Abstract
Purpose
Male cancer survivors have a higher risk of cancer than the general population, which might be caused by an increased prevalence of obesity or susceptibility to obesity-related carcinogenesis. We assessed the effects of obesity before the diagnosis of a first cancer on the development of secondary primary cancers (SPCs).
Methods
The study population consisted of 239,615 Korean male cancer survivors between January 2003 and December 2010. Incident SPCs were assessed throughout follow-up until December 2011. Cox proportional hazards models were used to calculate the hazard ratios of SPCs associated with prediagnosis body mass index (BMI), which were compared with those of first cancers in all cohort participants.
Results
After 1,614,583 person-years of follow-up, we observed 4,799 patients with SPC. The age-standardized incidence rate of cancer in cancer survivors was 1.1 times higher than that of the general population. We found positive linear trends between prediagnosis BMI and risk of all-combined, colorectal, liver, lymphoma, biliary tract, kidney, and obesity-related SPCs. The magnitude of the BMI-SPC risk association in male cancer survivors was stronger than that for first cancers in the general population, whereas the mean BMI was similar in both groups. In the severely obese category (BMI ≥ 30 kg/m2), the adjusted hazard ratios for SPCs among cancer survivors (1.41; 95% CI, 1.15 to 1.74) were significantly higher than those for first cancers among all cohort participants (1.12; 95% CI, 1.09 to 1.16; Pheterogeneity < .01).
Conclusion
Prediagnosis obesity is a risk factor for overall and individual SPCs, and the strength of the BMI-cancer association is slightly stronger in male cancer survivors than in the general population.
INTRODUCTION
Although improvements in cancer screening and treatment have led to continuously increasing survival,1,2 these advances have been offset by an increased risk of secondary primary cancers (SPCs). Many previous studies have demonstrated that the risk of developing an SPC among cancer survivors might be as great as or greater than that among the general population.3-6 SPCs could be due to not only the late carcinogenic effects of cancer treatment but also the influence of shared behavioral risk factors or increased susceptibility to carcinogenesis.
Among shared behavioral risk factors, most studies have focused on the associations between tobacco5,7-9 and alcohol10,11 use and the risk of SPCs. Although obesity is a well-known risk factor for cancer in the general population,12-15 previous studies on body weight and SPC risk have been largely confined to survivors of breast cancer16 or colorectal cancer (CRC)17; there are few studies about these associations among male cancer survivors. In addition, few studies have examined how obesity might contribute to increased SPC risk. Only one study, by Gibson et al,17 suggested that elevated cancer risk in survivors of CRC compared with the general population might be caused by an increased prevalence of obesity rather than increased susceptibility to obesity-related tumorigenesis. However, in that study, the number of secondary obesity-related cancers was too small (n = 224) to examine associations between body mass index (BMI) and specific SPCs. In addition, because their participants were mainly white, little is known about these associations among other races or ethnicities.
Because body weight is influenced by cancer,18 an ideal approach to assess the association between BMI and SPC risk would involve a large, longitudinal study with detailed data on exposures before the diagnosis of the first cancer. We therefore examined associations between prediagnosis BMI and SPC risk by examining merged data from the Korean National Health Insurance Service (NHIS) and National Cancer Registry, in which the total number of male participants was greater than 11 million. During the 8 years of follow-up, more than 230,000 men were diagnosed with a first cancer. From this cohort of male cancer survivors, we identified the role of prediagnosis BMI in the development of SPCs. We also compared these risk estimates with those calculated for first cancers in all cohort participants.
METHODS
Study Population
The study population consisted of Korean male cancer survivors who received medical examinations provided by the NHIS before their first cancer diagnosis between January 1, 2003, and December 31, 2010. The Korean National Cancer Registry data were constructed by the Ministry of Health and Welfare in Korea in 1980. More than 180 malignant tumors have been registered, including at least 95% of newly diagnosed malignant tumors in Korea.19 Since 1995, the Korean NHIS, the single insurer of the Korean public health insurance sector, has provided national health examinations for all Koreans, including height and weight measurements, laboratory tests, and behavioral surveys. The overall participation rate is 65.3% of the eligible population.20 The study protocol was approved by the Institutional Review Board of the National Cancer Center in Korea (NCC2015-2017). The need for participant consent was waived by the Ethics Committee because this study involved routinely collected medical data that were anonymously managed at all stages, including data cleaning and statistical analyses.
Among the male participants who were 18 years of age or older and went to the Korean national health examination service at least once between 2003 and 2010 (n = 11,358,043), we excluded individuals who had no information on prediagnosis BMI and who were dying or diagnosed with their first incident of cancer within 1 year after their baseline national health examination (n = 153,671). Of the remaining 11,204,372 participants, only men with their first incident of cancer diagnosed between 2003 and 2010 with no previously diagnosed cancer were included in the study (N = 239,615). The primary end point of this study was a newly diagnosed SPC, defined as a cancer diagnosed at least 2 months later than the first cancer and of a different topology using the four-digit International Classification of Diseases code.21
Assessment of Prediagnosis Exposure and Covariates
Participants were asked to complete self-reported questionnaires on medical history, current health status, family history, tobacco and alcohol consumption, dietary preferences, and leisure-time physical activities. Height and weight were measured directly by trained personnel. Medical comorbidities, location of residence, and insurance premium (proxy for socioeconomic status) were obtained from the merged database of the NHIS.
Prediagnosis exposure and covariate variables were obtained from the data of one follow-up cycle before the first cancer diagnosis between 2003 and 2010. BMI was calculated as prediagnosis weight divided by adult height squared (kg/m2) and categorized according to the WHO criteria for Asian populations. A BMI between 18.6 and 22.9 kg/m2 was classified as normal, 23 to 24.9 kg/m2 as overweight, 25 to 29.9 kg/m2 as obese, and > 30 kg/m2 as severely obese.22
Statistical Analysis
Accumulated person-years of risk for each patient were calculated, beginning with the date of diagnosis of the first primary cancer and ending with the date of diagnosis of an SPC, death, or December 31, 2011, whichever came first. We defined obesity-related cancers as cancers known to have a dose-response relationship with obesity (colorectal, kidney, pancreas, and esophageal adenocarcinoma).17,23
To compare cancer incidence between the general population and the cancer survivors, we calculated age-standardized incidence rates (IRs) by a direct standardization method. We used Cox proportional hazards analysis to estimate age-adjusted and multivariable adjusted hazard ratios (aHRs) for SPC development related to BMI before diagnosis of the first primary tumor. For multivariable analyses, we used the following categories: fasting serum glucose level (< 100, 100-125.9, 126-139.9, or ≥ 140 mg/dL, or history of diabetes),24 fasting serum cholesterol level (< 200, 200-239.9, 240-259.9, or ≥ 260 mg/dL, or history of hyperlipidemia), frequency of exercise (none, 1-2, 3-4, 5-6, or 7 times/week), smoking (never, former, or current smoker), alcohol drinking (none, < 2, 3-4, or ≥ 5 times/week), and history of comorbidity (hypertension, osteoporosis, heart disease, liver disease, or cerebrovascular disease). Tests for trends were performed by assigning median values for BMI and treating the new variables as continuous terms in the models.
In addition, we performed several sensitivity analyses. First, we excluded those diagnosed with other primary cancers within the first 1 to 2 years since the first cancer diagnosis to minimize detection bias. We also performed additional analyses with an adjustment for follow-up time. Second, we stratified associations by smoking history, age at first cancer diagnosis, first cancer stage, place of residence, insurance premium, and disability status. Third, we assessed the BMI-SPC risk association in survivors of an obesity-related cancer.
To compare the different strengths of the associations of BMI with risk of cancer among male cancer survivors and the cancer-free cohort population, we performed a similar Cox proportional analysis of the first cancer among cancer-free male participants. In the analysis of first cancer risk, 11,204,372 cancer-free participants who were 18 years of age or older and went to the Korean national health examination service at least once between 2003 and 2010 were included. Exposure variables were used from their first health examination between 2003 and 2010. Follow-up duration extended from the date of the first national health examination and continued until the diagnosis of a first cancer, death, or December 31, 2011, whichever came first. P values for heterogeneity were calculated using the Q statistic. To account for the potential impact of death as a competing risk, the Poisson regression method of Fine and Gray25 was used to assess the ratio of subdistribution hazards. All data were analyzed using SAS for Windows (version 9.3; SAS Institute, Cary, NC), and P values < .05 were considered statistically significant.
RESULTS
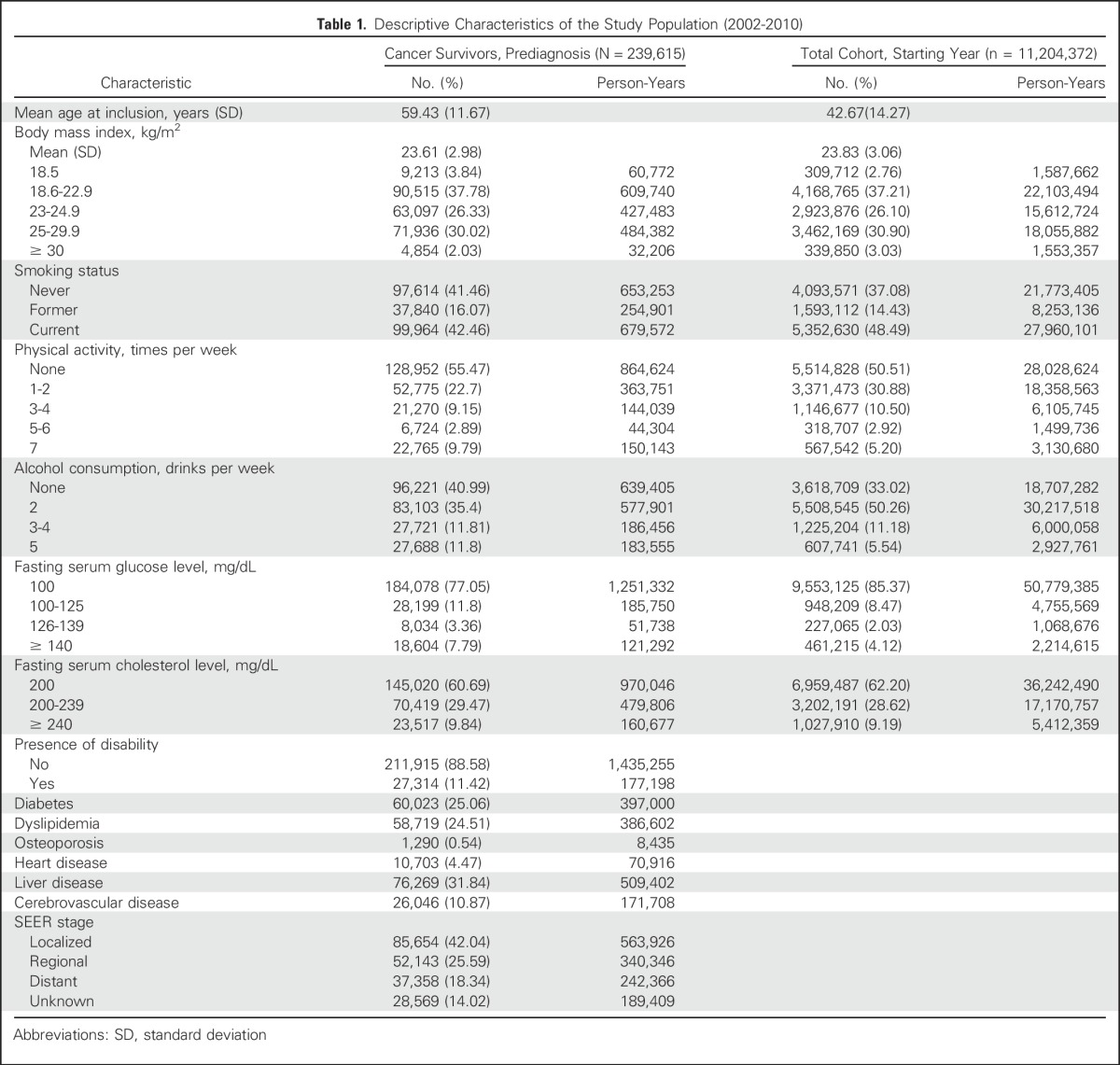
The mean age of the cancer survivors was 59.4 years. Table 1 lists the baseline characteristics. After 1,614,583 person-years of follow-up, we observed 4,799 patients with SPCs. When we compared baseline characteristics, cancer survivors were less likely to smoke than the general population, whereas the mean BMI was similar in both groups. The proportions of the obese population (BMI ≥ 25 kg/m2) were 32.05% for cancer survivors and 33.93% for the entire cohort.
Table 1.
Descriptive Characteristics of the Study Population (2002-2010)
Age-Standardized IRs of First Primary Cancers and SPCs
The overall age-standardized IR of SPCs was 353.8 occurrences per 100,000 person-years (Table 2), which was approximately 1.1 times higher than that of first primary cancers (321.9 occurrences per 100,000 person-years; P < .001). In stratified analysis by BMI, the age-standardized cancer IR was 23% higher for SPCs (391.9 occurrences per 100,000 person-years) than for the first primary cancer (318.3 occurrences per 100,000 person-years) among men with a BMI of at least 25 kg/m2. Among the obese population, the increased age-standardized IR of obesity-related cancers was more prominent for SPCs (105.1 occurrences per 100,000 person-years) than for the first primary cancer (81.4 occurrences per 100,000 person-years; P < .001).
Table 2.
Age-Standardized IRs of the First and Second Primary Cancers

Associations Between Prediagnosis BMI and SPCs
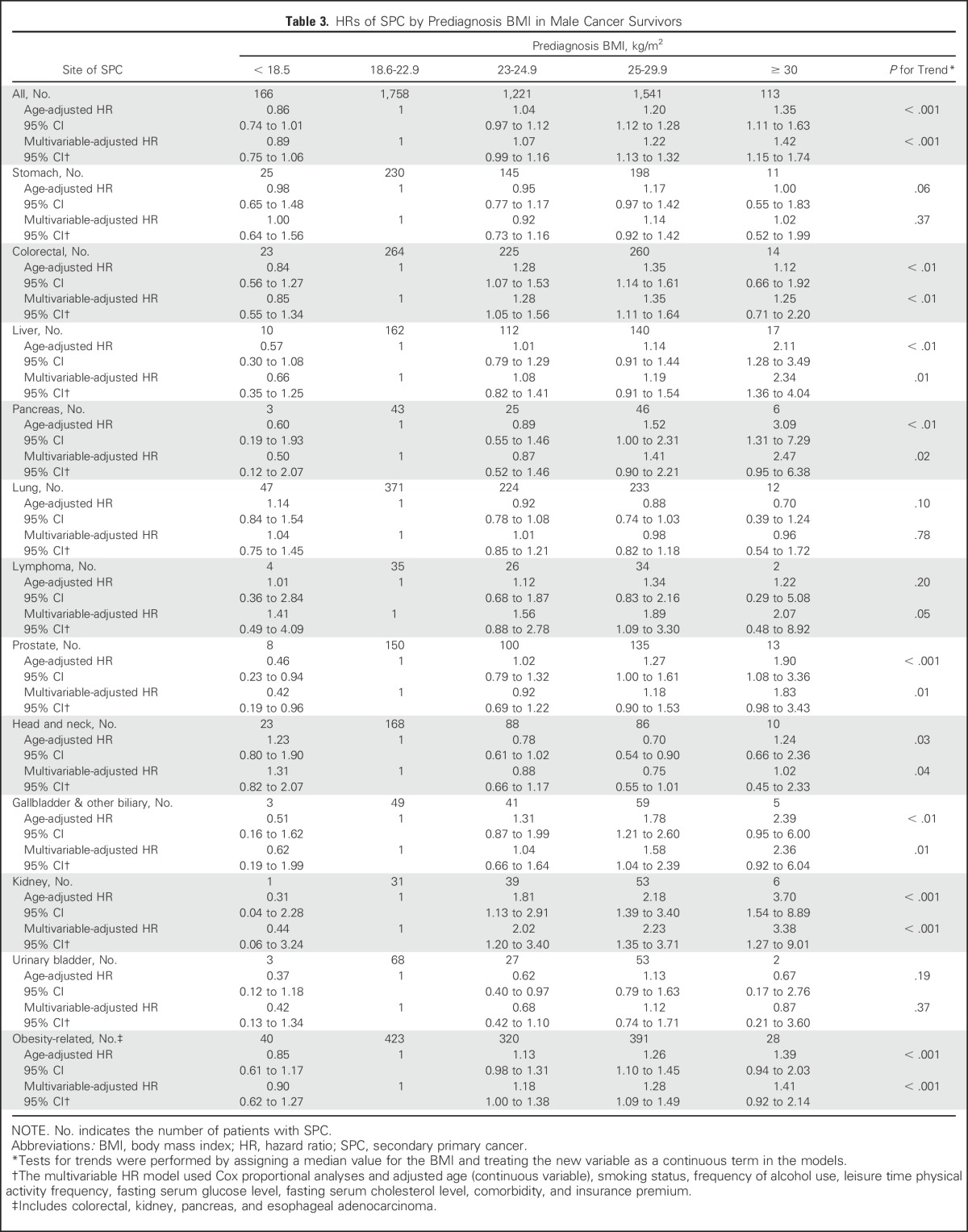
We found positive linear trends between prediagnosis BMI and risk for all-combined, colorectal, liver, biliary tract, thyroid, prostate, and kidney cancers (Ptrend < .01; Table 3). Compared with patients with a BMI between 18.6 and 22.9 kg/m2, obese cancer survivors (BMI ≥ 25 and < 30 kg/m2) had elevated SPC risk for all-combined (aHR, 1.22; 95% CI, 1.13 to 1.32), colorectal (aHR, 1.35; 95% CI, 1.11 to 1.64), lymphoma (aHR, 1.89; 95% CI, 1.09 to 3.30), biliary tract (aHR, 1.58; 95% CI, 1.04 to 2.39), kidney (aHR, 2.23; 95% CI, 1.35 to 3.71), and obesity-related (aHR, 1.28; 95% CI, 1.09 to 1.49) cancers. In addition, severely obese cancer survivors (BMI ≥ 30 kg/m2) were more likely to develop an SPC for all-combined (aHR, 1.42; 95% CI, 1.15 to 1.74), liver (aHR, 2.34; 95% CI, 1.36 to 4.04), and kidney (aHR, 3.38; 95% CI, 1.27 to 9.01) cancers.
Table 3.
HRs of SPC by Prediagnosis BMI in Male Cancer Survivors
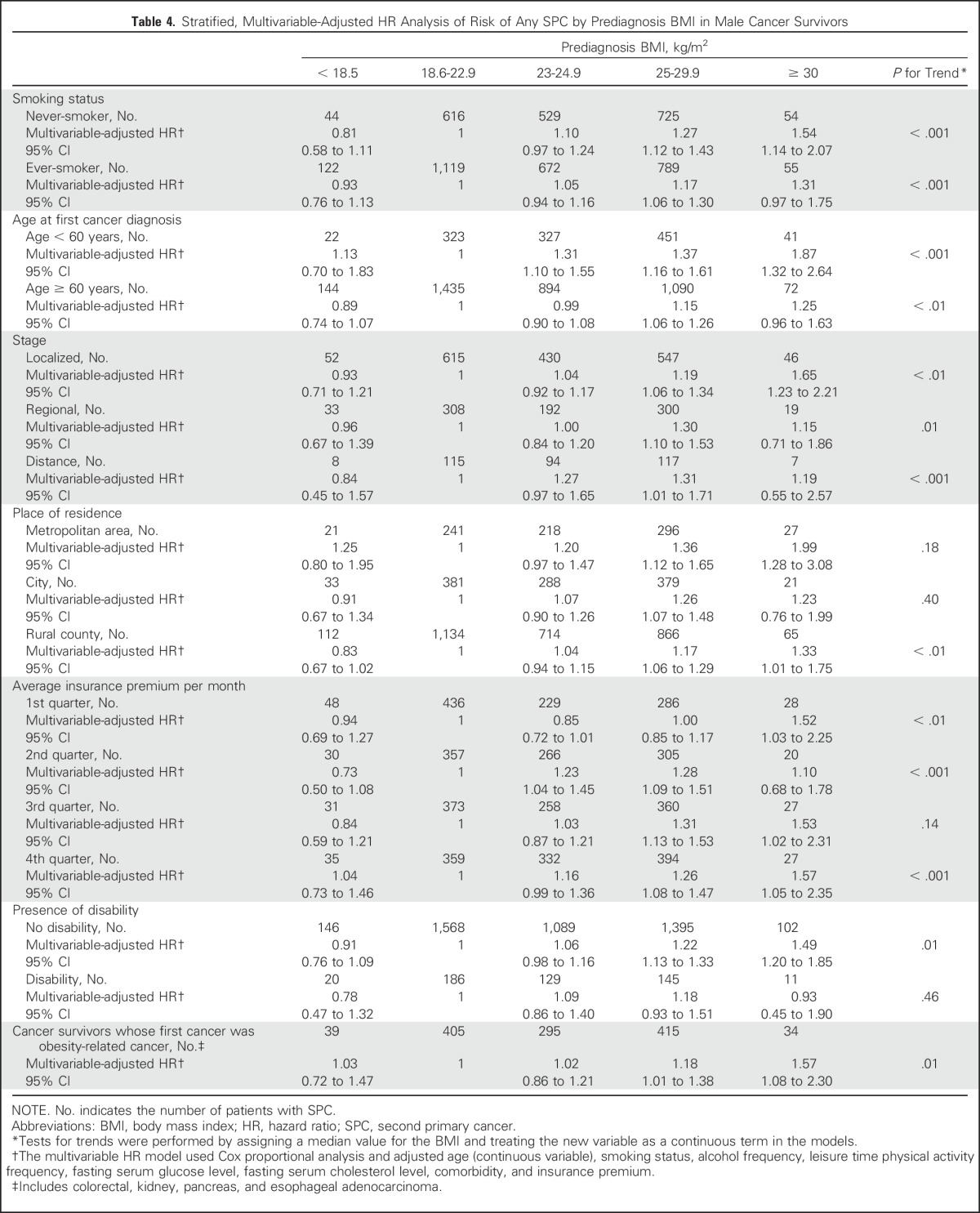
When we performed a sensitivity analysis after excluding SPCs within 1 to 2 years after diagnosis of the first cancer, the association between SPC risk and prediagnosis BMI was generally similar to that from the primary analysis (data not shown). In analyses of adjustments for follow-up time, the BMI-SPC risk association showed a similar trend (aHR, for BMI ≥ 30 kg/m2 category, 1.36; 95% CI, 1.07 to 1.73; Ptrend < .001). This association was similar to that identified for smoking history (Table 4). According to age at the time of the first cancer diagnosis, the BMI-SPC risk association was more prominent in those who developed their first cancer before 60 years of age: The aHR of all-combined SPCs for a BMI of at least 30 kg/m2 was 1.87 (95% CI, 1.32 to 2.64; Ptrend < .001). In a stratified analysis by first cancer stage, the strength of the association between BMI and SPC risk was higher among cancer survivors with a localized stage (aHR for BMI ≥ 30 kg/m2 category, 1.65; 95% CI, 1.23 to 2.21; Ptrend < .001) than among those with a regional or advanced stage. When we assessed the BMI-SPC risk association among survivors of obesity-related cancer, overall trends were generally similar to those from the primary analysis (Table 4).
Table 4.
Stratified, Multivariable-Adjusted HR Analysis of Risk of Any SPC by Prediagnosis BMI in Male Cancer Survivors
In analyses of SPC risk allowing for death as a competing risk, subdistribution HRs for SPC in overweight (aHR, 1.09; 95% CI, 1.07 to 1.11) cancer survivors were similar to cause-specific HRs, as listed in Table 3, whereas SPC risk was slightly attenuated in the obese (aHR, 1.13; 95% CI, 1.11 to 1.15) and severely obese groups (aHR, 1.05; 95% CI, 0.99 to 1.11). Five-year cumulative mortality among male cancer survivors was lower in the overweight (41.6%), obese (38.1%), and severely obese (39.2%) groups compared with the normal (49.4%) and underweight (65.1%) groups. Compared with the groups with a BMI between 18.6 and 22.9 kg/m2, the severely obese group had a subdistribution HR for death of 0.81 (95% CI, 0.80 to 0.83; Ptrend < .001).
Comparison of the Association Between BMI and Risk of First Cancer in All Cohort Participants
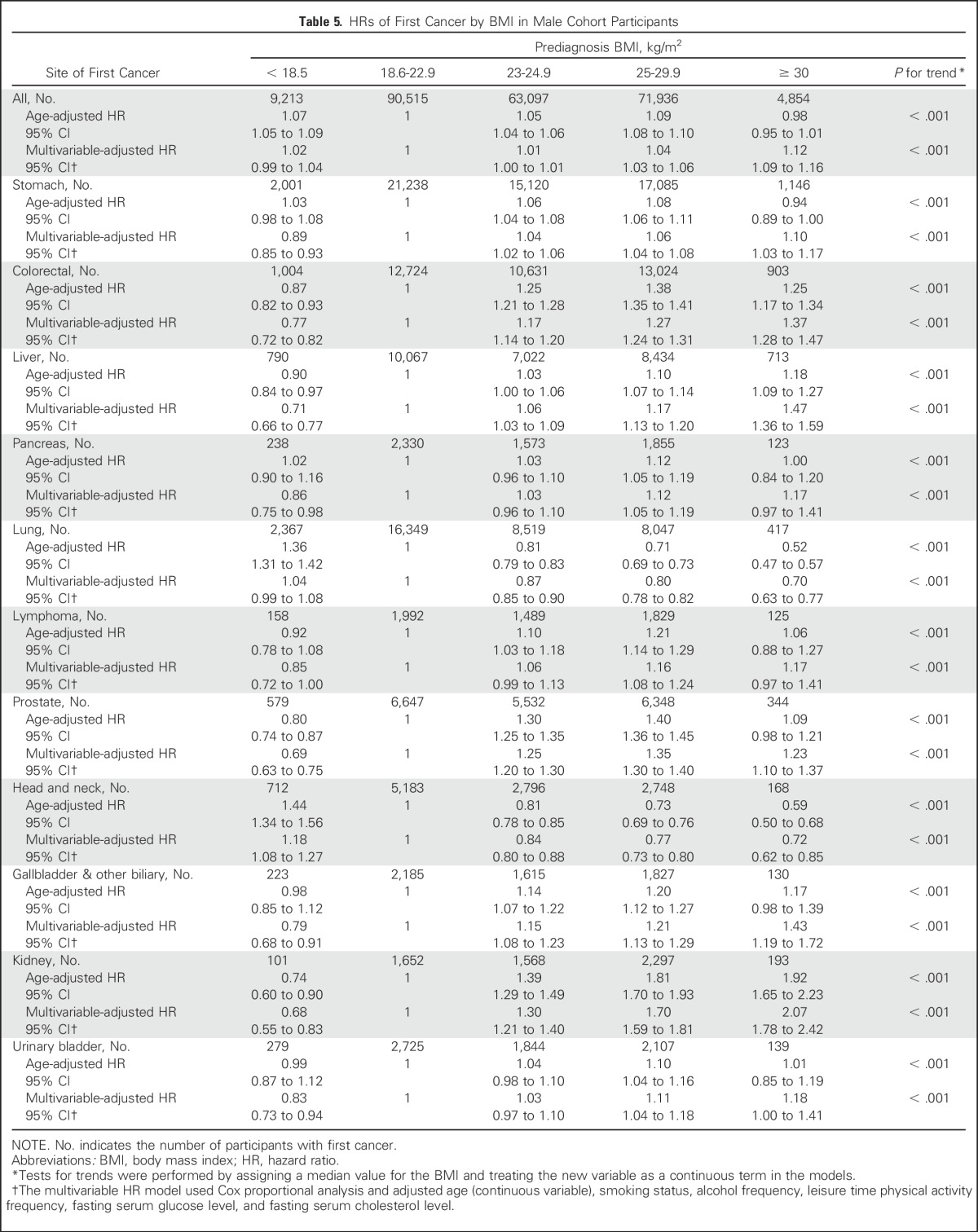
We compared the magnitude of the association between obesity and risk of overall SPC among male cancer survivors with the association between BMI and risk of first cancer among all cohort participants without a cancer history. After 58,913,119 person-years of follow-up, we documented 239,738 patients with primary cancer. Overall, dose-response trends between BMI and first cancer risk were similar to those observed for SPC risk among male cancer survivors (Table 5). However, the magnitudes of the BMI-cancer associations were slightly higher in male cancer survivors than in the general population. In the severely obese category (BMI ≥ 30 kg/m2), the aHRs for SPCs among cancer survivors (1.42; 95% CI, 1.15 to 1.74) were significantly higher than those for first cancers among all cohort participants (aHR, 1.12; 95% CI, 1.09 to 1.16, Pheterogeneity < .010). In a similar comparison in the obese category (BMI ≥ 25 and < 30 kg/m2), the magnitude of the BMI-SPC risk association was slightly higher for SPCs (aHR, 1.27; 95% CI, 1.12 to 1.43) than for first cancers (aHR, 1.04; 95% CI, 1.03 to 1.06, Pheterogeneity < .01).
Table 5.
HRs of First Cancer by BMI in Male Cohort Participants
DISCUSSION
In this large male cancer survivor cohort study, we showed that individuals who were obese before their cancer diagnosis had an increased risk of subsequent overall cancer, as well as increased risk of second primary colorectal, liver, lymphoma, biliary tract, kidney, and obesity-related cancers. In addition, the magnitude of the association between obesity and SPC risk among male cancer survivors was slightly stronger than that for first primary cancer risk in the overall cohort.
Obesity is a well-known risk factor for first cancers, including colorectal,12,13 liver,26 and kidney,14 in the general population; these findings are consistent with our observation of an association between obesity and risk of first cancer. We also found significant dose-dependent relationships between prediagnosis BMI and subsequent overall, obesity-related, and individual SPCs. Some studies have shown obesity to be a risk factor for recurrent colorectal, prostate, and breast cancer.27-29 Other studies of the effects of obesity on SPC risk at discordant sites have been mainly confined to female breast cancer survivors.30 As for male cancer survivors, little is known about the association between obesity and SPC risk. In one study of CRC survivors, 60% of whom were male,17 obesity was significantly associated with secondary cancers. In another cohort study,5 which enrolled only government employees and teachers, male cancer survivors who were obese had a significantly elevated risk of colorectal SPCs, whereas the number of cancer survivors (n = 14,181) and patients with SPC (n = 204) was insufficient to examine the association between BMI and other SPCs. To our knowledge, this is the first large study with a sufficient number of male cancer survivors (n = 239,738) to suggest that prediagnosis obesity may be a risk factor for subsequent overall and individual SPCs.
When we performed subgroup analyses, the HRs for SPCs were more prominent among cancer survivors whose age at their first cancer diagnosis was younger than 60 years or whose first cancer stage was localized. Because younger cancer survivors with a localized first cancer stage might have a greater chance for long-term survival, our findings highlight the need for long-term cancer survivorship programs for early SPC detection.
The observation that obesity increases the risk of SPCs at sites for which a relationship is well established for first primary cancers suggests that these associations may partially result from lifelong complications from excess body weight.17 Causative mechanisms, such as a chronic inflammatory state or increased blood levels of sex steroids, insulin, insulin-like growth factor, and other growth factors, could be involved, which could influence cancer development or growth.31,32 However, the magnitude of the association between obesity and SPC risk among male cancer survivors was slightly stronger than that for first primary malignancy risk in the overall cohort, whereas the prevalence of prediagnosis obesity among cancer survivors did not exceed that of the general population. In addition, among men with a BMI of at least 25 kg/m2, the age-standardized IR of cancers was 23% higher for secondary than for first primary cancers, whereas there was little difference in men with a BMI < 25 kg/m2. When we focused on the age-standardized IR of obesity-related cancers among the obese population, it was more prominent for SPCs than for the first primary cancer. These findings suggest that elevated cancer risk in cancer survivors cannot be fully explained by an increased prevalence of obesity at baseline. In addition to the role of shared behavioral risk factors,1 there could be additional susceptibility to obesity-related carcinogenesis in cancer survivors, which may be related to genetic predisposition or the effects of cancer treatment. In analyses with adjustments for follow-up time, the overall trends did not significantly differ based on the time since the first cancer diagnosis, which might provide indirect evidence that the BMI-SPC risk association is not attributable to cancer treatments. However, because we did not include information about first cancer treatments, and this is an indirect assessment of susceptibility, further research is needed to clarify this point.
In analyses of SPC risk allowing for death as a competing risk, subdistribution aHRs for SPCs were slightly attenuated in the obese and severely obese groups. These findings might be partially due to an inverse association between prediagnosis BMI and mortality in cancer survivors. Although several previous studies have shown that obesity is associated with higher mortality in breast29 and colon17 cancers, other studies suggest that prediagnosis BMI could be a nutritional status marker in patients with cancer, with heavier patients with cancer having a better prognosis.33,34
The strengths of our study included the prospective cohort design, with approximately 240,000 male cancer survivors and a detailed assessment of prediagnosis behavioral and measurable risk factors. In particular, height and weight measurements, the main exposure variables in this study, were performed by trained personnel, which is preferable to self-reported data because people tend to under-report their weight.35,36
Our study had several limitations. First, although we excluded cancer survivors whose follow-up duration was less than 2 months after their first cancer diagnosis, there was some chance of SPC misclassification. However, when we performed sensitivity analyses, excluding patients with SPC that occurred within 1 to 2 years after the first cancer diagnosis, the overall trends remained similar. Second, information about the first cancer treatment could not be included, and we could not therefore consider the effects of cancer treatment on SPCs. Third, overall participation in national health examinations is 65.3% of all eligible Koreans. It is possible that cancer IRs might differ between those who did and did not participate, which may limit the generalizability of our results on the basis of selection bias.
Our study shows that prediagnosis obesity is a risk factor for overall and individual SPCs among male cancer survivors, and the strength of the BMI-cancer association was slightly stronger among male cancer survivors than among the general population. These findings suggest that increased risk of cancer among cancer survivors might be partially due to an increased prevalence of obesity or an increased susceptibility to obesity-related carcinogenesis among cancer survivors compared with the general population.
Footnotes
Supported by National Cancer Center Grant Nos. 1310252 and 1532200.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Sang Min Park, Young Ho Yun, Young Ae Kim
Collection and assembly of data: Young Ho Yun, Young Ae Kim, Minkyung Jo, Young-Joo Won, Joung Hwan Back
Data analysis and interpretation: Sang Min Park, Young Ho Yun, Young Ae Kim, Minkyung Jo
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Prediagnosis Body Mass Index and Risk of Secondary Primary Cancer in Male Cancer Survivors: A Large Cohort Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Sang Min Park
No relationship to disclose
Young Ho Yun
No relationship to disclose
Young Ae Kim
No relationship to disclose
Minkyung Jo
No relationship to disclose
Young-Joo Won
No relationship to disclose
Joung Hwan Back
No relationship to disclose
Eun-Sook Lee
No relationship to disclose
REFERENCES
- 1.Travis LB, Demark Wahnefried W, Allan JM, et al. Aetiology, genetics and prevention of secondary neoplasms in adult cancer survivors. Nat Rev Clin Oncol. 2013;10:289–301. doi: 10.1038/nrclinonc.2013.41. [DOI] [PubMed] [Google Scholar]
- 2.Wood ME, Vogel V, Ng A, et al. Second malignant neoplasms: Assessment and strategies for risk reduction. J Clin Oncol. 2012;30:3734–3745. doi: 10.1200/JCO.2012.41.8681. [DOI] [PubMed] [Google Scholar]
- 3.Travis LB, Curtis RE, Storm H, et al. Risk of second malignant neoplasms among long-term survivors of testicular cancer. J Natl Cancer Inst. 1997;89:1429–1439. doi: 10.1093/jnci/89.19.1429. [DOI] [PubMed] [Google Scholar]
- 4.Henderson TO, Amsterdam A, Bhatia S, et al. Systematic review: Surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med. 2010;152:444–455. doi: 10.1059/0003-4819-152-7-201004060-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SM, Lim MK, Jung KW, et al. Prediagnosis smoking, obesity, insulin resistance, and second primary cancer risk in male cancer survivors: National Health Insurance Corporation Study. J Clin Oncol. 2007;25:4835–4843. doi: 10.1200/JCO.2006.10.3416. [DOI] [PubMed] [Google Scholar]
- 6.Youlden DR, Baade PD. The relative risk of second primary cancers in Queensland, Australia: A retrospective cohort study. BMC Cancer. 2011;11:83. doi: 10.1186/1471-2407-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Do KA, Johnson MM, Doherty DA, et al. Second primary tumors in patients with upper aerodigestive tract cancers: Joint effects of smoking and alcohol (United States) Cancer Causes Control. 2003;14:131–138. doi: 10.1023/a:1023060315781. [DOI] [PubMed] [Google Scholar]
- 8.van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiotherapy and smoking in lung cancer following Hodgkin’s disease. J Natl Cancer Inst. 1995;87:1530–1537. doi: 10.1093/jnci/87.20.1530. [DOI] [PubMed] [Google Scholar]
- 9.Tabuchi T, Ito Y, Ioka A, et al. Tobacco smoking and the risk of subsequent primary cancer among cancer survivors: A retrospective cohort study. Ann Oncol. 2013;24:2699–2704. doi: 10.1093/annonc/mdt279. [DOI] [PubMed] [Google Scholar]
- 10.Druesne-Pecollo N, Keita Y, Touvier M, et al. Alcohol drinking and second primary cancer risk in patients with upper aerodigestive tract cancers: A systematic review and meta-analysis of observational studies. Cancer Epidemiol Biomarkers Prev. 2014;23:324–331. doi: 10.1158/1055-9965.EPI-13-0779. [DOI] [PubMed] [Google Scholar]
- 11.Knight JA, Bernstein L, Largent J, et al. Alcohol intake and cigarette smoking and risk of a contralateral breast cancer: The Women’s Environmental Cancer and Radiation Epidemiology Study. Am J Epidemiol. 2009;169:962–968. doi: 10.1093/aje/kwn422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 13.Ma Y, Yang Y, Wang F, et al. Obesity and risk of colorectal cancer: A systematic review of prospective studies. PLoS One. 2013;8:e53916. doi: 10.1371/journal.pone.0053916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang F, Xu Y. Body mass index and risk of renal cell cancer: A dose-response meta-analysis of published cohort studies. Int J Cancer. 2014;135:1673–1686. doi: 10.1002/ijc.28813. [DOI] [PubMed] [Google Scholar]
- 15.Qin Q, Xu X, Wang X, et al. Obesity and risk of bladder cancer: A meta-analysis of cohort studies. Asian Pac J Cancer Prev. 2013;14:3117–3121. doi: 10.7314/apjcp.2013.14.5.3117. [DOI] [PubMed] [Google Scholar]
- 16.Druesne-Pecollo N, Touvier M, Barrandon E, et al. Excess body weight and second primary cancer risk after breast cancer: A systematic review and meta-analysis of prospective studies. Breast Cancer Res Treat. 2012;135:647–654. doi: 10.1007/s10549-012-2187-1. [DOI] [PubMed] [Google Scholar]
- 17.Gibson TM, Park Y, Robien K, et al. Body mass index and risk of second obesity-associated cancers after colorectal cancer: A pooled analysis of prospective cohort studies. J Clin Oncol. 2014;32:4004–4011. doi: 10.1200/JCO.2014.56.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satia JA, Campbell MK, Galanko JA, et al. Longitudinal changes in lifestyle behaviors and health status in colon cancer survivors. Cancer Epidemiol Biomarkers Prev. 2004;13:1022–1031. [PubMed] [Google Scholar]
- 19.Shin HR, Won YJ, Jung KW, et al. Nationwide cancer incidence in Korea, 1999∼2001; first result using the national cancer incidence database. Cancer Res Treat. 2005;37:325–331. doi: 10.4143/crt.2005.37.6.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korean National Health Insurance Corporation . Analysis of 2009 national health screening program. Seoul, Korea, National Health Insurance Corporation: 2010. [Google Scholar]
- 21.Curtis RE, Freedman DM, Ron E, et al. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. Bethesda, MD: NIH; 2006. [Google Scholar]
- 22.Western Pacific Region WHO . International Association for the Study of Obesity, and International Obesity Taskforce: The Asian-Pacific Perspective: Redefining Obesity and Its Treatment. Geneva, Switzerland: WHO Western Pacific Region; 2000. [Google Scholar]
- 23.Marmot M, Atinmo T, Byers T, et al. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: World Cancer Research Fund/American Institute for Cancer Research; 2007. [Google Scholar]
- 24. American Diabetes Association: Diagnosis and classification of diabetes mellitus. Diabetes Care 37:S81-S90, 2014 (suppl 1) [DOI] [PubMed]
- 25.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 26.Tanaka K, Tsuji I, Tamakoshi A, et al. Obesity and liver cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn J Clin Oncol. 2012;42:212–221. doi: 10.1093/jjco/hyr198. [DOI] [PubMed] [Google Scholar]
- 27.Meyerhardt JA, Catalano PJ, Haller DG, et al. Influence of body mass index on outcomes and treatment-related toxicity in patients with colon carcinoma. Cancer. 2003;98:484–495. doi: 10.1002/cncr.11544. [DOI] [PubMed] [Google Scholar]
- 28.Freedland SJ, Aronson WJ, Kane CJ, et al. Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: A report by the Shared Equal Access Regional Cancer Hospital database study group. J Clin Oncol. 2004;22:446–453. doi: 10.1200/JCO.2004.04.181. [DOI] [PubMed] [Google Scholar]
- 29.Jiralerspong S, Kim ES, Dong W, et al. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. 2013;24:2506–2514. doi: 10.1093/annonc/mdt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trentham-Dietz A, Newcomb PA, Nichols HB, et al. Breast cancer risk factors and second primary malignancies among women with breast cancer. Breast Cancer Res Treat. 2007;105:195–207. doi: 10.1007/s10549-006-9446-y. [DOI] [PubMed] [Google Scholar]
- 31.Ricceri F, Fasanelli F, Giraudo MT, et al. Risk of second primary malignancies in women with breast cancer: Results from the European prospective investigation into cancer and nutrition (EPIC) Int J Cancer. 2015;137:940–948. doi: 10.1002/ijc.29462. [DOI] [PubMed] [Google Scholar]
- 32.Nagaraju GP, Aliya S, Alese OB. Role of adiponectin in obesity related gastrointestinal carcinogenesis. Cytokine Growth Factor Rev. 2015;26:83–93. doi: 10.1016/j.cytogfr.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 33.Park SM, Lim MK, Shin SA, et al. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol. 2006;24:5017–5024. doi: 10.1200/JCO.2006.07.0243. [DOI] [PubMed] [Google Scholar]
- 34.Choi Y, Park B, Jeong BC, et al. Body mass index and survival in patients with renal cell carcinoma: A clinical-based cohort and meta-analysis. Int J Cancer. 2013;132:625–634. doi: 10.1002/ijc.27639. [DOI] [PubMed] [Google Scholar]
- 35.Hill A, Roberts J. Body mass index: A comparison between self-reported and measured height and weight. J Public Health Med. 1998;20:206–210. doi: 10.1093/oxfordjournals.pubmed.a024744. [DOI] [PubMed] [Google Scholar]
- 36.Danubio ME, Miranda G, Vinciguerra MG, et al. Comparison of self-reported and measured height and weight: Implications for obesity research among young adults. Econ Hum Biol. 2008;6:181–190. doi: 10.1016/j.ehb.2007.04.002. [DOI] [PubMed] [Google Scholar]


