Abstract
Purpose
Under-representation of elderly, women, and racial/ethnic minority patients with cancer in clinical trials is of national concern. The goal of this study was to characterize enrollment trends and disparities by age, sex, and race/ethnicity in lung cancer trials.
Methods
We analyzed data for 23,006 National Cancer Institute cooperative group lung cancer trial participants and 578,476 patients with lung cancer from the SEER registry from 1990 to 2012. The enrollment disparity difference (EDD) and enrollment disparity ratio (EDR) were calculated on the basis of the proportion of each subgroup in the trial population and the US lung cancer population. Annual percentage changes (APCs) in the subgroup proportions in each population were compared over time.
Results
Enrollment disparity for patients ≥ 70 years of age with non–small-cell lung cancer improved from 1990 to 2012 (test of parallelism, P = .020), with a remaining EDD of 0.22 (95% CI, 0.19 to 0.25) and EDR of 1.65 (95% CI, 1.51 to 1.82) in 2010 to 2012. No improvement was seen for elderly patients with small-cell lung cancer (SCLC), with an APC of 0.20 (P = .714) among trial participants, despite a rising proportion of elderly patients with SCLC in the US population (APC, 0.32; P = .020). Enrollment disparity for women with lung cancer improved overall, with the gap closing by 2012 (EDD, 0.03 [95% CI, 0.00 to 0.06]; EDR, 1.07 [95% CI, 1.00 to 1.16]). Enrollment disparities persisted without significant improvement for elderly women, blacks, Asians/Pacific Islanders, and Hispanics.
Conclusion
Under-representation in lung cancer trials improved significantly from 1990 to 2012 for elderly patients with non–small-cell lung cancer and for women, but ongoing efforts to improve the enrollment of elderly patients with SCLC and minorities are needed. Our study highlights the importance of addressing enrollment disparities by demographic and disease subgroups to better target under-represented groups of patients with lung cancer.
INTRODUCTION
Lung cancer is the most common cause of cancer death in the United States, accounting for > 26% of all cancer deaths.1 It is a disease of the elderly, with a median age at diagnosis of 70 years.2 Despite the growing population of older adults in the United States and worldwide, many cancer treatments are studied primarily in younger, fit patients, with results extrapolated to older adults.3,4 In addition, women and racial/ethnic minorities are under-represented in clinical trials, prompting the National Institutes of Health (NIH) Revitalization Act of 1993, which mandated the inclusion of women and minorities in all NIH-funded research. To enhance the generalizability of lung cancer trial results, diverse patient representation is necessary.5
Enrollment disparities in clinical trials have been recognized for many years.6 For example, the Southwest Oncology Group (SWOG) reported under-representation of elderly patients with lung cancer in SWOG trials from 1993 to 1996; the proportion of patients ≥ 65 years of age in the trial was 39%, whereas the proportion in the US lung cancer population was 66%.7 Similar findings were described for women and blacks. Older adults, women, and racial/ethnic minorities were also less likely to participate in National Cancer Institute (NCI)–sponsored trials from 2000 to 2002.8 However, these older studies examined trends over relatively short time periods with fewer clinical trials and do not reflect recent trends.6-8 In addition, prior studies did not investigate differences by lung cancer subtype or extent of disease.
To characterize clinical trial enrollment disparities in lung cancer over time, we conducted analyses of enrollment disparities in NCI-sponsored cooperative group lung cancer trials from 1990 to 2012 compared with the US lung cancer population, which is captured by SEER registry data. We determined whether enrollment disparities existed for age, sex, and race/ethnicity during each year by tumor and treatment subgroups; we also assessed for temporal changes.
METHODS
Data Sources
We identified 210 lung cancer treatment trials in adults from 1990 to 2012 conducted by NCI-sponsored cooperative groups (American College of Surgeons Oncology Group, Cancer and Leukemia Group B, Eastern Cooperative Oncology Group [ECOG], North Central Cancer Treatment Group, Radiation Therapy Oncology Group, and SWOG). Treatment trials were defined as clinical trials investigating a lung cancer therapy with the goal of demonstrating clinical benefit and safety. Trials activated between January 1, 1990, and December 31, 2010 (with accruals through December 2012), were included in this analysis. We excluded trials with unpublished results or immature data by December 2014, those with difficult-to-extract data, and those with accruals of < 15% of target or with < 35 participants, resulting in a final analysis cohort of 131 trials that enrolled 17,485 patients with non–small-cell lung cancer (NSCLC) and 5,521 patients with small-cell lung cancer (SCLC; Data Supplement). Contingency tables of included and excluded trials by study phase and time period are given in the Data Supplement. The proportions of enrolled elderly patients, women, racial/ethnic minorities; extent of disease; and lung cancer type were computed. Elderly patients were defined as those ≥ 70 years of age on the basis of the median age at diagnosis for lung cancer (Data Supplement). Cancer incidence data of the same time period were estimated from the SEER registry9; see Data Supplement for details.
Statistical Analysis
We examined trends by demographic and lung cancer subgroups. To analyze trends of both SEER registry and trial enrollment, we calculated the annual percentage of change (APC) and performed the test of parallelism using the method of joinpoint regression analysis (Joinpoint version 4.2.0.2, June 2015).10 Joinpoint regression fits a piecewise linear regression model, which is a special case of linear spline. We modeled the relationship between years and the logarithmic proportion with zero change point on the basis of our model selection procedure; see Data Supplement. The APC for each trend was estimated, and P values for the test of zero APC were calculated. The study period of 1990 to 2012 was divided into 4-year intervals, with the exception of the last interval, which covered 3 years.
For each subgroup of interest, we calculated (1) the enrollment disparity difference (EDD), the absolute difference between the estimated subgroup proportion among the US lung cancer population and the subgroup proportion among trial participants, and (2) the enrollment disparity ratio (EDR), the estimated subgroup proportion among the US lung cancer population divided by the subgroup proportion among trial participants. The EDD and the EDR allowed us to measure enrollment disparity in absolute and in relative terms. Two-sided 95% CIs of these two measures were calculated using a nonparametric percentile bootstrapping procedure with 10,000 resamples.
The proportions of patients in each lung cancer subgroup enrolled in trials versus those in the SEER registry were compared over time using the test of parallelism. A permutation distribution of the test statistic under the null hypothesis was used to obtain the P value10; a significant P value indicates that the two slopes differ. A sensitivity analysis using age ≥ 65 years to define elderly was also performed. Two-sided tests with a P value < .05 were considered statistically significant. The P values reported were not adjusted for multiple testing. SAS (version 9.4; SAS Institute, Cary, NC) and R (version 3.2.2) software were used for data management and analyses. See Data Supplement for additional details of statistical methods.
RESULTS
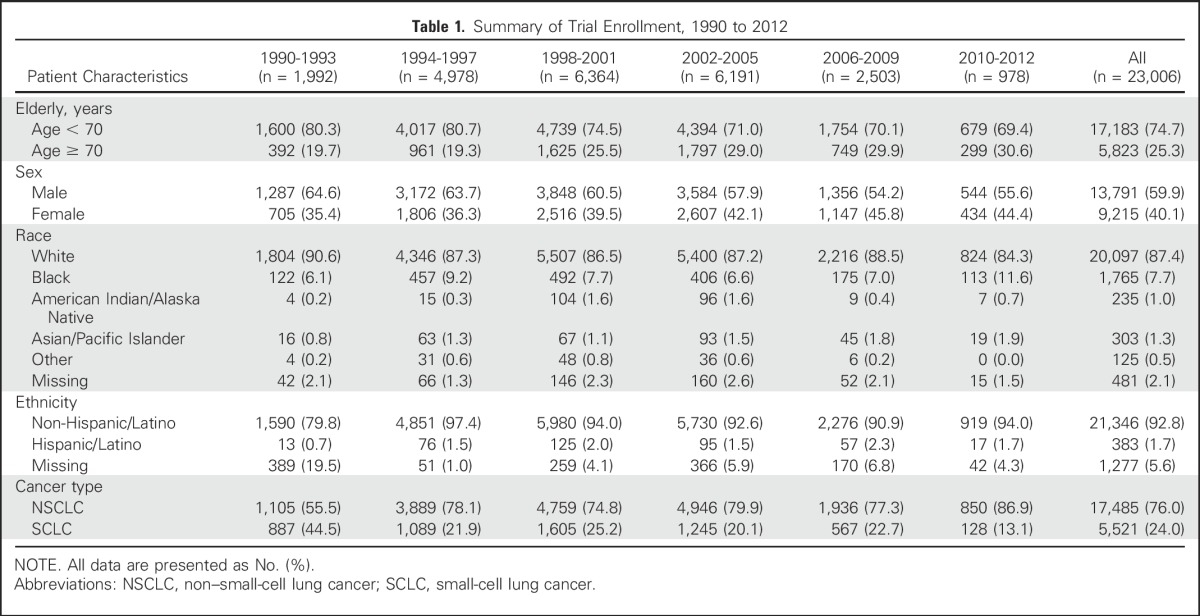
Our study population included 23,006 patients with lung cancer enrolled in NCI cancer cooperative group treatment trials from 1990 to 2012 (Table 1) and 578,476 patients with lung cancer enrolled in the SEER registry (Data Supplement). Table 1 and the Data Supplement list patient demographics by year group.
Table 1.
Summary of Trial Enrollment, 1990 to 2012
Elderly and Women Patients
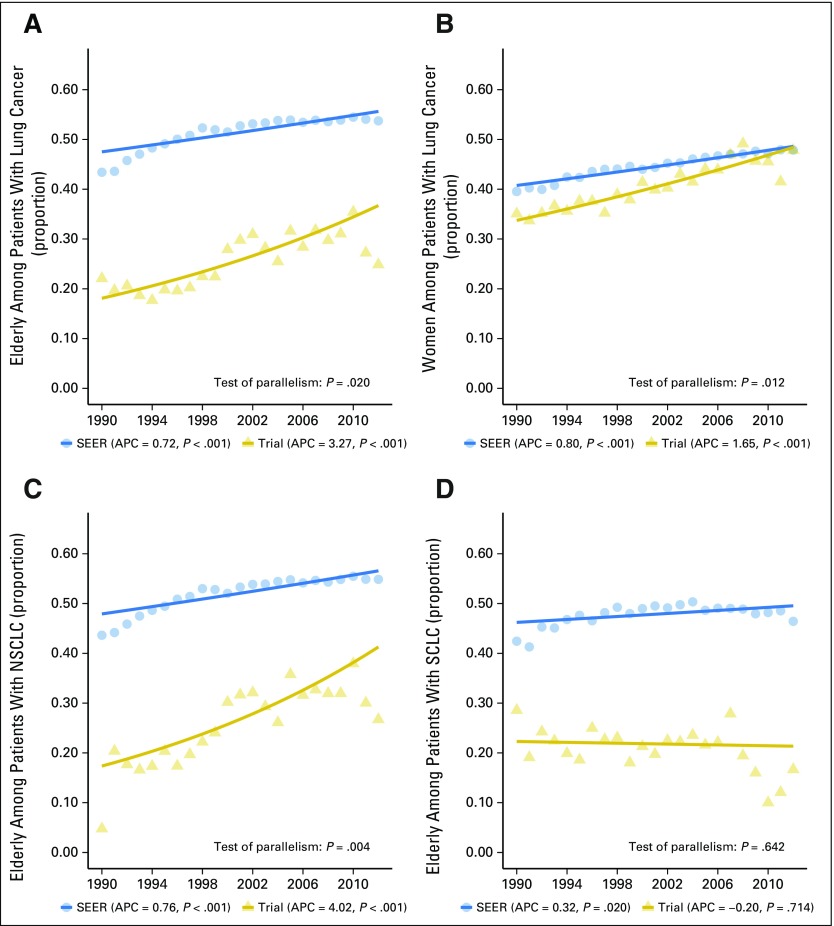
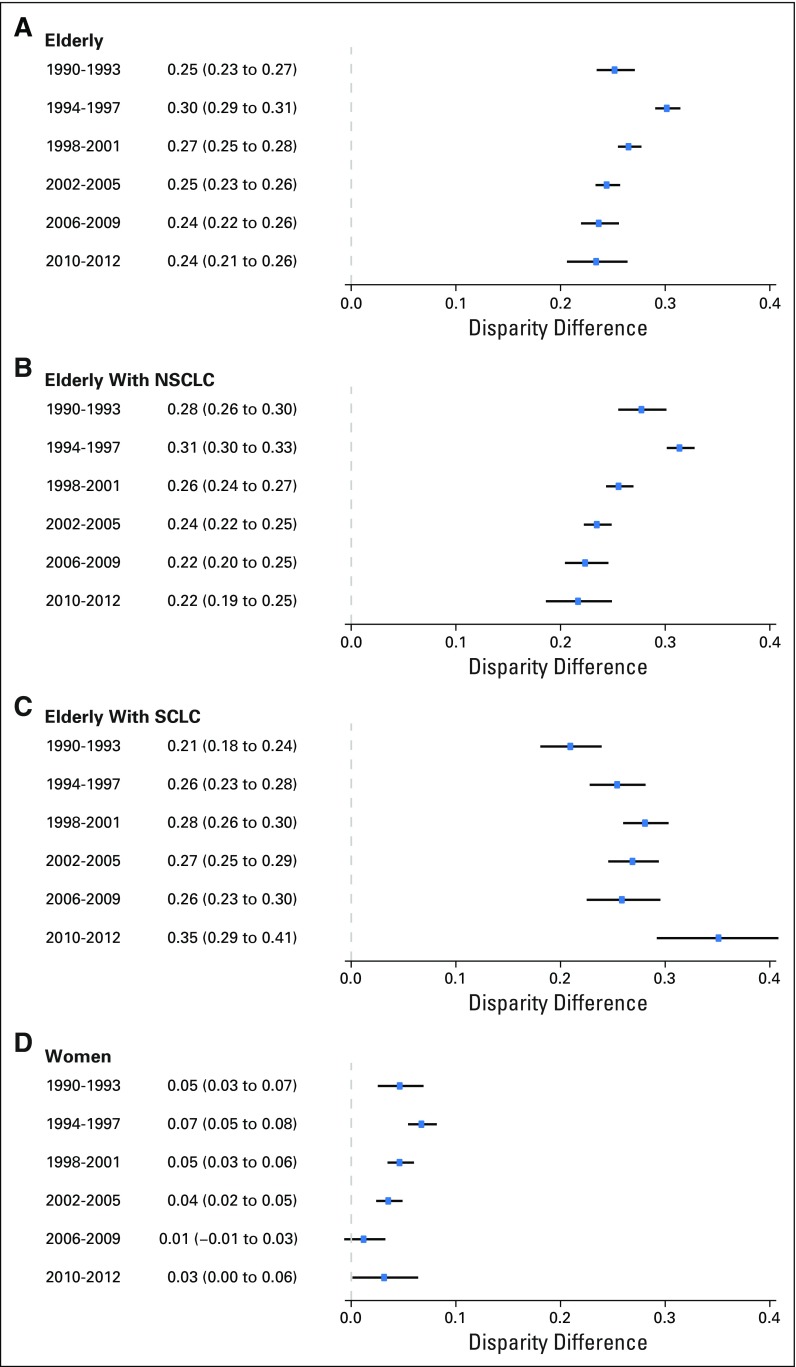
The gaps between the proportions of elderly and women patients with lung cancer among trial participants versus in the US population in Figure 1 demonstrate an under-representation of these subgroups of patients with lung cancer from 1990 to 2012 (Figs 1A and 1B). During the study period, the proportions of elderly patients with lung cancer in the United States and among trial participants both increased steadily. The APCs from 1990 to 2012 were 3.27 (95% CI, 2.21 to 4.34; P < .001) for trial participants and 0.72 (95% CI, 0.51 to 0.93; P < .001) for the US population. The enrollment disparity for elderly patients improved over the study period (test of parallelism, P = .020; Fig 1A) but the EDD remained 0.24 in the period from 2010 to 2012 (95% CI, 0.21 to 0.26; Fig 2A) and the EDR, 1.77 (95% CI, 1.62 to 1.95; Data Supplement). Sensitivity analysis using age ≥ 65 years showed similar results (data not shown).
Fig 1.
Proportion of elderly and women among patients with lung cancer: trial participants and US population, 1990 to 2012. (A) Elderly among patients with lung cancer. (B) Women among patients with lung cancer. (C) Elderly among patients with non–small-cell lung cancer (NSCLC). (D) Elderly among patients with small-cell lung cancer (SCLC). The annual percentage change (APC) P value corresponds to testing whether the APC is different from 0. The solid lines represent the fitted values of the joinpoint regression.
Fig 2.
Enrollment disparity difference with 95% CIs, 1990 to 2012. (A) Elderly among patients with lung cancer. (B) Elderly among patients with non–small-cell lung cancer (NSCLC). (C) Elderly among patients with small-cell lung cancer (SCLC). (D) Women among patients with lung cancer. The last year group consists of only 3 years because the study period was from 1990 to 2012. The definition of enrollment disparity difference is the absolute difference between the estimated subgroup proportion among the US lung cancer population and the subgroup proportion among trial participants. The solid lines are the bootstrapped 95% CIs.
Under-representation of women with lung cancer in trials improved as well, with the enrollment gap closed by the end of the study period in 2010 to 2012 (EDD, 0.03 [95% CI, 0.00 to 0.06]; Fig 2D; EDR, 1.07 [95% CI, 1.00 to 1.16]; Data Supplement). The APCs were 1.65 (95% CI, 1.31 to 2.00), P < .001, for trial participants and 0.80 (95% CI, 0.71 to 0.90), P < .001, for the US population. The enrollment disparity for women was larger among elderly women compared with nonelderly women (Data Supplement) for both the earlier (1990 to 2001) and later (2002 to 2012) time periods (Data Supplement).
Racial/Ethnic Minority Patients
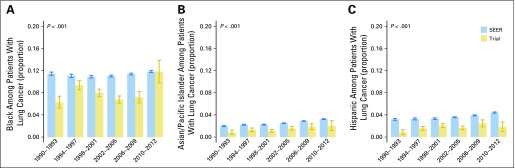
The proportions of black, Asian/Pacific Islander, and Hispanic patients with lung cancer among trial participants and in the US population are illustrated in Figure 3. There was a consistent under-representation of blacks enrolled in trials for all year groups, with the exception of 2010 to 2012, where the 95% CI is wide because of the smaller sample size for the 3-year interval. Enrollment disparity among blacks persisted during study period; for example, in 2006 to 2009 (EDD, 0.04 [95% CI, 0.03 to 0.05]; EDR, 1.58 [95% CI, 1.38 to 1.85]; see Data Supplement). Similar to the enrollment disparity among blacks, the disparity for Asian/Pacific Islander and Hispanic patients also persisted over the study period (Data Supplement). Because of the small sample size, there was no clear trend for American Indian/Alaska Native patients (Data Supplement).
Fig 3.
Proportion of black, Asian/Pacific Islander, and Hispanic among patients with lung cancer: trial participants and the US population, 1990 to 2012. (A) Black. (B) Asian/Pacific Islander. (C) Hispanic. The last year group consists of only 3 years because the study period was from 1990 to 2012. The solid lines are the bootstrapped 95% CIs. The P value corresponds to the test for equality of proportions between SEER and the trial for 1990 to 2012.
Elderly Patients by Lung Cancer Type
For elderly patients with NSCLC, a trend similar to that of the overall elderly population was observed. Both trends increased, with APCs of 4.02 (95% CI, 2.64 to 5.41), P < .001, for trial participants and 0.76 (95% CI, 0.55 to 0.97), P < .001, for the US population (Fig 1C). The enrollment disparity gap decreased over the study period (Figs 1C and 1D; test of parallelism P = .004). However, the EDD and EDR for elderly patients with NSCLC in trials were still high in the period from 2010 to 2012, with an absolute difference of 0.22 (95% CI, 0.19 to 0.25; Fig 2B) and a ratio of 1.65 (95% CI, 1.51 to 1.82; Data Supplement). The results were consistent for both of the major histologic subtypes of NSCLC, adenocarcinoma and squamous cell carcinoma (Data Supplement). For elderly patients with SCLC, there was no change in the proportion of trial participants over time (APC, −0.20 [95% CI, −1.29 to 0.91]; P = .714) despite an increase in the proportion of elderly patients with SCLC in the US population (APC, 0.32 [95% CI, 0.06 to 0.58]; P = .020). The EDD and EDR for elderly patients with SCLC in trials in 2010 to 2012 were high, at 0.35 (95% CI, 0.29 to 0.41) and 3.82 (95% CI, 2.59 to 6.88), respectively (Fig 2C and Data Supplement).
Elderly and Women Patients by Extent of Disease
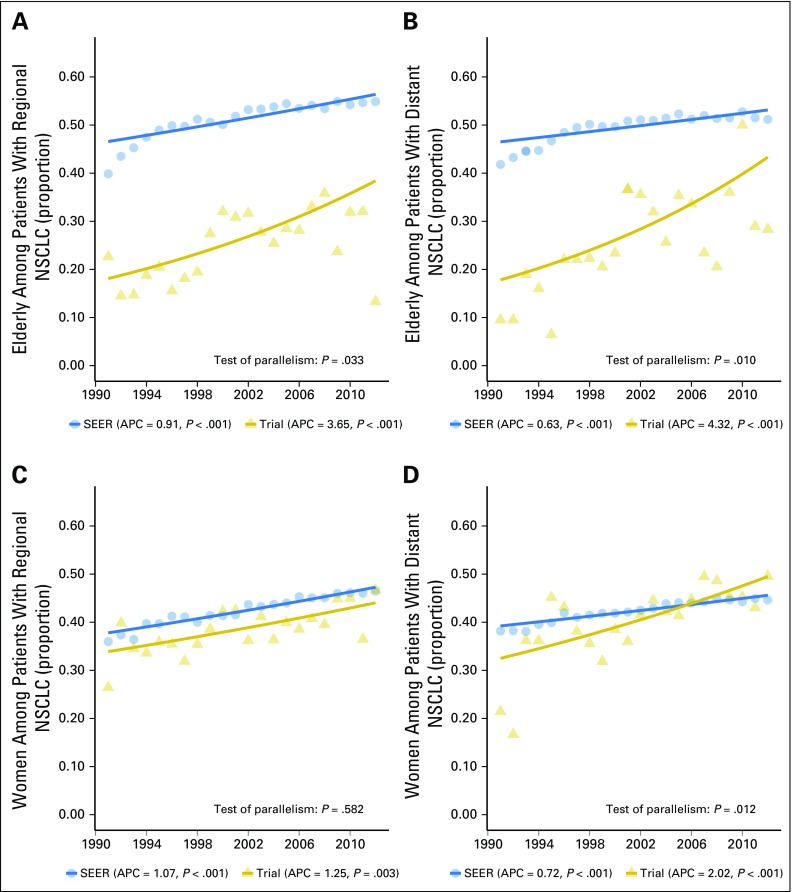
We further subdivided patients with NSCLC into those with regional or distant disease. The trends for elderly patients with regional or distant NSCLC disease were similar to those of the overall elderly NSCLC subgroup (Figs 4A and 4B), with APCs of 0.91 (95% CI, 0.66 to 1.17), P < .001, for regional disease and 0.63 (95% CI, 0.40 to 0.88), P < .001, for distant disease in the US population during 1990 to 2012. The APCs were higher for trial participants, at 3.65 (95% CI, 1.83 to 5.50), P < .001, for regional disease and 4.32 (95% CI, 1.99 to 6.70), P = .001, for distant disease. The decreasing enrollment disparity gap among elderly patients with NSCLC with regional (P = .033) and distant disease (P = .010) was supported by the test of parallelism.
Fig 4.
Proportion of elderly and women patients: trial participants and US population, 1990 to 2012. (A) Elderly among patients with regional non–small-cell lung cancer (NSCLC). (B) Elderly among patients with distant NSCLC. (C) Women among patients with regional NSCLC. (D) Women among patients with distant NSCLC. The annual percentage change (APC) P value corresponds to testing whether the APC is different from 0. The solid lines represent the fitted values of the joinpoint regression. The year 1990 was excluded from analysis because of the small number of trial participants with regional or distant NSCLC.
Representation of women with regional NSCLC disease showed significant increases, with APCs of 1.25 (95% CI, 0.48 to 2.03), P = .001, for trial participants and 1.07 (95% CI, 0.92 to 1.22), P < .001, for the US population (Figs 4C and 4D). However, the enrollment disparity gap for women with regional NSCLC disease remained stable over the study period (test of parallelism P = .582). The representation of women with distant NSCLC disease also showed significant increases, with APCs of 2.02 (95% CI, 1.19 to 2.87), P < .001, for trial participants and 0.72 (95% CI, 0.60 to 0.84), P < .001, for the US population. The trend lines suggested that the proportion of women with distant NSCLC enrolled in trials overtook the US population in the latter years (test of parallelism P = .012).
Targeted Agents
Trials with targeted agents began enrolling patients in 2001. Twenty-one targeted therapy trials and 38 nontargeted therapy trials between 2001 and 2012 were investigated. The proportion of elderly patients enrolled in targeted therapy trials of 29.4% (95% CI, 28.0 to 31.1) was not significantly different from the 24.6% (95% CI, 28.4 to 30.5) for trials of nontargeted therapy (P = .98). A higher proportion of women was observed in erlotinib trials, at 55.6%, versus 43.4% of nonerlotinib trials conducted during the same time period (P < .001).
DISCUSSION
The lung cancer clinical trial enrollment disparity from 1990 to 2012 differed by subgroups, with the gap closing for women overall, women with distant NSCLC, elderly patients overall, and elderly patients with regional and distant NSCLC. Conversely, elderly patients with SCLC, elderly women, and black, Asian/Pacific Islander, and Hispanic patients showed minimal improvement.
The increase in the number of elderly patients enrolled in cancer trials in the latter half of the study period can be attributed partly to six trials designed specifically for older patients. However, these elderly-focused trials do not fully explain the increase in trial participation by older adults, because the trends remained significant even when these six trials were removed in a sensitivity analysis. When cancer type was examined, there was no improvement in the proportion of elderly patients with SCLC enrolled in trials despite an increase in the proportion of elderly patients with SCLC in the US population. SCLC studies during our study period were mostly smaller, phase II trials, which may be more susceptible to local enrollment patterns. In addition, the development of better tolerated therapies for SCLC has been slower than for NSCLC, which may limit the enrollment of older patients with SCLC who may have a higher risk of treatment toxicity.
The trial enrollment disparity for women with lung cancer lessened steadily and finally closed by 2012. These findings suggest a beneficial effect of the NIH Revitalization Act of 1993 that mandated the inclusion of women and minorities in all NIH-funded research. The improvement was driven largely by the increased enrollment of women with distant NSCLC. This can be explained partially by a higher proportion of women in erlotinib versus nonerlotinib trials conducted during the same time period. However, the trends for representation of women with regional NSCLC in trials and in the US population have remained parallel to each other, suggesting a persistent enrollment disparity. Older women also remained under-represented compared with younger female patients in the entire study period. Similar to a prior study, older men with lung cancer were more likely to enroll than were older women.8
Unlike the improvements seen in the enrollment disparity for elderly patients and women overall, there are no clear trends of improvement for racial/ethnic disparities in lung cancer clinical trials. Between 1990 and 2009, a significant enrollment disparity in lung cancer trials existed among black patients, Hispanics, and Asians/Pacific Islanders. When targeted trials were examined, the enrollment disparity for Asians was smaller in erlotinib trials compared with nonerlotinib trials, which would be expected because EGFR mutations are more common among Asian patients. However, this small improvement had little effect on the enrollment disparity for Asians overall. The lack of improvement in the enrollment disparities for these racial/ethnic minority groups highlights the critical need to better understand the barriers to trial awareness, opportunities to participate, and acceptance of enrollment.11,12 Proposed strategies to increase minority participation in cancer trials include targeting providers and patients by using diverse cancer care settings including community practices, culturally competent patient navigation, policy changes, and partnering with community members throughout the clinical trial process, from before protocol development, through participant recruitment, and completion of the study.13-18 The NCI Community Oncology Research Program aims to address this challenge by linking established research bases to community sites. It has supportive policies and practices to encourage the enrollment of minority and underserved patients and better physician attitudes toward trial participation.19 Other potential processes include the use of electronic medical records and the development of clinical trial screening logs that can assist in the identification of institution-specific accrual patterns.20,21
To our knowledge, this study provides the first comprehensive trend analysis of enrollment disparities in lung cancer treatment trials spanning more than two decades sponsored by the NCI-cooperative groups. Our clinical trial data represent approximately 84% of participants with lung cancer in NCI cooperative group treatment trials that were activated between 1990 and 2010, making our estimates of trial enrollment characteristics robust and representative. We also incorporated lung cancer subtypes and extent of disease in our analysis, which, to our knowledge, have not been investigated previously. The EDD and the EDR calculated in reference to the US lung cancer population eliminate the influence of the changing demographics of the lung cancer population, which offers an advantage over simply reporting the absolute number or percentage of patients. In addition, our enrollment disparity measures were based on the direct yearly comparison of trial patient enrollment with the incidence distribution of US patients with lung cancer derived from SEER and US Census data.7,8 This direct comparison allowed us to adjust for the impact of the changing proportion of lung cancer subgroups in the US population over time. Furthermore, our individual patient-level data for each trial, rather than relying on summary statistics only, allowed us to examine trends in enrollment disparities for important subgroups such as elderly women and elderly patients with SCLC.
Despite the novelty and significance of our findings, our study has several limitations. Our analysis was restricted to NCI-cooperative group clinical trials. Other lung cancer clinical trials, such as NCI-funded noncooperative group trials, trials sponsored by nonprofit organizations, and industry-sponsored trials, were not included. However, the NCI is the largest single sponsor of cancer clinical trials and it values broad patient representation and the conducting of trials at numerous community and academic cancer centers. It is unlikely that non-NCI sponsored trials would have different enrollment characteristics, because both the physician and patient populations are similar. A second limitation is that the small sample size for some minority racial groups such as American Indians/Alaskan Natives limited our power to detect enrollment disparities. Third, our incidence proportion estimate of the US population relied on SEER registry data, which include only a sample of all lung cancer cases in the United States. However, these data have been used widely to estimate the incidence of different types of cancer.22
Lessons may be learned from the design and study strategies used for lung cancer trials that can help address enrollment disparities in trials of other cancer types. Lower enrollment disparity in trials can improve the generalizability of the clinical trial results and provide valid subgroups for analyses.13 Examining specific subgroups can also deepen our understanding of age-, sex-, and race/ethnicity–based differences in prognosis and response to therapy.8,23,24 Past research on enrollment disparities has highlighted barriers that are specific to older adults.25,26 Conducting more elderly-specific or related trials can be a remedy, as we have seen in our primary data analysis. These trials can be enhanced by the use of comprehensive geriatric assessment, which can help identify fit older adults who can tolerate clinical trial participation and cancer treatment.27 Our study also highlights the need to investigate more refined subgroups, such as cancer subtypes and extent of disease, to better understand enrollment disparities and allow policy makers to prioritize their goals. In the era of personalized and precision medicine, not only must we identify subgroups that would benefit from therapies the most, but our study designs and research policies should also target appropriate subgroups of individuals to yield more effective outcomes and continue to close the enrollment disparity gap.
In conclusion, patient under-representation in lung cancer trials improved significantly between 1990 and 2012 for older adults and for women overall, with the enrollment disparity largely being eliminated for women with distant NSCLC and decreased by 20% for elderly patients with NSCLC. However, other important enrollment disparities, especially for older patients with SCLC, elderly women, and racial/ethnic minorities, continue to persist and require ongoing work to eliminate under-representation in lung cancer treatment trials.
ACKNOWLEDGMENT
We thank Suzanne Dahlberg (ECOG-ACRIN), James Dignam (NRG Oncology), Stephen L. George (Duke), Robert J. Gray (ECOG-ACRIN), Lin Gu, MS (Duke), Yating Gu (Duke), Michael LeBlanc (Southwest Oncology Group), Jeffrey P. Meyer, BS (Mayo Clinic), James Moon, MS (Southwest Oncology Group), and Rebecca Paulus, BS (NRG Oncology).
Footnotes
Supported by Grant No. R21-AG042894 from the National Institutes of Health National Institute on Aging and the Health and Medical Research Fund of Hong Kong, and by the National Institute on Aging (Grant No.T32-AG000212 to M.L.W.).
The sponsors had no role in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; the preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Authors’ disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
AUTHOR CONTRIBUTIONS
Conception and design: Herbert H. Pang, Xiaofei Wang, Thomas E. Stinchcombe, Melisa L. Wong, Apar Kishor Ganti, Daniel J. Sargent, Richard L. Schilsky, Harvey J. Cohen, Everett E. Vokes
Collection and assembly of data: Herbert H. Pang, Xiaofei Wang, Ying Zhang, Chen Hu, Mary W. Redman, Judith B. Manola, Jeffrey D. Bradley, Alex A. Adjei, David Gandara, Suresh S. Ramalingam, Everett E. Vokes
Data analysis and interpretation: Herbert H. Pang, Xiaofei Wang, Thomas E. Stinchcombe, Melisa L. Wong, Perry Cheng, Apar Kishor Ganti, Daniel J. Sargent, Ying Zhang, Chen Hu, Sumithra J. Mandrekar, Richard L. Schilsky, Harvey J. Cohen, Everett E. Vokes
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Enrollment Trends and Disparity Among Patients With Lung Cancer in National Clinical Trials, 1990 to 2012
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Herbert H. Pang
No relationship to disclose
Xiaofei Wang
No relationship to disclose
Thomas E. Stinchcombe
Consulting or Advisory Role: Eli Lilly, Helsinn Therapeutics (US), Bristol-Myers Squibb, Boehringer Ingelheim, Abbvie
Research Funding: Genentech, EMD Serono, Bristol-Myers Squibb, Abbvie
Melisa L. Wong
No relationship to disclose
Perry Cheng
No relationship to disclose
Apar Kishor Ganti
Consulting or Advisory Role: Otsuka, Boehringer Ingelheim, Biodesix, Pfizer
Research Funding: Pfizer (Inst), Amgen (Inst), NewLink Genetics (Inst), ARIAD Pharmaceuticals (Inst), Astex Pharmaceuticals (Inst), Bristol-Myers Squibb (Inst), Merck Serono (Inst), Merck (Inst), Janssen Biotech (Inst)
Daniel J. Sargent
Consulting or Advisory Role: Abbvie, Acerta Pharma, ARIAD Pharmaceuticals, Astellas Pharma, AstraZeneca/MedImmune, Biothera, Celldex, Exelixis, Genentech, Incyte, Kyowa Hakko Kirin, Medivation, Merck, Merrimack, Nektar, Novartis, Pharmacyclics, Pique, Spiration, XBiotech
Research Funding: Celgene (Inst), Genentech (Inst)
Travel, Accommodations, Expenses: Celgene
Ying Zhang
No relationship to disclose
Chen Hu
No relationship to disclose
Sumithra J. Mandrekar
No relationship to disclose
Mary W. Redman
No relationship to disclose
Judith B. Manola
No relationship to disclose
Richard L. Schilsky
No relationship to disclose
Harvey J. Cohen
No relationship to disclose
Jeffrey D. Bradley
Honoraria: ViewRay
Consulting or Advisory Role: ViewRay (Inst), Varian Medical Systems
Research Funding: Mevion Medical Systems (Inst), ViewRay (Inst)
Travel, Accommodations, Expenses: Mevion Medical Systems
Alex A. Adjei
No relationship to disclose
David Gandara
Consulting or Advisory Role: ARIAD Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, Celgene, Clovis Oncology, Genentech, Guardant Health, Eli Lilly, Merck, Mirati Therapeutics, Novartis, Pfizer
Research Funding: AstraZeneca/MedImmune (Inst), Bristol-Myers Squibb (Inst), Clovis Oncology (Inst), Genentech (Inst), Johnson & Johnson (Inst), Eli Lilly (Inst), Merck (Inst), Novartis (Inst)
Suresh S. Ramalingam
Honoraria: Abbvie, Amgen, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Genentech, Eli Lilly, Merck, Novartis
Everett E. Vokes
Stock or Other Ownership: McKesson
Consulting or Advisory Role: Eli Lilly
REFERENCES
- 1. National Cancer Institute: SEER cancer statistics review, 1975-2012. http://seer.cancer.gov/csr/1975_2012/
- 2. National Cancer Institute: SEER stat fact sheets: Lung and bronchus cancer. http://seer.cancer.gov/statfacts/html/lungb.html.
- 3.Hurria A, Naylor M, Cohen HJ. Improving the quality of cancer care in an aging population: Recommendations from an IOM report. JAMA. 2013;310:1795–1796. doi: 10.1001/jama.2013.280416. [DOI] [PubMed] [Google Scholar]
- 4.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 5.Oh SS, Galanter J, Thakur N, et al. Diversity in clinical and biomedical research: A promise yet to be fulfilled. PLoS Med. 2015;12:e1001918. doi: 10.1371/journal.pmed.1001918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis JH, Kilgore ML, Goldman DP, et al. Participation of patients 65 years of age or older in cancer clinical trials. J Clin Oncol. 2003;21:1383–1389. doi: 10.1200/JCO.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 8.Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: Race-, sex-, and age-based disparities. JAMA. 2004;291:2720–2726. doi: 10.1001/jama.291.22.2720. [DOI] [PubMed] [Google Scholar]
- 9. SEER research data record description: Cases diagnoses in 1973-2012. http://seer.cancer.gov/data/seerstat/nov2014/TextData.FileDescription.pdf.
- 10.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Ford JG, Howerton MW, Lai GY, et al. Barriers to recruiting underrepresented populations to cancer clinical trials: A systematic review. Cancer. 2008;112:228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 12. doi: 10.1002/cncr.28574. Durant RW, Wenzel JA, Scarinci IC, et al: Perspectives on barriers and facilitators to minority recruitment for clinical trials among cancer center leaders, investigators, research staff, and referring clinicians: Enhancing minority participation in clinical trials (EMPaCT). Cancer 120:1097-1105, 2014 (suppl 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. doi: 10.1002/cncr.28575. Chen MS Jr, Lara PN, Dang JH, et al: Twenty years post-NIH Revitalization Act: Enhancing Minority Participation in Clinical Trials (EMPaCT): Laying the groundwork for improving minority clinical trial accrual: Renewing the case for enhancing minority participation in cancer clinical trials. Cancer 120:1091-1096, 2014 (suppl 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heller C, Balls-Berry JE, Nery JD, et al. Strategies addressing barriers to clinical trial enrollment of underrepresented populations: A systematic review. Contemp Clin Trials. 2014;39:169–182. doi: 10.1016/j.cct.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. doi: 10.1002/cncr.28570. Ghebre RG, Jones LA, Wenzel JA, et al: State-of-the-science of patient navigation as a strategy for enhancing minority clinical trial accrual. Cancer 120:1122-1130, 2014 (suppl 7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks SE, Muller CY, Robinson W, et al. Increasing minority enrollment onto clinical trials: Practical strategies and challenges emerge from the NRG Oncology Accrual Workshop. J Oncol Pract. 2015;11:486–490. doi: 10.1200/JOP.2015.005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Salman A, Nguyen C, Lee YH, Cooksey-James T. A review of barriers to minorities' participation in cancer clinical trials: Implications for future cancer research. J Immigr Minor Health 18:447-453, 2016. [DOI] [PubMed]
- 18.Fouad MN, Acemgil A, Bae S, et al. Patient navigation as a model to increase participation of African Americans in cancer clinical trials. J Oncol Pract. 2016;12:556–563. doi: 10.1200/JOP.2015.008946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs SR, Weiner BJ, Reeve BB, et al. Organizational and physician factors associated with patient enrollment in cancer clinical trials. Clin Trials. 2014;11:565–575. doi: 10.1177/1740774514536000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko NY, Fu JL, Lane SC, et al. Cancer clinical trial enrollment of diverse and underserved patients within an urban safety net hospital. J Community Support Oncol. 2015;13:429–435. doi: 10.12788/jcso.0181. [DOI] [PubMed] [Google Scholar]
- 21.St Germain D, Denicoff AM, Dimond EP, et al. Use of the National Cancer Institute Community Cancer Centers Program screening and accrual log to address cancer clinical trial accrual. J Oncol Pract. 2014;10:e73–e80. doi: 10.1200/JOP.2013.001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Welch HG, Gorski DH, Albertsen PC. Trends in metastatic breast and prostate cancer: Lessons in cancer dynamics. N Engl J Med. 2015;373:1685–1687. doi: 10.1056/NEJMp1510443. [DOI] [PubMed] [Google Scholar]
- 23.Dignam JJ, Ye Y, Colangelo L, et al. Prognosis after rectal cancer in blacks and whites participating in adjuvant therapy randomized trials. J Clin Oncol. 2003;21:413–420. doi: 10.1200/JCO.2003.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Johnson RH, Chien FL, Bleyer A. Incidence of breast cancer with distant involvement among women in the United States, 1976 to 2009. JAMA. 2013;309:800–805. doi: 10.1001/jama.2013.776. [DOI] [PubMed] [Google Scholar]
- 25.Townsley CA, Selby R, Siu LL. Systematic review of barriers to the recruitment of older patients with cancer onto clinical trials. J Clin Oncol. 2005;23:3112–3124. doi: 10.1200/JCO.2005.00.141. [DOI] [PubMed] [Google Scholar]
- 26.Mills EJ, Seely D, Rachlis B, et al. Barriers to participation in clinical trials of cancer: A meta-analysis and systematic review of patient-reported factors. Lancet Oncol. 2006;7:141–148. doi: 10.1016/S1470-2045(06)70576-9. [DOI] [PubMed] [Google Scholar]
- 27.Hurria A, Cirrincione CT, Muss HB, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. J Clin Oncol. 2011;29:1290–1296. doi: 10.1200/JCO.2010.30.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]