Abstract
Purpose
The 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins) have activity in one of the pathways influenced by nitrogen-containing bisphosphonates, which are associated with improved survival in multiple myeloma (MM). To understand the benefit of statins in MM, we evaluated the association between statin use and mortality in a large cohort of patients with MM.
Patients and Methods
From the Veterans Administration Central Cancer Registry, we identified patients diagnosed with MM between 1999 and 2013. We defined statin use as the presence of any prescription for a statin within 3 months before or any time after MM diagnosis. Cox proportional hazards regression assessed the association of statin use with mortality, while controlling for known MM prognostic factors.
Results
We identified a cohort of 4,957 patients, of whom 2,294 received statin therapy. Statin use was associated with a 21% decrease in all-cause mortality (adjusted hazard ratio, 0.79; 95% CI, 0.73 to 0.86; P < .001) as well as a 24% decrease in MM-specific mortality (adjusted hazard ratio, 0.76; 95% CI, 0.67 to 0.86; P < .001). This association remained significant across all sensitivity analyses. In addition to reductions in mortality, statin use was associated with a 31% decreased risk of developing a skeletal-related event.
Conclusion
In this cohort study of US veterans with MM, statin therapy was associated with a reduced risk of both all-cause and MM-specific mortality. Our findings suggest a potential role for statin therapy in patients with MM. The putative benefit of statin therapy in MM should be corroborated in prospective studies.
INTRODUCTION
Statins, 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors, are widely used for treatment of dyslipidemia and prevention of coronary heart disease. In a recent trial of patients with perceived life expectancy of 1 to 12 months, there was no difference in 60-day mortality between patients randomly assigned to discontinue pre-existing statin therapy, and those randomly assigned to continue statin therapy.1 Of the 381 patients enrolled, half of them had cancer as the principal diagnosis. Subgroup analyses of this population were not done, and cancer-specific outcomes were not assessed.
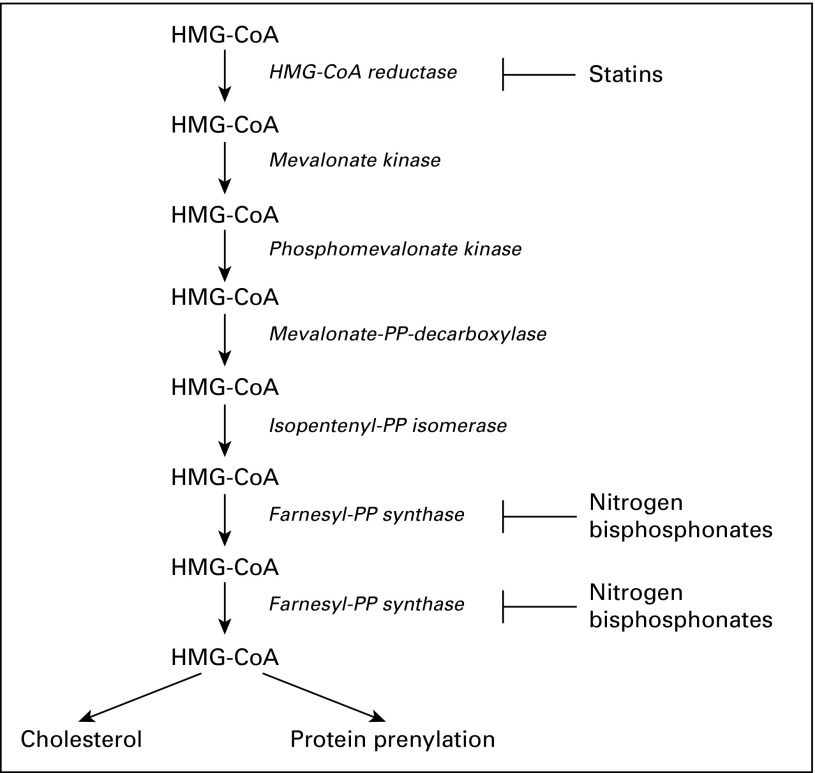
Because statins directly inhibit HMG-CoA reductase, the rate-limiting enzyme of the mevalonate pathway,2 they may have activity in multiple myeloma (MM).3 Nitrogen-containing bisphosphonates, an adjunct therapy in MM, act downstream of statins in the mevalonate pathway through inhibition of farnesyl-diphosphate synthase (Fig 1). Modification of this pathway in a prospective, randomized trial found a 16% reduction in the risk of mortality in patients with MM randomly assigned to receive zoledronic acid (a nitrogen-containing bisphosphonate) versus clodronate (a non-nitrogen–containing bisphosphonate).4 A similar association between zoledronic acid and survival was found in separate studies.4,5 Preclinical data suggest this improvement is related to the nitrogen-containing class inhibition of the mevalonate pathway leading to a reduction in protein prenylation,3 a process crucial for MM cell growth and survival.6,7
Fig 1.
Statin and nitrogen-containing bisphosphonate mechanism of action on the mevalonate pathway. HMG-CoA, 3-hydroxy-3-methylglutaryl-coenzyme A; PP, pyrophosphate.
In vivo and in vitro studies show acceleration of MM cell apoptosis and cell growth arrest with statin therapy.8-12 Statins decrease angiogenesis13 and reduce the metastatic potential of tumor cells.14,15 In addition, statins have been shown to stimulate bone formation.16 A randomized trial of lovastatin in MM found improved progression-free survival,17 but enrolled only 91 patients and this practice has not been adopted. Thus, to understand the putative benefit of statins in MM, we quantified the association between statin use and mortality in a large cohort of patients with MM.
PATIENTS AND METHODS
Study Population
Patients diagnosed with MM within the Veterans Health Administration (VHA) between September 1, 1999, and December 31, 2013, were identified in the Veterans Administration (VA) Central Cancer Registry using International Classification of Diseases (ICD)–03 code 9732/3. The cohort was followed through October 2014. To remove patients who had monoclonal gammopathy of undetermined significance, solitary plasmacytoma, or smoldering MM, we excluded patients who did not receive treatment within 6 months of MM diagnosis. Before cohort assembly, the St Louis VHA Medical Center and Washington University School of Medicine institutional review boards approved this study.
Measurements and Covariates
International Classification of Diseases, 9th revision–Clinical Modification (ICD-9-CM) codes, Pharmacy Benefits Management records, and laboratory data were obtained using the VA Informatics and Computing Infrastructure platform. Records from Pharmacy Benefits Management included dates of administration of all MM-directed therapy, bisphosphonates, and statins (Appendix Table A1, online only). Height and weight were assessed within 1 month of diagnosis. Data on comorbidities present at the time of MM diagnosis were obtained using ICD-9-CM diagnostic codes for ischemic heart disease, diabetes mellitus, chronic kidney disease, peripheral vascular disease, cerebrovascular disease, and dyslipidemia.
Using ICD-9-CM codes, we calculated the Romano adaptation of the Charlson comorbidity index18 for each patient at the time of diagnosis. Patients with skeletal-related events were identified using a combination of ICD-9-CM and Current Procedural Terminology codes, using previously described methods.19,20 Hemoglobin and albumin levels were measured at the time closest to, but within 2 months of diagnosis, and before treatment initiation. We calculated the estimated glomerular filtration rate (eGFR) with the Chronic Kidney Disease Epidemiology Collaboration formula,21 using data from immediately before treatment initiation.
Survival was measured from the date of MM diagnosis until death. Date of death was obtained using the VA vital status file (a VA database that combines death dates from the VA Beneficiary Identification and Records Locator Subsystem Death File, the Social Security Administration-Death Master File, the Medicare Vital Status File, and the Medical SAS Inpatient Datasets).22,23 Cause-specific death information, including death from MM, was available for patients diagnosed up to December 31, 2009, and was obtained from the VHA National Death Index.
Statin Use
Statins available through the VHA during the study period included atorvastatin, fluvastatin, lovastatin, pitavastatin, pravastatin, rosuvastatin, and simvastatin. Patients were identified as statin users if they had any prescriptions for statins in the 3 months before or any time after their date of MM diagnosis. To account for variation in statin use over time and adjust for immortal time bias,24,25 we assessed statin use as a time-varying covariate (except in sensitivity analysis). To remove bias from medication changes that may occur at the end of life, we used a 3-month lag for the statin start and stop dates.26,27 To account for differences in statin dosing and potency, we calculated the daily defined dose (DDD) as specified by the WHO.28 The DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults.
Statistical Analyses
Baseline patient characteristics were compared between statin users and nonusers using χ2 and Cochrane-Mantel-Haenszel tests for categorical variables. Unpaired Student t tests were used for continuous variables. A two-tailed α significance level of 0.05 was used for all analyses. Statistical analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
Primary Analyses
Our primary objective was to evaluate the association between statin use and all-cause mortality in patients with MM. A secondary objective of the study was to assess the association between statin use and MM-specific mortality. Cox proportional hazards regression controlled for the following baseline MM prognostic factors in both mortality analyses: body mass index (BMI), age, race, Charlson comorbidity index score, year of diagnosis, hemoglobin level, creatinine clearance, albumin level, and MM treatment (zoledronic acid, pamidronate, dexamethasone monotherapy, bortezomib, lenalidomide, thalidomide, and autologous stem cell transplantation [ASCT]). Dexamethasone monotherapy was defined as treatment with dexamethasone alone without receipt of other MM-specific therapies; for the remaining treatment categories, patients could receive any combination of MM-directed therapy. All prognostic factors were defined a priori. We used the same methods when quantifying the association between statin use and development of skeletal-related events.
Sensitivity Analyses
All sensitivity analyses were adjusted for the same MM prognostic factors as in the primary analyses. To assess the impact of race on the association between statin use and mortality, we stratified our initial results by race (white v black race).
To adjust for immortal time bias, we assessed the association between statin use and both all-cause and MM-specific mortality with a 12-month landmark analysis.29,30 Patients were included in the landmark analysis if they survived at least 12 months from MM diagnosis, with statin users defined as those who were on statin therapy at MM diagnosis, or who started within 12 months of MM diagnosis. All other patients in the cohort were considered nonstatin users. In addition, we performed a 12-month landmark analysis and assessed the association between cumulative duration of statin use and mortality by 3-month increments of increasing duration of statin use. Statin use for all landmark analyses was limited to the 12-month period following MM diagnosis.
Adjusting for variations in statin potency and dosing, we quantified the association between statin use and both all-cause and MM-specific mortality by DDD. In these analyses, we dichotomized patients into two groups: those who had received at least 365 DDDs and those who had received < 365 DDDs. To control for immortal time bias in the DDD analyses, we assessed statin use as a time-varying covariate.
Propensity score–matched analyses were used to adjust for baseline differences between statin users and nonusers.31 Propensity scores were generated using logistic regression to estimate the probability that each patient in the cohort would be prescribed statin therapy conditional on baseline covariates.
Last, given the prognostic implications of cytogenetics and fluorescent in situ hybridization (FISH), we used Current Procedural Terminology codes to identify patients with results in either category. We then manually abstracted the results of testing in this population. Differences between the prevalence of high-, intermediate-, and standard-risk results between statin users and nonusers were assessed.
RESULTS
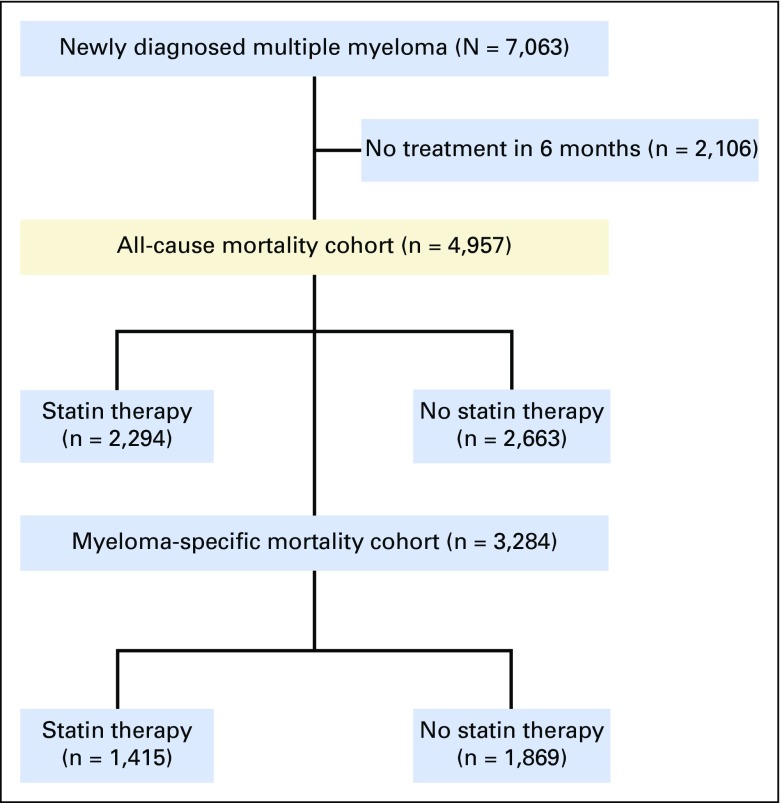
A total of 4,957 patients with MM met entry criteria into the all-cause mortality analytic cohort. Of these patients, 2,294 had prescriptions for statin therapy, and 2,663 patients did not (Appendix Fig A1, online only). The median follow-up for statin and nonstatin users was 34 months and 26 months, respectively. A total of 3,284 patients met entry criteria into the MM-specific mortality analytic cohort, of whom 1,415 patients received statin therapy (Appendix Fig A1). The median follow-up for statin and nonstatin users was 38 months and 24 months, respectively. In the MM-specific mortality cohort, 69% of patients died as a result of myeloma; remaining causes of death are listed in Appendix Table A2 (online only).
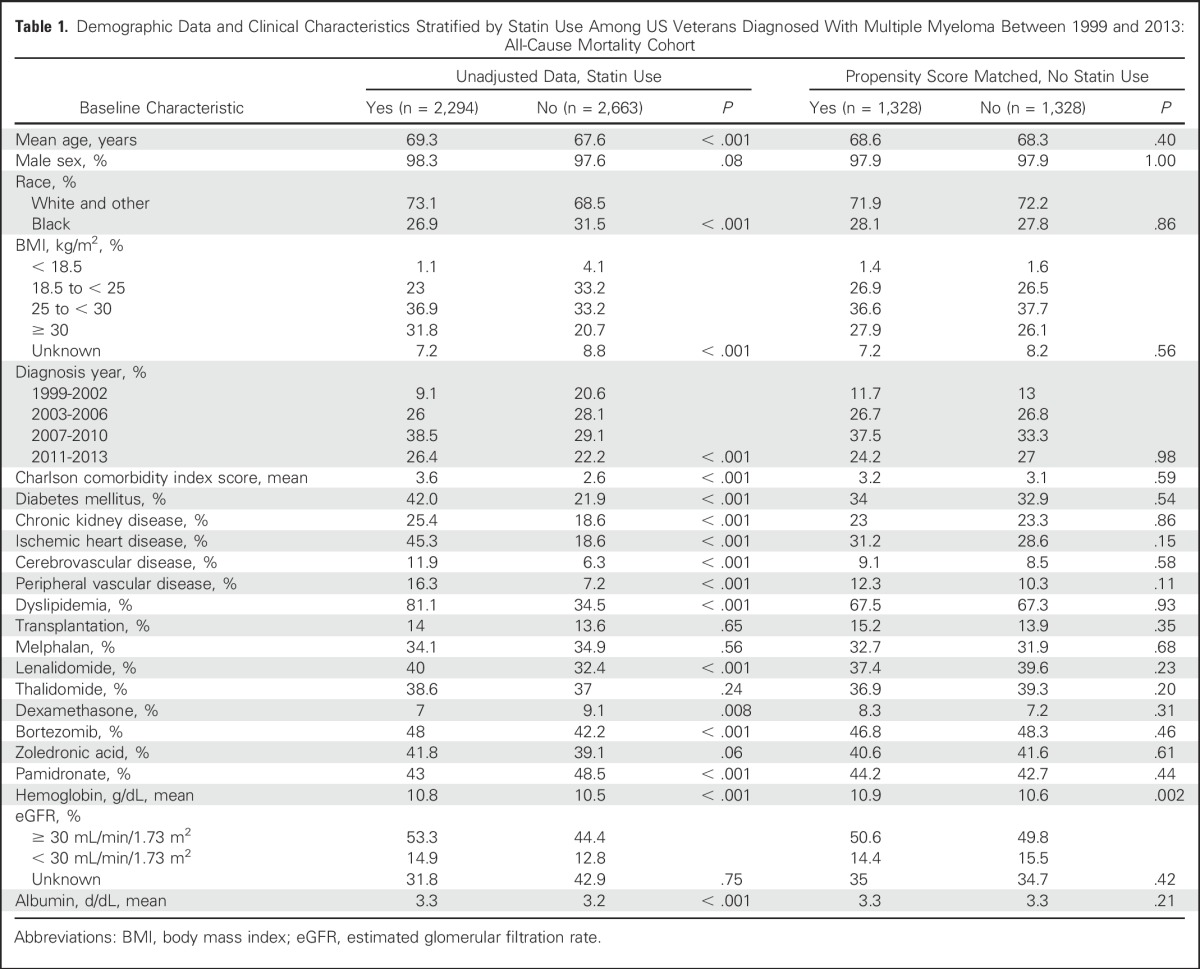
At baseline, patients in the all-cause mortality cohort receiving statin therapy were significantly older, more likely to be white, had higher BMIs, were more likely to be diagnosed after 2006, and had differences in baseline albumin and hemoglobin levels. In addition, they were more likely to have medical comorbidities, including diabetes mellitus, chronic kidney disease, ischemic heart disease, cerebrovascular disease, peripheral vascular disease, and dyslipidemia. Compared with nonstatin users, statin users had a higher frequency of lenalidomide, bortezomib, and pamidronate use, and were less likely to be treated with dexamethasone monotherapy. After propensity score matching, demographic data and clinical characteristics were well balanced in the all-cause mortality cohort (except for baseline hemoglobin level; Table 1). Baseline differences in the MM-specific mortality cohort were similar to those in the all-cause mortality cohort, with the additional observation that statin users were more likely to receive treatment with zoledronic acid and thalidomide compared with nonstatin users. All demographic data and clinical characteristics were well balanced after propensity score matching in the MM-specific mortality cohort (Appendix Table A3, online only).
Table 1.
Demographic Data and Clinical Characteristics Stratified by Statin Use Among US Veterans Diagnosed With Multiple Myeloma Between 1999 and 2013: All-Cause Mortality Cohort
Primary Analyses
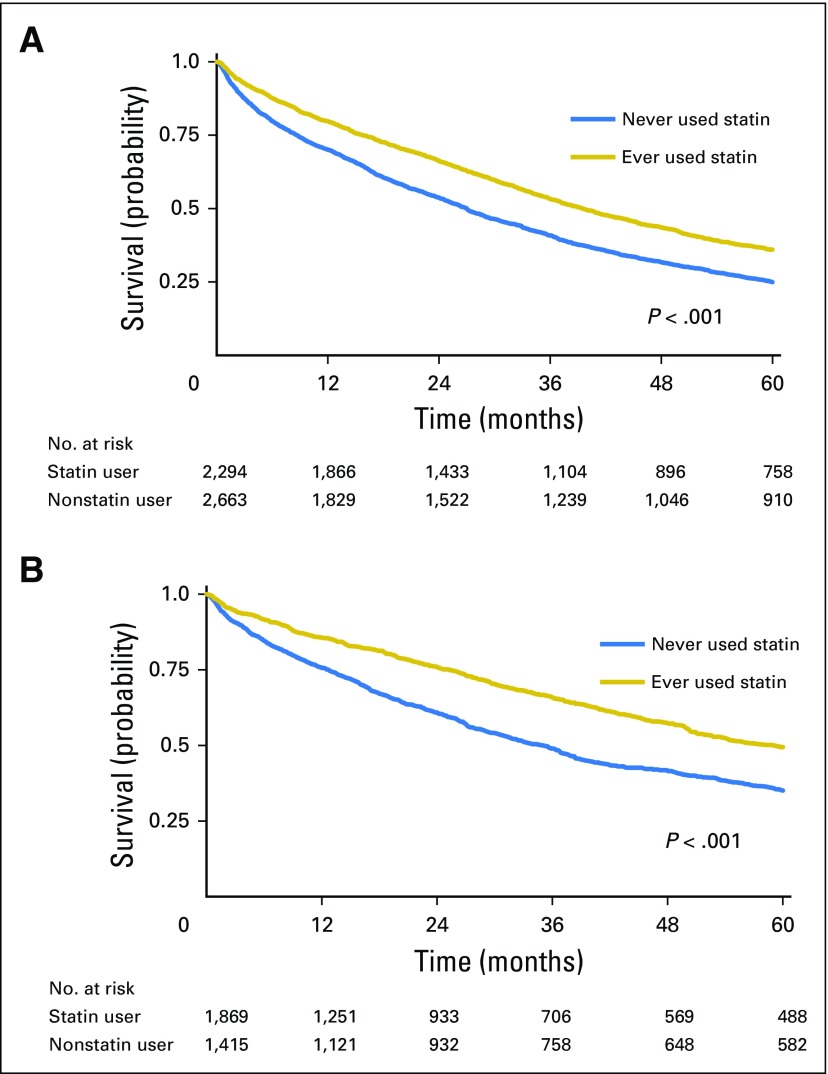
Patients with MM receiving statin therapy had a significant reduction in all-cause mortality (Fig 2A). The median survival among statin users was 39.5 months compared with 27 months in nonusers. After adjusting for potential confounders, statin users had a 21% reduction in the risk of all-cause mortality compared with nonusers (adjusted hazard ratio [aHR], 0.79; 95% CI, 0.73 to 0.86; P < .001; Table 2). Several factors were associated with increased all-cause mortality, including older age, BMI < 18.5 kg/m2, higher Charlson comorbidity index score, hemoglobin < 10 g/dL, eGFR < 30 mL/min/1.73 m2, albumin < 3 g/dL, and treatment with pamidronate. In contrast, ASCT, first-line treatment with a novel agent (lenalidomide, bortezomib, or thalidomide), treatment with zoledronic acid, diagnosis after 2006, black race, and BMI ≥ 25 kg/m2 were each associated with a reduced risk of all-cause mortality (Table 2).
Fig 2.
Kaplan-Meier curves. Survival for patients with multiple myeloma treated with statin versus no statin therapy. (A) All-cause survival; (B) multiple myeloma–specific survival.
Table 2.
Association Between Statin Use and All-Cause and Multiple Myeloma–Specific Mortality
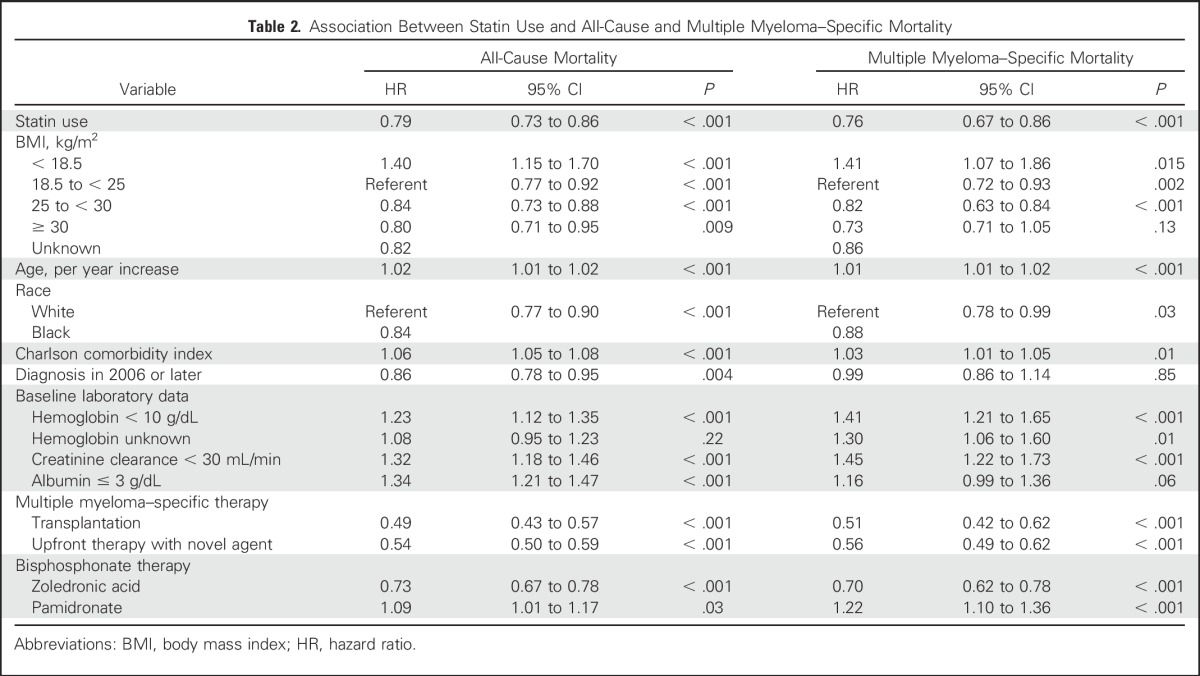
Patients with MM receiving statin therapy also had a significant reduction in MM-specific mortality (Fig 2B). Statin use was associated with a 24% reduction in the risk of MM-specific mortality (aHR, 0.76; 95% CI, 0.67 to 0.86; P < .001; Table 2). Older age, BMI < 18.5 kg/m2, higher comorbidity score, hemoglobin < 10 g/dL, eGFR < 30 mL/min/1.73 m2, and use of pamidronate were all associated with increased MM-specific mortality. First-line treatment with a novel agent, ASCT, treatment with zoledronic acid, black race, and BMI ≥ 25 kg/m2 were all associated with reduced MM-specific mortality.
Users of statin therapy had a 31% reduction in the risk of developing a skeletal-related event (aHR, 0.69; 95% CI, 0.59 to 0.80; P < .001). In this analysis, being a recipient of a stem-cell transplantation was associated with a decreased risk of skeletal-related events, whereas treatment with pamidronate was associated with an increased risk of skeletal-related events.
Sensitivity Analyses
When stratifying our results on the basis of race, statin use remained associated with a significant reduction in all-cause mortality in black (aHR, 0.73; 95% CI, 0.62 to 0.86; P < .001) as well as white (aHR, 0.83; 95% CI, 0.76 to 0.92; P < .001) patients. Similarly, statin use was associated with a reduced risk of MM-specific mortality in black (aHR, 0.67; 95% CI, 0.51 to 0.86; P = .002) and white (aHR, 0.81; 95% CI, 0.70 to 0.95; P = .007) patients.
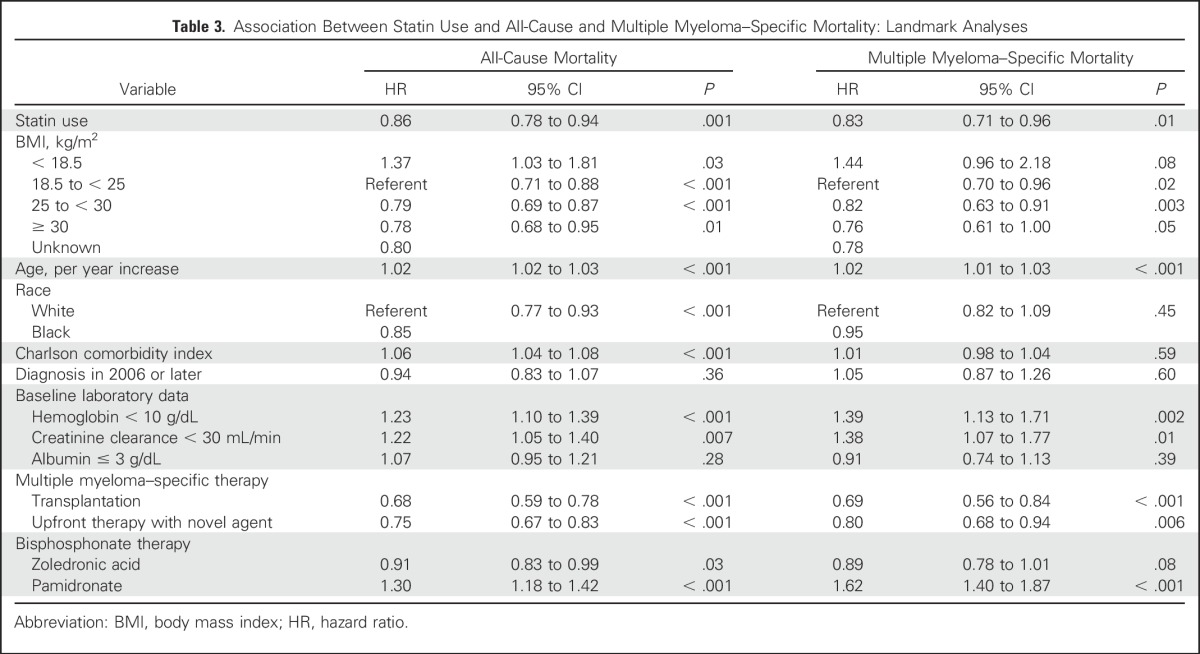
In the 12-month landmark analyses, statins remained associated with a significant reduction in risk of all-cause mortality (aHR, 0.86; 95% CI, 0.78 to 0.94; P = .001), as well as MM-specific mortality (aHR, 0.83; 95% CI, 0.71 to 0.96; P = .01; Table 3). In addition, patients prescribed statins for longer durations had greater reductions in all-cause mortality by 12% (95% CI, 0.80 to 0.96; P = .004), 16% (95% CI, 0.76 to 0.92; P < .001), and 18% (95% CI, 0.74 to 0.92; P = .003) for at least 3-, 6-, and 9-month use, respectively.
Table 3.
Association Between Statin Use and All-Cause and Multiple Myeloma–Specific Mortality: Landmark Analyses
Using DDD, patients with < 365 DDDs had a 20% reduction (aHR, 0.80; 95% CI, 0.73 to 0.89; P < .001), whereas patients with ≥ 365 DDDs of statin use had a 22% reduction in risk of all-cause mortality (aHR, 0.78; 95% CI, 0.70 to 0.87; P < .001; Appendix Table A4, online only). MM-specific mortality in patients with < 365 DDDs was reduced by 22% (aHR, 0.78; 95% CI, 0.68 to 0.91; P = .001), whereas patients with ≥ 365 DDDs of statin use had a 28% reduction in risk of death from MM (aHR, 0.72; 95% CI, 0.59 to 0.86; P < .001; Appendix Table A4, online only).
When further adjusting for baseline differences between statin users and nonusers with propensity score matching, statin use remained similarly associated with both a reduction in all-cause mortality (aHR, 0.78; 95% CI, 0.70 to 0.87; P < .001), as well as with MM-specific mortality (aHR, 0.79; 95% CI, 0.67 to 0.94; P = .007).
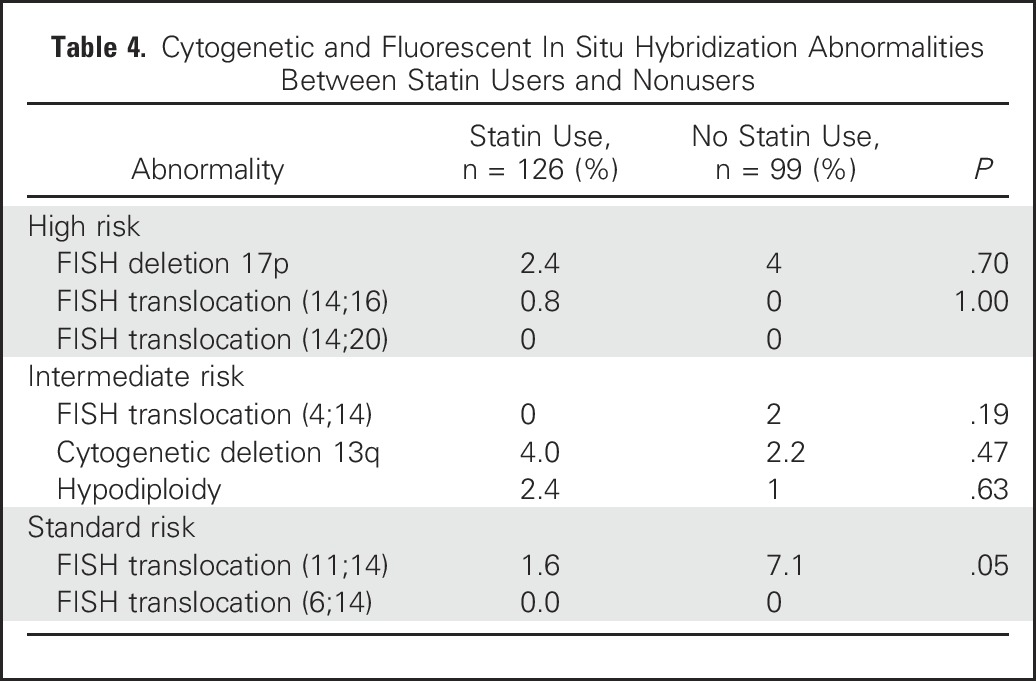
Cytogenetic and FISH data were not available for the majority of the cohort. A subgroup of 988 patients with results of either routine cytogenetic or FISH testing was identified. All 988 patients had cytogenetic testing results, whereas only 374 also had FISH testing results. There was no difference in the prevalence of high- or intermediate-risk results between statin users and nonusers (Table 4). There was a significant difference in the prevalence of translocation (11;14), a favorable FISH test result, with a higher prevalence noted in statin nonusers.
Table 4.
Cytogenetic and Fluorescent In Situ Hybridization Abnormalities Between Statin Users and Nonusers
DISCUSSION
In this multicenter, population-based cohort study of patients with newly diagnosed MM, statin use was associated with a 21% reduction in all-cause mortality and a 24% reduction in MM-specific mortality. These reductions were statistically significant across all analyses, with the reduction in mortality persisting after adjustment for immortal time bias and propensity score. In addition, statin use was associated with a decreased risk of developing skeletal-related events, even after adjusting for bisphosphonate use.
Statins directly inhibit HMG-CoA reductase, the rate-limiting enzyme of the mevalonate pathway.2 This enzyme is upstream of the mechanism of action of bisphosphonates on the same pathway. Bisphosphonates are associated with a reduction in the risk of mortality in MM.4,5 Our findings support an association between statin use and reduced risk of mortality even after adjusting for bisphosphonate use.
Interestingly, pamidronate (a nitrogen-containing bisphosphonate) was associated with increased risk of mortality in several analyses. One hypothesis to explain this observation is confounding by indication. In 1995, pamidronate was approved by the Food and Drug Administration for treatment of patients with MM with at least one lytic bone lesion. In 2002, the year zoledronic acid was approved for the same indication, ASCO released clinical treatment guidelines for bisphosphonates in MM.32 These guidelines reinforced the efficacy of bisphosphonates (either pamidronate or zoledronic acid) in patients with lytic bone disease in MM. However, they also suggested that use was reasonable in patients with MM with osteoporosis/osteopenia, expanding use of bisphosphonates. In 2007, the National Comprehensive Cancer Network released guidelines that recommended bisphosphonate use for any patient with active MM, regardless of lytic bone lesions.33 Thus, it is probable that patients who received pamidronate were more likely to have lytic bone disease at time of treatment compared with those treated with zoledronic acid. Lytic bone lesions have been associated with reduced survival in patients with MM because they are probably a sign of more advanced and/or aggressive disease.34
Three prior studies have investigated the role of statins in patients with MM.17,35,36 The first trial, containing 16 patients, found a decrease in treatment resistance with the addition of simvastatin to MM-directed therapy.36 The second study, a retrospective analysis, assessed statin use in 146 patients with MM undergoing ASCT.35 In that study, there was a trend toward increased overall response rates among patients taking a statin; however, there was no change in progression-free or overall survival. Last, a trial of 91 patients receiving thalidomide and dexamethasone with or without lovastatin found improved progression-free survival with lovastatin and a trend toward improved overall survival.17 Although these prior studies suggest a possible benefit of statins in MM, they are limited by small sample sizes. In addition, MM-specific mortality was not assessed.
Our population-based study has notable strengths and limitations. The VHA is the largest integrated health care system in the United States with facilities in all 50 states and Puerto Rico providing care to a diverse population of patients. Given the comprehensive benefits provided by the VHA, records of survival status are well maintained and accurate.23 Prior work has shown the accuracy of VHA vital status files for survival status, with > 97% of death events accurately captured in the database.22,23 Misclassification of statin use was possible as a result of patient noncompliance; however, the 3-month lag, and the duration-of-use analysis requiring multiple statin prescriptions, make misclassification of less concern. Additionally, misclassification of statin use, if present, would be expected to bias our results toward a null finding.
The robust data within the Veterans Administration Central Cancer Registry allowed for the adjustment of known prognostic factors associated with survival in MM, including baseline comorbidities, year of diagnosis, baseline laboratory data, MM-specific therapy, and transplantation status. Although we used propensity score analyses in an attempt to adjust for unmeasured confounding, it is plausible that there were residual, unmeasured differences between the two groups, resulting in confounding. Cytogenetic and FISH analyses were unavailable for the majority of the cohort. However, an analysis of the group of patients with cytogenetic information found no significant difference in the presence of high- or intermediate-risk cytogenetics between statin users and nonusers.
In conclusion, in this cohort study of United States veterans with MM, statin therapy was associated with a reduced risk of both all-cause and MM-specific mortality. Our findings suggest a potential role for statin therapy in patients with MM. The putative benefit of statin therapy in MM should be corroborated in a prospective study.
Appendix
Fig A1.
Strengthening the reporting of observational studies in epidemiology (STROBE) diagram.
Table A1.
Pharmacy Benefits Management: List of Variables Abstracted
Table A2.
Listed Causes of Death: Multiple Myeloma–Specific Mortality Cohort
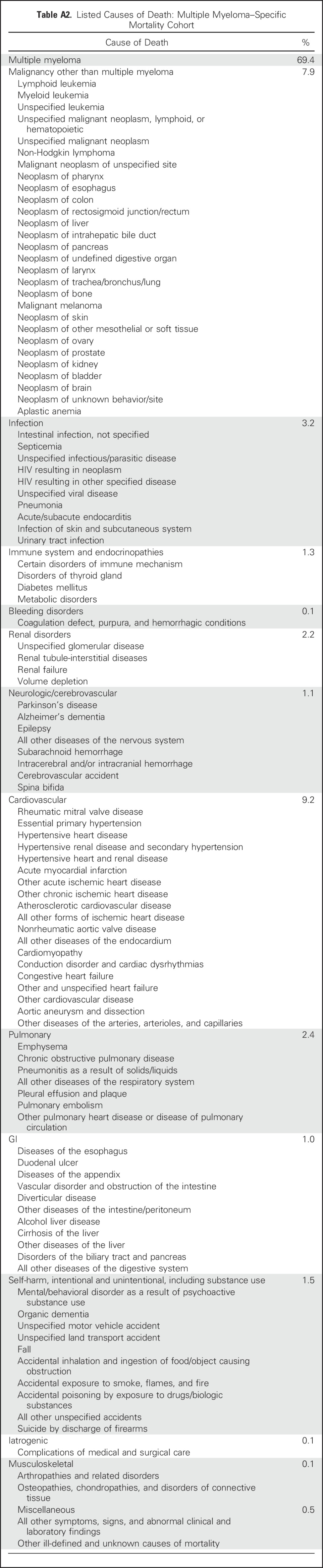
Table A3.
Demographic Data and Clinical Characteristics Stratified by Statin Use Among US Veterans Diagnosed with Multiple Myeloma Between 1999 and 2009: Multiple Myeloma–Specific Mortality Cohort
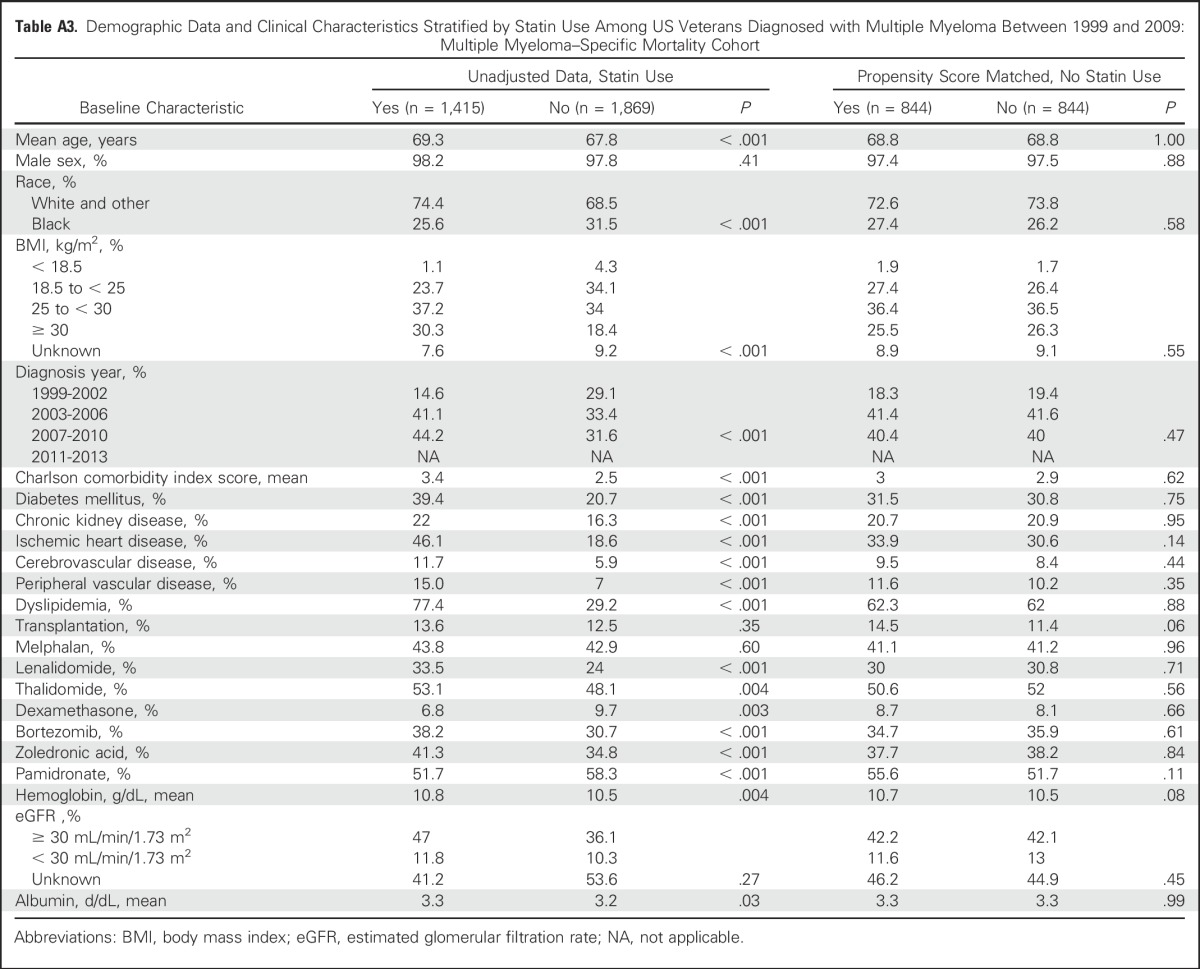
Table A4.
Association Between Statin Use and All-Cause and Multiple Myeloma–Specific Mortality Adjusted by Daily Defined Dose
Footnotes
Supported by National Institutes of Health, National Heart, Lung, and Blood Institute Grants No. 5K12HL087107 and U54 HL112303; the Barnes Jewish Foundation; and the American Cancer Society Institutional Research Grant No. 58-010-52.
Presented at the 57th Annual American Society of Hematology Annual Meeting and Exposition, Orlando, FL, December 5-8, 2015.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Kristen Marie Sanfilippo, Jesse Keller, Brian F. Gage, Gerard Moskowitz, Kenneth R. Carson
Administrative Support: Katiuscia O’Brian
Collection and assembly of data: Kristen Marie Sanfilippo, Suhong Luo, Jason Gumbel, Brandon Blue, Katiuscia O’Brian
Data analysis and interpretation: Kristen Marie Sanfilippo, Jesse Keller, Brian F. Gage, Suhong Luo, Tzu-Fei Wang, Kenneth R. Carson
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Statins Are Associated With Reduced Mortality in Multiple Myeloma
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Kristen Marie Sanfilippo
Speakers’ Bureau: Amgen
Jesse Keller
No relationship to disclose
Brian F. Gage
No relationship to disclose
Suhong Luo
No relationship to disclose
Tzu-Fei Wang
No relationship to disclose
Gerard Moskowitz
No relationship to disclose
Jason Gumbel
No relationship to disclose
Brandon Blue
No relationship to disclose
Katiuscia O’Brian
No relationship to disclose
Kenneth R. Carson
Consulting or Advisory Role: Celgene, Seattle Genetics
Research Funding: Millennium, Kyowa Hakko Kirin, Allos Therapeutics, Celgene, Bristol-Myers Squibb, Seattle Genetics
Expert Testimony: Pharmacia
Travel, Accommodations, Expenses: Flatiron Health
REFERENCES
- 1.Kutner JS, Blatchford PJ, Taylor DH, Jr, et al. Safety and benefit of discontinuing statin therapy in the setting of advanced, life-limiting illness: A randomized clinical trial. JAMA Intern Med. 2015;175:691–700. doi: 10.1001/jamainternmed.2015.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stancu C, Sima A. Statins: Mechanism of action and effects. J Cell Mol Med. 2001;5:378–387. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Russell RG, Xia Z, Dunford JE, et al. Bisphosphonates: An update on mechanisms of action and how these relate to clinical efficacy. Ann N Y Acad Sci. 2007;1117:209–257. doi: 10.1196/annals.1402.089. [DOI] [PubMed] [Google Scholar]
- 4.Morgan GJ, Davies FE, Gregory WM, et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): A randomised controlled trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanfilippo KM, Gage B, Luo S, et al. Comparative effectiveness on survival of zoledronic acid versus pamidronate in multiple myeloma. Leuk Lymphoma. 2015;56:615–621. doi: 10.3109/10428194.2014.924117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shipman CM, Rogers MJ, Apperley JF, et al. Bisphosphonates induce apoptosis in human myeloma cell lines: A novel anti-tumour activity. Br J Haematol. 1997;98:665–672. doi: 10.1046/j.1365-2141.1997.2713086.x. [DOI] [PubMed] [Google Scholar]
- 7.Guenther A, Gordon S, Tiemann M, et al. The bisphosphonate zoledronic acid has antimyeloma activity in vivo by inhibition of protein prenylation. Int J Cancer. 2010;126:239–246. doi: 10.1002/ijc.24758. [DOI] [PubMed] [Google Scholar]
- 8.Jánosi J, Sebestyén A, Bocsi J, et al. Mevastatin-induced apoptosis and growth suppression in U266 myeloma cells. Anticancer Res. 2004;24(3a):1817–1822. [PubMed] [Google Scholar]
- 9.Otsuki T, Sakaguchi H, Hatayama T, et al. Effects of an HMG-CoA reductase inhibitor, simvastatin, on human myeloma cells. Oncol Rep. 2004;11:1053–1058. [PubMed] [Google Scholar]
- 10.van de Donk NW, Kamphuis MM, van Kessel B, et al. Inhibition of protein geranylgeranylation induces apoptosis in myeloma plasma cells by reducing Mcl-1 protein levels. Blood. 2003;102:3354–3362. doi: 10.1182/blood-2003-03-0970. [DOI] [PubMed] [Google Scholar]
- 11.Dai Y, Khanna P, Chen S, et al. Statins synergistically potentiate 7-hydroxystaurosporine (UCN-01) lethality in human leukemia and myeloma cells by disrupting Ras farnesylation and activation. Blood. 2007;109:4415–4423. doi: 10.1182/blood-2006-09-047076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidmaier R, Baumann P, Simsek M, et al. The HMG-CoA reductase inhibitor simvastatin overcomes cell adhesion-mediated drug resistance in multiple myeloma by geranylgeranylation of Rho protein and activation of Rho kinase. Blood. 2004;104:1825–1832. doi: 10.1182/blood-2003-12-4218. [DOI] [PubMed] [Google Scholar]
- 13.Park HJ, Kong D, Iruela-Arispe L, et al. 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors interfere with angiogenesis by inhibiting the geranylgeranylation of RhoA. Circ Res. 2002;91:143–150. doi: 10.1161/01.res.0000028149.15986.4c. [DOI] [PubMed] [Google Scholar]
- 14.Wong B, Lumma WC, Smith AM, et al. Statins suppress THP-1 cell migration and secretion of matrix metalloproteinase 9 by inhibiting geranylgeranylation. J Leukoc Biol. 2001;69:959–962. [PubMed] [Google Scholar]
- 15.Kort WJ, Hülsmann WC, Stehman TE. Modulation of metastatic ability by inhibition of cholesterol synthesis. Clin Exp Metastasis. 1989;7:517–523. doi: 10.1007/BF01753812. [DOI] [PubMed] [Google Scholar]
- 16.Mundy G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 17.Hus M, Grzasko N, Szostek M, et al. Thalidomide, dexamethasone and lovastatin with autologous stem cell transplantation as a salvage immunomodulatory therapy in patients with relapsed and refractory multiple myeloma. Ann Hematol. 2011;90:1161–1166. doi: 10.1007/s00277-011-1276-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: Differing perspectives. J Clin Epidemiol. 1993;46:1075–1079, discussion 1081-1090. doi: 10.1016/0895-4356(93)90103-8. [DOI] [PubMed] [Google Scholar]
- 19.Velde NV, Wu EQ, Guo A, et al. The benefits of timely intervention with zoledronic acid in patients with metastatic prostate cancer to bones: A retrospective study of the US Veterans Affairs population. Prostate Cancer Prostatic Dis. 2011;14:79–84. doi: 10.1038/pcan.2010.49. [DOI] [PubMed] [Google Scholar]
- 20.Krupski TL, Foley KA, Baser O, et al. Health care cost associated with prostate cancer, androgen deprivation therapy and bone complications. J Urol. 2007;178:1423–1428. doi: 10.1016/j.juro.2007.05.135. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savas LS, del Junco DJ, Bastian LA, et al. Mortality ascertainment of women veterans: A comparison of sources of vital status information, 1979-2002. Med Care. 2009;47:125–128. doi: 10.1097/MLR.0b013e3181809125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sohn MW, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, et al. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 25.Mantel N, Byar DP. Evaluation of response-time data involving transient states: An illustration using heart-transplant data. J Am Stat Assoc. 1974;69:81–86. [Google Scholar]
- 26.Rothman KJ. Induction and latent periods. Am J Epidemiol. 1981;114:253–259. doi: 10.1093/oxfordjournals.aje.a113189. [DOI] [PubMed] [Google Scholar]
- 27.Chubak J, Boudreau DM, Wirtz HS, et al. Threats to validity of nonrandomized studies of postdiagnosis exposures on cancer recurrence and survival. J Natl Cancer Inst. 2013;105:1456–1462. doi: 10.1093/jnci/djt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO Collaborating Centre for Drug Statistics Methodology (WHOCC) DDD Definition and general considerations. http://www.whocc.no/ddd/definition_and_general_considera/
- 29.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 30.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31:2963–2969. doi: 10.1200/JCO.2013.49.5283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berenson JR, Hillner BE, Kyle RA, et al. American Society of Clinical Oncology clinical practice guidelines: The role of bisphosphonates in multiple myeloma. J Clin Oncol. 2002;20:3719–3736. doi: 10.1200/JCO.2002.06.037. [DOI] [PubMed] [Google Scholar]
- 33.Terpos E, Morgan G, Dimopoulos MA, et al. International Myeloma Working Group recommendations for the treatment of multiple myeloma-related bone disease. J Clin Oncol. 2013;31:2347–2357. doi: 10.1200/JCO.2012.47.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saad F, Lipton A, Cook R, et al. Pathologic fractures correlate with reduced survival in patients with malignant bone disease. Cancer. 2007;110:1860–1867. doi: 10.1002/cncr.22991. [DOI] [PubMed] [Google Scholar]
- 35.Hamadani M, Hade E, Benson DM, Jr, et al. The effect of statin use at the time of autologous transplant on response and survival in multiple myeloma. Biol Blood Marrow Transplant. 2008;14:351–352. doi: 10.1016/j.bbmt.2007.12.489. [DOI] [PubMed] [Google Scholar]
- 36.Schmidmaier R, Baumann P, Bumeder I, et al. First clinical experience with simvastatin to overcome drug resistance in refractory multiple myeloma. Eur J Haematol. 2007;79:240–243. doi: 10.1111/j.1600-0609.2007.00902.x. [DOI] [PubMed] [Google Scholar]