In this perspective, we provide a holistic management approach to middle-aged and older men with functional hypogonadism.
Abstract
Context:
Middle-aged and older men (≥50 years), especially those who are obese and suffer from comorbidities, not uncommonly present with clinical features consistent with androgen deficiency and modestly reduced testosterone levels. Commonly, such men do not demonstrate anatomical hypothalamic–pituitary–testicular axis pathology but have functional hypogonadism that is potentially reversible.
Evidence Acquisition:
Literature review from 1970 to October 2016.
Evidence Synthesis:
Although definitive randomized controlled trials are lacking, evidence suggests that in such men, lifestyle measures to achieve weight loss and optimization of comorbidities, including discontinuation of offending medications, lead to clinical improvement and a modest increase in testosterone. Also, androgen deficiency–like symptoms and end-organ deficits respond to targeted treatments (such as phosphodiesterase-5 inhibitors for erectile dysfunction) without evidence that hypogonadal men are refractory. Unfortunately, lifestyle interventions remain difficult and may be insufficient even if successful. Testosterone therapy should be considered primarily for men who have significant clinical features of androgen deficiency and unequivocally low testosterone levels. Testosterone should be initiated either concomitantly with a trial of lifestyle measures, or after such a trial fails, after a tailored diagnostic work-up, exclusion of contraindications, and appropriate counseling.
Conclusions:
There is modest evidence that functional hypogonadism responds to lifestyle measures and optimization of comorbidities. If achievable, these interventions may have demonstrable health benefits beyond the potential for increasing testosterone levels. Therefore, treatment of underlying causes of functional hypogonadism and of symptoms should be used either as an initial or adjunctive approach to testosterone therapy.
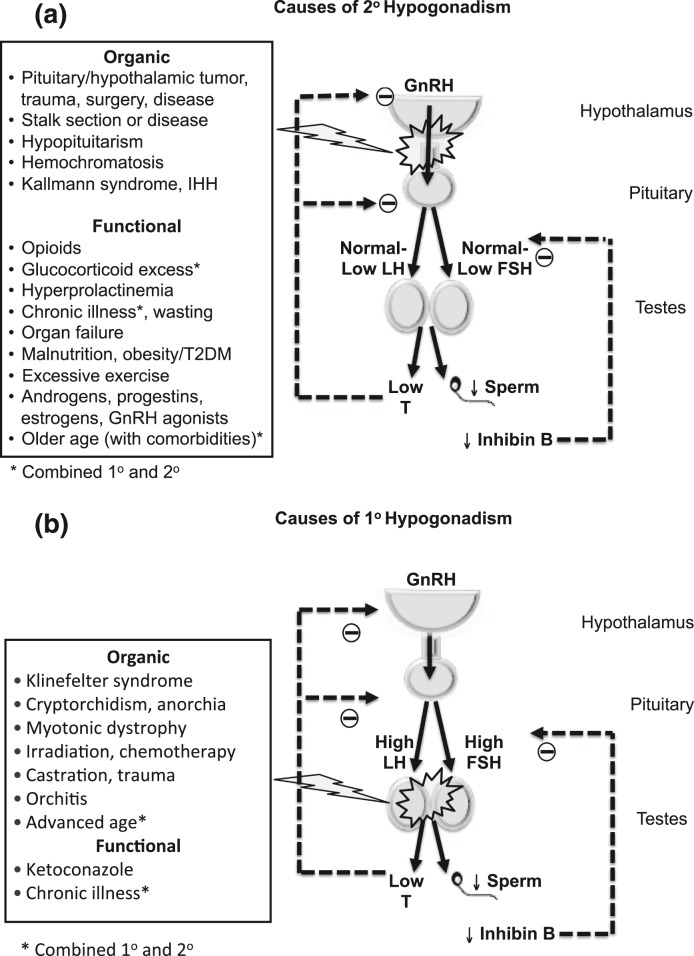
Male hypogonadism is a syndromic diagnosis based on consistent clinical symptoms and signs of androgen deficiency and repeatedly low serum testosterone levels (1, 2). Hypogonadism that is caused by intrinsic structural, destructive, or congenital pathology of the hypothalamic–pituitary–testicular (HPT) axis (such as pituitary tumor or Klinefelter syndrome) is referred to as organic (also termed classical) hypogonadism (Fig. 1). Organic hypogonadism usually warrants testosterone replacement; it is an important diagnosis not to miss, as there is evidence that this condition is underdiagnosed and undertreated (3). Organic hypogonadism can manifest at any age, with some older men presenting with primary hypogonadism due to testicular failure, evidenced by high gonadotropin levels, reduced testicular response to human chorionic gonadotropin, and reduced Leydig cell mass (4, 5).
Figure 1.
Causes of hypogonadism. (a) Causes of secondary hypogonadism. (b) Causes of primary hypogonadism. In middle-aged and older men, functional (late-onset, age-related onset, or adult onset) hypogonadism is usually associated with low or normal gonadotropin levels. In contrast to organic secondary hypogonadism due to structural, destructive, or congenital pathology, functional hypogonadism is due to functional HPT axis suppression. Whereas organic hypogonadism typically presents with clinically and biochemically severe androgen deficiency and is not usually reversible, functional hypogonadism often presents with less severe androgen deficiency, is potentially reversible, and is more common than organic hypogonadism. IHH, idiopathic hypogondadotropic hypogonadism; GnRH, gonadotropin-releasing hormone; FSH, follicle-stimulating hormone; T, testosterone; T2DM, type 2 diabetes mellitus. Adapted from Matsumoto (12).
In contrast, many middle-aged and older men (defined here as aged 50 years or older), especially when obese and suffering from comorbid illness, present with clinical features resembling organic androgen deficiency and modestly to occasionally severely low testosterone levels, yet they do not have recognizable intrinsic structural HPT pathology. In most such men, gonadotropin levels are not elevated (6), and hypogonadism is caused by functional HPT axis suppression in the presence of an intact HPT axis (analogous to functional amenorrhea in women). Provided that specific pathologic etiologies of functional hypogonadism such as hyperprolactinemia or endogenous Cushing syndrome [Fig. 1(a)] have been excluded, the unexplained low serum testosterone concentrations could be due to functional HPT axis suppression caused by excess adiposity, comorbid illness, and/or medications such as opioids or glucocorticoids. In such men, androgen deficiency–like symptoms may be caused by, or at least contributed to by, their comorbid burden, instead of, or in addition to, their low testosterone levels (Table 1) (7). For our perspective, we define functional hypogonadism as the coexistence of androgen deficiency–like features and low serum testosterone concentrations occurring in the absence of both intrinsic structural HPT axis pathology and of specific pathologic conditions suppressing the HPT axis (such as microprolactinoma, endogenous Cushing syndrome) in middle-aged or older men. The community prevalence estimates of potentially functional hypogonadism in middle-aged and older men (also referred to as late-onset, age-related, or adult-onset hypogonadism) vary from 2.1% to 12.3% (8–10) (Supplemental Fig. 1).
Table 1.
Organic Hypogonadism Versus Functional Hypogonadism in Middle-Aged and Older Men
| Organic Hypogonadism | Functional Hypogonadism | |
|---|---|---|
| Condition | Proven HPT axis pathology (structural, destructive, or congenital disease) | No recognizable structural intrinsic HPT axis pathology. No specific pathologic etiologies of functional hypogonadism (diagnosis of exclusion) |
| Reversibility | Established disease state, organic and generally irreversible HPT axis pathology | HPT axis suppression is functional and may be reversible |
| Symptoms/signs | Specific: eunuchoidism. More specific/objective: low libido, small testes, loss of male hair, gynecomastia | Less specific: erectile dysfunction, low energy and mood |
| Testosterone levels | Unequivocally, consistently, and severely low | Borderline low, fluctuating around the lower limit of assay range, occasionally severely low |
| Gonadotropin levels | Elevated (primary hypogonadism) or low/inappropriately normal (secondary hypogonadism) | Usually in the normal range, occasionally low (secondary hypogonadism) |
| Association of low T with symptoms | Causal | Uncertain, symptoms may be predominantly or partially due to comorbid illness |
| Testosterone therapy | Replacement | Replacement? |
| Benefits of therapy | Marked symptomatic and somatic response (except fertility) | Symptomatic and somatic response less well established |
| Risks of therapy | Considered low relative to benefits | Unknown |
Adapted in part from Basaria (7).
In this perspective, we discuss the evidence supporting a holistic approach to the management of middle-aged and older men who present with functional hypogonadism, with a focus on the evidence for therapeutic strategies other than testosterone treatment. There are not enough clinical trial data to determine, when faced with such men, whether the low testosterone is a marker of poor health or part of a syndrome that can be rectified with testosterone treatment. However, we will present our opinion regarding who might benefit from testosterone treatment. We will not comprehensively review the risks and benefits of testosterone treatment, which are covered elsewhere (11, 12).
Characteristics of Functional Hypogonadism as a Rationale for Nontestosterone-Based Management
Several characteristics of functional hypogonadism provide a rationale for the use of therapeutic strategies other than, or in addition to, testosterone treatment (Table 1). First, the clinical features of androgen deficiency in middle-aged and older men are nonspecific. In the European Male Aging Study (EMAS) a cross-sectional survey of community-dwelling men, only nine of 32 candidate symptoms of androgen deficiency were associated with testosterone levels, and of these only three sexual symptoms clustered with low testosterone and had a syndromic association (10). In other observational studies of middle-aged and older men, symptoms and low testosterone also seldomly occurred together (Supplemental Fig. 1) (8–10). In EMAS, even among men with late-onset hypogonadism defined by stringent syndromic criteria, the association of low testosterone with symptoms was attenuated after adjustment for comorbidities (10). Therefore, symptoms in middle-aged and older men with confounding comorbidities may not confirm androgen deficiency even when testosterone levels are low. Second, testosterone levels in men with functional hypogonadism are usually only modestly reduced and fluctuate around the lower limit of normal. Even in stringently defined late-onset hypogonadism, 60% had testosterone levels ≥8.0 nmol/L (231 ng/day) (10). Therefore, therapeutic strategies that result in modest increases in testosterone may be sufficient to normalize circulating testosterone in many men. Third, the HPT axis suppression in men with functional hypogonadism is potentially reversible. In a 15-year longitudinal study of US men with symptomatic androgen deficiency, >50% remitted either because symptoms resolved or testosterone normalized (13). Moreover, as discussed later, the HPT axis remains responsive to lifestyle and pharmacological measures. Fourth, functional hypogonadism is relatively uncommon in healthy men. In EMAS, obesity increased the prevalence of late-onset hypogonadism by 13-fold and comorbidities by ninefold. Late-onset hypogonadism was uncommon in men who were lean (0.4%) or healthy (0.6%) and was relatively rare in men who were both (10). Given this coexistence, it is unclear whether the lowered testosterone drives ill health or whether, conversely, obesity and chronic disease cause poor outcomes. Whether low testosterone is a maladaptive, neutral, or even beneficial state remains unknown in the absence of definitive intervention trials.
In contrast to men with functional hypogonadism who have only modestly low testosterone levels, men with prostate cancer receiving androgen deprivation therapy develop very low testosterone and severely symptomatic androgen deficiency with osteo-sarcopenic obesity and increased insulin resistance (14). Thus, the maintenance of some minimum level of circulating testosterone is clearly important in older men, although the threshold levels below which adverse health outcomes manifest remain unknown. In the Testosterone Trials of stringently selected symptomatic men with a baseline testosterone averaging < 9.5 nmol/L (275 ng/dL) for no apparent reason other than age, testosterone treatment had a modest benefit in sexual function, and some benefit with respect to mood and depressive symptoms (15).
General Considerations for Management of Middle-Aged and Older Men With Functional Hypogonadism
Treatment other than testosterone treatment can involve lifestyle measures and optimization of chronic disease aimed at reversing the functional hypogonadism and targeting the specific presenting clinical manifestations. Optimization of lifestyle measures and comorbidities has the potential to improve all symptoms, as well as testosterone levels and general health. Targeted treatment of specific end-organ dysfunction also may improve specific clinical features and, as demonstrated with phosphodiesterase-5 (PDE5) inhibitors discussed later (16), may also increase testosterone slightly.
Evidence for Lifestyle Measures
In large prospective observational studies, weight gain and development of comorbidities were consistently associated with an acceleration of the age-related decline in testosterone levels (17, 18). A 4 to 5 kg⁄m2 increase in body mass index (BMI) or an incident chronic disease such as type 2 diabetes is associated with declines in testosterone levels equivalent to 10 years of aging (17). In the Framingham Heart Study, a high cardiovascular disease risk factor burden markedly increased the age-related testosterone decline (18). These data suggest that maintaining good health may prevent, or at least decelerate, the age-related decline in testosterone, although causality cannot be inferred from these observational studies.
Weight Loss
Obesity is the strongest risk factor associated with low testosterone levels middle-aged and older men (6). Typically, luteinizing hormone (LH) levels are not elevated (6). In a cross-sectional analysis among 1822 men from the Boston Area Community Health cohort, central adiposity was the strongest risk factor for symptomatic androgen deficiency, overriding age and overall health status (19). In EMAS, compared with lean weight, overweight (BMI, 25 to 30 kg/m2) was associated with a 2.3 nmol/L (66 ng/dL) reduction and obesity with a 5.1 nmol/L (147 ng/dL) reduction in total testosterone and, respectively, a 17.6 pmol/L (5.1 pg/mL) and 53.7 pmol/L (15.5 pg/mL) reduction in free testosterone (6).
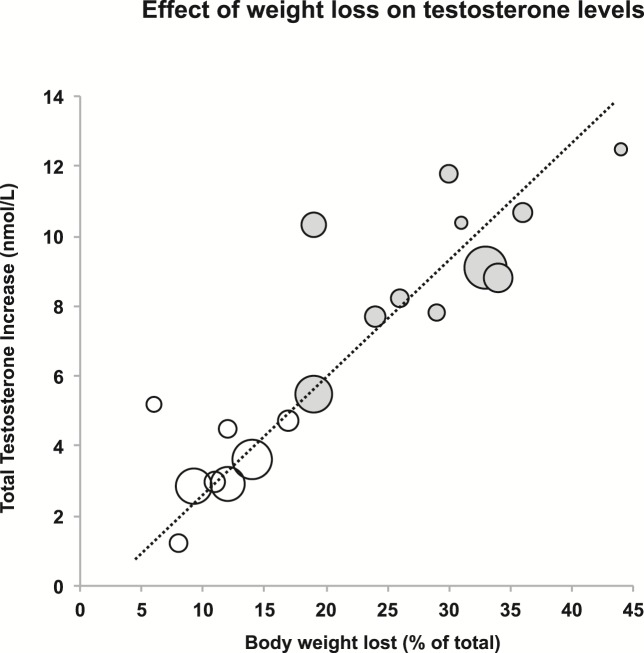
Consistent with these observational data, successful weight loss can lead to increases in testosterone in obese men (Fig. 2; Supplemental Table 1) (20). In a meta-analysis, diet-associated weight loss (mean 9.8%) increased testosterone by 2.9 nmol/L (84 ng/dL) and surgical weight loss (32%) by 8.7 nmol/L (251 ng/dL) (21). However, this meta-analysis was limited to small studies (total < 500 patients) with short-term follow-up and heterogeneous study designs (21). Longitudinal data from EMAS have reported similar findings in older less obese men (mean age 58 years; BMI, 27.6 kg/m2) (22). During 4.4 years, weight loss of 5% was associated a significant linear rise in serum sex hormone–binding globulin, probably because insulin resistance improved (22). This weight loss of 5% was associated with an increase in total testosterone [+2 nmol/L (+58 ng/dL)] but not in free testosterone. Weight gain was associated with opposite changes. Weight loss > 15% was associated with more marked increases in total testosterone [+5.7 nmol/L (+164 ng/dL)], LH (+2.2 IU/L), and free testosterone [+52 pmol/L (+52 pg/mL)] (22). In the aforementioned meta-analysis (21), LH similarly increased by 1.3 IU/L and free testosterone by 42 pmol/L (12.1 pg/mL) with weight loss (21). Therefore, significant weight loss can normalize the HPT axis, probably by amelioration of the obesity-associated hypothalamic–pituitary–gonadal axis suppression. Serum testosterone should therefore be remeasured when symptoms of androgen deficiency persist after significant weight loss. Re-evaluation for organic HPT pathology may be required if the testosterone concentration remains low despite successful weight loss.
Figure 2.
Effect of weight loss on testosterone levels. Each datum point refers to an individual study, and the size of the datum point is proportional to the size of the study, ranging from 10 to 293 men. Open circles represent studies where weight loss was achieved by diet and exercise, and filled circles by bariatric surgery. Updated from Grossmann (20). For individual studies, refer to Supplemental Table 1.
Lifestyle-achieved weight loss can also improve erectile dysfunction, a common symptom for which men with low testosterone seek treatment. In an intensive lifestyle randomized controlled trial (RCT) of relatively healthy obese middle-aged men, 15% weight loss increased the mean International Index of Erectile Function (IIEF) score by 3.10, which is clinically meaningful (23) and similar to the increase of 2.64 reported in the Testosterone Trials, although those men were older (15). In older diabetic men (24), weight loss (9.9%) increased the IIEF score by 1.3 relative to controls. This reduced efficacy in older diabetic men was likely due to higher rates of irreversible erectile dysfunction due to neurovascular disease (24). In a meta-analysis of four RCTs, lifestyle intervention increased the IIEF score by 2.66 (25). Although head-to-head studies are lacking, lifestyle interventions appear less effective than PDE5 inhibitors, which increased IIEF scores by 5.66 to 7.49 in a meta-analysis (26). Bariatric surgery studies have reported large improvements in sexual function and sexual quality of life. In a 2-year prospective controlled study of gastric bypass surgery in 64 men (age 49 years; BMI, 46 kg/m2), 30% weight loss increased testosterone by 10.8 nmol/L (311 ng/dL) and reduced sexual dissatisfaction by 7.5 on a 20-scale questionnaire (27). Mechanisms other than the testosterone increase, including psychosocial factors and body image, may play a role in improved sexual function after bariatric surgery. In one prospective study, the degree of weight loss and baseline erectile function predicted improved erectile function, whereas changes in reproductive hormones did not (28). Knowledge that obesity may be contributing to sexual symptoms and low testosterone levels could provide the motivation to lose weight in some patients.
Exercise
In middle-aged and older men, low testosterone is associated with reduced physical activity and several end-organ deficits such as sarcopenia, reduced bone mass, or the metabolic syndrome (10). Even modest exercise can lead to improvement in features of the metabolic syndrome (29) and to improvements in muscle mass, bone density, and physical function in such men (30). In obese men, exercise decreased body weight by 5.9 kg and improved erectile function (2.6 increase in IIEF-5 score) (31). Given multiple health benefits, exercise, similar to weight loss, should be encouraged routinely.
Optimization of Chronic Disease
Almost any acute or chronic disease can mimic or confound clinical features of androgen deficiency and lead to functional HPT axis suppression. Although intuitive, evidence that optimization of chronic disease might improve features of functional hypogonadism is relatively limited. Supplemental Fig. 2 shows testosterone reductions associated with selected chronic diseases or medications from large observational studies or meta-analyses (6, 32–34). Although such studies have potential for confounding, one may, analogous to weight loss effects, posit that potential testosterone increases achievable with stopping offending medications or optimizing comorbidities could range from 2 to 5 nmol/L (58 to 144 ng/dL).
Type 2 diabetes and obstructive sleep apnoea (OSA), commonly associated with functional hypogonadism, will be discussed as representative examples. Up to 50% of men with type 2 diabetes have low testosterone. The HPT axis and dysglycemia interact in a complex bidirectional relationship with multiple underlying potential mechanisms, including visceral adiposity and the body composition–independent effects implicated (20). Evidence for improvement in symptoms and testosterone levels following stabilization of poorly controlled diabetes is largely anecdotal and confounded by weight change. A longitudinal study in 265 men with a baseline hemoglobin A1c of 7.6% reported an inverse relationship between changes in glycemic control and testosterone levels. In those whose glycemic control improved over time, testosterone increased, whereas in those whose control worsened, testosterone decreased (35). This suggests that optimizing glycemic control may improve testosterone even in men with fairly well controlled diabetes, although such observational data do not prove causality.
OSA is also frequently associated low testosterone levels, but multiple additional mechanisms such as sleep fragmentation, shared cardiovascular risk factors, and obesity can explain symptoms such as sexual dysfunction or low energy (36). Indeed, continuous positive airway pressure (CPAP), the first-line treatment of OSA, improves erectile dysfunction, fatigue, as well as quality of life (36). However, in a recent meta-analysis including 232 men, CPAP had no consistent effect on testosterone levels (37). Although the precise effect of CPAP on serum testosterone levels requires further study, this suggests that mechanisms other than increasing testosterone, including improved hypoxia and endothelial function, may explain the benefit of CPAP (38). In a RCT of 67 men with OSA not treated with CPAP, testosterone treatment did not improve quality of life, neurocognitive function, or sexual function, except for a modest increase in sexual desire (39).
Targeting the Clinical Problem
Middle-aged and older men with lowered testosterone are a heterogeneous population, and the clinical presentation can differ among individuals. Multiple factors other than low testosterone can contribute to clinical features that are also caused by androgen deficiency. For example, severe osteoporosis should not be attributed to modestly low testosterone but requires a tailored work-up to exclude other causes. For most symptoms and end-organ deficits associated with lowered testosterone, specific first-line therapies targeting clinical features individually are available. Erectile dysfunction, a common symptom that men present with, and reduced bone density, a common end-organ deficit, are used as examples.
Erectile dysfunction
In a rigorous RCT of 140 middle-aged men (mean age, 55 years) with sexual dysfunction and testosterone < 11.4 nmol/L (329 ng/dL), erectile function was initially optimized with sildenafil in all men, leading to a large, clinically meaningful improvement in erectile function (+7.7 on the IIEF erectile function domain) (16). Thus, men with low to low-normal testosterone levels can respond well to PDE5 inhibitors, consistent with an analysis of 1075 men reporting that baseline testosterone levels did not influence PDE5 inhibitor responsiveness (40). In phase 2 of the RCT of sildenafil, men were subsequently randomized to testosterone treatment or placebo. Testosterone had no added benefit in combination with sildenafil on sexual function (16). Interestingly, sildenafil alone increased testosterone by 30% (16). Though sildenafil may have a direct action on the testes (41), this increase is also consistent with evidence that resumption of sexual activity by itself can increase testosterone by 30% (42). Although head-to-head studies are lacking, the efficacy reported for PDE5 inhibitors is greater than that reported for lifestyle intervention and testosterone treatment (15, 25). The American College of Physicians guidelines emphasize lifestyle and risk factor modification, given that erectile dysfunction is an early marker of cardiovascular risk, recommend PDE5 inhibitors as first-line pharmacotherapy, and judge the evidence for testosterone treatment insufficient to guide definitive recommendations (43).
Osteoporosis
Low testosterone levels are associated with reduced bone mineral density (44) and with increased fracture risk (45). Testosterone treatment increases bone mineral density in older men with low testosterone (46, 47). However, trials were too short and not designed to determine fracture rates. In 1199 men with osteoporosis, zoledronic acid decreased vertebral fracture risk by 67%, irrespective of baseline testosterone (48). In 1468 men with nonmetastatic prostate cancer receiving androgen deprivation therapy, who have castrate levels of sex steroids, denosumab reduced vertebral fracture risk by 62% (49). Therefore, men with very low testosterone levels are not refractory to conventional antiresorptive treatment. The Endocrine Society recommended that men with high fracture risk should receive an agent with proven antifracture activity irrespective of receiving testosterone or not (50).
Testosterone Therapy
In some men, measures to reverse functional hypogonadism may be unsuccessful, either because implementation is not feasible (e.g., cessation of opioids in chronic pain or methadone maintenance patients) or they are not achieved or maintained (e.g., sustained weight loss in obese patients). Significant clinical features consistent with androgen deficiency and/or consistently low testosterone may persist even despite successful implementation of these measures. Re-evaluation for organic HPT pathology should be considered, as this can be missed. Moreover, low testosterone may contribute to fatigue or poor motivation, reducing the ability to initiate healthy lifestyle measures. Therefore, testosterone treatment in middle-aged and older men with substantially low testosterone may increase their motivation to diet and exercise, although clinical studies supporting this concept are lacking. Finally, although few studies to date have tested this hypothesis, testosterone treatment may augment the benefits of successful lifestyle interventions. For example, an RCT in 100 middle-aged obese men with low testosterone subjected to a rigorous weight loss program has reported that although men receiving placebo lost both fat and lean body mass, testosterone treatment prevented the diet-associated loss of lean mass, and weight loss in testosterone-treated men was almost exclusively due to loss of body fat (51).
In situations where reversal of functional hypogonadism suppression is not successful, the question arises as to whether a trial of testosterone treatment is justified to determine whether there is clinical benefit. In RCTs of middle-aged and older men, where contraindications have been rigorously excluded and treatment was carefully monitored, short-term testosterone treatment has been associated with a low risk of adverse events (15). After testosterone initiation, symptoms should improve within 3 to 6 months if low testosterone contributed significantly to clinical manifestations, provided that adequate testosterone levels are achieved and maintained. Weakness (a symptom of severe hypogonadism) may not improve after 3 to 6 months of testosterone treatment.
If testosterone treatment is considered, contraindications must be excluded. We advise following the recommendations made in the Endocrine Society guidelines (1). These guideline recommendations are consistent with the exclusion criteria specified in the Testosterone Trials (15). Accordingly, we generally do not offer testosterone treatment to men with a history of, or at high risk for, prostate cancer, severe benign prostatic hyperplasia, or with high cardiovascular risk (1, 15). In the 12-month Testosterone Trials, the rates of adverse events were similar in the testosterone- and placebo-treated men. Although the trial was too small to reliably assess testosterone treatment-associated risks (15), it does provide some assurance about the potential relative short-term safety of testosterone treatment in men without the previously mentioned contraindications. The decision to treat with testosterone or not will take into account both the anticipated benefits and risks in the context of each individual patient. In men with functional hypogonadism, testosterone should therefore only be commenced after appropriate counseling. It is our practice to inform the patient with functional hypogonadism about the absence of high-level evidence regarding long-term benefits and risks. Clear patient-specific goals should be identified, and treatment should be stopped if these goals are not achieved. Monitoring for adverse events should follow consensus recommendations (1). Testosterone treatment should be targeted to men with more severe and specific symptoms and signs of androgen deficiency and unequivocally and repeatedly low testosterone levels. The benefit/risk ratio will be better in the younger leaner man with fewer comorbidities, who has more specific symptoms of androgen deficiency and consistently low testosterone (Supplemental Fig. 3).
Other Medications Intended to Increase Endogenous Testosterone Levels
Aromatase inhibitors and selective estrogen receptor modulators are prescribed off-label by some practitioners to antagonize the hypothalamic negative feedback of circulating estradiol on pituitary gonadotropin production, thereby stimulating endogenous gonadotropin and testosterone secretion. These agents require residual hypothalamic–pituitary responsiveness and are not indicated or are ineffective in organic hypogonadotrophic hypogonadism, and they will not work in primary hypogonadism. Although these agents can lead to robust increases in testosterone levels in men with functional HPT axis suppression, RCTs have been small and short-term, and clinical outcome data are very limited (Supplemental Table 2). Additionally, aromatase inhibitors decrease estradiol levels, and there is evidence that estradiol has important biological actions not only for maintenance of bone mass in men, but also for optimal sexual function and the prevention of fat gain (52). Also, RCTs of dehydroepiandrosterone, dihydrotestosterone, selective androgen receptor modulators, or human chorionic gonadotropin (Supplemental Table 2) have been relatively small, short-term, and have not been designed to provide definitive evidence for clinical use. More evidence is required to guide if and when such agents should be considered.
Conclusions
Middle-aged and older men with chronic disease and/or obesity commonly present with nonspecific symptoms and modestly low testosterone levels. This makes the diagnosis of androgen deficiency more difficult than in younger otherwise healthy men without comorbidities. Men with specific features of androgen deficiency and unequivocally low testosterone levels should be evaluated for an underlying pathological cause (particularly if testosterone levels are severely low), and it should not be assumed that their presentation is a nonspecific consequence of age-related comorbidities or obesity.
Increasing evidence suggests that in many middle-aged and older men low testosterone is due to functional HPT axis suppression. The nonspecific androgen deficiency-like symptoms may, at least in part, be mediated by comorbidities. Knowledge that obesity and comorbidities might be causing symptoms and why testosterone levels are low may motivate some men to more actively engage in lifestyle interventions. Although dedicated studies to assess the efficacy of lifestyle measures, such as weight loss, and optimization of comorbidities among stringently selected symptomatic men with low testosterone levels are lacking, the current evidence supports these measures as first-line management approaches, as they have the potential to improve androgen deficiency–like symptoms irrespective of their effect on testosterone levels, reverse the HPT axis suppression to a degree sufficient to normalize the often only modestly reduced testosterone levels, and improve overall health. Thus, these interventions may have demonstrable health benefits beyond the potential for increasing testosterone levels. These measures should be accompanied by evidence-based treatment targeting the main clinical problem (e.g., erectile dysfunction or osteoporosis).
However, lifestyle intervention, management of comorbid illnesses, and discontinuation of offending medications may be difficult and many times not possible. For example, substantial weight loss in obese men is required to achieve meaningful increases in testosterone levels. Even if successful, such measures may be insufficient to relieve symptoms and to normalize testosterone levels. In appropriately selected men, in the context of this holistic approach encompassing treatment of the underlying causes of functional HPT axis suppression and of symptoms, testosterone treatment could be started concomitantly or after these initial measures fail. In contrast, the use of other medications intended to increase testosterone levels cannot currently be recommended outside of clinical trials due to lack of evidence.
Supplementary Material
Acknowledgments
Disclosure Summary: M.G. has received research funding from Bayer Schering, Novartis, Weight Watchers, and Eli Lilly, and speaker’s honoraria from Besins Healthcare. A.M.M. has received research funding from AbbVie and GSK, consulting fees from AbbVie, Endo, Eli Lilly, Lipocine, USADA, and the Partnership for Clean Competition, and royalties from UpToDate.
Abbreviations:
- BMI
body mass index
- CPAP
continuous positive airway pressure
- EMAS
European Male Aging Study
- HPT
hypothalamic–pituitary–testicular
- IIEF
International Index of Erectile Function
- LH
luteinizing hormone
- OSA
obstructive sleep apnoea
- PDE5
phosphodiesterase-5
- RCT
randomized controlled trial
References
- 1. Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM; Task Force, Endocrine Society . Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2010;95(6):2536–2559. [DOI] [PubMed] [Google Scholar]
- 2. Wang C, Nieschlag E, Swerdloff R, Behre HM, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morales A, Morley JE, Schulman C, Thompson IM, Weidner W, Wu FC; International Society of Andrology (ISA); International Society for the Study of Aging Male (ISSAM); European Association of Urology (EAU); European Academy of Andrology (EAA); American Society of Andrology (ASA) . Investigation, treatment, and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA, and ASA recommendations. J Androl. 2009;30(1):1–9. [DOI] [PubMed] [Google Scholar]
- 3. Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003;88(2):622–626. [DOI] [PubMed] [Google Scholar]
- 4. Harman SM, Tsitouras PD. Reproductive hormones in aging men. I. Measurement of sex steroids, basal luteinizing hormone, and Leydig cell response to human chorionic gonadotropin. J Clin Endocrinol Metab. 1980;51(1):35–40. [DOI] [PubMed] [Google Scholar]
- 5. Neaves WB, Johnson L, Porter JC, Parker CR Jr, Petty CS. Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab. 1984;59(4):756–763. [DOI] [PubMed] [Google Scholar]
- 6. Tajar A, Forti G, O’Neill TW, Lee DM, Silman AJ, Finn JD, Bartfai G, Boonen S, Casanueva FF, Giwercman A, Han TS, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Vanderschueren D, Huhtaniemi IT, Wu FC; EMAS Group . Characteristics of secondary, primary, and compensated hypogonadism in aging men: evidence from the European Male Ageing Study. J Clin Endocrinol Metab. 2010;95(4):1810–1818. [DOI] [PubMed] [Google Scholar]
- 7. Basaria S. Testosterone therapy in older men with late-onset hypogonadism: a counter-rationale. Endocr Pract. 2013;19(5):853–863. [DOI] [PubMed] [Google Scholar]
- 8. Araujo AB, O’Donnell AB, Brambilla DJ, Simpson WB, Longcope C, Matsumoto AM, McKinlay JB. Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2004;89(12):5920–5926. [DOI] [PubMed] [Google Scholar]
- 9. Araujo AB, Esche GR, Kupelian V, O’Donnell AB, Travison TG, Williams RE, Clark RV, McKinlay JB. Prevalence of symptomatic androgen deficiency in men. J Clin Endocrinol Metab. 2007;92(11):4241–4247. [DOI] [PubMed] [Google Scholar]
- 10. Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O’Neill TW, Bartfai G, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Lean ME, Pendleton N, Punab M, Boonen S, Vanderschueren D, Labrie F, Huhtaniemi IT; EMAS Group . Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med. 2010;363(2):123–135. [DOI] [PubMed] [Google Scholar]
- 11. Spitzer M, Huang G, Basaria S, Travison TG, Bhasin S. Risks and benefits of testosterone therapy in older men. Nat Rev Endocrinol. 2013;9(7):414–424. [DOI] [PubMed] [Google Scholar]
- 12. Matsumoto AM. Testosterone administration in older men. Endocrinol Metab Clin North Am. 2013;42(2):271–286. [DOI] [PubMed] [Google Scholar]
- 13. Travison TG, Shackelton R, Araujo AB, Hall SA, Williams RE, Clark RV, O’Donnell AB, McKinlay JB. The natural history of symptomatic androgen deficiency in men: onset, progression, and spontaneous remission. J Am Geriatr Soc. 2008;56(5):831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grossmann M, Zajac JD. Management of side effects of androgen deprivation therapy. Endocrinol Metab Clin North Am. 2011;40(3):655–671, x. [DOI] [PubMed] [Google Scholar]
- 15. Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM, Barrett-Connor E, Swerdloff RS, Wang C, Ensrud KE, Lewis CE, Farrar JT, Cella D, Rosen RC, Pahor M, Crandall JP, Molitch ME, Cifelli D, Dougar D, Fluharty L, Resnick SM, Storer TW, Anton S, Basaria S, Diem SJ, Hou X, Mohler ER III, Parsons JK, Wenger NK, Zeldow B, Landis JR, Ellenberg SS; Testosterone Trials Investigators . Effects of testosterone treatment in older men. N Engl J Med. 2016;374(7):611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Spitzer M, Basaria S, Travison TG, Davda MN, Paley A, Cohen B, Mazer NA, Knapp PE, Hanka S, Lakshman KM, Ulloor J, Zhang A, Orwoll K, Eder R, Collins L, Mohammed N, Rosen RC, DeRogatis L, Bhasin S. Effect of testosterone replacement on response to sildenafil citrate in men with erectile dysfunction: a parallel, randomized trial. Ann Intern Med. 2012;157(10):681–691. [DOI] [PubMed] [Google Scholar]
- 17. Travison TG, Araujo AB, Kupelian V, O’Donnell AB, McKinlay JB. The relative contributions of aging, health, and lifestyle factors to serum testosterone decline in men. J Clin Endocrinol Metab. 2007;92(2):549–555. [DOI] [PubMed] [Google Scholar]
- 18. Haring R, Xanthakis V, Coviello A, Sullivan L, Bhasin S, Wallaschofski H, Murabito JM, Vasan RS. Clinical correlates of sex steroids and gonadotropins in men over the late adulthood: the Framingham Heart Study. Int J Androl. 2012;35(6):775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hall SA, Esche GR, Araujo AB, Travison TG, Clark RV, Williams RE, McKinlay JB. Correlates of low testosterone and symptomatic androgen deficiency in a population-based sample. J Clin Endocrinol Metab. 2008;93(10):3870–3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Grossmann M. Low testosterone in men with type 2 diabetes: significance and treatment. J Clin Endocrinol Metab. 2011;96(8):2341–2353. [DOI] [PubMed] [Google Scholar]
- 21. Corona G, Rastrelli G, Monami M, Saad F, Luconi M, Lucchese M, Facchiano E, Sforza A, Forti G, Mannucci E, Maggi M. Body weight loss reverts obesity-associated hypogonadotropic hypogonadism: a systematic review and meta-analysis. Eur J Endocrinol. 2013;168(6):829–843. [DOI] [PubMed] [Google Scholar]
- 22. Camacho EM, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, Lee DM, Tajar A, Bartfai G, Boonen S, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Keevil B, Lean ME, Pendleton N, Punab M, Vanderschueren D, Wu FC; EMAS Group . Age-associated changes in hypothalamic–pituitary–testicular function in middle-aged and older men are modified by weight change and lifestyle factors: longitudinal results from the European Male Ageing Study. Eur J Endocrinol. 2013;168(3):445–455. [DOI] [PubMed] [Google Scholar]
- 23. Esposito K, Giugliano F, Di Palo C, Giugliano G, Marfella R, D’Andrea F, D’Armiento M, Giugliano D. Effect of lifestyle changes on erectile dysfunction in obese men: a randomized controlled trial. JAMA. 2004;291(24):2978–2984. [DOI] [PubMed] [Google Scholar]
- 24. Wing RR, Rosen RC, Fava JL, Bahnson J, Brancati F, Gendrano Iii IN, Kitabchi A, Schneider SH, Wadden TA. Effects of weight loss intervention on erectile function in older men with type 2 diabetes in the Look AHEAD trial. J Sex Med. 2010;7(1 Pt 1):156–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gupta BP, Murad MH, Clifton MM, Prokop L, Nehra A, Kopecky SL. The effect of lifestyle modification and cardiovascular risk factor reduction on erectile dysfunction: a systematic review and meta-analysis. Arch Intern Med. 2011;171(20):1797–1803. [DOI] [PubMed] [Google Scholar]
- 26. Yuan J, Zhang R, Yang Z, Lee J, Liu Y, Tian J, Qin X, Ren Z, Ding H, Chen Q, Mao C, Tang J. Comparative effectiveness and safety of oral phosphodiesterase type 5 inhibitors for erectile dysfunction: a systematic review and network meta-analysis. Eur Urol. 2013;63(5):902–912. [DOI] [PubMed] [Google Scholar]
- 27. Hammoud A, Gibson M, Hunt SC, Adams TD, Carrell DT, Kolotkin RL, Meikle AW. Effect of Roux-en-Y gastric bypass surgery on the sex steroids and quality of life in obese men. J Clin Endocrinol Metab. 2009;94(4):1329–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mora M, Aranda GB, de Hollanda A, Flores L, Puig-Domingo M, Vidal J. Weight loss is a major contributor to improved sexual function after bariatric surgery. Surg Endosc. 2013;27(9):3197–3204. [DOI] [PubMed] [Google Scholar]
- 29. Pattyn N, Cornelissen VA, Eshghi SR, Vanhees L. The effect of exercise on the cardiovascular risk factors constituting the metabolic syndrome: a meta-analysis of controlled trials. Sports Med. 2013;43(2):121–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Villareal DT, Chode S, Parimi N, Sinacore DR, Hilton T, Armamento-Villareal R, Napoli N, Qualls C, Shah K. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364(13):1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Khoo J, Tian HH, Tan B, Chew K, Ng CS, Leong D, Teo RC, Chen RY. Comparing effects of low- and high-volume moderate-intensity exercise on sexual function and testosterone in obese men. J Sex Med. 2013;10(7):1823–1832. [DOI] [PubMed] [Google Scholar]
- 32. Bawor M, Bami H, Dennis BB, Plater C, Worster A, Varenbut M, Daiter J, Marsh DC, Steiner M, Anglin R, Coote M, Pare G, Thabane L, Samaan Z. Testosterone suppression in opioid users: a systematic review and meta-analysis. Drug Alcohol Depend. 2015;149:1–9. [DOI] [PubMed] [Google Scholar]
- 33. Kamischke A, Kemper DE, Castel MA, Lüthke M, Rolf C, Behre HM, Magnussen H, Nieschlag E. Testosterone levels in men with chronic obstructive pulmonary disease with or without glucocorticoid therapy. Eur Respir J. 1998;11(1):41–45. [DOI] [PubMed] [Google Scholar]
- 34. Corona G, Monami M, Rastrelli G, Aversa A, Tishova Y, Saad F, Lenzi A, Forti G, Mannucci E, Maggi M. Testosterone and metabolic syndrome: a meta-analysis study. J Sex Med. 2011;8(1):272–283. [DOI] [PubMed] [Google Scholar]
- 35. Grossmann M, Thomas MC, Panagiotopoulos S, Sharpe K, Macisaac RJ, Clarke S, Zajac JD, Jerums G. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J Clin Endocrinol Metab. 2008;93(5):1834–1840. [DOI] [PubMed] [Google Scholar]
- 36. Wittert G. The relationship between sleep disorders and testosterone. Curr Opin Endocrinol Diabetes Obes. 2014;21(3):239–243. [DOI] [PubMed] [Google Scholar]
- 37. Zhang XB, Jiang XT, Du YP, Yuan YT, Chen B. Efficacy of continuous positive airway pressure on testosterone in men with obstructive sleep apnea: a meta-analysis. PLoS One. 2014;9(12):e115033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoyos CM, Melehan KL, Liu PY, Grunstein RR, Phillips CL. Does obstructive sleep apnea cause endothelial dysfunction? A critical review of the literature. Sleep Med Rev. 2015;20:15–26. [DOI] [PubMed] [Google Scholar]
- 39. Melehan KL, Hoyos CM, Yee BJ, Wong KK, Buchanan PR, Grunstein RR, Liu PY. Increased sexual desire with exogenous testosterone administration in men with obstructive sleep apnea: a randomized placebo-controlled study. Andrology. 2016;4(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mulhall JP, Brock GB, Glina S, Baygani S, Donatucci CF, Maggi M. Impact of baseline total testosterone level on successful treatment of sexual dysfunction in men taking once-daily tadalafil 5 mg for lower urinary tract symptoms and benign prostatic hyperplasia: an integrated analysis of three randomized controlled trials. J Sex Med. 2016;13(5):843–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spitzer M, Bhasin S, Travison TG, Davda MN, Stroh H, Basaria S. Sildenafil increases serum testosterone levels by a direct action on the testes. Andrology. 2013;1(6):913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jannini EA, Screponi E, Carosa E, Pepe M, Lo Giudice F, Trimarchi F, Benvenga S. Lack of sexual activity from erectile dysfunction is associated with a reversible reduction in serum testosterone. Int J Androl. 1999;22(6):385–392. [DOI] [PubMed] [Google Scholar]
- 43. Qaseem A, Snow V, Denberg TD, Casey DE Jr, Forciea MA, Owens DK, Shekelle P; Clinical Efficacy Assessment Subcommittee of the American College of Physicians . Hormonal testing and pharmacologic treatment of erectile dysfunction: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2009;151(9):639–649. [DOI] [PubMed] [Google Scholar]
- 44. Tajar A, Huhtaniemi IT, O’Neill TW, Finn JD, Pye SR, Lee DM, Bartfai G, Boonen S, Casanueva FF, Forti G, Giwercman A, Han TS, Kula K, Labrie F, Lean ME, Pendleton N, Punab M, Vanderschueren D, Wu FC; EMAS Group . Characteristics of androgen deficiency in late-onset hypogonadism: results from the European Male Aging Study (EMAS). J Clin Endocrinol Metab. 2012;97(5):1508–1516. [DOI] [PubMed] [Google Scholar]
- 45. LeBlanc ES, Nielson CM, Marshall LM, Lapidus JA, Barrett-Connor E, Ensrud KE, Hoffman AR, Laughlin G, Ohlsson C, Orwoll ES; Osteoporotic Fractures in Men Study Group . The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab. 2009;94(9):3337–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Holmes JH, Dlewati A, Staley J, Santanna J, Kapoor SC, Attie MF, Haddad JG Jr, Strom BL. Effect of testosterone treatment on bone mineral density in men over 65 years of age. J Clin Endocrinol Metab. 1999;84(6):1966–1972. [DOI] [PubMed] [Google Scholar]
- 47. Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab. 2004;89(2):503–510. [DOI] [PubMed] [Google Scholar]
- 48. Boonen S, Reginster JY, Kaufman JM, Lippuner K, Zanchetta J, Langdahl B, Rizzoli R, Lipschitz S, Dimai HP, Witvrouw R, Eriksen E, Brixen K, Russo L, Claessens F, Papanastasiou P, Antunez O, Su G, Bucci-Rechtweg C, Hruska J, Incera E, Vanderschueren D, Orwoll E. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367(18):1714–1723. [DOI] [PubMed] [Google Scholar]
- 49. Smith MR, Egerdie B, Hernández Toriz N, Feldman R, Tammela TL, Saad F, Heracek J, Szwedowski M, Ke C, Kupic A, Leder BZ, Goessl C; Denosumab HALT Prostate Cancer Study Group . Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361(8):745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Watts NB, Adler RA, Bilezikian JP, Drake MT, Eastell R, Orwoll ES, Finkelstein JS; Endocrine Society . Osteoporosis in men: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(6):1802–1822. [DOI] [PubMed] [Google Scholar]
- 51. Ng Tang Fui M, Prendergast LA, Dupuis P, Raval M, Strauss BJ, Zajac JD, Grossmann M. Effects of testosterone treatment on body fat and lean mass in obese men on a hypocaloric diet: a randomised controlled trial. BMC Med. 2016;14(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Finkelstein JS, Lee H, Burnett-Bowie SA, Pallais JC, Yu EW, Borges LF, Jones BF, Barry CV, Wulczyn KE, Thomas BJ, Leder BZ. Gonadal steroids and body composition, strength, and sexual function in men. N Engl J Med. 2013;369(11):1011–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.