014;60:1580–4. doi: 10.1016/j.jvs.2014.08.071. [DOI] [PubMed] [Google Scholar]
- 400.Connors G, Todoran TM, Engelson BA, et al. Percutaneous revascularization of long femoral artery lesions for claudication: patency over 2.5 years and impact of systematic surveillance. Catheter Cardiovasc Interv. 2011;77:1055–62. doi: 10.1002/ccd.22802. [DOI] [PubMed] [Google Scholar]
- 401.Brumberg RS, Back MR, Armstrong PA, et al. The relative importance of graft surveillance and warfarin therapy in infrainguinal prosthetic bypass failure. J Vasc Surg. 2007;46:1160–6. doi: 10.1016/j.jvs.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 402.Calligaro KD, Doerr K, McAffee-Bennett S, et al. Should duplex ultrasonography be performed for surveillance of femoropopliteal and femorotibial arterial prosthetic bypasses? Ann Vasc Surg. 2001;15:520–4. doi: 10.1007/s10016-001-0006-y. [DOI] [PubMed] [Google Scholar]
- 403.Stone PA, Armstrong PA, Bandyk DF, et al. Duplex ultrasound criteria for femorofemoral bypass revision. J Vasc Surg. 2006;44:496–502. doi: 10.1016/j.jvs.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 404.Subherwal S, Patel MR, Chiswell K, et al. Clinical trials in peripheral vascular disease: pipeline and trial designs: an evaluation of the ClinicalTrials.gov database. Circulation. 2014;130:1812–9. doi: 10.1161/CIRCULATIONAHA.114.011021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Darling JD, McCallum JC, Soden PA, et al. Predictive ability of the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system following infrapopliteal endovascular interventions for critical limb ischemia. J Vasc Surg. 2016;64:616–22. doi: 10.1016/j.jvs.2016.03.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406.Causey MW, Ahmed A, Wu B, et al. Society for Vascular Surgery limb stage and patient risk correlate with outcomes in an amputation prevention program. J Vasc Surg. 2016;63:1563–73. doi: 10.1016/j.jvs.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 407.Beropoulis E, Stavroulakis K, Schwindt A, et al. Validation of the Wound, Ischemia, foot Infection (WIfI) classification system in nondiabetic patients treated by endovascular means for critical limb ischemia. J Vasc Surg. 2016;64:95–103. doi: 10.1016/j.jvs.2016.01.040. [DOI] [PubMed] [Google Scholar]
- 408.Zhan LX, Branco BC, Armstrong DG, et al. The Society for Vascular Surgery lower extremity threatened limb classification system based on Wound, Ischemia, and foot Infection (WIfI) correlates with risk of major amputation and time to wound healing. J Vasc Surg. 2015;61:939–44. doi: 10.1016/j.jvs.2014.11.045. [DOI] [PubMed] [Google Scholar]
- 409.Mills JL, Conte MS, Armstrong DG, et al. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: risk stratification based on wound, ischemia, and foot infection (WIfI) J Vasc Surg. 2014;59:220–34. doi: 10.1016/j.jvs.2013.08.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Evidence Table 1. Nonrandomized Trials, Observational Studies, and/or Registries of History for Clinical Assessment for PAD–Section 2.1.
Evidence Table 2. Nonrandomized Trials, Observational Studies, and/or Registries of Physical Examination for Clinical Assessment for PAD–Section 2.1.
Evidence Table 3. RCTs of Resting ABI for Diagnosing PAD–Section 3.1.
Evidence Table 4. Nonrandomized Trials, Observational Studies, and/or Registries of Resting ABI for Diagnosing PAD–Section 3.1.
Evidence Table 5. Nonrandomized Trials, Observational Studies, and/or Registries of Physiological Testing–Section 3.2.
Evidence Table 6. Nonrandomized Trials, Observational Studies, and/or Registries of Imaging for Anatomic Assessment (Ultrasound, CTA, MRA, Angiography)–Section 3.3.
Evidence Table 7. RCTs of Imaging for Anatomic Assessment (Ultrasound, CTA, MRA, Angiography)–Section 3.3.
Evidence Table 8. Nonrandomized Trials, Observational Studies, and/or Registries for Abdominal Aortic Aneurysm–Section 4.1.
Evidence Table 9. Nonrandomized Trials, Observational Studies, and/or Registries of Coronary Artery Disease Screening in PAD–Section 4.2.
Evidence Table 10. RCTs for CAD Screening in PAD–Section 4.2.
Evidence Table 11. Nonrandomized Trials, Observational Studies, and/or Registries of Screening in Carotid Artery Disease–Section 4.3.
Evidence Table 12. Nonrandomized Trials, Observational Studies, and/or Registries for Renal Artery Disease–Section 4.4.
Evidence Table 13. RCTs Evaluating Antiplatelet Agents– Section 5.1.
Evidence Table 14. Nonrandomized Trials, Observational Studies, and/or Registries of Antiplatelet Agents–Section 5.2.
Evidence Table 15. Randomized Trials Comparing Statin Agents–Section 5.2.
Evidence Table 16. Nonrandomized Trials, Observational Studies, and/or Registries of Statin Agents–Section 5.2.
Evidence Table 17. RCTs for Antihypertensive Agents– Section 5.3.
Evidence Table 18. Nonrandomized Trials, Observational Studies, and/or Registries of Antihypertensive Agents–Section 5.3.
Evidence Table 19. RCTs for Smoking Cessation–Section 5.4.
Evidence Table 20. Nonrandomized Trials, Observational Studies, and/or Registries of Smoking Cessation–Section 5.4.
Evidence Table 21. RCTs Evaluating Glycemic Control in Patients with PAD and Diabetes Mellitus–Section 5.5.
Evidence Table 22. Nonrandomized Trials, Observational Studies, and/or Registries of Glycemic Control–Section 5.5.
Evidence Table 23. RCTs Evaluating Oral Anticoagulation–Section 5.6.
Evidence Table 24. Nonrandomized Trials, Observational Studies, and/or Registries of Oral Anticoagulation–Section 5.6.
Evidence Table 25. RCTs and Observational Studies of Cilostazol–Section 5.7.
Evidence Table 26. Nonrandomized Trials, Observational Studies, and/or Registries of Pentoxifylline–Section 5.8.
Evidence Table 27. Systematic Review of Chelation Therapy–Section 5.9.
Evidence Table 28. Nonrandomized Trials, Observational Studies, and/or Registries of Homocysteine Lowering Therapy for Lower Extremity PAD in Patients with Diabetes Mellitus–Section 5.10.1.
Evidence Table 29. RCTs Comparing Additional Medical Therapies of Homocysteine Lowering Therapy for Lower Extremity PAD–Section 5.10.1.
Evidence Table 30. RCTs for Influenza Vaccination–Section 5.10.2.
Evidence Table 31. Nonrandomized Trials for Influenza Vaccination–Section 5.10.2.
Evidence Table 32. RCTs for Exercise Therapy–Section 6.
Evidence Table 33. Nonrandomized Trials, Observational Studies, and/or Registries for Exercise Therapy–Section 6.
Evidence Table 34. Nonrandomized Trials and Observational Studies of Minimizing Tissue Loss in Patients with PAD–Section 7.
Data Supplement 34a. Functions of a Multidisciplinary Foot Care / Amputation Prevention Team–Section 7.
Evidence Table 35. RCTs Comparing Endovascular Treatment and Endovascular Versus Noninvasive Treatment of Claudication–Section 8.1.
Evidence Table 36. Nonrandomized Trials, Observational Studies, and/or Registries of Endovascular and Endovascular Versus Noninvasive Treatment of Claudication–Section 8.1.
Evidence Table 37. RCTs Evaluating Surgical Treatment for Claudication–Section 8.1.2.
Evidence Table 38. Nonrandomized Trials, Observational Studies, and/or Registries of Surgical Treatment for Claudication–Section 8.1.2.
Evidence Table 39. RCTs Comparing Endovascular Revascularization for Chronic CLI–Section 8.2.
Evidence Table 40. Nonrandomized Trials, Observational Studies, and/or Registries of Endovascular Revascularization for Chromic CLI–Section 8.2.1.
Evidence Table 41. RCTs of Surgical Revascularization for Chronic CLI–Section 8.2.
Evidence Table 42. Nonrandomized Trials, Observational Studies, and/or Registries for Surgical Revascularization for Chronic CLI–Section 8.2.
Evidence Table 43. RCT Comparing Prostanoids for End-Stage Peripheral Artery Disease–Section 8.2.3.
Evidence Table 44. Nonrandomized Trials, Observational Studies, and/or Registries for Would Healing Therapies for CLI–Section 8.2.3.
Evidence Table 45. Nonrandomized Trials, Observational Studies, and/or Registries of Acute Limb Ischemia–Section 9.1.
Evidence Table 46. Nonrandomized Trials, Observational studies, and/or Registries Comparing Evaluating Noninvasive Testing and Angiography for ALI–Section 9.1.
Evidence Table 47. RCTs of Revascularization Strategy for ALI–Section 9.2.2.
Evidence Table 48. Nonrandomized Trials, Observational Studies, and/or Registries of Clinical Presentation of ALI–Section 9.2.2.
Evidence Table 49. Nonrandomized Trials, Observational Studies, and/or Registries of Diagnostic Evaluation of the Cause of ALI–Section 9.2.2.
Evidence Table 50. Nonrandomized Trials, Observational Studies, and/or Registries of Revascularization Strategy for ALI–Section 9.2.2.
Evidence Table 51. RCTs for Longitudinal Follow-Up–Section 10.
Evidence Table 52. Nonrandomized Trials, Observational Studies, and/or Registries for Longitudinal Follow-Up–Section 10.
References.


